Anti-Thrombotic Activity of 3-Deoxysappanchalcone via Inhibiting Platelet Aggregation and Thrombin (FIIa)/Activated Factor X (FXa) Activity
Abstract
1. Introduction
2. Results
2.1. Effects of 3-DSC on Clotting Time and Bleeding Times
2.2. Effects of 3-DSC on Thrombin-Catalyzed Platelet Aggregation and Fibrin Polymerization and Cellular Viability
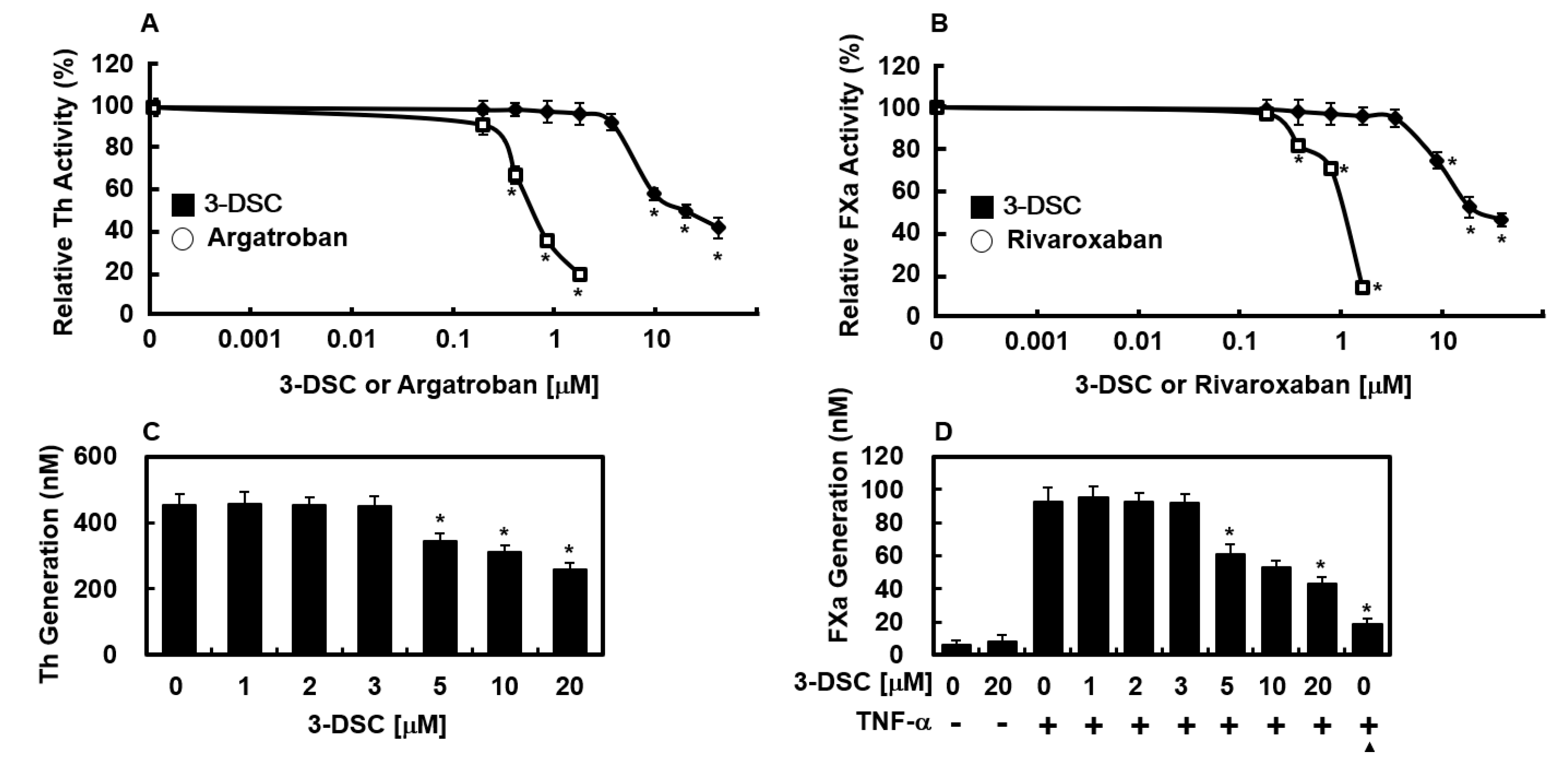
2.3. Effects of 3-DSC on the Activities of Thrombin and FXa
2.4. Effects of 3-DSC on Production of Thrombin and FXa
2.5. Effects of 3-DSC on Secretion of PAI-1 or t-PA Protein
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Reagents Isolation of Human Plasma
4.3. Anticoagulation Assay
4.4. Platelet Aggregation Assay
4.5. Thrombin-Catalyzed Fibrin Polymerization
4.6. Cell Culturemin
4.7. Animals and Husbandry
4.8. Cell Viability Assay
4.9. Factor Xa Production on the Surfaces of HUVECs
4.10. Thrombin Production on the Surfaces of HUVECs
4.11. Thrombin Activity Assay
4.12. Factor Xa (FXa) Activity Assay
4.13. In Vivo Bleeding Time
4.14. Ex Vivo Clotting Time
4.15. ELISA for PAI-1 and t-PA
4.16. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, J.; Wang, J.; Xu, T.; Yao, H.; Yu, L.; Huang, D. Hemostasis strategies and recent advances in nanomaterials for hemostasis. Molecules 2023, 28, 5264. [Google Scholar] [CrossRef] [PubMed]
- LaPelusa, A.; Dave, H.D. Physiology, hemostasis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Raskob, G.E.; Angchaisuksiri, P.; Blanco, A.N.; Buller, H.; Gallus, A.; Hunt, B.J.; Hylek, E.M.; Kakkar, A.; Konstantinides, S.V.; McCumber, M.; et al. Thrombosis: A major contributor to the global disease burden. J. Thromb. Haemost. 2014, 12, 1580–1590. [Google Scholar] [CrossRef]
- Abdel-Aziz, S.; Aeron, A.; Kahil, T. Health benefits and possible risks of herbal medicine. In Microbes in Food and Health; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Fu, L.C.; Huang, X.A.; Lai, Z.Y.; Hu, Y.J.; Liu, H.J.; Cai, X.L. A new 3-benzylchroman derivative from sappan lignum (Caesalpinia sappan). Molecules 2008, 13, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lee, S.O.; Kwak, A.W.; Chae, S.B.; Cho, S.S.; Yoon, G.; Kim, K.T.; Choi, Y.H.; Lee, M.H.; Joo, S.H.; et al. 3-deoxysappanchalcone inhibits cell growth of gefitinib-resistant lung cancer cells by simultaneous targeting of egfr and met kinases. Biomol. Ther. 2023, 31, 446–455. [Google Scholar] [CrossRef]
- Badami, S.; Moorkoth, S.; Rai, S.R.; Kannan, E.; Bhojraj, S. Antioxidant activity of Caesalpinia sappan heartwood. Biol. Pharm. Bull. 2003, 26, 1534–1537. [Google Scholar] [CrossRef]
- Jung, E.G.; Han, K.I.; Kwon, H.J.; Patnaik, B.B.; Kim, W.J.; Hur, G.M.; Nam, K.W.; Han, M.D. Anti-inflammatory activity of sappanchalcone isolated from Caesalpinia sappan L. in a collagen-induced arthritis mouse model. Arch. Pharmacal Res. 2015, 38, 973–983. [Google Scholar] [CrossRef]
- Kim, C.; Kim, B. Anti-cancer natural products and their bioactive compounds inducing er stress-mediated apoptosis: A review. Nutrients 2018, 10, 1021. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.L.; Shu, S.H.; Qin, H.L.; Lee, S.M.; Wang, Y.T.; Du, G.H. In vitro anti-influenza viral activities of constituents from Caesalpinia sappan. Planta Medica 2009, 75, 337–339. [Google Scholar] [CrossRef]
- Yodsaoue, O.; Cheenpracha, S.; Karalai, C.; Ponglimanont, C.; Tewtrakul, S. Anti-allergic activity of principles from the roots and heartwood of Caesalpinia sappan on antigen-induced beta-hexosaminidase release. Phytother. Res. 2009, 23, 1028–1031. [Google Scholar] [CrossRef]
- Lee, J.; Han, G.; Bae, J.-S. 3-deoxysappanchalcone inhibited high mobility group box protein 1-mediated severe inflammatory responses. Pharmaceuticals 2025, 18, 731. [Google Scholar] [CrossRef]
- Sireeratawong, S.; Piyabhan, P.; Singhalak, T.; Wongkrajang, Y.; Temsiririrkkul, R.; Punsrirat, J.; Ruangwises, N.; Saraya, S.; Lerdvuthisopon, N.; Jaijoy, K. Toxicity evaluation of sappan wood extract in rats. J. Med. Assoc. Thail. 2010, 93 (Suppl. 7), S50–S57. [Google Scholar]
- Costantini, T.W.; Kornblith, L.Z.; Pritts, T.; Coimbra, R. The intersection of coagulation activation and inflammation after injury: What you need to know. J. Trauma Acute Care Surg. 2024, 96, 347–356. [Google Scholar] [CrossRef]
- Esmon, C.T. The interactions between inflammation and coagulation. Br. J. Haematol. 2005, 131, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Diehl, K.H.; Hull, R.; Morton, D.; Pfister, R.; Rabemampianina, Y.; Smith, D.; Vidal, J.M.; van de Vorstenbosch, C. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J. Appl. Toxicol. 2001, 21, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Sugo, T.; Nakamikawa, C.; Tanabe, S.; Matsuda, M. Activation of prothrombin by factor xa bound to the membrane surface of human umbilical vein endothelial cells: Its catalytic efficiency is similar to that of prothrombinase complex on platelets. J. Biochem. 1995, 117, 244–250. [Google Scholar] [CrossRef]
- Rao, L.V.; Rapaport, S.I.; Lorenzi, M. Enhancement by human umbilical vein endothelial cells of factor xa-catalyzed activation of factor VII. Blood 1988, 71, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Ezban, M.; Persson, E.; Pendurthi, U.; Hedner, U.; Rao, L.V. Activity and regulation of factor VIIa analogs with increased potency at the endothelial cell surface. J. Thromb. Haemost. 2007, 5, 336–346. [Google Scholar] [CrossRef]
- Philip-Joet, F.; Alessi, M.C.; Philip-Joet, C.; Aillaud, M.; Barriere, J.R.; Arnaud, A.; Juhan-Vague, I. Fibrinolytic and inflammatory processes in pleural effusions. Eur. Respir. J. 1995, 8, 1352–1356. [Google Scholar] [CrossRef]
- Schleef, R.R.; Bevilacqua, M.P.; Sawdey, M.; Gimbrone, M.A., Jr.; Loskutoff, D.J. Cytokine activation of vascular endothelium. Effects on tissue-type plasminogen activator and type 1 plasminogen activator inhibitor. J. Biol. Chem. 1988, 263, 5797–5803. [Google Scholar] [CrossRef]
- Hamaguchi, E.; Takamura, T.; Shimizu, A.; Nagai, Y. Tumor necrosis factor-alpha and troglitazone regulate plasminogen activator inhibitor type 1 production through extracellular signal-regulated kinase- and nuclear factor-kappaB-dependent pathways in cultured human umbilical vein endothelial cells. J. Pharmacol. Exp. Ther. 2003, 307, 987–994. [Google Scholar] [CrossRef]
- Davie, E.W. Biochemical and molecular aspects of the coagulation cascade. Thromb. Haemost. 1995, 74, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Davie, E.W.; Fujikawa, K.; Kisiel, W. The coagulation cascade: Initiation, maintenance, and regulation. Biochemistry 1991, 30, 10363–10370. [Google Scholar] [CrossRef] [PubMed]
- Quinn, C.; Hill, J.; Hassouna, H. A guide for diagnosis of patients with arterial and venous thrombosis. Clin. Lab. Sci. 2000, 13, 229–238. [Google Scholar]
- Lopez, S.; Peiretti, F.; Bonardo, B.; Juhan-Vague, I.; Nalbone, G. Effect of atorvastatin and fluvastatin on the expression of plasminogen activator inhibitor type-1 in cultured human endothelial cells. Atherosclerosis 2000, 152, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Shirinsky, V.P. Vascular endothelium at the molecular level: From fundamental knowledge toward medical implementation. Biomedicines 2024, 13, 2. [Google Scholar] [CrossRef]
- Schönichen, C.; Sun, S.; Middelveld, H.; Huskens, D.; de Groot, P.G.; Heemskerk, J.W.M.; Roest, M.; de Laat, B. Functionally distinct anticoagulant mechanisms of endothelial cells. Thromb. Res. 2024, 244, 109208. [Google Scholar] [CrossRef]
- Man, C.; An, Y.; Wang, G.X.; Mao, E.Q.; Ma, L. Recent advances in pathogenesis and anticoagulation treatment of sepsis-induced coagulopathy. J. Inflamm. Res. 2025, 18, 737–750. [Google Scholar] [CrossRef]
- Zhang, H.; Dhalla, N.S. The role of pro-inflammatory cytokines in the pathogenesis of cardiovascular disease. Int. J. Mol. Sci. 2024, 25, 1082. [Google Scholar] [CrossRef]
- Sillen, M.; Declerck, P.J. A narrative review on plasminogen activator inhibitor-1 and its (patho)physiological role: To target or not to target? Int. J. Mol. Sci. 2021, 22, 2721. [Google Scholar] [CrossRef]
- Dockerill, M.; Ford, D.J.; Angerani, S.; Alwis, I.; Dowman, L.J.; Ripoll-Rozada, J.; Smythe, R.E.; Liu, J.S.T.; Pereira, P.J.B.; Jackson, S.P.; et al. Development of supramolecular anticoagulants with on-demand reversibility. Nat. Biotechnol. 2025, 43, 186–193. [Google Scholar] [CrossRef]
- Ieko, M.; Tarumi, T.; Nakabayashi, T.; Yoshida, M.; Naito, S.; Koike, T. Factor xa inhibitors: New anti-thrombotic agents and their characteristics. Front. Biosci. 2006, 11, 232–248. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fareed, J.; Thethi, I.; Hoppensteadt, D. Old versus new oral anticoagulants: Focus on pharmacology. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 79–99. [Google Scholar] [CrossRef] [PubMed]
- Harbrecht, U. Old and new anticoagulants. Hamostaseologie 2011, 31, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Alquwaizani, M.; Buckley, L.; Adams, C.; Fanikos, J. Anticoagulants: A review of the pharmacology, dosing, and complications. Curr. Emerg. Hosp. Med. Rep. 2013, 1, 83–97. [Google Scholar] [CrossRef]
- Balkrishna, A.; Sharma, N.; Srivastava, D.; Kukreti, A.; Srivastava, S.; Arya, V. Exploring the safety, efficacy, and bioactivity of herbal medicines: Bridging traditional wisdom and modern science in healthcare. Future Integr. Med. 2024, 3, 35–49. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Wang, L.; Liu, Q.; Yang, S.; Wang, C. Advancing herbal medicine: Enhancing product quality and safety through robust quality control practices. Front. Pharmacol. 2023, 14, 1265178. [Google Scholar] [CrossRef]
- Lee, W.; Lee, S.; Choi, J.; Park, J.H.; Kim, K.M.; Jee, J.G.; Bae, J.S. Antithrombotic properties of jj1, a potent and novel thrombin inhibitor. Sci. Rep. 2017, 7, 14862. [Google Scholar] [CrossRef]
- Kim, N.; Jeon, C.; Kim, C.; Ryu, S.H.; Lee, W.; Bae, J.S. Inhibition of factor xa activity, platelet aggregation, and experimentally induced thrombosis by sparstolonin B. Phytomedicine 2022, 99, 153987. [Google Scholar] [CrossRef]
- Ku, S.K.; Bae, J.S. Antithrombotic activities of sulforaphane via inhibiting platelet aggregation and fiia/fxa. Arch. Pharmacal Res. 2014, 37, 1454–1463. [Google Scholar] [CrossRef]
- Despotis, G.J.; Gravlee, G.; Filos, K.; Levy, J. Anticoagulation monitoring during cardiac surgery: A review of current and emerging techniques. Anesthesiology 1999, 91, 1122–1151. [Google Scholar] [CrossRef]
- Jauch, E.C.; Saver, J.L.; Adams, H.P., Jr.; Bruno, A.; Connors, J.J.; Demaerschalk, B.M.; Khatri, P.; McMullan, P.W., Jr.; Qureshi, A.I.; Rosenfield, K.; et al. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke 2013, 44, 870–947. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Huang, H.; Choi, B.Y.; Liu, X.; Zhang, M.; Zhou, S.; Song, M.; Yin, F.; Chen, H.; Shim, J.H.; et al. Cell growth inhibition by 3-deoxysappanchalcone is mediated by directly targeting the topk signaling pathway in colon cancer. Phytomedicine 2019, 61, 152813. [Google Scholar] [CrossRef]
- Nowak, P.; Zbikowska, H.M.; Ponczek, M.; Kolodziejczyk, J.; Wachowicz, B. Different vulnerability of fibrinogen subunits to oxidative/nitrative modifications induced by peroxynitrite: Functional consequences. Thromb. Res. 2007, 121, 163–174. [Google Scholar] [CrossRef]
- Baek, D.H.; Kim, G.O.; Choi, H.J.; Yun, M.Y.; Park, D.H.; Song, G.Y.; Bae, J.S. Inhibitory activities of gdx-365 on hmgb1-mediated septic responses. Biotechnol. Bioprocess Eng. 2023, 28, 623–631. [Google Scholar] [CrossRef]
- Hahn, D.; Kim, M.J.; Kwon, Y.; Kim, E.; Park, D.H.; Bae, J.-S. Natural products ameliorating the adverse health effects by air particulate matter. Biotechnol. Bioprocess Eng. 2024, 29, 1–24. [Google Scholar] [CrossRef]
- Suo, S.K.; Zhao, Y.Q.; Wang, Y.M.; Pan, X.Y.; Chi, C.F.; Wang, B. Seventeen novel angiotensin converting enzyme (ace) inhibitory peptides from the protein hydrolysate of Mytilus edulis: Isolation, identification, molecular docking study, and protective function on huvecs. Food Funct. 2022, 13, 7831–7846. [Google Scholar] [CrossRef] [PubMed]
- Dejana, E.; Callioni, A.; Quintana, A.; de Gaetano, G. Bleeding time in laboratory animals. II—A comparison of different assay conditions in rats. Thromb. Res. 1979, 15, 191–197. [Google Scholar] [CrossRef]
- Kim, T.H.; Ku, S.K.; Bae, J.S. Antithrombotic and profibrinolytic activities of eckol and dieckol. J. Cell. Biochem. 2012, 113, 2877–2883. [Google Scholar] [CrossRef]


| In vitro coagulant assay | |||||
| Sample | Dose | aPTT (s) | PT (s) | PT (INR) | |
| Control | saline | 24.8 ± 0.3 | 12.1 ± 0.6 | 1.00 | |
| 3-DSC | 1 μM | 24.9 ± 0.3 | 12.2 ± 0.2 | 1.02 | |
| 2 μM | 25.1 ± 0.4 | 12.3 ± 0.4 | 1.04 | ||
| 3 μM | 25.2 ± 0.3 | 12.3 ± 0.2 | 1.04 | ||
| 5 μM | 29.5 ± 0.4 * | 15.9 ± 0.3 * | 1.82 * | ||
| 10 μM | 37.7 ± 0.5 * | 19.4 ± 0.4 * | 2.83 * | ||
| 20 μM | 51.7 ± 0.3 * | 25.9 ± 0.3 * | 5.33 * | ||
| Heparin | 20 μM | 61.8 ± 0.8 * | 30.1 ± 0.4 * | 7.43 * | |
| Warfarin | 20 μM | 58.3 ± 1.2 * | 31.2 ± 0.8 * | 8.04 * | |
| In vivo bleeding time | |||||
| Sample | Dose | Tail Bleeding time (s) | n | ||
| Control | Saline | 31.4 ± 0.8 | 5 | ||
| 3-DSC | 0.02 mg/kg | 30.9 ± 1.1 | 5 | ||
| 0.04 mg/kg | 31.9 ± 0.5 | 5 | |||
| 0.06 mg/kg | 32.1 ± 0.5 | 5 | |||
| 0.1 mg/kg | 48.1 ± 1.3 * | 5 | |||
| 0.2 mg/kg | 63.1 ± 1.1 * | 5 | |||
| 0.4 mg/kg | 82.3 ± 0.8 * | 5 | |||
| Heparin | 0.4 mg/kg | 96.2 ± 1.8 * | 5 | ||
| Warfarin | 0.4 mg/kg | 89.7 ± 1.0 * | 5 | ||
| Sample | Dose | aPTT (s) | PT (s) | PT (INR) |
|---|---|---|---|---|
| Control | saline | 28.7 ± 0.8 | 13.2 ± 0.3 | 1.00 |
| 3-DSC | 0.02 mg/kg | 29.1 ± 0.9 | 13.4 ± 0.9 | 1.03 |
| 0.04 mg/kg | 28.8 ± 0.7 | 13.5 ± 0.8 | 1.05 | |
| 0.06 mg/kg | 30.5 ± 1.0 | 13.7 ± 0.7 | 1.09 | |
| 0.1 mg/kg | 35.4 ± 0.9 * | 23.8 ± 0.8 * | 3.66 * | |
| 0.2 mg/kg | 38.5 ± 0.7 * | 28.7 ± 1.1 * | 5.22 * | |
| 0.4 mg/kg | 47.9 ± 0.8 * | 29.4 ± 1.2 * | 5.82 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, G.; Lee, J.; Bae, J.-S. Anti-Thrombotic Activity of 3-Deoxysappanchalcone via Inhibiting Platelet Aggregation and Thrombin (FIIa)/Activated Factor X (FXa) Activity. Molecules 2025, 30, 2580. https://doi.org/10.3390/molecules30122580
Han G, Lee J, Bae J-S. Anti-Thrombotic Activity of 3-Deoxysappanchalcone via Inhibiting Platelet Aggregation and Thrombin (FIIa)/Activated Factor X (FXa) Activity. Molecules. 2025; 30(12):2580. https://doi.org/10.3390/molecules30122580
Chicago/Turabian StyleHan, Gyuri, Jinhee Lee, and Jong-Sup Bae. 2025. "Anti-Thrombotic Activity of 3-Deoxysappanchalcone via Inhibiting Platelet Aggregation and Thrombin (FIIa)/Activated Factor X (FXa) Activity" Molecules 30, no. 12: 2580. https://doi.org/10.3390/molecules30122580
APA StyleHan, G., Lee, J., & Bae, J.-S. (2025). Anti-Thrombotic Activity of 3-Deoxysappanchalcone via Inhibiting Platelet Aggregation and Thrombin (FIIa)/Activated Factor X (FXa) Activity. Molecules, 30(12), 2580. https://doi.org/10.3390/molecules30122580









