2,4-Dichlorophenoxyacetic Acid in the Gas and Crystal Phases and Its Intercalation in Montmorillonite—An Experimental and Theoretical Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Structure Calculations
2.1.1. Molecule
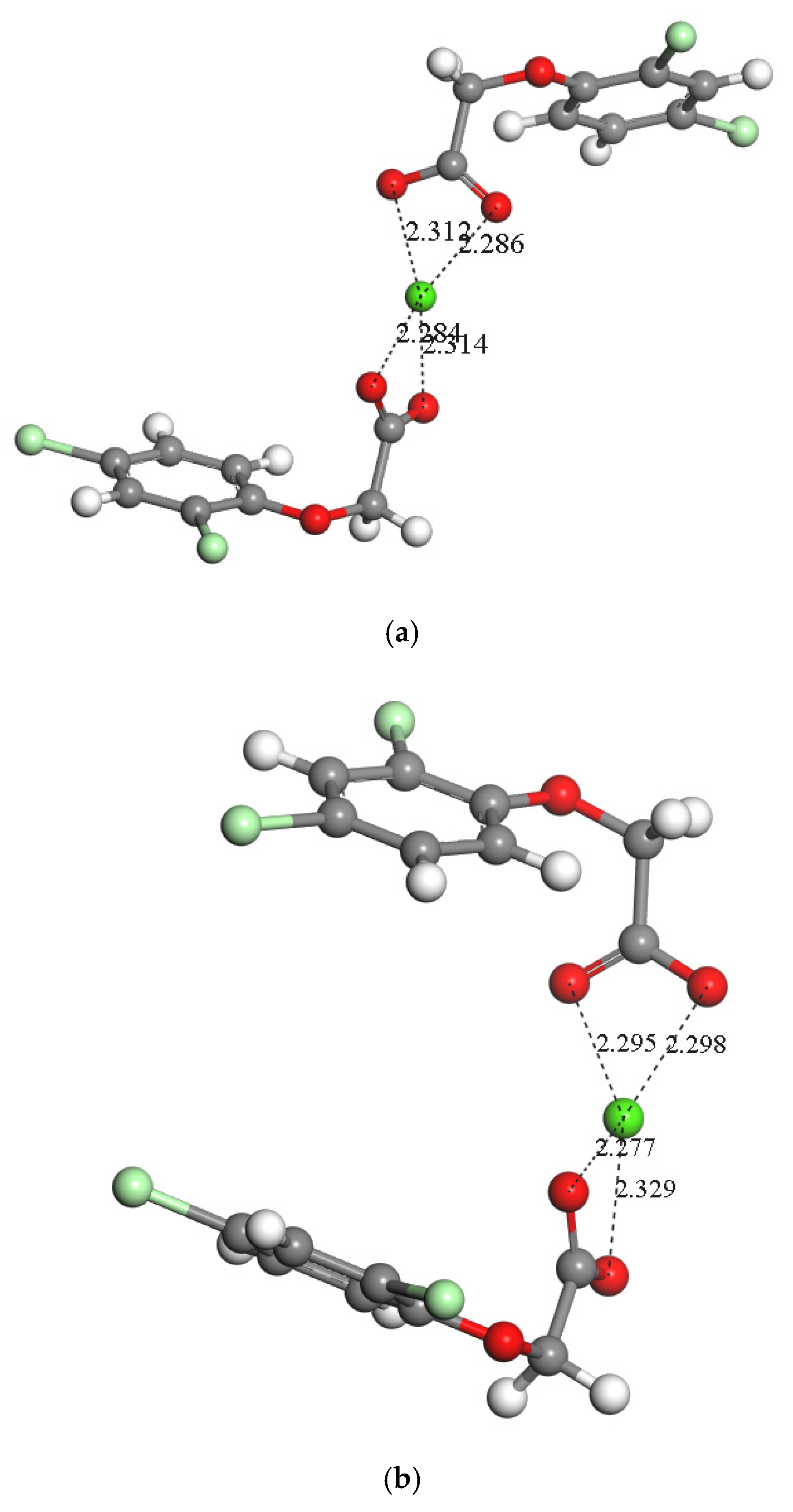
2.1.2. Dimer
2.1.3. Crystal Structure
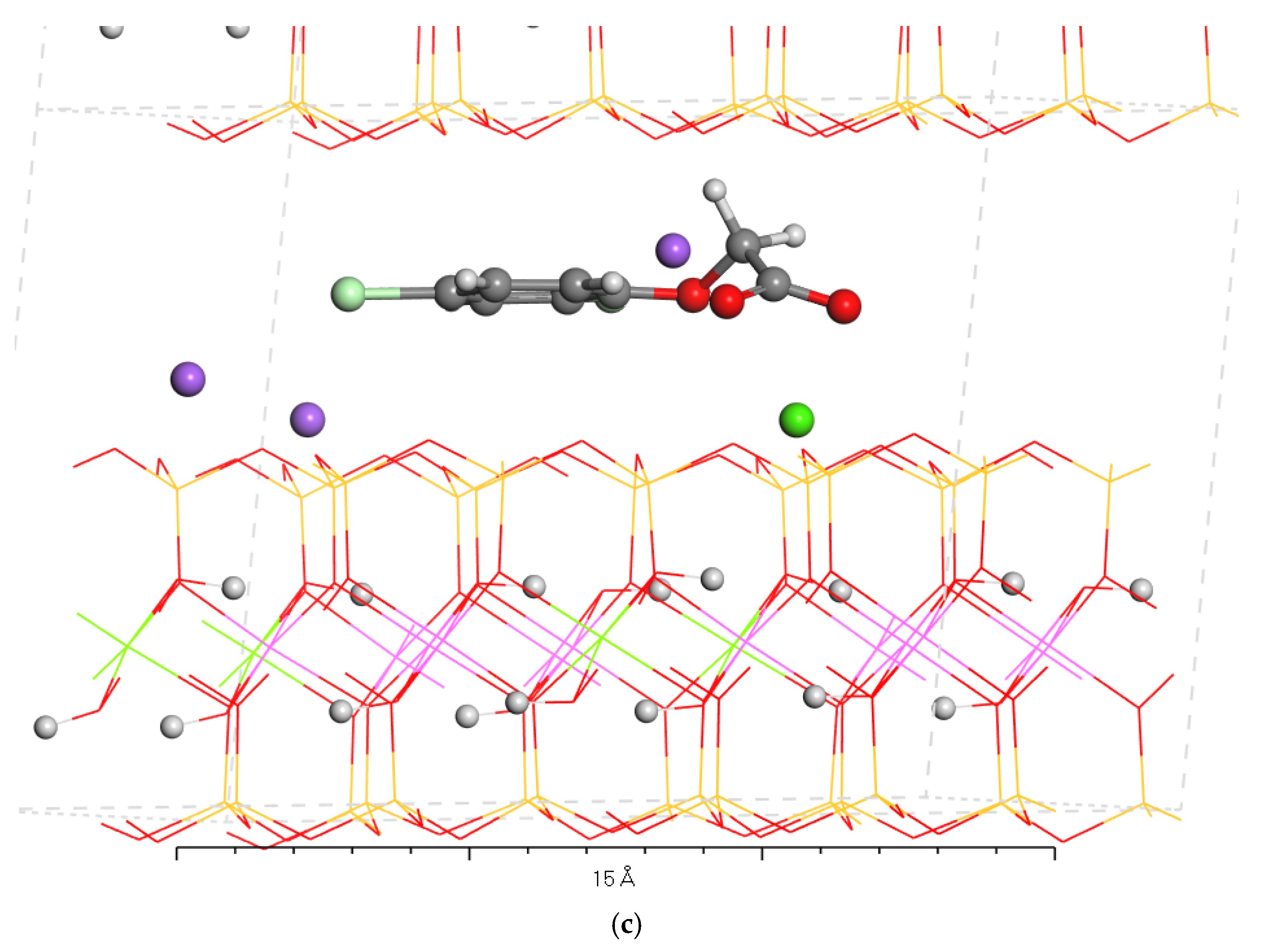
2.1.4. Intercalation of 2,4-D into Montmorillonite
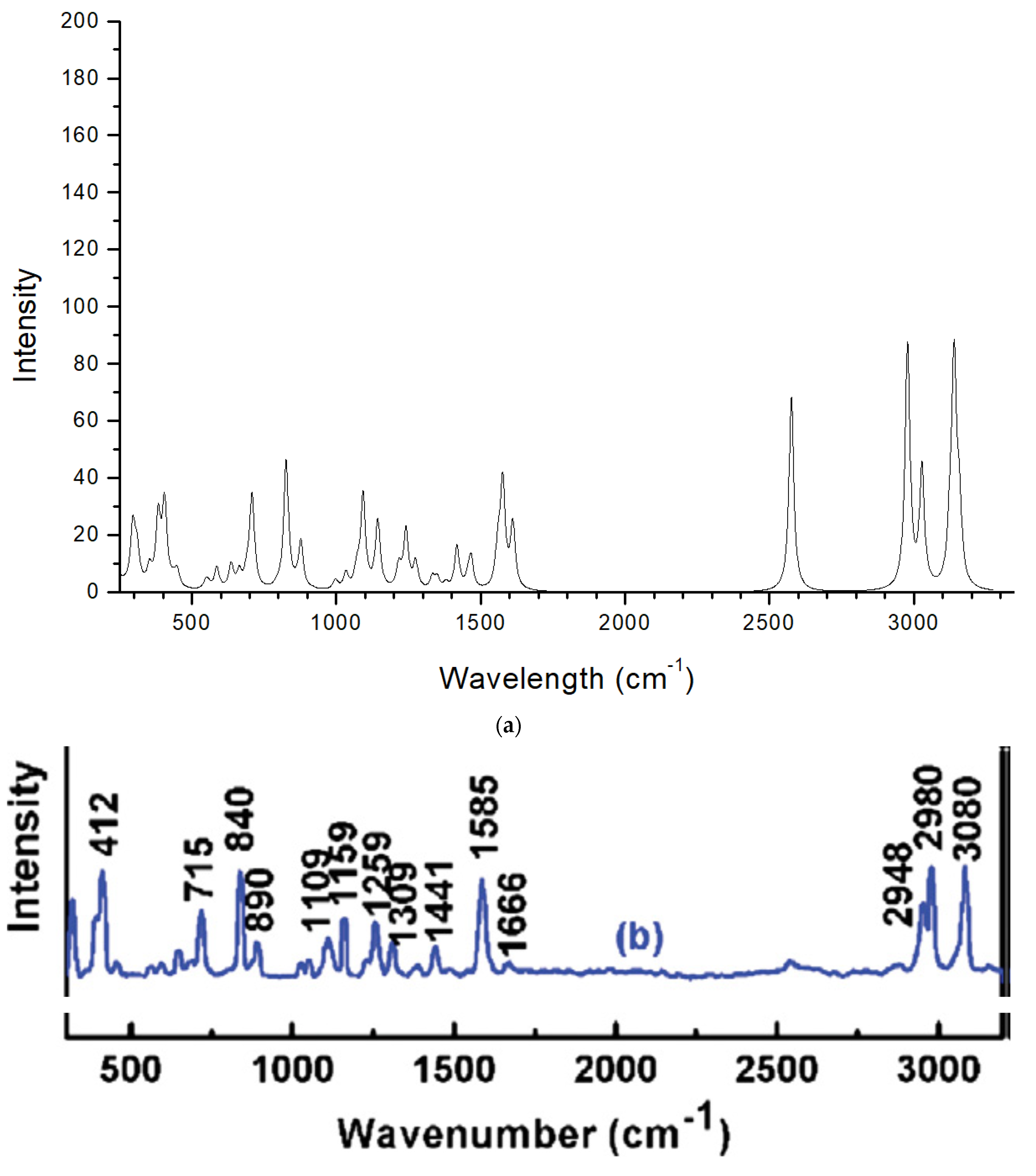
2.2. Vibrational Analysis: IR Frequencies
2.2.1. Region 4000–1900 cm−1
2.2.2. Region 1800–1200 cm−1
2.3. Raman Spectrum
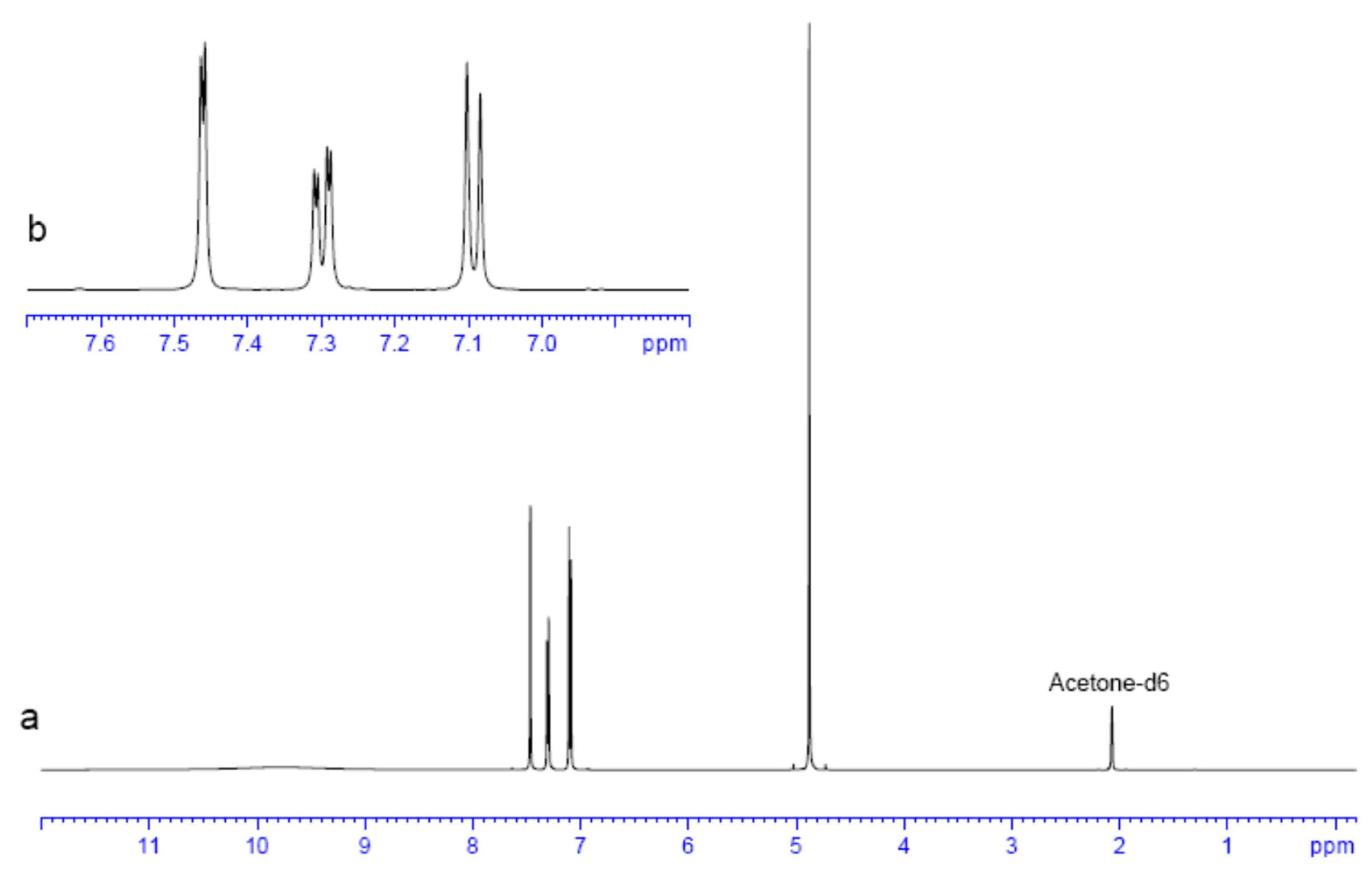
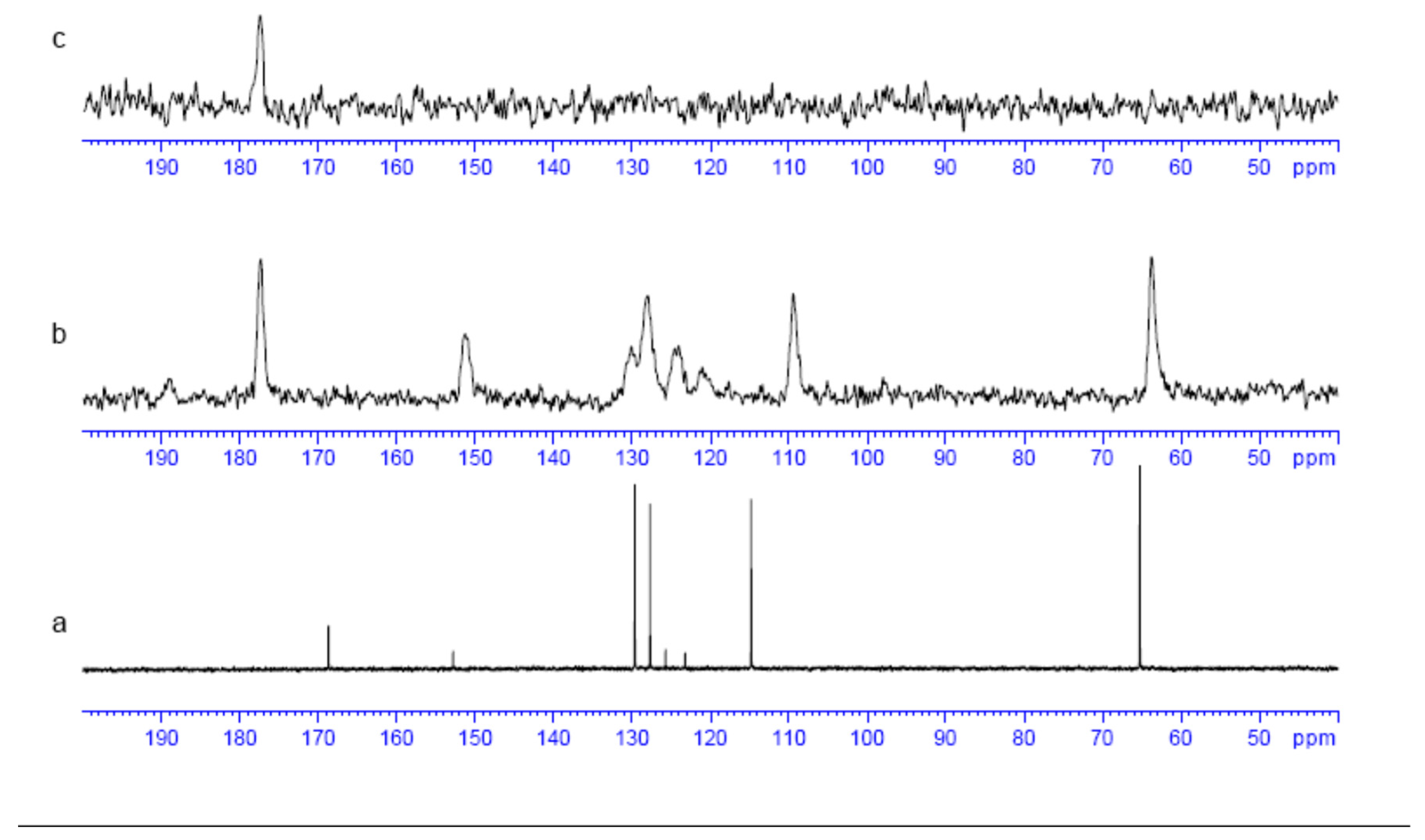
2.4. Experimental NMR Analysis
3. Methods
3.1. Computational Details
3.2. 1H and 13C NMR Spectra
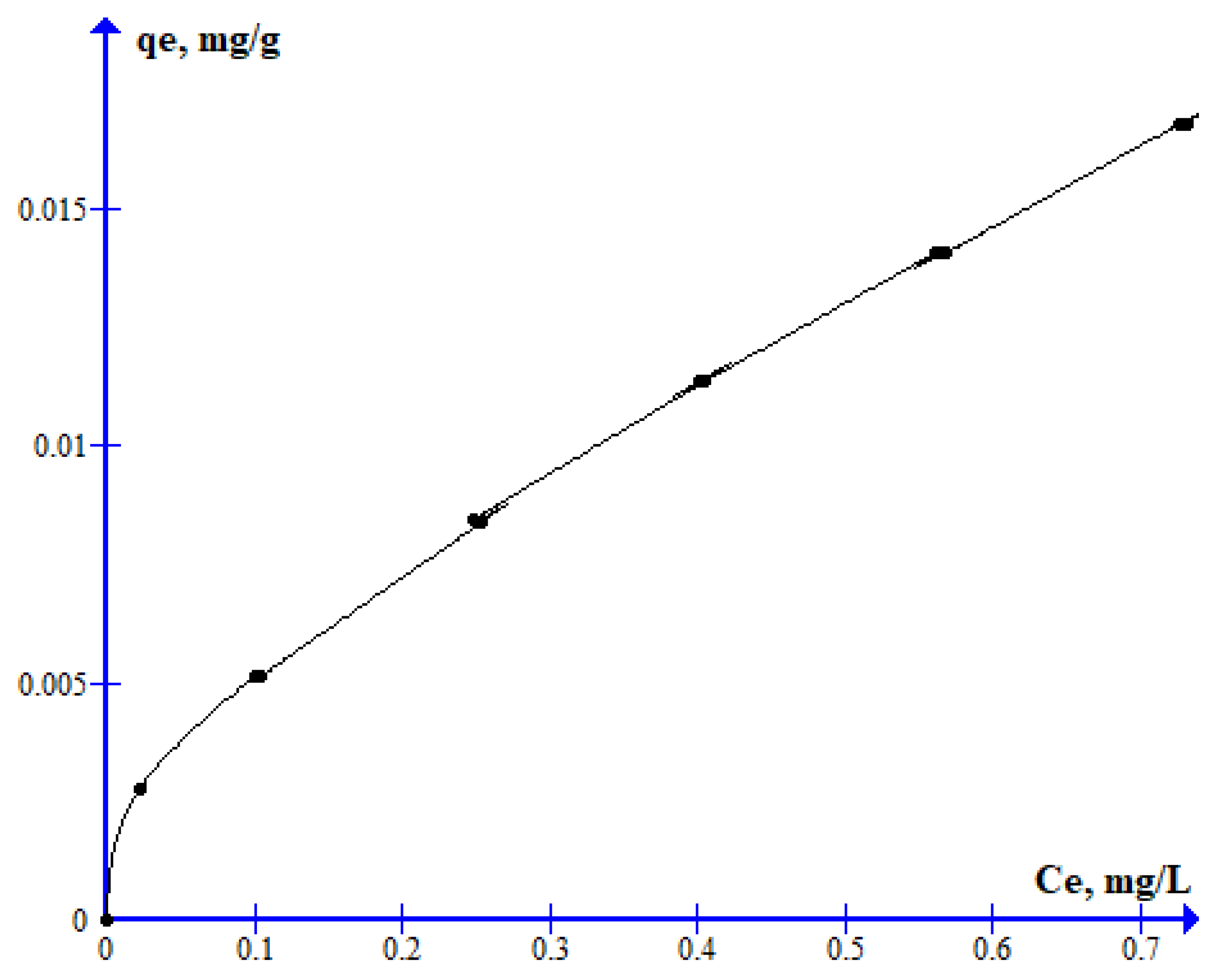
3.3. Experimental Sorption of 2,4-D on Clay-Rich Soils
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eriksen, S.H.; Jensen, B.; Schneider, I.; Kaasgaard, S.; Olsen, J. Uptake of phenoxyacetic acid by Penicilliumchrysogenum. Appl. Microbiol. Biotech. 1995, 42, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Santagostino, A.; Leone, M.P.; Maci, R.; Casale, A.; Marabini, L. Effects of Phenoxyacetic Acid Herbicides on Chicken Embryo Liver Drug Metabolizing Enzymes. Pharmacol. Toxicol. 1991, 68, 110–114. [Google Scholar] [CrossRef]
- Arnold, E.K.; Beasley, V.R. The pharmacokinetics of chlorinated phenoxy acid herbicides: A literature review. Vet. Hum. Toxicol. 1989, 31, 121–125. [Google Scholar] [PubMed]
- Gehring, P.J.; Betso, J.E. Phenoxy Acids: Effects and Fate in Mammals. In Ecological Bulletins 27, Chlorinated Phenoxy Acids and Their Dioxins; Ramel, C., Ed.; Oikos Editorial Office: Stockholm, Sweden, 1978; pp. 122–133. [Google Scholar]
- Munro, I.C.; Carlo, G.L.; Orr, J.C.; Sund, K.G.; Wilson, R.M.; Kennelpohl, E.; Lunch, B.S.; Jablinske, M.; Lee, N.L. A comprehensive, integrated review and evaluation of the scientific evidence relating to the safety of the herbicide 2,4-D. J. Am. Coll. Toxicol. 1992, 11, 559–664. [Google Scholar] [CrossRef]
- Cheah, U.B.; Ooi, G.G. Potential approaches towards degradation of 2,4-D. In Proceedings of the National Seminar and Workshop on Rice Field Weed Management, Penang, Malaysia, 7–9 June 1988; pp. 237–243. [Google Scholar]
- Turker, L. AM1 Treatment of Some Phenoxyacetic Acid Herbicides. Turk. J. Biol. 2000, 24, 291–298. [Google Scholar]
- Gruzdyev, G.S.; Zinchenko, V.A.; Kalinin, V.A.; Slovtsov, R.I. The Chemical Protection of Plants; Mir Publishers: Moscow, Russia, 1983. [Google Scholar]
- Cremlyn, R. Pesticides: Preparation and Mode of Action; John Wiley: New York, NY, USA, 1979. [Google Scholar]
- United States Environmental Protection Agency. National Primary Drinking Water Regulation. 2015. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations (accessed on 20 May 2020).
- Muszynski, P.; Brodowska, M.S.; Paszko, T. Occurrence and transformation of phenoxy acids in aquatic environment and photochemical methods of their removal: A review. Environ. Sci. Pollut. Res. 2019, 27, 1276–1293. [Google Scholar] [CrossRef]
- Loos, R.; Locoro, G.; Comero, S.; Contini, S.; Schewesig, D.; Werres, F.; Balsaa, P.; Gans, O.; Weiss, S.; Blaha, L.; et al. Pan–European survey on the occurrence of selected polar organic persistent pollutants in ground water. Water Res. 2010, 44, 4115–4126. [Google Scholar] [CrossRef]
- Loos, R.; Gawlik, B.M.; Locoro, G.; Rimaviciute, E.; Contini, S.; Bidoqlio, G. EU-wide survey of polar organic persistent pollutants in European river waters. Environ. Pollut. 2009, 157, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Romero, J.M.; Jorge, N.L.; Grand, A.; Hernández-Laguna, A. Hydrolysis reaction of 2,4-dichlorophenoxyacetic acid. A kinetic and computational study. Chem. Phys. Lett. 2015, 639, 57–62. [Google Scholar] [CrossRef]
- Andino, M.G.; Profeta, M.I.; Romero, J.M.; Jorge, N.L.; Castro, E.A. Theoretical Studies on the Structure and Spectroscopic Properties of 2,4-D (2,4-Diclorofenoxiacetic Acid). In Methodologies and Applications for Chemoinformatics and Chemical Engineering; IGI Global: Hershey, PA, USA, 2013; pp. 180–190. [Google Scholar]
- Badawi, H.M. Molecular structure and vibrational assignments of 2,4-dichlorophenoxyacetic acid herbicide. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 77, 24–27. [Google Scholar] [CrossRef]
- Sundaraganesan, N.; Meganathan, C.; Karthikeyan, B. FT-IR, FT-Raman spectra and quantum chemical calculations of some chloro substituted phenoxy acetic acids. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 70, 430–438. [Google Scholar] [CrossRef]
- Karthikeyan, B.; Saravanan, B. Raman spectral analysis of phenoxyacetic acid and some chloro substituted phenoxyacetic acids. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 63, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Molinelli, A.; O’Mahony, J.; Nolan, K.; Smyth, M.R.; Jakusch, M.; Mizaikoff, B. Analyzing the Mechanisms of Selectivity in Biomimetic Self-Assemblies via IR and NMR Spectroscopy of Prepolymerization Solutions and Molecular Dynamics Simulations. Anal. Chem. 2005, 77, 5196–5204. [Google Scholar] [CrossRef]
- Smith, G.; Kennard, C.H.L.; White, A.H. Herbicides. Part I. Crystal structure of 2,4-D (2,4-dichlorophenoxyacetic acid). J. Chem. Soc. Perkin Trans. 1976, 2, 791–792. [Google Scholar] [CrossRef]
- Mehmood, Z.; Williamson, M.P.; Kelly, D.E.; Kelly, S.L. Human cytochrome P450 3A4 is involved in the biotransformation of the herbicide 2,4-dichlorophenoxyacetic acid. Environ. Toxicol. Pharmacol. 1996, 2, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Aquino, A.J.A.; Tunega, D.; Haberhauer, G.; Gerzabek, M.H.; Lischka, H. Quantum Chemical Adsorption Studies on the (110) Surface of the Mineral Goethite. J. Phys. Chem. C 2007, 111, 877–885. [Google Scholar] [CrossRef]
- Meunier, A. Clays; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Sieron, L.; Kobylecka, J.; Turek, A. Crystal Packing and Supramolecular Motifs in Four Phenoxyalkanoic Acid Herbicides—Low-Temperature Redeterminations. Org. Chem. Int. 2011, 4, 608165. [Google Scholar] [CrossRef]
- NIST Standard Reference Data Program Collection. U.S. Secretary of Commerce, USA. 2018. Available online: https://www.nist.gov/srd (accessed on 20 May 2020).
- SDBSWeb. National Institute of Advanced Industrial Science and Technology. 2010. Available online: https://sdbs.db.aist.go.jp/ (accessed on 20 May 2020).
- Naz, N.; Sirajuddin, M.; Harder, A.; Mustansar-Abbas, S.; Ali, S.; Wadood, A.; Ghufran, M.; Rehman, G.; Mirza, B. Synthesis, characterization, biological screenings and molecular docking study of Organotin(IV) derivatives of 2,4-dichlorophenoxyacetic acid. J. Mol. Struct. 2019, 1179, 662–671. [Google Scholar] [CrossRef]
- Trivedi, N.S.; Kharkar, R.A.; Mandavgane, S.A. Uttilization of cotton plant ash and char for removal 4-dichlorophenoxyacetic acid. Resour. Effic. Technol. 2016, 2, S39–S46. [Google Scholar]
- Jia, J.L.; Jin, X.Y.; Liu, Q.L.; Liang, W.L.; Lin, M.S.; Xu, H.H. Preparation, Characterization and Intracellular Imaging of 2,4-Dichlorophenoxyacetic Acid Conjugated Gold Nanorods. J. Nanosci. Nanotechnol. 2016, 16, 4936–4942. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate spin dependent electron liquid correlation energies for local spin density calculation: A critical analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z = 11−18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Frisch, M.J.; Truck, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Frisch, A.; Nielson, A.B.; Holder, A.J. GAUSSVIEW User Manual; Gaussian. Inc.: Pittsburgh, PA, USA, 2000. [Google Scholar]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Zeits. Kristall. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 2002, 14, 2717–2744. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Vanderbilt, D. Soft Self-Consistent Pseudopotentials in a Generalized Eigenvalue Formalism. Phys. Rev. B 1990, 41, 7892–7895. [Google Scholar] [CrossRef]
- Tkatchenko, A.; Scheffler, M. Accurate Molecular Van Der Waals Interactions from Ground-State Electron Density and Free-Atom Reference Data. Phys. Rev. Lett. 2009, 102, 073005. [Google Scholar] [CrossRef]
- Sainz-Díaz, C.I.; Palin, E.J.; Hernández-Laguna, A.; Dove, M.T. Octahedral cation ordering of illite and smectite. Theoretical exchange potential determination and Monte Carlo simulations. Phys. Chem. Miner. 2003, 30, 382–392. [Google Scholar] [CrossRef]
- Ortega-Castro, J.; Hernández-Haro, N.; Dove, M.T.; Hernández-Laguna, A.; Sainz-Díaz, C.I. Density functional theory and Monte Carlo study of octahedral cation ordering of Al/Fe/Mg cations in dioctahedral 2: 1 phyllosilicates. Am. Mineral. 2010, 95, 209–220. [Google Scholar] [CrossRef]
- Baker, J.; Kessi, A.; Delley, B.K. The generation and use of delocalized internal coordinates in geometry optimization. J. Chem. Phys. 1996, 105, 192–212. [Google Scholar] [CrossRef]
- Delley, B.K. Hardness conserving semilocal pseudopotentials. Phys. Rev. B 2002, 66, 155125. [Google Scholar] [CrossRef]
- Biovia. Materials Studio v2020; Dassault Inc.: Waltham, MA, USA, 2020. [Google Scholar]
- Delley, B. The conductor-like screening model for polymers and surfaces. Mol. Simul. 2006, 32, 117–123. [Google Scholar] [CrossRef]
- Jorge, N.L.; Garrafa, M.V.; Romero, J.M.; Jorge, M.J.; Jorge, L.C.; Delfino, M.R.; Meruvia-Rojas, Y.V.; Hernández-Laguna, A.; Sainz-Díaz, C.I. Adsorption of Ciprofloxacin on Clay Minerals in Argentinian Santa Rosa-Corrientes Soils. Molecules 2024, 29, 1760. [Google Scholar] [CrossRef]
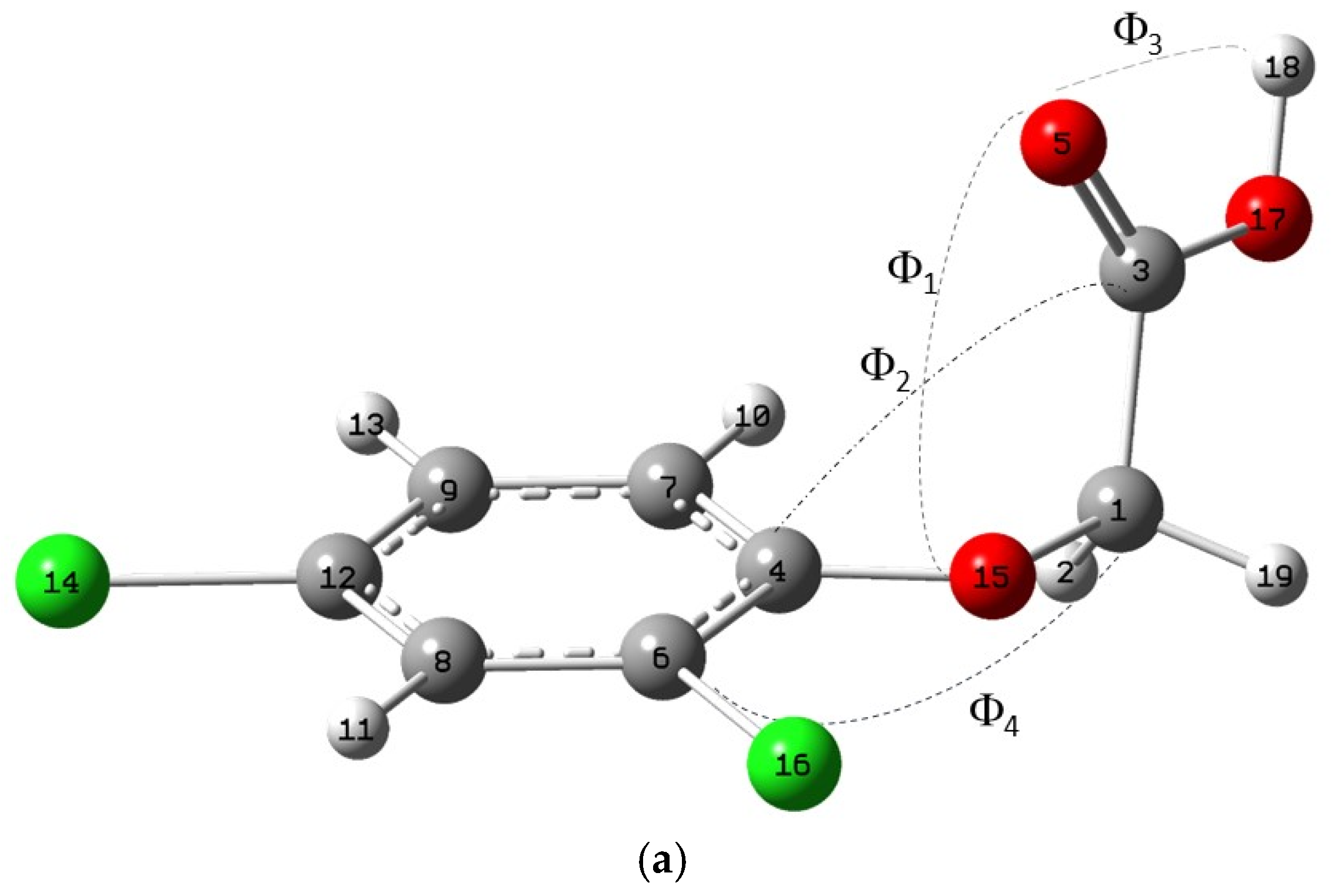
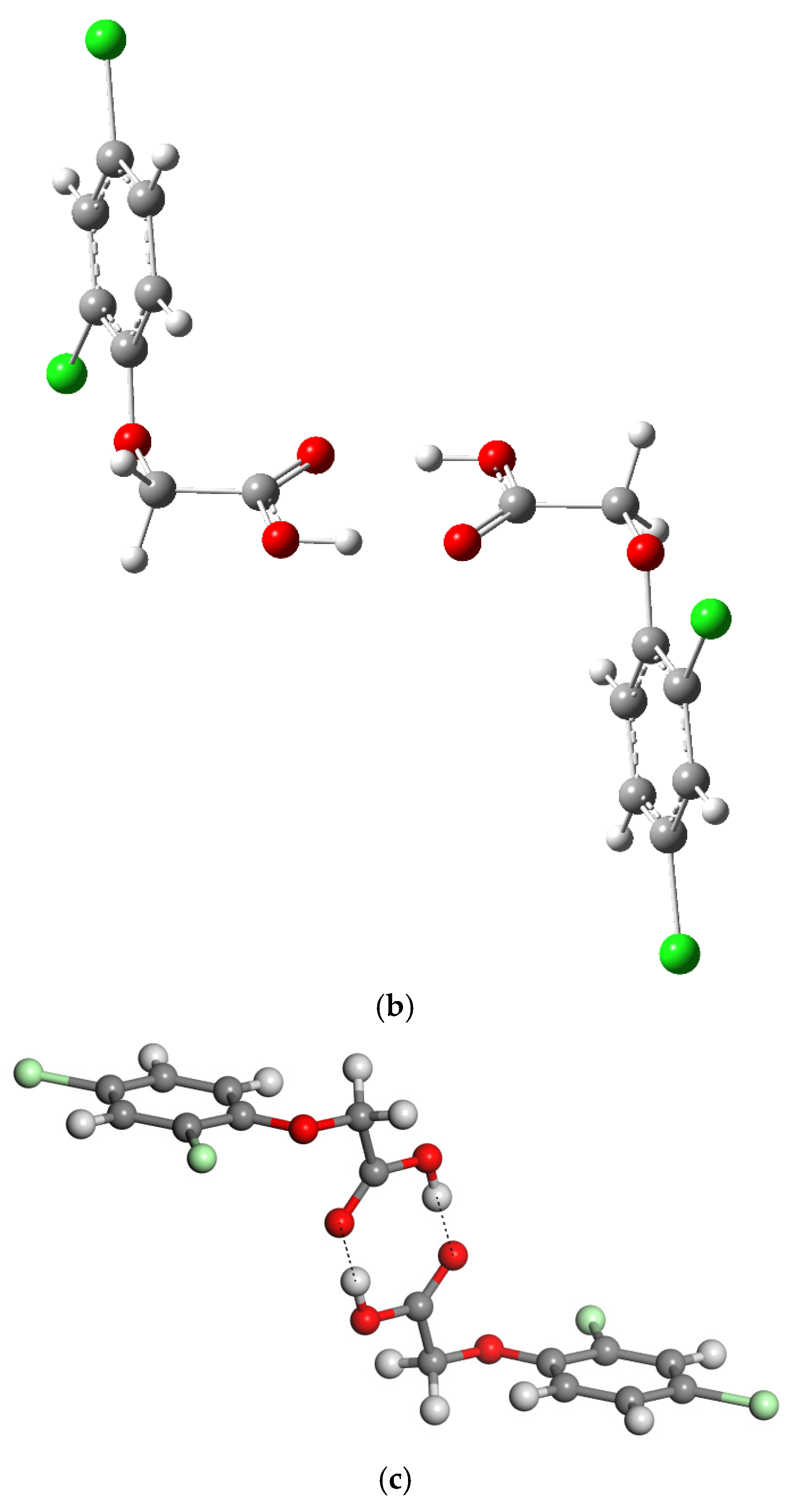
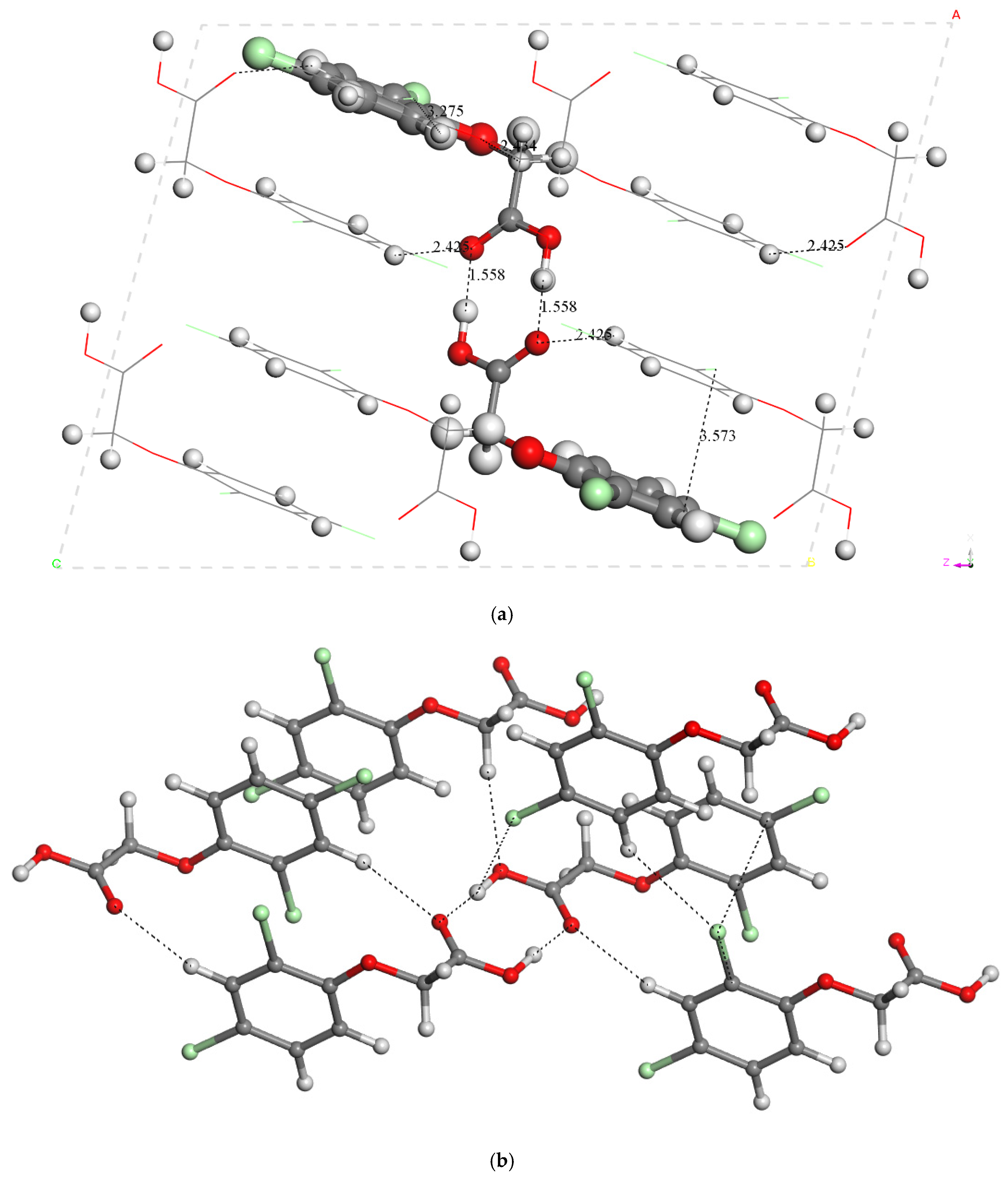
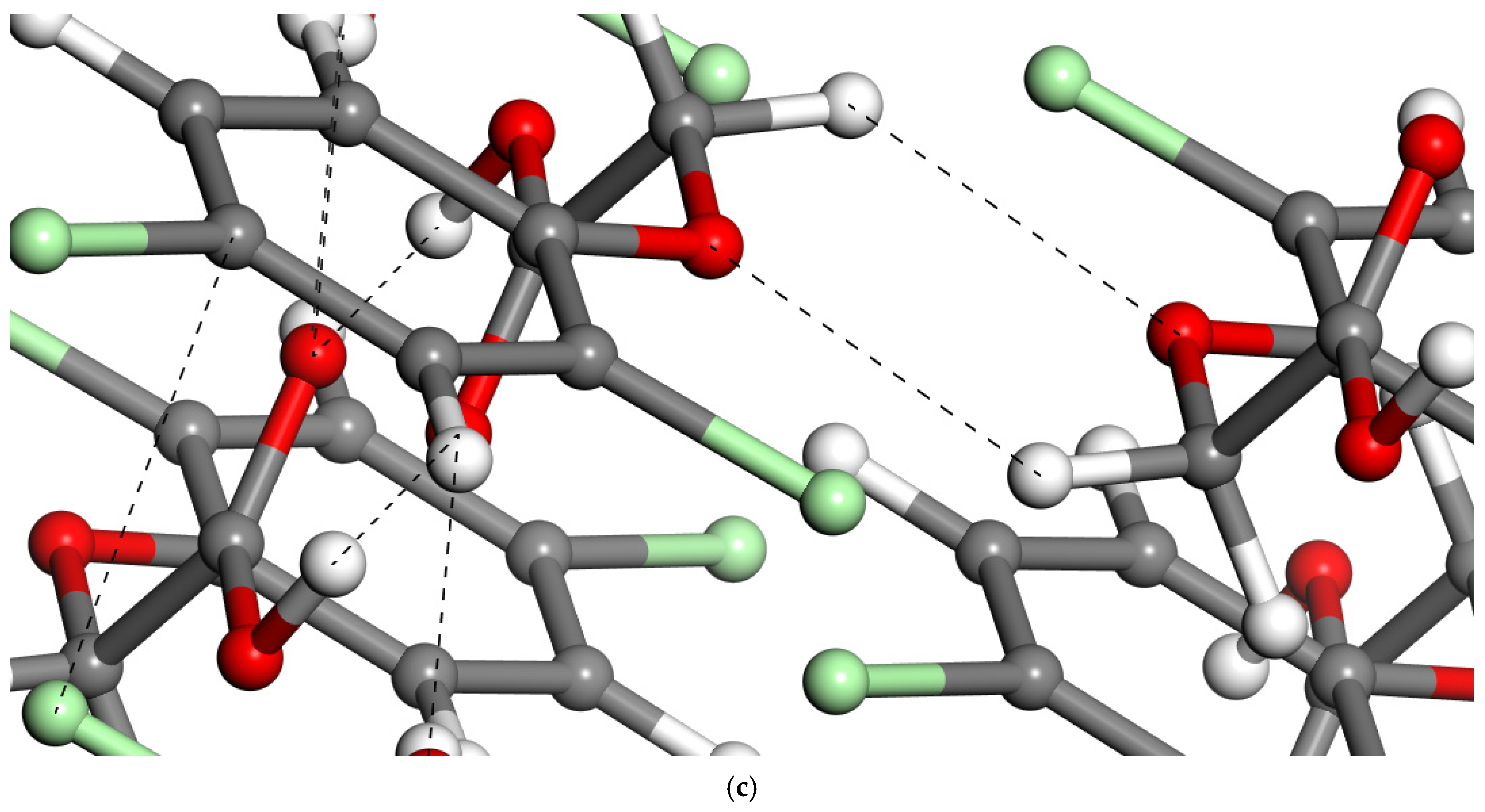
| Energy (Ha) | Molecule | Dimer-I |
|---|---|---|
| &E | −1454.7351857 | −2909.496049 |
| #E | −1454.6069160 | −2909.237675 |
| *E | −2909.494342 |
| Parameters | a | b | c | α | β | γ |
|---|---|---|---|---|---|---|
| experimental | 7.12 | 7.84 | 9.0 | 90.7 | 104.6 | 110.5 |
| Method A a | 7.31 | 7.99 | 9.03 | 90.2 | 104.6 | 111.6 |
| Method B b | 7.19 | 7.86 | 8.95 | 90.6 | 104.0 | 110.7 |
| Method C c | 7.20 | 7.87 | 8.97 | 90.6 | 103.9 | 110.7 |
| Freundlich | Langmuir | Temkin | ||||||
|---|---|---|---|---|---|---|---|---|
| Kfdes (L g−1) | 1/ndes | R2 | KL (L g−1) | qm (m g−1) | R2 | KT (L g−1) | B (mg g−1) | R2 |
| 0.019 | 0.526 | 0.988 | 1.856 | 0.028 | 0.946 | 20.02 | 0.006 | 0.954 |
| Experimental | Molecule b | Dimer b | Crystal b | Assignments c | ||
|---|---|---|---|---|---|---|
| Gas a | dissolution | solid | ||||
| 3580 | 3251 d (molecule) | 3636 | ν(OH) | |||
| 3080 | 3075 e | 3141 f, 3136 g, 3120 h | 3140 f, 3136 g, 3121 h | 3157 f, 3138 g, 3126 h | ν(CH) ring | |
| 2940 | 2979 e | 3019 | 3023 | 3028 | ν(CH2)as OCH2 | |
| 2953 e | 2955 | 2961 | 2980 | ν(CH2)s, OCH2 | ||
| 2495 d (dimer) | 2700–2550 i | 2782s, 2628as | 2691s, 2576as | ν(OH)s, with H bond with C=O. | ||
| 1820, 1760 | 1739 d | 1733 e,1735 i, 1667 j | 1777 | 1681 | 1660s, 1611as | ν(C=O) |
| 1580 | 1595, 1580 e | 1571, 1554 | 1569, 1555 | 1572,1560 | ν(C=C) + δ(CH) ring | |
| 1486–1479 e | 1455, 1375 | 1373 | 1458, 1379 | δ(CH) (ring) | ||
| 1431 | 1466, 1441 | δ(OH) | ||||
| 1480 i | δ(CH2)s + δ(OH) | |||||
| 1480 | 1449 e, 1435 k | 1419 | 1412 | 1420 | δ(CH2)s | |
| 1420 | 1288, 1253 | δ(CH) (ring) + δ(OH) + δ(CH2) | ||||
| 1431 e | 1420 | δ(CH2)twist OCH2 | ||||
| 1250 | 1393–1298 e | 1355s, 1224as | 1339s, (1274, 1226, 1212)as | 1357s, (1282, 1238, 1212)as | δ(CH2)wagging | |
| 1320 | 1235 e, 1234 i | 1234, 1136, 1086, 1032 | 1135, 1089 | 1246, 1144, 1092 | δ(CH) (ring) | |
| 1280 | 1145 e | 1104 | δ(OH) + δ(CH2) | |||
| 1105 e, 1089 k | 1068 | 1073 | 1069 | ν(C-O) + δ(CH) ring + δ(OH) | ||
| 994 | 993 | 999 | γ(CH2) OCH2 | |||
| 1037 | 1028 | γ(OH) | ||||
| 880, 760 | 873–796 e | 856, 779 | 908, 858, 777 | 926, 861, 779 | γ(CH) ring | |
| 850 | 838 e | 885 | ν(C-CH2) | |||
| C number (Figure 1) | δ 1H (ppm) | δ 13C (ppm) |
|---|---|---|
| 3 (C=O) | 168.7, 170.5 b | |
| 1 (CH2) | 4.87, 4.83 a | 65.3, 68.6 b |
| 4 (O-Carom) | 152.9, 154.2 b | |
| 6 (Cl-Carom) | 123.3, 122.2 b | |
| 8 (H-Carom) | 7.47, 7.48 b | 129.6, 129.2 b |
| 12 (Cl-Carom) | 125.8, 127.9 b | |
| 9 (H-Carom) | 7.29, 7.27 b | 127.7, 127.1 b |
| 7 (H-Carom) | 7.09, 6.86 b | 114.8, 115.6 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sainz-Díaz, C.I.; Jorge, N.L.; Romero, J.M.; Grand, A.; Hernández-Laguna, A. 2,4-Dichlorophenoxyacetic Acid in the Gas and Crystal Phases and Its Intercalation in Montmorillonite—An Experimental and Theoretical Study. Molecules 2025, 30, 367. https://doi.org/10.3390/molecules30020367
Sainz-Díaz CI, Jorge NL, Romero JM, Grand A, Hernández-Laguna A. 2,4-Dichlorophenoxyacetic Acid in the Gas and Crystal Phases and Its Intercalation in Montmorillonite—An Experimental and Theoretical Study. Molecules. 2025; 30(2):367. https://doi.org/10.3390/molecules30020367
Chicago/Turabian StyleSainz-Díaz, Claro Ignacio, Nelly L. Jorge, Jorge M. Romero, André Grand, and Alfonso Hernández-Laguna. 2025. "2,4-Dichlorophenoxyacetic Acid in the Gas and Crystal Phases and Its Intercalation in Montmorillonite—An Experimental and Theoretical Study" Molecules 30, no. 2: 367. https://doi.org/10.3390/molecules30020367
APA StyleSainz-Díaz, C. I., Jorge, N. L., Romero, J. M., Grand, A., & Hernández-Laguna, A. (2025). 2,4-Dichlorophenoxyacetic Acid in the Gas and Crystal Phases and Its Intercalation in Montmorillonite—An Experimental and Theoretical Study. Molecules, 30(2), 367. https://doi.org/10.3390/molecules30020367





