Kaempferol 3-O-Rutinoside, a Flavone Derived from Tetrastigma hemsleyanum Diels et Gilg, Reduces Body Temperature through Accelerating the Elimination of IL-6 and TNF-α in a Mouse Fever Model
Abstract
1. Introduction
2. Results
2.1. Determination and Analysis of Components of T. hemsleyanum Extracts
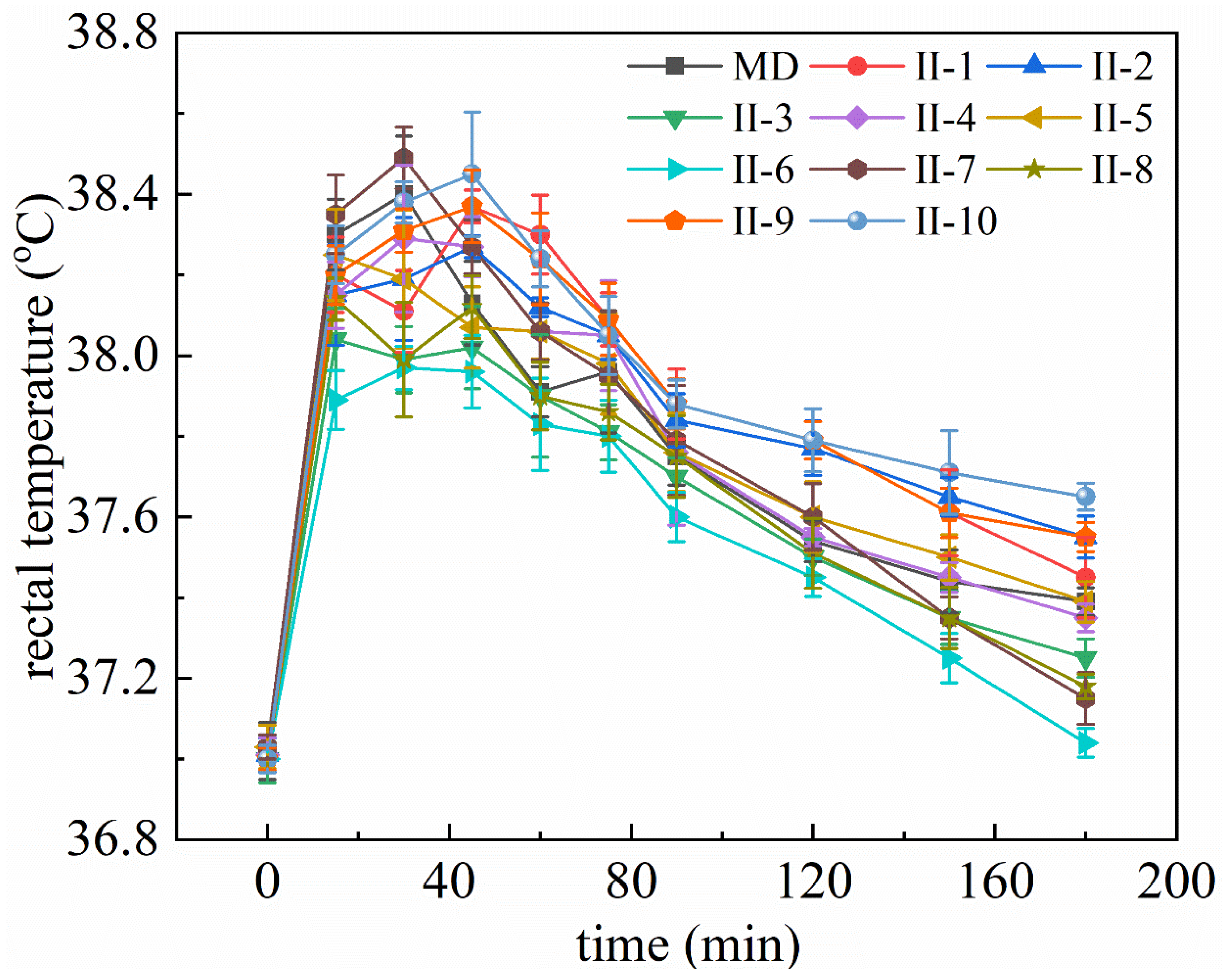
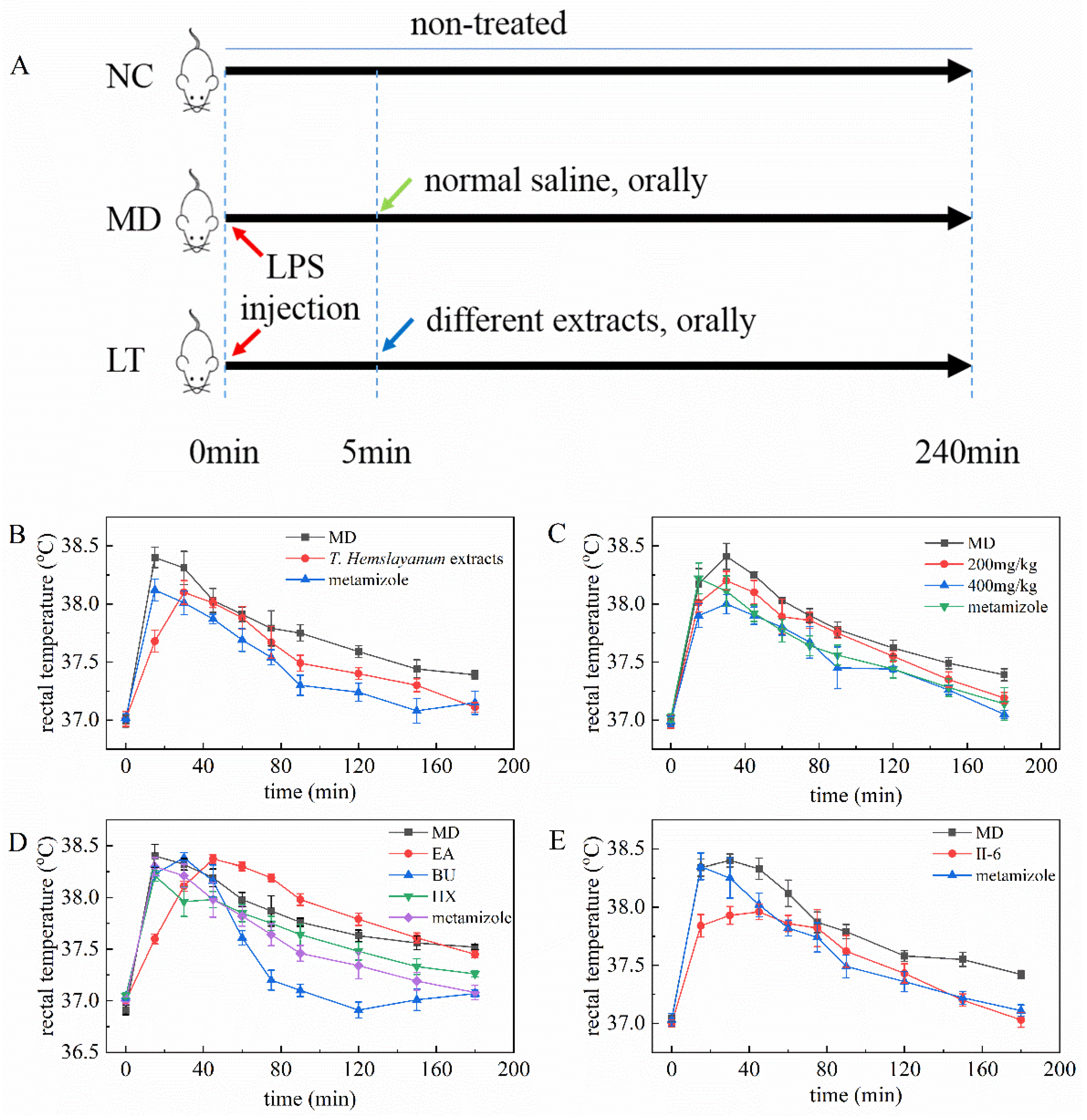
2.2. T. hemsleyanum Extracts and Components Reduce Rectal Temperature in Fever Model Mice
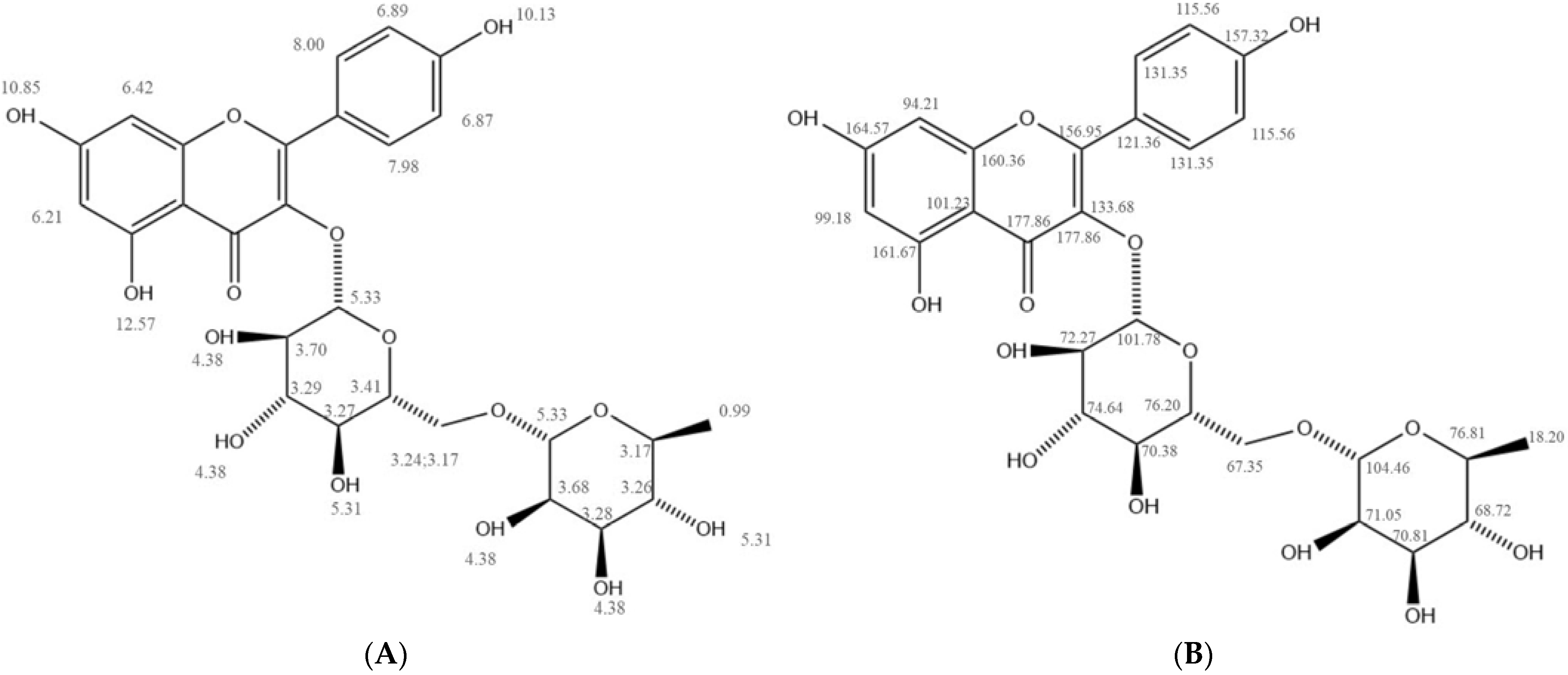
2.3. Structural Characterization of II-6
2.4. The Effect of Different Concentration of K3OR on the Rectal Temperature of Fever Model Mice
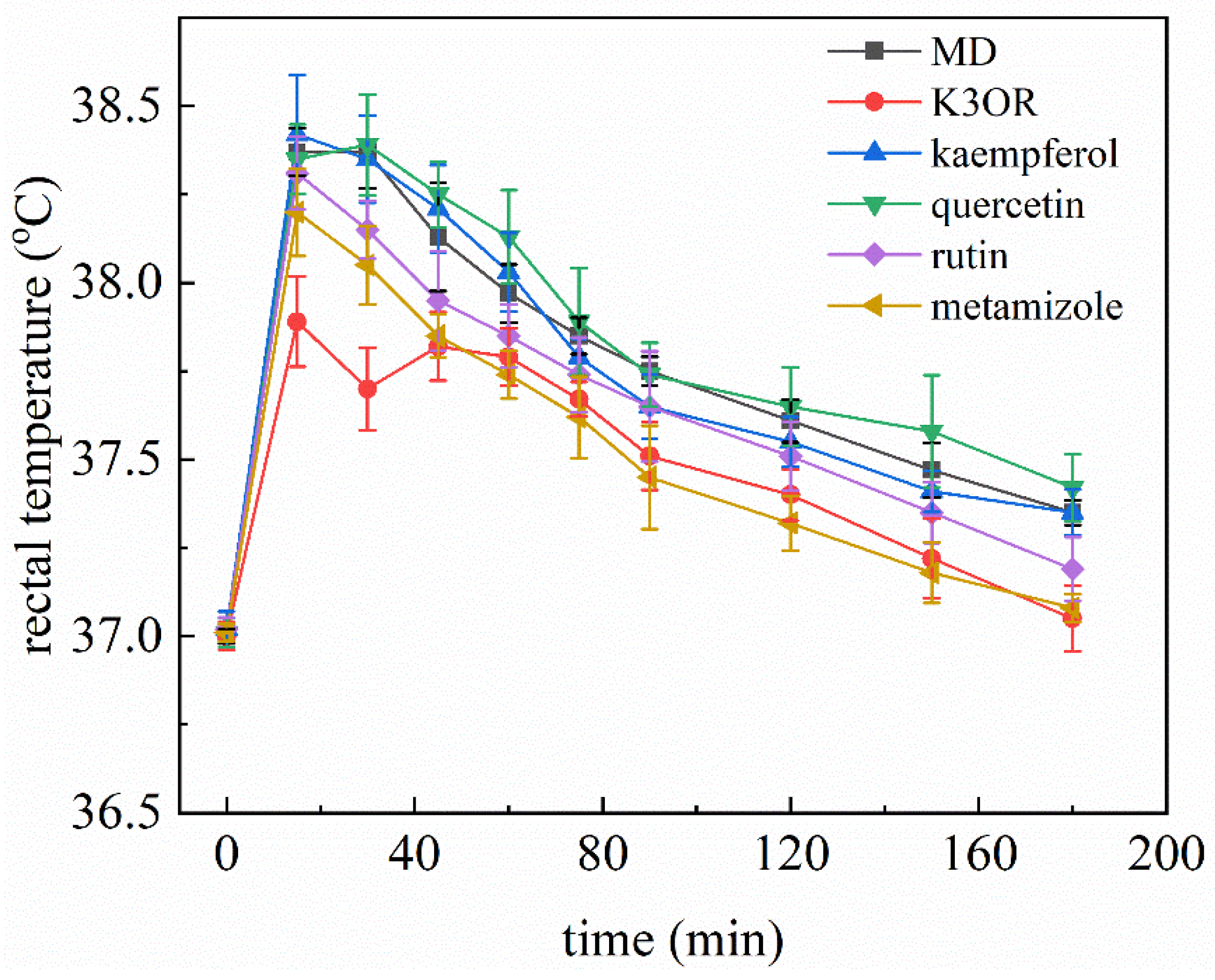
2.5. Comparison of the Effects of K3OR, Ibuprofen, and Acetaminophen
2.6. Fever-Associated Gene Expression Profiles among NC, MD, and LT Groups
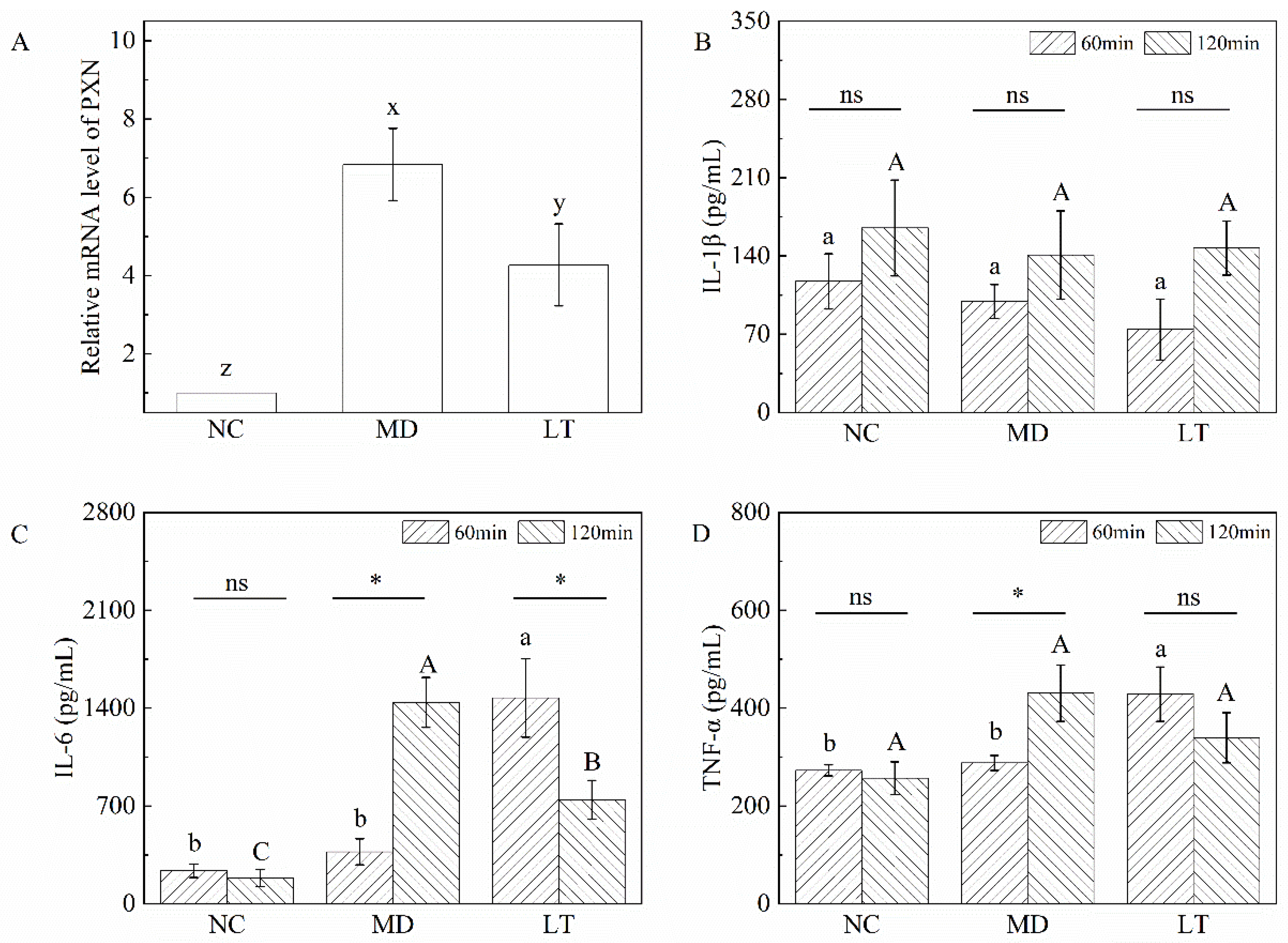
2.7. Validation of qRT-PCR and ELISA Assay
3. Discussion
4. Materials and Methods
4.1. Preparation of T. hemsleyanum Extracts and Separation of Constituents
4.2. Animal Experiment
4.3. Separation and Purification of Chemicals from BU Extract
4.4. RNA Sequencing Analysis
4.5. RNA Extraction and Real-Time Quantitative RT-PCR Analysis
- PXN forward: 5′-ACGTCTACAGCTTCCCCAACAA-3′;
- PXN reverse: 5′- CTCGATTCGGCTTCATCTGC-3′;
- β-actin forward: 5′-GTATCCTGACCCTGAAGTAC-3′;
- β-actin reverse: 5′-CCAGAGGCATACAGGGACAG-3′.
- Data were analyzed using the 2−∆∆Ct method.
4.6. Measurement of Serum Cytokines
4.7. Analysis Methods
- (1)
- The molecular weight was determined using UHPLC–MS. HPLC–HRESIMS (Agilent Technologies 1260 Infinity II/6224, Santa Clara, CA, USA) was utilized, and the analysis was conducted on a ZORBAX RRHD chromatography column (2.1 mm × 100 mm, 1.8 µm). The elution condition employed was as follows: 55–90% acetonitrile/water over a period of 0–20 min.
- (2)
- NMR was utilized to determine the chemical composition of the purified samples. Both 1H NMR and 13C NMR were recorded on a Bruker 400 MHz spectrometer. The samples were dissolved in DMSO-d6 for analysis.
- (3)
- FTIR was employed to determine the chemical group of the purified samples. A Fourier transform infrared spectrometer (iS10, Thermo Nicolet, Waltham, MA, USA) was used for data recording. Each sample was mixed with 200 mg of KBr under anhydrous conditions. Subsequently, the mixture was pressed into pellets, which underwent scanning and analysis using the FTIR spectroscope. The spectra were recorded within the frequency range of 4000–400 cm−1 at a resolution of 4 cm−1.
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TCMs | Traditional Chinese medicines |
| T. hemsleyanum | Tetrastigma hemsleyanum Diels et Gilg |
| HPLC | High performance liquid chromatography |
| UHPLC–MS | Ultra-high performance liquid chromatography–mass spectrometer |
| NMR | Nuclear magnetic resonance |
| FTIR | Fourier transform infrared spectroscopy |
| ELISA | Enzyme-linked immunosorbent assay |
| HX | n-hexane |
| EA | Ethyl acetate |
| CHL | Chloroform |
| BU | n-butanol |
| DEGs | Differentially expressed genes |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| DNP | 2,4-dinitrophenol |
| RSEM | RNA-Seq by expectation-maximization |
| FPKM | Fragments per kilo base per million mapped reads |
| LPS | Lipopolysaccharide |
Appendix A



References
- Atkins, E. Pathogenesis of fever. Physiol. Rev. 1960, 40, 580–646. [Google Scholar] [CrossRef] [PubMed]
- Walter, E.J.; Hanna-Jumma, S.; Carraretto, M.; Forni, L. The pathophysiological basis and consequences of fever. Crit. Care 2016, 20, 200. [Google Scholar] [CrossRef]
- Hasday, J.D.; Thompson, C.; Singh, I.S. Fever, immunity, and molecular adaptations. Compr. Physiol. 2014, 4, 109–148. [Google Scholar]
- Brune, K.; Renner, B.; Tiegs, G. Acetaminophen/paracetamol: A history of errors, failures and false decisions. Eur. J. Pain. 2015, 19, 953–965. [Google Scholar] [CrossRef]
- Ghanem, C.I.; Pérez, M.J.; Manautou, J.E.; Mottino, A.D. Acetaminophen from liver to brain: New insights into drug pharmacological action and toxicity. Pharmacol. Res. 2016, 109, 119–131. [Google Scholar] [CrossRef]
- Cendejas-Hernandez, J.; Sarafian, J.T.; Lawton, V.G.; Palkar, A.; Anderson, L.G.; Larivière, V.; Parker, W. Paracetamol (acetaminophen) use in infants and children was never shown to be safe for neurodevelopment: A systematic review with citation tracking. Eur. J. Pediatr. 2022, 181, 1835–1857. [Google Scholar] [CrossRef] [PubMed]
- Rainsford, K.D. Ibuprofen: Pharmacology, efficacy and safety. Inflammopharmacology 2009, 17, 275–342. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-H.; Yang, C.-C.; Li, P.-C.; Chen, W.-C.; Chien, C.-T. Therapeutic Potential of Traditional Chinese Medicine on Inflammatory Diseases. J. Tradit. Complement. Med. 2013, 3, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-H.; Morris-Natschke, S.; Qian, K.; Dong, Y.; Yang, X.; Zhou, T.; Belding, E.; Wu, S.-F.; Wada, K.; Akiyama, T. Recent Progress of Research on Herbal Products Used in Traditional Chinese Medicine: The Herbs belonging to The Divine Husbandman’s Herbal Foundation Canon (神農本草經 Shén Nóng Běn Cǎo Jīng). J. Tradit. Complement. Med. 2012, 2, 6–26. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Fei, S.; Pei, Y.; Chen, Q.; Wang, J.; Jiang, H. Ziqi Dihuang decoction ameliorates thrombosis in septic rats by inhitbiting plasminogen activator inhibitor-1. J. Tradit. Complement. Med. 2023, 13, 531–537. [Google Scholar] [CrossRef]
- Zhu, R.; Xu, X.; Ying, J.; Cao, G.; Wu, X. The Phytochemistry, Pharmacology, and Quality Control of Tetrastigma hemsleyanum Diels & Gilg in China: A Review. Front. Pharmacol. 2020, 11, 550497. [Google Scholar]
- Chen, X.; Tao, L.; Ru, Y.; Weng, S.; Chen, Z.; Wang, J.; Guo, L.; Lin, Z.; Pan, W.; Qiu, B. Antibacterial mechanism of Tetrastigma hemsleyanum Diels et Gilg’s polysaccharides by metabolomics based on HPLC/MS. Int. J. Biol. Macromol. 2019, 140, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zheng, Y.; Xia, P.; Liang, Z. The research progresses and future prospects of Tetrastigma hemsleyanum Diels et Gilg: A valuable Chinese herbal medicine. J. Ethnopharmacol. 2021, 271, 113836. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Guo, F.; Peng, X.; Cheng, K.; Xiao, L.; Zhang, H.; Li, H.; Jiang, L.; Deng, Z. Metabolism of Phenolics of Tetrastigma hemsleyanum Roots under In Vitro Digestion and Colonic Fermentation as Well as Their In Vivo Antioxidant Activity in Rats. Foods 2021, 10, 2123. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Liu, J.; Du, R.; Yu, Q.; Gong, L.; Jiang, H.; Rong, R. Qualitative and Quantitative Analysis for the Chemical Constituents of Tetrastigma hemsleyanum Diels et Gilg Using Ultra-High Performance Liquid Chromatography/Hybrid Quadrupole-Orbitrap Mass Spectrometry and Preliminary Screening for Anti-Influenza Virus Components. Evid.-Based Complement. Altern. Med. 2019, 2019, 9414926. [Google Scholar]
- Ru, Y.; Chen, X.; Wang, J.; Guo, L.; Lin, Z.; Peng, X.; Qiu, B.; Wong, W.L. Structural characterization, hypoglycemic effects and mechanism of a novel polysaccharide from Tetrastigma hemsleyanum Diels et Gilg. Int. J. Biol. Macromol. 2019, 123, 775–783. [Google Scholar] [CrossRef]
- Zhou, F.; Lu, Y.; Sun, T.; Sun, L.; Wang, B.; Lu, J.; Li, Z.; Zhu, B.; Huang, S.; Ding, Z. Antitumor effects of polysaccharides from Tetrastigma hemsleyanum Diels et Gilg via regulation of intestinal flora and enhancing immunomodulatory effects in vivo. Front. Immunol. 2022, 13, 1009530. [Google Scholar] [CrossRef] [PubMed]
- Romanovsky, A.A. Thermoregulation: Some concepts have changed. Functional architecture of the thermoregulatory system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R37–R46. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.H.; Wu, S.C. Fever—An update. J. Am. Podiat. Med. Assn. 2010, 100, 281–290. [Google Scholar]
- Conti, B.; Tabarean, I.; Andrei, C.; Bartfai, T. Cytokines and fever. Front. Biosci. 2004, 9, 1433–1449. [Google Scholar] [CrossRef] [PubMed]
- Ayertey, F.; Ofori-Attah, E.; Antwi, S.; Amoa-Bosompem, M.; Djameh, G.; Lartey, N.L.; Ohashi, M.; Kusi, K.A.; Appiah, A.A.; Appiah-Opong, R.; et al. Anti-inflammatory activity and mechanism of action of ethanolic leaf extract of Morinda lucida Benth. J. Tradit. Complement. Med. 2021, 11, 249–258. [Google Scholar] [CrossRef]
- Blatteis, C.M. The onset of fever: New insights into its mechanism. Prog. Brain Res. 2007, 162, 3–14. [Google Scholar] [PubMed]
- Varrassi, G.; Pergolizzi, J.V.; Dowling, P.; Paladini, A. Ibuprofen Safety at the Golden Anniversary: Are all NSAIDs the Same? A Narrative Review. Adv. Ther. 2020, 37, 61–82. [Google Scholar] [CrossRef]
- Busson, M. Update on ibuprofen: Review article. J. Int. Med. Res. 1986, 14, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Konstan, M.W.; Hoppel, C.L.; Chai, B.L.; Davis, P.B. Ibuprofen in children with cystic fibrosis: Pharmacokinetics and adverse effects. J. Pediatr. 1991, 118, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Aminoshariae, A.; Khan, A. Acetaminophen: Old drug, new issues. J. Endod. 2015, 41, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.S.; Wang, Z.; Lee, J.M.; Lee, J.Y.; Lim, S.S. Screening of Korean Natural Products for Anti-Adipogenesis Properties and Isolation of Kaempferol-3-O-rutinoside as a Potent Anti-Adipogenetic Compound from Solidago virgaurea. Molecules 2016, 21, 226. [Google Scholar] [CrossRef]
- Shahlehi, S.; Petalcorin, M.I.R. Activation of cholinergic pathway induced vasodilation in rat aorta using aqueous and methanolic leaf extracts of Gynura procumbens. Biomed. Pharmacother. 2021, 143, 112066. [Google Scholar] [CrossRef]
- Habtemariam, S. A-glucosidase inhibitory activity of kaempferol-3-O-rutinoside. Nat. Prod. Commun. 2011, 6, 201–203. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, X.; Wang, Y.; Zheng, X.; Chu, Q. Kaempferol-3-O-rutinoside, a flavone derived from Tetrastigma hemsleyanum, suppresses lung adenocarcinoma via the calcium signaling pathway. Food Funct. 2021, 12, 8351–8365. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Sun, J.; Sun, C.; Zhao, H.; Li, X.; Yao, J.; Su, J.; Xu, X.; Xu, X.; Hu, J.; et al. Total flavonoids from the dried root of Tetrastigma hemsleyanum Diels et Gilg inhibit colorectal cancer growth through PI3K/AKT/mTOR signaling pathway. Phytother. Res. 2022, 36, 4263–4277. [Google Scholar] [CrossRef] [PubMed]
- Mesas, C.; Martínez, R.; Ortíz, R.; Galisteo, M.; López-Jurado, M.; Cabeza, L.; Perazzoli, G.; Melguizo, C.; Porres, J.M.; Prados, J. Antitumor Effect of the Ethanolic Extract from Seeds of Euphorbia lathyris in Colorectal Cancer. Nutrients 2021, 13, 566. [Google Scholar] [CrossRef]
- Li, Y.; Piao, D.; Zhang, H.; Kim, T.; Lee, S.H.; Chang, H.W.; Woo, M.H.; Son, J.K. Quality evaluation of Carthami Flos by HPLC-UV. Arch. Pharm. Res. 2015, 38, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Liu, Q.; Xie, Y.; Zeng, H.; Zhang, L.; Jiang, X.; Chen, X. Separation of five flavonoids from tartary buckwheat (Fagopyrum tataricum (L.) Gaertn) grains via off-line two dimensional high-speed counter-current chromatography. Food Chem. 2015, 186, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Jiang, Q.; Lin, J.; Li, X.; Sun, C.; Chen, K. Physicochemical characterisation of four cherry species (Prunus spp.) grown in China. Food Chem. 2015, 173, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Budzianowski, J. Kaempferol glycosides from Hosta ventricosa. Phytochemistry 1990, 29, 3643–3647. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Han, Y.; He, Y.; Fu, Y.; Yang, H.; Chen, Y.; Shi, Y. Kaempferol Improves Breast Cancer-Related Depression through the COX-2/PGE2 Pathway. Front. Biosci. Landmark 2023, 28, 311. [Google Scholar] [CrossRef] [PubMed]
- Ur Rashid, H.; Xu, Y.; Ahmad, N.; Muhammad, Y.; Wang, L. Promising anti-inflammatory effects of chalcones via inhibition of cyclooxygenase, prostaglandin E(2), inducible NO synthase and nuclear factor κb activities. Bioorganic Chem. 2019, 87, 335–365. [Google Scholar] [CrossRef]
- Hämäläinen, M.; Nieminen, R.; Asmawi, M.Z.; Vuorela, P.; Vapaatalo, H.; Moilanen, E. Effects of flavonoids on prostaglandin E2 production and on COX-2 and mPGES-1 expressions in activated macrophages. Planta Med. 2011, 77, 1504–1511. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.X.; Guo, W.T. Toxicological study on Tetrastigma hemsleyanum. J. Chin. Med. Res. 2005, 5, 774–776. [Google Scholar]
- Chen, H.; Liao, S.B.; Chen, D.; Xie, P.; Huang, J. Study on the acute toxicity of the anti-inflammatory extract from the aerial part of Tetrastigmna Hemsleyanum. J. Fujian Med. Uni. 2017, 51, 287–290. [Google Scholar]
- Xie, P.; Yu, W.J.; Chen, D.; Hong, L.T.; Liu, X.M.; Xiong, Z.D.; Huang, X.P. Acute toxicity test of Tetrastigma Hemsleyanum aerial part formula granules. Fujian J. Tradit. Chin. Med. 2019, 60, 63–65. [Google Scholar]
- Brown, M.C.; Turner, C.E. Paxillin: Adapting to change. Physiol. Rev. 2004, 84, 1315–1339. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.E. Paxillin and focal adhesion signalling. Nat. Cell Biol. 2000, 2, E231–E236. [Google Scholar] [CrossRef] [PubMed]
- Luheshi, G.; Rothwell, N. Cytokines and fever. Int. Arch. Allergy Immunol. 1996, 109, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.M.; Figueiredo, M.J.; Martins, J.M.; Machado, R.R.; Sorgi, C.; Faciolli, L.H.; Alves-Filho, J.C.; Cunha, F.Q.; Souza, G.E. A crucial role for IL-6 in the CNS of rats during fever induced by the injection of live E. coli. Med. Microbiol. Immunol. 2012, 201, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Klir, J.J.; Roth, J.; Szelényi, Z.; McClellan, J.L.; Kluger, M.J. Role of hypothalamic interleukin-6 and tumor necrosis factor-alpha in LPS fever in rat. Am. J. Physiol. 1993, 265, R512–R517. [Google Scholar] [CrossRef] [PubMed]


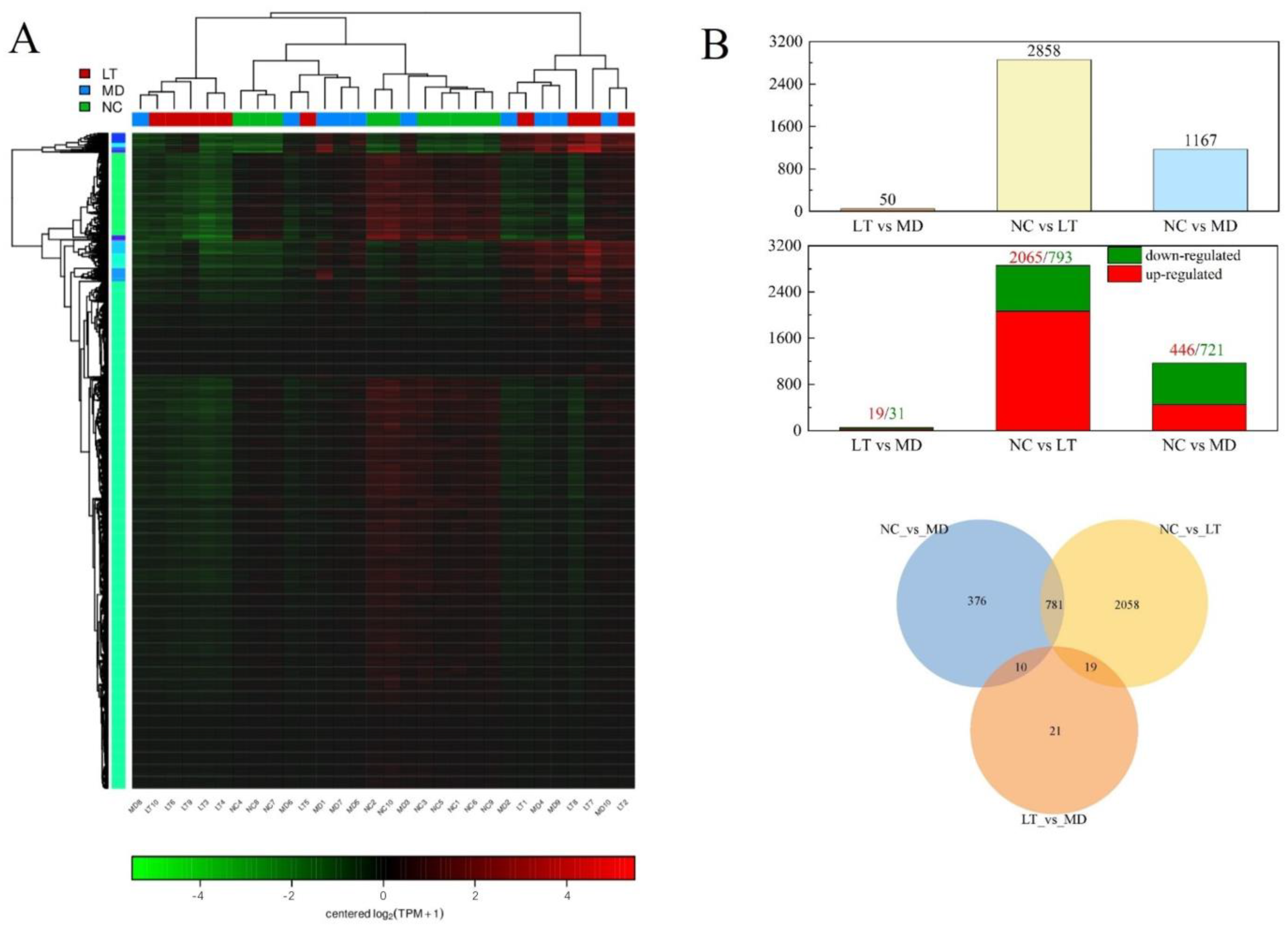

| ID | Description | Count | geneID |
|---|---|---|---|
| hsa04510 | Focal adhesion | 4 | PIK3CD/PXN/XIAP/ACTN1 |
| hsa05203 | Viral carcinogenesis | 4 | PIK3CD/PXN/UBR4/ACTN1 |
| hsa04670 | Leukocyte transendothelial migration | 3 | PIK3CD/PXN/ACTN1 |
| hsa04210 | Apoptosis | 3 | PIK3CD/XIAP/CTSD |
| Treatment Comparison | Gene Count | Gene Information |
|---|---|---|
| MD vs. NC upregulated and LT vs. MD downregulated | 3 | Bag6, Pxn, Arid5a |
| MD vs. NC downregulated and LT vs. MD upregulated | 5 | Wbp1l, Rapgef6, Ifit1bl2, Ice1, Epb41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, W.; Wang, H.; Wang, X.; Li, X.; Hu, J.; Zi, X.; Zhou, Y.; Pan, D.; Fu, Y. Kaempferol 3-O-Rutinoside, a Flavone Derived from Tetrastigma hemsleyanum Diels et Gilg, Reduces Body Temperature through Accelerating the Elimination of IL-6 and TNF-α in a Mouse Fever Model. Molecules 2024, 29, 1641. https://doi.org/10.3390/molecules29071641
Zheng W, Wang H, Wang X, Li X, Hu J, Zi X, Zhou Y, Pan D, Fu Y. Kaempferol 3-O-Rutinoside, a Flavone Derived from Tetrastigma hemsleyanum Diels et Gilg, Reduces Body Temperature through Accelerating the Elimination of IL-6 and TNF-α in a Mouse Fever Model. Molecules. 2024; 29(7):1641. https://doi.org/10.3390/molecules29071641
Chicago/Turabian StyleZheng, Weilong, Haina Wang, Xue Wang, Xin Li, Jiahuan Hu, Xiangyu Zi, Yufeng Zhou, Duotao Pan, and Yongqian Fu. 2024. "Kaempferol 3-O-Rutinoside, a Flavone Derived from Tetrastigma hemsleyanum Diels et Gilg, Reduces Body Temperature through Accelerating the Elimination of IL-6 and TNF-α in a Mouse Fever Model" Molecules 29, no. 7: 1641. https://doi.org/10.3390/molecules29071641
APA StyleZheng, W., Wang, H., Wang, X., Li, X., Hu, J., Zi, X., Zhou, Y., Pan, D., & Fu, Y. (2024). Kaempferol 3-O-Rutinoside, a Flavone Derived from Tetrastigma hemsleyanum Diels et Gilg, Reduces Body Temperature through Accelerating the Elimination of IL-6 and TNF-α in a Mouse Fever Model. Molecules, 29(7), 1641. https://doi.org/10.3390/molecules29071641






