A Comparative Analysis of the Polyphenolic Content and Identification of New Compounds from Oenothera biennis L. Species from the Wild Flora
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Collection of Vegetal Material
- Vatra Dornei, Romania; altitude 926 m; GPS coordinates 47° 20′ 30.2136′′ N, 25° 19′ 55.0128′′ E; the samples were registered with voucher number 1256 in the Herbarium of the Plant Systematics discipline, part of the Department of Biology and Life Sciences at the “Vasile Goldiș” Western University of Arad (Romania) (Figure S1);
- Macea, Romania; altitude 99 m; GPS coordinates 46° 23′ 17.6676′′ N, 21° 18′ 7.7934′′ E; the samples were registered with voucher number 1257 in the Herbarium of the Plant Systematics discipline, part of the Department of Biology and Life Sciences at the “Vasile Goldiș” Western University of Arad (Romania) (Figure S2).
4.2. Preparation of the Hydroalcoholic Extract
4.3. Preparation of the Aqueous Extract
4.4. Liquid Chromatography—Mass Spectrometry Analysis
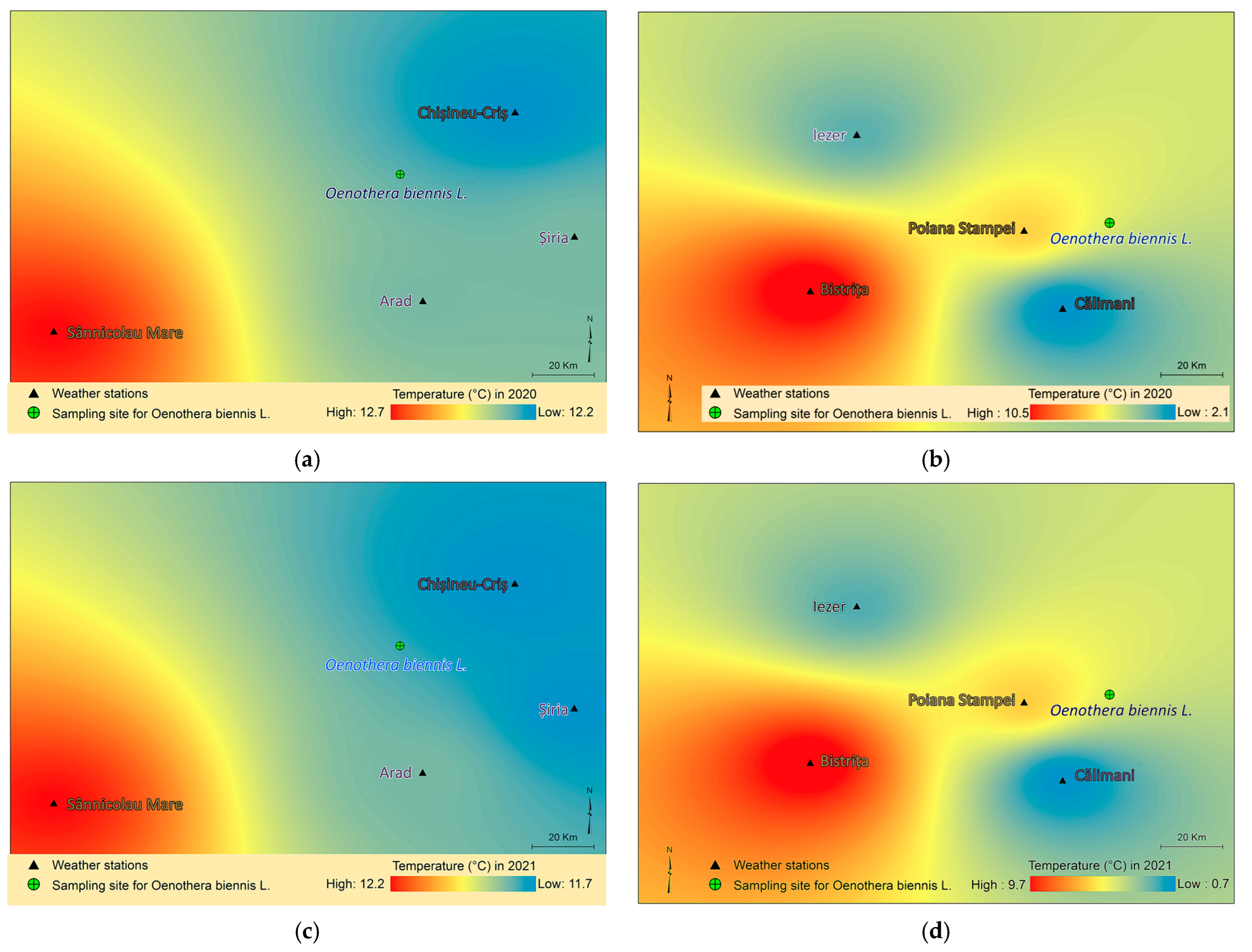
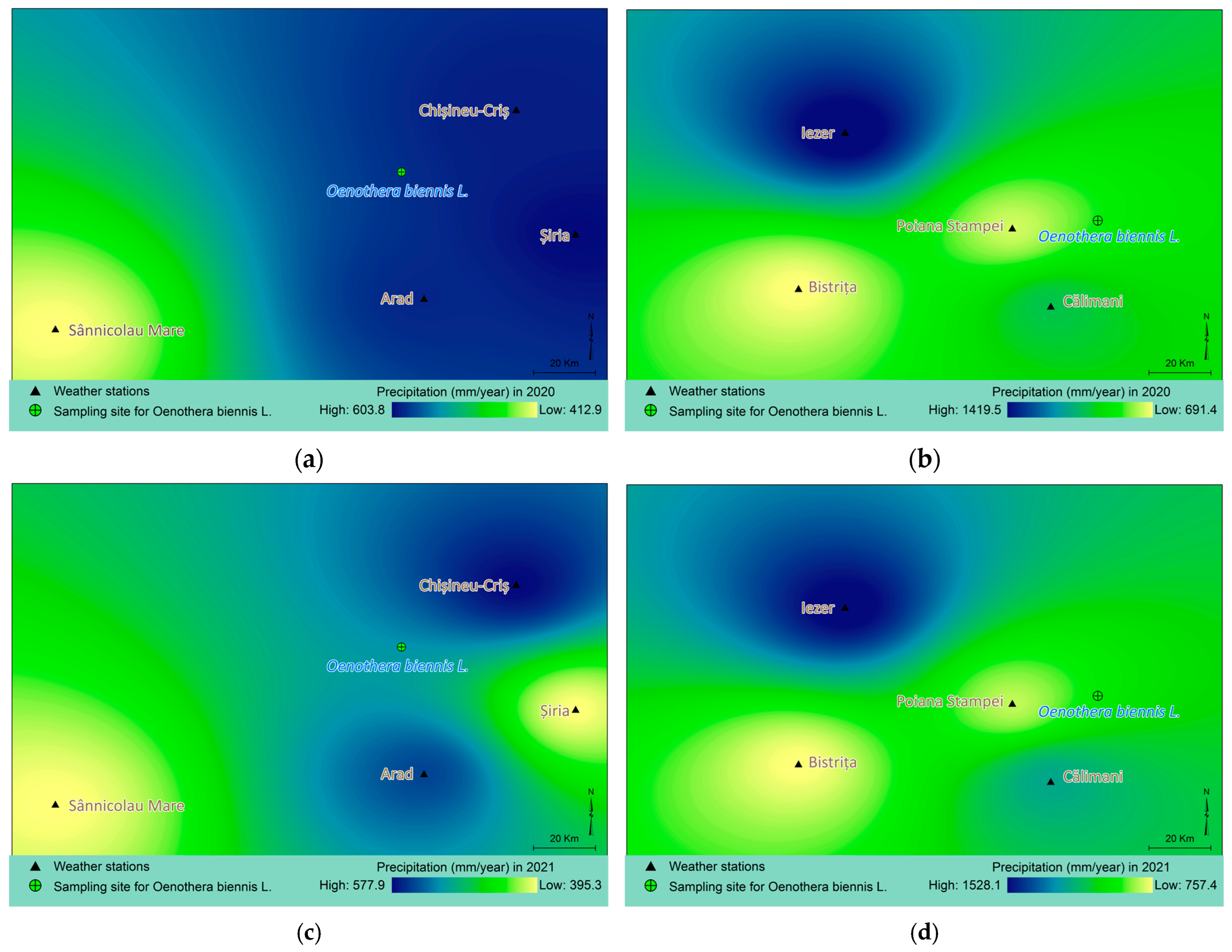
4.5. Spatial Modelling and Thematic Mapping of Climatic Parameters
4.6. Bibliographic Study
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WFO [World Flora Online]: Oenothera biennis L. Available online: https://www.worldfloraonline.org/taxon/wfo-0000389564 (accessed on 2 October 2023).
- Steckel, L.E.; Sosnoskie, L.M.; Steckel, S.J. Common evening-primrose (Oenothera biennis L.). Weed Technol. 2019, 33, 757–760. [Google Scholar] [CrossRef]
- Calapai, G.; Minciullo, P.L.; Miroddi, M.; Chinou, I.; Gangemi, S.; Schmidt, R.J. Contact dermatitis as an adverse reaction to some topically used European herbal medicinal products-Part 3: Mentha × piperita-Solanum dulcamara. Contact Dermat. 2016, 74, 131–144. [Google Scholar] [CrossRef]
- Dietrich, W.; Wagner, W.L.; Raven, P.H. Systematic Botany Monographs; The American Society of Plant Taxonomists: Laramie, WY, USA, 1997; Volume 50, pp. 20–39. [Google Scholar]
- Săvulescu, T. Genul Oenothera L. Oenothera biennis L. In Flora Republicae Popularis Romanicae; Ghișa, E., Grințescu, I., Gușuleac, M., Morariu, I., Nyárády, E.I., Prodan, I., Eds.; Editio Academiae Republicae Popularis Romanicae: Bucharest, Romania, 1957; Volume V, pp. 514–516. [Google Scholar]
- Sârbu, I.; Ștefan, N.; Oprea, A.; Bortaș, V. Plante Vasculare din România: Determinator Ilustrat de Teren; Editura Victor B Victor: Bucharest, Romania, 2013. [Google Scholar]
- Balch, A. Evaluation of Native Evening Primrose for Gamma-Linolenic Acid. Ph.D Thesis, Texas Tech University, Lubbock, TX, USA, August 2000. [Google Scholar]
- Kuhn, M.A.; Wiston, D. Herbal Therapy & Supplements: A Scientific & Traditional Approach, 2nd ed.; Wolters Kluwer Health|Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; pp. 181–185. [Google Scholar]
- Stonemetz, D. A Review of the Clinical Efficacy of Evening Primrose. Holist. Nurs. Pract. 2008, 22, 171–174. [Google Scholar] [CrossRef]
- Howard, G.Z.; Mabry, T.J.; Raven, P.H. Distribution of flavonoids in twenty-one species of Oenothera. Phytochemistry 1972, 11, 289–291. [Google Scholar] [CrossRef]
- Zinsmeister, H.D.; Bartl, S. The phenolic compounds of Oenothera. Phytochemistry 1971, 10, 3129–3132. [Google Scholar] [CrossRef]
- Yoshida, T.; Tong, C.; Muneto, M.; Taeko, Y.; Kuzufumi, Y.; Tsutomu, H.; Aya, N.; Takuo, O. Woodfordin D and Oenothein A, Trimeric Hydrolyzable Tannins of Macro-Ring Structure with Antitumor Activity. Chem. Pharm. Bull. 1991, 39, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.T.J.; Agrawal, A.A.; Maron, J.L.; Salminen, J.-P. Heritability, covariation and natural selection on 24 traits of common evening primrose (Oenothera biennis) from a field experiment. J. Evol. Biol. 2009, 22, 1295–1307. [Google Scholar] [CrossRef]
- Weglarz, Z.; Kosakowska, L.; Pelc, M.; Geszprych, A.; Przybyl, J.L.; Baczek, K. Intraspecific variability of fireweed (Chamaenerion angustifolium/L./Scop.) and evening primrose (Oenothera biennis L.) in respect of sterol content. Herba Pol. 2011, 57, 7–15. [Google Scholar]
- Lakshman, J.A.; Shasidhar, M.; Ravi Kumar, A. Preliminary phytochemical and toxicity studies of Oenothera biennis. Indian J. Res. Pharm. Biotechnol. 2014, 2, 1114–1118. [Google Scholar]
- Anstett, D.N.; Cheval, I.; D’Souza, C.; Salminen, J.-P.; Johnson, M.T.J. Ellagitannins from the Onagraceae Decrease the Performance of Generalist and Specialist Herbivores. J. Chem. Ecol. 2019, 45, 86–94. [Google Scholar] [CrossRef]
- Agrawal, A.A.; Hastings, A.P.; Johnson, M.T.J.; Maron, J.L.; Salminen, J.-P. Insect Herbivores Drive Real-Time Ecological and Evolutionary Change in Plant Populations. Science 2012, 338, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Anstett, D.N.; Ahern, J.R.; Glinos, J.; Nawar, N.; Salminen, J.-P.; Johnson, M.T.J. Can Genetically Based Clines in Plant Defence Explain Greater Herbivory at Higher Latitudes? Edited by Ted Turlings. Ecol. Lett. 2015, 18, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Noge, K.; Tamogami, S. Isovaleronitrile co-induced with its precursor, l-leucine, by herbivory in the common evening primrose stimulates foraging behavior of the predatory blue shield bug. Biosci. Biotechnol. Biochem. 2018, 82, 395–406. [Google Scholar] [CrossRef]
- Dong, Z.Y.; Yin, X.S.; Liu, S.L.; Zhang, T.H.; Ren, H.; Tan, H.Y. Ultrasonic-assisted Extraction, Antioxidant Activity and Structural Characterization of Polysaccharides from Oenothera biennis L. Leaves. E3S Web Conf. 2020, 189, 02025. [Google Scholar] [CrossRef]
- Patan, A.; Saranya, M.; Vignesh, S.; Bharathi, A.; Vikram, G.; Yuvaraj, P.; Vijeyaanandhi, M. Qualitative Phytochemical Analysis in Determination of Antioxidant Activity of Methanolic Extract of Oenothera biennis by GC-MS–A Preliminary Research Study. Res. J. Pharm. Technol. 2021, 14, 3744–3750. [Google Scholar] [CrossRef]
- Mukherjee, K.D.; Kiewitt, I. Formation of gamma-linolenic acid in the higher plant evening primrose (Oenothera biennis L.). J. Agric. Food Chem. 1987, 35, 1009–1012. [Google Scholar] [CrossRef]
- Krzaczek, T.; Bogucka-Kocka, A. P21 chromatographical analysis of phenolic acids in some species genus Oenothera L. Eur. J. Pharm. Sci. 1994, 2, 117–194. [Google Scholar]
- Krzaczek, T.; Bogucka-Kocka, A.; Śnieżko, R. The phenolic acids of some species of the Oenothera L. genus. Acta Soc. Bot. Pol. 1995, 64, 41–44. [Google Scholar] [CrossRef]
- Granica, S.; Bazylko, A.; Kiss, A.K. Determination of Macrocyclic Ellagitannin Oenothein B in Plant Materials by HPLC-DAD-MS: Method Development and Validation. Phytochem. Anal. 2012, 23, 582–587. [Google Scholar] [CrossRef]
- Granica, S.; Czerwinska, M.E.; Piwowarski, J.P.; Ziaja, M.; Kiss, A.K. Chemical composition, antioxidative and anti-inflammatory activity of extracts prepared from aerial parts of Oenothera biennis L. and Oenothera paradoxa Hudziok obtained after seed cultivation. J. Agric. Food Chem. 2013, 61, 801–810. [Google Scholar] [CrossRef]
- Fecker, R.; Buda, V.; Alexa, E.; Avram, S.; Pavel, I.Z.; Muntean, D.; Cocan, I.; Watz, C.; Minda, D.; Dehelean, C.A.; et al. Phytochemical and Biological Screening of Oenothera biennis L. Hydroalcoholic Extract. Biomolecules 2020, 10, 818. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Ikeda, H.; Hiromu, K. The constituents of the essential oil from Oenothera biennis L. Nippon. Nōgeikagaku Kaishi 1978, 52, 449–455. [Google Scholar] [CrossRef]
- Shukla, Y.N.; Srivastava, A.; Kumar, S. Aryl, lipid and triterpenoid constituents from Oenothera biennis. Indian J. Chem. 1999, 38, 705–708. [Google Scholar]
- Shukla, Y.N.; Ranganathan, T.; Kumar, S.; Srivastava, A.; Khanuja, S.P.S.; Gupta, V.K.; Kumar, S. Antibacterial Composition Comprising Oenostacin from Oenothera biennis. US Patent 6.451.356 B1, 17 September 2002. [Google Scholar]
- Ahmad, A.; Singh, D.K.; Fatima, K.; Tandon, S.; Luqman, S. New constituents from the roots of Oenothera biennis and their free radical scavenging and ferric reducing activity. Ind. Crop Prod. 2014, 58, 125–132. [Google Scholar] [CrossRef]
- Singh, S.; Dubey, V.; Singh, D.K.; Fatima, K.; Ahmad, A.; Luqman, S. Antiproliferative and antimicrobial efficacy of the compounds isolated from the roots of Oenothera biennis L. J. Pharm. Pharmacol. 2017, 69, 1230–1243. [Google Scholar] [CrossRef]
- Sultana, N.S.; Ali, N.M.; Rasool, S. Chemical Constituents From the Roots of Oenothera biennis L. Pharm. Biosci. J. 2018, 6, 29–35. [Google Scholar] [CrossRef]
- Ambrin, D.G.; Bakht, J.; Adil, M. Phytotoxic, insecticidal and cytotoxic activities of Ziziphus mauritiana var. spontanea Edgew. and Oenothera biennis L. Pak. J. Bot. 2020, 52, 2191–2195. [Google Scholar] [CrossRef]
- Hudson, B.J.F. Evening primrose (Oenothera spp.) oil and seed. J. Am. Oil Chem. Soc. 1984, 61, 540–543. [Google Scholar] [CrossRef]
- Ratnayake, W.M.N.; Matthews, D.G.; Ackman, R.G. Triacylglycerols of evening primrose Oenothera biennis seed oil. J. Am. Oil Chem. Soc. 1989, 66, 966–969. [Google Scholar] [CrossRef]
- Court, W.A.; Hendel, J.G.; Pocs, R. Determination of the fatty acids and oil content of evening primrose (Oenothera biennis L.). Food Res. Int. 1993, 26, 181–186. [Google Scholar] [CrossRef]
- Ntsourankoua, H.; Artaud, J. Determination and identification of triterpenic alcohols in borage, blackcurrant and evening primrose oils. Ol. Corps Gras Lipides 1997, 4, 147–151. [Google Scholar]
- Zadernowski, R.; Naczk, M.; Nowak-Polakowska, H. Phenolic acids of borage (Borago officinalis L.) and evening primrose (Oenothera biennis L.). J. Am. Oil Chem. Soc. 2002, 79, 335–338. [Google Scholar] [CrossRef]
- Aitani, M.; Kimura, H.; Yasuhiro, A.; Soyama, H.; Murakami, H.; Zhang, H.L.; Sugishita, T.; Konishi, Y. Effect of an Extract from Evening-Primrose Seeds on Postprandial Blood Glucose Level and Its Active Components. Nippon Shokuhin Kagaku Kogaku Kaishi 2003, 50, 180–187. [Google Scholar] [CrossRef]
- Zaugg, J.; Olivier, P.; Plescher, A.; Bernd, H.; Hamburger, M. Quantitative Analysis of Anti-inflammatory and Radical Scavenging Triterpenoid Esters in Evening Primrose Seeds. J. Agric. Food Chem. 2006, 54, 6623–6628. [Google Scholar] [CrossRef] [PubMed]
- Eskin, N.A.M. Borage and evening primrose oil. Eur. J. Lipid Sci. Technol. 2008, 110, 651–654. [Google Scholar] [CrossRef]
- Nogala-Kalucka, M.; Rudzinska, M.; Zadernowski, R.; Siger, A.; Krzyzostaniak, I. Phytochemical content and antioxidant properties of seeds of unconventional oil plants. J. Am. Oil Chem. Soc. 2010, 87, 1481–1487. [Google Scholar] [CrossRef]
- Montserrat-de la Paz, S.; Fernández-Arche, Á.; Ángel-Martín, M.; García-Giménez, M.D. The sterols isolated from Evening Primrose oil modulate the release of proinflammatory mediators. Phytomed 2012, 19, 1072–1076. [Google Scholar] [CrossRef]
- Montserrat-de la Paz, S.; Fernández-Arche, M.A.; Ángel-Martín, M.; García-Giménez, M.D. Phytochemical characterization of potential nutraceutical ingredients from Evening Primrose oil (Oenothera biennis L.). Phytochem. Lett. 2013, 8, 158–162. [Google Scholar] [CrossRef]
- Montserrat-de la Paz, S.; García-Giménez, M.D.; Ángel-Martín, M.; Pérez-Camino, M.C.; Fernández Arche, A. Long-chain fatty alcohols from evening primrose oil inhibit the inflammatory response in murine peritoneal macrophages. J. Ethnopharmacol. 2014, 151, 131–136. [Google Scholar] [CrossRef]
- Pająk, P.; Socha, R.; Broniek, J.; Królikowska, K.; Fortuna, T. Antioxidant properties, phenolic and mineral composition of germinated chia, golden flax, evening primrose, phacelia and fenugreek. Food Chem. 2019, 275, 69–76. [Google Scholar] [CrossRef]
- Zhao, B.; Gong, H.; Li, H.; Zhang, Y.; Deng, J.; Chen, Z. Fatty Acid, Triacylglycerol and Unsaponifiable Matters Profiles and Physicochemical Properties of Chinese Evening Primrose Oil. J. Oleo Sci. 2019, 68, 719–728. [Google Scholar] [CrossRef]
- Ghasemnezhad, A.; Honermeier, B. Seed yield, oil content and fatty acid composition of Oenothera biennis L. affected by harvest date and harvest method. Ind. Crops Prod. 2007, 25, 274–281. [Google Scholar] [CrossRef]
- Ghatas, Y.; Mohamed, Y. Influence of some phosphorus sources and biofertilizers (em and phosphorein) on vegetative growth, fixed oil productivity and chemical constituents of Oenothera biennis L. plant. Sci. J. Flowers Ornam. Plants 2020, 7, 247–268. [Google Scholar] [CrossRef]
- Zielińska, A.; Kubasiewicz, K.; Wójcicki, K.; Silva, A.M.; Nunes, F.M.; Szalata, M.; Słomski, R.; Eder, P.; Souto, E.B. Two- and Three-Dimensional Spectrofluorimetric Qualitative Analysis of Selected Vegetable Oils for Biomedical Applications. Molecules 2020, 25, 5608. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Z.; Zuo, G.; Lim, S.S.; Yan, H. Defatted Seeds of Oenothera biennis as a Potential Functional Food Ingredient for Diabetes. Foods 2021, 10, 538. [Google Scholar] [CrossRef]
- Sumara, A.; Stachniuk, A.; Trzpil, A.; Bartoszek, A.; Montowska, M.; Fornal, E. LC–MS Metabolomic Profiling of Five Types of Unrefined, Cold-Pressed Seed Oils to Identify Markers to Determine Oil Authenticity and to Test for Oil Adulteration. Molecules 2023, 28, 4754. [Google Scholar] [CrossRef]
- Šardzíková, I.; Schmidt, Š.; Farkaš, P.; Sekretar, S.; Kovac, M. Isolation and Antioxidant Activity of Phenolic Compounds of Evening Primrose (Oenothera biennis L.). Bulletin Potravinárskeho Výskumu (Bull. Food Res.) Roè. 2002, 41, 241–253. [Google Scholar]
- Zahradníková, L.; Schmidt, Š.; Sékelyová, Z.; Sekretár, S. Fractionation and identification of some phenolics extracted from evening primrose seed meal. Czech J. Food Sci. 2008, 26, 58–64. [Google Scholar] [CrossRef]
- Ratz-Łyko, A.; Arct, J.; Pytkowska, K.; Majewski, S.; Bregisz, M. Effect of enzymatic hydrolysis on the antioxidant properties of alcoholic extracts of oilseed cakes. Food Technol. Biotechnol. 2013, 51, 539–546. [Google Scholar]
- Wettasinghe, M.; Shahidi, F.; Amarowicz, R. Identification and quantification of low molecular weight phenolic antioxidants in seeds of evening primrose (Oenothera biennis L.). J. Agr. Food Chem. 2002, 50, 1267–1271. [Google Scholar] [CrossRef]
- Hadidi, M.; Ibarz, A.; Pouramin, S. Optimization of extraction and deamidation of edible protein from evening primrose (Oenothera biennis L.) oil processing by-products and its effect on structural and techno-functional properties. Food Chem. 2021, 334, 127613. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, S.Y.; Chung, D.K. Effect of the Oral Administration of Common Evening Primrose Sprout (Oenothera biennis L.) Extract on Skin Function Improvement in UVB-irradiated Hairless Mice. Pharmaceuticals 2021, 14, 222. [Google Scholar] [CrossRef]
- Kim, T.H.; Shin, H.Y.; Park, S.Y.; Kim, H.; Chung, D.K. Development and Validation of a Method for Determining the Quercetin-3-O-glucuronide and Ellagic Acid Content of Common Evening Primrose (Oenothera biennis) by HPLC-UVD. Molecules 2021, 26, 267. [Google Scholar] [CrossRef]
- Farag, M.A.; Reda, A.; Nabil, M.; Elimam, D.M.; Zayed, A. Evening primrose oil: A comprehensive review of its bioactives, extraction, analysis, oil quality, therapeutic merits, and safety. Food Funct. 2023, 14, 8049–8070. [Google Scholar] [CrossRef]
- Kowalewski, Z.; Kowalska, M.; Skrzypezakowa, L. Flavonoids of Oenothera biennis. Diss. Pharm. Pharmacol. 1968, 20, 573–575. [Google Scholar]
- Anstett, D.N.; Chen, W.; Johnson, M.T.J. Latitudinal Gradients in Induced and Constitutive Resistance against Herbivores. J. Chem. Ecol. 2016, 42, 772–781. [Google Scholar] [CrossRef]
- Parker, J.D.; Salminen, J.-P.; Agrawal, A.A. Evolutionary Potential of Root Chemical Defense: Genetic Correlations with Shoot Chemistry and Plant Growth. J. Chem. Ecol. 2012, 38, 992–995. [Google Scholar] [CrossRef]
- Van Doormaal, J.J.; Idema, I.G.; Muskiet, F.A.J.; Martini, I.A.; Doorenbos, H. Effects of Short-Term High Dose Intake of Evening Primrose Oil on Plasma and Cellular Fatty Acid Compositions, α-Tocopherol Levels, and Erythropoiesis in Normal and Type 1 (Insulin-Dependent) Diabetic Men. Diabetologia 1988, 31, 576–584. [Google Scholar] [CrossRef]
- Singer, P.; Moritz, V.; Wirth, M.; Berger, I.; Forster, D. Blood Pressure and Serum Lipids from SHR after Diets Supplemented with Evening Primrose, Sunflowerseed or Fish Oil. Prostaglandins Leukot. Essent. Fat. 1990, 40, 17–20. [Google Scholar] [CrossRef]
- Zaitone, S.A.; Moustafa, Y.M.; Mosaad, S.M.; El-Orabi, N.F. Effect of evening primrose oil and ω-3 polyunsaturated fatty acids on the cardiovascular risk of celecoxib in rats. J. Cardiovasc. Pharmacol. 2011, 58, 72–79. [Google Scholar] [CrossRef]
- Uccella, R.; Contini, A.; Sartorio, M. L’azione dell’olio di “evening primrose” sui fattori di rischio cardiovascolare dei diabetici insulino-dipendenti [Action of evening primrose oil on cardiovascular risk factors in insulin-dependent diabetics]. La Clin. Ter. 1989, 129, 381–388. [Google Scholar]
- De La Cruz, J.P.; Martin-Romero, M.; Carmona, J.A.; Villalobos, M.A.; De La Cuesta, F.S. Effect of Evening Primrose Oil On Platelet Aggregation in Rabbits Fed an Atherogenic Diet. Thromb. Res. 1997, 87, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.Y.; Chapkin, R.S.; Ramos, K.S. Dietary lipid source alters murine macrophage/vascular smooth muscle cell interactions in vitro. J. Nutr. 1996, 126, 2083–2088. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.Y.; Ramos, K.S.; Chapkin, R.S. Dietary gamma-linolenic acid suppresses aortic smooth muscle cell proliferation and modifies atherosclerotic lesions in apolipoprotein E knockout mice. J. Nutr. 2001, 131, 1675–1681. [Google Scholar] [CrossRef]
- Villalobos, M.A.; De La Cruz, J.P.; Martín-Romero, M.; Carmona, J.A.; Smith-Agreda, J.M.; Sánchez de la Cuesta, F. Effect of dietary supplementation with evening primrose oil on vascular thrombogenesis in hyperlipemic rabbits. Thromb. Haemost. 1998, 80, 696–701. [Google Scholar] [PubMed]
- Sadi, A.M.; Toda, T.; Oku, H.; Hokama, S. Dietary effects of corn oil, oleic acid, perilla oil, and evening [corrected] primrose oil on plasma and hepatic lipid level and atherosclerosis in Japanese quail. Exp. Ani 1996, 45, 55–62. [Google Scholar] [CrossRef]
- Mosaad, S.M.; Zaitone, S.A.; Ahmed, A.A.; Abo-Elmatty, D.M.; El-Baz, A.A.; Moustafa, Y.M. Evening primrose oil or forskolin ameliorates celecoxib-enhanced upregulation of tissue factor expression in mice subjected to lipopolysaccharide-induced endotoxemia. Naunyn Schmiedebergs Arch. Pharmacol. 2017, 390, 483–492. [Google Scholar] [CrossRef]
- Andjic, M.; Draginic, N.; Radoman, K.; Jeremic, J.; Turnic, T.N.; Srejovic, I.; Zivkovic, V.; Kovacevic, M.; Bolevich, S.; Jakovljevic, V. Flaxseed and evening primrose oil slightly affect systolic and diastolic function of isolated heart in male but not in female rats. Int. J. Vitam. Nutr. Res. 2021, 91, 99–107. [Google Scholar] [CrossRef]
- Jack, A.M.; Keegan, A.; Cotter, M.A.; Cameron, N.E. Effects of diabetes and evening primrose oil treatment on responses of aorta, corpus cavernosum and mesenteric vasculature in rats. Life Sci. 2002, 71, 1863–1877. [Google Scholar] [CrossRef]
- Stevens, E.J.; Carrington, A.L.; Tomlinson, D.R. Prostacyclin release in experimental diabetes: Effects of evening primrose oil. Prostaglandins Leukot. Essent. Fat. 1993, 49, 699–706. [Google Scholar] [CrossRef]
- Singer, P.; Hoffmann, P.; Beitz, J.; Förster, W.; Wirth, M.; Gödicke, W. Serum triglycerides and HDL cholesterol from SHR after evening primrose oil and other polyunsaturated fats. Prostaglandins Leukot. Med. 1986, 22, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Sugano, M.; Ide, T.; Ishida, T.; Yoshida, K. Hypocholesterolemic effect of gamma-linolenic acid as evening primrose oil in rats. Ann. Nutr. Metab. 1986, 30, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Fragoso, Y.D.; Skinner, E.R. The effect of gammalinolenic acid on the subfractions of plasma high-density lipoprotein of the rabbit. Biochem. Pharmacol. 1992, 44, 1085–1090. [Google Scholar] [CrossRef]
- Mert, H.; İrak, K.; Çibuk, S.; Yıldırım, S.; Mert, N. The effect of evening primrose oil (Oenothera biennis) on the level of adiponectin and some biochemical parameters in rats with fructose-induced metabolic syndrome. Arch. Physiol. Biochem. 2022, 128, 1539–1547. [Google Scholar] [CrossRef]
- Zeng, G.; Ju, Y.; Shen, H.; Zhou, N.; Huang, L. Immunopotentiating activities of the purified polysaccharide from evening primrose in H22 tumor-bearing mice. Int. J. Biol. Macromol. 2013, 52, 280–285. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, M.J.; Woo, K.J.; Hong, J.; Kim, S.H.; Kim, T.J. Evening Primrose Extracts Inhibit PDGF-BB-Induced Vascular Smooth Muscle Cell Proliferation and Migration by Regulating Cell-Cycle-Related Proteins. Curr. Issues Mol. Biol. 2022, 44, 1928–1940. [Google Scholar] [CrossRef]
- Liu, W.; Yin, D.; Li, N.; Hou, X.; Wang, D.; Li, D.; Liu, J. Influence of Environmental Factors on the Active Substance Production and Antioxidant Activity in Potentilla fruticosa L. and Its Quality Assessment. Sci. Rep. 2016, 6, 28591. [Google Scholar] [CrossRef]
- Chelghoum, M.; Guenane, H.; Tahri, D.; Laggoun, I.; Marfoua, F.Z.; Rahmani, F.Z.; Khenifer, F.; Yousfi, M. Influence of Altitude, Precipitation, and Temperature Factors on the Phytoconstituents, Antioxidant, and α-Amylase Inhibitory Activities of Pistacia atlantica. J. Food Meas. Charact. 2021, 15, 4411–4425. [Google Scholar] [CrossRef]
- Wulff, A.; Anttonen, S.; Pellinen, R.; Savonen, E.M.; Sutinen, M.L.; Heller, W.; Sandermann, H.J.; Kangasjärvi, J. Birch (Betula pendula Roth.) responses to high UV-B radiation. Boreal. Environ. Res. 1999, 4, 77–88. [Google Scholar]
- Dong, J.; Ma, X.; Wei, Q.; Peng, S.; Zhang, S. Effects of growing location on the contents of secondary metabolites in the leaves of four selected superior clones of Eucommia ulmoides. Ind. Crops Prod. 2011, 3, 1607–1614. [Google Scholar] [CrossRef]
- Suyal, R.; Rawat, S.; Rawal, R.S.; Bhatt, I.D. Variability in morphology, phytochemicals, and antioxidants in Polygonatum verticillatum (L.) All. populations under different altitudes and habitat conditions in Western Himalaya, India. Environ. Monit. Assess. 2020, 191 (Suppl. 3), 783. [Google Scholar] [CrossRef] [PubMed]
- ANM [Administrația Națională de Meteorologie]. Clima României; Editura Academiei Române: Bucharest, Romania, 2008. [Google Scholar]
- Păcurar, F. Specii Indicator Pentru Evaluarea și Elaborarea Managementului Sistemelor de Pajiști cu Înaltă Valoare Naturală–HNV; Casa Cărții de Știință: Cluj-Napoca, Romania, 2020; pp. 16–18. [Google Scholar]
- Ielciu, I.; Filip, G.A.; Oniga, I.; Olah, N.-K.; Bâldea, I.; Olteanu, D.; Burtescu, R.F.; Turcuș, V.; Sevastre-Berghian, A.C.; Benedec, D.; et al. Oxidative Stress and DNA Lesion Reduction of a Polyphenolic Enriched Extract of Thymus marschallianus Willd. in Endothelial Vascular Cells Exposed to Hyperglycemia. Plants 2021, 10, 2810. [Google Scholar] [CrossRef] [PubMed]
- Mihali, C.V.; Petrescu, C.M.; Ciolacu-Ladasiu, C.F.; Mathe, E.; Popescu, C.; Bota, V.; Mizeranschi, A.E.; Ilie, D.E.; Neamț, R.I.; Turcus, V. Assessing Phenotypic Variability in Some Eastern European Insular Populations of the Climatic Relict Ilex aquifolium L. Plants 2022, 11, 2022. [Google Scholar] [CrossRef]
- Bota, V.B.; Neamtu, A.-A.; Olah, N.-K.; Chișe, E.; Burtescu, R.F.; Pripon Furtuna, F.R.; Nicula, A.-S.; Neamtu, C.; Maghiar, A.-M.; Ivănescu, L.-C.; et al. A Comparative Analysis of the Anatomy, Phenolic Profile, and Antioxidant Capacity of Tussilago farfara L. Vegetative Organs. Plants 2022, 11, 1663. [Google Scholar] [CrossRef]
- Chytrý, M.; Tichý, L.; Dřevojan, P.; Sádlo, J.; Zelený, D. Ellenberg-type indicator values for the Czech flora. Preslia 2018, 90, 83–103. [Google Scholar] [CrossRef]
- Kosakowska, O.; Węglarz, Z.; Przybył, J.L. Chemical and genetic diversity of evening primrose (Oenothera biennis L.) occurring in the eastern area of Poland. Acta Hortic. 2008, 765, 151–156. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, W.J.; Park, S.Y.; Kim, H.; Chung, D.K. In Vitro Anti-Wrinkle and Skin-Moisturizing Effects of Evening Primrose (Oenothera biennis) Sprout and Identification of Its Active Components. Processes 2021, 9, 145. [Google Scholar] [CrossRef]
- Anstett, D.N.; Ahern, J.R.; Johnson, M.T.J.; Salminen, J.P. Testing for latitudinal gradients in defense at the macroevolutionary scale. Evolution 2018, 72, 2129–2143. [Google Scholar] [CrossRef]
- Yan, J.; Yu, L.; Xu, S.; Gu, W.; Zhu, W. Apigenin accumulation and expression analysis of apigenin biosynthesis relative genes in celery. Sci. Hortic. 2014, 165, 218–224. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The Role of Polyphenols in Abiotic Stress Response: The Influence of Molecular Structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Smith, G.J.; Markham, K.R. Tautomerism of flavonol glucosides: Relevance to plant UV protection and flower colour. J. Photochem. Photobiol. A Chem. 1988, 118, 99–105. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, Q.; Li, X. Esculetin: A review of its pharmacology and pharmacokinetics. Phytother. Res. 2021, 36, 279–298. [Google Scholar] [CrossRef] [PubMed]
- Tattini, M.; Di Ferdinando, M.; Brunetti, C.; Goti, A.; Pollastri, S.; Bellasio, C.; Giordano, C.; Fini, A.; Agati, G. Esculetin and esculin (esculetin 6-O-glucoside) occur as inclusions and are differentially distributed in the vacuole of palisade cells in Fraxinus ornus leaves: A fluorescence microscopy analysis. J. Photochem. Photobiol. B 2014, 140, 28–35. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, Z.; Li, Q.; Zhang, S.; Li, Y.; Li, X.; Kong, L.; Luo, J. The parallel biosynthesis routes of hyperoside from naringenin in Hypericum monogynum. Hortic. Res. 2023, 10, uhad166. [Google Scholar] [CrossRef]
- Hara, M.; Furukawa, J.; Sato, A.; Mizoguchi, T.; Miura, K. Abiotic Stress and Role of Salicylic Acid in Plants. In Abiotic Stress Responses in Plants; Ahmad, P., Prasad, M., Eds.; Springer: New York, NY, USA, 2012. [Google Scholar] [CrossRef]
- Ozfidan-Konakci, C.; Yildiztugay, E.; Alp, F.N.; Kucukoduk, M.; Turkan, I. Naringenin induces tolerance to salt/osmotic stress through the regulation of nitrogen metabolism, cellular redox and ROS scavenging capacity in bean plants. Plant Physiol. Biochem. 2020, 157, 264–275. [Google Scholar] [CrossRef]
- Legea Plantelor Medicinale și Aromatice, nr. 491/2003. Available online: https://lege5.ro/Gratuit/gq3denbx/legea-nr-491-2003-privind-plantele-medicinale-si-aromatice-precum-si-produsele-stupului (accessed on 25 June 2020).
- Ordonața de Urgență 57/2007 Privind Regimul Ariilor Naturale Protejate, Conservarea Habitatelor Naturale, a Florei și Faunei Sălbatice. Monitorul Oficial al României. 2007. Available online: https://legislatie.just.ro/Public/DetaliiDocument/83289 (accessed on 25 June 2020).
- Directive 92/43/EEC. Available online: https://eur-lex.europa.eu/legal-content/en/NIM/?uri=CELEX:31992L0043 (accessed on 25 June 2020).
- Pirnă, I.; Voicu, E.; Radu, M.; Ciupercă, R.; Muscalu, A. Ghid. Tehnologii de Recoltare a Plantelor Medicinale Și Aromatice Din Zona Călărași-Silistra. Institutul Național de Cercetare-Dezvoltare pentru Științe Biologice. 2012. Available online: https://proiecte.incdsb.ro/medplanet/doc/Ghid%20tehnologii%20de%20recoltare%20RO.pdf (accessed on 25 June 2020).
- European Directorate for the Quality of Medicines & HealthCare (EDQM). European Pharmacopoeia; EDQM IX; Council of Europe: Strasbourg, France, 2021. [Google Scholar]
- Homöopathische Arzneibuch; HAB 2021; Deutscher Apotheker Verlag: Stuttgart, Germany, 2021.
- Editura Medicala. Romanian Pharmacopoeia; RPh. X; Editura Medicala: Bucharest, Romania, 1993; pp. 1035–1041. [Google Scholar]
- Meteomanz Database. Available online: http://www.meteomanz.com/ (accessed on 10 January 2022).
- QGIS Development Team. QGIS Geographic Information System. Version 3.40 “Bratislava”; Open Source Geospatial Foundation Project: 2024. Available online: https://qgis.org (accessed on 25 September 2025).
- Philip, G.M.; Watson, D.F. A Precise Method for Determining Contoured Surfaces. APEA J. 1982, 22, 205–212. [Google Scholar] [CrossRef]
- Watson, D.F.; Philip, G.M. A Refinement of Inverse Distance Weighted Interpolation. Geoprocessing 1985, 2, 315–327. [Google Scholar]
- Goodchild, M.F. Citizens as sensors: The world of volunteered geography. GeoJournal 2007, 69, 211–221. [Google Scholar] [CrossRef]
- Longley, P.A.; Goodchild, M.F.; Maguire, D.J.; Rhind, D.W. Geographic Information Science and Systems, 5th ed.; Wiley: Hoboken, NJ, USA, 2021. [Google Scholar]
- Crampton, J.W. Mapping: A Critical Introduction to Cartography and GIS; Wiley-Blackwell: Chichester, UK, 2010. [Google Scholar]
- Nistor, M.M.; Nicula, A.-S.; Cervi, F.; Man, T.-C.; Irimuș, I.-A.; Surdu, I. Groundwater vulnerability GIS models in the Carpathian Mountains under climate and land cover changes. Appl. Ecol. Environ. Res. 2018, 16, 5095–5116. [Google Scholar] [CrossRef]
- Nicula, A.-S.; Stoica, M.-S.; Bîrsănuc, E.-M.; Man, T.-C. Why do Romanians take to the streets? A spatial analysis of Romania’s 2016–2017 protests. Rom. J. Political Sci. 2019, 19, 201–222. [Google Scholar]
- Nicula, A.-S.; Boțan, C.N.; Gligor, V.; Cociș, E.-A. Celebrating the Great Union through smart digital solutions: Lessons from Alba Iulia, Romania. J. Urban Hist. 2022, 48, 425–443. [Google Scholar] [CrossRef]
- Nicula, A.-S.; Stoica, M.-S.; Ilovan, O.-R. The cultural-historical and political spheres of influence of Alba Iulia. Transylv. Rev. 2017, 26 (Suppl. 2), 299–315. [Google Scholar]
- Bîrsănuc, E.-M.; Cociș, E.-A.; Gligor, V.; Man, T.-C.; Nicula, A.-S.; Stoica, M.-S. Performing democracy: An analysis of church-based electoral capital in Romania. Transylv. Rev. 2020, 29 (Suppl. 2), 291–309. [Google Scholar] [CrossRef]
- Nicula, A.-S.; Nistor, M.M. Application of GIS Technology for Tourism Flow Modelling in the United Kingdom. Geogr. Tech. 2021, 16, 1–12. [Google Scholar] [CrossRef]
- Nistor, M.M.; Nicula, A.-S.; Dezsi, S.; Petrea, D.; Kamarajugedda, S.A.; Carebia, I.-A. GIS-based kernel analysis for tourism flow mapping. J. Settl. Spat. Plan. 2020, 11, 137–145. [Google Scholar] [CrossRef]
- Marosi, Z.; Adorean, E.-C.; Ilovan, O.-R.; Gligor, V.; Voicu, C.-G.; Nicula, A.-S.; Dulamă, M.-E. Living the urban cultural landscapes in the city centre of Cluj Napoca/Kolozsvár/Klausenburg, Romania. MÖGG 2019, 161, 117–160. [Google Scholar] [CrossRef]
- Nistor, M.M.; Nicula, A.-S.; Haidu, I.; Surdu, I.; Carebia, I.-A.; Petrea, D. GIS Integration Model of Metropolitan Area Sustainability Index (MASI). The Case of Paris Metropolitan Area. J. Settl. Spat. Plan. 2019, 10, 39–48. [Google Scholar] [CrossRef]
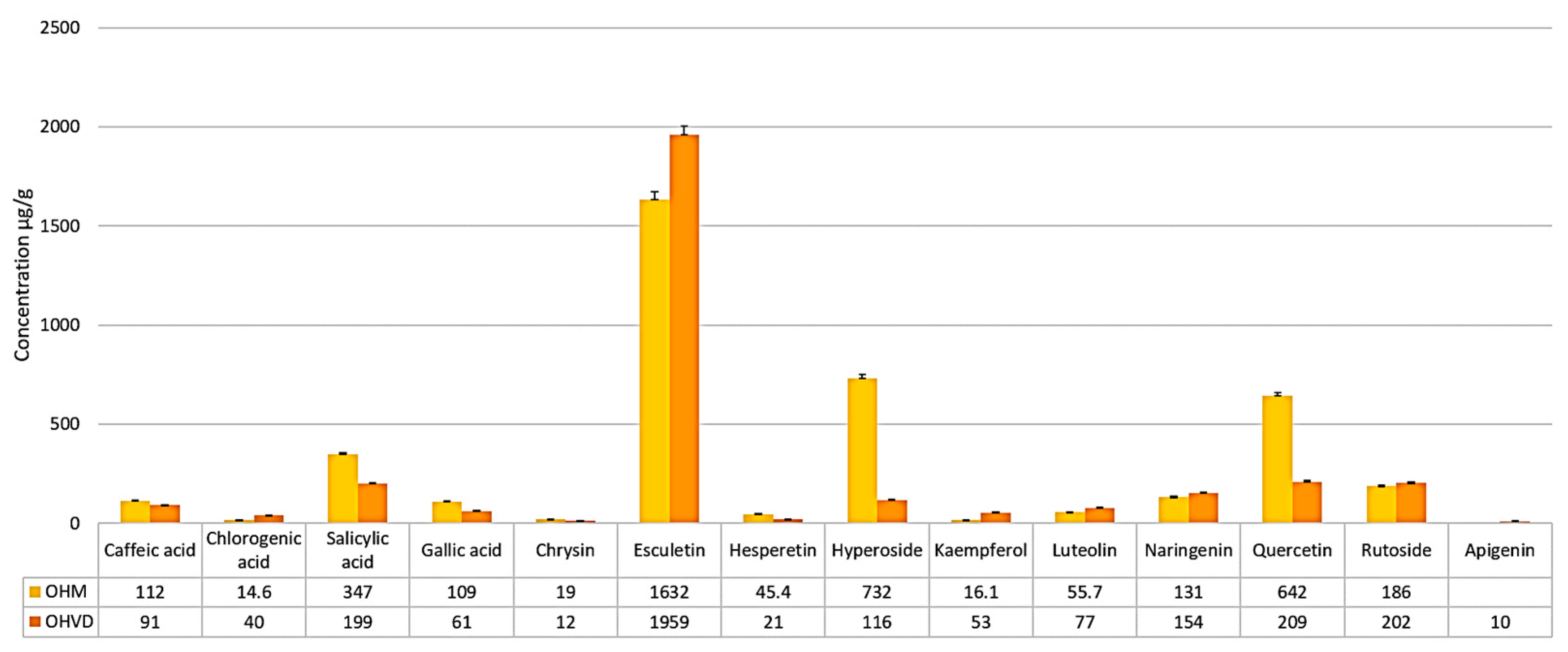
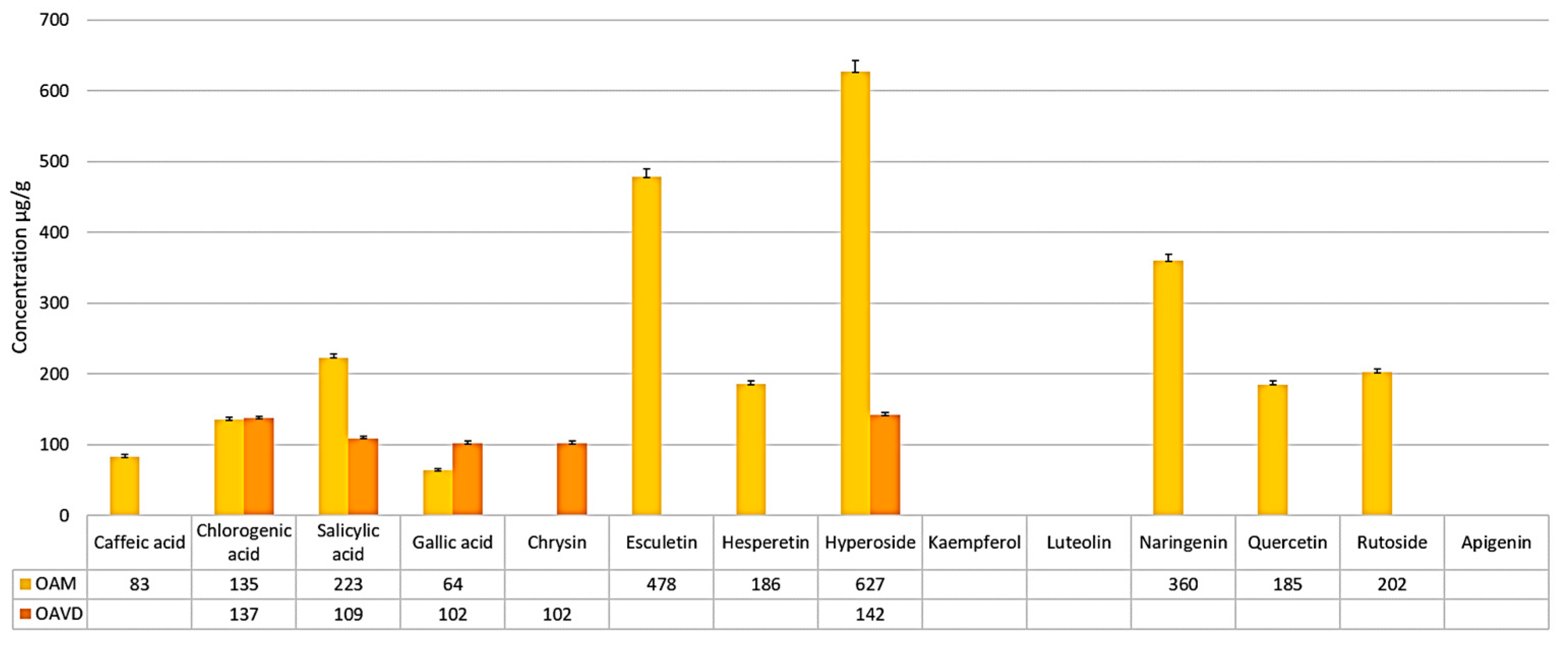
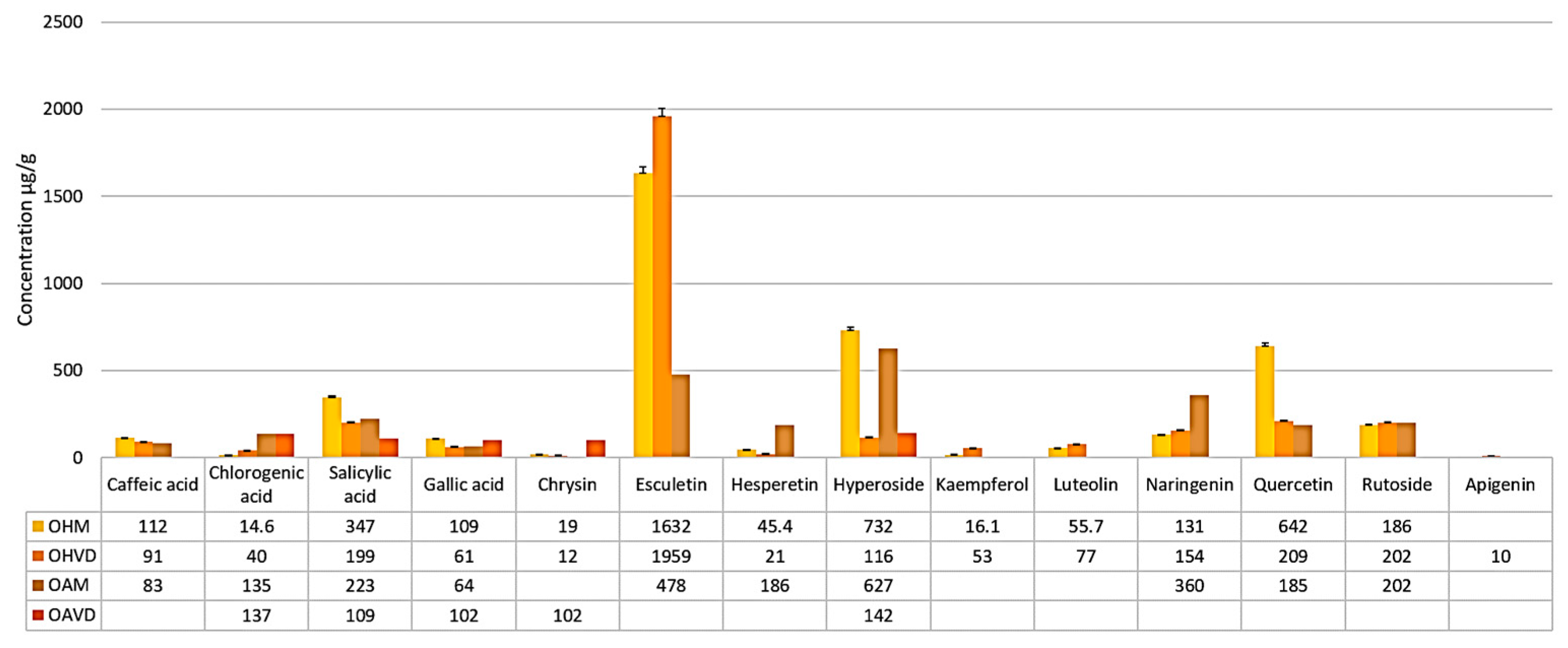
| Sample Compounds | OHM Sample [μg/g Dry Plant Weight] | OHVD Sample [μg/g Dry Plant Weight] |
|---|---|---|
| Caffeic acid | 112 ± 2.8 | 91 ± 2.4 |
| Chlorogenic acid | 14.6 ± 0.8 | 40 ± 1.0 |
| Salicylic acid | 347 ± 8.7 | 199 ± 4.9 |
| Gallic acid | 109 ± 2.7 | 61 ± 1.6 |
| Apigenin | <QL | 10 ± 0.6 |
| Chrysin | 19 ± 1.1 | 12 ± 0.7 |
| Esculetin | 1632 ± 38.5 | 1959 ± 46.3 |
| Hesperetin | 45.4 ± 1.3 | 21± 1.2 |
| Hyperoside | 732 ± 18.3 | 116 ± 2.9 |
| Kaempferol | 16.1 ± 0.9 | 53 ± 1.4 |
| Luteolin | 55.7 ± 1.5 | 77 ± 2.0 |
| Naringenin | 131 ± 3.2 | 154 ± 3.8 |
| Quercetin | 642 ± 16.0 | 209 ± 5.2 |
| Rutoside | 186 ± 4.6 | 202 ± 5.0 |
| Sample Compounds | OAM Sample [μg/g Dry Plant Weight] | OAVD Sample [μg/g Dry Plant Weight] |
|---|---|---|
| Caffeic acid | 83 ± 3.0 | <QL |
| Chlorogenic acid | 135 ± 3.3 | 137 ± 3.2 |
| Salicylic acid | 223 ± 5.5 | 109 ± 2.7 |
| Gallic acid | 102 ± 2.6 | 64 ± 1.7 |
| Apigenin | <QL | <QL |
| Chrysin | <QL | 102 ± 2.6 |
| Esculetin | 478 ± 11.9 | <QL |
| Hesperetin | 186± 4.6 | <QL |
| Hyperoside | 627 ± 15.6 | 142 ± 3.5 |
| Kaempferol | <QL | <QL |
| Luteolin | <QL | <QL |
| Naringenin | 360 ± 9.0 | <QL |
| Quercetin | 185 ± 4.6 | <QL |
| Rutoside | 202 ± 5.0 | <QL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bota, V.B.; Oláh, N.-K.; Chișe, E.; Burtescu, R.-F.; Furtună, F.-R.P.; Ivănescu, L.-C.; Zamfirache, M.-M.; Máthé, E.; Turcuș, V. A Comparative Analysis of the Polyphenolic Content and Identification of New Compounds from Oenothera biennis L. Species from the Wild Flora. Molecules 2025, 30, 4059. https://doi.org/10.3390/molecules30204059
Bota VB, Oláh N-K, Chișe E, Burtescu R-F, Furtună F-RP, Ivănescu L-C, Zamfirache M-M, Máthé E, Turcuș V. A Comparative Analysis of the Polyphenolic Content and Identification of New Compounds from Oenothera biennis L. Species from the Wild Flora. Molecules. 2025; 30(20):4059. https://doi.org/10.3390/molecules30204059
Chicago/Turabian StyleBota, Viviane Beatrice, Neli-Kinga Oláh, Elisabeta Chișe, Ramona-Flavia Burtescu, Flavia-Roxana Pripon Furtună, Lăcrămioara-Carmen Ivănescu, Maria-Magdalena Zamfirache, Endre Máthé, and Violeta Turcuș. 2025. "A Comparative Analysis of the Polyphenolic Content and Identification of New Compounds from Oenothera biennis L. Species from the Wild Flora" Molecules 30, no. 20: 4059. https://doi.org/10.3390/molecules30204059
APA StyleBota, V. B., Oláh, N.-K., Chișe, E., Burtescu, R.-F., Furtună, F.-R. P., Ivănescu, L.-C., Zamfirache, M.-M., Máthé, E., & Turcuș, V. (2025). A Comparative Analysis of the Polyphenolic Content and Identification of New Compounds from Oenothera biennis L. Species from the Wild Flora. Molecules, 30(20), 4059. https://doi.org/10.3390/molecules30204059







