Cu2+ Intercalation and Structural Water Enhance Electrochemical Performance of Cathode in Zinc-Ion Batteries
Abstract
1. Introduction
2. Results and Discussion
2.1. DFT Calculations
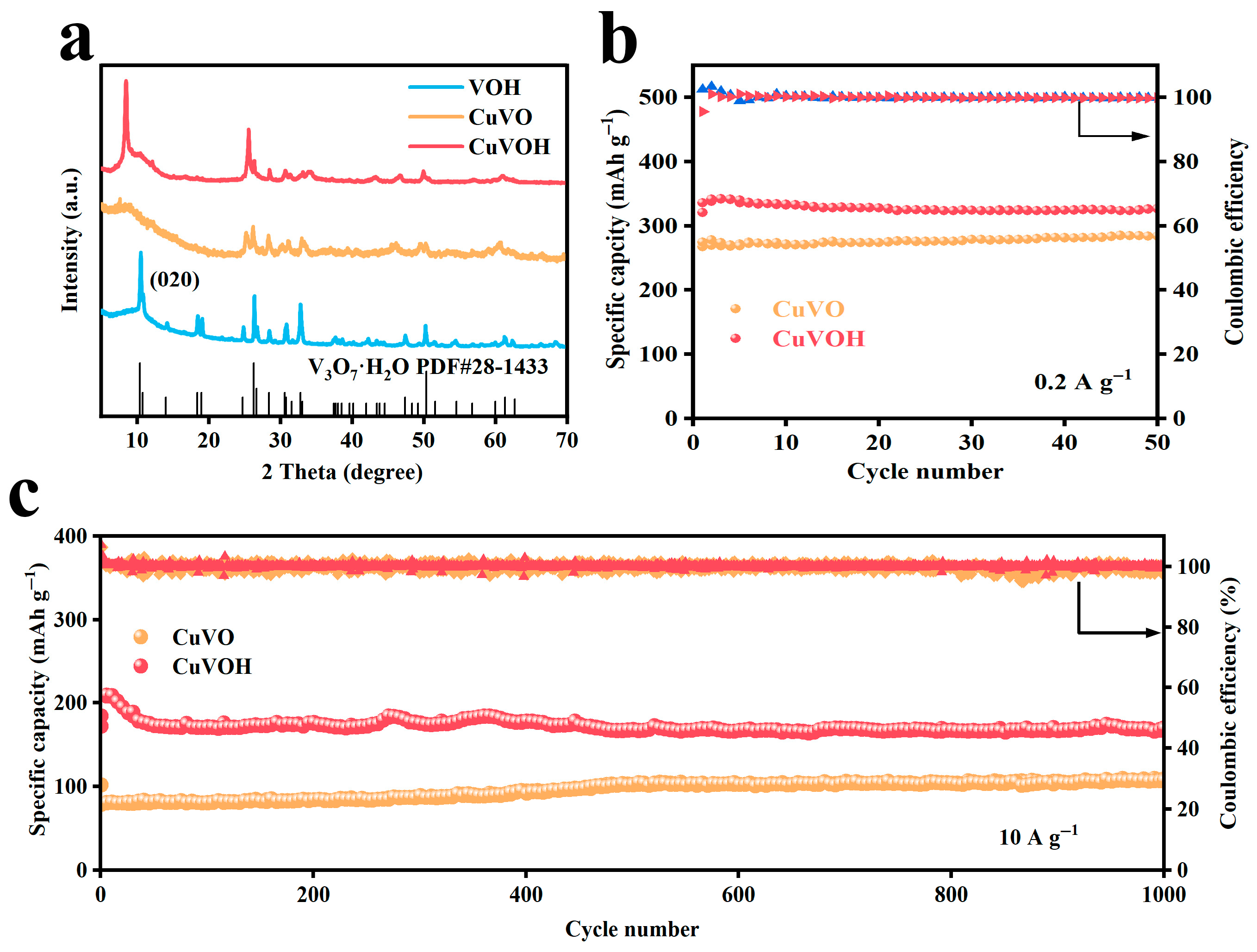
2.2. Morphological Characterization
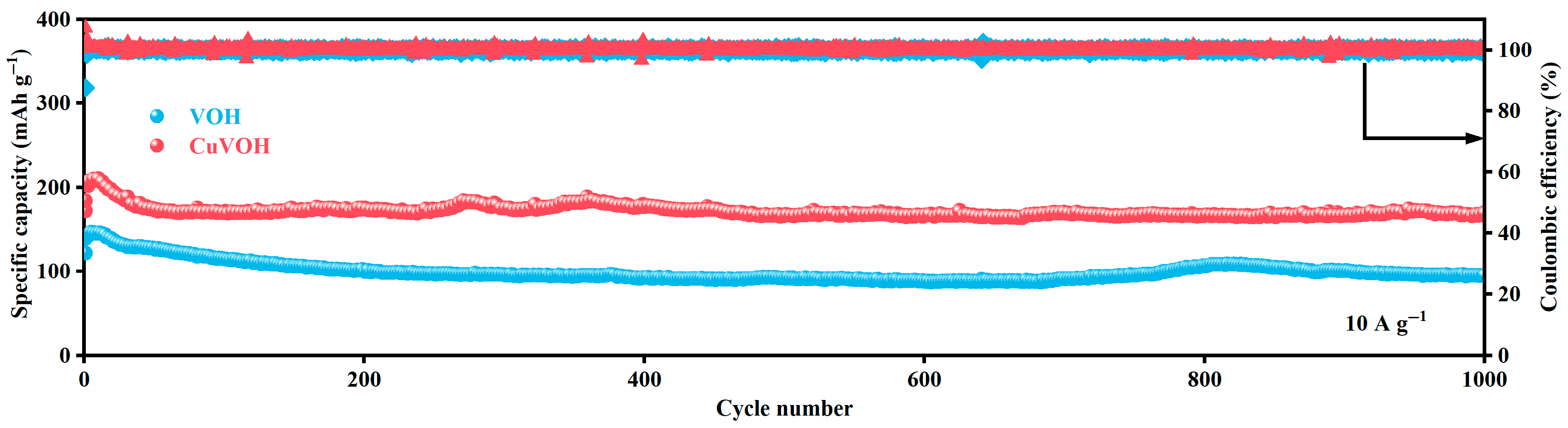
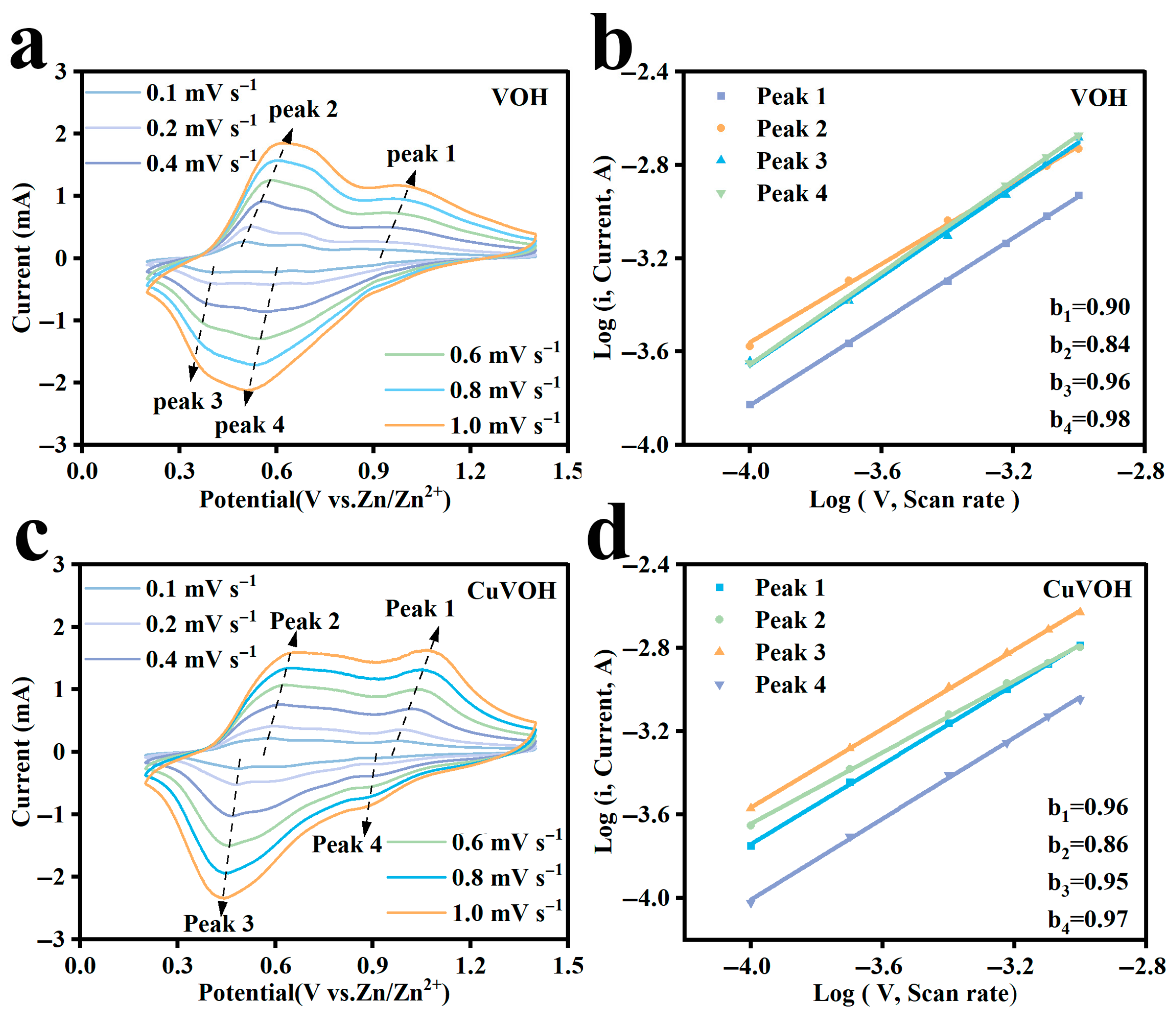
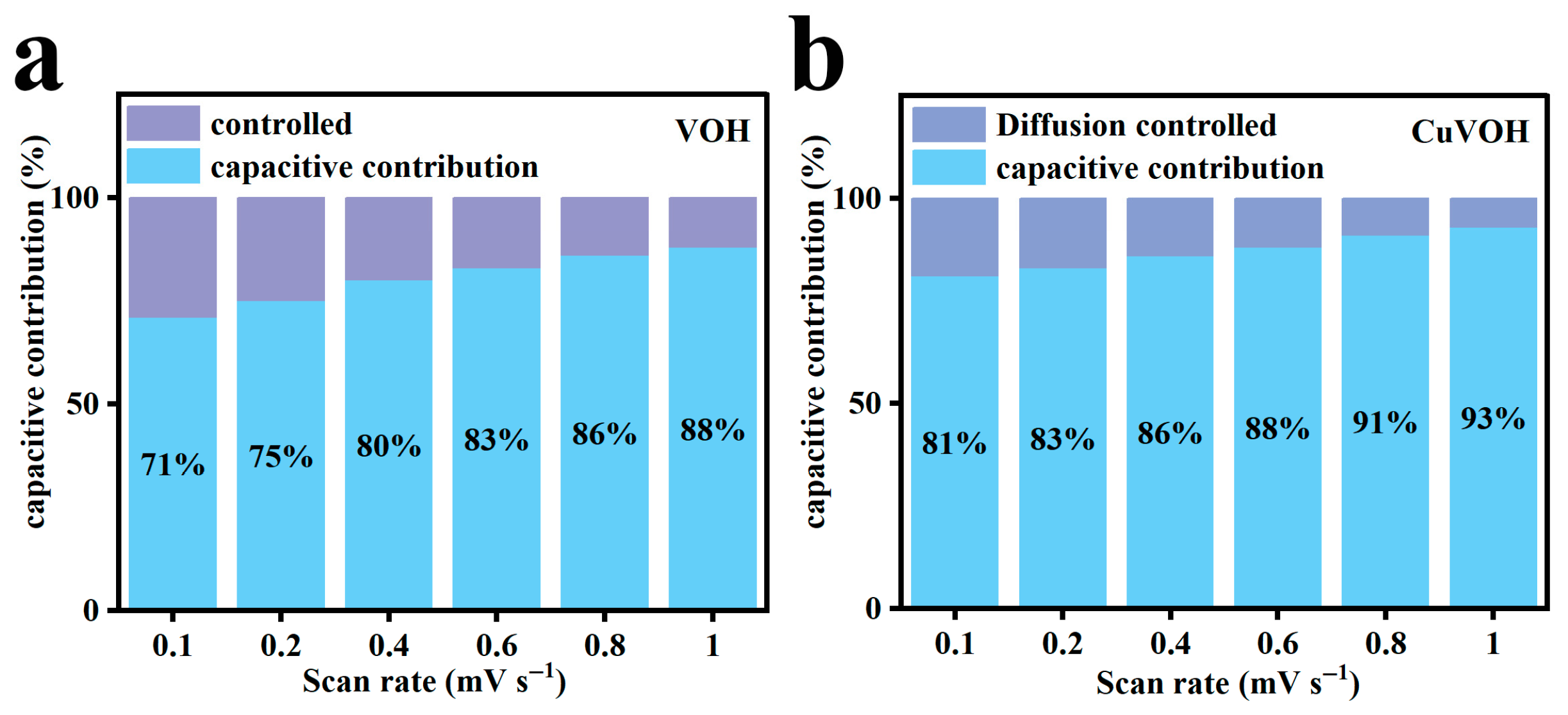
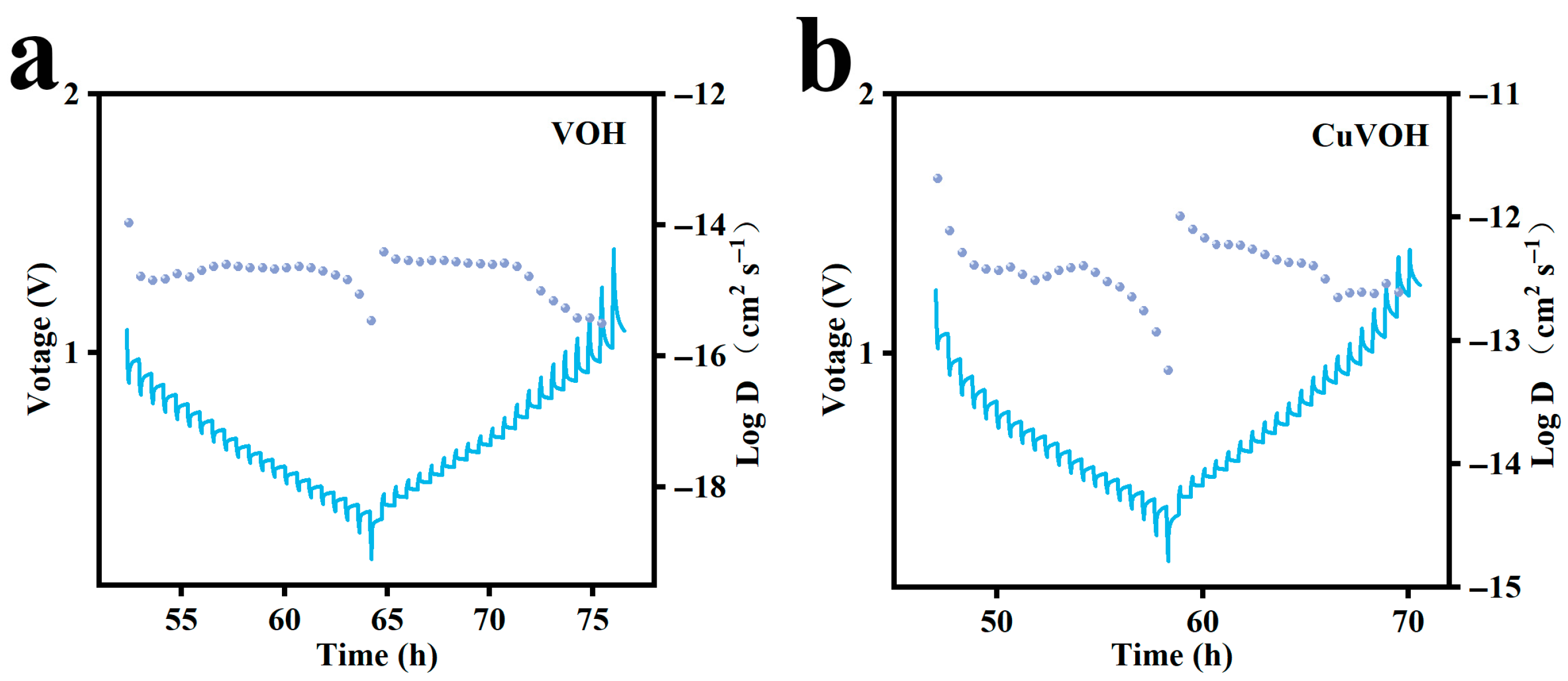
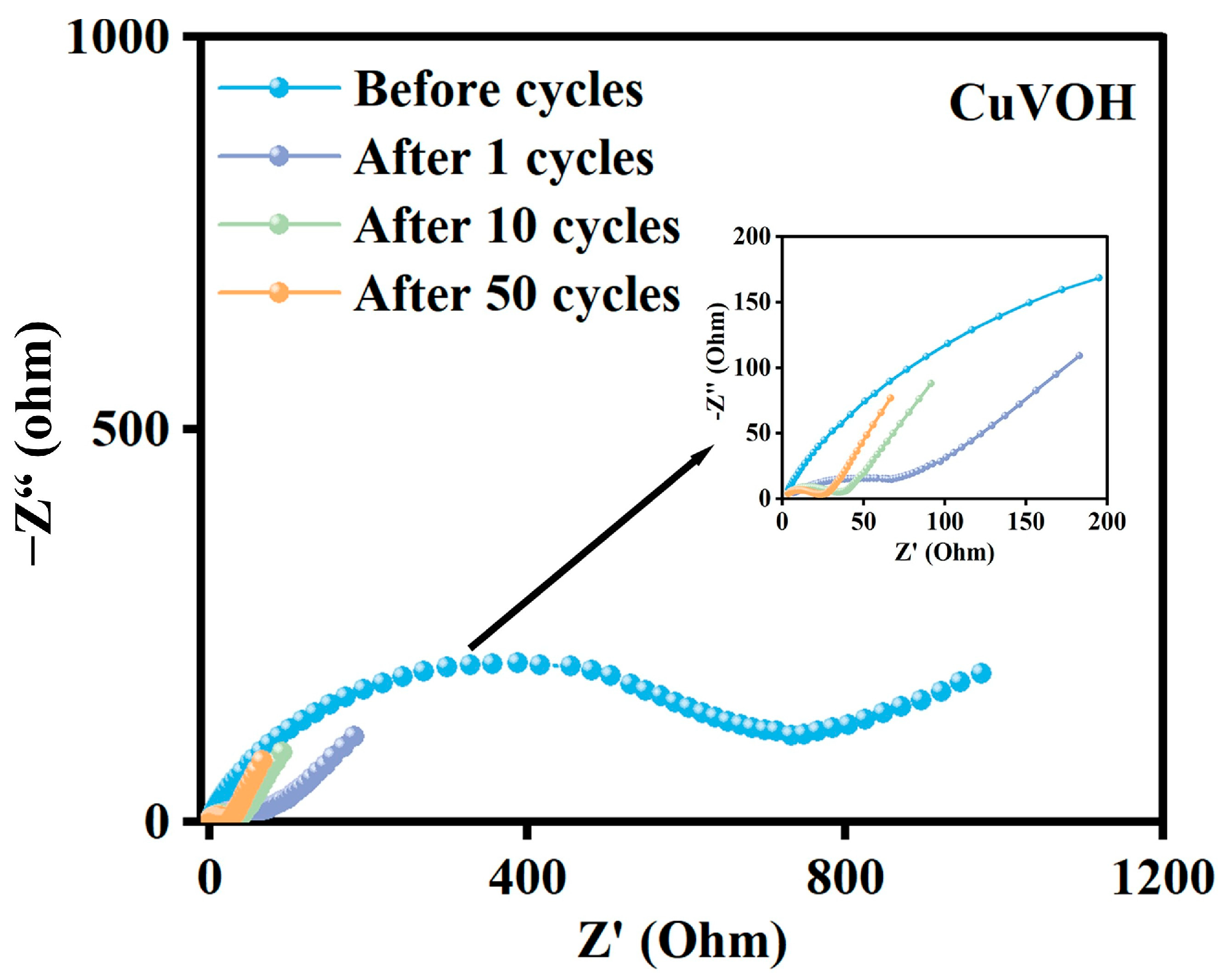
2.3. Electrochemical Properties Characterization
3. Materials and Methods
3.1. Calculation Method
3.2. Preparation of Material
3.3. Materials Characterization
3.4. Electrode Preparation and Battery Assembly
3.5. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Algarni, S.; Tirth, V.; Alqahtani, T.; Alshehery, S.; Kshirsagar, P. Contribution of renewable energy sources to the environmental impacts and economic benefits for sustainable development. Sustain. Energy Technol. Assess. 2023, 56, 103098. [Google Scholar] [CrossRef]
- Jia, X.; Liu, C.; Neale, Z.G.; Yang, J.; Cao, G. Active materials for aqueous zinc ion batteries: Synthesis, crystal structure, morphology, and electrochemistry. Chem. Rev. 2020, 120, 7795–7866. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, H.; Liu, X.H.; Yang, Z.; He, X.X.; Li, L.; Qiao, Y.; Chou, S.L. Low-cost polyanion-type sulfate cathode for sodium-ion battery. Adv. Energy Mater. 2021, 11, 2101751. [Google Scholar] [CrossRef]
- Zeng, Y.; Lai, Z.; Han, Y.; Zhang, H.; Xie, S.; Lu, X. Oxygen-vacancy and surface modulation of ultrathin nickel cobaltite nanosheets as a high-energy cathode for advanced Zn-ion batteries. Adv. Mater. 2018, 30, 1802396. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Ru, Y.; Xue, H.; Pang, H.; Xu, Q. Vanadium-based materials as positive electrode for aqueous zinc-ion batteries. Adv. Sustain. Syst. 2020, 4, 2000178. [Google Scholar] [CrossRef]
- Xu, J.; Cai, X.; Cai, S.; Shao, Y.; Hu, C.; Lu, S.; Ding, S. High-energy lithium-ion batteries: Recent progress and a promising future in applications. Energy Environ. Mater. 2023, 6, e12450. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, T.; Zhang, M. Advances in intelligent regeneration of cathode materials for sustainable lithium-ion batteries. Adv. Energy Mater. 2022, 12, 2201526. [Google Scholar] [CrossRef]
- Shadike, Z.; Tan, S.; Wang, Q.C.; Lin, R.; Hu, E.; Qu, D.; Yang, X.Q. Review on organosulfur materials for rechargeable lithium batteries. Mater. Horiz. 2021, 8, 471–500. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Huo, W.; Dong, X.; Sun, Z.; Lu, Y.; Wu, X.; Dai, L.; Wang, L.; Lin, H.; Liu, H.; et al. A review on 3D zinc anodes for zinc ion batteries. Small 2022, 6, 2200597. [Google Scholar] [CrossRef] [PubMed]
- Sari, D.; Rutt, A.; Kim, J.; Chen, Q.; Hahn, N.T.; Kim, H.; Persson, K.A.; Ceder, G. Alkali-ion-assisted activation of ε-VOPO4 as a cathode material for Mg-ion batteries. Adv. Sci. 2024, 11, 2307838. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liang, G.; Cui, X.; Liu, X.; Zhong, H.; Zhi, C.; Yang, Y. Engineering hosts for Zn anodes in aqueous Zn-ion batteries. Energy Environ. Sci. 2024, 17, 369–385. [Google Scholar] [CrossRef]
- Yi, Y.; Xing, Y.; Wang, H.; Zeng, Z.; Sun, Z.; Li, R.; Lin, H.; Ma, Y.; Pu, X.; Li, M.M.J.; et al. Deciphering anion-modulated solvation structure for calcium intercalation into graphite for Ca-ion batteries. Angew. Chem. Int. Ed. 2024, 63, e202317177. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhao, F.; Tao, B.; Wang, Z.; Wang, Y.; Sheng, J.; Tang, G.; Wang, Y.; Guo, X.; Li, J.; et al. Anode-free aqueous aluminum ion batteries. Small 2024, 20, 2402025. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.L.; Wei, J.X.; Liu, Y.; Zaman, F.; Rehman, W.; Hou, L.R.; Yuan, C.Z. V2CTx MXene and its derivatives: Synthesis and recent progress in electrochemical energy storage applications. Rare Met. 2022, 41, 775–797. [Google Scholar] [CrossRef]
- Chen, R.; Luo, R.; Huang, Y.; Wu, F.; Li, L. Advanced high energy density secondary batteries with multi-electron reaction materials. Adv. Sci. 2016, 3, 1600051. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Wang, Y.; Xie, Y.; Yuan, S.; Ding, K.; Begin, E.J.; Zhang, Y.; Bao, J.L.; Wang, Y. Zinc-coordinated imidazole-based ionic liquid as liquid salt for all-temperature aqueous zinc-ion batteries. Adv. Funct. Mater. 2024, 34, 2316788. [Google Scholar] [CrossRef]
- Xiao, Y.; Ren, J.; Li, M.; Xiao, K.; Wang, Y. Charge-tuning mediated rapid kinetics of zinc ions in aqueous Zn-ion battery. Chem. Eng. J. 2023, 474, 145801. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Li, X.; Fan, L.; Shuai, Y.; Zhang, N. Loosening zinc ions from separator boosts stable Zn plating/striping behavior for aqueous zinc ion batteries. Adv. Energy Mater. 2023, 13, 2302126. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, J.; Han, X.; Wang, Y.; Ni, Q.; Hu, Z.; Wu, C.; Bai, Y. Stabilizing Zn metal anodes via cation/anion regulation toward high energy density Zn-ion batteries. Adv. Energy Mater. 2023, 13, 2203542. [Google Scholar] [CrossRef]
- Lee, Y.H.; Jeoun, Y.; Kim, J.H.; Shim, J.; Ahn, K.S.; Yu, S.H.; Sung, Y.E. Selective ion transport layer for stable aqueous zinc-ion batteries. Adv. Funct. Mater. 2024, 34, 2310884. [Google Scholar] [CrossRef]
- Wang, M.; Meng, Y.; Li, X.; Qi, J.; Li, A.; Huang, S. Challenges and strategies for zinc anodes in aqueous Zinc-ion batteries. Chem. Eng. J. 2025, 507, 160615. [Google Scholar] [CrossRef]
- Ma, L.; Liu, X.; Fan, J.; Yu, X.; Cao, L.; Zhao, C. Mass production of robust hydrogel electrolytes for high-performance zinc-ion batteries. Mater. Horiz. 2025, 12, 2736–2744. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yin, J.; Xu, X.; Cheng, Y. Pseudocapacitive storage in cathode materials of aqueous zinc ion batteries toward high power and energy density. J. Mater. Chem. A 2022, 10, 9773–9787. [Google Scholar] [CrossRef]
- Li, L.; Jia, S.; Yue, S.; Wang, C.; Qiu, H.; Ji, Y.; Cao, M.; Zhang, D. Hydrogel-stabilized zinc ion batteries: Progress and outlook. Green Chem. 2024, 26, 6404–6422. [Google Scholar] [CrossRef]
- Lee, D.; Kim, H.I.; Kim, W.Y.; Cho, S.K.; Baek, K.; Jeong, K.; Ahn, D.B.; Park, S.; Kang, S.J.; Lee, S.Y. Water-repellent ionic liquid skinny gels customized for aqueous Zn-ion battery anodes. Adv. Funct. Mater. 2021, 31, 2103850. [Google Scholar] [CrossRef]
- Pang, X.; An, B.; Zheng, S.; Wang, B. Cathode materials of metal-ion batteries for low-temperature applications. J. Alloys Compd. 2022, 912, 165142. [Google Scholar] [CrossRef]
- Lv, T.; Peng, Y.; Zhang, G.; Liang, S.; Yang, Z.; Yang, S.; Pang, H. How about vanadium-based compounds as cathode materials for aqueous zinc ion batteries? Adv. Sci. 2023, 10, 2206907. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhao, P.; Li, X.; Liu, J.; Liu, H.; Sun, B.; Pan, K.; Song, K.; Cheng, H. Heterojunction tunnelled vanadium-based cathode materials for high-performance aqueous zinc ion batteries. J. Colloid Interface Sci. 2024, 665, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Dong, Y.; Li, J.; Li, Y.; Fan, Q.; Kuang, Q.; Zhao, Y. In situ electrochemical activation strategy toward organic cation preintercalated layered vanadium-based oxide cathode for high-performance aqueous zinc-ion batteries. ACS Appl. Mater. Interfaces 2025, 17, 16791–16801. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, H.; Liu, J.-H.; Gao, Y.; Cao, X.; Zhan, C.; Wang, Y.; Wang, S.; Chou, S.-L.; Dou, S.-X.; et al. Vanadium-based cathodes for aqueous zinc-ion batteries: Mechanism, design strategies and challenges. Energy Storage Mater. 2022, 50, 21–46. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X. Strategies for constructing manganese-based oxide electrode materials for aqueous rechargeable zinc-ion batteries. Chin. Chem. Lett. 2022, 33, 1236–1244. [Google Scholar] [CrossRef]
- Sun, Q.Q.; Du, J.Y.; Sun, T.; Zhuang, Z.B.; Xie, Z.L.; Xie, H.M.; Huang, G.; Zhang, X.B. Spatial structure design of thioether-linked naphthoquinone cathodes for high-performance aqueous zinc–organic batteries. Adv. Mater. 2024, 36, 2313388. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Chen, Z.; Tao, Z.; Wang, A.; Lai, S.; Ho, S.; Yang, Y. Anion electrostatic insertion boosts efficient zinc ferrocyanide cathode for aqueous dual-ion battery. Energy Storage Mater. 2024, 67, 103274. [Google Scholar] [CrossRef]
- Beitia, J.; Ahedo, I.; Paredes, J.I.; Goikolea, E.; Larramendi, I.R. Exploring zinc-doped manganese hexacyanoferrate as cathode for aqueous zinc-ion batteries. Nanomaterials 2024, 14, 1092. [Google Scholar] [CrossRef] [PubMed]
- Ruo, H.; Chen, L.; Huang, J.; Lv, C.; Bai, J.; Xu, S.; Chen, J.; Zhang, D.; Yang, H. Constructing low-cost stable zinc-ion batteries with sodium-rich monoclinic manganese hexacyanoferrate cathode. Surf. Interfaces 2024, 51, 104594. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, W.; Yang, T.; Gao, R.; Luo, D.; Park, H.W.; Hu, Y.; Yu, A. Vacancy and growth modulation of cobalt hexacyanoferrate by porous MXene for zinc ion batteries. Adv. Energy Mater. 2024, 14, 2400543. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, H.J.; Luo, H.; Xue, Y. Advances of vanadium-based cathodes for aqueous zinc ion batteries. Chem. Eur. J. 2025, 31, e202500219. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Gao, H.; Ji, D.; Zhao, K.; Wang, S.; Cheng, F. Vanadium-based cathodes for aqueous zinc-ion batteries: From crystal structures, diffusion channels to storage mechanisms. J. Mater. Chem. A 2021, 9, 5258–5275. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, X.; Hu, X.; Wang, T.; Parkin, I.P.; Wang, M.; Boruah, B.D. Polyaniline and water pre-intercalated V2O5 cathodes for high-performance planar zinc-ion micro-batteries. Chem. Eng. J. 2024, 487, 150384. [Google Scholar] [CrossRef]
- Xu, Q.; Chu, N.; Wang, Y.; Wang, H.; Xu, T.; Li, X.; Huang, S.; Li, X.; Luo, Y.; Yang, H.Y.; et al. 3D printed low-tortuosity and ultra-thick hierarchical porous electrodes for high-performance wearable quasi-solid-state Zn-VOH batteries. Adv. Sci. 2025, 12, 2401660. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cui, P.; Wang, X.; Chang, M.; Yu, Y.; You, J.; Hu, F.; Wu, Y.; Zhu, K. Co-substitution engineering boosting the kinetics and stablity of VO2 for Zn ion batteries. Adv. Funct. Mater. 2024, 34, 2407925. [Google Scholar] [CrossRef]
- Wu, Z.; Yao, J.; Chen, C.; Chen, X.; Pan, X.; Zheng, J.; Gan, Y.; Li, J.; Liu, X.; Xia, C.; et al. Ammonium intercalation engineering regulated structural stability of V6O13 cathodes for durable zinc ion batteries. Chem. Eng. J. 2024, 479, 147889. [Google Scholar] [CrossRef]
- Jiang, H.; Yue, P.; Gao, Q.; Zhang, S.; Gao, M.; Wang, J.; Liu, Y.; Hou, L.; Chen, M.; Yuan, C. Construction of nano ZnV2O4/N-doped porous carbon composites with optimized ionic and electronic conductivities as competitive cathodes toward zinc-ion capacitors. Nanomaterials 2024, 10, e202400445. [Google Scholar] [CrossRef]
- Wu, M.; Shi, C.; Yang, J.; Zong, Y.; Chen, Y.; Ren, Z.; Zhao, Y.; Li, Z.; Zhang, W.; Wang, L.; et al. The LiV3O8 superlattice cathode with optimized zinc ion insertion chemistry for high mass-loading aqueous zinc-ion batteries. Adv. Mater. 2024, 36, 2310434. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Sun, C.; Yu, Y.; Zhao, K.; Zhang, Z.; Voyles, P.M.; Chen, G.; Wei, Y.; Wang, X. H2V3O8 nanowire/graphene electrodes for aqueous rechargeable zinc ion batteries with high rate capability and large capacity. Adv. Energy Mater. 2018, 8, 1800144. [Google Scholar] [CrossRef]
- Kuhn, A.; Pérez-Flores, J.C.; Prado-Gonjal, J.; Morán, E.; Hoelzel, M.; Díez-Gómez, V.; Sobrados, I.; Sanz, J.; García-Alvarado, F. Lithium intercalation mechanism and critical role of structural water in layered H2V3O8 high-capacity cathode material for lithium-ion batteries. Chem. Mater. 2022, 34, 694–705. [Google Scholar] [CrossRef]
- Lv, T.; Zhu, G.; Dong, S.; Kong, Q.; Peng, Y.; Jiang, S.; Zhang, G.; Yang, Z.; Yang, S.; Dong, X.; et al. Co-intercalation of dual charge carriers in metal-ion-confining layered vanadium oxide nanobelts for aqueous zinc-ion batteries. Angew. Chem. Int. Ed. 2023, 62, e202216089. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Wu, T.; Huang, K. A high-voltage activated high-performance cathode for aqueous Zn-ion batteries. Energy Storage Mater. 2021, 38, 473–481. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
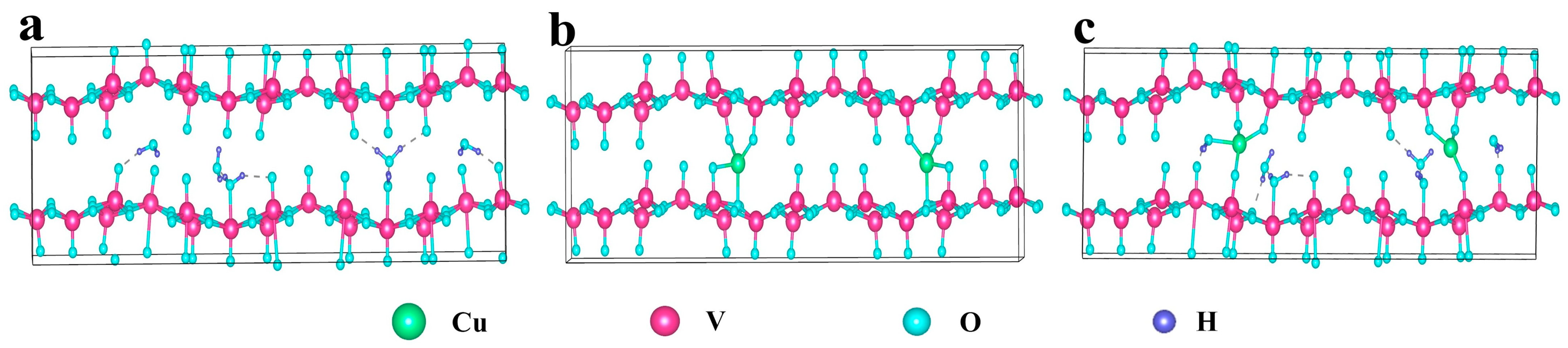
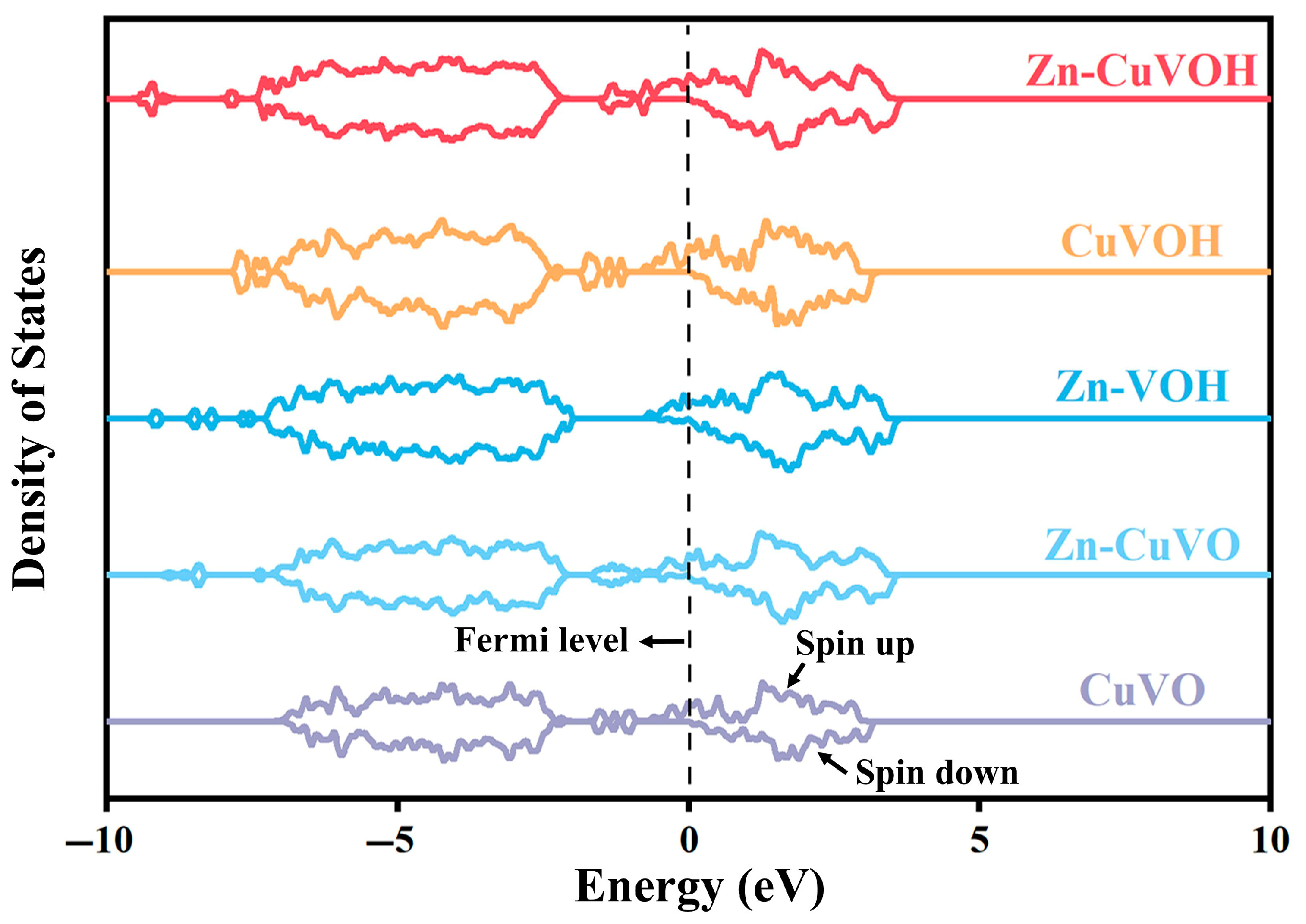
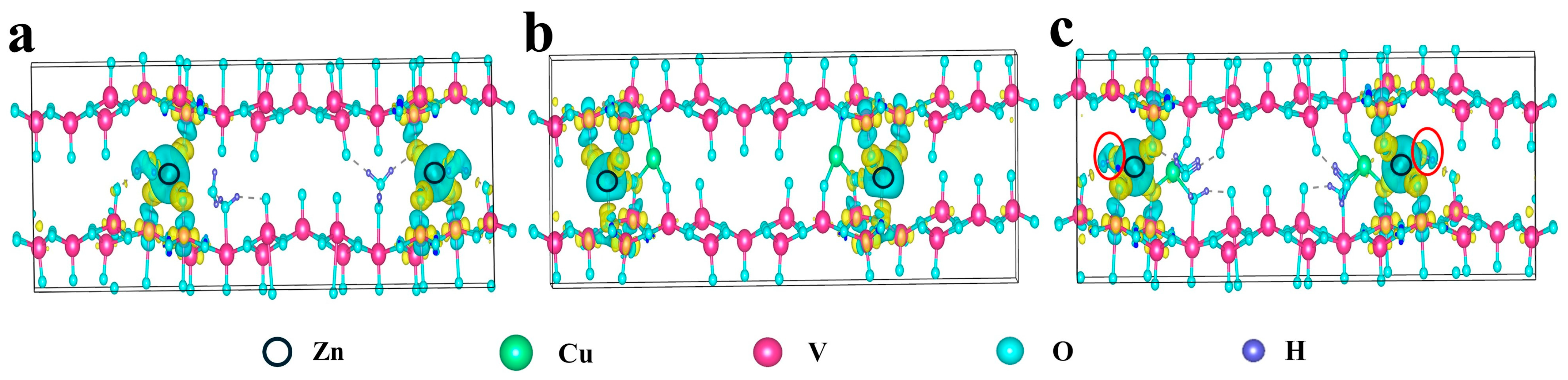
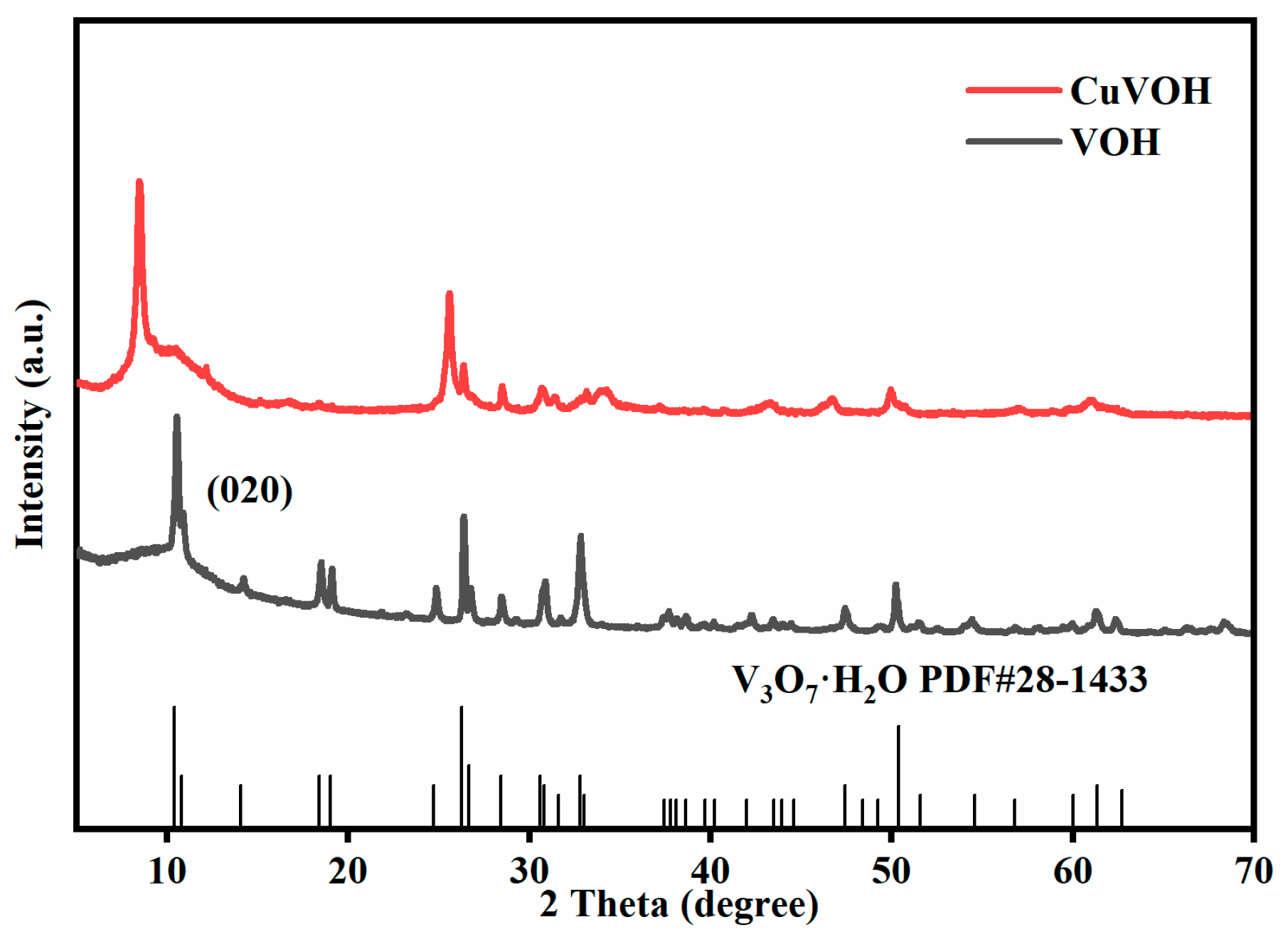
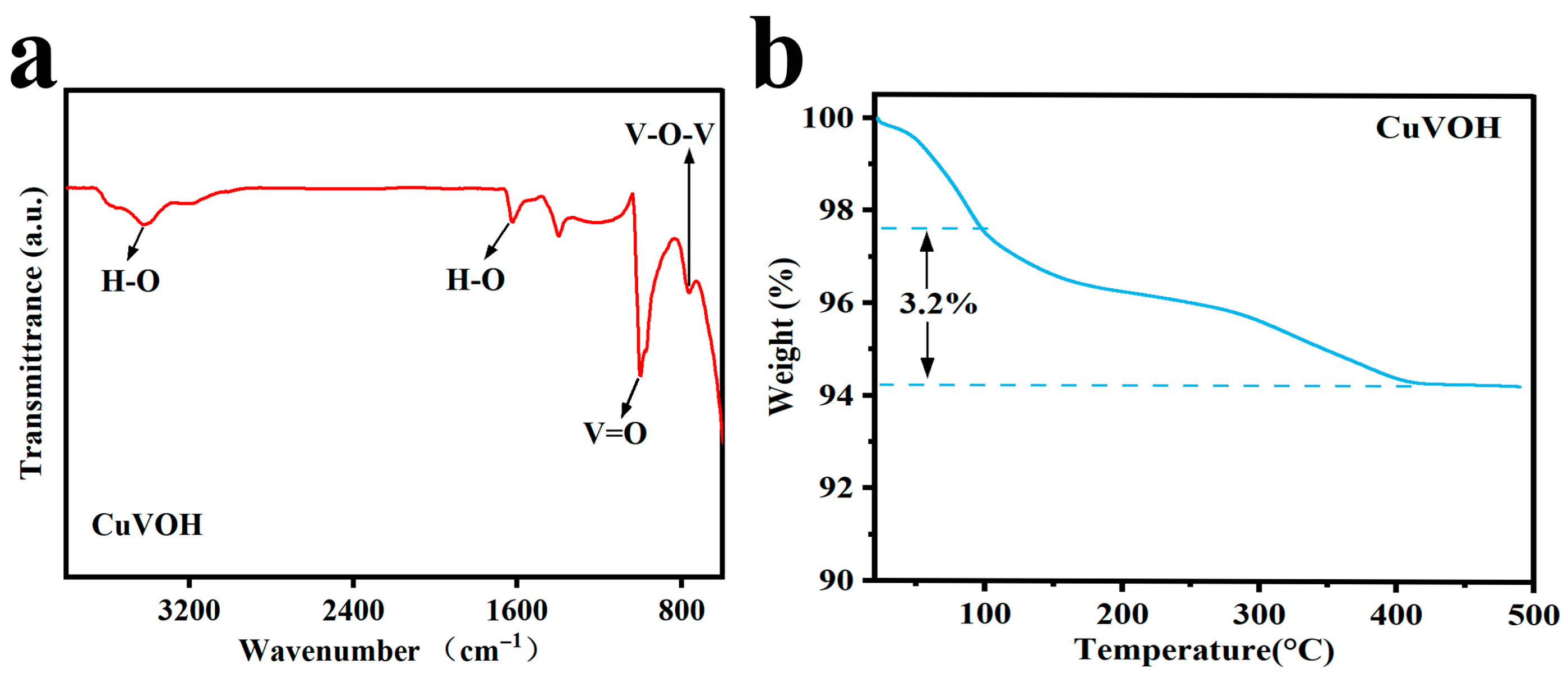
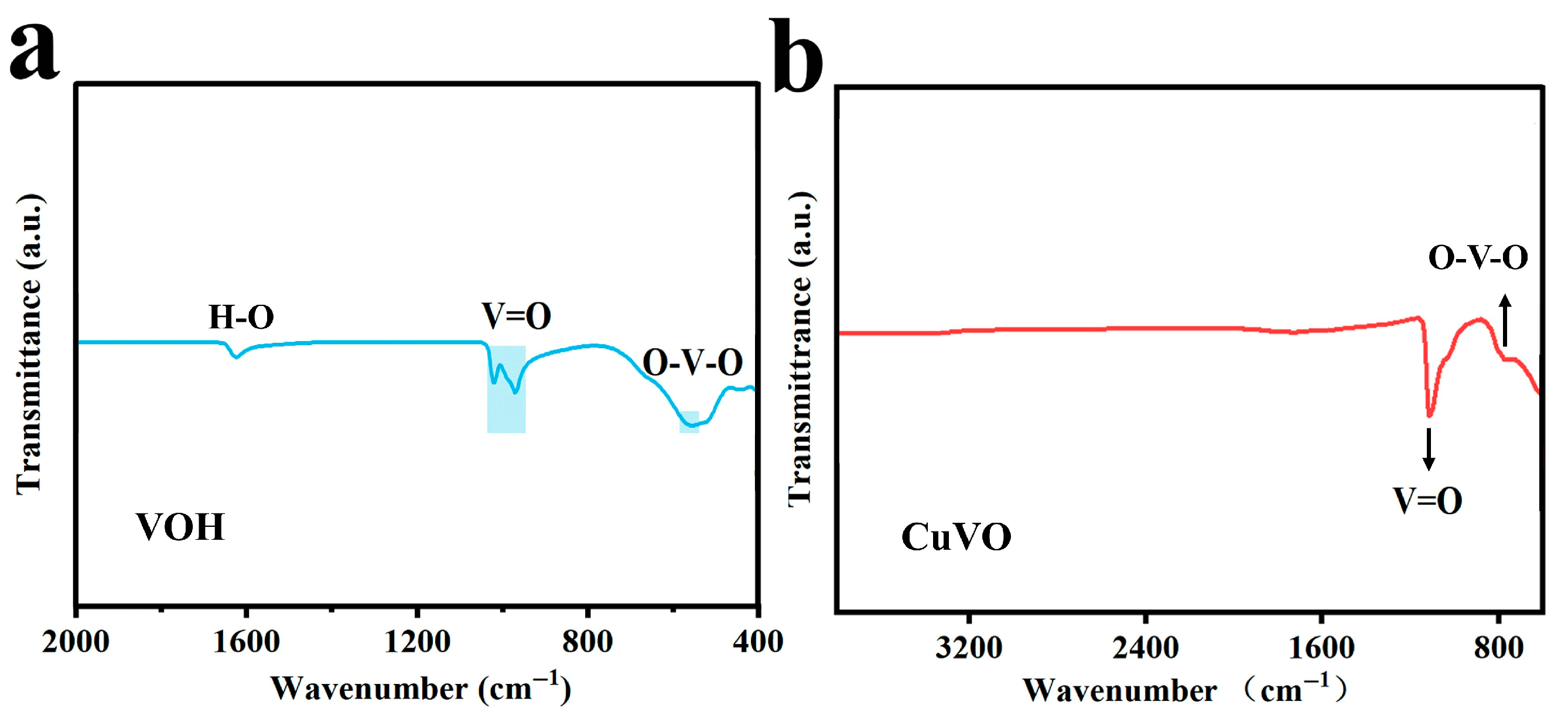
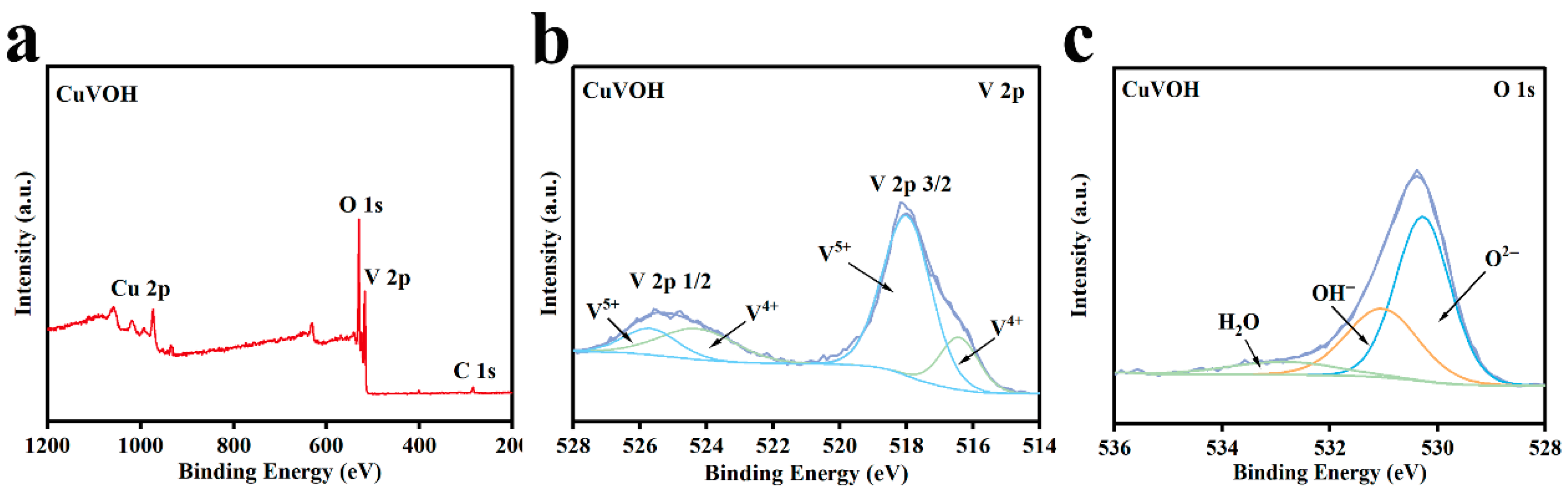
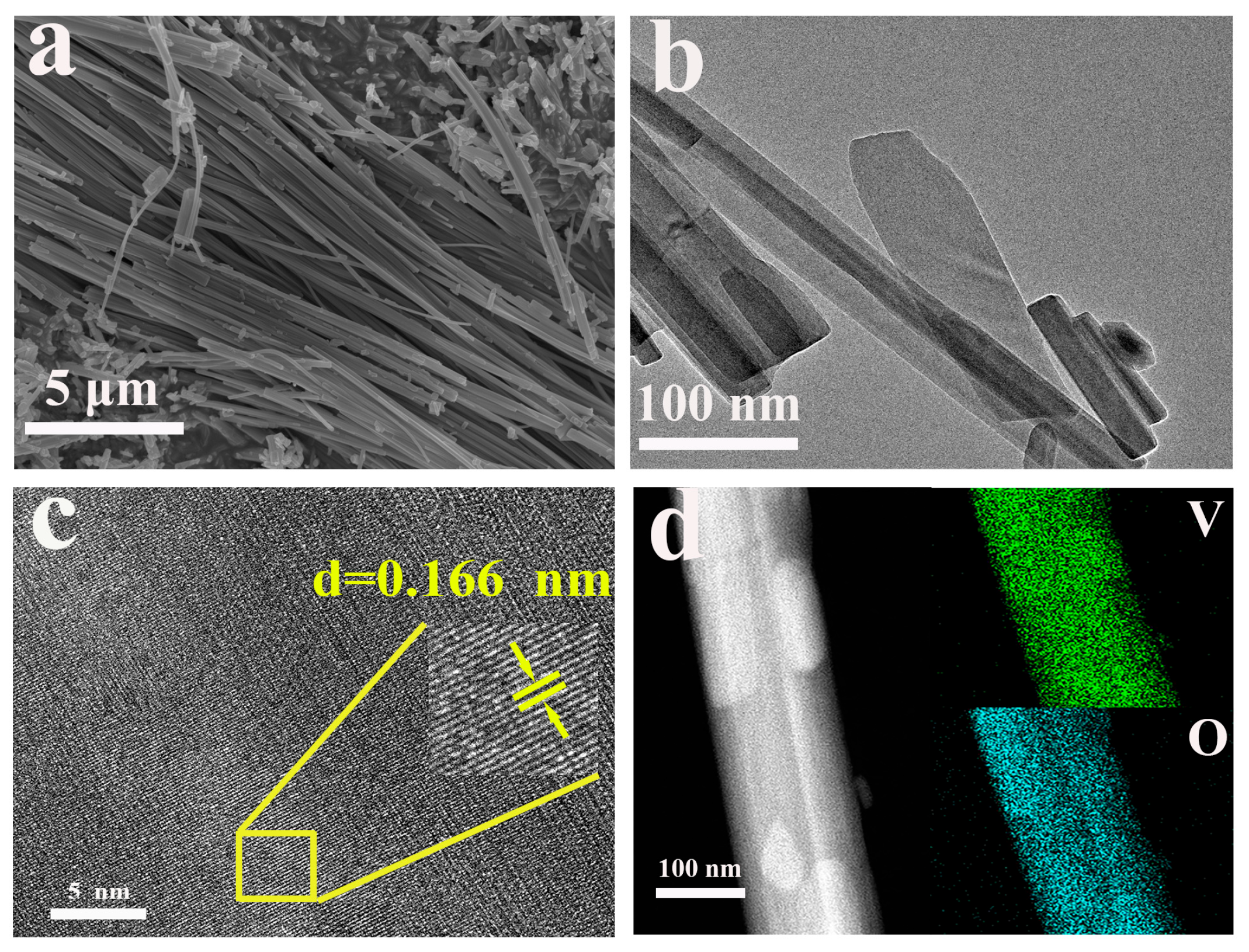
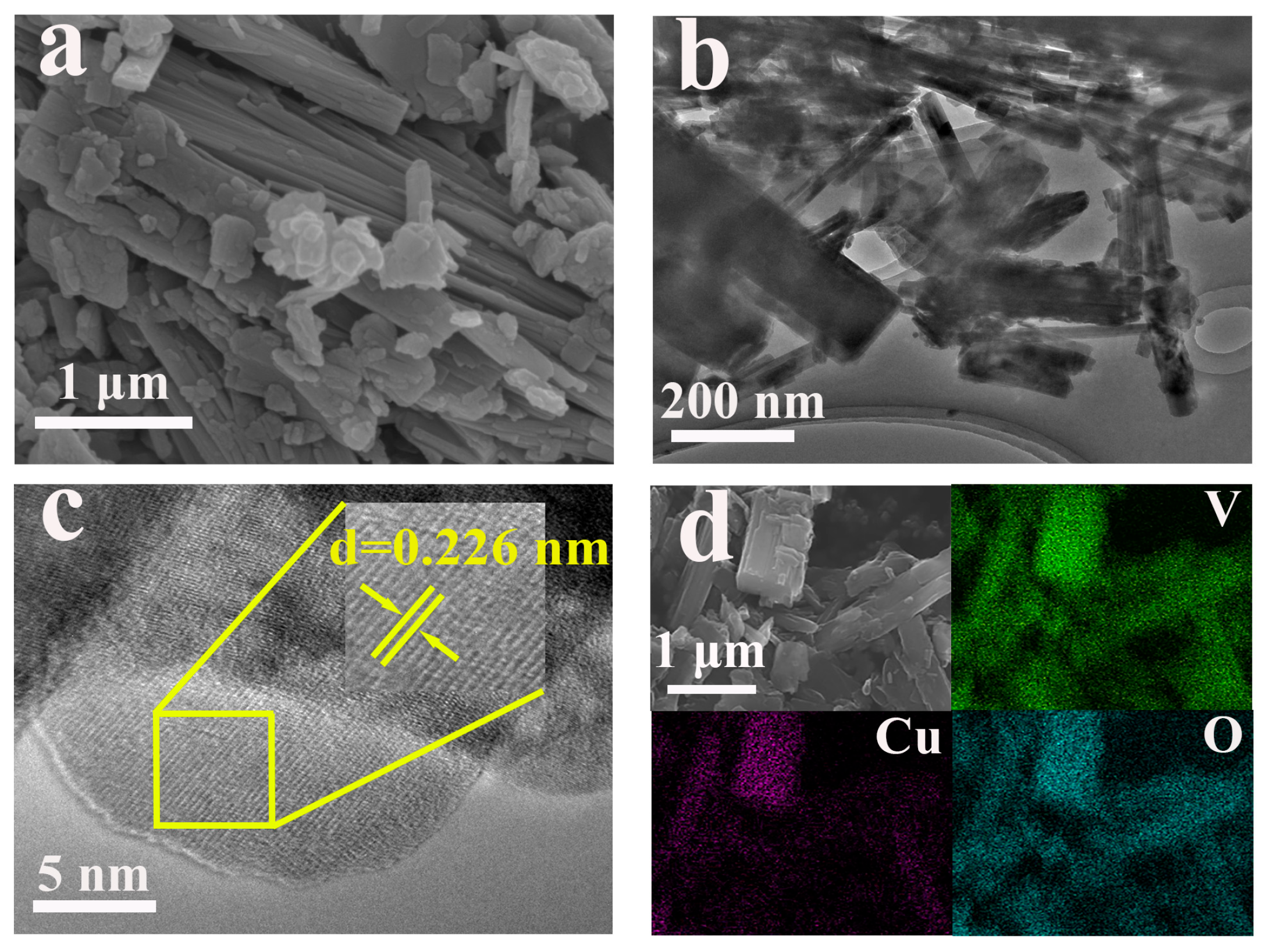
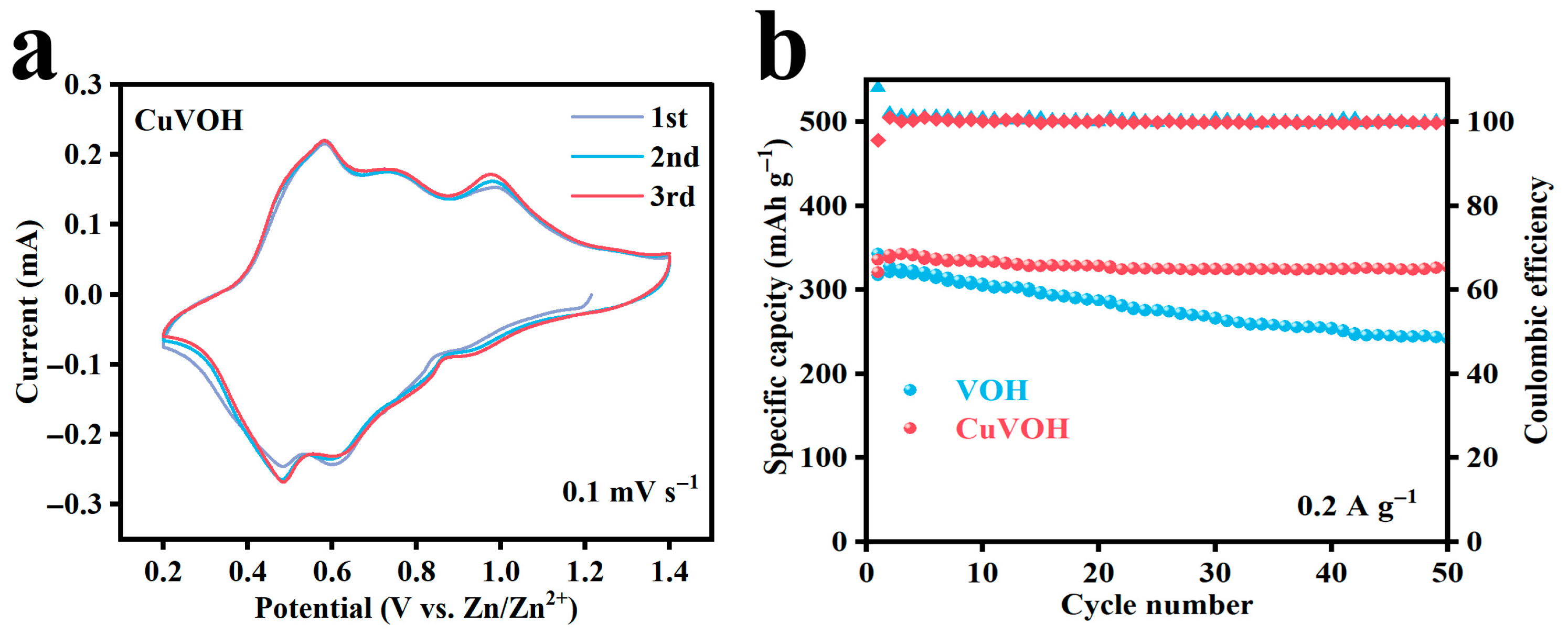
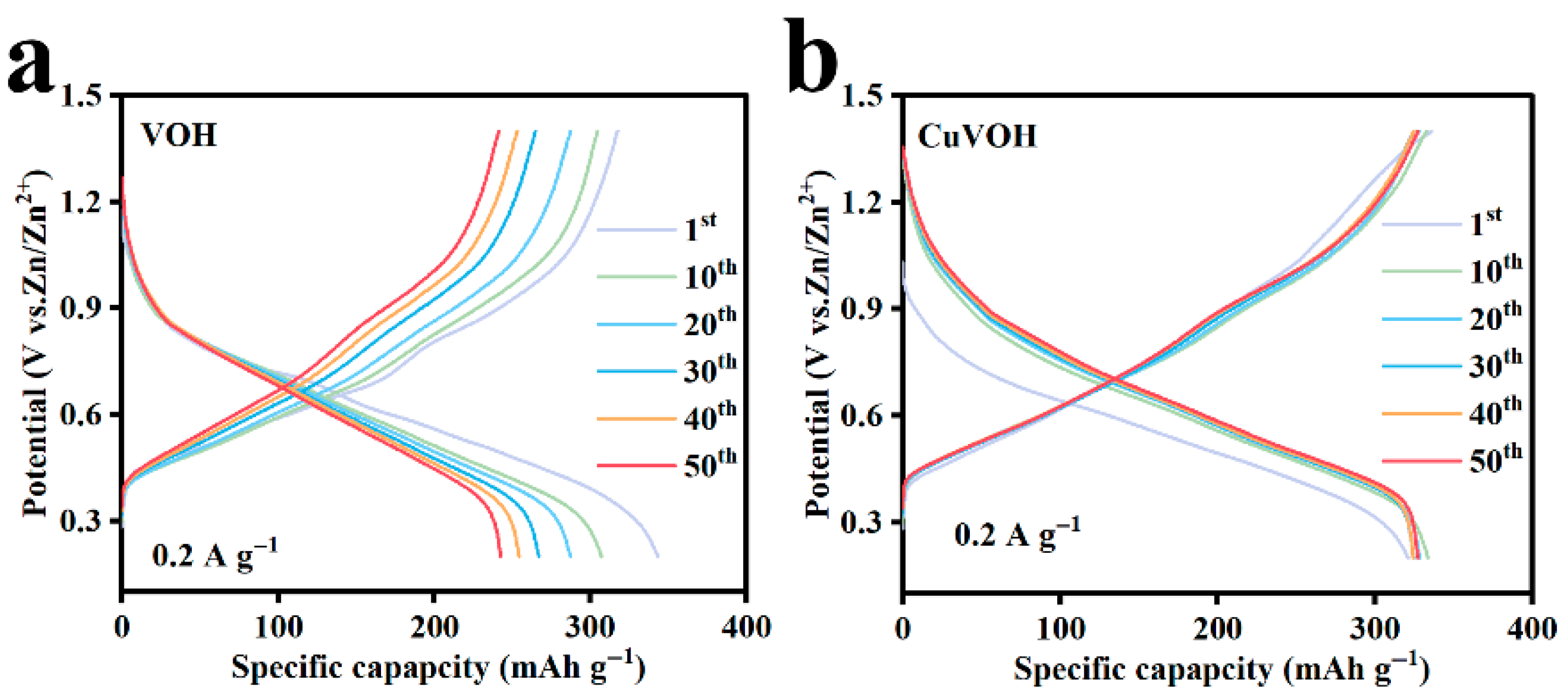
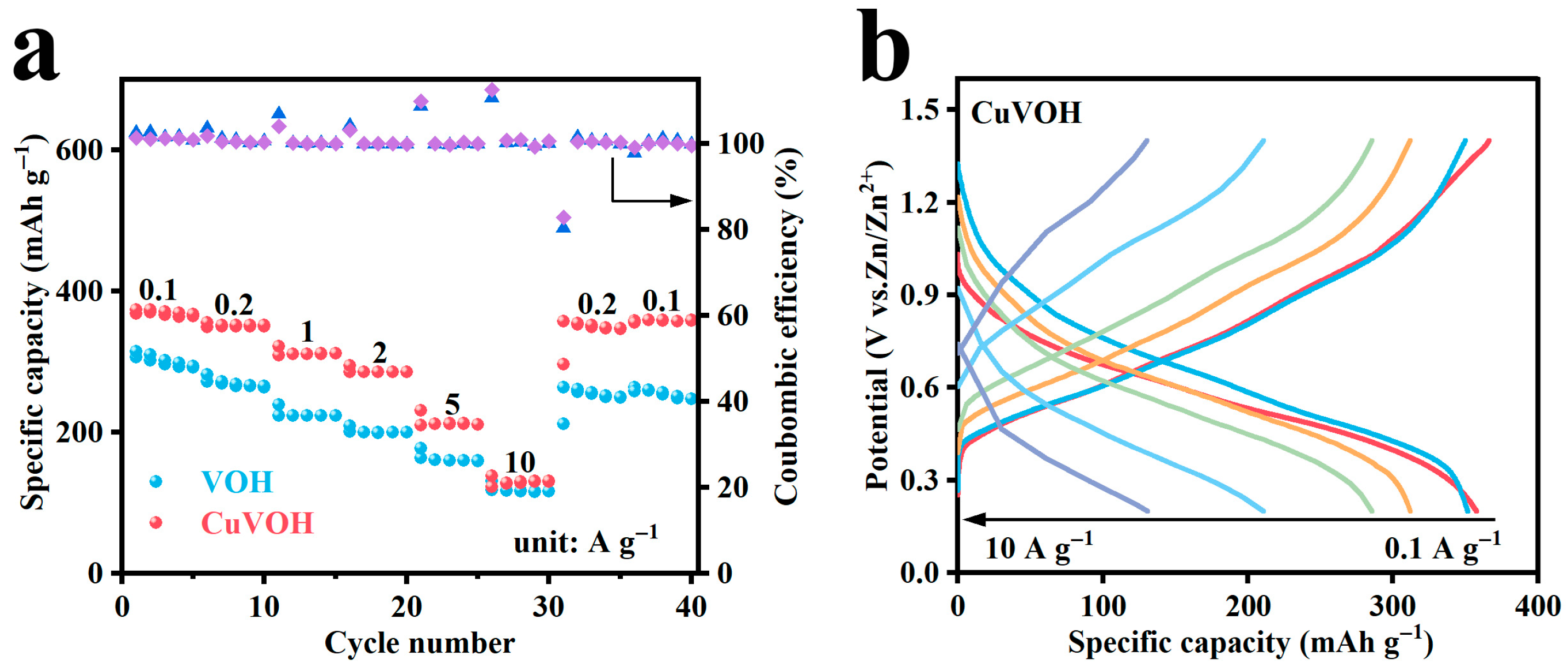
| Element | Mass Content (mg kg−1) | Molar Amount (mmol kg−1) |
|---|---|---|
| Copper (Cu) | 39,965 | 628.91 |
| Vanadium (V) | 567,432 | 11,138.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.; Wei, M.; Zhang, Y. Cu2+ Intercalation and Structural Water Enhance Electrochemical Performance of Cathode in Zinc-Ion Batteries. Molecules 2025, 30, 3092. https://doi.org/10.3390/molecules30153092
Lin H, Wei M, Zhang Y. Cu2+ Intercalation and Structural Water Enhance Electrochemical Performance of Cathode in Zinc-Ion Batteries. Molecules. 2025; 30(15):3092. https://doi.org/10.3390/molecules30153092
Chicago/Turabian StyleLin, He, Mengdong Wei, and Yu Zhang. 2025. "Cu2+ Intercalation and Structural Water Enhance Electrochemical Performance of Cathode in Zinc-Ion Batteries" Molecules 30, no. 15: 3092. https://doi.org/10.3390/molecules30153092
APA StyleLin, H., Wei, M., & Zhang, Y. (2025). Cu2+ Intercalation and Structural Water Enhance Electrochemical Performance of Cathode in Zinc-Ion Batteries. Molecules, 30(15), 3092. https://doi.org/10.3390/molecules30153092






