Stereoisomeric Effects of Diammoniumcyclohexane Counterions on the Self-Assembly of Amino Acid-Based Surfactants
Abstract
1. Introduction
2. Results and Discussion
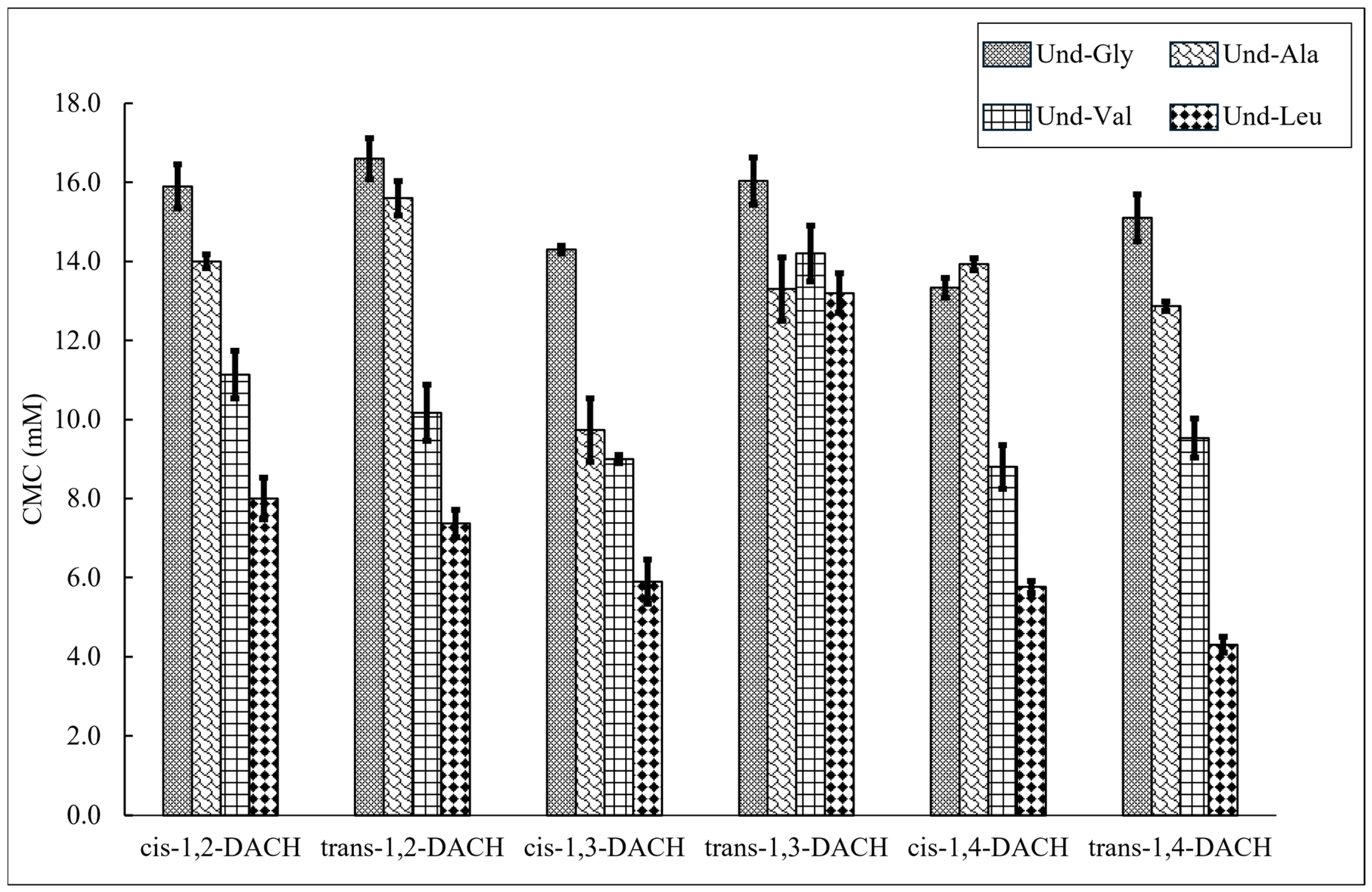
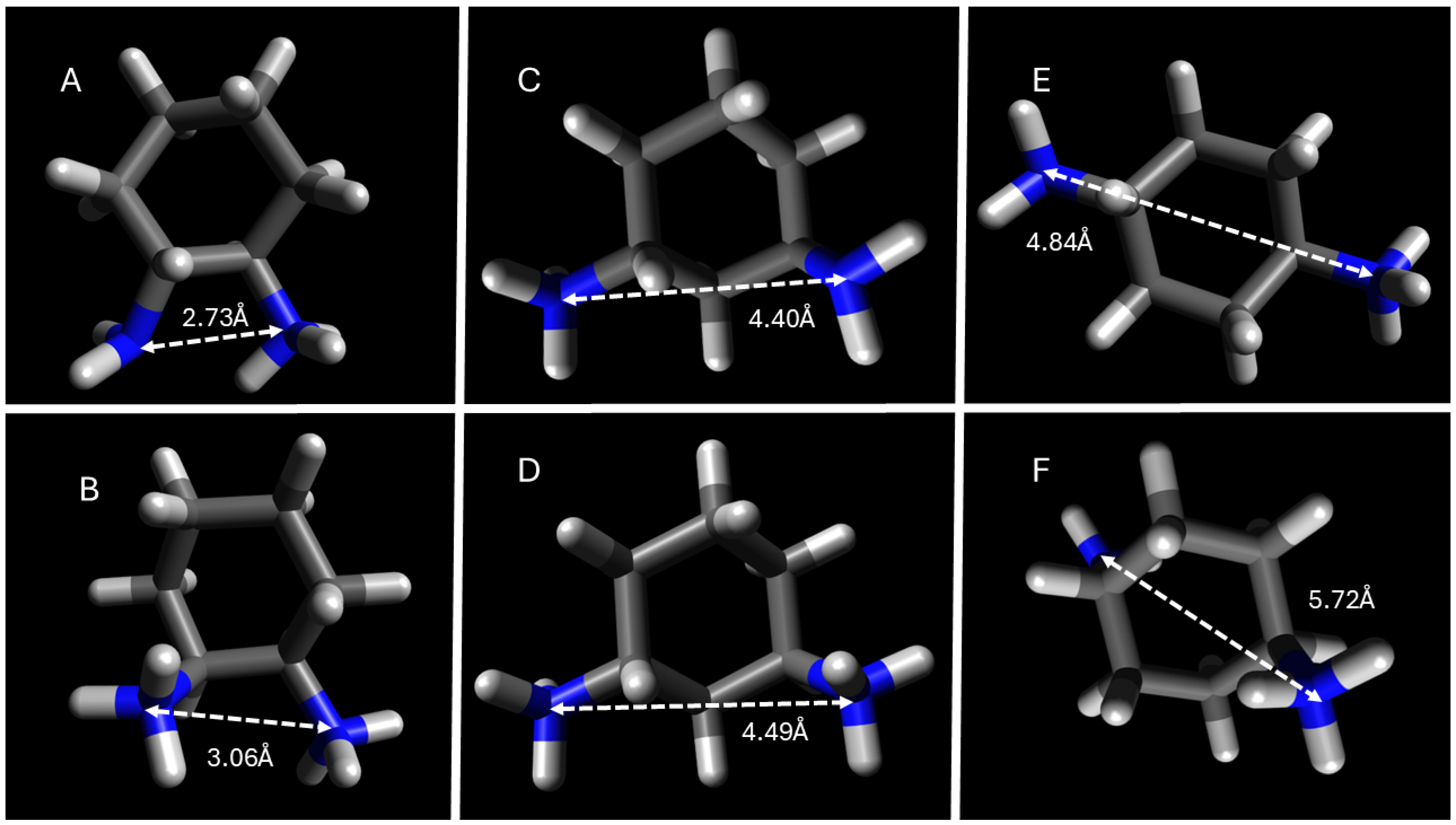
2.1. Structural Effects on Critical Micelle Concentration
2.2. Factors Affecting Micellar Hydrodynamic Diameter
2.3. Degree of Counterion Binding
2.4. pH Measurements and Calculations
3. Materials and Methods
3.1. Materials
3.2. Analytical Techniques
3.3. Synthesis of AABSs and Their Respective DACH Counterions
3.4. Variable Concentration Conductivity Measurements
3.5. Photon Correlation Spectroscopy/DLS Size Measurements
3.6. Degree of Counterion Binding Calculations
3.7. Density Functional Theory Calculations
3.8. pH Measurements and Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AABS | Amino Acid-Based Surfactant |
| DLS | Dynamic Light Scattering |
| CMC | Critical Micelle Concentration |
| DACH | Diamino Cyclo Hexane |
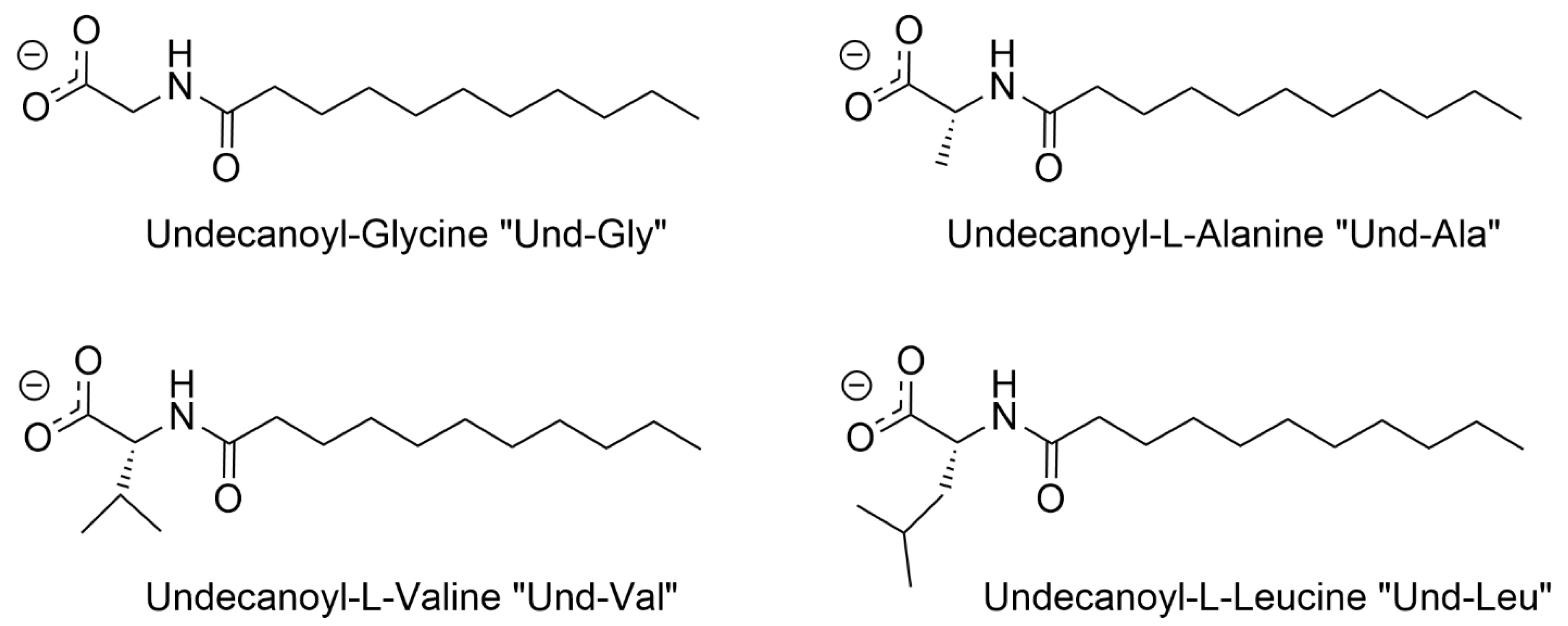
| Und-Gly | Undecanoyl-Glycine |
| Und-Ala | Undecanoyl-L-Alanine |
| Und-Val | Undecanoyl-L-Valine |
| Und-Leu | Undecanoyl-L-Leucine |
| DFT | Density Functional Theory |
References
- Badmus, S.O.; Amusa, H.K.; Oyehan, T.A.; Saleh, T.A. Environmental Risks and Toxicity of Surfactants: Overview of Analysis, Assessment, and Remediation Techniques. Environ. Sci. Pollut. Res. 2021, 28, 60987–61012. [Google Scholar] [CrossRef]
- Raju, S.; Sivamurugan, M.; Gunasagaran, K.; Subramani, T.; Natesan, M. Preliminary Studies on the Occurrence of Nonylphenol in the Marine Environments, Chennai—A Case Study. J. Basic Appl. Zool. 2018, 79, 52. [Google Scholar] [CrossRef]
- García, M.T.; Ribosa, I.; Guindulain, T.; Sánchez-Leal, J.; Vives-Rego, J. Biodegradability and Aquatic Toxicity of Quaternary Ammonium-Based Gemini Surfactants: Effect of the Spacer on Their Ecological Properties. Chemosphere 2016, 154, 155–160. [Google Scholar] [CrossRef]
- Kim, S.; Ji, K.; Shin, H.; Park, S.; Kho, Y.; Park, K.; Kim, K.; Choi, K. Occurrences of Benzalkonium Chloride in Streams near a Pharmaceutical Manufacturing Complex in Korea and Associated Ecological Risk. Chemosphere 2020, 256, 127084. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Kim, Y.; Oh, S.; Ahn, B.; Jo, H.; Choi, K. Toxicity of Perfluorooctane Sulfonic Acid and Perfluorooctanoic Acid on Freshwater Macroinvertebrates (Daphnia magna and Moina macrocopa) and Fish (Oryzias latipes). Environ. Toxicol. Chem. 2008, 27, 2159–2168. [Google Scholar] [CrossRef]
- Nagtode, V.S.; Cardoza, C.; Yasin, H.K.A.; Mali, S.N.; Tambe, S.M.; Roy, P.; Singh, K.; Goel, A.; Amin, P.D.; Thorat, B.R.; et al. Green Surfactants (Biosurfactants): A Petroleum-Free Substitute for Sustainability—Comparison, Applications, Market, and Future Prospects. ACS Omega 2023, 8, 11674–11699. [Google Scholar] [CrossRef]
- Guo, J.; Sun, L.; Zhang, F.; Sun, B.; Xu, B.; Zhou, Y. Review: Progress in Synthesis, Properties and Application of Amino Acid Surfactants. Chem. Phys. Lett. 2022, 794, 139499. [Google Scholar] [CrossRef]
- Bordes, R.; Holmberg, K. Amino Acid-Based Surfactants—Do They Deserve More Attention? Adv. Colloid Interface Sci. 2015, 222, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Ananthapadmanabhan, K.P. Amino-Acid Surfactants in Personal Cleansing (Review). Tenside Surfactants Deterg. 2019, 56, 378–386. [Google Scholar] [CrossRef]
- Ansari, N.H.; Shahid, S.; Khan, M.S.; Rizvi, N.Z.; Iqubal, S.M.S.; Bahafi, A. Amino Acid-Based Biosurfactants: Promising and Ecofriendly Biomolecules for Attaining Sustainable Agriculture and Environmental Safety. Colloid J. 2025, 87, 78–100. [Google Scholar] [CrossRef]
- Takassi, M.A.; Zargar, G.; Madani, M.; Zadehnazari, A. The Preparation of an Amino Acid-Based Surfactant and Its Potential Application as an EOR Agent. Petrol. Sci. Technol. 2017, 35, 385–391. [Google Scholar] [CrossRef]
- Mayer, J.D.; Rauscher, R.M.; Fritz, S.R.; Fang, Y.; Billiot, E.J.; Billiot, F.H.; Morris, K.F. An Investigation of the Effect of pH on Micelle Formation by a Glutamic Acid-Based Biosurfactant. Colloids Interfaces 2024, 8, 38. [Google Scholar] [CrossRef]
- Bhatt, J.; Rai, A.K.; Gupta, M.; Vyas, S.; Ameta, R.; Ameta, S.C.; Chavoshani, A.; Hashemi, M. Surfactants: An Emerging Face of Pollution. In Micropollutants and Challenges; Chavoshani, A., Hashemi, M., Amin, M.M., Ameta, S.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 145–178. [Google Scholar] [CrossRef]
- Abooali, D.; Soleimani, R. Structure-Based Modeling of Critical Micelle Concentration (CMC) of Anionic Surfactants in Brine Using Intelligent Methods. Sci. Rep. 2023, 13, 13361. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yue, Z.; Xie, J.; Wang, W.; Zhu, H.; Zhang, E.; Cao, Z. Micelles with Ultralow Critical Micelle Concentration as Carriers for Drug Delivery. Nat. Biomed. Eng. 2018, 2, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, E.; Yang, J.; Cao, Z. Strategies to Improve Micelle Stability for Drug Delivery. Nano Res. 2018, 11, 4985–4998. [Google Scholar] [CrossRef]
- Torchilin, V.P. Structure and Design of Polymeric Surfactant-Based Drug Delivery Systems. J. Control. Release 2001, 73, 137–172. [Google Scholar] [CrossRef]
- Wang, Q.; Atluri, K.; Tiwari, A.K.; Babu, R.J. Exploring the Application of Micellar Drug Delivery Systems in Cancer Nanomedicine. Pharmaceuticals 2023, 16, 433. [Google Scholar] [CrossRef]
- Maboudi, A.H.; Lotfipour, M.H.; Rasouli, M.; Azhdari, M.H.; MacLoughlin, R.; Bekeschus, S.; Doroudian, M. Micelle-Based Nanoparticles with Stimuli-Responsive Properties for Drug Delivery. Nanotechnol. Rev. 2024, 13, 20230218. [Google Scholar] [CrossRef]
- Farhoudi, L.; Hosseinikhah, S.M.; Vahdat-Lasemi, F.; Sukhorukov, V.N.; Kesharwani, P.; Sahebkar, A. Polymeric Micelles Paving the Way: Recent Breakthroughs in Camptothecin Delivery for Enhanced Chemotherapy. Int. J. Pharm. 2024, 659, 124292. [Google Scholar] [CrossRef]
- Alvarez, M.A.; Black, N.; Blanco, S.E.; Reid, K.R.; Billiot, E.J.; Billiot, F.H.; Morris, K.F. Influence of Linear Diamine Counterions on the Self-Assembly of Glycine-, Alanine-, Valine-, and Leucine-Based Amphiphiles. Molecules 2024, 29, 4436. [Google Scholar] [CrossRef]
- Maynard-Benson, A.; Alekisch, M.; Wall, A.; Billiot, E.J.; Billiot, F.H.; Morris, K.F. Characterization of Micelle Formation by the Single Amino Acid-Based Surfactants Undecanoic L-Isoleucine and Undecanoic L-Norleucine in the Presence of Diamine Counterions with Varying Chain Lengths. Colloids Interfaces 2023, 7, 28. [Google Scholar] [CrossRef]
- Fletcher, J.; Mahant, G.; Witzleb, T.; Busche, R.; Garcia, M.; Fang, Y.; Billiot, E.J.; Billiot, F.H.; Morris, K.F. NMR Investigation of Counterion Binding to Undecyl LL-Leucinevalanate Micelles. J. Dispers. Sci. Technol. 2022, 45, 284–295. [Google Scholar] [CrossRef]
- Garcia, M.; Black, N.; Billiot, E.; Billiot, F.; Morris, K.; Fang, Y. Chiral Recognition of Dansyl Derivatives with an Amino Acid-Based Molecular Micelle: A Molecular Dynamics Investigation. Open J. Phys. Chem. 2021, 11, 64–86. [Google Scholar] [CrossRef]
- Ramos, Z.; Rothbauer, G.A.; Turner, J.; Lewis, C.; Morris, K.; Billiot, E.; Billiot, F.; Fang, Y. Comparison of Chiral Recognition of Binaphthyl Derivatives with L-Undecyl-Leucine Surfactants in the Presence of Arginine and Sodium Counterions. J. Chromatogr. Sci. 2019, 57, 54–62. [Google Scholar] [CrossRef]
- Rothbauer, G.A.; Rutter, E.A.; Reuter-Seng, C.; Vera, S.; Billiot, E.J.; Fang, Y.; Billiot, F.H.; Morris, K.F. Nuclear Magnetic Resonance Investigation of the Effect of pH on Micelle Formation by the Amino Acid-Based Surfactant Undecyl L-Phenylalaninate. J. Surfactants Deterg. 2018, 21, 139–153. [Google Scholar] [CrossRef]
- Lewis, C.; Hughes, B.H.; Vasquez, M.; Wall, A.M.; Northrup, V.L.; Witzleb, T.J.; Billiot, E.J.; Fang, Y.; Billiot, F.H.; Morris, K.F. Effect of pH on the Binding of Sodium, Lysine, and Arginine Counterions to L-Undecyl Leucinate Micelles. J. Surfactants Deterg. 2016, 19, 1175–1188. [Google Scholar] [CrossRef]
- Ropers, M.H.; Czichocki, G.; Brezesinski, G. Counterion Effect on the Thermodynamics of Micellization of Alkyl Sulfates. J. Phys. Chem. B 2003, 107, 5281–5288. [Google Scholar] [CrossRef]
- Benrraou, M.; Bales, B.L.; Zana, R. Effect of the Nature of the Counterion on the Properties of Anionic Surfactants. 1. CMC, Ionization Degree at the CMC and Aggregation Number of Micelles of Sodium, Cesium, Tetramethylammonium, Tetraethylammonium, Tetrapropylammonium, and Tetrabutylammonium Dodecyl Sulfates. J. Phys. Chem. B 2003, 107, 13432–13440. [Google Scholar] [CrossRef]
- Talapatra, S.K.; Talapatra, B. Basic Concepts in Organic Stereochemistry; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Rossini, F.D.; Pitzer, K.S. Relabeling of the Cis and Trans Isomers of 1,3-Dimethylcyclohexane. Science 1947, 105, 647–648. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Feng, Y. Thermo-Switchable Surfactant Gel. Chem. Commun. 2011, 47, 7191–7193. [Google Scholar] [CrossRef]
- Xie, H.; Asad Ayoubi, M.; Lu, W.; Wang, J.; Huang, J.; Wang, W. A Unique Thermo-Induced Gel-to-Gel Transition in a pH-Sensitive Small-Molecule Hydrogel. Sci. Rep. 2017, 7, 8459. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.Z.; Petricevic, M.; Sobala, L.; Davies, G.J.; Williams, S.J. Exploration of Strategies for Mechanism-Based Inhibitor Design for Family GH99 Endo-α-1,2-Mannanases. ACS Chem. Biol. 2023, 18, 1474–1482. [Google Scholar] [CrossRef] [PubMed]
- Dubin, P.L.; Chew, C.-H.; Davis, R.M. Competition between Monovalent and Divalent Counterions in Micelle Binding. J. Colloid Interface Sci. 1996, 179, 157–167. [Google Scholar] [CrossRef]
- Sammalkorpi, M.; Karttunen, M.; Haataja, M. Ionic Surfactant Aggregates in Saline Solutions: Sodium Dodecyl Sulfate (SDS) in the Presence of Excess Sodium Chloride (NaCl) or Calcium Chloride (CaCl2). J. Phys. Chem. B 2009, 113, 5863–5870. [Google Scholar] [CrossRef]
- Lapidot, Y.; Rappoport, S.; Wolman, Y. Use of Esters of N-Hydroxysuccinimide in the Synthesis of N-Acylamino Acids. J. Lipid Res. 1967, 8, 142–145. [Google Scholar] [CrossRef]
- Lewis, R.N.A.H.; McElhaney, R.N.; Pabst, G.; Hodzic, A.; Prenner, E.J. Temperature-Dependent Miscibility Behavior of Model Membrane Systems Consisting of a Phosphatidylcholine and a Saturated N-Acyl Amino Acid. J. Surfactants Deterg. 2016, 19, 1259–1274. [Google Scholar] [CrossRef]
- Zana, R. Critical Micellization Concentration of Surfactants in Aqueous Solution and Free Energy of Micellization. Langmuir 1996, 12, 1208–1211. [Google Scholar] [CrossRef]
- Jiang, N.; Li, P.; Wang, Y.; Wang, J.; Yan, H.; Thomas, R.K. Micellization of Cationic Gemini Surfactants with Various Counterions and Their Interaction with DNA in Aqueous Solution. J. Phys. Chem. B 2004, 108, 15385–15391. [Google Scholar] [CrossRef]
- Ono, Y.; Kawasaki, H.; Annaka, M.; Maeda, H. Effects of Micelle-to-Vesicle Transitions on the Degree of Counterion Binding. J. Colloid Interface Sci. 2005, 287, 685–693. [Google Scholar] [CrossRef]
- Bakshi, M.S.; Singh, J.; Kaur, J. Estimation of Degree of Counterion Binding and Thermodynamic Parameters of Ionic Surfactants from Cloud Point Measurements by Using Triblock Polymer as Probe. J. Colloid Interface Sci. 2005, 287, 704–711. [Google Scholar] [CrossRef]
- Aggrawal, R.; Kumari, S.; Gangopadhyay, S.; Saha, S.K. Role of Different States of Solubilized Water on Solvation Dynamics and Rotational Relaxation of Coumarin 490 in Reverse Micelles of Gemini Surfactants, Water/12-s-12.2Br–(s = 5, 6, 8)/n-Propanol/Cyclohexane. ACS Omega 2020, 5, 6738–6753. [Google Scholar] [CrossRef]
- Neese, F. The ORCA Program System. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA Quantum Chemistry Program Package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.C. Quantitative Chemical Analysis, 9th ed.; Macmillan: New York, NY, USA, 2011. [Google Scholar]






| Critical Micelle Concentration (CMC) (mM) | ||||
|---|---|---|---|---|
| Counterion (Conformer) α | Surfactant | |||
| Und-Gly | Und-Ala | Und-Val | Und-Leu | |
| cis-1,2-DACH (ae) | 15.9 ± 0.6 | 14.0 ± 0.2 | 11.1 ± 0.6 | 8.0 ± 0.5 |
| trans-1,2-DACH (ee) | 16.6 ± 0.5 | 15.6 ± 0.4 | 10.2 ± 0.7 | 7.4 ± 0.4 |
| cis-1,3-DACH (ee) | 14.3 ± 0.1 | 9.7 ± 0.8 | 9.0 ± 0.1 | 5.9 ± 0.6 |
| trans-1,3-DACH (ae) | 16.0 ± 0.6 | 13.3 ± 0.8 | 14.2 ± 0.7 | 13.2 ± 0.5 |
| cis-1,4-DACH (ae) | 13.3 ± 0.3 | 13.9 ± 0.2 | 8.8 ± 0.6 | 5.8 ± 0.2 |
| trans-1,4-DACH (ee) | 15.1 ± 0.7 β | 12.9 ± 0.1 | 9.5 ± 0.5 | 4.3 ± 0.2 |
| Size (nm) | ||||
|---|---|---|---|---|
| Counterion | Surfactant | |||
| Und-Gly | Und-Ala | Und-Val | Und-Leu | |
| cis-1,2-DACH | 2.3 ± 0.2 | 2.0 ± 0.2 | 2.0 ± 0.4 | 2.7 ± 0.6 |
| trans-1,2-DACH | 1.7 ± 0.2 | 1.7 ± 0.3 | 2.0 ± 0.2 | 2.3 ± 0.2 |
| cis-1,3-DACH | 2.0 ± 0.6 | 2.0 ± 0.2 | 2.7 ± 0.2 | 2.7 ± 0.2 |
| trans-1,3-DACH | 1.7 ± 0.2 | 1.7 ± 0.2 | 2.3 ± 0.3 | 2.3 ± 0.4 |
| cis-1,4-DACH | 2.0 ± 0.2 | 1.7 ± 0.2 | 2.7 ± 0.3 | 2.7 ± 0.3 |
| trans-1,4-DACH | 4.3 ± 0.3 α | 2.7 ± 0.2 | 2.7 ± 0.2 | 3.1 ± 0.2 |
| Degree of Counterion Binding (β) | ||||
|---|---|---|---|---|
| Counterion | Surfactant | |||
| Und-Gly | Und-Ala | Und-Val | Und-Leu | |
| cis-1,2-DACH | 0.53 ± 0.02 | 0.58 ± 0.02 | 0.42 ± 0.01 | 0.47 ± 0.01 |
| cis-1,3-DACH | 0.66 ± 0.02 | 0.65 ±0.03 | 0.44 ± 0.01 | 0.59 ± 0.02 |
| cis-1,4-DACH | 0.69 ± 0.01 | 0.59 ± 0.02 | 0.49 ± 0.02 | 0.51 ± 0.05 |
| trans-1,2-DACH | 0.50 ± 0.01 | 0.52 ± 0.01 | 0.43 ± 0.01 | 0.47 ± 0.05 |
| trans-1,3-DACH | 0.54 ± 0.02 | 0.56 ± 0.02 | 0.56 ± 0.05 | 0.66 ± 0.04 |
| trans-1,4-DACH | α 0.75 ± 0.05 | 0.61 ± 0.01 | 0.48 ± 0.02 | 0.64 ± 0.02 |
| Surfactant | DACH Isomer | pH | pKa1 | pKa2 | +2% | +1% | 0% |
|---|---|---|---|---|---|---|---|
| Und-Gly | cis-1,2-DACH | 9.25 ± 0.05 | 6.13 [23] | 9.93 [23] | 0.06 | 82.67 | 17.27 |
| Und-Ala | 9.24 ± 0.09 | 0.06 | 82.99 | 16.94 | |||
| Und-Val | 9.44 ± 0.02 | 0.04 | 75.52 | 24.44 | |||
| Und-Leu | 9.27 ± 0.02 | 0.06 | 82.00 | 17.91 | |||
| Und-Gly | trans-1,2-DACH | 9.66 ± 0.01 | 6.47 [23] | 9.94 [23] | 0.04 | 65.55 | 34.4 |
| Und-Ala | 9.62 ± 0.01 | 0.05 | 67.6 | 32.35 | |||
| Und-Val | 9.71 ± 0.00 | 0.04 | 62.92 | 37.05 | |||
| Und-Leu | 9.61 ± 0.02 | 0.05 | 68.1 | 31.85 | |||
| Und-Gly | cis-1,3-DACH | 10.33 ± 0.04 | 8.29 [34] | 10.3 [34] | 0.44 | 48.06 | 51.5 |
| Und-Leu | 10.21 ± 0.07 | 0.66 | 54.8 | 44.54 | |||
| Und-Val | 10.31 ± 0.03 | 0.47 | 49.19 | 50.34 | |||
| Und-Ala | 10.33 ± 0.13 | 0.44 | 48.06 | 51.5 | |||
| Und-Gly | trans-1,3-DACH | 9.72 ± 0.02 | 8.54 [34] | 10.36 [34] | 5.10 | 77.21 | 17.69 |
| Und-Ala | 9.55 ± 0.03 | 7.80 | 79.83 | 12.36 | |||
| Und-Val | 9.90 ± 0.04 | 3.14 | 71.92 | 24.94 | |||
| Und-Leu | 10.03 ± 0.01 | 2.16 | 66.66 | 31.18 | |||
| Und-Gly | cis-1,4-DACH | 9.26 ± 0.07 | 9.4 [23] | 10.8 [23] | 57.30 | 41.51 | 1.2 |
| Und-Ala | 9.24 ± 0.10 | 58.45 | 40.44 | 1.11 | |||
| Und-Val | 9.44 ± 0.05 | 46.63 | 51.13 | 2.23 | |||
| Und-Leu | 9.28 ± 0.03 | 56.13 | 42.58 | 1.29 | |||
| Und-Gly * | trans-1,4-DACH | 9.4 [23] | 10.8 [23] | ||||
| Und-Ala | 9.93 ± 0.07 | 20.64 | 69.93 | 9.43 | |||
| Und-Val | 10.07 ± 0.07 | 15.27 | 71.43 | 13.3 | |||
| Und-Leu | 9.86 ± 0.07 | 23.72 | 68.42 | 7.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco, S.E.; Black, N.; Alvarez, M.A.; Morris, K.F.; Olson, M.A.; Billiot, E.J.; Billiot, F.H. Stereoisomeric Effects of Diammoniumcyclohexane Counterions on the Self-Assembly of Amino Acid-Based Surfactants. Molecules 2025, 30, 4114. https://doi.org/10.3390/molecules30204114
Blanco SE, Black N, Alvarez MA, Morris KF, Olson MA, Billiot EJ, Billiot FH. Stereoisomeric Effects of Diammoniumcyclohexane Counterions on the Self-Assembly of Amino Acid-Based Surfactants. Molecules. 2025; 30(20):4114. https://doi.org/10.3390/molecules30204114
Chicago/Turabian StyleBlanco, Saylor E., Nathan Black, Margarita A. Alvarez, Kevin F. Morris, Mark A. Olson, Eugene J. Billiot, and Fereshteh H. Billiot. 2025. "Stereoisomeric Effects of Diammoniumcyclohexane Counterions on the Self-Assembly of Amino Acid-Based Surfactants" Molecules 30, no. 20: 4114. https://doi.org/10.3390/molecules30204114
APA StyleBlanco, S. E., Black, N., Alvarez, M. A., Morris, K. F., Olson, M. A., Billiot, E. J., & Billiot, F. H. (2025). Stereoisomeric Effects of Diammoniumcyclohexane Counterions on the Self-Assembly of Amino Acid-Based Surfactants. Molecules, 30(20), 4114. https://doi.org/10.3390/molecules30204114








