Stability and Ultrafast Dynamics of Luminescent Biquinoxen-Bis-σH-Adducts
Abstract
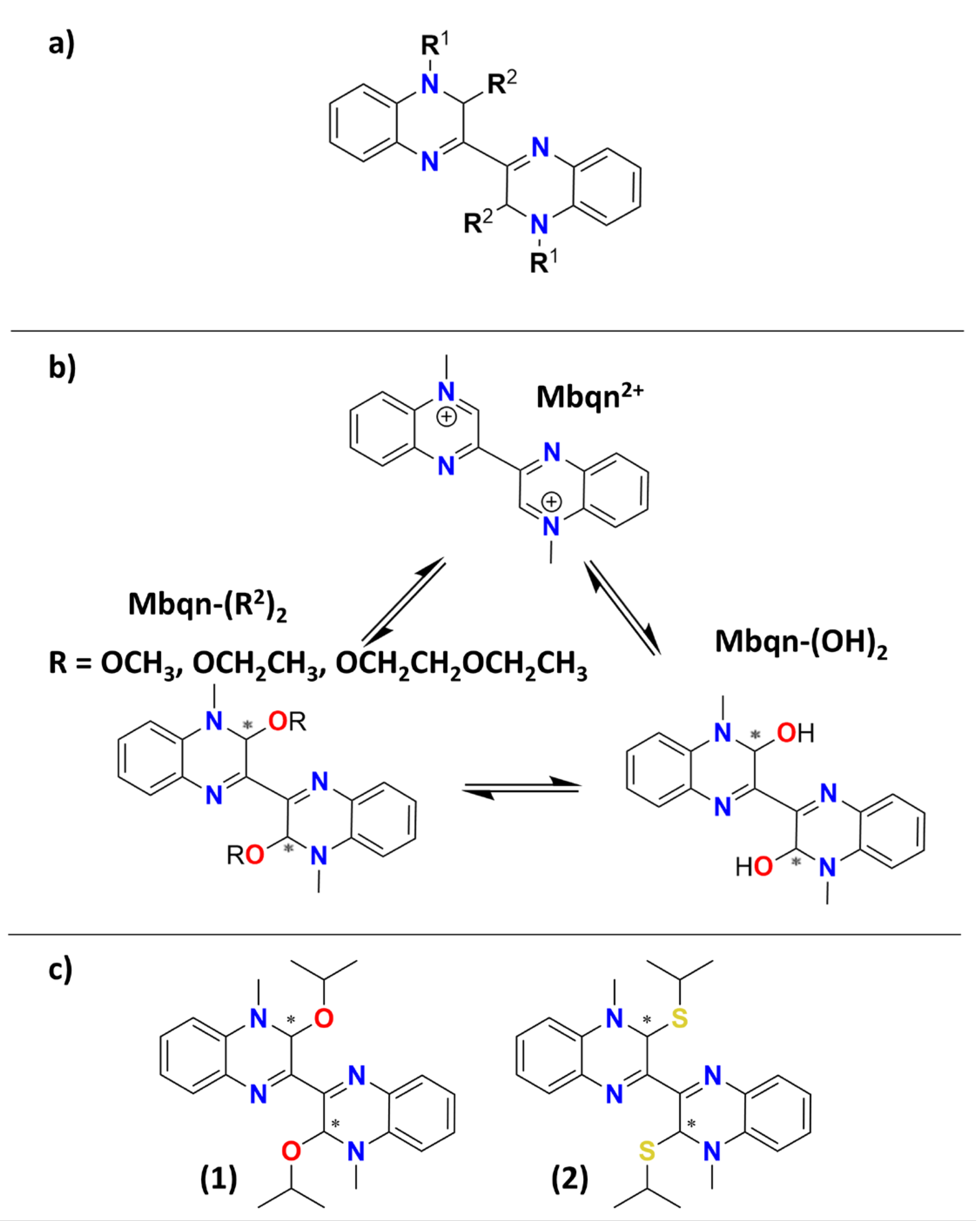
1. Introduction
2. Results
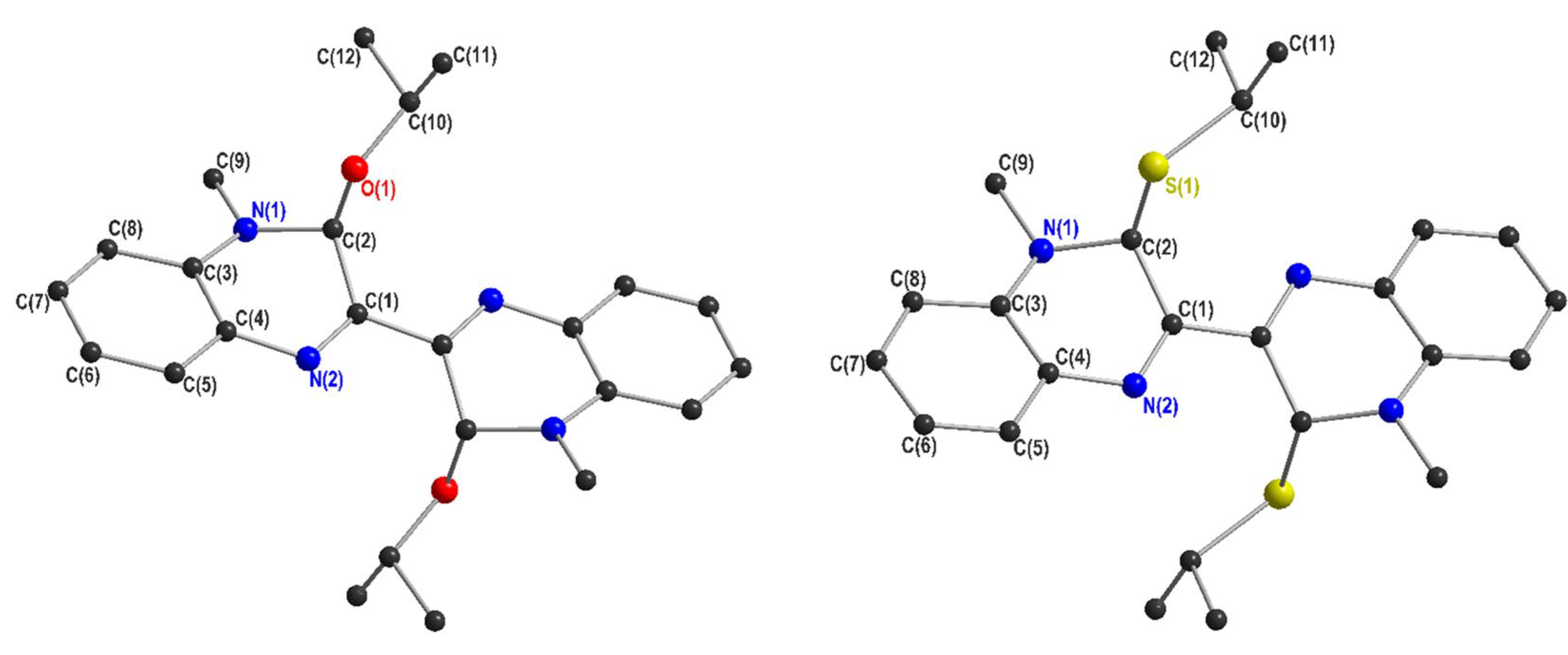
2.1. Crystallography
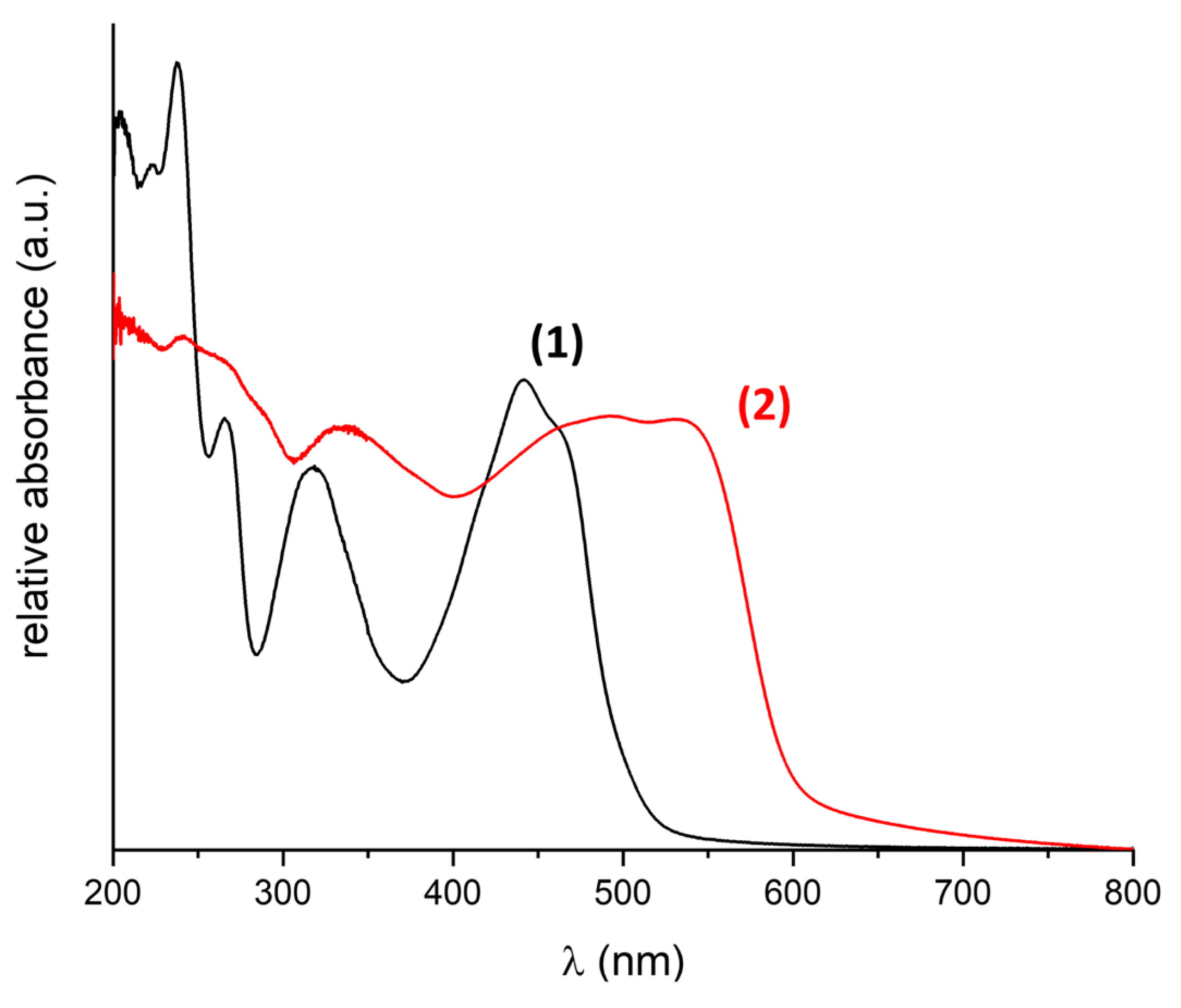
2.2. Steady-State UV-Vis Absorption Spectroscopy
2.3. Quantum Chemical Calculations
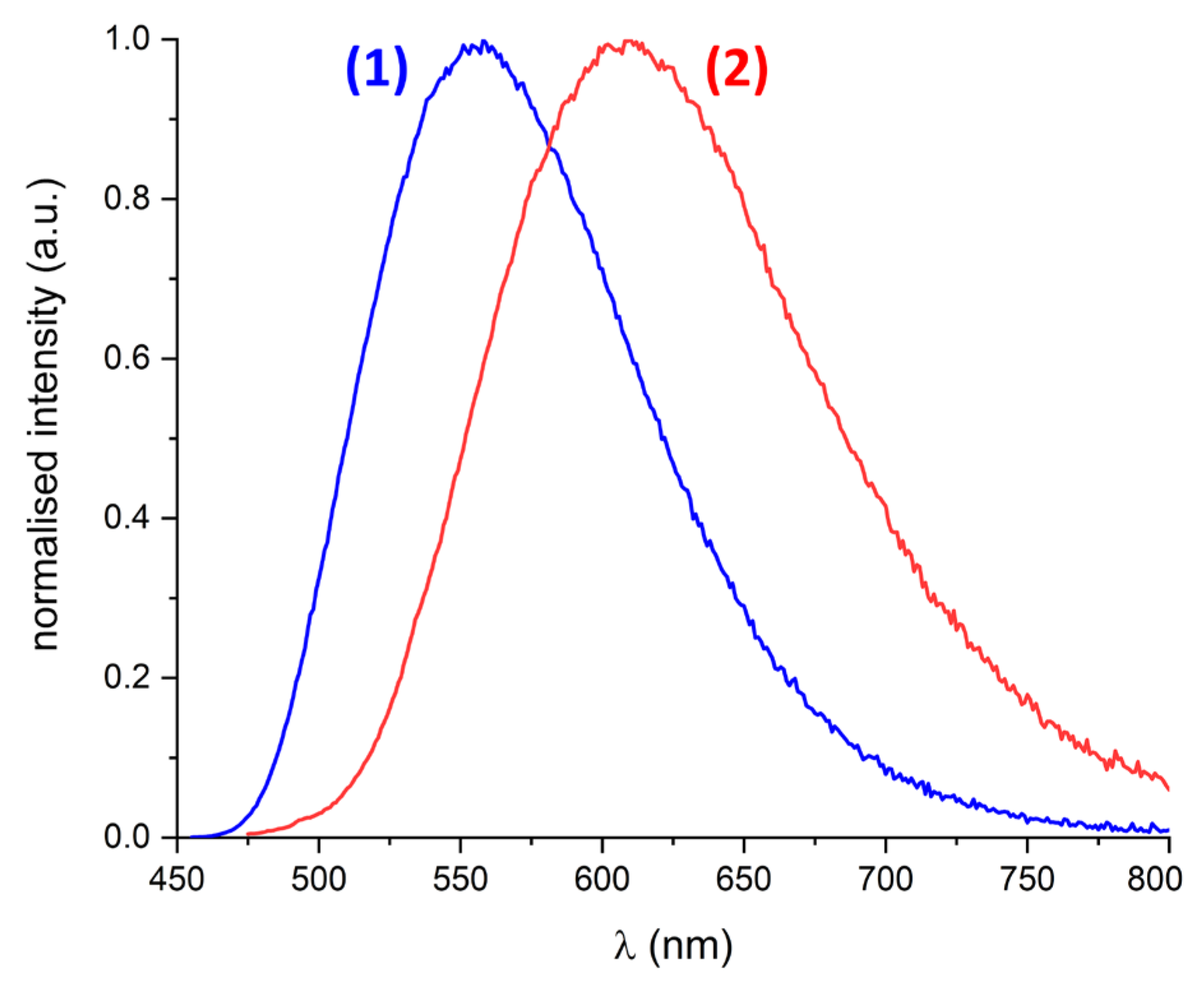
2.4. Emission Spectroscopy
2.5. Quantum Yields and Equilibrium in Solution
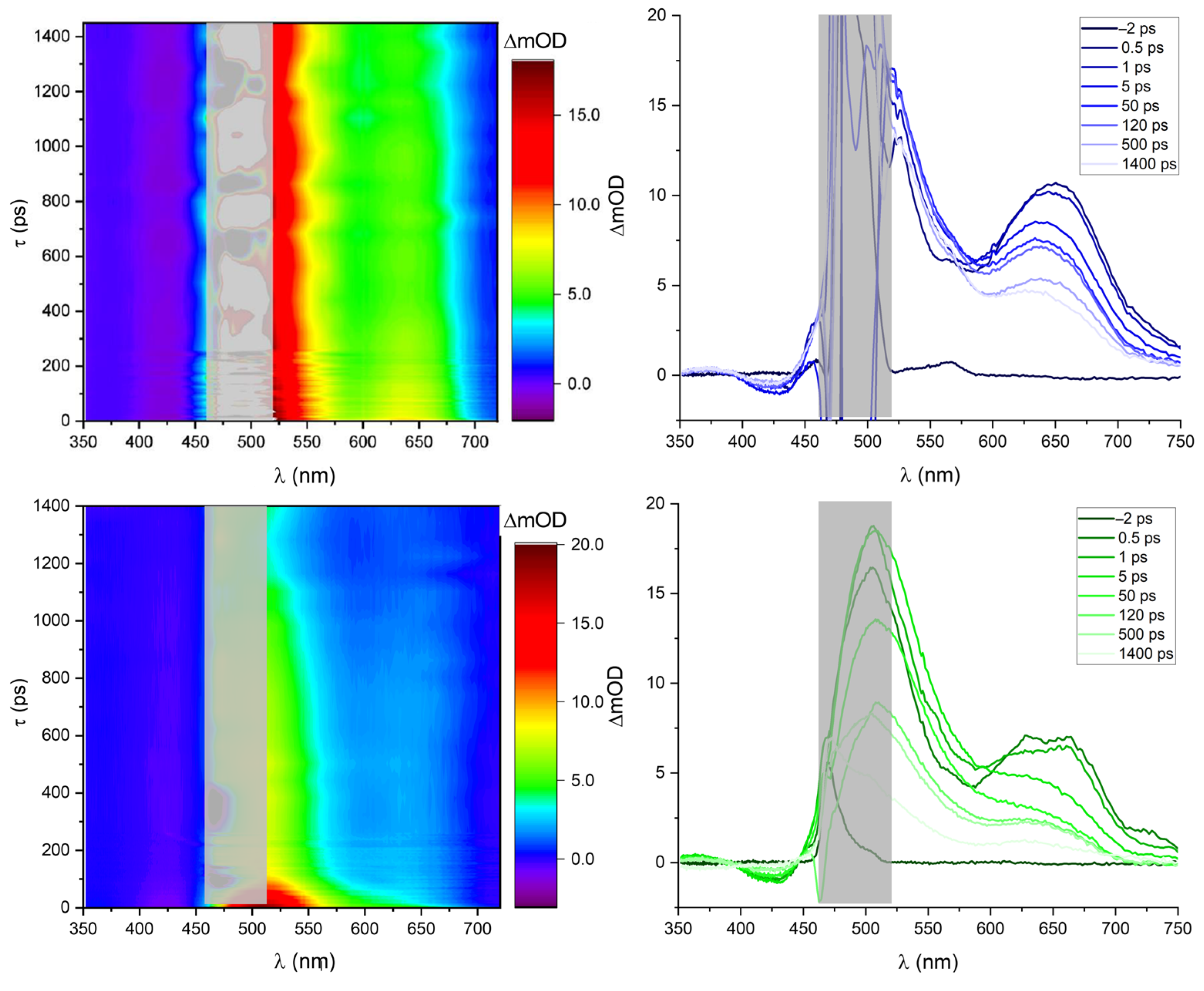
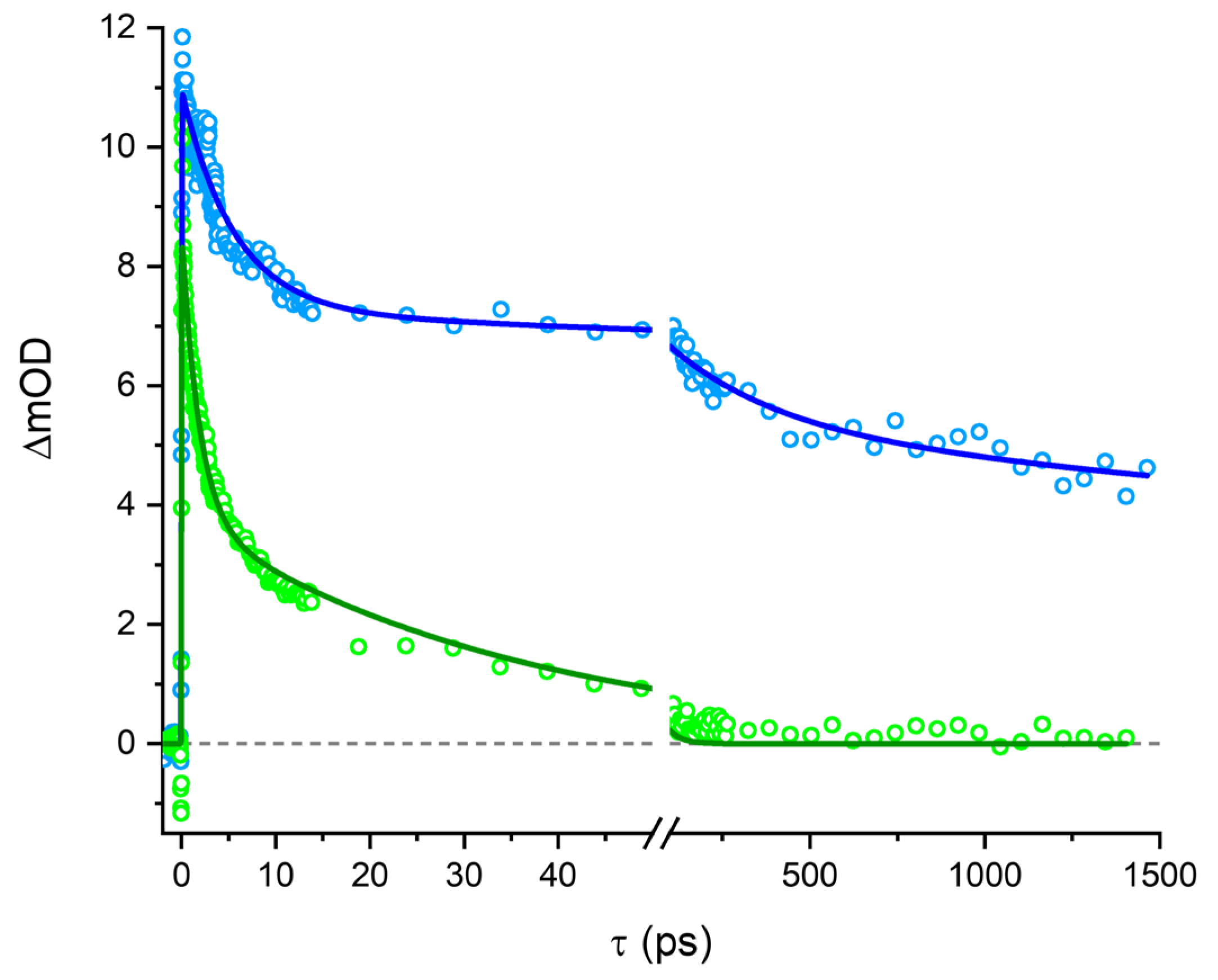
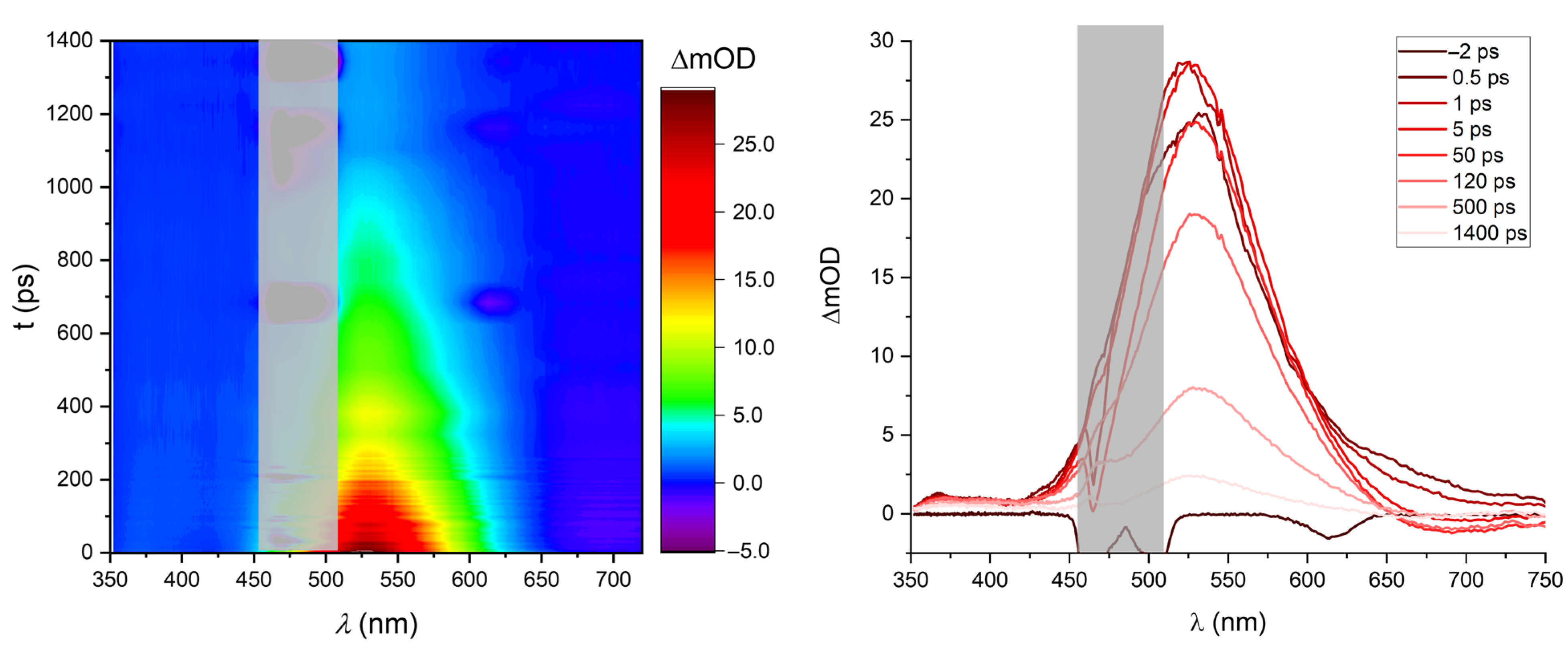
2.6. Transient Absorption Spectroscopy
2.7. Oxidation of Mbqn-(OiPr)2
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ishikita, H.; Knapp, E.W. Function of redox-active tyrosine in photosystem II. Biophys. J. 2006, 90, 3886–3896. [Google Scholar] [CrossRef]
- Benkó, T.; Lukács, D.; Li, M.; Pap, J.S. Redox-active ligands in artificial photosynthesis: A review. Environ. Chem. Lett. 2022, 20, 3657–3695. [Google Scholar] [CrossRef]
- Sies, H.; Mailloux, R.J.; Jakob, U. Fundamentals of redox regulation in biology. Nat. Rev. Mol. Cell Biol. 2024, 25, 701–719. [Google Scholar] [CrossRef] [PubMed]
- Chirik, P.J.; Wieghardt, K. Radical Ligands Confer Nobility on Base-Metal Catalysts. Science 2010, 327, 794–795. [Google Scholar] [CrossRef] [PubMed]
- Luca, O.R.; Crabtree, R.H. Redox-active ligands in catalysis. Chem. Soc. Rev. 2013, 42, 1440–1459. [Google Scholar] [CrossRef]
- Cabanero, D.C.; Rovis, T. Low-energy photoredox catalysis. Nat. Rev. Chem. 2025, 9, 28–45. [Google Scholar] [CrossRef]
- Dong, H.; Kang, N.; Li, L.; Li, L.; Yu, Y.; Chou, S. Versatile Nitrogen-Centered Organic Redox-Active Materials for Alkali Metal-Ion Batteries. Adv. Mater. 2024, 36, 2311401. [Google Scholar] [CrossRef]
- Kang, F.; Yan, L.; Chen, Z.; Zhang, Y.; Gu, Q.; Yang, J.; Xu, S.; Wang, X.; Lee, C.S.; Wang, Y.; et al. Multiple Redox-Active Centers in an Azatriangulenetrione-Based Covalent Organic Framework for High-Capacity, High-Rate and Ultra-Stable Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2025, 64, e202417779. [Google Scholar] [CrossRef]
- Lee, C.K.; Gangadharappa, C.; Fahrenbach, A.C.; Kim, D.J. Harnessing Radicals: Advances in Self-Assembly and Molecular Machinery. Adv. Mater. 2024, 36, e2408271. [Google Scholar] [CrossRef]
- Leblanc, N.; Mercier, N.; Zorina, L.; Simonov, S.; Auban-Senzier, P.; Pasquier, C. Large spontaneous polarization and clear hysteresis loop of a room-temperature hybrid ferroelectric based on mixed-halide [BiI3Cl2] polar chains and methylviologen dication. J. Am. Chem. Soc. 2011, 133, 14924–14927. [Google Scholar] [CrossRef]
- Evanko, B.; Yoo, S.J.; Chun, S.E.; Wang, X.; Ji, X.; Boettcher, S.W.; Stucky, G.D. Efficient Charge Storage in Dual-Redox Electrochemical Capacitors through Reversible Counterion-Induced Solid Complexation. J. Am. Chem. Soc. 2016, 138, 9373–9376. [Google Scholar] [CrossRef]
- Ding, J.; Zheng, C.; Wang, L.; Lu, C.; Zhang, B.; Chen, Y.; Li, M.; Zhai, G.; Zhuang, X. Viologen-inspired functional materials: Synthetic strategies and applications. J. Mater. Chem. A 2019, 7, 23337–23360. [Google Scholar] [CrossRef]
- Madasamy, K.; Velayutham, D.; Suryanarayanan, V.; Kathiresan, M.; Ho, K.-C. Viologen-based electrochromic materials and devices. J. Mater. Chem. C 2019, 7, 4622–4637. [Google Scholar] [CrossRef]
- Matheis, W.; Poppe, J.; Kaim, W.; Zalis, S. Diquaternired Heterocycles with Strong Electronic Coupling between a Metal-chelating Site and a Methylviologen-type Redox Function: EPR/ENDOR Detected Coordination of Metal Ions and Complexes by Radical Cation Intermediates. J. Chem. Soc. Perkin Trans. 2 1994, 9, 1923–1928. [Google Scholar] [CrossRef]
- Rinehart, J.D.; Fang, M.; Evans, W.J.; Long, J.R. Strong exchange and magnetic blocking in N23--radical-bridged lanthanide complexes. Nat. Chem. 2011, 3, 538–542. [Google Scholar] [CrossRef]
- Demir, S.; Zadrozny, J.M.; Nippe, M.; Long, J.R. Exchange coupling and magnetic blocking in bipyrimidyl radical-bridged dilanthanide complexes. J. Am. Chem. Soc. 2012, 134, 18546–18549. [Google Scholar] [CrossRef]
- Demir, S.; Jeon, I.-R.; Long, J.R.; Harris, T.D. Radical ligand-containing single-molecule magnets. Coord. Chem. Rev. 2015, 289–290, 149–176. [Google Scholar] [CrossRef]
- Ma, X.; Suturina, E.A.; De, S.; Negrier, P.; Rouzieres, M.; Clerac, R.; Dechambenoit, P. A Redox-Active Bridging Ligand to Promote Spin Delocalization, High-Spin Complexes, and Magnetic Multi-Switchability. Angew. Chem. Int. Ed. 2018, 57, 7841–7845. [Google Scholar] [CrossRef]
- Ma, X.; Suturina, E.A.; Rouzieres, M.; Platunov, M.; Wilhelm, F.; Rogalev, A.; Clerac, R.; Dechambenoit, P. Using Redox-Active pi Bridging Ligand as a Control Switch of Intramolecular Magnetic Interactions. J. Am. Chem. Soc. 2019, 141, 7721–7725. [Google Scholar] [CrossRef]
- Leblanc, N.; Sproules, S.; Fink, K.; Sanguinet, L.; Aleveque, O.; Levillain, E.; Rosa, P.; Powell, A.K. A fascinating multifaceted redox-active chelating ligand: Introducing the N,N′-dimethyl-3,3′-biquinoxalinium “methylbiquinoxen” platform. Chem. Sci. 2016, 7, 3820–3828. [Google Scholar] [CrossRef]
- Leblanc, N.; Sproules, S.; Powell, A.K. An alternative method to access diverse N,N′-diquaternised-3,3′-biquinoxalinium “biquinoxen” dications. New J. Chem. 2017, 41, 2949–2954. [Google Scholar] [CrossRef]
- Leblanc, N.; Genovese, D.; De Cola, L.; Powell, A.K. A platform with connections in many directions-further remarkable facets to the multifaceted methylbiquinoxen dication. Phys. Chem. Chem. Phys. 2017, 19, 6981–6988. [Google Scholar] [CrossRef]
- Scott, G.W.; Talley, L.D.; Anderson, R.W. Excited state absorption spectra and intersystem crossing kinetics in diazanaphthalenes. J. Chem. Phys. 1980, 72, 5002–5013. [Google Scholar] [CrossRef]
- Boldridge, D.W.; Scott, G.W. Excited singlet state absorption spectra and relaxation kinetics of the azanaphthalenes. J. Chem. Phys. 1986, 84, 6790–6798. [Google Scholar] [CrossRef]
- Etinski, M.; Marian, C.M. A theoretical study of low-lying singlet and triplet excited states of quinazoline, quinoxaline and phthalazine: Insight into triplet formation. Phys. Chem. Chem. Phys. 2017, 19, 13828–13837. [Google Scholar] [CrossRef] [PubMed]
- Akash, M.S.H.; Rehman, K. Comprehensive Insights into UV-VIS Spectrophotometry. In Essentials of Pharmaceutical Analysis, 2nd ed.; Springer: Singapore, 2025; Volume 2, pp. 95–160. [Google Scholar]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Chai, J.D.; Head-Gordon, M. Systematic optimization of long-range corrected hybrid density functionals. J. Chem. Phys. 2008, 128, 084106. [Google Scholar] [CrossRef]
- Rappoport, D.; Furche, F. Property-optimized gaussian basis sets for molecular response calculations. J. Chem. Phys. 2010, 133, 134105. [Google Scholar] [CrossRef]
- TURBOMOLE. Available online: http://www.turbomole.com (accessed on 3 July 2025).
- Brouwer, A.M. Standards for photoluminescence quantum yield measurements in solution (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-integrated space-group and crystal-structure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- De Mello, J.C.; Wittmann, H.F.; Friend, R.H. An improved experimental determination of external photoluminescence quantum efficiency. Adv. Mater. 1997, 9, 230–232. [Google Scholar] [CrossRef]
- Schweigert, C.; Babii, O.; Afonin, S.; Schober, T.; Leier, J.; Michenfelder, N.C.; Komarov, I.V.; Ulrich, A.S.; Unterreiner, A.-N. Real-Time Observation of Diarylethene-Based Photoswitches in a Cyclic Peptide Environment. ChemPhotoChem 2019, 3, 403–410. [Google Scholar] [CrossRef]
- Leier, J.; Michenfelder, N.C.; Unterreiner, A.-N.; Olzmann, M. Indications for an intermolecular photo-induced excited-state proton transfer of p-nitrophenol in water. Mol. Phys. 2021, 119, e1975051. [Google Scholar] [CrossRef]
- Krause, K.; Klopper, W. Implementation of the Bethe-Salpeter equation in the TURBOMOLE program. J. Comput. Chem. 2017, 38, 383–388. [Google Scholar] [CrossRef]
- Holzer, C.; Klopper, W. Communication: A hybrid Bethe-Salpeter/time-dependent density-functional-theory approach for excitation energies. J. Chem. Phys. 2018, 149, 101101. [Google Scholar] [CrossRef]
- Holzer, C.; Klopper, W. Ionized, electron-attached, and excited states of molecular systems with spin-orbit coupling: Two-component GW and Bethe-Salpeter implementations. J. Chem. Phys. 2019, 150, 204116. [Google Scholar] [CrossRef]
- Hellweg, A.; Rappoport, D. Development of new auxiliary basis functions of the Karlsruhe segmented contracted basis sets including diffuse basis functions (def2-SVPD, def2-TZVPPD, and def2-QVPPD) for RI-MP2 and RI-CC calculations. Phys. Chem. Chem. Phys. 2015, 17, 1010–1017. [Google Scholar] [CrossRef]


| λmax,abs (nm) | λmax,em (nm) | Stokes Shift (cm−1) | τ1 (ns) | τ2 (ns) | QY1 | |
|---|---|---|---|---|---|---|
| 1 | 439 | 558 | 4858 | 6.9 | - | 0.97 |
| 2 | 469 | 610 | 4929 | 0.4 | 6.7 | 0.07 |
| Storage Time of Solution | QY |
|---|---|
| 0 h 1 | 0.97 |
| 2 h | 0.643 |
| 3 h | 0.517 |
| 5 h | 0.469 |
| 1 d | 0.062 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braun, J.; Leier, J.; Khorenko, M.; Leblanc, N.; Anson, C.E.; Klopper, W.; Feldmann, C.; Bizzarri, C.; Unterreiner, A.-N.; Powell, A.K. Stability and Ultrafast Dynamics of Luminescent Biquinoxen-Bis-σH-Adducts. Molecules 2025, 30, 4115. https://doi.org/10.3390/molecules30204115
Braun J, Leier J, Khorenko M, Leblanc N, Anson CE, Klopper W, Feldmann C, Bizzarri C, Unterreiner A-N, Powell AK. Stability and Ultrafast Dynamics of Luminescent Biquinoxen-Bis-σH-Adducts. Molecules. 2025; 30(20):4115. https://doi.org/10.3390/molecules30204115
Chicago/Turabian StyleBraun, Jonas, Julia Leier, Mikhail Khorenko, Nicolas Leblanc, Christopher E. Anson, Wim Klopper, Claus Feldmann, Claudia Bizzarri, Andreas-Neil Unterreiner, and Annie K. Powell. 2025. "Stability and Ultrafast Dynamics of Luminescent Biquinoxen-Bis-σH-Adducts" Molecules 30, no. 20: 4115. https://doi.org/10.3390/molecules30204115
APA StyleBraun, J., Leier, J., Khorenko, M., Leblanc, N., Anson, C. E., Klopper, W., Feldmann, C., Bizzarri, C., Unterreiner, A.-N., & Powell, A. K. (2025). Stability and Ultrafast Dynamics of Luminescent Biquinoxen-Bis-σH-Adducts. Molecules, 30(20), 4115. https://doi.org/10.3390/molecules30204115








