Purification, Structural Characteristics, Bioactive Properties, and Applications of Naematelia aurantialba Polysaccharides: A Comprehensive Review
Abstract
1. Introduction
2. Extraction and Purification of NAPs
2.1. Extraction and Preparation of NAPs
2.1.1. Extraction from Fruiting Bodies
2.1.2. Mycelium Submerged Fermentation Pathway
2.1.3. Spore Fermentation Pathway
2.2. Purification of NAPs
3. Structural Characterizations of NAPs
3.1. Relative Molecular Weight
3.2. Monosaccharide Composition
3.3. Structural Characteristics
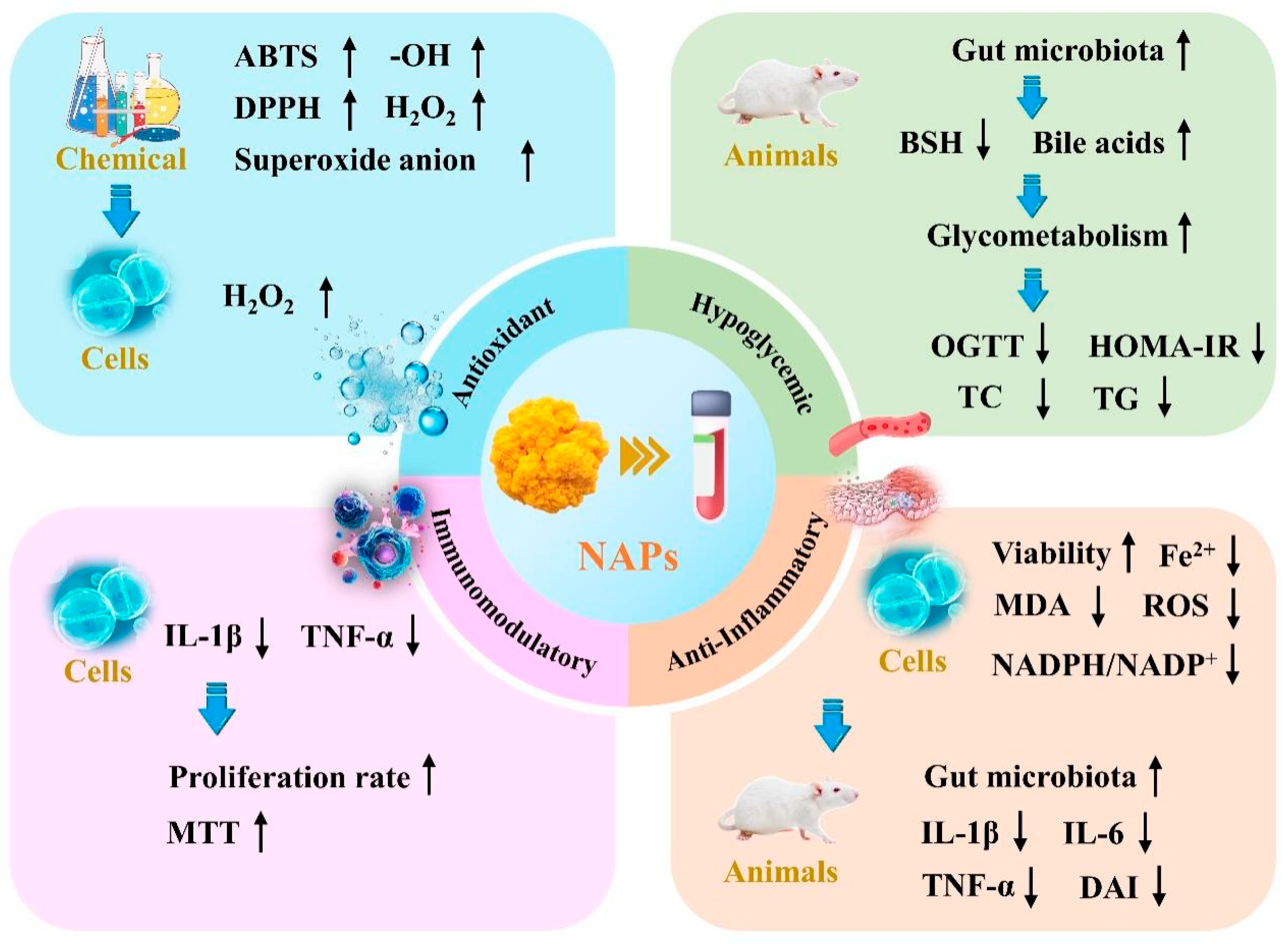
4. Biological Activity of NAPs
4.1. Antioxidant Activity
4.2. Hypoglycemic Activity
4.3. Immunomodulatory Activity
4.4. Other Activity
5. Application of NAPs
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAE | Alkali-assisted extraction |
| ABTS | 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) |
| ALT | Alanine transaminase |
| Ara | Arabinose |
| AST | Aspartate transaminase |
| BSH | Bile salt hydrolase |
| CAT | Catalase |
| CD | Circular dichroism |
| CE | Capillary electrophoresis |
| DAI | Disease activity index |
| DAO | Diamine oxidase |
| D-LA | D-lactic acid |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| DSC | Differential scanning calorimetry |
| EAE | Enzyme-assisted extraction |
| EAE/UAE | Enzyme and ultrasonic-assisted extraction |
| Fru | Fructose |
| FT-IR | Fourier transform infrared spectroscopy |
| Gal | Galactose |
| GalA | Galacturonic acid |
| GC | Glutathione reductase |
| GC-MS | Gas chromatography-mass spectrometer |
| Glc | Glucose |
| GlcA | Glucose acid |
| GlcN | Glucosamine hydrochloride |
| GPC | Gel permeation chromatography |
| GSH | Glutathione |
| GSH-Px | Glutathione peroxidase |
| H&E | Hematoxylin-eosin |
| HOMA-IR | Homeostasis model assessment of the insulin resistance index |
| HPAEC | High-performance anion-exchange chromatography |
| HPAEC-PAD | High-performance anion-exchange pulsed-amperometric detection chromatography |
| HPGFC | High-performance gel filtration chromatography |
| HPGPC | High-performance gel permeation chromatography |
| HPLC | High-performance liquid chromatography |
| HPLC-SEC | High-performance liquid chromatography-size exclusion chromatography |
| HWE | Hot water extraction |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| ITT | Insulin tolerance test |
| Man | Mannose |
| Man-N | Epichitosamine |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MDA | Malondialdehydes |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| MW | Molecular weight |
| NADP+ | Nicotinamide adenine dinucleotide phosphate |
| NADPH | Nicotinamide adenine dinucleotide phosphate hydrogen |
| N. aurantialba | Naematelia aurantialba |
| NAPs | Naematelia aurantialba polysaccharides |
| NMR | Nuclear magnetic resonance spectroscopy |
| NO | Nitric oxide |
| OGTT | Oral glucose tolerance test |
| PAGE | Polyacrylamide gel electrophoresis |
| PDA | Potato dextrose agar |
| Rha | Rhamnose |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| RT-PCR | Reverse transcription-polymerase chain reaction |
| SOD | Superoxide dismutase |
| SSU | Cucumis sativus polysaccharide extracted by the ultrasound-assisted method |
| SSW | Cucumis sativus polysaccharide extracted by the hot water method |
| STZ | Streptozotocin |
| T. aurantialba | Tremella aurantialba |
| T2D | Type 2 diabetes mellitus |
| T-AOC | Total antioxidant activities |
| TC | Total cholesterol |
| TFPs | Tremella fuciformis polysaccharides |
| TG | Total triglyceride |
| TNF-α | Tumor necrosis factor-α |
| UAE | Ultrasound-assisted extraction |
| UC | Ulcerative colitis |
| WB | Western blot |
| XRD | X-ray diffraction spectrum |
| Xyl | Xylose |
References
- Wang, Z.; Huang, K.; Pu, K.L.; Li, L.; Jiang, W.X.; Wu, J.; Kawagishi, H.; Li, M.L.; Qi, J.Z. Naematelia aurantialba: A comprehensive review of its medicinal, nutritional, and cultivation aspects. Food Med. Med. Homol. 2025, 2, 9420072. [Google Scholar] [CrossRef]
- Elkhateeb, W.A.; Daba, G.M. Mycotherapy of the good and the tasty medicinal mushrooms Lentinus, Pleurotus, and Tremella. Pharm. Pharmacol. Res. 2021, 4, 01–06. [Google Scholar] [CrossRef]
- Zhu, B.H.; Jing, S.; Nian, S.W.; Xi, Y.F.; Xu, H.D.; Mao, Y.M.; Qian, D.; Kou, L.P. Moisture state and volatile flavor behavior characterization of Naematelia aurantialba during postharvest in modified atmosphere packaging storage after treated with ultraviolet radiation C. Postharvest Biol. Technol. 2025, 227, 113611. [Google Scholar] [CrossRef]
- Yan, Y.H.; Wang, M.T.; Chen, N.; Wang, X.; Fu, C.H.; Li, Y.M.; Gan, X.R.; Lv, P.; Zhang, Y. Isolation, structures, bioactivities, application and future prospective for polysaccharides from Tremella aurantialba: A review. Front. Immunol. 2022, 13, 1091210. [Google Scholar] [CrossRef]
- Yan, Y.H.; Wang, M.T.; Gan, X.R.; Wang, X.; Fu, C.H.; Li, Y.M.; Chen, N.; Lv, P.; Zhang, Y. Evaluation of pharmacological activities and active components in Tremella aurantialba by instrumental and virtual analyses. Front. Nutr. 2022, 9, 1083581. [Google Scholar] [CrossRef]
- Geng, X.R.; Yang, D.X.; Zhang, Q.Y.; Chang, M.C.; Xu, L.J.; Cheng, Y.F.; Wang, H.X.; Meng, J.L. Good hydrolysis activity on raffinose family oligosaccharides by a novel α-galactosidase from Tremella aurantialba. Int. J. Biol. Macromol. 2020, 150, 1249–1257. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, Y.X.; Jiang, H.; Yang, K.; Wang, S.Y.; Wang, R.; Li, S.; Lei, P.; Xu, H.; Qiu, Y.; et al. Whole genome sequencing and annotation of Naematelia aurantialba (Basidiomycota, Edible-Medicinal Fungi). J. Fungi 2021, 8, 6. [Google Scholar] [CrossRef]
- Lan, J.Q.; Zhang, Y.K.; Cai, Y.L.; Shi, X.F.; Zhang, K.X.; Huang, J.C.; Yang, C.M.; He, X.H.; Yu, F.Q.; Liu, W. Spatial ratio of two fungal genotypes content of Naematelia aurantialba and Stereum hirsutum in nutritional growth substrate and fruiting bodies reveals their potential parasitic life cycle characteristics. J. Agr. Food. Res. 2025, 22, 102101. [Google Scholar] [CrossRef]
- Li, G.Z.; Wang, X.; Na, Z.G.; Xiu, W.Y.; Wang, J.Y.; Guan, Y.; Guo, J.J.; Ma, Y.Q. The effects of different cooking methods on nutrients, bioactive components and antioxidant activities of Naematelia aurantialba were revealed by simulating in vitro digestion. Food Res. Int. 2024, 198, 115342. [Google Scholar] [CrossRef]
- Guo, Y.J.; Deng, G.F.; Xu, X.R.; Wu, S.; Li, S.; Xia, E.Q.; Li, F.; Chen, F.; Ling, W.H.; Li, H.B. Antioxidant capacities, phenolic compounds and polysaccharide contents of 49 edible macro-fungi. Food Funct. 2012, 3, 1195–1205. [Google Scholar] [CrossRef]
- Sun, T.; Jiang, H.; Yang, K.; Li, X.K.; Wang, S.Y.; Yao, H.Y.; Wang, R.; Li, S.; Gu, Y.; Lei, P.; et al. Nutritional function and flavor evaluation of a new soybean beverage based on Naematelia aurantialba fermentation. Foods 2022, 11, 272. [Google Scholar] [CrossRef]
- Zeng, Y.K.; Shen, Z.H.; Cao, Y.; Luo, X.Y.; Yang, L.L.; Li, R.P.; Lu, Q.Q.; Li, R.C. Aeronautical mutagenesis and whole-genome resequencing reveal the genetic basis of color change in Naematelia aurantialba. Mycobiology 2025, 53, 539–549. [Google Scholar] [CrossRef]
- Feng, H.; Luo, L.F.; Wang, L.Y.; Ding, Y.Y.; Sun, L.P.; Zhuang, Y.L. Effects of Tremella aurantialba on physical properties, in vitro glucose release, digesta rheology, and microstructure of bread. J. Food Sci. 2023, 88, 4853–4866. [Google Scholar] [CrossRef]
- Yang, Y.; Dong, C.H. Fruiting body heterogeneity, dimorphism and haustorium-like structure of Naematelia aurantialba (Jin er mushroom). J. Fungi 2024, 10, 557. [Google Scholar] [CrossRef]
- Xie, C.; Sun, Q.L.; Chen, J.L.; Yang, B.S.; Lu, H.W.; Liu, Z.P.; Li, Y.C.; Li, K.; Tang, B.; Lin, L.J. Cu-Tremella fuciformis polysaccharide-based tumor microenvironment-responsive injectable gels for cuproptosis-based synergistic osteosarcoma therapy. Int. J. Biol. Macromol. 2024, 270, 132029. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Xiao, H.H.; Li, X.Y.; Huang, J.; Zhang, B.; Zeng, M.H. Recent advances in the anti-aging effects of natural polysaccharides: Sources, structural characterization, action mechanisms and structure-activity relationships. Trends Food Sci. Technol. 2025, 160, 105000. [Google Scholar] [CrossRef]
- Yin, Z.H.; Liang, Z.H.; Li, C.Q.; Wang, J.M.; Ma, C.Y.; Kang, W.Y. Immunomodulatory effects of polysaccharides from edible fungus: A review. Food Sci. Hum. Well. 2021, 10, 393–400. [Google Scholar] [CrossRef]
- Wang, Z.J.; Zeng, Y.W.; Luo, D.H. Structure elucidation of a non-branched and entangled heteropolysaccharide from Tremella sanguinea Peng and its antioxidant activity. Carbohydr. Polym. 2016, 152, 33–40. [Google Scholar] [CrossRef]
- Guo, Y.X.; Chen, X.F.; Gong, P. Classification, structure and mechanism of antiviral polysaccharides derived from edible and medicinal fungus. Int. J. Biol. Macromol. 2021, 183, 1753–1773. [Google Scholar] [CrossRef]
- Ma, X.; Yang, M.; He, Y.; Zhai, C.T.; Li, C.L. A review on the production, structure, bioactivities and applications of Tremella polysaccharides. Int. J. Immunopath. Ph. 2021, 35, 20587384211000541. [Google Scholar] [CrossRef]
- Khondkar, P. Composition and partial structure characterization of Tremella polysaccharides. Mycobiology 2009, 37, 286–294. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, J.X.; Zhang, M.R.; Sun, Y.; Li, X.L.; Gao, K.; Liang, J. Naematelia aurantialba polysaccharide: Enhancing 3D printability of soy protein gel via rheology and interactions improvement. Int. J. Biol. Macromol. 2025, 319, 145415. [Google Scholar] [CrossRef]
- Du, X.J.; Zhang, J.S.; Lv, Z.W.; Ye, L.B.; Yang, Y.; Tang, Q.L. Chemical modification of an acidic polysaccharide (TAPA1) from Tremella aurantialba and potential biological activities. Food Chem. 2014, 143, 336–340. [Google Scholar] [CrossRef]
- Wu, Y.J.; Wei, Z.X.; Zhang, F.M.; Linhardt, R.J.; Sun, P.L.; Zhang, A.Q. Structure, bioactivities and applications of the polysaccharides from Tremella fuciformis mushroom: A review. Int. J. Biol. Macromol. 2019, 121, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Kan, J.T.; Wu, F.; Wang, F.J.; Zheng, J.H.; Cheng, J.R.; Li, Y.; Yang, Y.X.; Du, J. Phytonutrients: Sources, bioavailability, interaction with gut microbiota, and their impacts on human health. Front. Nutr. 2022, 9, 960309. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, X.J.; Nie, S.P. Purification, structural characterization, and in vitro fermentation properties of okra polysaccharides. Bioact. Carbohydr. Diet. Fibre 2025, 34, 100481. [Google Scholar] [CrossRef]
- Baker, M.T.; Lu, P.; Parrella, J.A.; Leggette, H.R. Consumer acceptance toward functional foods: A scoping review. Int. J. Environ. Res. Public Health 2022, 19, 1217. [Google Scholar] [CrossRef]
- Yu, D.M.; Wang, W.X.; Hou, S.T.; Chang, M.C.; Cheng, Y.F.; Meng, J.L.; Feng, C.P.; Xu, L.J.; Geng, X.R.; Wang, S.R.; et al. The effect of sequential extraction on the physicochemical and rheological properties of Naematelia aurantialba polysaccharides. Int. J. Biol. Macromol. 2024, 265, 130777. [Google Scholar] [CrossRef]
- Guo, Y.H.; Qin, W.P.; Hou, Y.X.; Zhu, W.W.; Zhao, H.Y.; Zhang, X.K.; Jiao, K. Extraction, purification, structural characteristics and biological properties of the polysaccharides from Rubus L: A review. Food Chem. 2025, 478, 143711. [Google Scholar] [CrossRef]
- Wu, J.S.; Tan, C.; Li, H.Y.; Wang, S.Y.; Wang, X.K.; Wang, S.; Ning, C.; Li, W.X.; Guo, C.T. Extraction, purification, structural characterization, biological activity, structure-activity relationship, and applications of polysaccharides derived from Polygonatum sibiricum: A review. Trends Food Sci. Technol. 2025, 161, 105038. [Google Scholar] [CrossRef]
- Wang, L.; Mao, Y.G.; Zeng, X.; Liu, N.; Niu, C.F.; Li, X.X.; Ma, B.J.; Guo, L.P.; Yang, X.L. Structure and bioactivities of a novel polysaccharide extracted from Dendrobium huoshanense by subcritical Water. Front. Nutr. 2022, 9, 877871. [Google Scholar] [CrossRef]
- Li, X.X.; Zhang, Z.Q.; Wang, L.; Zhao, H.Q.; Jia, Y.H.; Ma, X.; Li, J.Z.; Wang, Y.; Ma, B.G. Three-phase extraction of polysaccharide from Stropharia rugosoannulata: Process optimization, structural characterization and bioactivities. Front. Immunol. 2023, 13, 994706. [Google Scholar] [CrossRef]
- Du, X.J.; Zhang, Y.; Mu, H.M.; Lv, Z.W.; Yang, Y.; Zhang, J.S. Structural elucidation and antioxidant activity of a novel polysaccharide (TAPB1) from Tremella aurantialba. Food Hydrocolloid. 2015, 43, 459–464. [Google Scholar] [CrossRef]
- Deng, C.; Sun, Y.Y.; Fu, H.T.; Zhang, S.X.; Chen, J.H.; Xu, X. Antioxidant and immunostimulatory activities of polysaccharides extracted from Tremella aurantialba mycelia. Mol. Med. Rep. 2016, 14, 4857–4864. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Wang, R.; Sun, D.F.; Li, S.; Xu, H.; Qiu, Y.B.; Lei, P.; Sun, L.; Xu, X.Q.; Zhu, Y.F. High-efficiency production of Tremella aurantialba polysaccharide through basidiospore fermentation. Bioresour. Technol. 2020, 318, 124268. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.X.; Zhang, X.D.; Ma, M.Y.; Long, T.; Xiao, C.L.; Zhang, J.; Liu, J.K.; Zhao, L.Y. Immunoenhancing glucuronoxylomannan from Tremella aurantialba Bandoni et Zang and its low-molecular-weight fractions by radical depolymerization: Properties, structures and effects on macrophages. Carbohydr. Polym. 2020, 238, 116184. [Google Scholar] [CrossRef]
- Du, X.J.; Zhang, J.S.; Yang, Y.; Tang, Q.J.; Jia, W.; Pan, Y.J. Purification, chemical modification and immunostimulating activity of polysaccharides from Tremella aurantialba fruit bodies. J. Zhejiang Univ. Sci. B 2010, 11, 437–442. [Google Scholar] [CrossRef]
- Du, X.J.; Zhang, J.S.; Yang, Y.; Ye, L.B.; Tang, Q.J.; Jia, W.; Liu, Y.F.; Zhou, S.; Hao, R.X.; Gong, C.Y.; et al. Structural elucidation and immuno-stimulating activity of an acidic heteropolysaccharide (TAPA1) from Tremella aurantialba. Carbohydr. Res. 2009, 344, 672–678. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Lian, B.; Huang, D.M.; Cui, F.J. Compare activities on regulating lipid-metabolism and reducing oxidative stress of diabetic rats of Tremella aurantialba broth’s extract (TBE) with its mycelia polysaccharides (TMP). J. Food Sci. 2009, 74, H15–H21. [Google Scholar] [CrossRef]
- Sun, L.; Jiang, J.; Jing, T.; Hu, D.J.; Zhu, J.; Zeng, Y.; Pang, Y.L.; Huang, D.C.; Cheng, S.J.; Cao, C.J. A polysaccharide NAP-3 from Naematelia aurantialba: Structural characterization and adjunctive hypoglycemic activity. Carbohydr. Polym. 2023, 318, 121124. [Google Scholar] [CrossRef]
- Huang, G.C.; Guo, Z.X.; Tan, J.N.; Xu, Q.R.; Wei, C.Y. Optimization of ultrasonic extraction, functional properties, and antioxidant activity of Naematelia aurantialba polysaccharides. Starch-Stärke 2025, 77, 2400141. [Google Scholar] [CrossRef]
- Peng, G.; Wang, S.S.; Zhang, H.S.; Xie, F.; Jiao, L.; Yuan, Y.; Ma, C.; Wu, H.; Meng, Z.L. Tremella aurantialba polysaccharides alleviate ulcerative colitis in mice by improving intestinal barrier via modulating gut microbiota and inhibiting ferroptosis. Int. J. Biol. Macromol. 2024, 281, 135835. [Google Scholar] [CrossRef]
- Pu, Y.L.; Yin, C.L.; Zhang, L.S.; Fang, X.; Zhong, X.H.; Tian, Y.J.; Tao, A.; Yang, Y.C.; Xia, C.L. Extraction, purification, structural characterization, pharmacological activities, and applications of Tricholoma polysaccharides: A review. J. Ethnopharmacol. 2025, 350, 120053. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zheng, J.F.; Zhang, T.; Chen, M.J.; Li, D.; Liu, R.; Li, X.Y.; Wang, H.W.; Sun, T.D. Review of polysaccharides from Citrus medica L. var. sarcodactylis. (Fingered citron): Their extraction, purification, structural characteristics, bioactivity and potential applications. Int. J. Biol. Macromol. 2024, 282, 136640. [Google Scholar] [CrossRef] [PubMed]
- Ai, X.P.; Yu, P.L.; Hou, Y.Q.; Shu, B.; Han, B.; Yang, M.; Fan, X.Y.; Wang, J. Research progress in the extraction, purification, structural features, biological activities, and structure-activity relationships from Prunella vulgaris polysaccharides. Int. J. Biol. Macromol. 2025, 307, 141957. [Google Scholar] [CrossRef] [PubMed]
- Ti, Y.R.; Zhang, Y.L.; Ban, Y.Q.; Wang, X.X.; Hou, Y.Q.; Song, Z.H. Polysaccharide from Hemerocallis citrina Borani by subcritical water with different temperatures and investigation of its physicochemical properties and antioxidant activity. Front. Nutr. 2022, 9, 982695. [Google Scholar] [CrossRef]
- Zhou, B.F.; Jiang, J.M.; Huo, Y.; Lin, J.Y.; Shang, C.H. Extraction, purification and antioxidant activity of Chlorella polysaccharide. Algal Res. 2025, 89, 104077. [Google Scholar] [CrossRef]
- Li, L.; Wang, Z.H.; Zhang, W.; Chen, L.X.; Yang, Y.Y.; Yang, W.; Li, M.K.; Yuan, C.H.; Zhang, L.M.; Wang, L.Q. Isolation, structures, and bioactivities of polysaccharides from Achyranthes bidentata: A review. Molecules 2025, 30, 2523. [Google Scholar] [CrossRef]
- Shi, L.T.; He, Q.; Li, J.; Liu, Y.L.; Cao, Y.L.; Liu, Y.Q.; Sun, C.D.; Pan, Y.J.; Li, X.; Zhao, X.Y. Polysaccharides in fruits: Biological activities, structures, and structure-activity relationships and influencing factors—A review. Food Chem. 2024, 451, 139408. [Google Scholar] [CrossRef]
- Feng, Y.Q.; Juliet, I.C.; Wen, C.T.; Duan, Y.Q.; Zhou, J.; He, Y.Q.; Zhang, H.H.; Ma, H.L. Effects of multi-mode divergent ultrasound pretreatment on the physicochemical and functional properties of polysaccharides from Sagittaria sagittifolia L. Food Biosci. Food Biosci. 2021, 42, 101145. [Google Scholar] [CrossRef]
- Xiao, W.W.; Zhou, P.F.; Wang, X.S.; Zhao, R.Z.; Wang, Y. Comparative characterization and immunomodulatory activities of polysaccharides extracted from the Radix of Platycodon grandiflorum with different extraction methods. Molecules 2022, 27, 4759. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Wang, X.S.; Wu, Y.Y.; Liu, L.Y.; Zhao, Y.; Zhao, R.Z. Comparative study on anti-inflammatory effect of polysaccharides from vinegar-baked Radix bupleuri using different methods. ACS Omega 2023, 8, 29253–29261. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.J.; Zhang, J.J.; Liu, X.C.; Yang, Q.H.; Dong, Y.H.; Jia, L. The antioxidant activities of alkalic-extractable polysaccharides from Coprinus comatus on alcohol-induced liver injury in mice. Sci. Rep. 2018, 8, 11695. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Q.; Wu, Y.D.; Chen, H. Comparative antioxidative characteristics of polysaccharide-enriched extracts from natural sclerotia and cultured mycelia in submerged fermentation of Inonotus obliquus. Food Chem. 2011, 127, 74–79. [Google Scholar] [CrossRef]
- Guo, J.; Tang, C.M.; Liu, Y.F.; Shi, J.; Vunduk, J.; Tang, C.H.; Feng, J.; Zhang, J.S. Innovative submerged directed fermentation: Producing high molecular weight polysaccharides from Ganoderma lucidum. Food Chem. 2025, 471, 142759. [Google Scholar] [CrossRef]
- Dai, C.X.; Huang, X.Y.; Huang, D.M.; Lv, R.Q.; Sun, J.; Zhang, Z.C.; Ma, M.; Aheto, J.H. Detection of submerged fermentation of Tremella aurantialba using data fusion of electronic nose and tongue. J. Food Process Eng. 2019, 42, e13002. [Google Scholar] [CrossRef]
- Zhu, H.; Cao, C.X.; Zhang, S.S.; Zhang, Y.; Zou, W.S. pH-control modes in a 5-L stirred-tank bioreactor for cell biomass and exopolysaccharide production by Tremella fuciformis spore. Bioresour. Technol. 2011, 102, 9175–9178. [Google Scholar] [CrossRef]
- Sun, T.; Xu, X.Y.; Ma, Y.H.; Jiang, H.; Yang, K.; Wang, R.; Gu, Y.; Li, S.; Qiu, Y.B.; Sun, D.F.; et al. Structure, rheology, and antifreeze property of the exopolysaccharide from Naematelia aurantialba through basidiospore fermentation. Food Hydrocolloid. 2023, 142, 108848. [Google Scholar] [CrossRef]
- Chen, J.F.; Wu, S.W.; Shi, Z.M.; Hu, B. Traditional Chinese medicine for colorectal cancer treatment: Potential targets and mechanisms of action. Chin. Med. UK 2023, 18, 14. [Google Scholar] [CrossRef]
- Sun, T.; Liang, X.N.; Xu, X.Y.; Wang, L.H.; Xiao, W.; Ma, Y.H.; Wang, R.; Gu, Y.A.; Li, S.; Qiu, Y.B.; et al. In vitro digestion and fecal fermentation of basidiospore-derived exopolysaccharides from Naematelia aurantialba. Int. J. Biol. Macromol. 2024, 261, 129756. [Google Scholar] [CrossRef]
- Liu, Z.X.; Wang, H.L. Research progress on the isolation, purification, structural characteristics and biological activity mechanism of Pleurotus citrinopileatus polysaccharides. Molecules 2025, 30, 2816. [Google Scholar] [CrossRef]
- Li, F.Y.; Jian, Z.M.; Rui, Y.H.; Tao, A.E. Extraction, purification, structural features and biological activities of the Opuntia dillenii polysaccharides: A review. Int. J. Biol. Macromol. 2025, 314, 144253. [Google Scholar] [CrossRef]
- Yu, A.Q.; Hu, W.J.; Bi, H.Z.; Fu, L.; Wang, Z.B.; Wang, M.; Kuang, H.X. Recent Advances in polysaccharides from Chaenomeles speciosa (sweet) Nakai.: Extraction, purification, structural characteristics, health benefits, and applications. Molecules 2024, 29, 2984. [Google Scholar] [CrossRef]
- Zhu, B.W.; Ni, F.; Xiong, Q.; Yao, Z. Marine oligosaccharides originated from seaweeds: Source, preparation, structure, physiological activity and applications. Crit. Rev. Food Sci. Nutr. 2020, 61, 60–74. [Google Scholar] [CrossRef]
- Sun, L.; Jiang, J.; Zeng, Y.; Zhu, J.; Wang, S.L.; Huang, D.C.; Cao, C.J. Polysaccharide NAP-3 synergistically enhances the efficiency of metformin in type 2 diabetes via bile acid/GLP-1 axis through gut microbiota remodeling. J. Agric. Food. Chem. 2024, 72, 21077–21088. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liang, J.; Gao, J.N.; Shen, Y.; Kuang, H.X.; Xia, Y.G. A novel LC-MS/MS method for complete composition analysis of polysaccharides by aldononitrile acetate and multiple reaction monitoring. Carbohyd. Polym. 2021, 272, 118478. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.X.; Liao, J.R.; Sun, Y.H.; Wang, Y.F.; Li, P.; Du, B. A review of advances in the extraction, structural characterization, gel properties, and biological activity mechanisms of Dendrobium officinale polysaccharides. Int. J. Biol. Macromol. 2025, 311, 143756. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.Z.; Teng, W.J.; Wang, J.Y.; Wang, X.Y.; Zhang, Z.J.; Wang, M. Recent developments in non-starch Ipomoea batatas (L.) Lam. polysaccharides: Extractions and purifications, structural characteristics, pharmacological activities, structure-activity relationships, and applications A review. Int. J. Biol. Macromol. 2025, 309, 142808. [Google Scholar] [CrossRef]
- Sun, T.; Jiang, H.; Xu, X.Y.; Ma, Y.H.; Liang, X.N.; Wang, R.; Gu, Y.; Li, S.; Qiu, Y.B.; Sun, D.F.; et al. Adaptive laboratory evolution of Naematelia aurantialba under high temperature for efficient production of exopolysaccharide. Int. J. Biol. Macromol. 2024, 263, 130425. [Google Scholar] [CrossRef]
- Fei, Z.Q.; Xie, H.Q.; Xie, D.C.; Wang, M.; Du, Q.Z.; Jin, P. Structural characterization and high-efficiency prebiotic activity of the polysaccharide from Tremella aurantialba endophytic bacteria. Int. J. Biol. Macromol. 2024, 260, 129347. [Google Scholar] [CrossRef]
- Yang, L.H.; Liu, J.; Xia, X.W.; Wong, I.N.; Chung, S.K.; Xu, B.J.; El-Seedi, H.R.; Wang, B.; Huang, R.M. Sulfated heteropolysaccharides from Undaria pinnatifida: Structural characterization and transcript-metabolite profiling of immunostimulatory effects on RAW264.7 cells. Food Chem. X 2022, 13, 100251. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wang, J.Y.; Bi, H.Z.; Zhang, Z.J.; Zhang, M.R.; Wang, M. Research progress in the extraction and purification, structural characteristics, pharmacological activities, structure-activity relationships, and applications from Alpinia oxyphylla Miq. polysaccharides. Int. J. Biol. Macromol. 2025, 315, 144387. [Google Scholar] [CrossRef]
- Liu, L.S.; Shi, X.G.; Jia, L.K.; Wang, R.; Liu, C.W. Natural compounds and health benefits of Ganoderma capense. Molecules 2025, 30, 2250. [Google Scholar] [CrossRef]
- Sun, M.H.; Zhang, Y.P.; Gao, W.Y.; He, Y.J.; Wang, Y.; Sun, Y.P.; Kuang, H.X. Polysaccharides from Porphyra haitanensis: A review of their extraction, modification, structures, and bioactivities. Molecules 2024, 29, 3105. [Google Scholar] [CrossRef]
- Zhang, L.M.; Li, X.Y.; Bi, B.; Sun, Y. Progress in the isolation, purification, structural characteristics and biological functions of polysaccharides from okra (Abelmoschus esculentus (L.) Moench): A review. Int. J. Biol. Macromol. 2025, 312, 144184. [Google Scholar] [CrossRef] [PubMed]
- Du, X.J.; Wang, X.D.; Chen, Y.; Tian, S.Y.; Lu, S.F. Antioxidant activity and oxidative injury rehabilitation of chemically modified polysaccharide (TAPA1) from Tremella aurantialba. Macromol. Res. 2018, 26, 479–483. [Google Scholar] [CrossRef]
- Kiho, T.; Morimoto, H.; Kobayashi, T.; Usui, S.; Ukai, S.; Aizawa, K.; Inakuma, T. Effect of a polysaccharides (TAP) from the fruiting bodies of Tremella aurantia on glucose metabolism in mouse liver. Biosci. Biotechnol. Biochem. 2000, 64, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Shangguan, H.Z.; Wang, X.; Liu, J.P.; Shi, Y.H.; Xu, X.Y.; Xie, Y.D. Extraction, purification, structural characterization, biological activity, mechanism of action and application of polysaccharides from Ganoderma lucidum: A review. Int. J. Biol. Macromol. 2025, 288, 138575. [Google Scholar] [CrossRef]
- Kiho, T.; Kochi, M.; Usui, S.; Hirano, K.; Aizawa, K.; Inakuma, T. Antidiabetic effect of an acidic polysaccharide (TAP) from Tremella aurantia and its degradation product (TAP-H). Biol. Pharm. Bull. 2001, 24, 1400–1403. [Google Scholar] [CrossRef]
- Yang, C.L.; Zhou, Y.Q.; Liu, H.J.; Xu, P. The Role of inflammation in cognitive impairment of obstructive sleep apnea syndrome. Brain Sci. 2022, 12, 1303. [Google Scholar] [CrossRef]
- Hao, J.B.; Liu, X.T.; Tang, J.; Chang, X.Y.; Jin, H.E.; Zhu, B.; Hao, L.R.; Zhang, L. The effect of allograft inflammatory factor-1 on inflammation, oxidative stress, and autophagy via mir-34a/ATG4B pathway in diabetic kidney disease. Oxid. Med. Cell. Longev. 2022, 2022, 1668000. [Google Scholar]
- Pinho, A.R.; Fortuna, A.; Falcão, A.; Santos, A.C.; Seiça, R.; Estevens, C.; Veiga, F.; Ribeiro, A.J. Comparison of ELISA and HPLC-MS methods for the determination of exenatide in biological and biotechnology-based formulation matrices. J. Pharm. Anal. 2019, 9, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Xie, Q.X.; Pan, X.H.; Zhang, R.N.; Zhang, X.Y.; Peng, G.; Zhang, Y.W.; Shen, S.M.; Tong, N.W. Type 2 diabetes mellitus in adults: Pathogenesis, prevention and therapy. Signal Transduct. Tar. 2024, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.Y.; Liu, H.Y.; Wang, H.G. Exogenous hydrogen sulfide plays an important role by regulating autophagy in diabetic-related diseases. Int. J. Mol. Sci. 2021, 22, 6715. [Google Scholar] [CrossRef]
- Liu, X.F.; Luo, D.H.; Guan, J.J.; Chen, J.; Xu, X.F. Mushroom polysaccharides with potential in anti-diabetes: Biological mechanisms, extraction, and future perspectives: A review. Front. Nutr. 2022, 9, 1087826. [Google Scholar] [CrossRef]
- Xu, L.; Wang, F.; Zhang, Z.C.; Terry, N. Optimization of polysaccharide production from Cordyceps militaris by solid-state fermentation on rice and its antioxidant activities. Foods 2019, 8, 590. [Google Scholar] [CrossRef]
- Jia, X.; Ma, L.; Xiao, M.; Atehli, D.; Zhang, Y.H.; Liu, Y.S.; Wang, W.; Wang, C.L.; Guo, Q.B. Characterization of salt brine sulfated polysaccharides: Immunomodulatory activity based on gut microbiota. Blue Biotechnol. 2024, 1, 7. [Google Scholar] [CrossRef]
- Lee, G.W.; Kim, H.Y.; Hur, H.; Lee, M.W.; Shim, M.J.; Lee, U.; Lee, T.S. Antitumor and immune-modulatory effect of crude polysaccharides from fruiting body of Tremella aurantia against mouse sarcoma 180. Kor. J. Mycol. 2008, 36, 66–74. [Google Scholar]
- O’Mahony, C.; Amamou, A.; Ghosh, S. Diet-microbiota interplay: An emerging player in macrophage plasticity and intestinal health. Int. J. Mol. Sci. 2022, 23, 3901. [Google Scholar] [CrossRef]
- Tu, A.B.; Zhao, X.; Shan, Y.Y.; Lü, X. Potential role of ovomucin and its peptides in modulation of intestinal health: A review. Int. J. Biol. Macromol. 2020, 162, 385–393. [Google Scholar] [CrossRef]
- Živković, L.; Bajić, V.; Topalović, D.; Bruić, M.; Spremo-Potparević, B. Antigenotoxic effects of biochaga and dihydroquercetin (taxifolin) on H2O2-induced DNA damage in human whole blood cells. Oxid. Med. Cell. Longev. 2019, 2019, 5039372. [Google Scholar] [CrossRef]
- Vasilopoulou, M.A.; Gioran, A.; Theodoropoulou, M.; Koutsaviti, A.; Roussis, V.; Ioannou, E.; Chondrogianni, N. Healthspan improvement and anti-aggregation effects induced by a marine-derived structural proteasome activator. Redox Biol. 2022, 56, 102462. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Liu, H.K.; Zhang, X.W.; Ao, Q. Effect of pressure grinding technology on the physicochemical and antioxidant properties of Tremella aurantialba powder. J. Food Process. Preserv. 2018, 42, e13833. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Su, C.X.; Wei, S.J.; Zhao, J.; Wei, F.; Liu, X.L.; Wang, H.B.; Wu, X.Y.; Feng, C.P.; Meng, J.L.; et al. The effects of Naematelia aurantialba on the pasting and rheological properties of starch and the research and development of soft candy. Foods 2024, 13, 247. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Yang, S.H.; Yang, Z.; Zheng, T.F.; Li, P.H.; Zhou, Q.W.; Cai, W.; Wang, Y.; Zhang, J.; Ji, X.Y.; et al. Effects of a novel microbial fermentation medium produced by Tremella aurantialba SCT-F3 on cigar filler leaf. Front. Microbiol. 2023, 14, 1267916. [Google Scholar] [CrossRef]

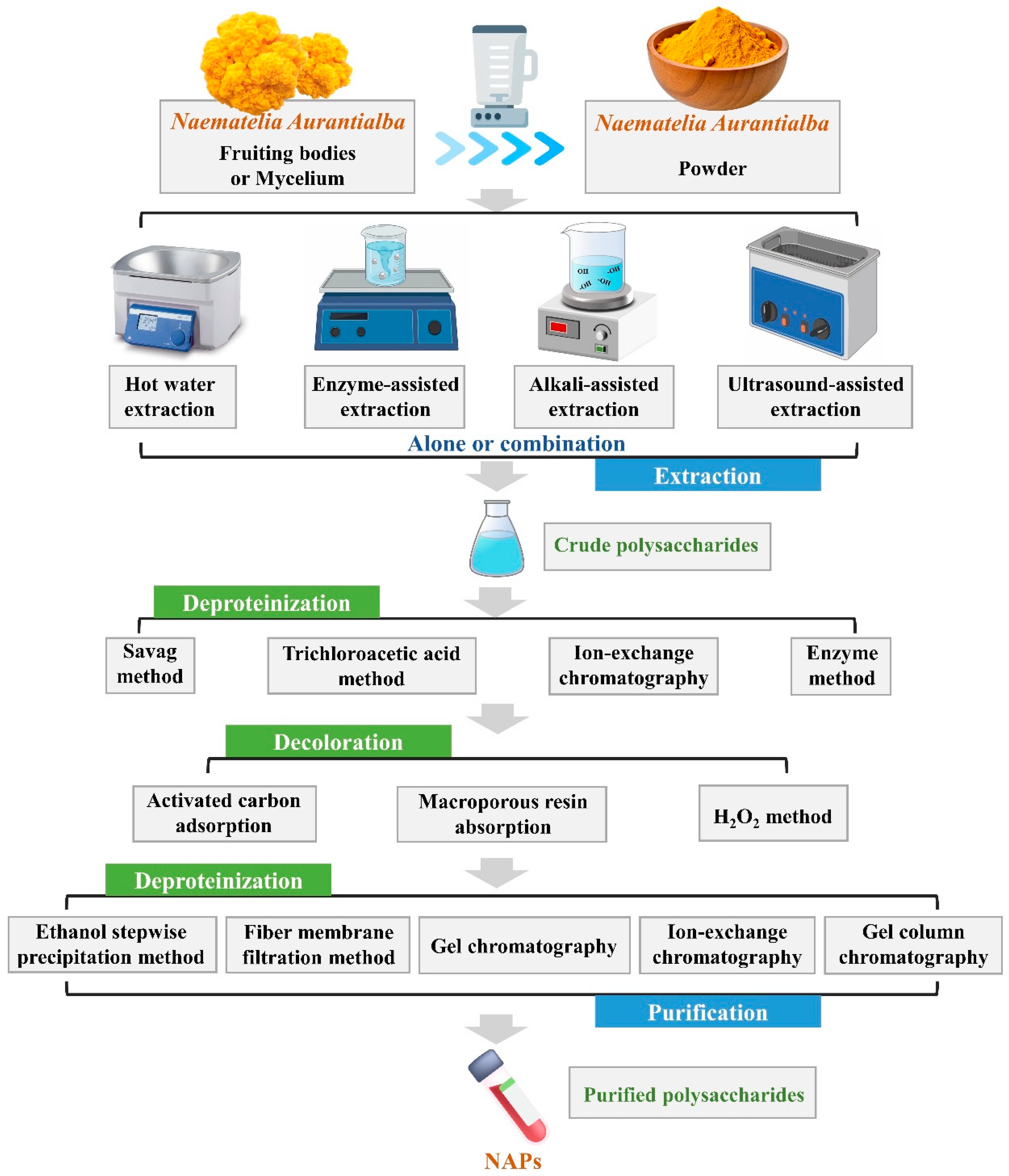
| Techniques | Principle | Extraction Conditions | Evaluation | References | |||||
|---|---|---|---|---|---|---|---|---|---|
| Sources | Time | Repetition | Temperature | Solid–Liquid Ratio | Yield | ||||
| HWE | NAPs have a higher solubility in hot water and a stable structure | Fruiting bodies | 3 h | Thrice | 95 °C | 1:40 | 50.60% | Advantages: Simple operation and equipment, low cost; Disadvantages: Long extraction time, low extraction rate, high temperature. | [36] |
| Fruiting bodies | 100 min | Thrice | 100 °C | 1:10 | 3.84% | [37] | |||
| Fruiting bodies | 2 h | Thrice | 100 °C | 1:10 | - | [38] | |||
| Mycelium | 4 h | Thrice | 100 °C | 1:10 | - | [39] | |||
| EAE | Biological enzymes can destroy the cell wall structure and accelerate the dissolution of NAPs | Fruiting bodies | 45 min | Once | 55 °C | 1:15 | - | Advantages: Mild conditions, low energy consumption, high efficiency; Disadvantages: expensive and prone to inactivation. | [35] |
| Fruiting bodies | 2 h | Thrice | 45 °C | 1:40 | 24.95% | [40] | |||
| UAE | Break down cell walls, and accelerate the precipitation rate of intracellular components | Fruiting bodies | 32 min | Once | - | 1:49 | 46.36% | Advantages: High efficiency, low energy consumption, simple operation. | [41] |
| Fruiting bodies | 4 h | Once | 75 °C | 1:20 | - | [42] | |||
| AAE | Acidic polysaccharides have a higher solubility in alkaline solutions | Mycelium | 4–6 h | Once | 25 °C | - | 1.53% | Advantages: High efficiency, high extraction rate; Disadvantage: Only suitable for acidic polysaccharides. | [34] |
| EAE/UAE | Increase extraction efficiency | Fruiting bodies | 1 h | Once | 80 °C | 1:80 | 50.15% | Advantages: High extraction rate, low energy consumption; Disadvantages: High cost, complex operation steps | [28] |
| Name | Carbohydrate Content | Molecular Weight (kDa) | Monosaccharide Composition | Structural Features | Analysis Technique | References |
|---|---|---|---|---|---|---|
| TAPS-E | - | 1130.4 | Man-N:Man:GlcA:Glc:Xyl = 3.3:52.0:4.1:2.4:1.8:36.4 | - | HPLC, GPC, FT-IR | [35] |
| TAPS-F | - | 2924.6 | Man-N:Man:GlcA:Glc:Xyl = 8.7:47.4:2.0:3.4:1.0:37.5 | - | ||
| NAPS-A | 93.4% | 2924.0 | Man:Xyl:GlcA:Glc:Gal = 59.75:31.73:4.20:2.51:1.81 | The structure is inferred as presented below: →3)-α-DManp, →2,3,4)-α-D-Manp-(1→, →3)-α-D-Manp-(1→, β-DManp-(1→, →2)-β-D-Xylp-(1→, β-D-Xylp-(1→, and →3)-β-DXylp-(1→ sugar residues | GPC, HPLC, FT-IR, GC-MS, NMR, SEM, AFM, XRD, DSC, TG | [58] |
| NAPS-B | 90.6% | 1763.0 | Man:Xyl:GlcA:Glc:Gal = 38.17:26.40:7.08:7.79:20.56 | - | ||
| NAPS-25 | 91.0% | 2948.0 | Man:Xyl:GlcA:Glc:Gal = 59.17:32.26:4.42:1.39:2.76 | 2,3,4-Me3-Xylp:2,4-Me2-Xylp:3,4-Me2-Xylp:2,3,4,6-Me4-Manp:2,3,6-Me3-GlcAp:2,4,6-Me3-Manp:6-Me-Manp = 10.27:7.07:13.58:31.00:2.45:20.05:15.58 | HPLC, FT-IR, SEM | [69] |
| NAPS-30 | 90.9% | 4647.0 | Man:Xyl:GlcA:Glc:Gal = 61.99:30.06:4.65:1.44:1.86 | 2,3,4-Me3-Xylp:2,4-Me2-Xylp:3,4-Me2-Xylp:2,3,4,6- Me4-Manp:2,3,6-Me3-GlcAp:2,4,6-Me3-Manp:6-Me-Manp = 8.69:8.17:13.95:26.76:2.85:20.62:18.96 | ||
| TAPA1 | 98.7% | 1350.0 | Man:Xyl:GlcA = 5:4:1 | The sequences of 4 residues are as follows: →3)-α-D-Manp-(1→3)-α-D-Manp-(1→3)-α-D-Manp-(1→,4-β-D-Manp-(1→3)-β-D-Xylp-(1→4)-β-D-ClcAp-(1→2)-α-D-Manp-(1→,α-D-Manp-(1→4)-β-D-Xylp-(1→2)-α-D-Manp-(1→,β-D-Xylp-(1→2)-β-D-Xylp-(1→4)-α-D-Manp-(1→ | GPC, HPAEC-PAD, FT-IR, NMR | [38] |
| TAPB1 | 97.6% | 760.0 | Man:Xyl:GlcA = 3.1:2.9:1.2 | The sequences of 3 residues are as follows: →3)-α-D-Manp-(1→3)-α-D-Manp-(1→3)-α-D-Manp-(1→, β-D-ClcAp-(1→3)-β-D-Xylp-(1→2)-α-D-Manp-(1→, β-D-Xylp-(1→2)-β-D-Xylp-(1→4)-α-D-Manp-(1→ | FT-IR, HPAEC-PAD, GPC, GC-MS, NMR | [33] |
| NAPS-A | 93.4% | 2930.0 | Man:Xyl:GlcA:Glc:Gal = 1.08:0.57:0.08:0.01:0.01 | - | GPC, HPLC, FT-IR, SEM | [60] |
| TAP-3 | 61.8% | 624.0 | Man:Xyl:GlcA = 27.31:9.02:8.86 | 7 linkage forms: (1→3)-linked Xylp, terminal Xylp, (1→4)—linked GlcpA, terminal Manp, (1→3)—linked Manp, (1→ 2,3)—linked Manp and (1→ 2)—linked Manp. | HPGPC, GC-MS, NMR, SEM | [36] |
| NAP-3 | 93.4% | 428.0 | Man:Rha:Xyl = 67.39:7.87:22.91 | 5 types of residues: β-1,2,3-D-Manp, terminal β-DXylp, β-1, 4-D-Glcp, β-1,3-D-Manp, β-1, 4-D-Rhap | HPGPC, FT-IR, XRD, HPLC, SEM, NMR | [40] |
| NAP | 89.9% | 915.0 | Man:Xyl:GlcA:GalA:Glc = 59.04:23.89:14.07:2.12:0.76 | - | HPLC-SEC, FT-IR | [41] |
| TA 2-1 | - | 127.0 | Man:Xyl:GlcA:Glc:Fru:Rha = 59.2:23.1:13.9:1.6:1.7:0.4 | 1, 3-Man with branch chains of T-Xylp, 1,3Xylp, 1,4-GlcAp, and T-Manp at its O-2 position | HPLC-SEC, FT-IR, GC-MS | [42] |
| TABP | - | 5.4 | Ara:GlcN:Gal:Glc:Man = 0.073:0.145:0.406:0.182:0.195 | Linkage types: 1,5-Araf, 1,4- linked-GlcpN, 1,4-linked-Galp, 1,4-linked-Manp, 1,6-linked-Manp, 1,4,6-linked-Galp, 1,2,6-linked-Manp, and 1,3,5-Araf | HPGPC, HPAEC, FT-IR, GC-MS, NMR | [70] |
| Bioactivities | Sources | Models | Measurement Indicators | References |
|---|---|---|---|---|
| Antioxidant activity | Basidiospore | Chemical determination | ABTS, DPPH, OH, and superoxide anion radical scavenging. | [58] |
| Basidiospore | Chemical determination | DPPH and superoxide anion radical scavenging. | [69] | |
| Fruiting bodies | Chemical determination | Superoxide anion and H2O2 scavenging. | [76] | |
| Oxidative injury PC12 cells | Cell viability. | |||
| Fruiting bodies | Chemical determination | Superoxide anion and H2O2 scavenging. | [33] | |
| Fruiting bodies | Chemical determination | ABTS, DPPH, and hydroxyl radical scavenging. | [41] | |
| Hypoglycemic activity | Fruiting bodies | Type 2 diabetic mice | Weight, fasting blood glucose, food consumption; Metabolic phenotyping: OGTT, HOMA-IR; LP-1, BSH; Enzymes activity: CAT, GSH-Px, SOD, MDA; | [40] |
| Human HepG2 cells | Glucose consumption, cytotoxicity assay, ROS | |||
| Fruiting bodies | Type 2 diabetic mice | Weight, fasting blood glucose, food consumption; Metabolic phenotyping: OGTT, ITT, HOMA-IR, TC, TG; Liver function: AST, ALT; Antioxidant enzymes activity: CAT, GSH-Px, SOD, MDA; Histopathology: H&E staining; Intestinal permeability: D-LA, DAO; Gut microbiota: 16s rRNA gene sequencing; Genetic expression: RT-PCR. | [65] | |
| Fruiting bodies | STZ-induced diabetic mice | Key indicators in glycometabolism pathway: phofructokinase, glycogen, and plasma cholesterol. | [77] | |
| Fruiting bodies | ddY mice | Plasma glucose, water and food consumption. | [77,78] | |
| Fruiting bodies | Type 2 diabetic mice | Weight, food and water consumption; Plasma insulin, plasma lipid, and lipid in feces. | [79] | |
| Immunomodulatory activity | Fruiting bodies | RAW264.7 cells | Cell viability: MTT; Inflammation: IL-1β and TNF-α | [36] |
| Fruiting bodies | C57BL/6 male mice spleen cells | Proliferation rate | [37] | |
| Fruiting bodies | C57BL/6 male mice spleen cells | Proliferation rate | [38] | |
| Anti-colitis activity | Fruiting bodies | Erastin-induced Caco-2 cells | Viability, Fe2+, MDA, ROS, NADPH/NADP+ | [42] |
| DSS-induced colitis mice | Apparent indicators: body weight loss, fecal consistency, fecal blood test scores, and DAI; Pathological evaluation: H&E staining, immunohistochemistry, and immunofluorescence staining; Inflammatory cytokine levels: IL-6, TNF-α, and MCP-1. | |||
| Prebiotic activity | Fruiting bodies | Lacticaseibacillus paracasei, Lacticaseibacillus rhamnosus | Prebiotic activity: growth curve. | [70] |
| Hypolipidemic activity | Mycelium | Type 2 diabetic rats | blood glucose, weight, food consumption, plasma cholesterol, plasma phospholipids, plasma triglyceride, T-AOC, CAT, SOD, MDA, GSH-Px, GC | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, R.-N.; Zhu, Y.-Y.; Ma, R.-H.; Ni, Z.-J.; Deng, X.-J.; Thakur, K.; Wei, Z.-J. Purification, Structural Characteristics, Bioactive Properties, and Applications of Naematelia aurantialba Polysaccharides: A Comprehensive Review. Molecules 2025, 30, 4073. https://doi.org/10.3390/molecules30204073
Wu R-N, Zhu Y-Y, Ma R-H, Ni Z-J, Deng X-J, Thakur K, Wei Z-J. Purification, Structural Characteristics, Bioactive Properties, and Applications of Naematelia aurantialba Polysaccharides: A Comprehensive Review. Molecules. 2025; 30(20):4073. https://doi.org/10.3390/molecules30204073
Chicago/Turabian StyleWu, Ri-Na, Yun-Yang Zhu, Run-Hui Ma, Zhi-Jing Ni, Xiao-Juan Deng, Kiran Thakur, and Zhao-Jun Wei. 2025. "Purification, Structural Characteristics, Bioactive Properties, and Applications of Naematelia aurantialba Polysaccharides: A Comprehensive Review" Molecules 30, no. 20: 4073. https://doi.org/10.3390/molecules30204073
APA StyleWu, R.-N., Zhu, Y.-Y., Ma, R.-H., Ni, Z.-J., Deng, X.-J., Thakur, K., & Wei, Z.-J. (2025). Purification, Structural Characteristics, Bioactive Properties, and Applications of Naematelia aurantialba Polysaccharides: A Comprehensive Review. Molecules, 30(20), 4073. https://doi.org/10.3390/molecules30204073







