Development of Nanotechnological Approaches to Improving the Antimalarial Potential of Natural Substances
Abstract
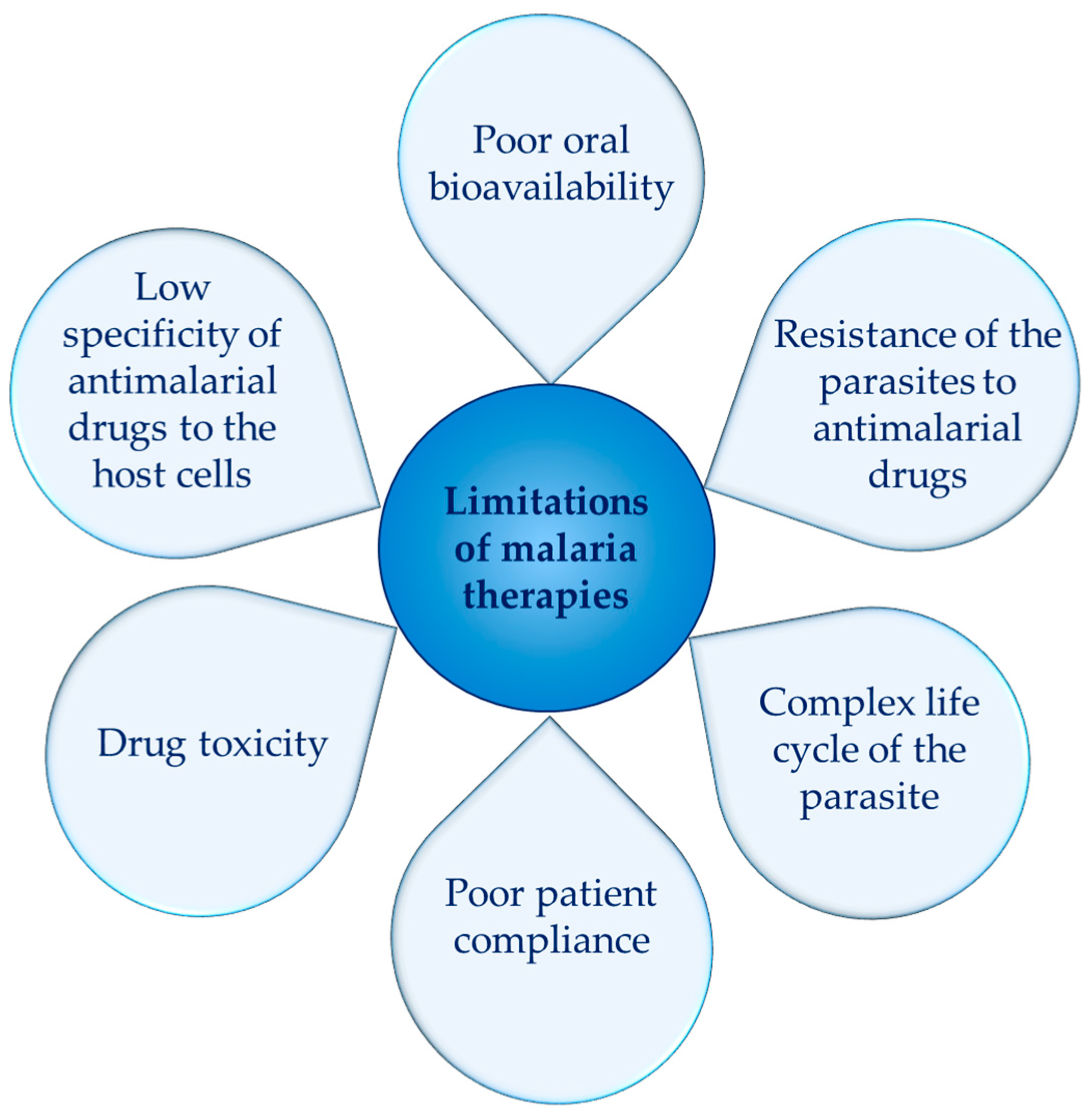
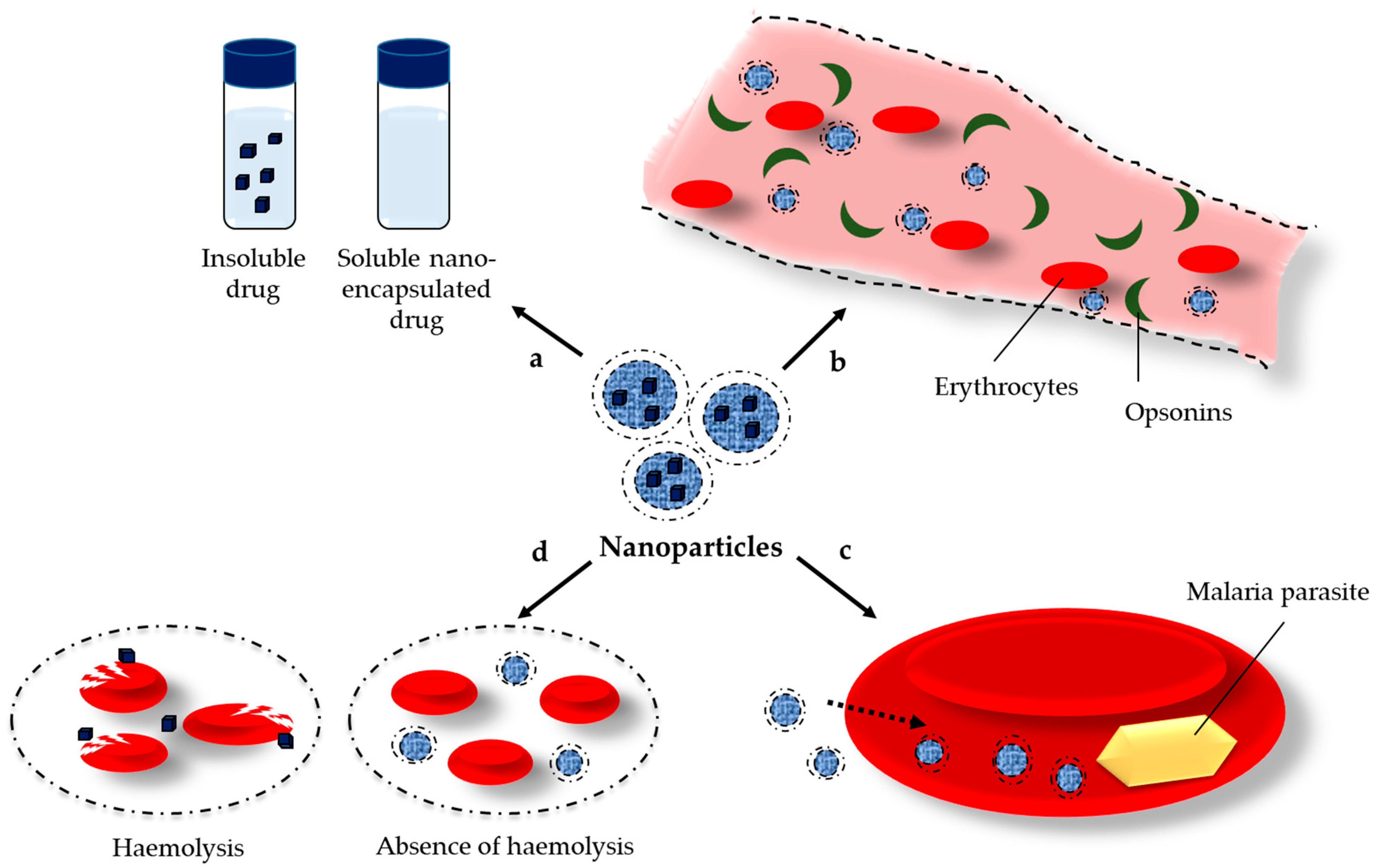
1. Introduction
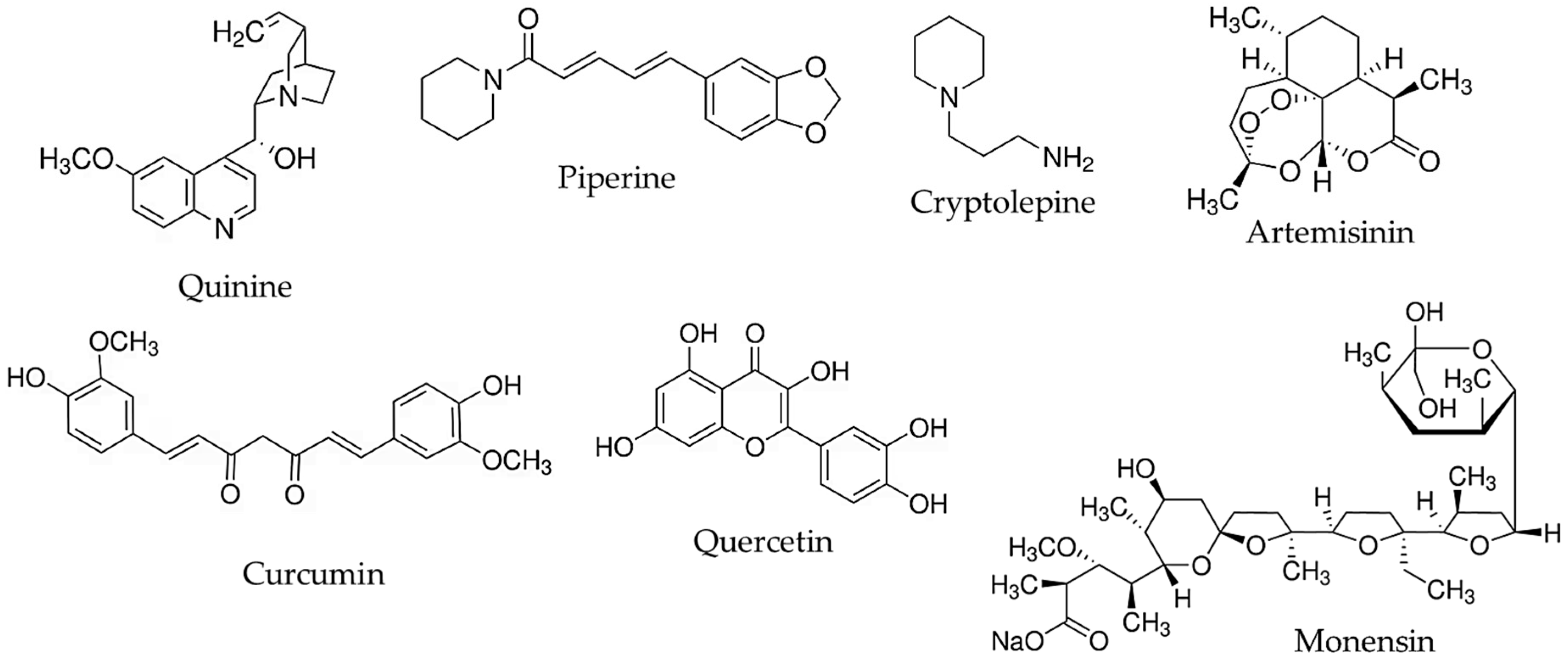
2. Alkaloids
2.1. Quinine
2.2. Piperine
2.3. Cryptolepine
3. Sesquiterpene Lactones
4. Curcuminoids
5. Flavonoids
5.1. Quercetin
5.2. Luteolin
6. Antibiotics
7. Metal Nanoparticles Obtained by Green Synthesis from Plant Extracts
7.1. Silver Nanoparticles (AgNP)
7.2. Gold Nanoparticles (AuNP)
7.3. Zinc Oxide Nanoparticles (ZnONP)
| Nanoparticle Type | Source | Achievement | Reference |
|---|---|---|---|
| AgNP | Azadirachta indica (Meliaceae) | Stronger antiplasmodial effect of AgNP compared to the plant extracts or amylase alone | [76] |
| Saraca asoca (Fabaceae) | |||
| Purified Alpha Amylase | |||
| AgNP | Indigofera oblongifolia (Fabaceae) | Suppression of the parasitemia in P. chabaudi-infected mice to approximately 98% | [66] |
| Downregulation of the expression of IL-1b, IL-10 and TNF-α | |||
| AgNP | Sargassum tenerrimum (Sargassaceae) | Antiplasmodial activity against P. falciparum and P. berghei | [68] |
| Significant reduction in the parasitemia in P. berghei-infected mice without adverse toxic effects | |||
| AgNP | Crataegus ambigua (Rosaceae) | Equal inhibition of P. falciparum (NF54 strain) with that of chloroquine | [69] |
| Strong antibacterial effect against seven bacterial strains | |||
| AgNP | Murraya koenigii (Rutaceae) | Antiplasmodial activity on chloroquine-sensitive P. falciparum (3D7) | [77] |
| AgNP | Azadirachta indica (Meliaceae) | Antiplasmodial activity against P. falciparum (3D7 and RKL9 strains) | [78] |
| AgNP | Euphorbia cotinifolia (Euphorbiaceae) | ROS production and disruption of the redox equilibrium of parasite Apoptosis of P. falciparum-infected erythrocytes | [79] |
| AgNP | Syzygium jambos (Myrtaceae) | Stronger in vitro antiplasmodial effect of AgNP compared to AuNP | [72] |
| AuNP | |||
| AuNP | Coccinia grandis (Cucurbitaceae) | Suppression of parasite by 88.75% | [73] |
| ZnONP | Lagenaria siceraria (Cucurbitaceae) | Antimalarial activity comparable to chloroquine | [74] |
| ZnONP | Rhazya stricta (Apocynaceae) | Antiplasmodial effect (IC50 of 3.41 μg/mL) | [75] |
| Minimum hemolytic activity |
8. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abumsimir, B.; Al-Qaisi, T.S. The next Generation of Malaria Treatments: The Great Expectations. Future Sci. OA 2023, 9, FSO834. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Malaria Report: Addressing Inequity in the Global Malaria Response; World Health Organization: Geneva, Switzerland, 2024; Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2024 (accessed on 22 September 2025).
- Avitabile, E.; Senes, N.; D’Avino, C.; Tsamesidis, I.; Pinna, A.; Medici, S.; Pantaleo, A. The Potential Antimalarial Efficacy of Hemocompatible Silver Nanoparticles from Artemisia Species Against P. falciparum Parasite. PLoS ONE 2020, 15, e0238532. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.P.; Tiyaboonchai, W.; Patankar, S.; Madhusudhan, B.; Souto, E.B. Curcuminoids-Loaded Lipid Nanoparticles: Novel Approach Towards Malaria Treatment. Colloids Surf. B Biointerfaces 2010, 81, 263–273. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Paralikar, P.; Gupta, I.; Medici, S.; Santos, C.A. Recent Advances in Use of Silver Nanoparticles as Antimalarial Agents. Int. J. Pharm. 2017, 526, 254–270. [Google Scholar] [CrossRef] [PubMed]
- Valissery, P.; Thapa, R.; Singh, J.; Gaur, D.; Bhattacharya, J.; Singh, A.P.; Dhar, S.K. Potent In Vivo Antimalarial Activity of Water-Soluble Artemisinin Nano-Preparations. RSC Adv. 2020, 10, 36201–36211. [Google Scholar] [CrossRef]
- Oliveira, A.B.; Dolabela, M.F.; Braga, F.C.; Jácome, R.L.R.P.; Varotti, F.P.; Póvoa, M.M. Plant-Derived Antimalarial Agents: New Leads and Efficient Phythomedicines. Part I. Alkaloids. An. Acad. Bras. Ciênc. 2009, 81, 715–740. [Google Scholar] [CrossRef]
- Uzor, P.F. Alkaloids from Plants with Antimalarial Activity: A Review of Recent Studies. Evid. Based Complement. Alternat. Med. 2020, 2020, 8749083. [Google Scholar] [CrossRef]
- Haas, S.E.; Bettoni, C.C.; de Oliveira, L.K.; Guterres, S.S.; Dalla Costa, T. Nanoencapsulation Increases Quinine Antimalarial Efficacy Against Plasmodium berghei In Vivo. Int. J. Antimicrob. Agents 2009, 34, 156–161. [Google Scholar] [CrossRef]
- Michels, L.R.; Maciel, T.R.; Nakama, K.A.; Teixeira, F.E.G.; de Carvalho, F.B.; Gundel, A.; de Araujo, B.V.; Haas, S.E. Effects of Surface Characteristics of Polymeric Nanocapsules on the Pharmacokinetics and Efficacy of Antimalarial Quinine. Int. J. Nanomed. 2019, 14, 10165–10178. [Google Scholar] [CrossRef]
- Gomes, G.S.; Maciel, T.R.; Piegas, E.M.; Michels, L.R.; Colomé, L.M.; Freddo, R.J.; de Ávila, D.S.; Gundel, A.; Haas, S.E. Optimization of Curcuma Oil/Quinine-Loaded Nanocapsules for Malaria Treatment. AAPS PharmSciTech 2018, 19, 551–564. [Google Scholar] [CrossRef]
- Amolegbe, S.A.; Hirano, Y.; Adebayo, J.O.; Ademowo, O.G.; Balogun, E.A.; Obaleye, J.A.; Krettli, A.U.; Yu, C.; Hayami, S. Mesoporous Silica Nanocarriers Encapsulated Antimalarials with High Therapeutic Performance. Sci. Rep. 2018, 8, 3078. [Google Scholar] [CrossRef] [PubMed]
- Izaguirry, A.P.; Pavin, N.F.; Soares, M.B.; Spiazzi, C.C.; Araújo, F.A.; Michels, L.R.; Leivas, F.G.; Brum, D.d.S.; Haas, S.E.; Santos, F.W. Effect of Quinine-Loaded Polysorbate-Coated Nanocapsules on Male and Female Reproductive Systems of Rats. Toxicol. Res. 2016, 5, 1561–1572. [Google Scholar] [CrossRef][Green Version]
- Nur Aisyah, A.; Cariri, P.H.R.; Kondorura, A.; Oktafiana, I.; Ramba, O.F.; Husain, M.P.R.; Arifin, A.A.; Megawati; Nur, S.; Lukman. Development of a Curcumin-Piperine Nanoparticle System Using Dissolving Microneedles for Transdermal Drug Delivery in Malaria Treatment: In Vitro Evaluation. Int. J. Pharm. 2025, 671, 125258. [Google Scholar] [CrossRef]
- Kuntworbe, N.; Al-Kassas, R. Design and In Vitro Haemolytic Evaluation of Cryptolepine Hydrochloride-Loaded Gelatine Nanoparticles as a Novel Approach for the Treatment of Malaria. AAPS PharmSciTech 2012, 13, 568–581. [Google Scholar] [CrossRef]
- Ibrahim, N.; Ibrahim, H.; Sabater, A.M.; Mazier, D.; Valentin, A.; Nepveu, F. Artemisinin Nanoformulation Suitable for Intravenous Injection: Preparation, Characterization and Antimalarial Activities. Int. J. Pharm. 2015, 495, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Isacchi, B.; Bergonzi, M.C.; Grazioso, M.; Righeschi, C.; Pietretti, A.; Severini, C.; Bilia, A.R. Artemisinin and Artemisinin plus Curcumin Liposomal Formulations: Enhanced Antimalarial Efficacy against Plasmodium berghei-Infected Mice. Eur. J. Pharm. Biopharm. 2012, 80, 528–534. [Google Scholar] [CrossRef]
- Gérard Yaméogo, J.B.; Mazet, R.; Wouessidjewe, D.; Choisnard, L.; Godin-Ribuot, D.; Putaux, J.-L.; Semdé, R.; Gèze, A. Pharmacokinetic Study of Intravenously Administered Artemisinin-Loaded Surface-Decorated Amphiphilic γ-Cyclodextrin Nanoparticles. Mater. Sci. Eng. C 2020, 106, 110281. [Google Scholar] [CrossRef]
- Memvanga, P.B.; Coco, R.; Préat, V. An Oral Malaria Therapy: Curcumin-Loaded Lipid-Based Drug Delivery Systems Combined with β-Arteether. J. Control. Release 2013, 172, 904–913. [Google Scholar] [CrossRef]
- Rashidzadeh, H.; Salimi, M.; Sadighian, S.; Rostamizadeh, K.; Ramazani, A. In Vivo Antiplasmodial Activity of Curcumin-Loaded Nanostructured Lipid Carriers. Curr. Drug Deliv. 2019, 16, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Martí Coma-Cros, E.; Biosca, A.; Lantero, E.; Manca, M.L.; Caddeo, C.; Gutiérrez, L.; Ramírez, M.; Borgheti-Cardoso, L.N.; Manconi, M.; Fernàndez-Busquets, X. Antimalarial Activity of Orally Administered Curcumin Incorporated in Eudragit®-Containing Liposomes. Int. J. Mol. Sci. 2018, 19, 1361. [Google Scholar] [CrossRef]
- Busari, Z.A.; Dauda, K.A.; Morenikeji, O.A.; Afolayan, F.; Oyeyemi, O.T.; Meena, J.; Sahu, D.; Panda, A.K. Antiplasmodial Activity and Toxicological Assessment of Curcumin PLGA-Encapsulated Nanoparticles. Front. Pharmacol. 2017, 8, 622. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, F.; Rizvi, M.M.A.; Kar, S.K. Oral Delivery of Curcumin Bound to Chitosan Nanoparticles Cured Plasmodium yoelii Infected Mice. Biotechnol. Adv. 2012, 30, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Velasques, K.; Maciel, T.R.; de Castro Dal Forno, A.H.; Teixeira, F.E.G.; da Fonseca, A.L.; Varotti, F.d.P.; Fajardo, A.R.; de Ávila, D.S.; Haas, S.E. Co-Nanoencapsulation of Antimalarial Drugs Increases Their In Vitro Efficacy Against Plasmodium falciparum and Decreases Their Toxicity to Caenorhabditis elegans. Eur. J. Pharm. Sci. 2018, 118, 1–12. [Google Scholar] [CrossRef]
- Oyeyemi, O.; Morenkeji, O.; Afolayan, F.; Dauda, K.; Busari, Z.; Meena, J.; Panda, A. Curcumin-Artesunate Based Polymeric Nanoparticle; Antiplasmodial and Toxicological Evaluation in Murine Model. Front. Pharmacol. 2018, 9, 562. [Google Scholar] [CrossRef]
- Hanif, H.; Abdollahi, V.; Javani Jouni, F.; Nikoukar, M.; Rahimi Esboei, B.; Shams, E.; Vazini, H. Quercetin Nano Phytosome: As a Novel Anti-Leishmania and Anti-Malarial Natural Product. J. Parasit. Dis. 2023, 47, 257–264. [Google Scholar] [CrossRef]
- Fulgheri, F.; Ramírez, M.; Román-Álamo, L.; Gasco, P.; Manconi, M.; Aroffu, M.; Rached, R.A.; Baroli, B.; Fernàndez-Busquets, X.; Manca, M.L. Preliminary Evaluation of the In Vitro and In Vivo Efficacy of a Novel Nanovesicle-Doped Nanoemulsion Co-Loading Artemisinin and Quercetin as a Promising Strategy to Improve the Oral Malaria Therapy. J. Drug Deliv. Sci. Technol. 2025, 107, 106828. [Google Scholar] [CrossRef]
- Rajendran, V.; Rohra, S.; Raza, M.; Hasan, G.M.; Dutt, S.; Ghosh, P.C. Stearylamine Liposomal Delivery of Monensin in Combination with Free Artemisinin Eliminates Blood Stages of Plasmodium falciparum in Culture and P. berghei Infection in Murine Malaria. Antimicrob. Agents Chemother. 2015, 60, 1304–1318. [Google Scholar] [CrossRef] [PubMed]
- Achan, J.; Talisuna, A.O.; Erhart, A.; Yeka, A.; Tibenderana, J.K.; Baliraine, F.N.; Rosenthal, P.J.; D’Alessandro, U. Quinine, an Old Anti-Malarial Drug in a Modern World: Role in the Treatment of Malaria. Malar. J. 2011, 10, 144. [Google Scholar] [CrossRef]
- White, N.J. Cardiotoxicity of Antimalarial Drugs. Lancet Infect. Dis. 2007, 7, 549–558. [Google Scholar] [CrossRef]
- Ekkapongpisit, M.; Giovia, A.; Follo, C.; Caputo, G.; Isidoro, C. Biocompatibility, Endocytosis, and Intracellular Trafficking of Mesoporous Silica and Polystyrene Nanoparticles in Ovarian Cancer Cells: Effects of Size and Surface Charge Groups. Int. J. Nanomed. 2012, 7, 4147–4158. [Google Scholar] [CrossRef]
- Thiengsusuk, A.; Muhamad, P.; Chaijaroenkul, W.; Na-Bangchang, K. Antimalarial Activity of Piperine. J. Trop. Med. 2018, 2018, 9486905. [Google Scholar] [CrossRef]
- Lin, Z.; Liao, Y.; Venkatasamy, R.; Hider, R.C.; Soumyanath, A. Amides from Piper nigrum L. with Dissimilar Effects on Melanocyte Proliferation In-Vitro. J. Pharm. Pharmacol. 2007, 59, 529–536. [Google Scholar] [CrossRef]
- Khairani, S.; Fauziah, N.; Wiraswati, H.L.; Panigoro, R.; Setyowati, E.Y.; Berbudi, A. Oral Administration of Piperine as Curative and Prophylaxis Reduces Parasitaemia in Plasmodium berghei ANKA-Infected Mice. J. Trop. Med. 2022, 2022, 5721449. [Google Scholar] [CrossRef] [PubMed]
- Wansri, R.; Lin, A.C.K.; Pengon, J.; Kamchonwongpaisan, S.; Srimongkolpithak, N.; Rattanajak, R.; Wilasluck, P.; Deetanya, P.; Wangkanont, K.; Hengphasatporn, K.; et al. Semi-Synthesis of N-Aryl Amide Analogs of Piperine from Piper nigrum and Evaluation of Their Antitrypanosomal, Antimalarial, and Anti-SARS-CoV-2 Main Protease Activities. Molecules 2022, 27, 2841. [Google Scholar] [CrossRef] [PubMed]
- Adegunloye, A.P.; Adebayo, J.O. Piperine Enhances Antimalarial Activity of Methyl Gallate and Palmatine Combination. Acta Parasitol. 2024, 69, 1244–1252. [Google Scholar] [CrossRef]
- Khairani, S.; Fauziah, N.; Lina Wiraswati, H.; Panigoro, R.; Salleh, A.; Yuni Setyowati, E.; Berbudi, A. Piperine Enhances the Antimalarial Activity of Curcumin in Plasmodium berghei ANKA-Infected Mice: A Novel Approach for Malaria Prophylaxis. Evid. Based Complement. Alternat. Med. 2022, 2022, 7897163. [Google Scholar] [CrossRef]
- Paulo, A.; Gomes, E.T.; Steele, J.; Warhurst, D.C.; Houghton, P.J. Antiplasmodial Activity of Cryptolepis Sanguinolenta Alkaloids from Leaves and Roots. Planta Med. 2000, 66, 30–34. [Google Scholar] [CrossRef]
- Cimanga, K.; De Bruyne, T.; Pieters, L.; Vlietinck, A.J.; Turger, C.A. In Vitro and In Vivo Antiplasmodial Activity of Cryptolepine and Related Alkaloids from Cryptolepis sanguinolenta. J. Nat. Prod. 1997, 60, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Lavrado, J.; Cabal, G.G.; Prudêncio, M.; Mota, M.M.; Gut, J.; Rosenthal, P.J.; Díaz, C.; Guedes, R.C.; dos Santos, D.J.V.A.; Bichenkova, E.; et al. Incorporation of Basic Side Chains into Cryptolepine Scaffold: Structure−Antimalarial Activity Relationships and Mechanistic Studies. J. Med. Chem. 2011, 54, 734–750. [Google Scholar] [CrossRef]
- Ortet, R.; Prado, S.; Mouray, E.; Thomas, O.P. Sesquiterpene lactones from the endemic Cape Verdean Artemisia gorgonum. Phytochemistry 2008, 69, 2961–2965. [Google Scholar] [CrossRef]
- Balaich, J.N.; Mathias, D.K.; Torto, B.; Jackson, B.T.; Tao, D.; Ebrahimi, B.; Tarimo, B.B.; Cheseto, X.; Foster, W.A.; Dinglasan, R.R. The Nonartemisinin Sesquiterpene Lactones Parthenin and Parthenolide Block Plasmodium falciparum Sexual Stage Transmission. Antimicrob. Agents Chemother. 2016, 60, 2108–2117. [Google Scholar] [CrossRef]
- Toyang, N.J.; Krause, M.A.; Fairhurst, R.M.; Tane, P.; Bryant, J.; Verpoorte, R. Antiplasmodial Activity of Sesquiterpene Lactones and a Sucrose Ester from Vernonia guineensis Benth. (Asteraceae). J. Ethnopharmacol. 2013, 147, 618–621. [Google Scholar] [CrossRef][Green Version]
- Ibrahim, N.; Ibrahim, H.; Dormoi, J.; Briolant, S.; Pradines, B.; Moreno, A.; Mazier, D.; Legrand, P.; Nepveu, F. Albumin-Bound Nanoparticles of Practically Water-Insoluble Antimalarial Lead Greatly Enhance Its Efficacy. Int. J. Pharm. 2014, 464, 214–224. [Google Scholar] [CrossRef]
- Cui, L.; Miao, J.; Cui, L. Cytotoxic Effect of Curcumin on Malaria Parasite Plasmodium falciparum: Inhibition of Histone Acetylation and Generation of Reactive Oxygen Species. Antimicrob. Agents Chemother. 2007, 51, 488–494. [Google Scholar] [CrossRef]
- Dohutia, C.; Chetia, D.; Gogoi, K.; Sarma, K. Design, In Silico and In Vitro Evaluation of Curcumin Analogues against Plasmodium falciparum. Exp. Parasitol. 2017, 175, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Karmodiya, K.; Surolia, N.; Surolia, A. Synthesis and Exploration of Novel Curcumin Analogues as Anti-Malarial Agents. Bioorg. Med. Chem. 2008, 16, 2894–2902. [Google Scholar] [CrossRef]
- Reddy, R.C.; Vatsala, P.G.; Keshamouni, V.G.; Padmanaban, G.; Rangarajan, P.N. Curcumin for Malaria Therapy. Biochem. Biophys. Res. Commun. 2005, 326, 472–474. [Google Scholar] [CrossRef]
- Jamil, S.N.H.; Ali, A.H.; Feroz, S.R.; Lam, S.D.; Agustar, H.K.; Mohd Abd Razak, M.R.; Latip, J. Curcumin and Its Derivatives as Potential Antimalarial and Anti-Inflammatory Agents: A Review on Structure–Activity Relationship and Mechanism of Action. Pharmaceuticals 2023, 16, 609. [Google Scholar] [CrossRef] [PubMed]
- Andromeda, S.E.; Berbudi, A. The Role of Curcumin as An Antimalarial Agent. Syst. Rev. Pharm. 2020, 11, 18–25. [Google Scholar]
- Ali, A.H.; Sudi, S.; Basir, R.; Embi, N.; Sidek, H.M. The Antimalarial Effect of Curcumin Is Mediated by the Inhibition of Glycogen Synthase Kinase-3β. J. Med. Food 2017, 20, 152–161. [Google Scholar] [CrossRef]
- Tjahjani, S.; Syafruddin; Tjokropranoto, R. Interaction of Alphamangostin and Curcumin with Dihydroartemisinin as Antimalaria In Vitro. IOP Conf. Ser. Earth Environ. Sci. 2018, 125, 012017. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Bhatt, D.; Washimkar, K.R.; Kumar, S.; Mugale, M.N.; Pal, A.; Bawankule, D.U. Naringin and Chloroquine Combination Mitigates Chloroquine-Resistant Parasite-Induced Malaria Pathogenesis by Attenuating the Inflammatory Response. Phytomedicine 2024, 133, 155943. [Google Scholar] [CrossRef]
- Oyinloye, E.O.; Murtala, A.A.; Oladoja, F.A.; Okunye, O.L.; Alabi, A.O.; Ogundeyi, K.J. Chemoprofiling and Antimalarial Potentials of Methanol Extract of Solanum dasyphyllum against Plasmodium berghei Infected Mice. Pharmacol. Res. Nat. Prod. 2025, 6, 100138. [Google Scholar] [CrossRef]
- Ganesh, D.; Fuehrer, H.-P.; Starzengrüber, P.; Swoboda, P.; Khan, W.A.; Reismann, J.A.B.; Mueller, M.S.K.; Chiba, P.; Noedl, H. Antiplasmodial Activity of Flavonol Quercetin and Its Analogues in Plasmodium falciparum: Evidence from Clinical Isolates in Bangladesh and Standardized Parasite Clones. Parasitol. Res. 2012, 110, 2289–2295. [Google Scholar] [CrossRef]
- Ali, A.H.; Sudi, S.; Shi-Jing, N.; Hassan, W.R.M.; Basir, R.; Agustar, H.K.; Embi, N.; Sidek, H.M.; Latip, J. Dual Anti-Malarial and GSK3β-Mediated Cytokine-Modulating Activities of Quercetin Are Requisite of Its Potential as a Plant-Derived Therapeutic in Malaria. Pharmaceuticals 2021, 14, 248. [Google Scholar] [CrossRef]
- Davoodi, M.; Ahmed, F.; Panizai, M.; Obeidavi, Z. Nano-Phytosome of Quercetin Could Protect Liver from Plasmodium berghei in Mouse Model. GMJ Med. 2018, 2, 58–64. [Google Scholar] [CrossRef]
- Tasdemir, D.; Lack, G.; Brun, R.; Rüedi, P.; Scapozza, L.; Perozzo, R. Inhibition of Plasmodium falciparum Fatty Acid Biosynthesis: Evaluation of FabG, FabZ, and FabI as Drug Targets for Flavonoids. J. Med. Chem. 2006, 49, 3345–3353. [Google Scholar] [CrossRef]
- Lehane, A.M.; Saliba, K.J. Common dietary flavonoids inhibit the growth of the intraerythrocytic malaria parasite. BMC Res. Notes 2008, 1, 26. [Google Scholar] [CrossRef]
- Puttappa, N.; Kumar, R.S.; Yamjala, K. Artesunate-Quercetin/Luteolin Dual Drug Nanofacilitated Synergistic Treatment for Malaria: A Plausible Approach to Overcome Artemisinin Combination Therapy Resistance. Med. Hypotheses 2017, 109, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, S.K.; Eberhard, M.; Bobbala, D.; Lang, F. Monensin Induced Suicidal Erythrocyte Death. Cell. Physiol. Biochem. 2010, 25, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, N.; Garcia-Domenech, R.; Galvez, J.; Farhati, K.; Franetich, J.-F.; Sauerwein, R.; Hannoun, L.; Derouin, F.; Danis, M.; Mazier, D. New Active Drugs against Liver Stages of Plasmodium Predicted by Molecular Topology. Antimicrob. Agents Chemother. 2008, 52, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Adovelande, J.; Schrével, J. Carboxylic Ionophores in Malaria Chemotherapy: The effects of Monensin and Nigericin on Plasmodium falciparum In Vitro and Plasmodium vinckei petteri In Vivo. Life Sci. 1996, 59, PL309–PL315. [Google Scholar] [CrossRef] [PubMed]
- Pina, J.R.S.; Silva-Silva, J.V.; Carvalho, J.M.; Bitencourt, H.R.; Watanabe, L.A.; Fernandes, J.M.P.; Souza, G.E.D.; Aguiar, A.C.C.; Guido, R.V.C.; Almeida-Souza, F.; et al. Antiprotozoal and Antibacterial Activity of Ravenelin, a Xanthone Isolated from the Endophytic Fungus Exserohilum rostratum. Molecules 2021, 26, 3339. [Google Scholar] [CrossRef]
- Al-Quraishy, S.; Murshed, M.; Delic, D.; Al-Shaebi, E.M.; Qasem, M.A.A.; Mares, M.M.; Dkhil, M.A. Plasmodium Chabaudi-Infected Mice Spleen Response to Synthesized Silver Nanoparticles from Indigofera oblongifolia Extract. Lett. Appl. Microbiol. 2020, 71, 542–549. [Google Scholar] [CrossRef]
- Al-Shaebi, E.A.; Dkhil, M.A.; Al-Quraishy, S. Indigofera oblongifolia Regulates the Hepatic Gene Expression Profile Induced by Blood Stage Malaria. Microb. Pathog. 2018, 119, 170–182. [Google Scholar] [CrossRef]
- Veeragoni, D.; Deshpande, S.S.; Singh, V.; Misra, S.; Mutheneni, S.R. In Vitro and In Vivo Antimalarial Activity of Green Synthesized Silver Nanoparticles Using Sargassum tenerrimum—A Marine Seaweed. Acta Trop. 2023, 245, 106982. [Google Scholar] [CrossRef]
- Ojemaye, M.O.; Okoh, S.O.; Okoh, A.I. Silver Nanoparticles (AgNPs) Facilitated by Plant Parts of Crataegus ambigua Becker AK Extracts and Their Antibacterial, Antioxidant and Antimalarial Activities. Green Chem. Lett. Rev. 2021, 14, 51–61. [Google Scholar] [CrossRef]
- Sannella, A.R.; Casini, A.; Gabbiani, C.; Messori, L.; Bilia, A.R.; Vincieri, F.F.; Majori, G.; Severini, C. New Uses for Old Drugs. Auranofin, a Clinically Established Antiarthritic Metallodrug, Exhibits Potent Antimalarial Effects In Vitro: Mechanistic and Pharmacological Implications. FEBS Lett. 2008, 582, 844–847. [Google Scholar] [CrossRef]
- Varela-Aramburu, S.; Ghosh, C.; Goerdeler, F.; Priegue, P.; Moscovitz, O.; Seeberger, P.H. Targeting and Inhibiting Plasmodium falciparum Using Ultra-Small Gold Nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 43380–43387. [Google Scholar] [CrossRef]
- Dutta, P.P.; Bordoloi, M.; Gogoi, K.; Roy, S.; Narzary, B.; Bhattacharyya, D.R.; Mohapatra, P.K.; Mazumder, B. Antimalarial Silver and Gold Nanoparticles: Green Synthesis, Characterization and In Vitro Study. Biomed. Pharmacother. 2017, 91, 567–580. [Google Scholar] [CrossRef]
- Anbarasan, K.; Zahir, A.A.; Karthick, V.; Rahuman, A.A.; Rao, K.V.B. Antimalarial Activity of Eco-Friendly Green Synthesis of Gold Nanoparticles Using Coccinia grandis (L.) Voigt. Against Plasmodium berghei. Asian Pac. J. Health Sci. 2022, 9, 148–155. [Google Scholar] [CrossRef]
- Kalpana, V.N.; Alarjani, K.M.; Rajeswari, V.D. Enhancing Malaria Control Using Lagenaria siceraria and Its Mediated Zinc Oxide Nanoparticles Against the Vector Anopheles Stephensi and Its Parasite Plasmodium falciparum. Sci. Rep. 2020, 10, 21568. [Google Scholar] [CrossRef] [PubMed]
- Najoom, S.; Fozia, F.; Ahmad, I.; Wahab, A.; Ahmad, N.; Ullah, R.; Gul, A.; Bari, A.; Khan, M.Y.; Khan, A.A. Effective Antiplasmodial and Cytotoxic Activities of Synthesized Zinc Oxide Nanoparticles Using Rhazya stricta Leaf Extract. Evid.-Based Complement. Altern. Med. ECAM 2021, 2021, 5586740. [Google Scholar] [CrossRef]
- Mishra, A.; Kaushik, N.K.; Sardar, M.; Sahal, D. Evaluation of Antiplasmodial Activity of Green Synthesized Silver Nanoparticles. Colloids Surf. B Biointerfaces 2013, 111, 713–718. [Google Scholar] [CrossRef]
- Kamaraj, C.; Balasubramani, G.; Siva, C.; Raja, M.; Balasubramanian, V.; Raja, R.K.; Tamilselvan, S.; Benelli, G.; Perumal, P. Ag Nanoparticles Synthesized Using β-Caryophyllene Isolated from Murraya koenigii: Antimalarial (Plasmodium falciparum 3D7) and Anticancer Activity (A549 and HeLa Cell Lines). J. Clust. Sci. 2017, 28, 1667–1684. [Google Scholar] [CrossRef]
- Hawadak, J.; Kojom Foko, L.P.; Pande, V.; Singh, V. In Vitro Antiplasmodial Activity, Hemocompatibility and Temporal Stability of Azadirachta indica Silver Nanoparticles. Artif. Cells Nanomed. Biotechnol. 2022, 50, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Kumar, R.; Devi, S.; Sharma, P.; Chaudhary, N.R.; Negi, S.; Tandel, N.; Marepally, S.; Pied, S.; Tyagi, R.K. Biogenically Synthesized Green Silver Nanoparticles Exhibit Antimalarial Activity. Discov. Nano 2024, 19, 136. [Google Scholar] [CrossRef]


| Natural Product | Nanocarrier | Achievement | Reference |
|---|---|---|---|
| Quinine | Poly(ɛ-caprolactone) nanocapsules | Increased intra-erythrocytic concentration | [9] |
| Polysorbate coated Eudragit RS 100 nanospheres and nanocapsules | Improved interaction between the drug and erythrocytic membrane | [10] | |
| Eudragit RS or poly(ɛ-caprolactone) nanocapsules (co-loaded with curcuma oil) | Decreased level of parasitemia and increased survival rate | [11] | |
| Mesoporous silica nanoparticles | Increased antimalarial activity and survival rate due to increased uptake | [12] | |
| Polysorbate-coated nanocapsules | Decreased toxicity on reproductive system | [13] | |
| Piperine | Chitosan-alginate nanoparticles (co-loaded with curcumin) | Enhanced antimalarial activity against P. falciparum No toxicity and hemolytic activity | [14] |
| Cryptolepine | Gelatine nanoparticles | Reduced hemolysis and longer exposition of the drug to erythrocytes | [15] |
| Artemisinin | Poly(ε-caprolactone) nanoparticles and liposomes | Increased aqueous solubility | [6] |
| Albumin nanoparticles | Increased solubility and bioavailability Parasite targeting and strong antimalarial effect | [16] | |
| Pegylated liposomes (alone or co-loaded with curcumin) | Less variability in plasma concentrations Longer blood circulation and contact with erythrocytes | [17] | |
| mPEG-decorated/γ-CD nanoreservoir system and polysorbate 80/γ-CD nanospheres | Longer blood circulation and higher mean plasma half-life | [18] | |
| Curcumin | Nanostructured lipid carriers | Controlled release, resulting in prolonged exposure of the parasites to the drug | [4] |
| Lipid nanoparticles (alone or co-loaded with subtherapeutic dose β-arteether) | Increased solubility and survival rate | [19] | |
| Lipid nanoparticles | Higher antimalarial activity against P. berghei | [20] | |
| Liposomes—Eudragit-hyaluronan liposomes and Eudragit-water-soluble dextrin | Increased survival rate | [21] | |
| Poly(D,L-lactic-co-glycolic acid) nanoparticles | Increased suppression of malarial parasite | [22] | |
| Chitosan nanoparticles | Higher stability and capacity of crossing the mucosal barrier Stronger activity in vivo against P. yoelii | [23] | |
| Polysorbate-coated polymeric nanocapsules (co-loaded with quinine) | Reduction in P. falciparum parasitemia Decreased toxicity on reproductive system of Caenorhabditis elegans | [24] | |
| Poly(D,L-lactic-co-glycolic acid) nanoparticles (co-loaded with artesunate) | Increased suppression of P. berghei compared to non-loaded combination of chloroquine and artesunate | [25] | |
| Quercetin | Phytosomes | Higher activity against P. falciparum compared to pure quercetin Absence of in vitro toxicity and hemolytic activity | [26] |
| Nanovesicle-doped nanoemulsions (quercetin alone or co-loaded with artemisinin) | Enhanced antimalarial effect on P. falciparum; Increased survival rate (co-loaded system); Low in vitro toxicity | [27] | |
| Monensin | PEGylated liposomes | Long blood circulation Increased interactions with erythrocytic membranes Preferential internalization in erythrocytes | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoncheva, Y.; Radeva, L.; Yoncheva, K. Development of Nanotechnological Approaches to Improving the Antimalarial Potential of Natural Substances. Molecules 2025, 30, 4133. https://doi.org/10.3390/molecules30204133
Yoncheva Y, Radeva L, Yoncheva K. Development of Nanotechnological Approaches to Improving the Antimalarial Potential of Natural Substances. Molecules. 2025; 30(20):4133. https://doi.org/10.3390/molecules30204133
Chicago/Turabian StyleYoncheva, Yoana, Lyubomira Radeva, and Krassimira Yoncheva. 2025. "Development of Nanotechnological Approaches to Improving the Antimalarial Potential of Natural Substances" Molecules 30, no. 20: 4133. https://doi.org/10.3390/molecules30204133
APA StyleYoncheva, Y., Radeva, L., & Yoncheva, K. (2025). Development of Nanotechnological Approaches to Improving the Antimalarial Potential of Natural Substances. Molecules, 30(20), 4133. https://doi.org/10.3390/molecules30204133







