Iron(II) and Manganese(II) Coordination Chemistry Ligated by Coplanar Tridentate Nitrogen-Donor Ligand, 2,6-bis(5-isopropyl-1H-pyrazol-3-yl)pyridine
Abstract
1. Introduction
2. Results and Discussion
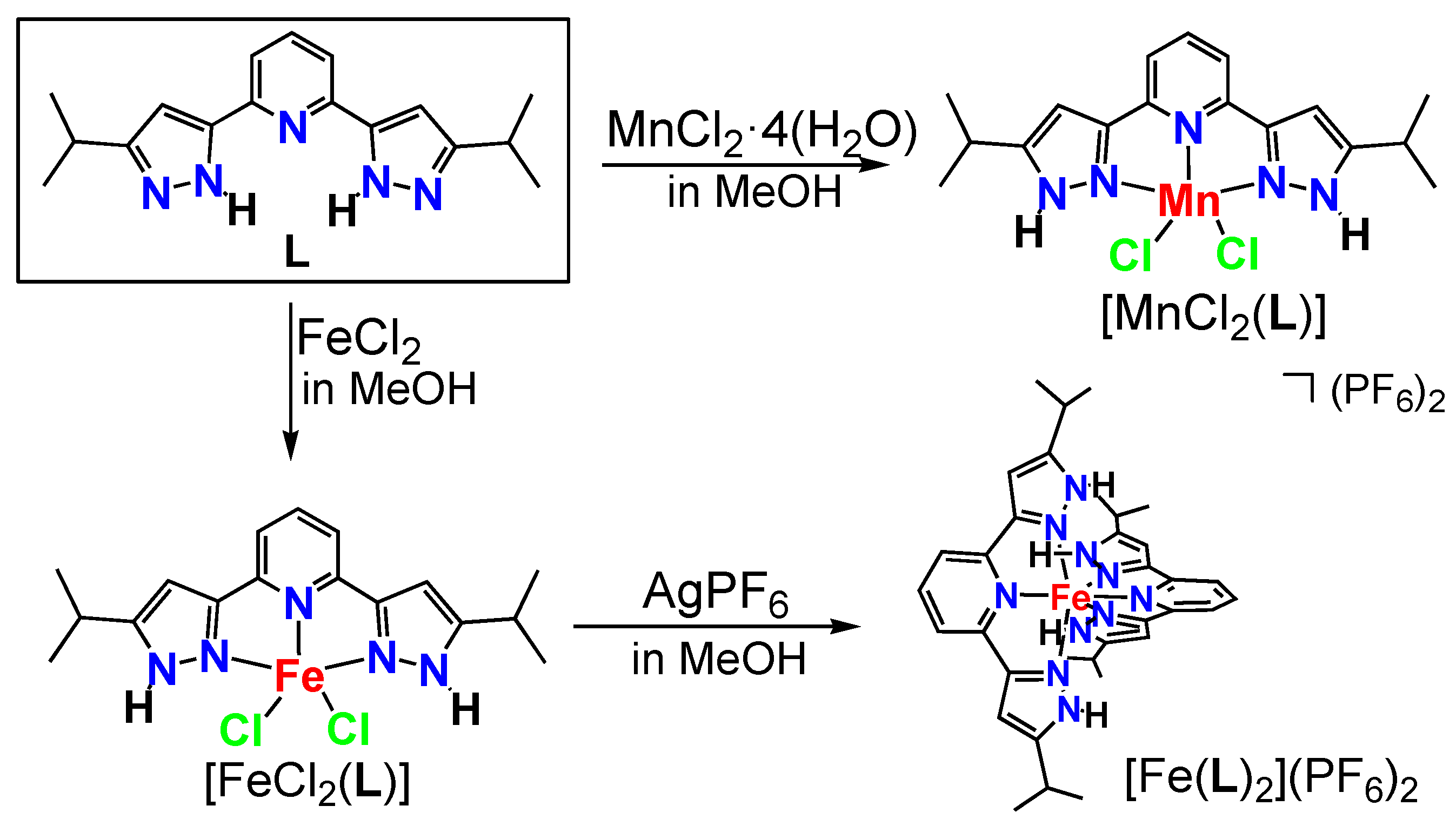
2.1. Synthesis of Complexes
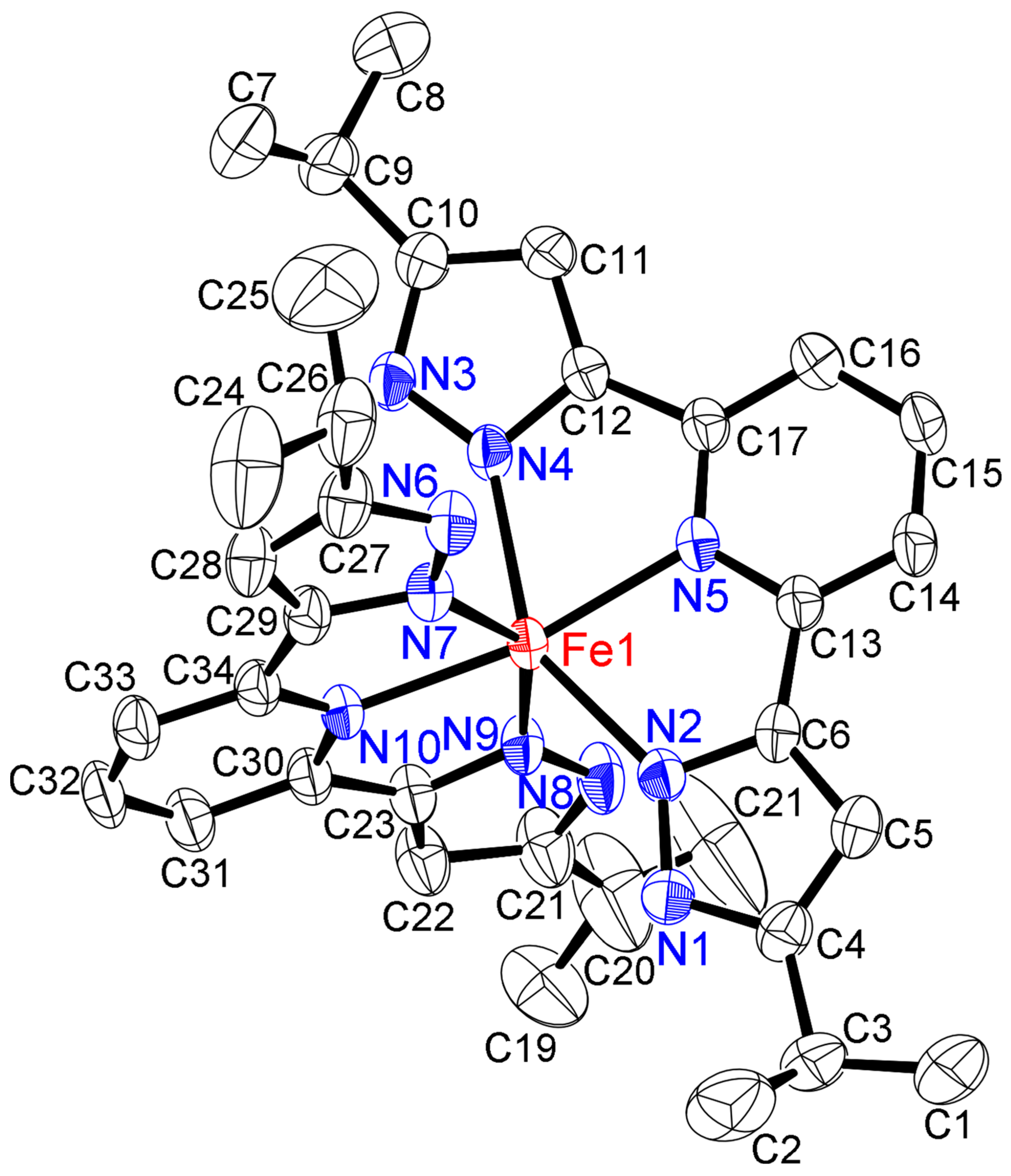
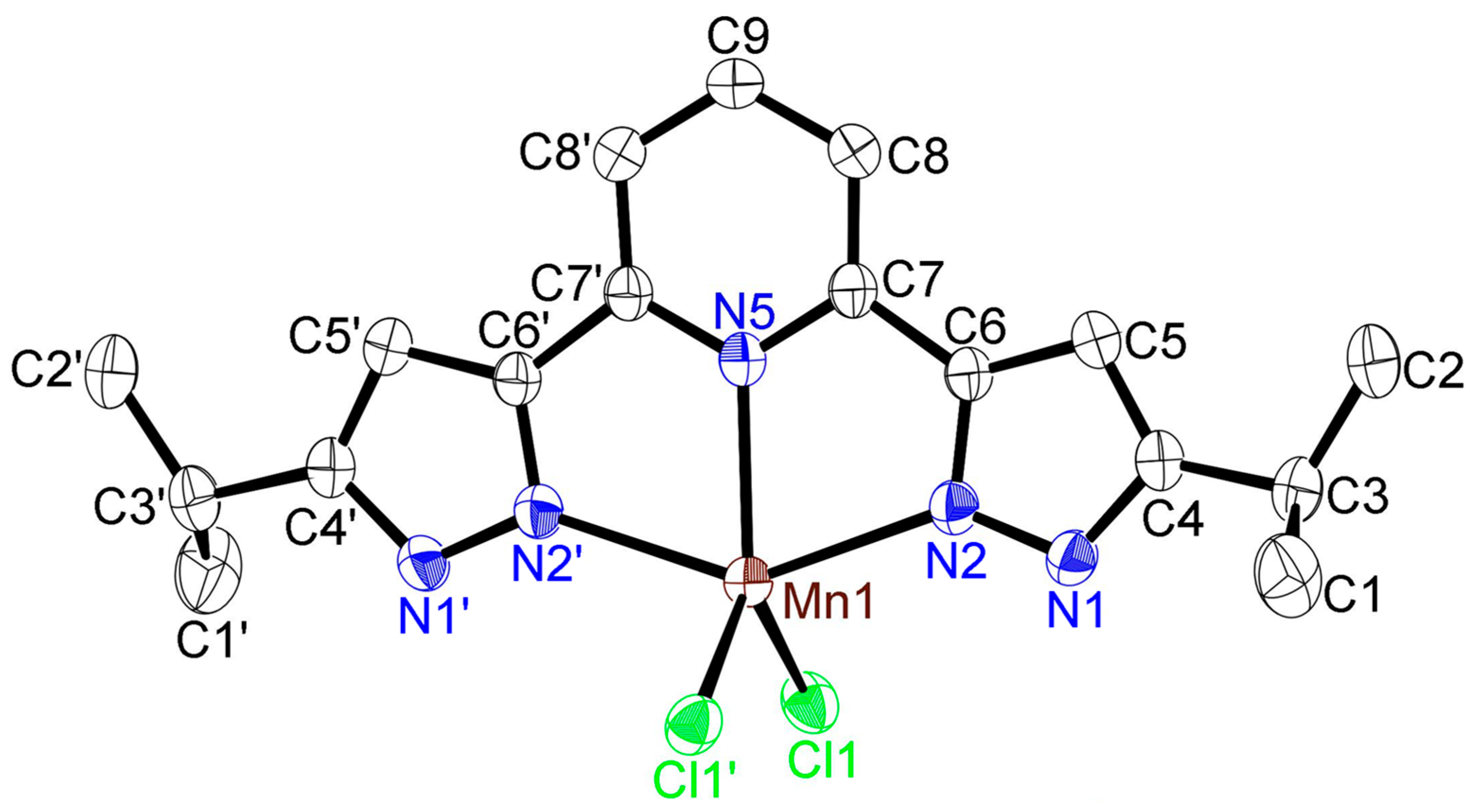
2.2. Solid-State Structures of Iron(II) and Manganese(II) Complexes
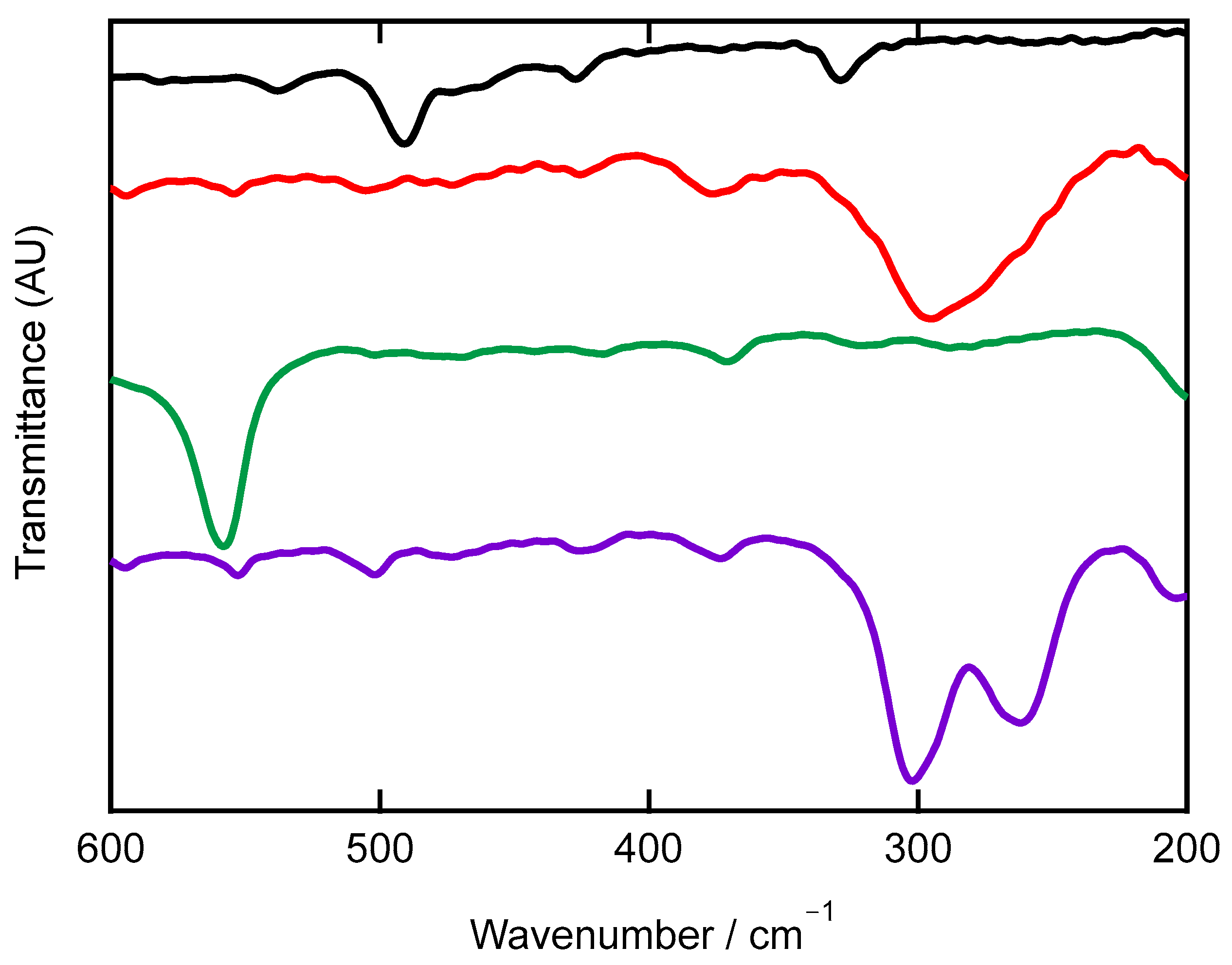
2.3. Infrared and Far-Infrared Spectroscopy
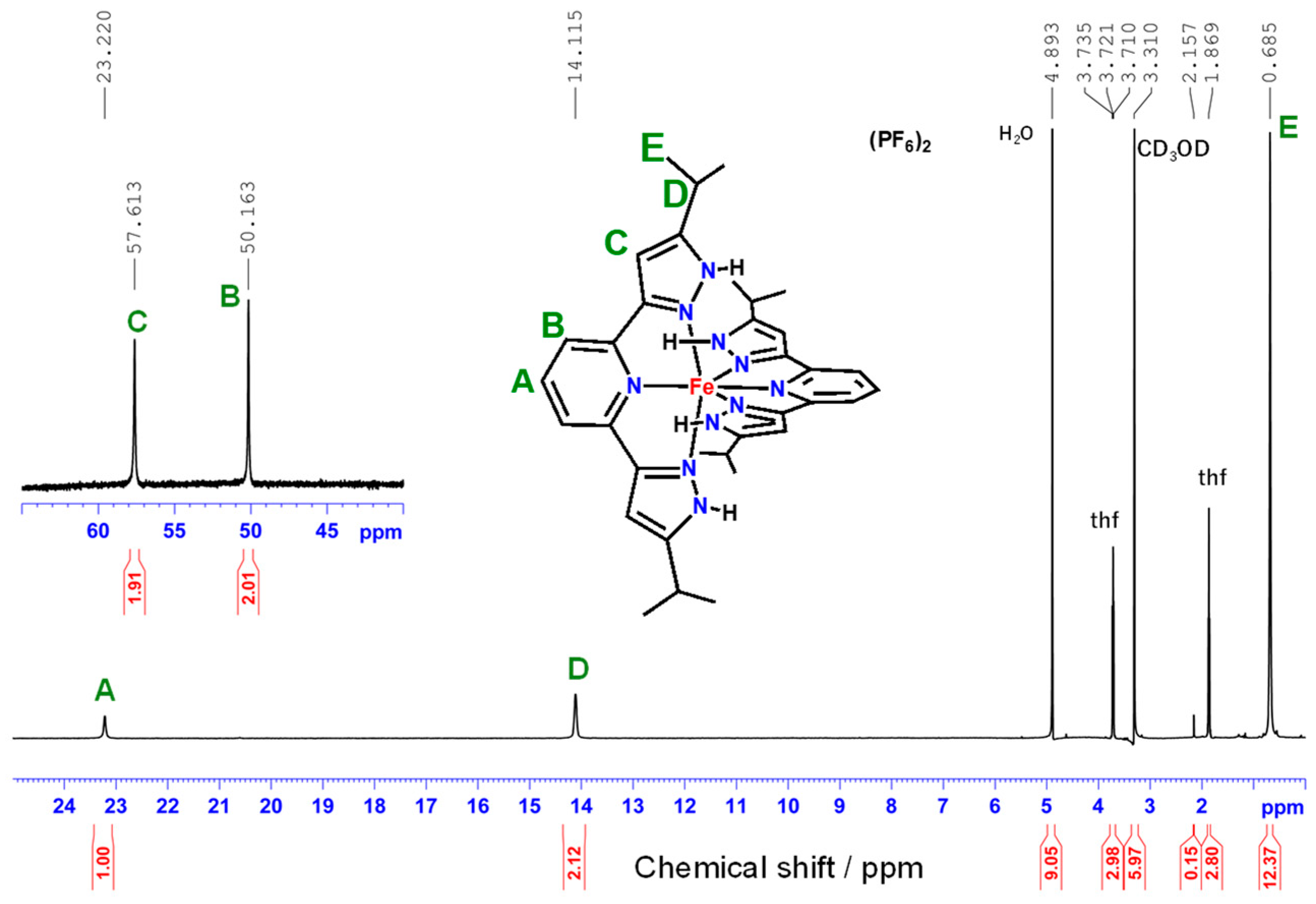
2.4. NMR Spectroscopy
2.5. UV-Vis Spectroscopy
3. Materials and Methods
3.1. Materials and General Techniques
3.2. Measurements
3.3. Preparation of Complexes
- [FeCl2(L)]
- [Fe(L)2](PF6)2
- [MnCl2(L)]
3.4. X-Ray Crystal Structure Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| bispzHpy | 2,6-bis(1H-pyrazol-3-yl)pyridine |
| bispzpy | 2,6-bis(N-pyrazol)pyridine |
| CCDC | Cambridge Crystallographic Data Centre |
| DR | Diffuse Reflectance |
| IR | Infrared |
| MeOH | Methanol |
| NMR | Nuclear Magnetic Resonance |
| ORTEP | Oak Ridge Thermal Ellipsoid Program |
| OTf− | Trifluoromethanesulfonate Anion |
| py | Pyridine |
| pz | Pyrazole |
| terpy | 2,2′:6′,2″-terpyridine |
| thf | Tetrahydrofuran |
| UV-Vis | Ultraviolet–Visible |
References
- Morgan, G.T.; Drew, H.D.K. CLXII—Researches on residual affinity and co-ordination. Part II. Acetylacetones of selenium and tellurium. J. Chem. Soc. Trans. 1920, 117, 1456–1465. [Google Scholar] [CrossRef]
- Thompson, A.M.W.C. The synthesis of 2,2′:6′,2″-terpyridine ligands–versatile building blocks for supramolecular chemistry. Coord. Chem. Rev. 1997, 160, 1–52. [Google Scholar] [CrossRef]
- Mukherjee, R. Coordination chemistry with pyrazole-based chelating ligands: Molecular structural aspects. Coord. Chem. Rev. 2000, 203, 151–218. [Google Scholar] [CrossRef]
- Baranoff, E.; Collin, J.-P.; Flamigni, L.; Sauvage, J.-P. From ruthenium(II) to iridium(III): 15 years of triads based on bis-terpyridine complexes. Chem. Soc. Rev. 2004, 33, 147–155. [Google Scholar] [CrossRef]
- Hofmeiera, H.; Schubert, U.S. Recent developments in the supramolecular chemistry of terpyridine–metal complexes. Chem. Soc. Rev. 2004, 33, 373–399. [Google Scholar] [CrossRef]
- Halcrow, M.A. The synthesis and coordination chemistry of 2,6-bis(pyrazolyl)pyridines and related ligands—Versatile terpyridine analogues. Coord. Chem. Rev. 2005, 249, 2880–2908. [Google Scholar] [CrossRef]
- Gibson, V.C.; Redshaw, C.; Solan, G.A. Bis(imino)pyridines: Surprisingly reactive ligands and a gateway to new families of catalysts. Chem. Rev. 2007, 107, 1745–1776. [Google Scholar] [CrossRef] [PubMed]
- Constable, E.C. 2,2′:6′,2″-Terpyridines: From chemical obscurity to common supramolecular motifs. Chem. Soc. Rev. 2007, 36, 246–253. [Google Scholar] [CrossRef]
- Olguín, J.; Brooker, S. Spin crossover active iron(II) complexes of selected pyrazole-pyridine/pyrazine ligands. Coord. Chem. Rev. 2011, 255, 203–240. [Google Scholar] [CrossRef]
- Lawrence, M.A.W.; Green, K.-A.; Nelson, P.N.; Lorraine, S.C. Review: Pincer ligands—Tunable, versatile and applicable. Polyhedron 2018, 143, 11–27. [Google Scholar] [CrossRef]
- Wei, C.; He, Y.; Shi, X.; Song, Z. Terpyridine-metal complexes: Applications in catalysis and supramolecular chemistry. Coord. Chem. Rev. 2019, 385, 1–19. [Google Scholar] [CrossRef]
- Mughal, M.U.; Mirzaei, M.; Sadiq, A.; Fatima, S.; Naseem, A.; Naeem, N.; Fatima, N.; Kausar, S.; Altaf, A.A.; Zafar, M.N.; et al. Terpyridine-metal complexes: Effects of different substituents on their physico-chemical properties and density functional theory studies. R. Soc. Open Sci. 2020, 7, 201208. [Google Scholar] [CrossRef]
- Winter, A.; Schubert, U.S. Metal-terpyridine complexes in catalytic application—A spotlight on the last decade. ChemCatChem 2020, 12, 2890–2941. [Google Scholar] [CrossRef]
- Lin, W.-S.; Kuwata, S. Recent developments in reactions and catalysis of protic pyrazole complexes. Molecules 2023, 28, 3529. [Google Scholar] [CrossRef]
- Fujisawa, K. A personal perspective on the discovery of dioxygen adducts of copper and iron by Nobumasa Kitajima. J. Biol. Inorg. Chem. 2017, 22, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, K.; Ono, T.; Ishikawa, Y.; Amir, N.; Miyashita, Y.; Okamoto, K.; Lehnert, O. Structural and electronic differences of copper(I) complexes with tris (pyrazolyl) methane and hydrotris (pyrazolyl) borate ligands. Inorg. Chem. 2006, 45, 1698–1713. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, K.; Yamada, A.; Koyama, M.; Young, D.J. The copper(II) coordination chemistry of alkyl substituted bispyrazole pyridine ligands: Structure and spectral properties. Inorg. Chim. Acta 2023, 555, 121567. [Google Scholar] [CrossRef]
- Fujisawa, K.; Minakawa, Y.; Tiekink, E.R.T. Crystal structure of dichlorido{2,6-bis(3,5-diisopropyl-N-pyrazolyl)pyridine}zinc(II), C23H33Cl2N5Zn. Z. Für Krist.-New Cryst. Struct. 2025; in press. [Google Scholar] [CrossRef]
- Fujisawa, K.; Minakawa, Y.; Young, D.J. Transition metal(II) coordination chemistry ligated by a new coplanar tridentate ligand, 2,6-bis(5-isopropyl-1H-pyrazol-3-yl)pyridine. Inorganics 2025, 13, 189. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Milutinović, M.M.; Bogojeski, J.V.; Klisurić, O.; Scheurer, A.; Elmrothd, S.K.C.; Bugarčić, Ž.D. Synthesis and structures of a pincer-type rhodium(III) complex: Reactivity toward biomolecules. Dalton Trans. 2016, 45, 15481–15491. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, W.; Wang, D. Mononuclear, dinuclear, hexanuclear, and one-dimensional polymeric silver complexes having ligand-supported and unsupported argentophilic interactions stabilized by pincer-like 2,6-bis(5-pyrazolyl)pyridine ligands. Dalton Trans. 2008, 1444–1453. [Google Scholar] [CrossRef]
- Cook, B.J.; Chen, C.-H.; Pink, M.; Lord, R.L.; Caulton, K.G. Coordination and electronic characteristics of a nitrogen heterocycle pincer ligand. Inorg. Chim. Acta 2016, 451, 82–91. [Google Scholar] [CrossRef]
- Sephton, H.E.; Watson, R.L.; Shahid, N.; Vasili, H.B.; Baker, D.L.; Saha, D.; Berdiell, I.C.; Pask, C.M.; Cespedes, O.; Halcrow, M.A. The impact of whole-molecule disorder on spin-crossover in a family of isomorphous molecular crystals. Chem. Sci. 2025, 16, 9203–9212. [Google Scholar] [CrossRef]
- Carey, M.C.; Adelman, S.L.; McCusker, J.K. Insights into the excited state dynamics of Fe(II) polypyridyl complexes from variable-temperature ultrafast spectroscopy. Chem. Sci. 2019, 10, 134–144. [Google Scholar] [CrossRef]
- Fatur, S.M.; Shepard, S.G.; Higgins, R.F.; Shores, M.P.; Damrauer, N.H. A Synthetically tunable system to control MLCT excited-state lifetimes and spin states in iron(II) polypyridines. J. Am. Chem. Soc. 2017, 139, 4493–4905. [Google Scholar] [CrossRef]
- Polezhaev, A.V.; Chen, C.-H.; Kinne, A.S.; Cabelof, A.C.; Lord, R.F.; Caulton, K.G. Ligand design toward multifunctional substrate reductive transformations. Inorg. Chem. 2017, 56, 9505–9514. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.S.; Ruben, M. Emerging trends in spin crossover (SCO) based functional materials and devices. Coord. Chem. Rev. 2017, 346, 176–205. [Google Scholar] [CrossRef]
- Cook, L.J.K.; Mohammed, R.; Sherborne, G.; Roberts, T.D.; Alvarez, S.; Halcrow, M.A. Spin state behavior of iron(II)/dipyrazolylpyridine complexes. New insights from crystallographic and solution measurements. Coord. Chem. Rev. 2015, 289–290, 2–12. [Google Scholar] [CrossRef]
- Roberts, T.D.; Little, M.A.; Cook, L.J.K.; Halcrow, M.A. Iron(II) complexes of 2,6-di(1H-pyrazol-3-yl)pyridine derivatives with hydrogen bonding and sterically bulky substituents. Dalton Trans. 2014, 43, 7577–7588. [Google Scholar] [CrossRef]
- Mohammed, R.; Chastanet, G.; Tuna, F.; Malkin, T.L.; Barrett, S.A.; Kilner, C.A.; Létard, J.-F.; Halcrow, M.A. Synthesis of 2,6-di(pyrazol-1-yl)pyrazine derivatives and the spin-state behavior of their iron(II) complexes. Eur. J. Inorg. Chem. 2013, 5–6, 819–831. [Google Scholar] [CrossRef]
- Roberts, T.D.; Little, M.A.; Tuna, F.; Kilner, C.A.; Halcrow, M.A. Isostructural salts of the same complex showing contrasting thermal spin-crossover mediated by multiple phase changes. Chem Commun. 2013, 49, 6280–6282. [Google Scholar] [CrossRef]
- Huang, T.-H.; Zhang, M.-H.; Gao, C.-Y.; Wang, L.-T. Synthesis, structures and characterization of metal complexes containing 4′-phenyl-2,2′:6′,2″-terpyridine ligands with extended π⋯π interactions. Inorg. Chim. Acta 2013, 408, 91–95. [Google Scholar] [CrossRef]
- Roberts, T.D.; Tuna, F.; Malkin, T.L.; Kilner, C.A.; Halcrow, M.A. An iron(II) complex exhibiting five anhydrous phases, two of which interconvert by spin-crossover with wide hysteresis. Chem. Sci. 2012, 3, 349–354. [Google Scholar] [CrossRef]
- Holland, J.M.; Barrett, S.A.; Kilner, C.A.; Halcrow, M.A. Control of the spin state of Fe(II) 2,6-di(pyrazol-1-yl)pyridine complexes by distal ligand substitution. Inorg. Chem. Commun. 2002, 5, 328–332. [Google Scholar] [CrossRef]
- Holland, J.M.; McAllister, J.A.; Lu, Z.; Kilner, C.A.; Thornton-Petta, M.; Halcrow, M.A. An unusual abrupt thermal spin-state transition in [FeL2][BF4]2 [L = 2,6-di(pyrazol-1-yl)pyridine]. Chem Commun. 2001, 577–578. [Google Scholar] [CrossRef]
- Gütlich, P.; Garciaa, Y.; Goodwin, H.A. Spin crossover phenomena in Fe(II) complexes. Chem. Soc. Rev. 2000, 29, 419–427. [Google Scholar] [CrossRef]
- Baker, A.T.; Goodwin, H.A. Crystal structure of bis(2,2′:6′,2″-terpyridine)iron(II) bis(perchlorate) hydrate. Aust. J. Chem. 1985, 38, 207–214. [Google Scholar] [CrossRef]
- Irving, H.; Williams, R.J.P. 637. The stability of transition-metal complexes. J. Chem. Soc. 1953, 3192–3210. [Google Scholar] [CrossRef]
- Gorelsky, S.I.; Basumallick, L.; Vura-Weis, J.; Sarangi, R.; Hodgson, K.O.; Hedman, B.; Fujisawa, K.; Solomon, E.I. Spectroscopic and DFT investigation of [M{HB(3,5-iPr2pz)3}(SC6F5)] (M = Mn, Fe, Co, Ni, Cu, and Zn) model complexes: periodic trends in metal−thiolate bonding. Inorg. Chem. 2005, 44, 4947–4960. [Google Scholar] [CrossRef]
- Matsunaga, Y.; Fujisawa, K.; Ibi, N.; Miyashita, Y.; Okamoto, K. Structural and spectroscopic characterization of first-row transition metal(II) substituted blue copper model complexes with hydrotris(pyrazolyl)borate. Inorg. Chem. 2005, 44, 325–335. [Google Scholar] [CrossRef]
- Danopoulos, A.A.; Tsoureas, N.; Wright, J.A.; Light, M.E. N-Heterocyclic pincer dicarbene complexes of iron(II): C-2 and C-5 Metalated carbenes on the same metal center. Organometallics 2004, 23, 166–168. [Google Scholar] [CrossRef]
- Pavel, E.G.; Kitajima, N.; Solomon, E.I. Magnetic circular dichroism spectroscopic studies of mononuclear non-heme ferrous model complexes. correlation of excited- and ground-state electronic structure with geometry. J. Am. Chem. Soc. 1998, 120, 3949–3962. [Google Scholar] [CrossRef]
- CrystalClear, 2.1 b29, Data Collection and Processing Software. Rigaku Corporation: Tokyo, Japan, 2013.
- CrysAlisPro, 41.118a, Data Collection and Processing Software. Rigaku Corporation: Tokyo, Japan, 2021.
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; De Caro, L.; Giacovazzo, C.; Polidori, G.; Siliqi, D.; Spagna, R. IL MILIONE: A suite of computer programs for crystal structure solution of proteins. J. Appl. Crystallogr. 2007, 40, 609–613. [Google Scholar] [CrossRef]
- Crystal Structure, version 4.3, Crystal Structure Analysis Package. Rigaku Corporation: Tokyo, Japan, 2003.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. 2015, 71, 9–18. [Google Scholar] [CrossRef]



| Complexes a | Temp /K | d (Fe–Ncenteral) (Avg.)/Å | d (Fe–Nterminal) (Avg.)/Å | ∠ (Ncenteral–Fe– Ncenteral)/° | ∠ (Nterminal–Fe– Nterminal) (Avg.)/° b | Spin State | Ref |
|---|---|---|---|---|---|---|---|
| Type b a | |||||||
| Fe(L)2](PF6)2 (Rb = iPr) | 223 | 2.140 (3) | 2.203 (3) | 170.21 (10) | 147.4 (1) | high-spin | tw c |
| 140 | 2.138 (2) | 2.194 (3) | 169.93 (9) | 147.52 (9) | high-spin | tw c | |
| Fe(Lb1)2](ClO4)2 (Rb = Me) | 150 | 1.955 (3) | 2.009 (3) | 178.63 (11) | 158.16 (11) | low-spin | 32 |
| Fe(Lb1)2](BF4)2 (Rb = Me) | 300 | 2.109 (5) | 2.171 (3) | 180 | 148.8 (2) | high-spin | 34 |
| 150 | 1.984 (6) | 2.033 (4) | 180 | 154.9 (2) | low-spin | 34 | |
| Fe(Lb2)2](ClO4)2 (Rb = NH2) | 150 | 2.1567 (12) | 2.191 (2) | 177.33 (6) | 146.87 (5) | high-spin | 30 |
| Fe(Lb3)2](BF4)2 (Rb = HNC(O)tBu) | 150 | 2.145 (2) | 2.197 (2) | 158.30 (4) | 147.32 (4) | high-spin | 30 |
| Type a a | |||||||
| Fe(terpy)2](PF6)2 (Ra = H) | 294 | 1.891 (5) | 1.988 (6) | 178.6 (3) | 161.1 (3) | low-spin | 38 |
| Fe(La1)2](PF6)2 (Ra = Ph) | 296 | 1.881 (2) | 1.977 (2) | 175.19 (7) | 161.97 (7) | low-spin | 33 |
| Fe(La2)2](BF6)2 (X = Cl) | 120 | 2.080 (3) | 2.272 (3) | 175.4 (2) | 149.8 (2) | high-spin | 26 |
| Fe(La3)2](BF6)2 (X = Br) | 100 | 2.075 (5) | 2.305 (5) | 168.4 (2) | 149.8 (2) | high-spin | 26 |
| Type c a | |||||||
| Fe(Lc1)2](ClO4)2 (Rc = H) | 290 | 2.126 (2) | 2.184 (3) | 173.15 (10) | 146.83 (9) | high-spin | 36 |
| Fe(Lc1)2](ClO4)2 (Rc = H) | 240 | 1.899 (3) | 1.976 (4) | 178.25 (18) | 160.04 (13) | low-spin | 36 |
| Fe(Lc2)2](ClO4)2 (Rc = Mes) | 150 | 1.894 (2) | 1.997 (2) | 178.98 (8) | 168.62 (8) | low-spin | 35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujisawa, K.; Minakawa, Y.; Young, D.J. Iron(II) and Manganese(II) Coordination Chemistry Ligated by Coplanar Tridentate Nitrogen-Donor Ligand, 2,6-bis(5-isopropyl-1H-pyrazol-3-yl)pyridine. Molecules 2025, 30, 4128. https://doi.org/10.3390/molecules30204128
Fujisawa K, Minakawa Y, Young DJ. Iron(II) and Manganese(II) Coordination Chemistry Ligated by Coplanar Tridentate Nitrogen-Donor Ligand, 2,6-bis(5-isopropyl-1H-pyrazol-3-yl)pyridine. Molecules. 2025; 30(20):4128. https://doi.org/10.3390/molecules30204128
Chicago/Turabian StyleFujisawa, Kiyoshi, Yurika Minakawa, and David James Young. 2025. "Iron(II) and Manganese(II) Coordination Chemistry Ligated by Coplanar Tridentate Nitrogen-Donor Ligand, 2,6-bis(5-isopropyl-1H-pyrazol-3-yl)pyridine" Molecules 30, no. 20: 4128. https://doi.org/10.3390/molecules30204128
APA StyleFujisawa, K., Minakawa, Y., & Young, D. J. (2025). Iron(II) and Manganese(II) Coordination Chemistry Ligated by Coplanar Tridentate Nitrogen-Donor Ligand, 2,6-bis(5-isopropyl-1H-pyrazol-3-yl)pyridine. Molecules, 30(20), 4128. https://doi.org/10.3390/molecules30204128








