The Properties of the Monolayers of Sorbitan Lipids as Informative Factors on the Hydrophilic–Lipophilic Balance Value of Their Mixtures, Proposed for Dermatological Applications
Abstract
1. Introduction
2. Results
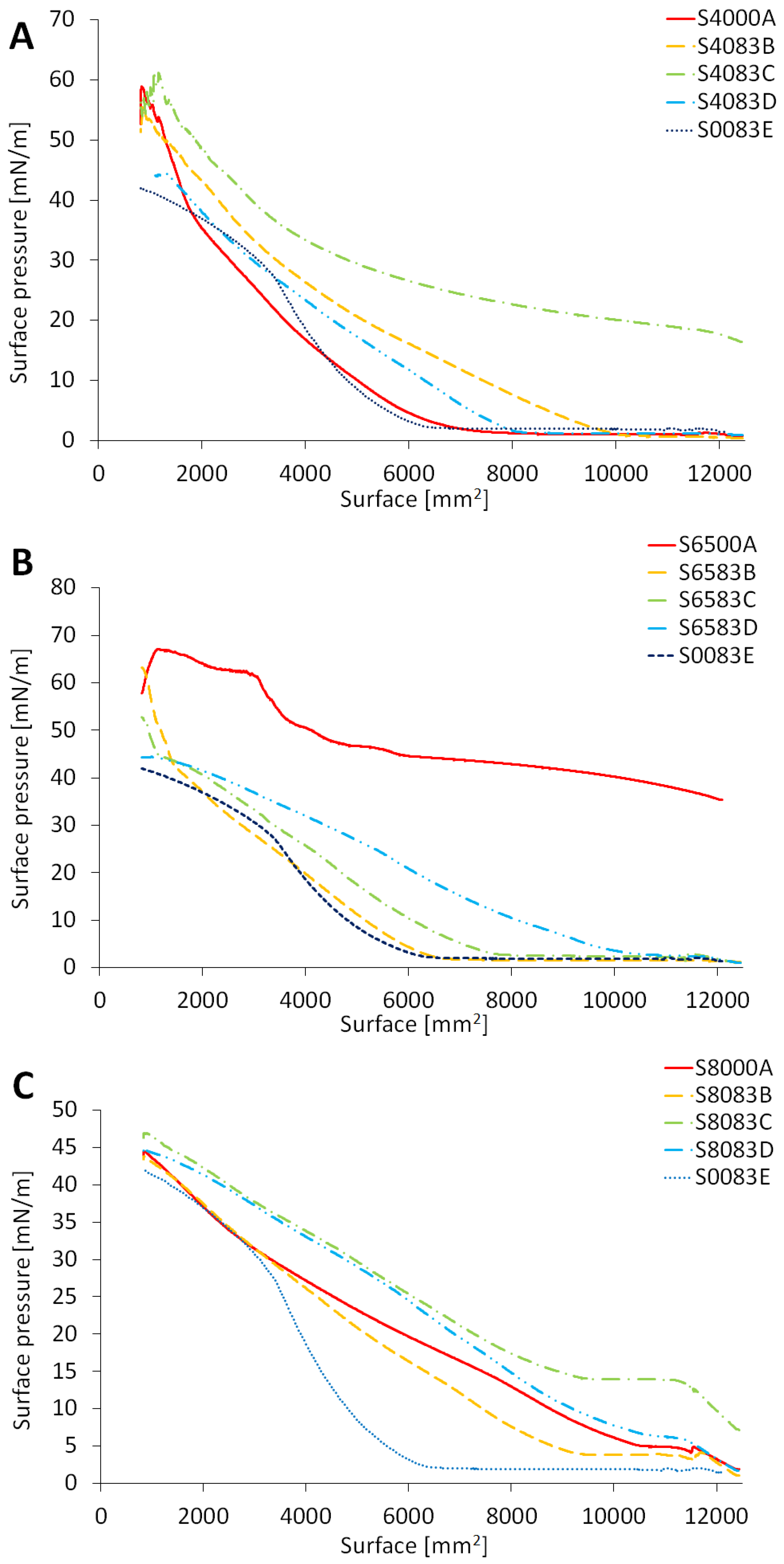
2.1. Recorded Surface Pressure of Evaluated Monolayers
2.2. The Stages of Compression Identified by Linear Sections of the Plots
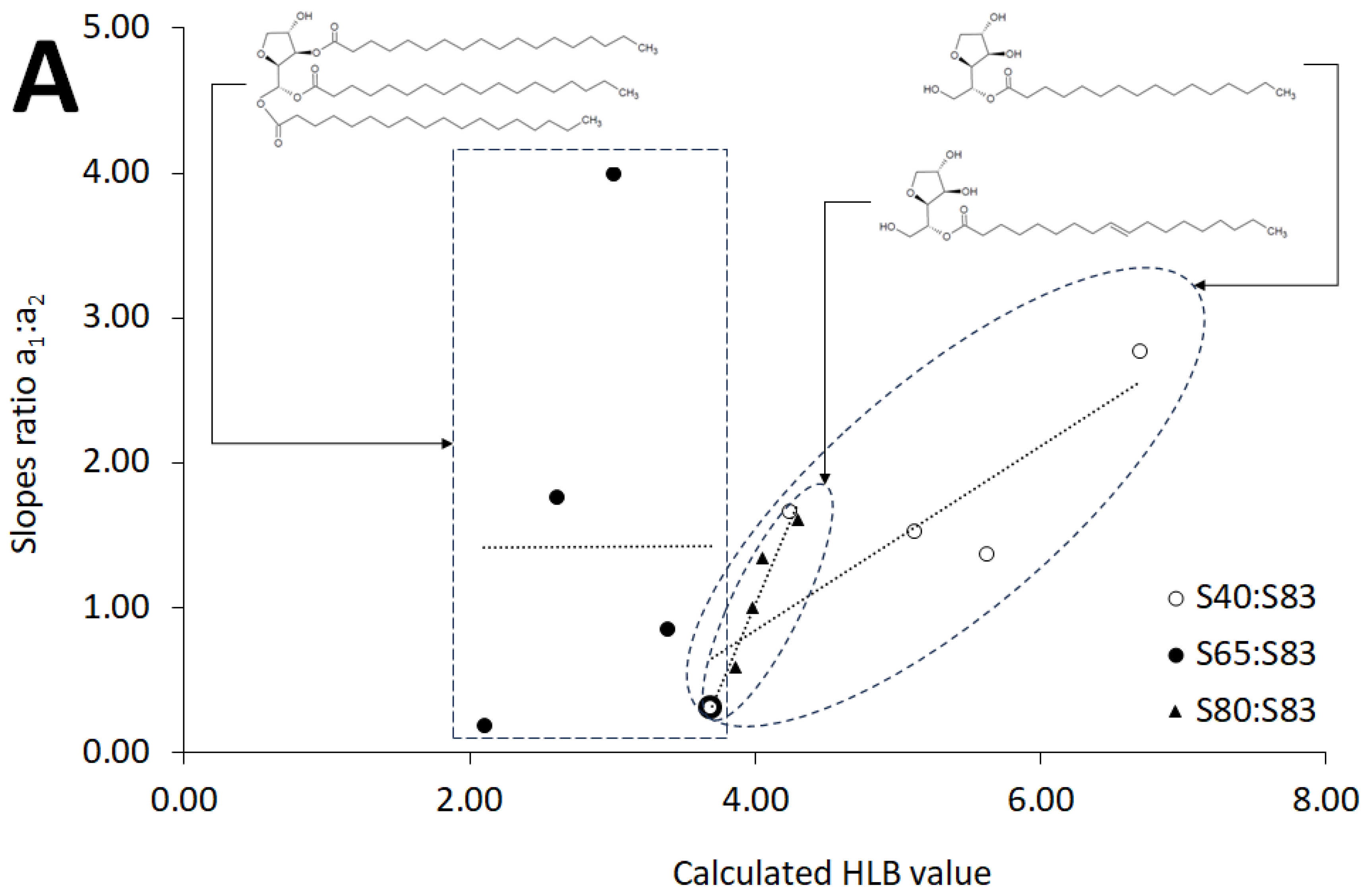
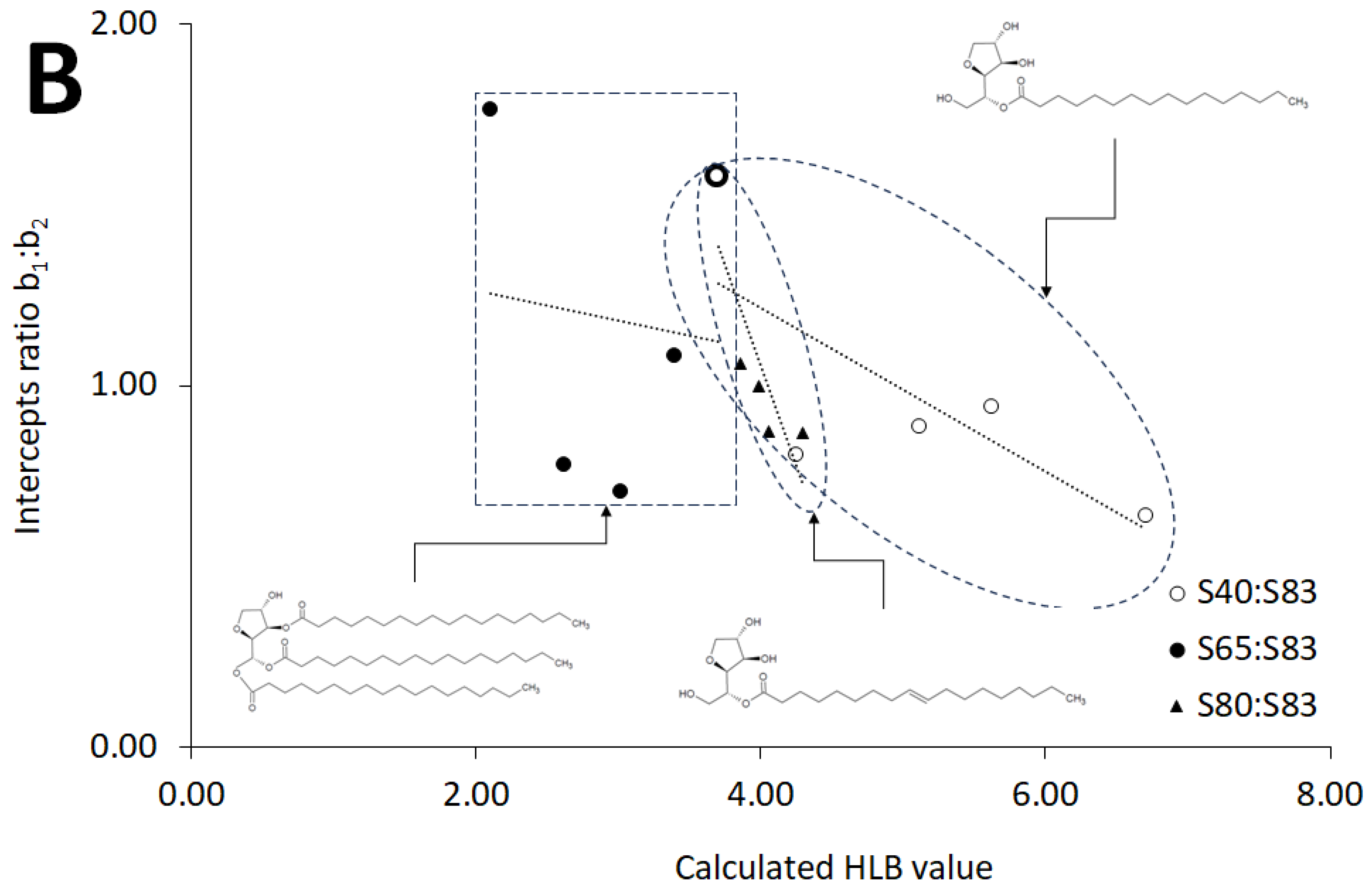
2.3. HLB Values of Evaluated Systems
3. Discussion
3.1. Recorded Surface Pressure of Evaluated Monolayers
3.2. Calculated HLB
3.3. The Stages of Compression Identified by Linear Sections of the Plots in the Context of HLB Values
4. Materials and Methods
4.1. Materials
4.2. Composition of the Evaluated Mixtures of Surfactants
4.3. Surface Pressure Measurements
4.4. Calculation of the Selected Parameters of the Evaluated π-A Isotherms
4.5. Calculation of HLB of the Assessed Surfactants Mixtures
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- James-Smith, M.A.; Hellner, B.; Annunziato, N.; Mitragotri, S. Effect of surfactant mixtures on skin structure and barrier properties. Ann. Biomed. Eng. 2011, 39, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Pokhrel, D.R.; Sah, M.K.; Gautam, B.; Basak, H.K.; Bhattarai, A.; Chatterjee, A. A recent overview of surfactant-drug interactions and their importance. RSC Adv. 2023, 26, 17685–17704. [Google Scholar] [CrossRef]
- Juhász, Á.; Ungor, D.; Berta, K.; Seres, L.; Csapó, E. Spreadsheet-based nonlinear analysis of in vitro release properties of a model drug from colloidal carriers. J. Mol. Liq. 2021, 328, 115405. [Google Scholar] [CrossRef]
- Strati, F.; Neubert, R.H.H.; Opálka, L.; Kerth, A.; Brezesinski, G. Non-ionic surfactants as innovative skin penetration enhancers: Insight in the mechanism of interaction with simple 2D stratum corneum model system. Eur. J. Pharm. Sci. 2021, 157, 105620. [Google Scholar] [CrossRef] [PubMed]
- Lanigan, R.S.; Yamarik, T.A.; Cosmetic Ingredient Review Expert panel. Final report on the safety assessment of sorbitan caprylate, sorbitan cocoate, sorbitan diisostearate, sorbitan dioleate, sorbitan distearate, sorbitan isostearate, sorbitan olivate, sorbitan sesquiisostearate, sorbitan sesquistearate, and sorbitan triisostearate. Int. J. Toxicol. 2002, 21, 93–112. [Google Scholar]
- Badmus, S.O.; Amusa, H.K.; Oyehan, T.A.; Saleh, T.A. Environmental risks and toxicity of surfactants: Overview of analysis, assessment, and remediation techniques. Environ. Sci. Pollut. Res. Int. 2021, 28, 62085–62104. [Google Scholar] [CrossRef]
- Korhonen, M.; Hirvonen, J.; Peltonen, L.; Antikainen, O.; Yrjänäinen, L.; Yliruusi, J. Formation and characterization of three-component-sorbitan monoester surfactant, oil and water-creams. Int. J. Pharm. 2004, 269, 227–239. [Google Scholar] [CrossRef]
- Fornasier, M.; Krautforst, K.; Kulbacka, J.; Jönsson, P.; Murgia, S.; Bazylińska, U. Cubosomes and hexosomes stabilized by sorbitan monooleate as biocompatible nanoplatforms against skin metastatic human melanoma. J. Colloid Interface Sci. 2024, 677, 842–852. [Google Scholar] [CrossRef]
- Abdelkader, H.; El-Wahab, A.A.; El-Gendy, A.O.; Abou-Taleb, H.A. Formulation and optimization of lipid- and Poloxamer-tagged niosomes for dermal delivery of terbinafine: Preparation, evaluation, and in vitro antifungal activity. Pharm. Dev. Technol. 2023, 28, 803–810. [Google Scholar] [CrossRef]
- Sonwai, S.; Podchong, P.; Rousseau, D. Crystallization kinetics of cocoa butter in the presence of sorbitan esters. Food Chem. 2017, 214, 497–506. [Google Scholar] [CrossRef]
- Asarch, A.; Scheinman, P.L. Sorbitan sesquioleate, a common emulsifier in topical corticosteroids, is an important contact allergen. Dermatitis 2008, 19, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Stingeni, L.; Foti, C.; Guarneri, F.; Corazza, M.; Cristaudo, A.; Ferrucci, S.M.; Gallo, R.; Martina, E.; Musumeci, M.L.; Napolitano, M.; et al. Contact allergy to SIDAPA baseline series allergens in patients with eyelid dermatitis: An Italian multicentre study. Contact Dermat. 2024, 90, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Boyd, J.; Parkinson, C.; Sherman, P. Factors affecting emulsion stability, and the HLB concept. J. Colloid Interface Sci. 1972, 41, 359–370. [Google Scholar] [CrossRef]
- Kunieda, H.; Shinoda, K. Evaluation of the hydrophile-lipophile balance (HLB) of nonionic surfactants. I. Multisurfactant systems. J. Colloid Interface Sci. 1985, 107, 107–121. [Google Scholar] [CrossRef]
- Pasquali, R.C.; Sacco, N.; Bregni, C. The studies on hydrophilic-lipophilic balance (HLB): Sixty years after William C. Griffin’s pioneer work (1949–2009). Lat. Am. J. Pharm 2009, 28, 313–317. [Google Scholar]
- Foo, K.S.; Bavoh, C.B.; Lal, B.; Mohd Shariff, A. Rheology impact of various hydrophilic-hydrophobic balance (HLB) index non-ionic surfactants on cyclopentane hydrates. Molecules 2020, 25, 3725. [Google Scholar] [CrossRef]
- Michor, E.L.; Ponto, B.S.; Berg, J.C. Effects of reverse micellar structure on the particle charging capabilities of the span surfactant series. Langmuir 2016, 32, 10328–10333. [Google Scholar] [CrossRef]
- Farooq, A.; Shafaghat, H.; Jae, J.; Jung, S.C.; Park, Y.K. Enhanced stability of bio-oil and diesel fuel emulsion using Span 80 and Tween 60 emulsifiers. J. Environ. Manag. 2019, 231, 694–700. [Google Scholar] [CrossRef]
- Jiao, J.; Burgess, D.J. Rheology and stability of water-in-oil-in-water multiple emulsions containing Span 83 and Tween 80. AAPS PharmSci 2003, 5, 62–73. [Google Scholar] [CrossRef]
- Satapathy, D.; Sagiri, S.S.; Pal, K.; Pramanik, K.J.D.M. Development of mustard oil-and groundnut oil-based span 40 organogels as matrices for controlled drug delivery. Des. Monomers Polym. 2014, 17, 545–556. [Google Scholar] [CrossRef]
- Sakai, T.; Kurosawa, H.; Okada, T.; Mishima, S. Vesicle formation in mixture of a PEO-PPO-PEO block copolymer (Pluronic P123) and a nonionic surfactant (Span 65) in water. Colloids Surf. A Physicochem. Eng. Asp. 2011, 389, 82–89. [Google Scholar] [CrossRef]
- Nakata, H.; Miyazaki, T.; Iwasaki, T.; Nakamura, A.; Kidani, T.; Sakayama, K.; Masumoto, J.; Miura, H. Development of tumor-specific caffeine-potentiated chemotherapy using a novel drug delivery system with Span 80 nano-vesicles. Oncol. Rep. 2015, 33, 1593–1598. [Google Scholar] [CrossRef] [PubMed]
- Fujihira, A.; Shimizu, N. The effects of internal and receptor pH on the rate of drug release from water-in-oil emulsions. Chem. Pharm. Bull. 2014, 62, 64–71. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zapolski, R.; Musiał, W. The Response Surface Methodology for Assessment of HLB Values of Mixtures of Non-Ionic Surfactants Using Parameters from Their π-A Isotherms. Molecules 2024, 29, 2351. [Google Scholar] [CrossRef]
- Fainerman, V.B.; Vollhardt, D. Equation of state for monolayers with additional phase transition between condensed phases of different compressibility. J. Phys. Chem. B 2009, 113, 6311–6313. [Google Scholar] [CrossRef]
- Pan, W.; Chen, H.; Wen, G.; Giaouzi, D.; Pispas, S.; Zuo, J. Surface micelle structures and monolayer compression moduli of double hydrophilic block copolymer. J. Phys. Chem. C 2020, 124, 17150–17157. [Google Scholar] [CrossRef]
- Keller, S.L. Miscibility transitions and lateral compressibility in liquid phases of lipid monolayers. Langmuir 2003, 19, 1451–1456. [Google Scholar] [CrossRef]
- Vollhardt, D.; Fainerman, V.B. Progress in characterization of Langmuir monolayers by consideration of compressibility. Adv. Colloid Interface Sci. 2006, 127, 83–97. [Google Scholar] [CrossRef]
- Schmidts, T.; Dobler, D.; Guldan, A.C.; Paulus, N.; Runkel, F. Multiple W/O/W emulsions—Using the required HLB for emulsifier evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2010, 372, 48–54. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M. The relevance HLB of surfactants on the stability of asphalt emulsion. Colloids Surf. A Physicochem. Eng. Asp. 2002, 204, 73–83. [Google Scholar] [CrossRef]
- Mahjoob, R.; Hakimzadeh, V.; Salehi, E.A.; Farmani, J. The interaction of polyglycerol esters with sorbitan tristearate, and sorbitan monostearate in structuring a low-saturated fat. J. Food Meas. Character 2022, 16, 4174–4184. [Google Scholar] [CrossRef]
- Anton, N.; Pierrat, P.; Lebeau, L.; Vandamme, T.F.; Bouriat, P. A study of insoluble monolayers by deposition at a bubble interface. Soft Matter 2013, 9, 10081–10091. [Google Scholar] [CrossRef]
- Rosen, M.J.; Zhou, Q. Surfactant-surfactant interactions in mixed monolayer and mixed micelle formation. Langmuir 2001, 17, 3532–3537. [Google Scholar] [CrossRef]
- Lu, D.; Burgess, D.J.; Rhodes, D.G. Nonideality in mixed monolayers of sorbitan oleates is enhanced by elevated ionic strength. Langmuir 2000, 16, 10329–10333. [Google Scholar] [CrossRef]
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Leblanc, J.C. Re-evaluation of sorbitan monostearate (E 491), sorbitan tristearate (E 492), sorbitan monolaurate (E 493), sorbitan monooleate (E 494) and sorbitan monopalmitate (E 495) when used as food additives. EFSA J. 2017, 15, e04788. [Google Scholar]
- Erni, P.; Fischer, P.; Windhab, E.J. Sorbitan tristearate layers at the air/water interface studied by shear and dilatational interfacial rheology. Langmuir 2005, 21, 10555–10563. [Google Scholar] [CrossRef]
- Griffin, W.C. Classification of surface-active agents by HLB. J. Soc. Cosmet. Chem. 1949, 1, 311–326. [Google Scholar]
- Pasquali, R.C.; Taurozzi, M.P.; Bregni, C. Some considerations about the hydrophilic–lipophilic balance system. Int. J. Pharm. 2008, 356, 44–51. [Google Scholar] [CrossRef]
- Yu, Z.W.; Jin, J.; Cao, Y. Characterization of the liquid-expanded to liquid-condensed phase transition of monolayers by means of compressibility. Langmuir 2002, 18, 4530–4531. [Google Scholar] [CrossRef]
- Kita-Tokarczyk, K.; Itel, F.; Grzelakowski, M.; Egli, S.; Rossbach, P.; Meier, W. Monolayer interactions between lipids and amphiphilic block copolymers. Langmuir 2009, 25, 9847–9856. [Google Scholar] [CrossRef]
- Brezesinski, G.; Möhwald, H. Langmuir monolayers to study interactions at model membrane surfaces. Adv. Colloid Interface Sci. 2003, 100–102, 563–584. [Google Scholar] [CrossRef] [PubMed]
- Fainerman, V.B.; Vollhardt, D. Penetration of Langmuir monolayers by soluble amphiphilic molecules. Langmuir 1999, 15, 1784–1790. [Google Scholar] [CrossRef]
- Harris, J.; Rice, S.A. A molecular dynamics study of the structure of a model Langmuir monolayer of amphiphile molecules. J. Chem. Phys. 1988, 89, 5898–5908. [Google Scholar] [CrossRef]
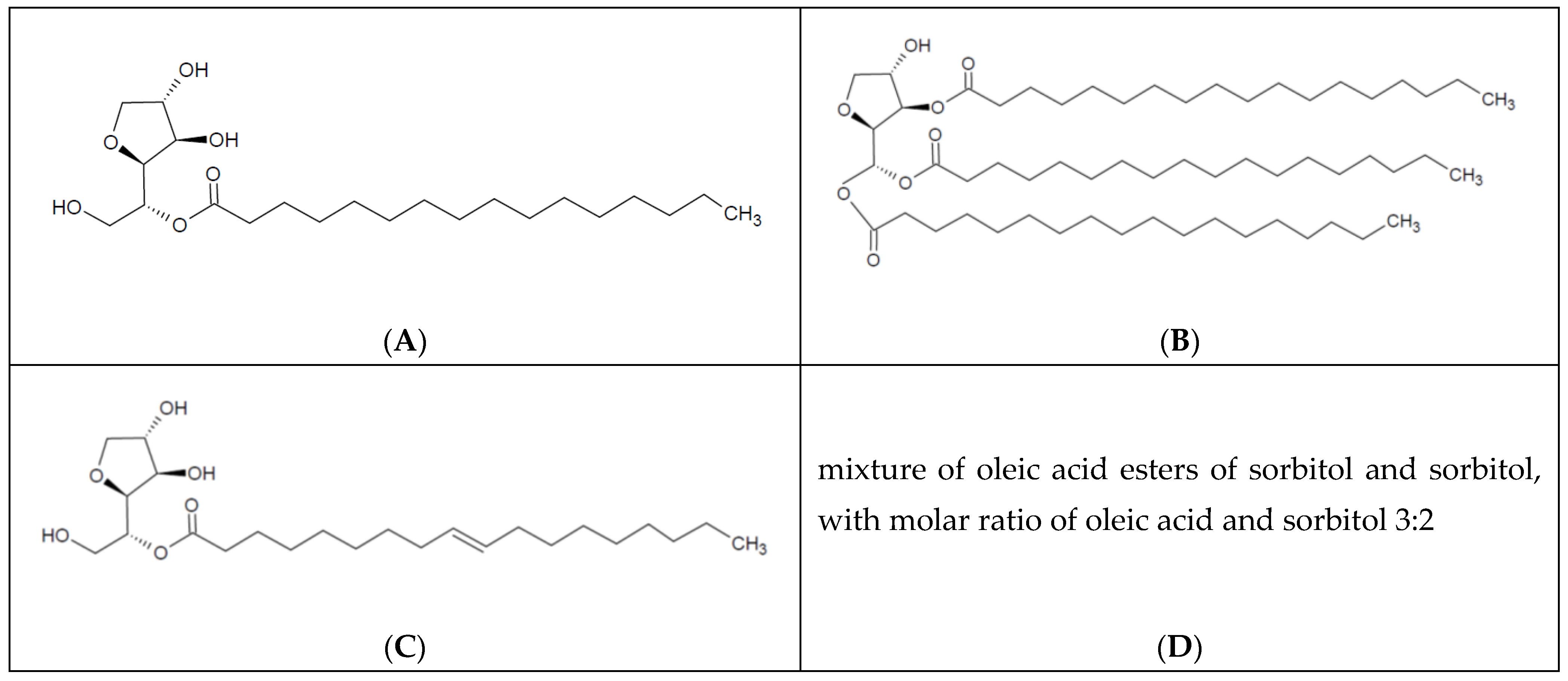
| AN | CSN | PG | NPG | NCC | NC | DB | M |
|---|---|---|---|---|---|---|---|
| S40 | Sorbitan monopalmitate | 1,4-sorbitan | Palmitic acid | 16 | 1 | N | 402.57 |
| S65 | Sorbitan tristearate | 1,4-sorbitan | Stearic acid | 18 | 3 | N | 963.54 |
| S80 | Sorbitan monooleate | 1,4-sorbitan | Oleic acid | 18 | 1 | Y | 428.6 |
| S83 | Sorbitan sesquioleate * | 1,4-sorbitan | Oleic acid | 18 | 1.5 | Y | 1175.7 |
| Surfactants Mixture | Slope (a1) | Intercept (b1) | x1 Intercept for y = 0 | R² | Slope (a2) | Intercept (b2) | x2 Intercept for y = 0 | R² | Slopes Ratio a1:a2 | Intercepts Ratio b1:b2 |
|---|---|---|---|---|---|---|---|---|---|---|
| S4000A | −9.50 × 10−3 | 54.12 | 5.70 × 103 | 0.9999 | −2.63 × 10−2 | 84.24 | 3.20 × 103 | 0.9986 | 2.77 | 0.64 |
| S4083B | −9.90 × 10−3 | 62.97 | 6.36 × 103 | 0.999 | −1.36 × 10−2 | 66.87 | 4.92 × 103 | 0.9834 | 1.37 | 0.94 |
| S4083C | −1.13 × 10−2 | 71.21 | 6.30 × 103 | 0.9975 | −1.72 × 10−2 | 79.99 | 4.65 × 103 | 0.9852 | 1.52 | 0.89 |
| S4083D | −5.70 × 10−3 | 46.14 | 8.09 × 103 | 0.9995 | −9.50 × 10−3 | 57.02 | 6.00 × 103 | 0.9996 | 1.67 | 0.81 |
| S6500A | −2.16 × 10−2 | 127.42 | 5.90 × 103 | 0.9947 | −4.00 × 10−3 | 72.17 | 1.80 × 104 | 0.9731 | 0.19 | 1.77 |
| S6583B | −2.94 × 10−2 | 85.031 | 2.89 × 103 | 0.9914 | −5.19 × 10−2 | 108.58 | 2.09 × 103 | 0.9932 | 1.77 | 0.78 |
| S6583C | −7.80 × 10−3 | 56.482 | 7.24 × 103 | 0.9994 | −3.12 × 10−2 | 79.535 | 2.55 × 103 | 0.9877 | 4.00 | 0.71 |
| S6583D | −5.90 × 10−3 | 56.184 | 9.52 × 103 | 0.9985 | −5.00 × 10−3 | 51.78 | 1.04 × 104 | 0.9998 | 0.85 | 1.09 |
| S8000A | −4.10 × 10−3 | 43.63 | 1.06 × 104 | 0.9994 | −6.60 × 10−3 | 50.30 | 7.62 × 103 | 0.9996 | 1.61 | 0.87 |
| S8083B | −4.40 × 10−3 | 42.94 | 9.76 × 103 | 0.9995 | −5.90 × 10−3 | 49.25 | 8.35 × 103 | 0.9992 | 1.34 | 0.87 |
| S8083C | −4.20 × 10−3 | 50.56 | 1.20 × 104 | 0.9981 | −4.20 × 10−3 | 50.58 | 1.20 × 104 | 0.9932 | 1.00 | 1.00 |
| S8083D | −4.10 × 10−3 | 49.64 | 1.21 × 104 | 0.9995 | −2.40 × 10−3 | 46.68 | 1.95 × 104 | 0.9964 | 0.59 | 1.06 |
| S0083E | −1.33 × 10−2 | 71.68 | 5.39 × 103 | 0.9992 | −4.00 × 10−3 | 45.45 | 1.14 × 104 | 0.9975 | 0.30 | 1.58 |
| Monolayer type | S40:S83 | HLB | S65:S83 | HLB | S80:S83 | HLB |
|---|---|---|---|---|---|---|
| Evaluated samples | S4000A | 6.70 | S6500A | 2.10 | S8000A | 4.30 |
| S4083B | 5.62 | S6583B | 2.61 | S8083B | 4.06 | |
| S4083C | 5.11 | S6583C | 3.01 | S8083C | 3.98 | |
| S4083D | 4.25 | S6583D | 3.39 | S8083D | 3.86 | |
| S0083E * | 3.70 | S0083E * | 3.70 | S0083E * | 3.70 |
| Type of Composition | Slopes Ratio a1:a2 v. HLB | Intercepts Ratio b1:b2 v. HLB | ||
|---|---|---|---|---|
| Equation | Determination Coefficient | Equation | Determination Coefficient | |
| S40:S83 | y = 0.6349x − 1.6969 | 0.7204 | y = −0.227x + 2.1244 | 0.5585 |
| S65:S83 | y = 0.0004x + 1.4185 | 3.0 × 10−8 | y = −0.0848x + 1.4352 | 0.0129 |
| S80:S83 | y = 2.3215x − 8.272 | 0.9468 | y = −1.1015x + 5.4595 | 0.7118 |
| Monolayer Type | S40:S83 | Monolayer Type | S65:S83 | Monolayer Type | S80:S83 | |||
|---|---|---|---|---|---|---|---|---|
| Components | Molar Fraction | Components | Molar Fraction | Components | Molar Fraction | |||
| S40 | S83 | S65 | S83 | S80 | S83 | |||
| S4000A | 1.00 | 0.00 | S6500A | 1.00 | 0.00 | S8000A | 1.00 | 0.00 |
| S4083B | 0.84 | 0.16 | S6583B | 0.73 | 0.28 | S8083B | 0.80 | 0.20 |
| S4083C | 0.72 | 0.28 | S6583C | 0.48 | 0.52 | S8083C | 0.71 | 0.29 |
| S4083D | 0.39 | 0.61 | S6583D | 0.23 | 0.77 | S8083D | 0.50 | 0.50 |
| S0083E * | 0.00 | 1.00 | S0083E * | 0.00 | 1.00 | S0083E * | 0.00 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zapolski, R.; Gasztych, M.; Jastrząb-Miśkiewicz, B.; Jankowska-Konsur, A.; Musiał, W. The Properties of the Monolayers of Sorbitan Lipids as Informative Factors on the Hydrophilic–Lipophilic Balance Value of Their Mixtures, Proposed for Dermatological Applications. Molecules 2025, 30, 1841. https://doi.org/10.3390/molecules30081841
Zapolski R, Gasztych M, Jastrząb-Miśkiewicz B, Jankowska-Konsur A, Musiał W. The Properties of the Monolayers of Sorbitan Lipids as Informative Factors on the Hydrophilic–Lipophilic Balance Value of Their Mixtures, Proposed for Dermatological Applications. Molecules. 2025; 30(8):1841. https://doi.org/10.3390/molecules30081841
Chicago/Turabian StyleZapolski, Remigiusz, Monika Gasztych, Beata Jastrząb-Miśkiewicz, Alina Jankowska-Konsur, and Witold Musiał. 2025. "The Properties of the Monolayers of Sorbitan Lipids as Informative Factors on the Hydrophilic–Lipophilic Balance Value of Their Mixtures, Proposed for Dermatological Applications" Molecules 30, no. 8: 1841. https://doi.org/10.3390/molecules30081841
APA StyleZapolski, R., Gasztych, M., Jastrząb-Miśkiewicz, B., Jankowska-Konsur, A., & Musiał, W. (2025). The Properties of the Monolayers of Sorbitan Lipids as Informative Factors on the Hydrophilic–Lipophilic Balance Value of Their Mixtures, Proposed for Dermatological Applications. Molecules, 30(8), 1841. https://doi.org/10.3390/molecules30081841







