Synthesis of an Azido-Substituted 8-Membered Ring Laddersiloxane and Its Application in Catalysis
Abstract
1. Introduction
2. Results and Discussion
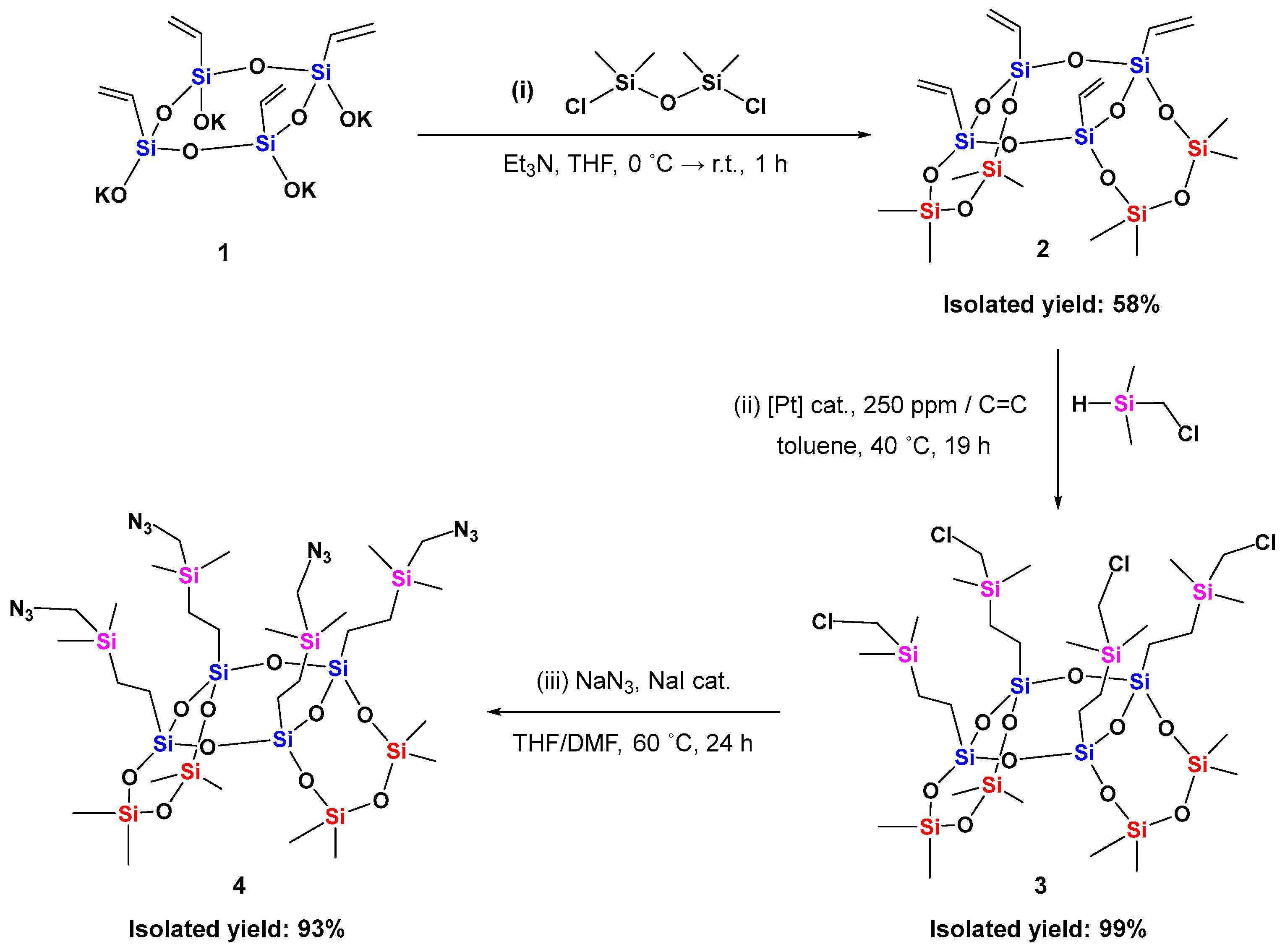
2.1. Synthesis of 8-8-8 Laddersiloxane with Four Azido Groups
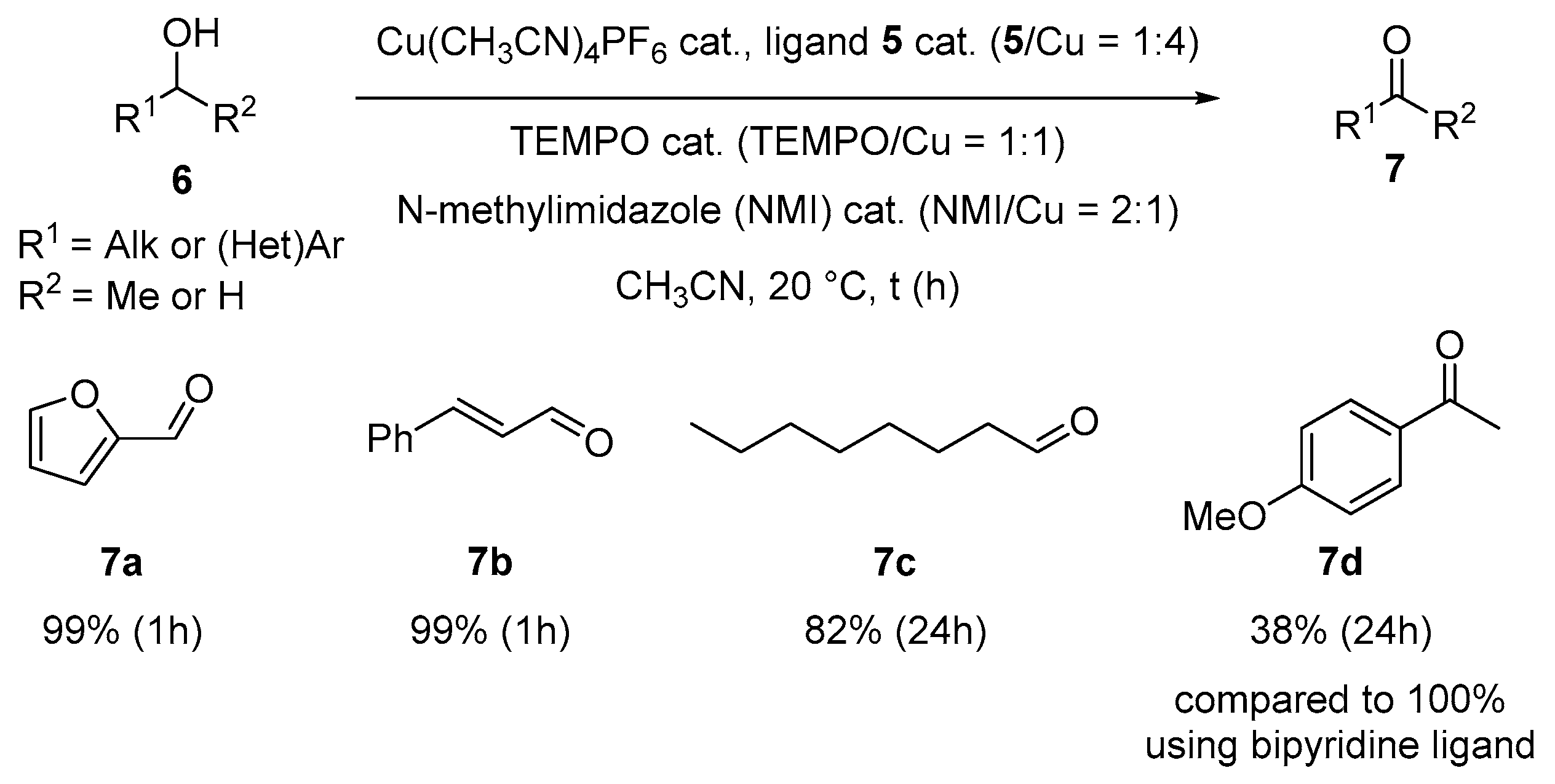
2.2. Synthesis of 8-8-8 Laddersiloxane Ligand and Application in Copper-Catalyzed Oxidative Dehydrogenation of Alcohols
3. Materials and Methods
3.1. General Considerations
3.2. Experimental Procedures and Characterization Data for Synthetic Compounds 2–5
- 1H NMR (600.17 MHz, CDCl3): δ = 0.10 (s, 24H), 0.11 (s, 12H), 0.15 (s, 12H), 0.48–0.53 (m, 8H), 0.63–0.68 (m, 8H), 2.79 (s, 8H) ppm.
- 13C{1H} NMR (150.91 MHz, CDCl3): δ = −4.96, 0.79, 0.89, 5.23, 5.30, 30.02 ppm.
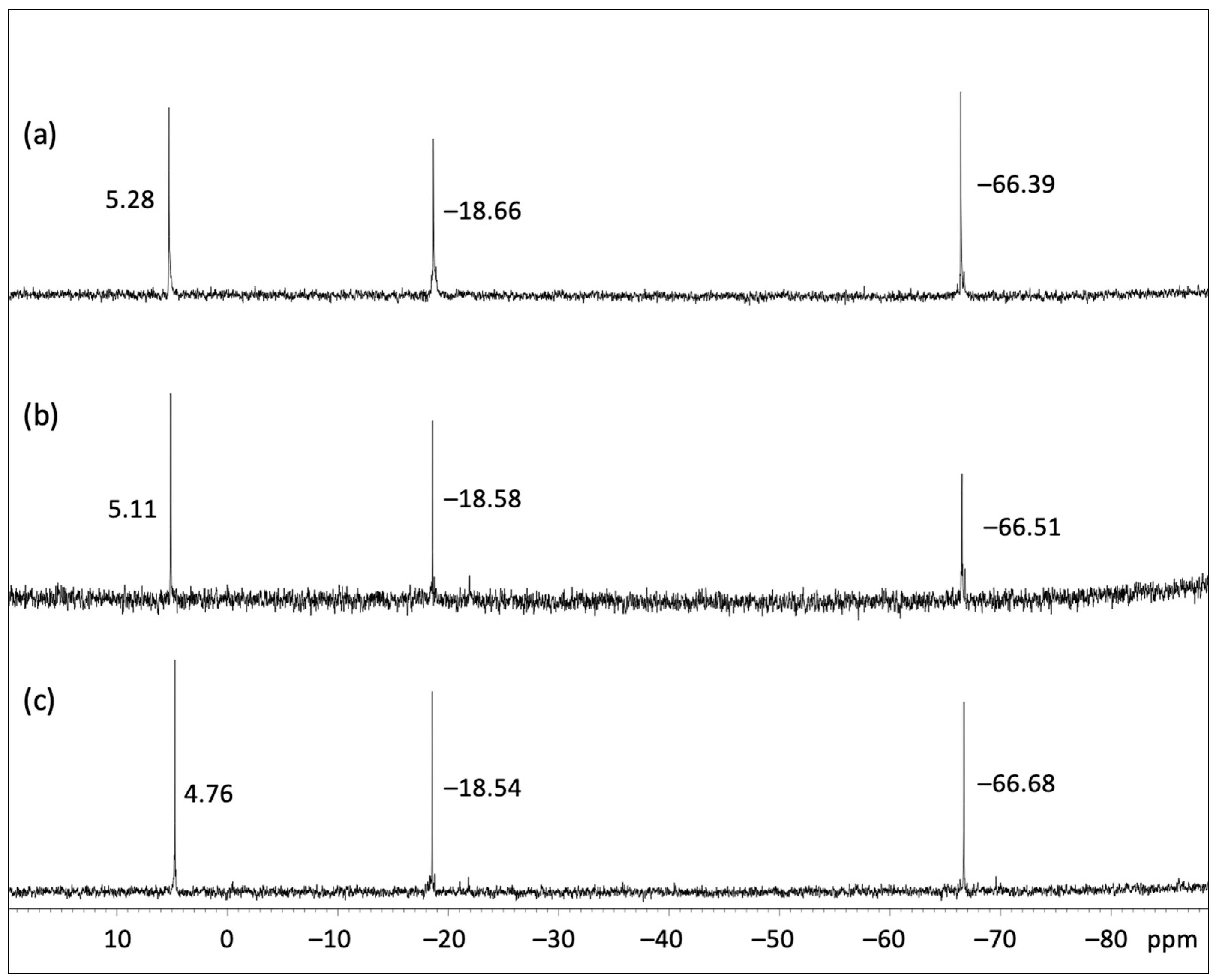
- 29Si{1H} NMR (119.24 MHz, CDCl3): δ = −66.39, −18.66, 5.28 ppm.
- MALDI-TOF MS (m/z): 1068.88 ([M+Na]+, calcd. 1069.10).
- Elemental analysis: Calcd for C28H72Cl4O10Si12: C, 32.10; H, 6.93; Found: C, 31.70; H, 7.16.
- 1H NMR (600.17 MHz, CDCl3): δ = 0.10 (s, 24H), 0.11 (s, 12H), 0.15 (s, 12H), 0.48–0.52 (m, 8H), 0.60–0.64 (m, 8H), 2.79 (s, 8H) ppm.
- 13C{1H} NMR (150.91 MHz, CDCl3): δ = −4.62, 0.78, 0.89, 5.24, 5.79, 40.71 ppm.
- 29Si{1H} NMR (119.24 MHz, CDCl3): δ = −66.51, −18.58, 5.11 ppm.
- Elemental analysis: Calcd for C28H72N12O10Si12: C, 31.31; H, 6.76; N, 15.65; Found: C, 31.59; H, 7.03; N, 13.84.
- 1H NMR (600.17 MHz, CDCl3): δ = 0.05 (s, 12 H), 0.11 (s, 36H), 0.52–0.55 (m, 8H), 0.68–0.71 (m, 8H), 3.98 (s, 8H), 7.14–7.16 (m, 4H), 7.69–7.70 (m, 4H), 8.00 (s, 4H), 8.11 (d, J = 7.9 Hz, 4H), 8.52 (d, J = 4.1 Hz, 4H) ppm.
- 13C{1H} NMR (150.91 MHz, CDCl3): δ = −4.43, 0.74, 0.89, 5.14, 5.91, 40.75, 120.17, 122.72, 122.88, 136.92, 148.17, 149.39, 150.66 ppm.
- 29Si{1H} NMR (119.24 MHz, CDCl3): δ = −66.68, −18.54, 4.76 ppm.
- MALDI-TOF MS (m/z): 1507.48 ([M+Na]+, calcd. 1507.43); 1523.40 ([M+K]+, calcd. 1523.41).
- Elemental analysis: Calcd for C56H92N16O10Si12: C, 45.25; H, 6.24; N, 15.08; Found: C, 45.45; H, 6.63; N, 14.10.
3.3. Experimental Procedure for Catalytic Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baney, R.H.; Itoh, M.; Sakakibara, T. Silsesquioxanes. Chem. Rev. 1995, 95, 1409–1430. [Google Scholar] [CrossRef]
- Kickelbick, G. Silsesquioxanes. In Functional Molecular Silicon Compounds I; Scheschkewitz, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 155, pp. 1–28. [Google Scholar]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent Developments in the Chemistry of Cubic Polyhedral Oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef] [PubMed]
- Laine, R.M.; Roll, M.F. Polyhedral Phenylsilsesquioxanes. Macromolecules 2011, 44, 1073–1109. [Google Scholar] [CrossRef]
- Zhou, H.; Ye, Q.; Xu, J. Polyhedral oligomeric silsesquioxane-based hybrid materials and their applications. Mater. Chem. Front. 2017, 1, 212–230. [Google Scholar] [CrossRef]
- Chen, F.; Lin, F.; Zhang, Q.; Cai, R.; Wu, Y.; Ma, X. Polyhedral Oligomeric Silsesquioxane Hybrid Polymers: Well-Defined Architectural Design and Potential Functional Applications. Macromol. Rapid Commun. 2019, 40, 1900101. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Liu, H. Cage-like silsesquioxanes-based hybrid materials. Dalton Trans. 2020, 49, 5396–5405. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, C.; Aprile, C.; Gruttadauria, M.; Giacalone, F. POSS nanostructures in catalysis. Catal. Sci. Technol. 2020, 10, 7415–7447. [Google Scholar] [CrossRef]
- Dudziec, B.; Marciniec, B. Double-decker Silsesquioxanes: Current Chemistry and Applications. Curr. Org. Chem. 2017, 21, 2794–2813. [Google Scholar] [CrossRef]
- Unno, M.; Suto, A.; Matsumoto, T. Laddersiloxanes−silsesquioxanes with defined ladder structure. Russ. Chem. Rev. 2013, 82, 289–302. [Google Scholar] [CrossRef]
- Kim, M.J.; Heo, Y.M.; Cho, J.H. Ladder-type silsesquioxane copolymer gate dielectrics for gating solution-processed IGZO field-effect transistors. Org. Electron. 2017, 43, 41–46. [Google Scholar] [CrossRef]
- Brown, J.F., Jr.; Vogt, L.H., Jr.; Katchman, A.; Eustance, J.W.; Kiser, K.M.; Krantz, K.W. Double Chain Polymers of Phenylsilsesquioxane. J. Am. Chem. Soc. 1960, 82, 6194–6195. [Google Scholar] [CrossRef]
- Endo, H.; Takeda, N.; Unno, M. Synthesis and Properties of Phenylsilsesquioxanes with Ladder and Double-Decker Structures. Organometallics 2014, 33, 4148–4151. [Google Scholar] [CrossRef]
- Liu, Y.; Onodera, K.; Takeda, N.; Ouali, A.; Unno, M. Synthesis and Characterization of Functionalizable Silsesquioxanes with Ladder-type Structures. Organometallics 2019, 38, 4373–4376. [Google Scholar] [CrossRef]
- Liu, Y.; Endo, A.; Zhang, P.; Takizawa, A.; Takeda, N.; Oua li, A.; Unno, M. Synthesis, Characterization, and Reaction of Divinyl-substituted Laddersiloxanes. Silicon 2022, 14, 2723–2730. [Google Scholar] [CrossRef]
- Liu, Y.; Katano, M.; Yingsukkamol, P.; Takeda, N.; Unno, M.; Ouali, A. Tricyclic 6-8-6 laddersiloxanes derived from all-cis-tetravinylcyclotetrasiloxanolate: Synthesis, characterization and reactivity. J. Organomet. Chem. 2022, 959, 122213. [Google Scholar] [CrossRef]
- Liu, Y.; Tokuda, M.; Takeda, N.; Ouali, A.; Unno, M. New Janus Tricyclic Laddersiloxanes: Synthesis, Characterization, and reactivity. Molecules 2023, 28, 5699. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kigure, M.; Okawa, R.; Takeda, N.; Unno, M.; Ouali, A. Synthesis and characterization of tetrathiol-substituted double-decker or ladder silsesquioxane nano-cores. Dalton Trans. 2021, 50, 3473–3478. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Shiba, H.; Yoshikawa, M.; Wada, H.; Shimojima, A.; Kuroda, K. Synthesis of Polycyclic and Cage Siloxanes by Hydrolysis and Intramolecular Condensation of Alkoxysilylated Cyclosiloxanes. Chem. Eur. J. 2019, 25, 2764–2772. [Google Scholar] [CrossRef] [PubMed]
- Chaiprasert, T.; Liu, Y.; Intaraprecha, P.; Kunthom, R.; Takeda, N.; Unno, M. Synthesis of Tricyclic Laddersiloxane with Various Ring Sizes (Bat Siloxane). Macromol. Rapid Commun. 2021, 42, 2000608. [Google Scholar] [CrossRef]
- Seki, H.; Abe, Y.; Gunji, T. Stereochemistry of the reaction of cis, trans, cis-2,4,6,8-tetraisocyanato-2,4,6,8-tetramethylcyclotetrasiloxane with triphenylsilanol and 1,1,3,3-tetraphenyldisiloxane-1,3-diol. J. Organomet. Chem. 2011, 696, 846–851. [Google Scholar] [CrossRef]
- Unno, M.; Tanaka, R.; Tanaka, S.; Takeuchi, T.; Kyushin, S.; Matsumoto, H. Oligocyclic Ladder Polysiloxanes: Alternative Synthesis by Oxidation. Organometallics 2005, 24, 765–768. [Google Scholar] [CrossRef]
- Du, Y.; Unno, M.; Liu, H. Hybrid Nanoporous Materials Derived from Ladder- and Cage-Type Silsesquioxanes for Water Treatment. ACS Appl. Nano Mater. 2020, 3, 1535–1541. [Google Scholar] [CrossRef]
- Guan, J.; Sun, Z.; Ansari, R.; Liu, Y.; Endo, A.; Unno, M.; Ouali, A.; Mahbub, S.; Furgal, J.C.; Yodsin, N.; et al. Conjugated Copolymers That Shouldn’t Be. Angew. Chem. Int. Ed. 2021, 60, 11115–11119. [Google Scholar] [CrossRef] [PubMed]
- Shoffelen, S.; Meldal, M. Catalytic Click Reactions, Chapter 32. In Applied Homogeneous Catalysis with Organometallic Compounds: A Comprehensive Handbook; Cornils, B., Herrmann, W.A., Beller, M., Paciello, R., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Hoboken, NJ, USA, 2018; Volume 4, ISBN 9783527328970/9783527651733. [Google Scholar] [CrossRef]
- Ervithayasuporn, V.; Kwanplod, K.; Boonmak, J.; Youngme, S.; Sangtrirutnugul, P. Homogeneous and heterogeneous catalysts of organopalladium functionalized-polyhedral oligomeric silsesquioxanes for Suzuki-Miyaura reaction. J. Catal. 2015, 332, 62–69. [Google Scholar] [CrossRef]
- Zheng, W.; Lu, G.; Yang, G.; Chen, Z.; Nie, J. POSS supported diarylprolinol silyl ether as an efficient and recyclable organocatalyst for asymmetric Michael addition reactions. Catal. Commun. 2015, 62, 34–38. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, G.; Lu, C.; Nie, J.; Chen, Z.; Ren, J. POSS supported C2-symmetric bisprolinamide as a recyclable chiral catalyst for asymmetric Aldol reaction. Catal. Commun. 2016, 75, 23–27. [Google Scholar] [CrossRef]
- Liu, Y.; Koizumi, K.; Takeda, N.; Unno, M.; Ouali, A. Synthesis of Octachloro- and Octaazido-Functionalized T8-Cages and Application to Recyclable Palladium Catalyst. Inorg. Chem. 2022, 61, 1495–1503. [Google Scholar] [CrossRef]
- Heyl, D.; Rikowski, E.; Hoffmann, R.C.; Schneider, J.J.; Fessner, W.-D. A “Clickable” Hybrid Nanocluster of Cubic Symmetry. Chem. Eur. J. 2010, 16, 5544–5548. [Google Scholar] [CrossRef]
- Fabritz, S.; Heyl, D.; Bagutski, V.; Empting, M.; Rikowski, E.; Frauendorf, H.; Balog, I.; Fessner, W.-D.; Schneider, J.J.; Avrutina, O.; et al. Towards click bioconjugations on cube-octameric silsesquioxane scaffolds. Org. Biomol. Chem. 2010, 8, 2212–2218. [Google Scholar] [CrossRef] [PubMed]
- Trastoy, B.; Bonsor, D.A.; Pérez-Ojeda, M.E.; Jimeno, M.L.; Méndez-Ardoy, A.; García Fernández, J.M.; Sundberg, E.J.; Chiara, J.L. Synthesis and Biophysical Study of Disassembling Nanohybrid Bioconjugates with a Cubic Octasilsesquioxane Core. Adv. Funct. Mater. 2012, 22, 3191–3201. [Google Scholar] [CrossRef]
- Pérez-Ojeda, M.E.; Trastoy, B.; Rol, Á.; Chiara, M.D.; García-Moreno, I.; Chiara, J.L. Controlled Click-Assembly of Well-Defined Hetero-Bifunctional Cubic Silsesquioxanes and Their Application in Targeted Bioimaging. Chem. Eur. J. 2013, 19, 6630–6640. [Google Scholar] [CrossRef] [PubMed]
- Rahimifard, M.; Ziarani, G.M.; Badiel, A.; Yazdian, F. Synthesis of Polyhedral Oligomeric Silsesquioxane (POSS) with Multifunctional Sulfonamide Groups Through Click Chemistry. J. Inorg. Organomet. Polym. 2017, 27, 1037–1044. [Google Scholar] [CrossRef]
- Ak, M.; Gacal, B.; Kiskan, B.; Yagci, Y.; Toppare, L. Enhancing electrochromic properties of polypyrrole by silsesquioxane nanocages. Polymer 2008, 49, 2202–2210. [Google Scholar] [CrossRef]
- Pérez-Ojeda, M.E.; Trastoy, B.; López-Arbeloa, Í.; Banuelos, J.; Costela, Á.; García-Moreno, I.; Chiara, J.L. Click Assembly of Dye-Functionalized Octasilsesquioxanes for Highly Efficient and Photostable Photonic Systems. Chem. Eur. J. 2011, 17, 13258–13268. [Google Scholar] [CrossRef]
- Chaiprasert, T.; Liu, Y.; Takeda, N.; Unno, M. Vinyl-Functionalized Janus Ring Siloxane: Potential Precursors to Hybrid Functional Materials. Materials 2021, 14, 2014. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kigure, M.; Koizumi, K.; Takeda, N.; Unno, M.; Ouali, A. Synthesis of Tetrachloro, Tetraiodo, and Tetraazido Double-Decker Siloxanes. Inorg. Chem. 2020, 59, 15478–15486. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Yagafarov, N.; Xu, Z.; Ouali, A.; Takeda, N.; Liu, Y.; Unno, M. BINOL and triazole-containing Janus rings and 29-8-29-membered tricyclic ladder-type hybridized siloxane: Application in the fluorescence sensing of anions. Dalton Trans. 2023, 52, 10298–10304. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jerca, V.V.; Hoogenboom, R. Self-healing Metallo-Supramolecular Hydrogel Based on Specific Ni2+ Coordination Interactions of Poly(ethylene glycol) with Bistriazole Pyridine Ligands in the Main Chain. Macromol. Rapid Commun. 2020, 41, 1900457. [Google Scholar] [CrossRef]
- Romero, T.; Orenes, R.A.; Espinosa, A.; Tarraga, A.; Molina, P. Synthesis, Structural Characterization, and Electrochemical and Optical Properties of Ferrocene-Triazole-Pyridine Triads. Inorg. Chem. 2011, 50, 8214–8224. [Google Scholar] [CrossRef]
- Juricek, M.; Felici, M.; Contreras-Carballada, P.; Lauko, J.; Rodriguez Bou, S.; Kouwer, P.H.J.; Brouwer, A.M.; Rowan, A.E. Triazole-pyridine ligands: A novel approach to chromophoric iridium arrays. J. Mater. Chem. 2011, 21, 2104–2111. [Google Scholar] [CrossRef]
- Felici, M.; Contreras-Carballada, P.; Vida, Y.; Smits, J.M.M.; Nolte, R.J.M.; De Cola, L.; Williams, R.M.; Feiters, M.C. IrIII and RuII Complexes Containing Triazole-Pyridine Ligands: Luminescence Enhancement upon Substitution with beta-Cyclodextrin. Chem. Eur. J. 2009, 15, 13124–13134. [Google Scholar] [CrossRef] [PubMed]
- Guven, N.; Sultanova, H.; Ozer, B.; Yucel, B.; Camurlu, P. Tuning of electrochromic properties of electrogenerated polythiophenes through Ru(II) complex tethering and backbone derivatization. Electrochimica Acta 2020, 329, 135134. [Google Scholar] [CrossRef]
- Kreofsky, N.W.; Dillenburg, M.D.; Villa, E.M.; Fletcher, J.T. Ru(II) coordination compounds of N-N bidentate chelators with 1,2,3 triazole and isoquinoline subunits: Synthesis, spectroscopy and antimicrobial properties. Polyhedron 2020, 177, 114259. [Google Scholar] [CrossRef]
- Leckie, L.; Mapolie, S.F. Triazole complexes of ruthenium immobilized on mesoporous silica as recyclable catalysts for octane oxidation. Catal. Commun. 2019, 131, 105803. [Google Scholar] [CrossRef]
- Chowdhury, B.; Khatua, S.; Dutta, R.; Chakraborty, S.; Ghosh, P. Bis-Heteroleptic Ruthenium(II) Complex of a Triazole Ligand as a Selective Probe for Phosphates. Inorg. Chem. 2014, 53, 8061–8070. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Y.; Wen, X.; Ding, C.; Li, Y. Dual-functional click-triazole: A metal chelator and immobilization linker for the construction of a heterogeneous palladium catalyst and its application for the aerobic oxidation of alcohols. Chem. Commun. 2012, 48, 2979–2981. [Google Scholar] [CrossRef]
- Schweinfurth, D.; Su, C.-Y.; Wei, S.-C.; Braunstein, P.; Sarkar, B. Nickel complexes with “click”-derived pyridyl-triazole ligands: Weak intermolecular interactions and catalytic ethylene oligomerisation. Dalton Trans. 2012, 41, 12984–12990. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kameda, T.; Hoshino, M.; Fujii, N.; Ohno, H.; Oishi, S. Fe(II)-Complexation of tripodal hexapeptide ligands with three bidentate triazolylpyridines: Induction of metal-centred chirality by peptide macrocyclization. Dalton Trans. 2017, 46, 13673–13676. [Google Scholar] [CrossRef] [PubMed]
- Parmeggiani, C.; Cardona, F. Transition metal based catalysts in the aerobic oxidation of alcohols. Green Chem. 2012, 14, 547–564. [Google Scholar] [CrossRef]
- Kopylovich, M.N.; Ribeiro, A.P.C.; Alegria, E.C.B.A.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Catalytic oxidation of alcohols: Recent advances. Adv. Organomet. Chem. 2015, 63, 91–174. [Google Scholar]
- Silva, T.F.S.; Martins, L.M.D.R.S. Recent Advances in Copper Catalyzed Alcohol Oxidation in Homogeneous Medium. Molecules 2020, 25, 748. [Google Scholar] [CrossRef]
- Jana, N.C.; Herchel, R.; Bagh, B. Cu(II) Coordination Polymers for the Selective Oxidation of Biomass-derived Veratryl Alcohol in Green Solvents: A Sustainable Catalytic Approach. Inorg. Chem. 2024, 63, 18615–18631. [Google Scholar] [CrossRef]
- Hoover, J.M.; Stahl, S.S. Highly Practical Copper(I)/TEMPO Catalyst System for Chemoselective Aerobic Oxidation of Primary Alcohols. J. Am. Chem. Soc. 2011, 133, 16901–16910. [Google Scholar] [CrossRef] [PubMed]
- Hoover, J.M.; Ryland, B.L.; Stahl, S.S. Mechanism of copper(I)/TEMPO-Catalyzed Aerobic Alcohol Oxidation. J. Am. Chem. Soc. 2013, 135, 2357–2367. [Google Scholar] [CrossRef] [PubMed]
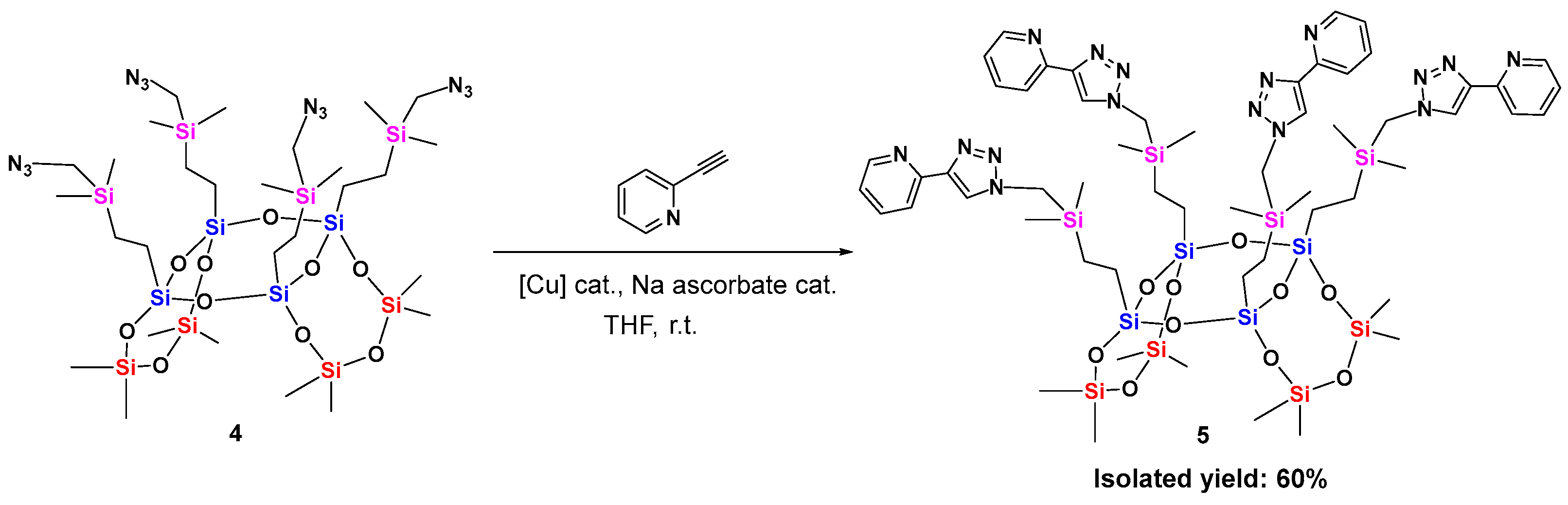
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Yagafarov, N.; Shimamura, K.; Takeda, N.; Unno, M.; Ouali, A. Synthesis of an Azido-Substituted 8-Membered Ring Laddersiloxane and Its Application in Catalysis. Molecules 2025, 30, 373. https://doi.org/10.3390/molecules30020373
Liu Y, Yagafarov N, Shimamura K, Takeda N, Unno M, Ouali A. Synthesis of an Azido-Substituted 8-Membered Ring Laddersiloxane and Its Application in Catalysis. Molecules. 2025; 30(2):373. https://doi.org/10.3390/molecules30020373
Chicago/Turabian StyleLiu, Yujia, Niyaz Yagafarov, Koki Shimamura, Nobuhiro Takeda, Masafumi Unno, and Armelle Ouali. 2025. "Synthesis of an Azido-Substituted 8-Membered Ring Laddersiloxane and Its Application in Catalysis" Molecules 30, no. 2: 373. https://doi.org/10.3390/molecules30020373
APA StyleLiu, Y., Yagafarov, N., Shimamura, K., Takeda, N., Unno, M., & Ouali, A. (2025). Synthesis of an Azido-Substituted 8-Membered Ring Laddersiloxane and Its Application in Catalysis. Molecules, 30(2), 373. https://doi.org/10.3390/molecules30020373







