Anti-Neuroinflammatory Effects of Prenylated Indole Alkaloids from the Antarctic Fungus Aspergillus sp. Strain SF-7367
Abstract
1. Introduction
2. Results
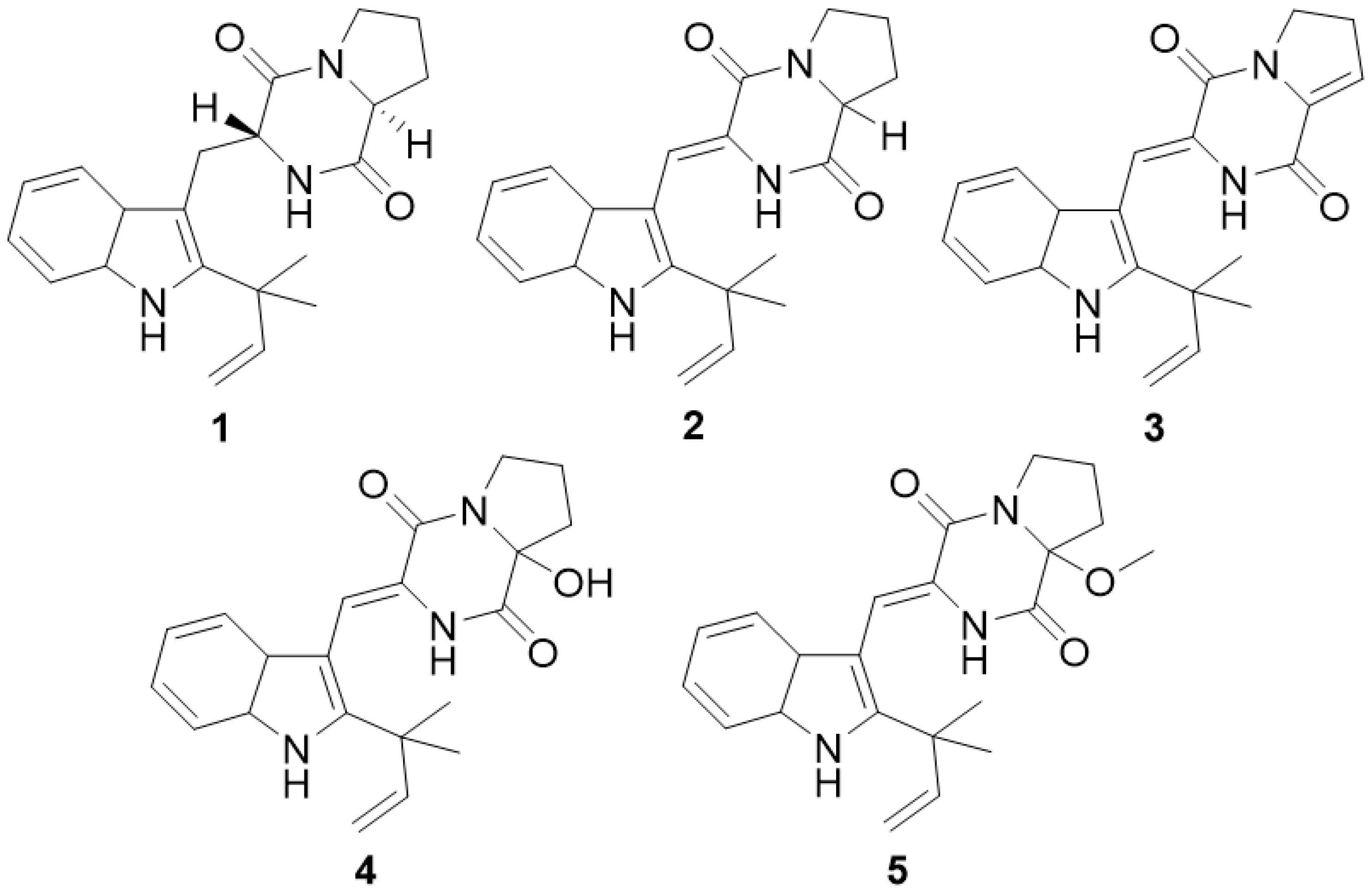
2.1. Isolation and Structure Determination of Five Metabolites
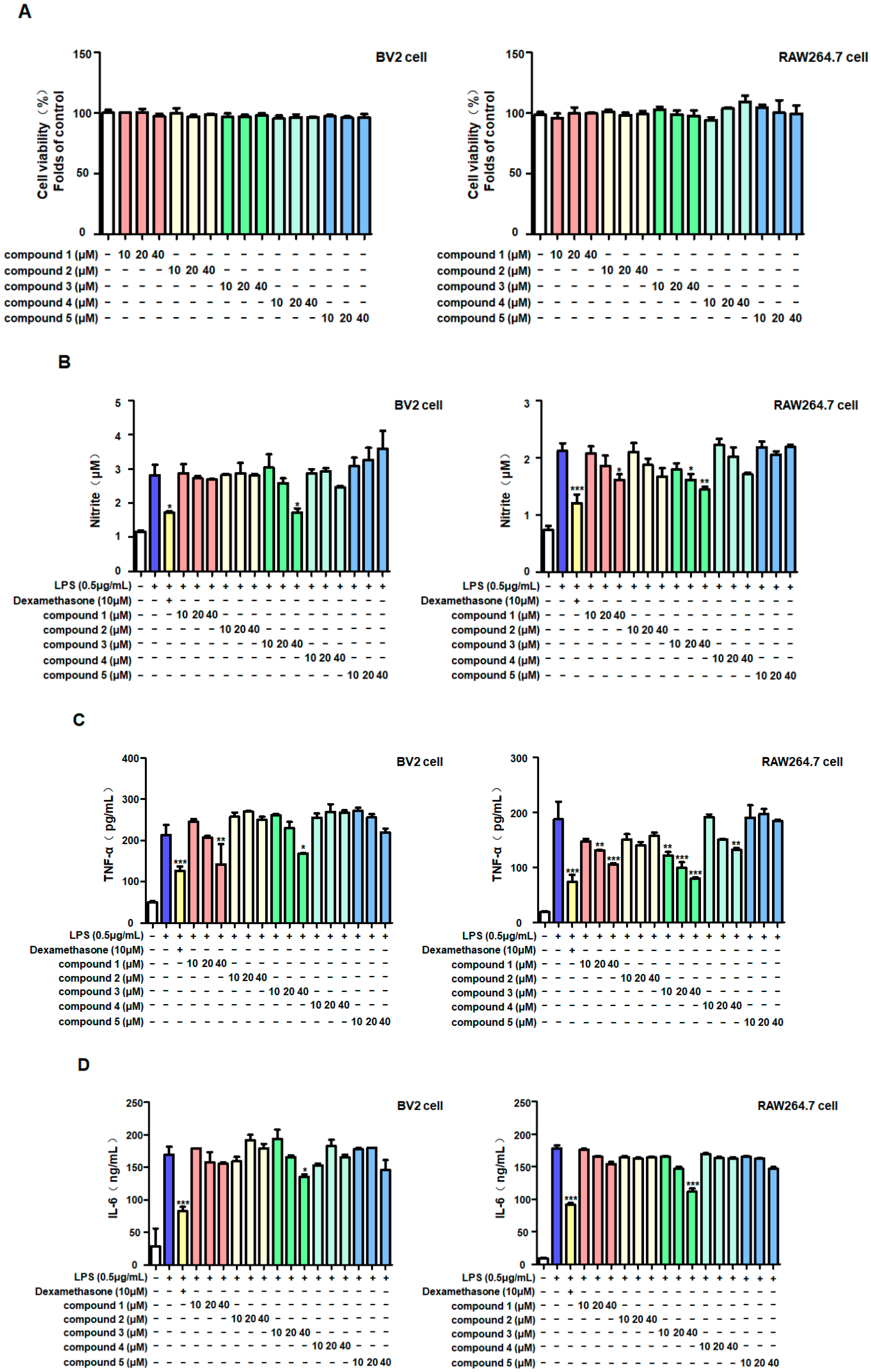
2.2. Anti-Inflammatory Effects of Five Metabolites in LPS-Stimulated BV2 and RAW264.7 Cells
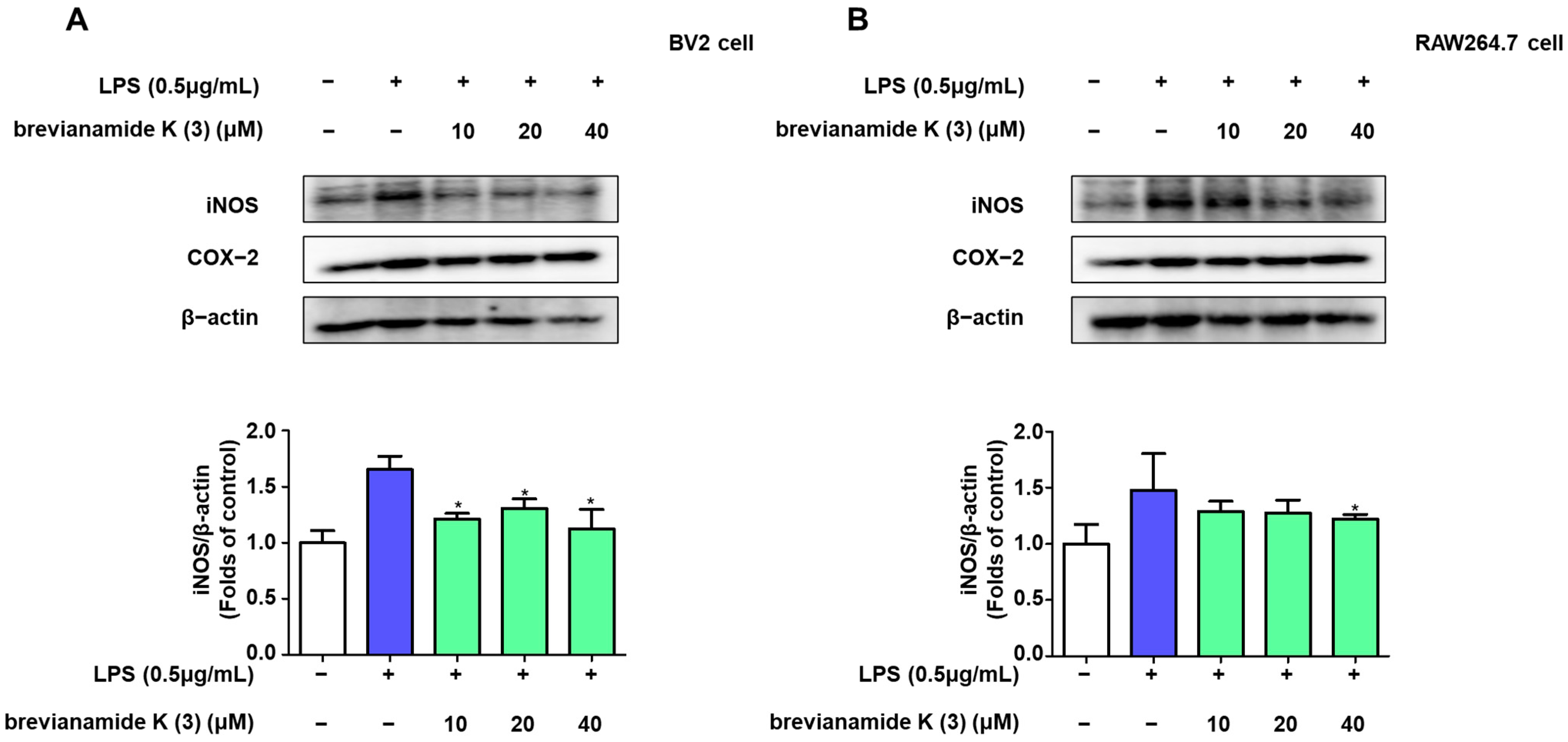
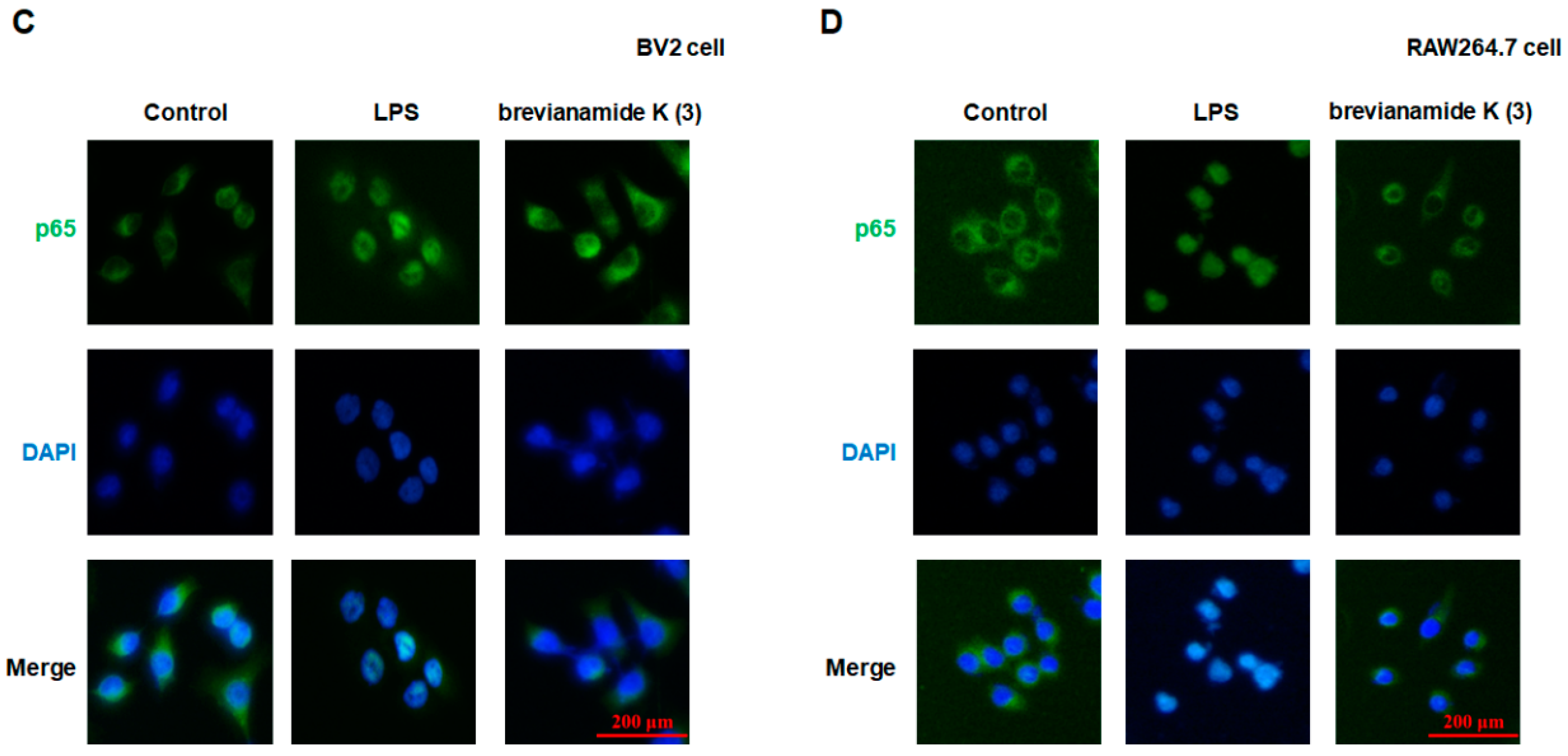
2.3. Inhibitory Effect of Brevianamide K (3) on NF-κB Activation
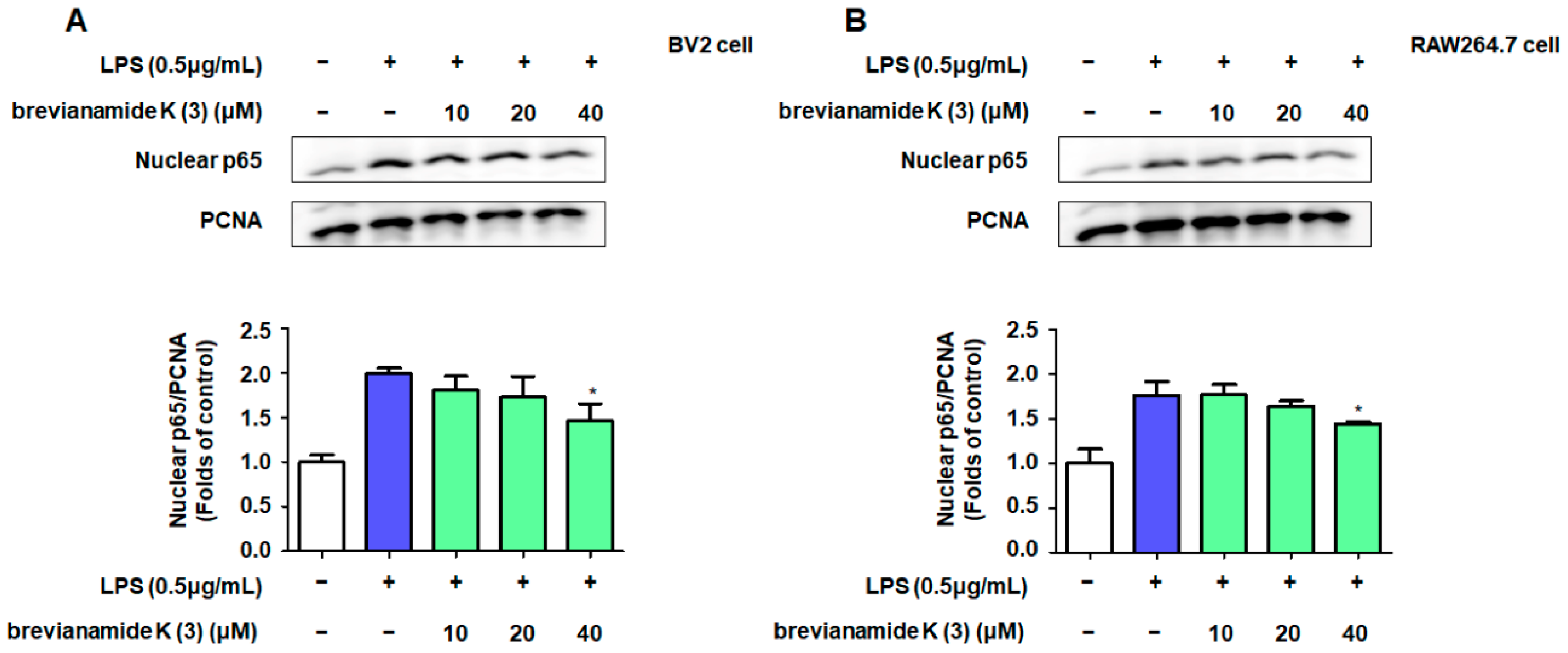
2.4. Role of NF-κB Activation in the Inflammation Inhibitory Effect of Brevianamide K (3)
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Fungal Material
4.3. Fungal Culture, Extraction, and Isolation
4.4. Cell Culture and Viability
4.5. Determination of Nitrite Levels
4.6. Determination of Inflammatory Factor Levels
4.7. Western Blot Analysis
4.8. Synergistic Effect Detection of JSH-23 (p65 Nuclear Inhibitor)
4.9. Molecular Docking
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, J.Z.; Zainab, S.R.; Rehman, M.U.; Abid, M.; Mazhar, M.U.; Shah, F.A.; Tipu, M.K. Chronic stress intensify PTZ-induced seizures by triggering neuroinflammation and oxidative stress. Biochem. Biophys. Res. Commun. 2024, 729, 150333. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Deng, S.; Wu, Y.; Ye, B. The effects of Ganoderma leucocontextum triterpenoids treatment on the D-galactose and aluminum chloride-induced Alzheimer-like pathology in mouse brain. J. Ethnopharmacol. 2024, 334, 118530. [Google Scholar] [CrossRef] [PubMed]
- Asahina, R.; Takahashi, M.; Takano, H.; Yao, R.; Abe, M.; Goshima, Y.; Ohshima, T. The role of CRMP4 in LPS-induced neuroinflammation. Brain Res. 2024, 1841, 149094. [Google Scholar] [CrossRef]
- Manzhulo, I.; Tyrtyshnaia, A.; Egoraeva, A.; Ivashkevich, D.; Girich, A.; Manzhulo, O. Anti-inflammatory and anti-apoptotic activity of synaptamide improves the morphological state of neurons in traumatic brain injury. Neuropharmacology 2024, 258, 110094. [Google Scholar] [CrossRef] [PubMed]
- Kandpal, M.; Baral, B.; Varshney, N.; Jain, A.K.; Chatterji, D.; Meena, A.K.; Pandey, R.K.; Jha, H.C. Gut-brain axis interplay via STAT3 pathway: Implications of Helicobacter pylori derived secretome on inflammation and Alzheimer’s disease. Virulence 2024, 15, 2303853. [Google Scholar] [CrossRef]
- Wang, S.; Wu, L.; Xie, Y.; Ge, S.; Wu, Y.; Chen, L.; Yi, L.; Yang, J.; Duan, F.; Huang, L. Erjingpill bionic cerebrospinal fluid alleviates LPS-induced inflammatory response in BV2 cells by inhibiting glycolysis via mTOR. J. Ethnopharmacol. 2024, 333, 118412. [Google Scholar] [CrossRef] [PubMed]
- Titisari, N.; Fauzi, A.; Abdul Razak, I.S.; Mohd Noor, M.H.; Samsulrizal, N.; Ahmad, H. Dietary menhaden fish oil supplementation suppresses lipopolysaccharide-induced neuroinflammation and cognitive impairment in diabetic rats. Pharm. Biol. 2024, 62, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xiang, S.; Chen, X.; Rong, Y.; Huang, L.; Chen, Z.; Yao, K.; Chen, W.; Deng, C.; Wang, J. Irisin attenuates acute glaucoma-induced neuroinflammation by activating microglia-integrin alphaVbeta5/AMPK and promoting autophagy. Int. Immunopharmacol. 2024, 138, 112545. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, X.; Zhang, W.; Fu, J.; Jiang, K.; Shen, Y.; Li, C.; Gao, H. Modulation of choline and lactate metabolism by basic fibroblast growth factor mitigates neuroinflammation in type 2 diabetes: Insights from (1)H-NMR metabolomics analysis. Neuropharmacology 2024, 257, 110049. [Google Scholar] [CrossRef]
- Long, Y.; Liu, J.; Wang, Y.; Guo, H.; Cui, G. The complex effects of miR-146a in the pathogenesis of Alzheimer’s disease. Neural Regen. Res. 2025, 20, 1309–1323. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, H.; Wang, X.; Cheng, H.; Zhu, G.; Yang, S. Novel mechanisms of Anshen Dingzhi prescription against PTSD: Inhibiting DCC to modulate synaptic function and inflammatory responses. J. Ethnopharmacol. 2024, 333, 118425. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Li, T.; Ji, K.; Zhou, X.; Yao, W.; Zhou, L.; Huang, P.; Zhong, K. Safranal alleviates pentetrazole-induced epileptic seizures in mice by inhibiting the NF-kappaB signaling pathway and mitochondrial-dependent apoptosis through GSK-3beta inactivation. J. Ethnopharmacol. 2024, 333, 118408. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Turak, A.; Li, B.; Aisa, H.A. Guaianolide sesquiterpene lactones from Cichorium glandulosum and their anti-neuroinflammation activities. Phytochemistry 2024, 226, 114223. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Vinh, L.B.; Tuan, N.Q.; Lee, H.; Kim, E.; Kim, Y.C.; Sohn, J.H.; Yim, J.H.; Lee, H.J.; Lee, D.S.; et al. Macrosphelides from Antarctic fungus Pseudogymnoascus sp. (strain SF-7351) and their neuroprotective effects on BV2 and HT22 cells. Chem. Biol. Interact. 2023, 385, 110718. [Google Scholar] [CrossRef]
- Papale, M.; Fazi, S.; Severini, M.; Scarinci, R.; Dell’Acqua, O.; Azzaro, M.; Venuti, V.; Fazio, B.; Fazio, E.; Crupi, V.; et al. Structural properties and microbial diversity of the biofilm colonizing plastic substrates in Terra Nova Bay (Antarctica). Sci. Total Environ. 2024, 943, 173773. [Google Scholar] [CrossRef]
- Hidalgo-Arias, A.; Muñoz-Hisado, V.; Valles, P.; Geyer, A.; Garcia-Lopez, E.; Cid, C. Adaptation of the Endolithic Biome in Antarctic Volcanic Rocks. Int. J. Mol. Sci. 2023, 24, 13824. [Google Scholar] [CrossRef] [PubMed]
- Li, S.M. Prenylated indole derivatives from fungi: Structure diversity, biological activities, biosynthesis and chemoenzymatic synthesis. Nat. Prod. Rep. 2010, 27, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Huang, Z.H.; Shen, W.B.; Lu, X.H.; Zhang, X.X.; Ma, X.; Qi, S.H. Prenylated indole diketopiperazine alkaloids as phosphatase inhibitors from the marine-derived fungus Talaromyces purpureogenus. Phytochemistry 2024, 223, 114119. [Google Scholar] [CrossRef] [PubMed]
- Huan, M.; Liu, Y.D.; Zhong, R. Identifying initial transformation products during chlorination of the indole moiety and unveiling their formation mechanisms. Environ. Sci. Process Impacts. 2024, 26, 1629–1640. [Google Scholar] [CrossRef]
- Cui, B.; Zheng, Y.; Sun, H.; Shang, H.; Du, M.; Shang, Y.; Yavuz, C.T. Catalytic enantioselective intramolecular hydroamination of alkenes using chiral aprotic cyclic urea ligand on manganese (II). Nat. Commun. 2024, 15, 6647. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.H.; Liu, X.D.; Jin, M.H.; Sun, H.N.; Kwon, T. Role of NLRP3 inflammasome-mediated neuronal pyroptosis and neuroinflammation in neurodegenerative diseases. Inflamm. Res. 2023, 72, 1839–1859. [Google Scholar] [CrossRef]
- Biswas, K. Microglia mediated neuroinflammation in neurodegenerative diseases: A review on the cell signaling pathways involved in microglial activation. J. Neuroimmunol. 2023, 383, 578180. [Google Scholar] [CrossRef]
- Murillo, D.; Kamga, C.; Mo, L.; Shiva, S. Nitrite as a mediator of ischemic preconditioning and cytoprotection. Nitric Oxide 2011, 25, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Soares, N.L.; Paiva, I.; Bravo, J.; Queiroga, C.S.F.; Melo, B.F.; Conde, S.V.; Romão, C.C.; Summavielle, T.; Vieira, H.L.A. Carbon Monoxide Modulation of Microglia-Neuron Communication: Anti-Neuroinflammatory and Neurotrophic Role. Mol. Neurobiol. 2022, 59, 872–889. [Google Scholar] [CrossRef] [PubMed]
- Somensi, N.; Rabelo, T.K.; Guimarães, A.G.; Quintans-Junior, L.J.; de Souza Araújo, A.A.; Moreira, J.C.F.; Gelain, D.P. Carvacrol suppresses LPS-induced pro-inflammatory activation in RAW 264.7 macrophages through ERK1/2 and NF-kB pathway. Int. Immunopharmacol. 2019, 75, 105743. [Google Scholar] [CrossRef]
- Gupta, S.; Biswas, J.; Gupta, P.; Singh, A.; Tiwari, S.; Mishra, A.; Singh, S. Salubrinal attenuates nitric oxide mediated PERK: IRE1alpha: ATF-6 signaling and DNA damage in neuronal cells. Neurochem. Int. 2019, 131, 104581. [Google Scholar] [CrossRef]
- Ko, W.; Sohn, J.H.; Jang, J.H.; Ahn, J.S.; Kang, D.G.; Lee, H.S.; Kim, J.S.; Kim, Y.C.; Oh, H. Inhibitory effects of alternaramide on inflammatory mediator expression through TLR4-MyD88-mediated inhibition of NF-B and MAPK pathway signaling in lipopolysaccharide-stimulated RAW264.7 and BV2 cells. Chem. Biol. Interact. 2016, 244, 16–26. [Google Scholar] [CrossRef]
- Geng, Y.; Zhu, S.; Cheng, P.; Lu, Z.M.; Xu, H.Y.; Shi, J.S.; Xu, Z.H. Bioassay-guided fractionation of ethyl acetate extract from Armillaria mellea attenuates inflammatory response in lipopolysaccharide (LPS) stimulated BV-2 microglia. Phytomedicine 2017, 26, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.S.; Lee, H.; Liu, Z.; Lee, H.K.; Lee, D.S. Effects of Compounds Isolated from Lindera erythrocarpa on Anti-Inflammatory and Anti-Neuroinflammatory Action in BV2 Microglia and RAW264.7 Macrophage. Int. J. Mol. Sci. 2022, 23, 7122. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.Y.; Kim, D.B.; Ryu, G.R.; Ko, S.H.; Jeong, I.K.; Ahn, Y.B.; Jo, Y.H.; Kim, M.J. Exendin-4 inhibits iNOS expression at the protein level in LPS-stimulated Raw264.7 macrophage by the activation of cAMP/PKA pathway. J. Cell Biochem. 2013, 114, 844–853. [Google Scholar] [CrossRef]
- Wen, J.; Ribeiro, R.; Zhang, Y. Specific PKC isoforms regulate LPS-stimulated iNOS induction in murine microglial cells. J. Neuroinflammation 2011, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.; Baek, J.S.; Liu, Z.; Dong, L.; Kim, N.; Lee, H.; Yoon, C.S.; Kim, N.Y.; Kim, S.C.; Lee, D.S. Anti-Inflammatory Activity of 1,6,7-Trihydroxy-2-(1,1-dimethyl-2-propenyl)-3-methoxyxanthone Isolated from Cudrania tricuspidata via NF-κB, MAPK, and HO-1 Signaling Pathways in Lipopolysaccharide-Stimulated RAW 264.7 and BV2 Cells. Molecules 2023, 28, 7299. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yoon, C.S.; Lee, H.; Lee, H.K.; Lee, D.S. Linderone Isolated from Lindera erythrocarpa Exerts Antioxidant and Anti-Neuroinflammatory Effects via NF-kappaB and Nrf2 Pathways in BV2 and HT22 Cells. Int. J. Mol. Sci. 2023, 24, 7569. [Google Scholar] [CrossRef]
- Zhang, J.; Peng, B. NF-kappaB promotes iNOS and VEGF expression in salivary gland adenoid cystic carcinoma cells and enhances endothelial cell motility in vitro. Cell Prolif. 2009, 42, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, S.; Saghari, S.; Bassiri, F.; Raesi, R.; Zarrabi, A.; Hushmandi, K.; Sethi, G.; Tergaonkar, V. NF-kappaB as a regulator of cancer metastasis and therapy response: A focus on epithelial-mesenchymal transition. J. Cell Physiol. 2022, 237, 2770–2795. [Google Scholar] [CrossRef]
- Xiang, X.; Xin, X.; Hou, Y.; Deng, Y.; Liu, X.; Yu, W. Diosgenin alters LPS-induced macrophage polarization by activating PPARgamma/NF-kappaB signaling pathway. Int. Immunopharmacol. 2024, 126, 111270. [Google Scholar] [CrossRef]
- Wang, Q.; Dong, X.; Li, N.; Wang, Y.; Guan, X.; Lin, Y.; Kang, J.; Zhang, X.; Zhang, Y.; Li, X.; et al. JSH-23 prevents depressive-like behaviors in mice subjected to chronic mild stress: Effects on inflammation and antioxidant defense in the hippocampus. Pharmacol. Biochem. Behav. 2018, 169, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Kadioglu, O.; Jacob, S.; Bohnert, S.; Naß, J.; Saeed, M.E.; Khalid, H.; Merfort, I.; Thines, E.; Pommerening, T.; Efferth, T. Evaluating ancient Egyptian prescriptions today: Anti-inflammatory activity of Ziziphus spina-christi. Phytomedicine 2016, 23, 293–306. [Google Scholar] [CrossRef]


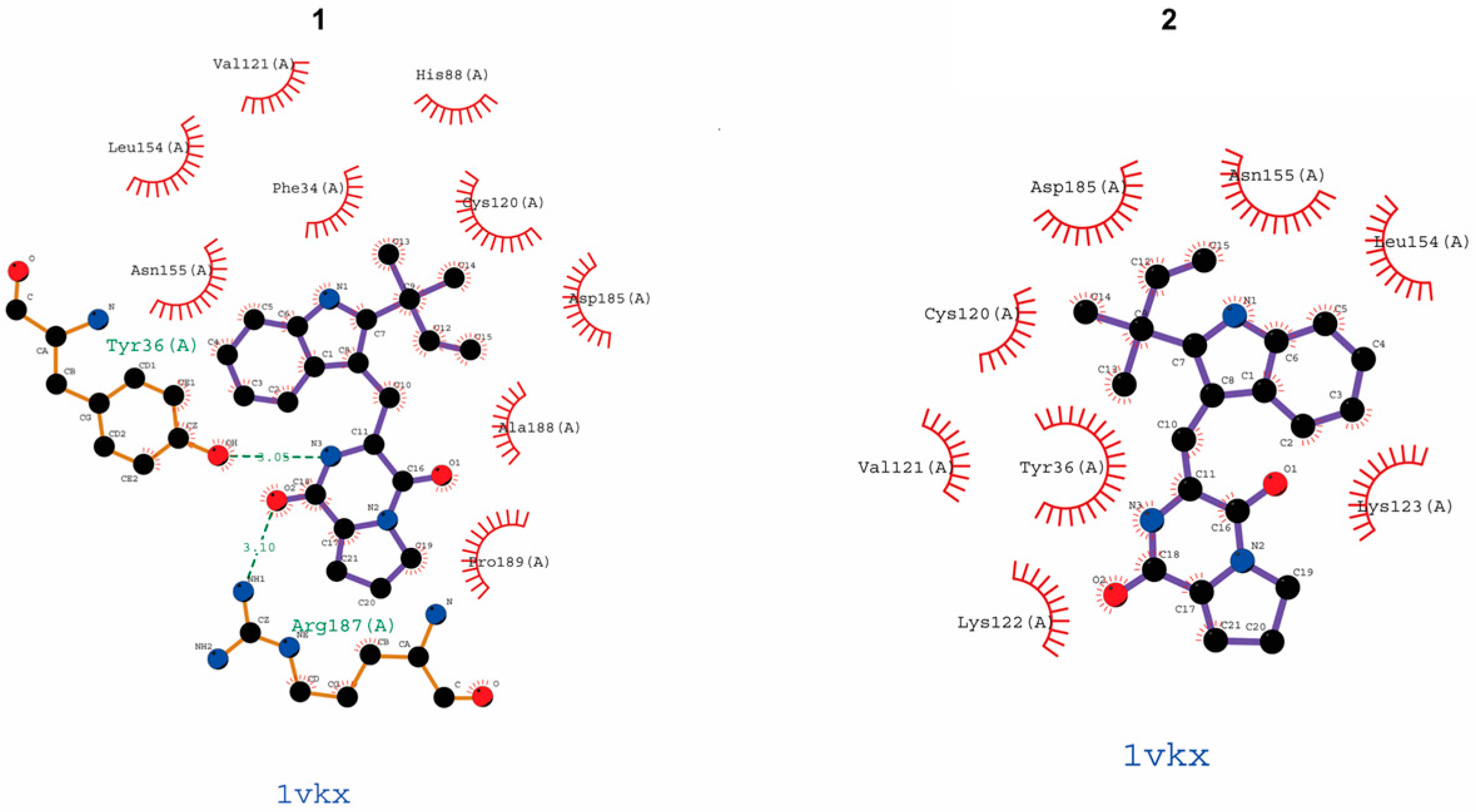
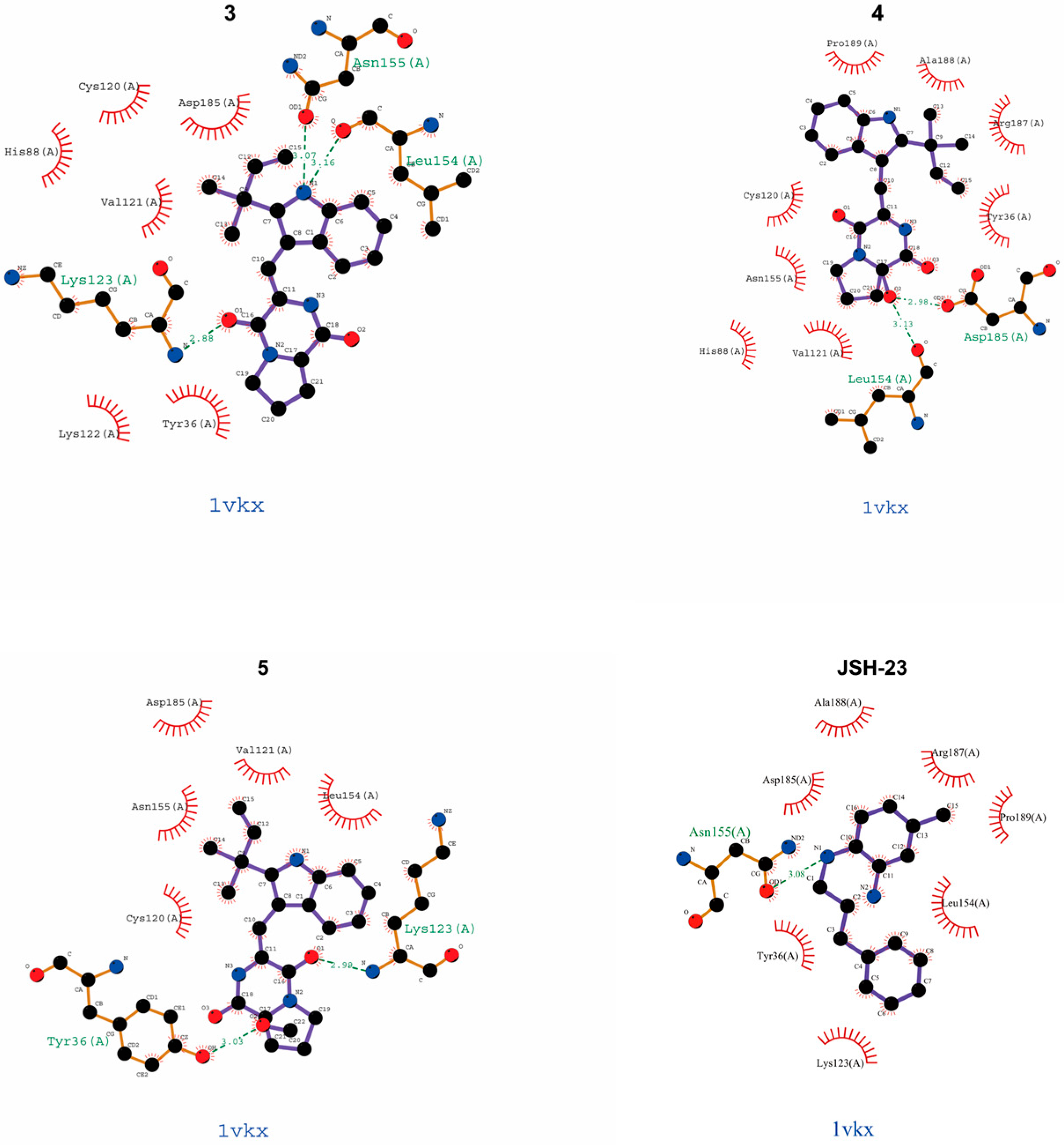
| Compound | Binding Site (Amino Acid Residues of p65) |
|---|---|
| epi-deoxybrevianamide E (1) | Try16 (A), Arg187 (A) |
| brevianamide V/W (2) | − |
| brevianamide K (3) | Asn155 (A), Leu154 (A), Lys123 (A) |
| brevianamide Q (4) | Leu154 (A), Asp185 (A) |
| brevianamide R (5) | Try36 (A), Lys123 (A) |
| JSH-23 | Asn155 (A) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Yoon, C.-S.; Cao, T.Q.; Lee, H.; Kim, I.-C.; Yim, J.H.; Sohn, J.H.; Lee, D.-S.; Oh, H. Anti-Neuroinflammatory Effects of Prenylated Indole Alkaloids from the Antarctic Fungus Aspergillus sp. Strain SF-7367. Molecules 2025, 30, 294. https://doi.org/10.3390/molecules30020294
Liu Z, Yoon C-S, Cao TQ, Lee H, Kim I-C, Yim JH, Sohn JH, Lee D-S, Oh H. Anti-Neuroinflammatory Effects of Prenylated Indole Alkaloids from the Antarctic Fungus Aspergillus sp. Strain SF-7367. Molecules. 2025; 30(2):294. https://doi.org/10.3390/molecules30020294
Chicago/Turabian StyleLiu, Zhiming, Chi-Su Yoon, Thao Quyen Cao, Hwan Lee, Il-Chan Kim, Joung Han Yim, Jae Hak Sohn, Dong-Sung Lee, and Hyuncheol Oh. 2025. "Anti-Neuroinflammatory Effects of Prenylated Indole Alkaloids from the Antarctic Fungus Aspergillus sp. Strain SF-7367" Molecules 30, no. 2: 294. https://doi.org/10.3390/molecules30020294
APA StyleLiu, Z., Yoon, C.-S., Cao, T. Q., Lee, H., Kim, I.-C., Yim, J. H., Sohn, J. H., Lee, D.-S., & Oh, H. (2025). Anti-Neuroinflammatory Effects of Prenylated Indole Alkaloids from the Antarctic Fungus Aspergillus sp. Strain SF-7367. Molecules, 30(2), 294. https://doi.org/10.3390/molecules30020294






