Thermosetting Resins Based on Poly(Ethylene Glycol Fumarate) and Acrylic Acid: Rheological and Thermal Analysis
Abstract
1. Introduction
2. Results and Discussion
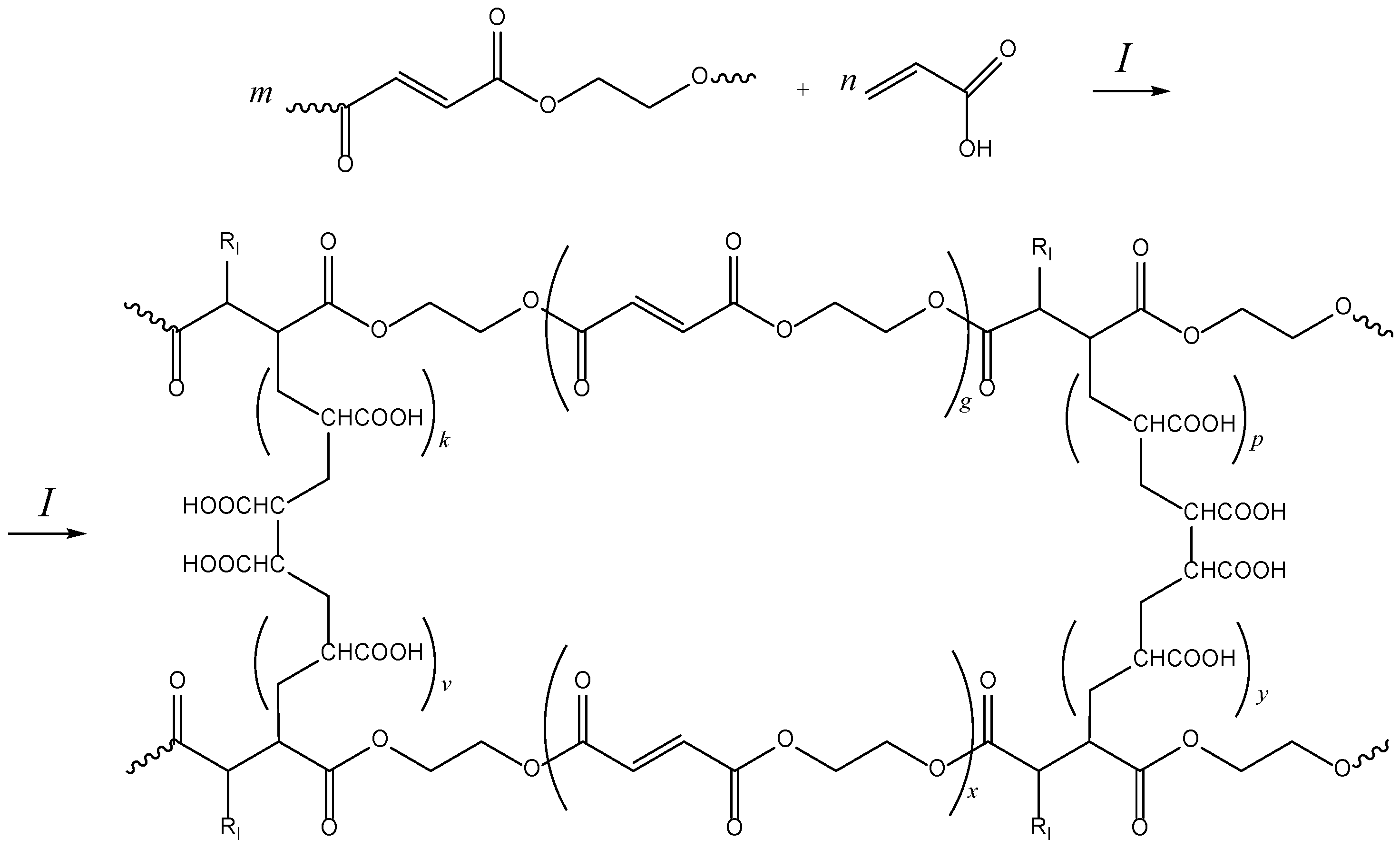
2.1. Curing of p-EGF-AA
2.2. Rheological Studies
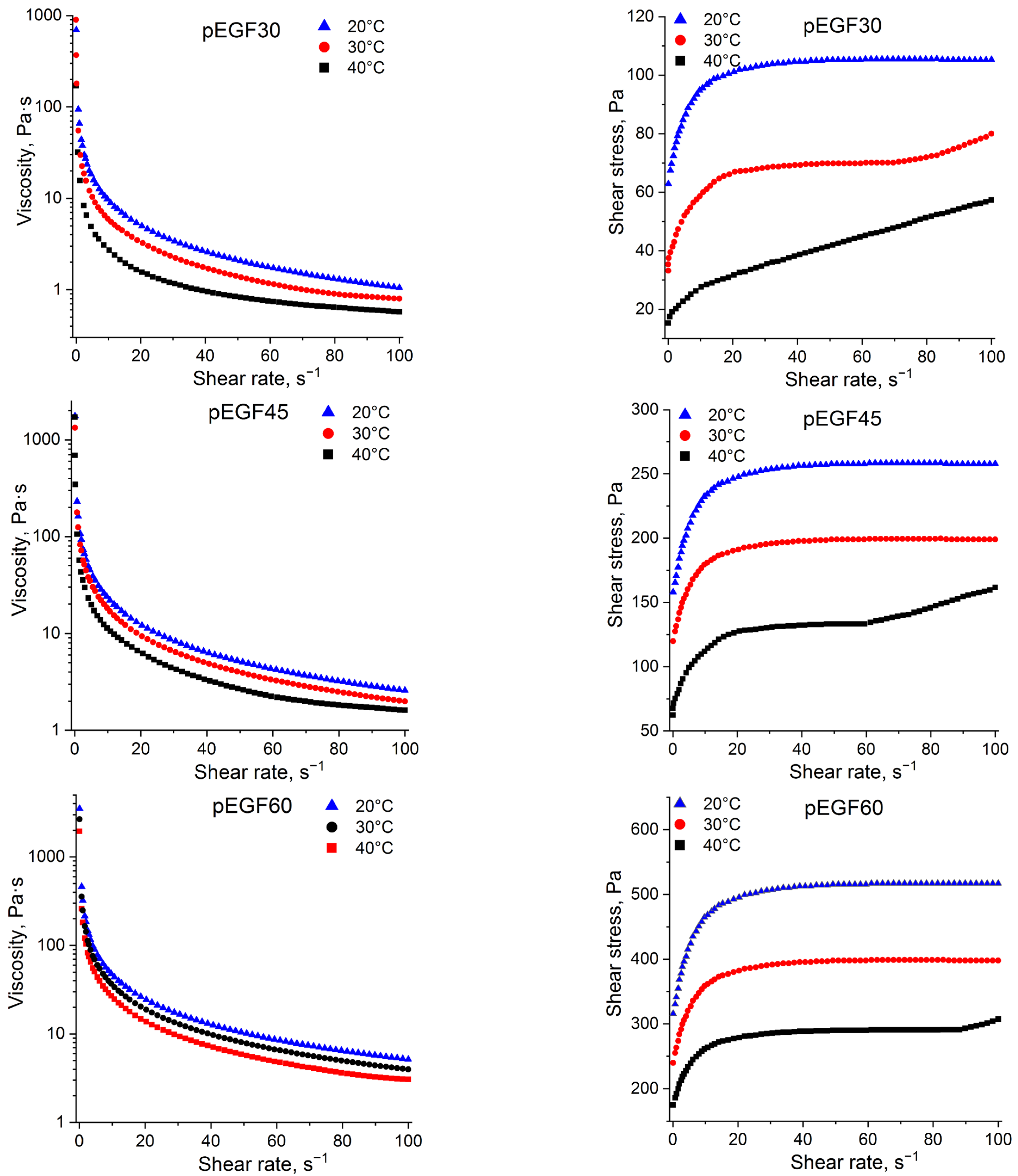
2.2.1. Flow Behavior of pEGF Solutions
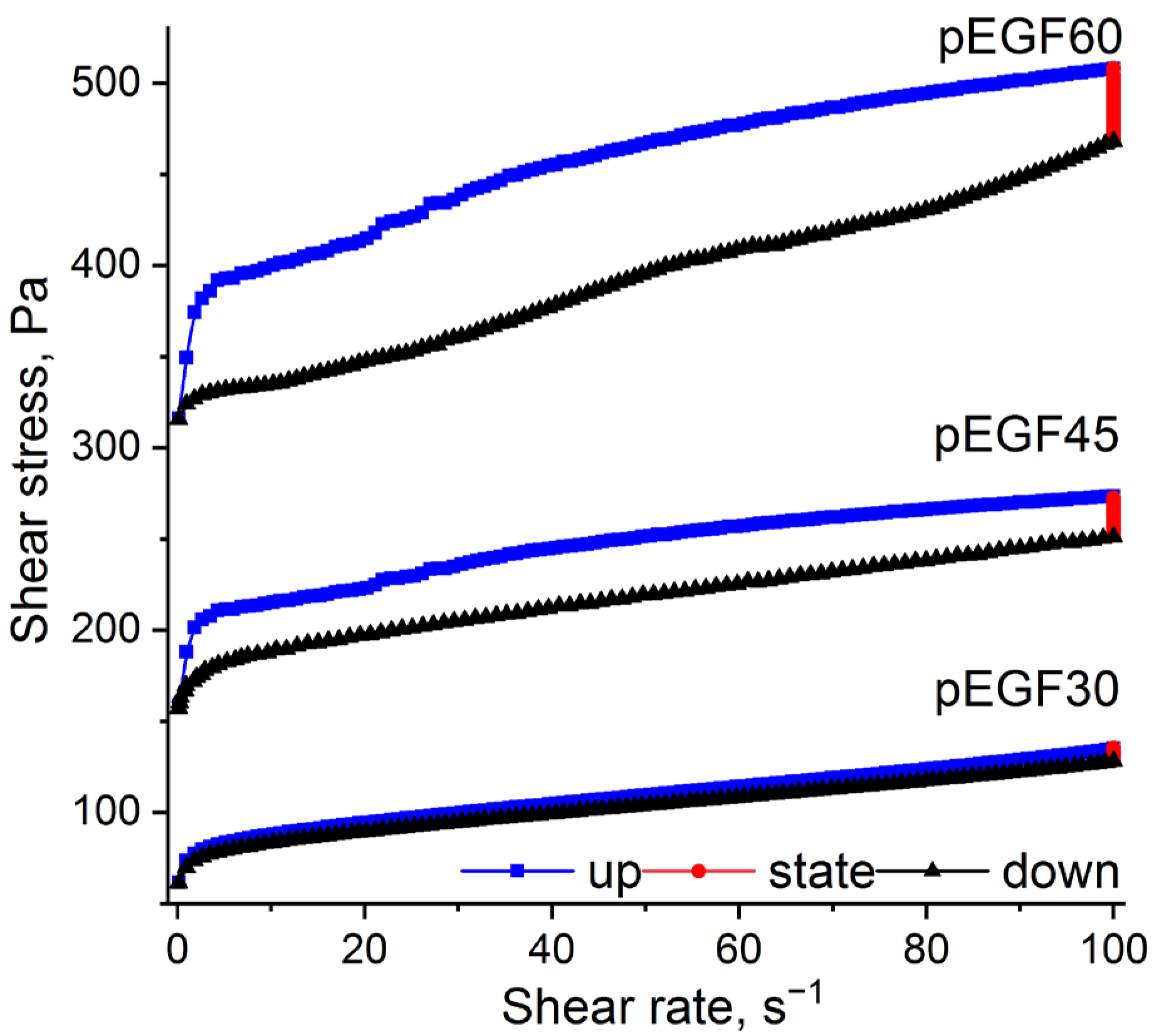
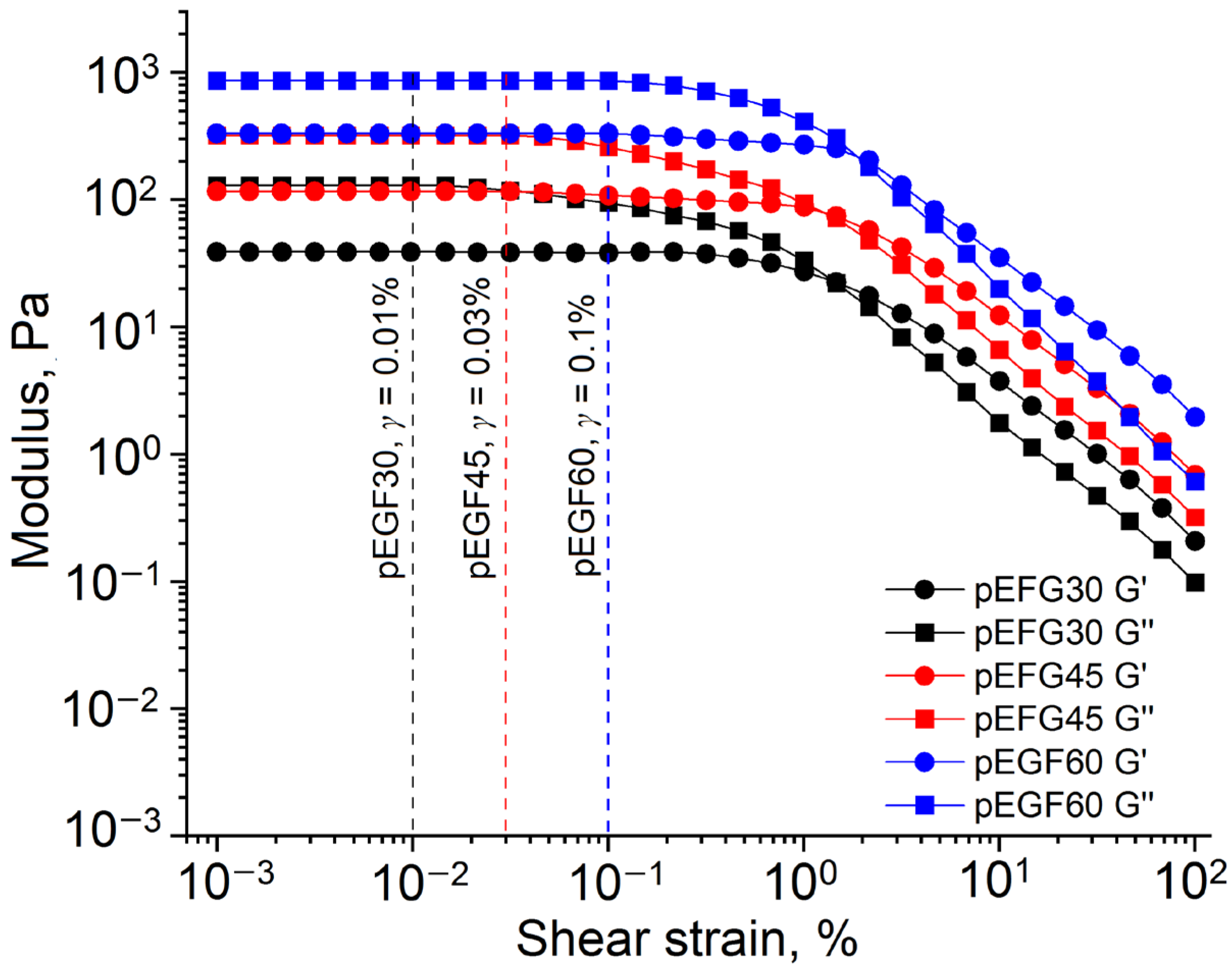
2.2.2. Investigation of Thixotropic Properties
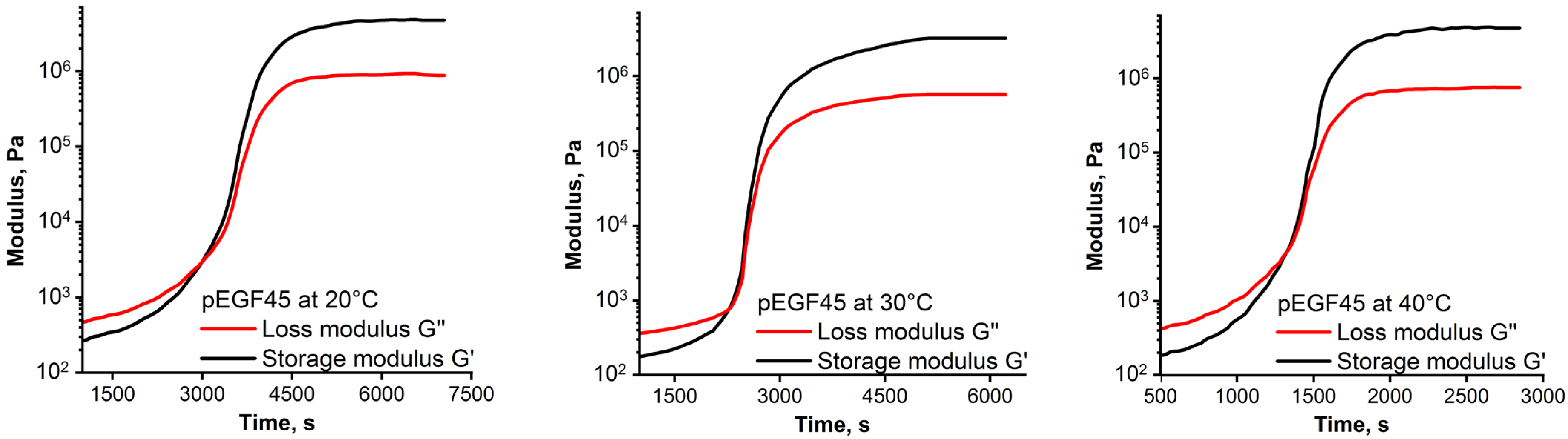
2.2.3. Rheological Analysis of Isothermal Curing
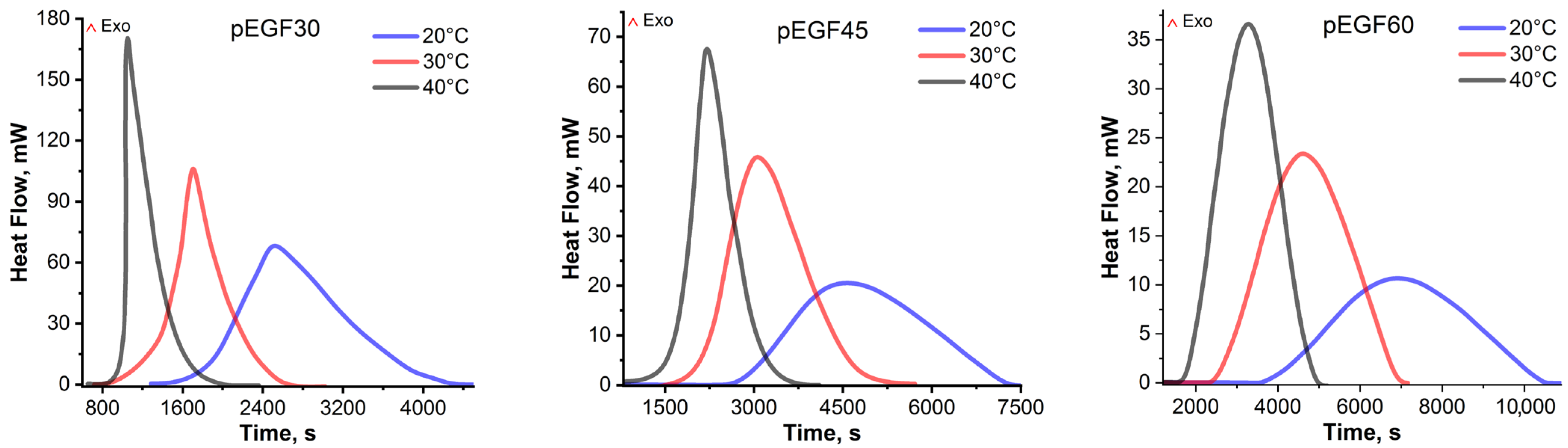
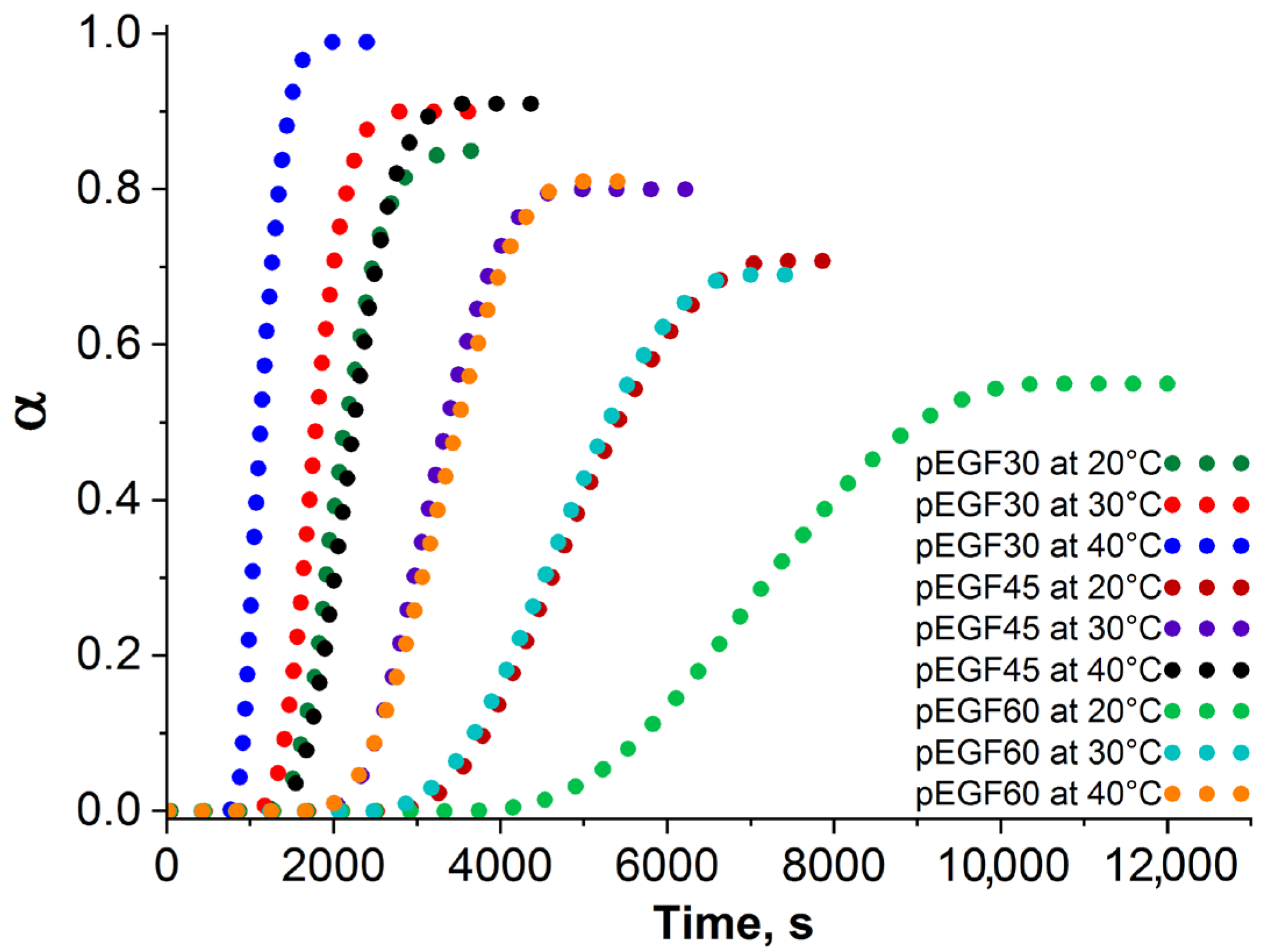
2.3. Isothermal and Dynamic DSC Results
2.4. Thermogravimetric Analysis (TGA)
3. Materials and Methods
3.1. Materials
3.2. Preparation of p-EGF
3.3. Curing of p-EGF
3.4. Methods
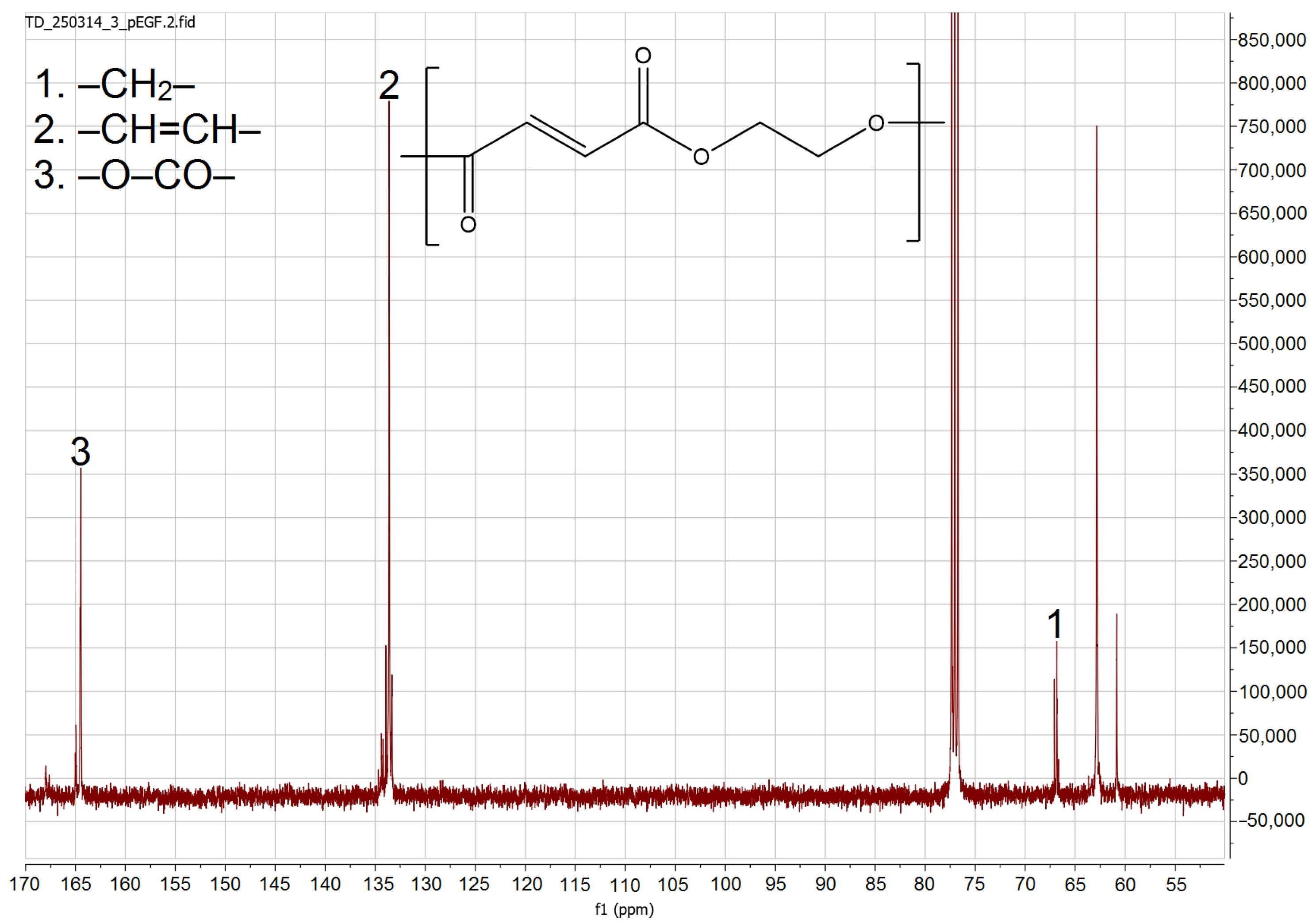
3.4.1. NMR Analysis
3.4.2. Gel-Permeation Chromatography
3.4.3. Rheological Analysis
3.4.4. Differential Scanning Calorimetry (DSC)
3.4.5. Thermogravimetric Analysis (TGA)
3.4.6. IR-Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | acrylic acid |
| BPO | benzoyl peroxide |
| DSC | differential scanning calorimetry |
| DMA | dimethyl aniline |
| LVR | linear viscoelastic region |
| PCM | polymer composite materials |
| pEGF | Poly(ethylene glycol fumarate) |
| TGA | thermogravimetric analysis |
| UP | unsaturated polyester |
| UPR | unsaturated polyester resins |
References
- Aisyah, H.; Paridah, M.; Sapuan, S.; Ilyas, R.; Khalina, A.; Nurazzi, N.; Lee, S.; Lee, C. A Comprehensive Review on Advanced Sustainable Woven Natural Fibre Polymer Composites. Polymers 2021, 13, 471. [Google Scholar] [CrossRef]
- Grujicic, M.; Sellappan, V.; Omar, M.; Seyr, N.; Obieglo, A.; Erdmann, M.; Holzleitner, J. An overview of the polymer-to-metal direct-adhesion hybrid technologies for load-bearing automotive components. J. Mater. Process. Technol. 2008, 197, 363–373. [Google Scholar] [CrossRef]
- Rzymski, W.; Radusch, H. New thermoplastic elastomers. Polimery 2005, 50, 249–254. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, X.; Bai, X.; Wang, G.; Rong, L.; Zhao, Y.; Li, X.; Zhu, J.; Mi, C. Phthalonitrile-etherified cardanol-phenol-formaldehyde resin: Synthesis, characterization and properties. Pigment Resin Technol. 2022, 51, 609–617. [Google Scholar] [CrossRef]
- Baghloul, R.; Babouri, L.; Hebhoub, H.; Boukhelf, F.; El Mendili, Y. Assessment of Mechanical Behavior and Microstructure of Unsaturated Polyester Resin Composites Reinforced with Recycled Marble Waste. Buildings 2024, 14, 3877. [Google Scholar] [CrossRef]
- Jones, F.R. Unsaturated polyester resins. In Brydson’s Plastics Materials, 8th ed.; Marianne, G., Ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 743–772. [Google Scholar] [CrossRef]
- Burkeyev, M.; Kudaibergen, G.; Tazhbayev, Y.; Burkeyeva, G.; Omasheva, A.; Yesentayeva, N.; Bolatbay, A. The number average and mass average molar masses of polyethylene (propylene) glycol fumarates. Bull. Univ. Karaganda Ser. Chem. 2018, 90, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Feng, X.; Zhang, K.; Hui, X.; Hou, X.; Ye, J. Effect of curing pressure on the curing behavior of an epoxy system: Curing kinetics and simulation verification. Polymer 2022, 256, 125162. [Google Scholar] [CrossRef]
- delaCaba, K.; Guerrero, P.; Eceiza, A.; Mondragon, I. Kinetic and rheological studies of an unsaturated polyester cured with different catalyst amounts. Polymer 1996, 37, 275–280. [Google Scholar] [CrossRef]
- Bartolomeo, P.; Chailan, J.; Vernet, J. Curing of cyanate ester resin: A novel approach based on FTIR spectroscopy and comparison with other techniques. Eur. Polym. J. 2001, 37, 659–670. [Google Scholar] [CrossRef]
- Fraga, F.; Burgo, S.; Núñez, E. Curing kinetic of the epoxy system BADGE n=0/1,2 DCH by Fourier Transform Infrared Spectroscopy (FTIR). J. Appl. Polym. Sci. 2001, 82, 3366–3372. [Google Scholar] [CrossRef]
- Arasa, M.; Ramis, X.; Salla, J.; Mantecón, A.; Serra, A. Kinetic study by FTIR and DSC on the cationic curing of a DGEBA/γ-valerolactone mixture with ytterbium triflate as an initiator. Thermochim. Acta 2008, 479, 37–44. [Google Scholar] [CrossRef]
- Garschke, C.; Parlevliet, P.; Weimer, C.; Fox, B. Cure kinetics and viscosity modelling of a high-performance epoxy resin film. Polym. Test. 2013, 32, 150–157. [Google Scholar] [CrossRef]
- Domínguez, J.; Alonso, M.; Oliet, M.; Rojo, E.; Rodríguez, F. Kinetic study of a phenolic-novolac resin curing process by rheological and DSC analysis. Thermochim. Acta 2010, 498, 39–44. [Google Scholar] [CrossRef]
- Sourour, S.; Kamal, M. Differential Scanning Calorimetry of Epoxy Cure–Isothermal Cure Kinetics. Thermochim. Acta 1976, 14, 41–59. [Google Scholar] [CrossRef]
- Lascano, D.; Lerma-Canto, A.; Fombuena, V.; Balart, R.; Montanes, N.; Quiles-Carrillo, L. Kinetic Analysis of the Curing Process of Biobased Epoxy Resin from Epoxidized Linseed Oil by Dynamic Differential Scanning Calorimetry. Polymers 2021, 13, 1279. [Google Scholar] [CrossRef]
- Vyazovkin, S. Thermal analysis. Anal. Chem. 2006, 78, 3875–3886. [Google Scholar] [CrossRef]
- Kamal, M.R. Thermoset characterization for moldability analysis. Polym. Eng. Sci. 1974, 14, 231–239. [Google Scholar] [CrossRef]
- Khanna, U.; Chanda, M. Kinetics of Anhydride Curing of Isophthalic Diglycidyl Ester Using Differential Scanning Calorimetry. J. Appl. Polym. Sci. 1993, 49, 319–329. [Google Scholar] [CrossRef]
- Fraga, F.; Soto, V.; Rodríguez-Núñez, E.; Martínez-Ageitos, J.; Rodríguez, V. Cure kinetic of the epoxy network diglycidyl ether of bisphenol A (BADGE n = 0)/Amantidine. J. Therm. Anal. Calorim. 2007, 87, 97–100. [Google Scholar] [CrossRef]
- Barakat, A.; Al Ghazal, M.; Fono Tamo, R.S.; Phadatare, A.; Unser, J.; Hagan, J.; Vaidya, U. Development of a Cure Model for Unsaturated Polyester Resin Systems Based on Processing Conditions. Polymers 2024, 16, 2391. [Google Scholar] [CrossRef]
- Rizzo, G.; Saitta, L.; Dattilo, S.; Tosto, C.; Pergolizzi, E.; Ivankovic, A.; Cicala, G. Thermomechanical Characterization of an Unsaturated Polyester Vitrimer Synthesized Using a Titanium Transesterification Catalyst. ACS Appl. Polym. Mater. 2023, 5, 8326–8338. [Google Scholar] [CrossRef]
- Farsane, M.; Lhasnaoui, S.; Anouar, A.; Dagdag, S.; Bouzziri, M. A Review of Measuring the Gelation Time in Unsaturated Polyester Resins. Mater. Tehnol. 2022, 56, 323–329. [Google Scholar] [CrossRef]
- Yang, Y.; Suspene, L. Curing of Unsaturated Polyester Resins: Viscosity Studies and Simulations in Pre-Gel State. Polym. Eng. Sci. 1991, 31, 321–332. [Google Scholar] [CrossRef]
- Vilas, J.; Laza, J.; Garay, M.; Rodríguez, M.; León, L. Unsaturated Polyester Resins Cure: Kinetic, Rheologic, and Mechanical-Dynamical Analysis. I. Cure Kinetics by DSC and TSR. J. Appl. Polym. Sci. 2001, 79, 447–457. [Google Scholar] [CrossRef]
- Spasojevic, P.; Seslija, S.; Markovic, M.; Pantic, O.; Antic, K.; Spasojevic, M. Optimization of Reactive Diluent for Bio-Based Unsaturated Polyester Resin: A Rheological and Thermomechanical Study. Polymers 2021, 13, 2667. [Google Scholar] [CrossRef]
- Akbari, S.; Root, A.; Skrifvars, M.; Ramamoorthy, S.; Åkesson, D. Novel Bio-based Branched Unsaturated Polyester Resins for High-Temperature Applications. J. Polym. Environ. 2024, 32, 2031–2044. [Google Scholar] [CrossRef]
- Malik, M.; Choudhary, V.; Varma, I. Current status of unsaturated polyester resins. J. Macromol. Sci.-Rev. Macromol. Chem. Phys. 2000, C40, 139–165. [Google Scholar] [CrossRef]
- Gao, Y.; Romero, P.; Zhang, H.; Huang, M.; Lai, F. Unsaturated polyester resin concrete: A review. Constr. Build. Mater. 2019, 228, 116709. [Google Scholar] [CrossRef]
- Kirshanov, K.; Toms, R.; Melnikov, P.; Gervald, A. Unsaturated Polyester Resin Nanocomposites Based on Post Consumer Polyethylene Terephthalate. Polymers 2022, 14, 1602. [Google Scholar] [CrossRef] [PubMed]
- Chabros, A.; Gawdzik, B. Methacrylate monomer as an alternative to styrene in typical polyester-styrene copolymers. J. Appl. Polym. Sci. 2019, 136, 47735. [Google Scholar] [CrossRef]
- Tank, R.; Gupta, D. Modification of styrene-divinyl benzene copolymers using monoacrylates as ter-monomer. J. Porous Mater. 2009, 16, 387–392. [Google Scholar] [CrossRef]
- Burkeyev, M.; Kovaleva, A.; Plocek, J.; Tazhbayev, E.; Burkeyeva, G.; Bolatbai, A.; Davrenbekov, S. Synthesis and Properties of Poly(Propylene Glycol Maleate Phthalate)-Styrene Copolymers as a Base of Composite Materials. Russ. J. Appl. Chem. 2018, 91, 1742–1749. [Google Scholar] [CrossRef]
- Burkeev, M.; Zhunissova, M.; Tazhbayev, Y.; Fomin, V.; Sarsenbekova, A.; Burkeyeva, G.; Kazhmuratova, A.; Zhumagalieva, T.; Zhakupbekova, E.; Khamitova, T. Influence of RAFT Agent on the Mechanism of Copolymerization of Polypropylene Glycol Maleinate with Acrylic Acid. Polymers 2022, 14, 1884. [Google Scholar] [CrossRef]
- Burkeev, M.; Kudaibergen, G.; Tazhbayev, Y.; Hranicek, J.; Burkeyeva, G.; Sarsenbekova, A. Synthesis and investigation of copolymer properties on the basis of poly(ethylene glycol) fumarate and methacrylic acid. Bull. Univ. Karaganda–Chem. 2019, 93, 32–38. [Google Scholar] [CrossRef]
- Burkeyev, M.; Kovaleva, A.; Burkeyeva, G.; Tazhbayev, Y.; Plocek, J. Polypropylene Glycol Maleate Phthalate Terpolymerization with Acrylamide and Acrylic Acid. Polym. Korea 2020, 44, 123–131. [Google Scholar] [CrossRef]
- Burkeyeva, G.; Kovaleva, A.; Tazhbayev, Y.; Ibrayeva, Z.; Zhaparova, L. Investigation of Curing Process and Thermal Behavior of Copolymers Based on Polypropylene Glycol Fumarate and Acrylic Acid Using the Methods of DSC and TGA. Polymers 2023, 15, 3753. [Google Scholar] [CrossRef] [PubMed]
- Jao, C.; Mao, H.; Shiu, J.; Chen, Y.; Chu, R.; Chen, C. Influence of Diol Structure on the Mechanical and Thermal Properties of Bio-Based UV-Curable Unsaturated Polyesters for 3D Printing. ACS Appl. Polym. Mater. 2025, 7, 11284–11299. [Google Scholar] [CrossRef]
- Li, L.; Cao, X.; Lee, L. Effect of dual-initiator on low temperature curing of unsaturated polyester resins. Polymer 2004, 45, 6601–6612. [Google Scholar] [CrossRef]
- Chirayil, C.; Mathew, L.; Hassan, P.; Mozetic, M.; Thomas, S. Rheological Behaviour of Nanocellulose Reinforced Unsaturated Polyester Nanocomposites. Int. J. Biol. Macromol. 2014, 69, 274–281. [Google Scholar] [CrossRef]
- Ewoldt, R.; Mckinley, G. Mapping Thixo-Elasto-Visco-Plastic Behavior. Rheol. Acta 2017, 56, 195–210. [Google Scholar] [CrossRef]
- Park, E.; Song, K. Rheological Evaluation of Petroleum Jelly as a Base Material in Ointment and Cream Formulations: Steady Shear Flow Behavior. Arch. Pharmacal Res. 2010, 33, 141–150. [Google Scholar] [CrossRef]
- Kuzdzal, E.; Cichy, B.; Kicko-Walczak, E.; Rymarz, G. Rheological and Fire Properties of a Composite of Unsaturated Polyester Resin and Halogen-Free Flame Retardants. J. Appl. Polym. Sci. 2017, 134, 44371. [Google Scholar] [CrossRef]
- Oleksy, M.; Galina, H. Thixotropic Unsaturated Polyester Resins of Prolonged Durability with Modified Smectites Added. Polimery 2000, 45, 541–547. [Google Scholar] [CrossRef]
- Sharma, S.; Shankar, V.; Joshi, Y. Viscoelasticity and Rheological Hysteresis. J. Rheol. 2023, 67, 139–155. [Google Scholar] [CrossRef]
- Wang, Y.; Ewoldt, R. Thixotropy, Antithixotropy, And Viscoelasticity in Hysteresis. J. Rheol. 2023, 67, 1199–1219. [Google Scholar] [CrossRef]
- Bhattacharyya, T.; Jacob, A.; Petekidis, G.; Joshi, Y. On The Nature of Flow Curve and Categorization of Thixotropic Yield Stress Materials. J. Rheol. 2023, 67, 461–477. [Google Scholar] [CrossRef]
- Si, J.; Li, Y.; Yu, X. Curing behavior and mechanical properties of an eco-friendly cold-mixed epoxy asphalt. Mater. Struct. 2019, 52, 81. [Google Scholar] [CrossRef]
- Ghriga, M.; Lebouachera, S.; Drouiche, N.; Grassl, B. Investigating the viscoelastic behavior of partially hydrolyzed polyacrylamide/polyethylenimine mixtures. J. Polym. Res. 2021, 28, 275. [Google Scholar] [CrossRef]
- Zlatanic, A.; Dunjic, B.; Djonlagic, J. Rheological study of the copolymerization reaction of acrylate-terminated unsaturated copolyesters with styrene. Macromol. Chem. Phys. 1999, 200, 2048–2058. [Google Scholar] [CrossRef]
- Macosko, C.W. Rheology: Principles, Measurements, and Applications; Wiley-VCH: New York, NY, USA, 1994; pp. 91–98. Available online: https://www.wiley.com/Rheology%3A+Principles%2C+Measurements%2C+and+Applications-p-9780471185758 (accessed on 27 September 2025).
- Dua, S.; Mccullough, R.; Palmese, G. Copolymerization Kinetics of Styrene/Vinyl-Ester Systems: Low Temperature Reactions. Polym. Compos. 1999, 20, 379–391. [Google Scholar] [CrossRef]
- Kamal, M.R.; Sourour, S. Kinetics and thermal characterization of thermoset cure. Polym. Eng. Sci. 1973, 13, 59–64. [Google Scholar] [CrossRef]
- Bruce Prime, R. Thermosets. In Thermal Characterization of Polymeric Materials; Turi, E.A., Ed.; Academic Press: New York, NY, USA, 1981; Volume 5, pp. 465–511. [Google Scholar] [CrossRef]
- Abenojar, J.; Encinas, N.; del Real, J.; Martínez, M. Polymerization kinetics of boron carbide/epoxy composites. Thermochim. Acta 2014, 575, 144–150. [Google Scholar] [CrossRef]
- Bashir, M. Cure Kinetics of Commercial Epoxy-Amine Products with Iso-Conversional Methods. Coatings 2023, 13, 592. [Google Scholar] [CrossRef]
- Teil, H.; Page, S.; Michaud, V.; Månson, J. TTT-cure diagram of an anhydride-cured epoxy system including gelation, vitrification, curing kinetics model, and monitoring of the glass transition temperature. J. Appl. Polym. Sci. 2004, 93, 1774–1787. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Achilias, D.; Fernandez-Francos, X.; Galukhin, A.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for analysis of thermal polymerization kinetics. Thermochim. Acta 2022, 714, 179243. [Google Scholar] [CrossRef]
- Khanna, U.; Chanda, M. A Kinetic Scheme for Anhydride Curing of Diglycidyl Ester with Tertiary Amine as Catalyst. J. Appl. Polym. Sci. 1993, 50, 1635–1644. [Google Scholar] [CrossRef]
- Simitzis, J.; Zoumpoulakis, L.; Soulis, S. DSC Curing Study of Catalytically Synthesized Maleic-Acid-Based Unsaturated Polyesters. Polym. Int. 2002, 51, 308–318. [Google Scholar] [CrossRef]
- Grunden, B.; Sung, C. Cure characterization of unsaturated polyester resin by near-IR and mid-IR spectroscopy. Macromolecules 2003, 36, 3166–3173. [Google Scholar] [CrossRef]
- Kicko-Walczak, E. Studies on the mechanism of thermal decomposition of unsaturated polyester resins with reduced flammability. Polym. Polym. Compos. 2004, 12, 127–134. [Google Scholar] [CrossRef]
- Shih, Y.; Jeng, R. Thermal degradation behaviour and kinetic analysis of unsaturated polyester-based composites and IPNs by conventional and modulated thermogravimetric analysis. Polym. Degrad. Stab. 2006, 91, 823–831. [Google Scholar] [CrossRef]
- Chung, A.; Tavsanli, B.; Gillies, E. Degradation of oligo[poly(ethylene glycol) fumarate] hydrogels through stimulus-mediated pendent group cyclization. Eur. Polym. J. 2023, 193, 112080. [Google Scholar] [CrossRef]
- Venkatesh, M.; Ravi, P.; Tewari, S. Isoconversional Kinetic Analysis of Decomposition of Nitroimidazoles: Friedman method vs Flynn-Wall-Ozawa Method. J. Phys. Chem. A 2013, 117, 10162–10169. [Google Scholar] [CrossRef]
- Mironi-Harpaz, I.; Narkis, M.; Siegmann, A. Peroxide crosslinking of a styrene-free unsaturated polyester. J. Appl. Polym. Sci. 2007, 105, 885–892. [Google Scholar] [CrossRef]
- Sbirrazzuoli, N. Is the Friedman method applicable to transformations with temperature dependent reaction heat? Macromol. Chem. Phys. 2007, 208, 1592–1597. [Google Scholar] [CrossRef]
- Farjas, J.; Roura, P. Exact analytical solution for the Kissinger equation: Determination of the peak temperature and general properties of thermally activated transformations. Thermochim. Acta 2014, 598, 51–58. [Google Scholar] [CrossRef]

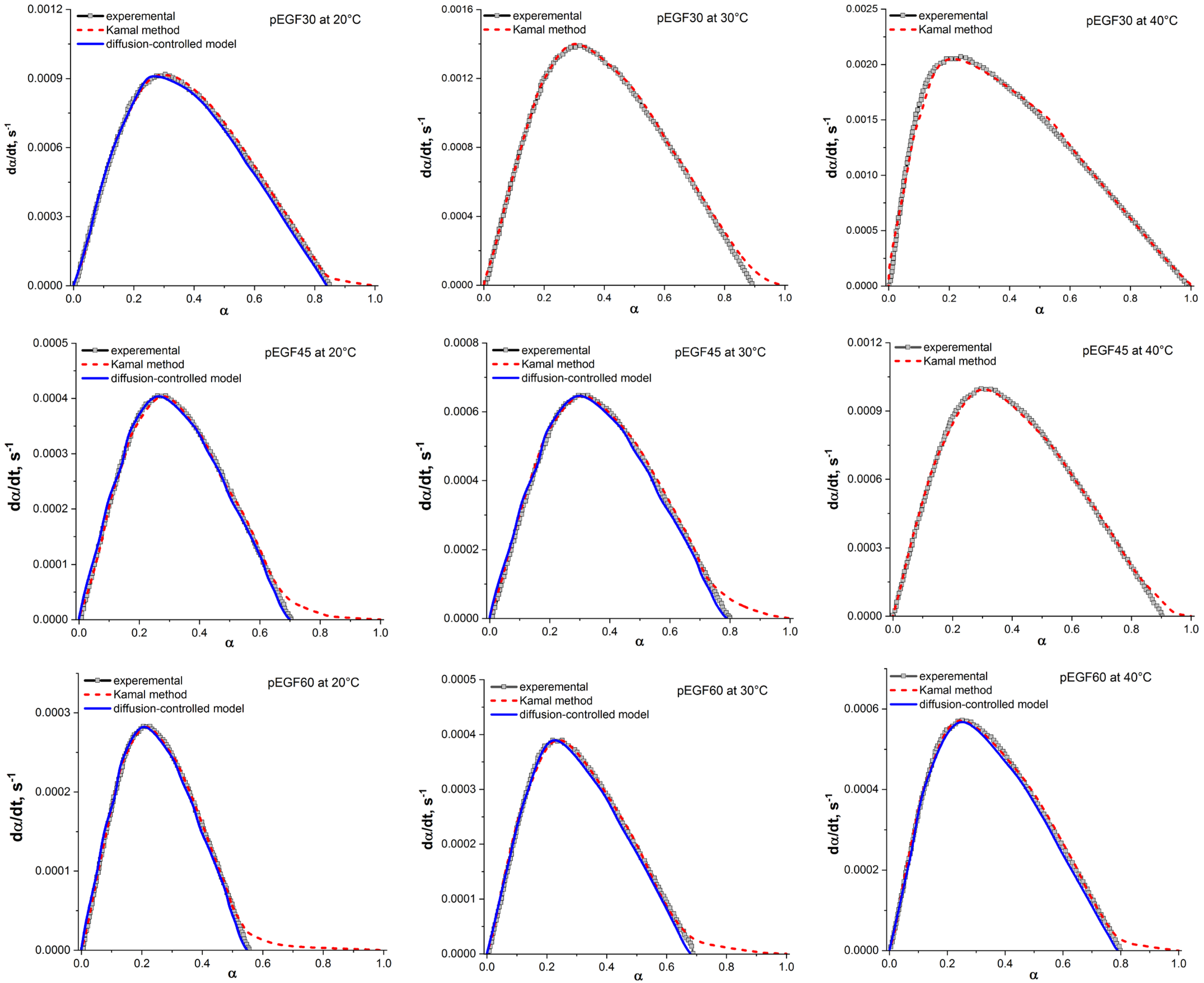
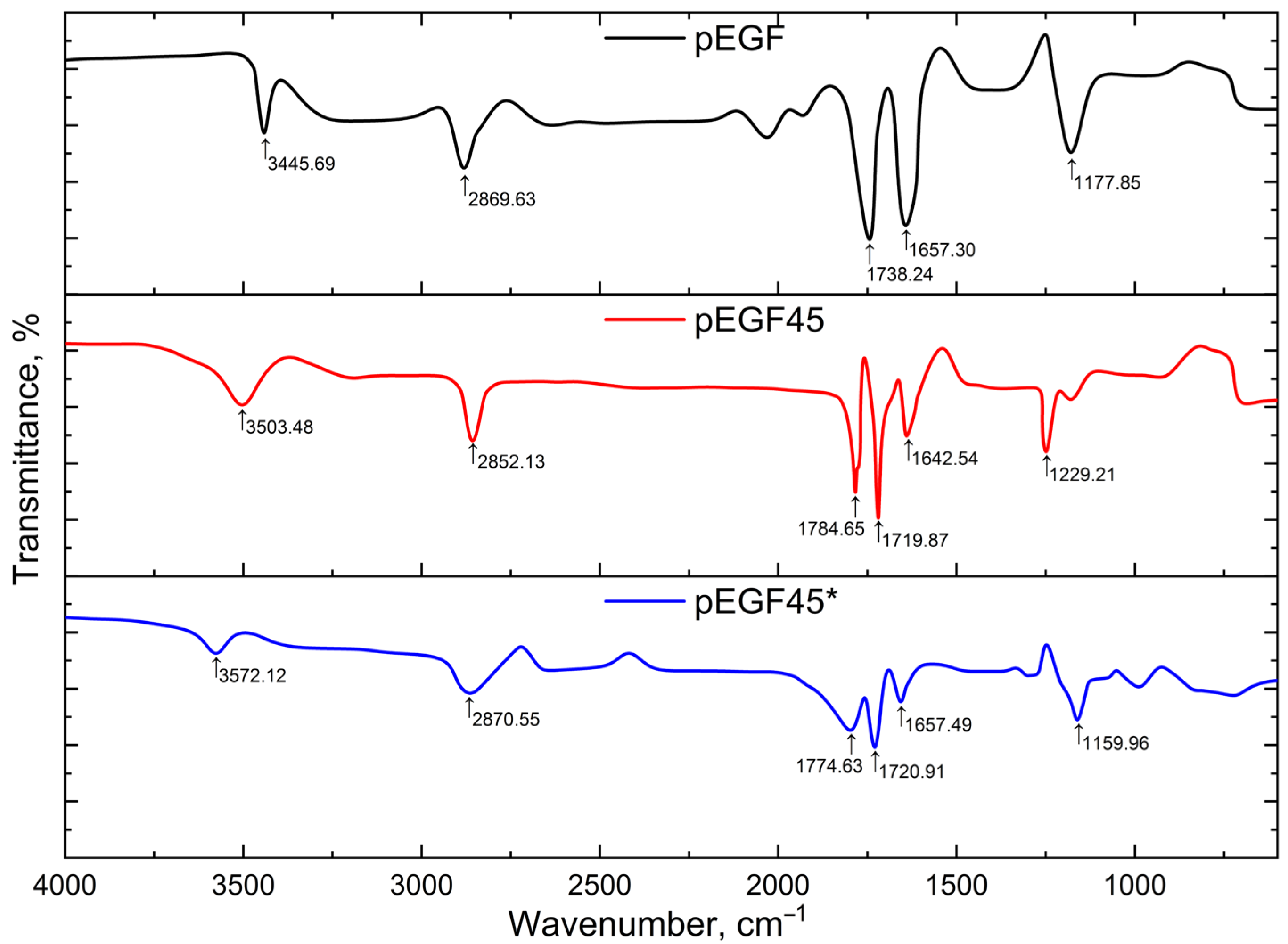


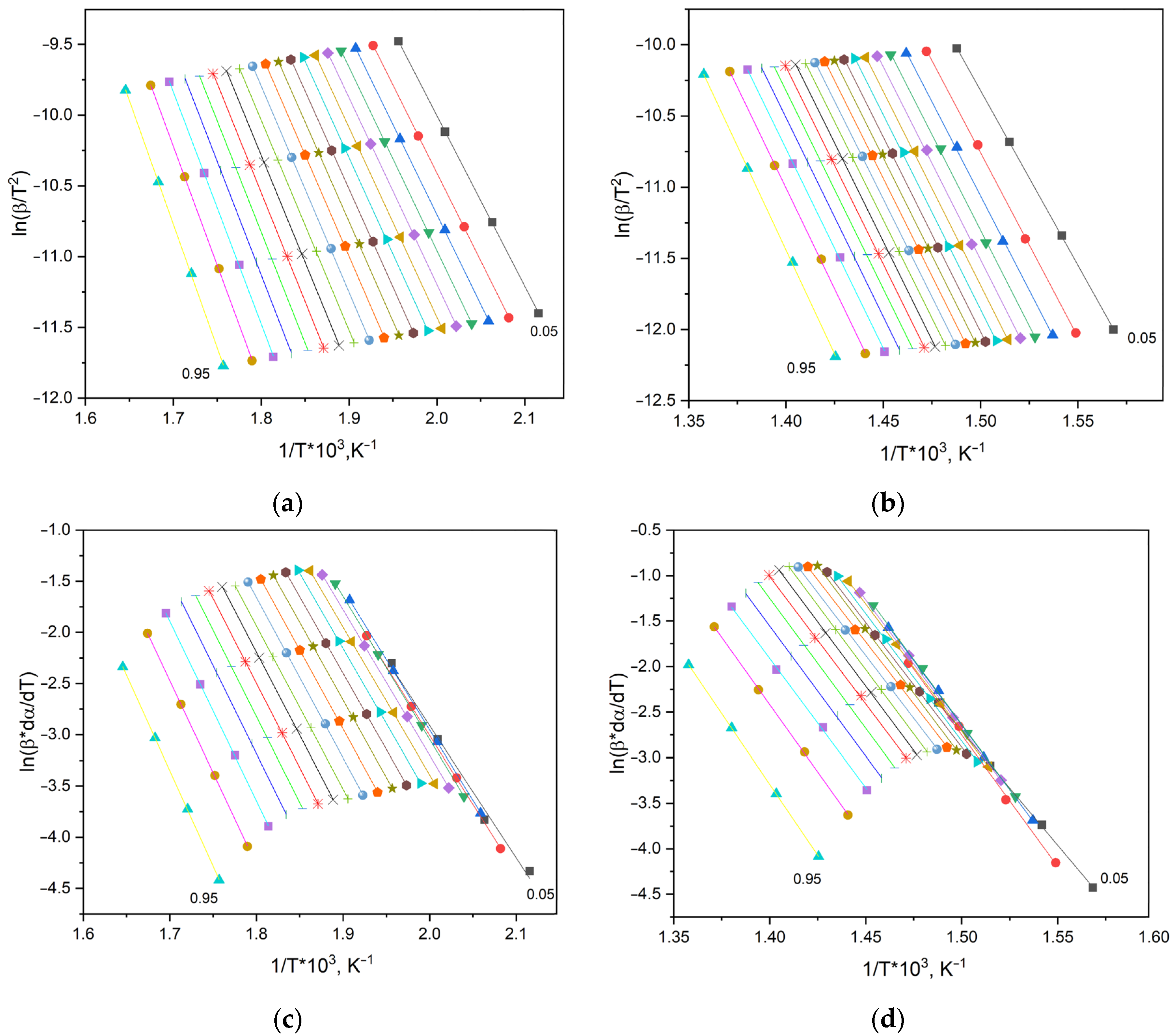
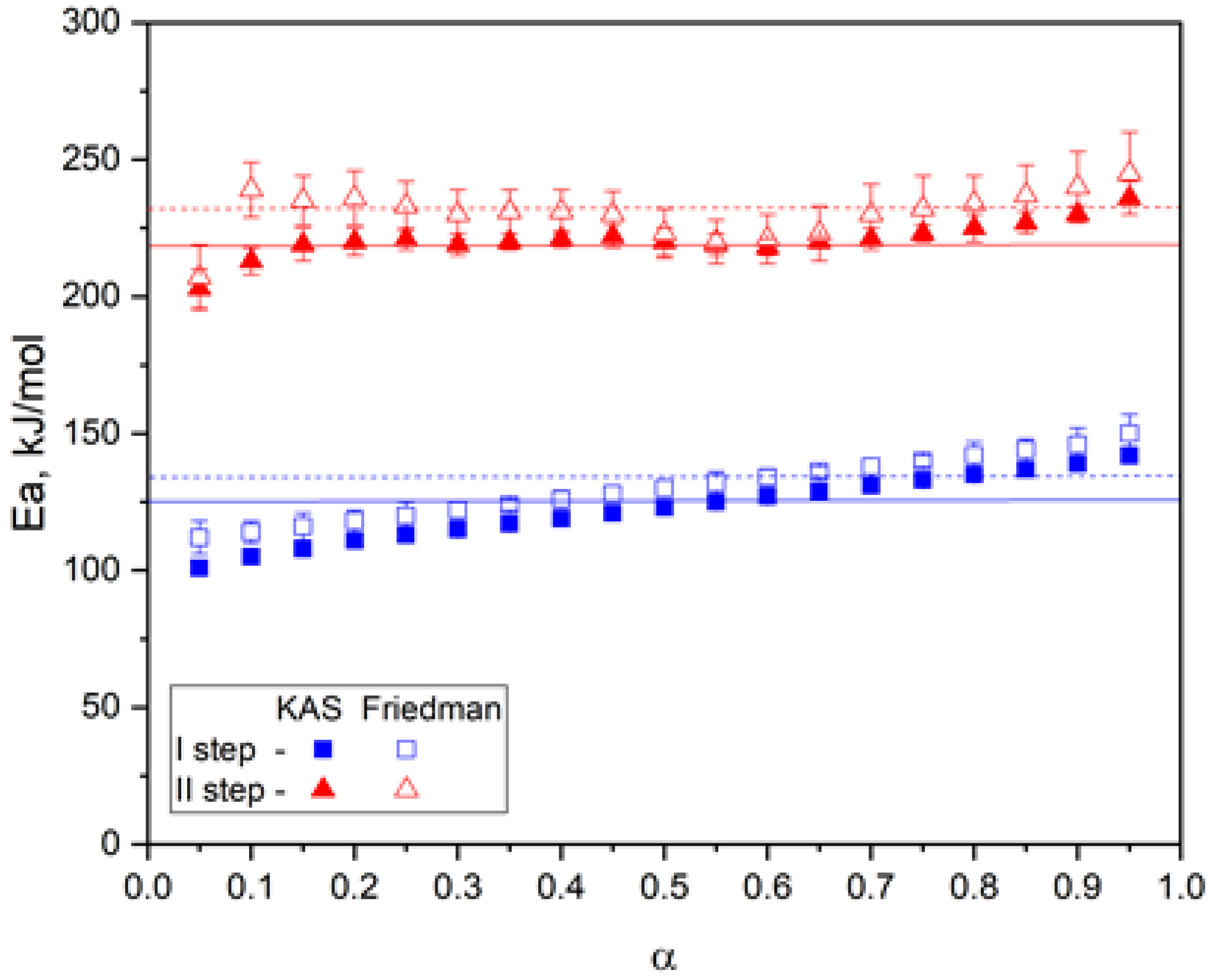
| Sample | Temperature, °C | , J/g | , J/g | , J/g |
|---|---|---|---|---|
| pEGF30 | 20 | 302.56 ± 6.96 | 52.90 ± 1.22 | 355.46 ± 7.21 |
| 30 | 340.44 ± 7.49 | 36.83 ± 0.74 | 371.27 ± 8.12 | |
| 40 | 386.12 ± 8.11 | 3.90 ± 0.08 | 390.02 ± 8.09 | |
| pEGF45 | 20 | 222.60 ± 5.12 | 95.56 ± 2.10 | 318.20 ± 7.21 |
| 30 | 264.18 ± 5.55 | 66.78 ± 1.34 | 330.96 ± 6.76 | |
| 40 | 310.85 ± 6.22 | 29.93 ± 0.60 | 340.78 ± 6.82 | |
| pEGF60 | 20 | 155.80 ± 3.90 | 130.23 ± 2.61 | 281.03 ± 6.41 |
| 30 | 199.92 ± 4.80 | 90.99 ± 1.82 | 290.91 ± 6.62 | |
| 40 | 245.96 ± 5.17 | 58.53 ± 1.17 | 304.49 ± 6.33 |
| Sample | Temperature, °C | max | ·10−6, s−1 | ·10−3, s−1 | R2 | , kJ/mol | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| pEGF30 | 20 | 0.85 | 2.003 | 1.863 | 0.599 | 1.112 | 1.711 | 24.7 | 0.83 | 0.9969 | 32.24 |
| 30 | 0.90 | 2.446 | 2.880 | 0.628 | 1.067 | 1.695 | - | - | - | ||
| 40 | 0.99 | 2.908 | 4.341 | 0.669 | 1.008 | 1.677 | - | - | - | ||
| pEGF45 | 20 | 0.70 | 1.407 | 0.789 | 0.469 | 0.953 | 1.422 | 22.3 | 0.67 | 0.9908 | 35.89 |
| 30 | 0.80 | 1.899 | 1.301 | 0.500 | 0.891 | 1.391 | 25.7 | 0.78 | 0.9959 | ||
| 40 | 0.91 | 2.301 | 2.022 | 0.533 | 0.828 | 1.361 | - | - | - | ||
| pEGF60 | 20 | 0.55 | 0.821 | 0.299 | 0.277 | 0.794 | 1.071 | 20.9 | 0.50 | 0.9926 | 40.01 |
| 30 | 0.69 | 1.198 | 0.519 | 0.330 | 0.735 | 1.065 | 22.8 | 0.66 | 0.9939 | ||
| 40 | 0.81 | 1.601 | 0.909 | 0.371 | 0.688 | 1.059 | 27.1 | 0.79 | 0.9971 |
| Sample | Curing Temperature T, °C | Temperature Range T, °C | Residue, % | |
|---|---|---|---|---|
| I Stage | II Stage | |||
| pEGF45 | 20 | 170~360 | 361~530 | 14.5 |
| 30 | 175~363 | 364~544 | 14.7 | |
| 40 | 177~365 | 366~550 | 15.1 | |
| Sample | p-EGF, mol.% | AA, mol.% | BPO, % | DMA, % |
|---|---|---|---|---|
| pEGF30 | 30.3 | 69.7 | 1 | 0.15 |
| pEGF45 | 45.4 | 54.6 | 1 | 0.15 |
| pEGF60 | 60.5 | 39.5 | 1 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burkeyeva, G.; Kovaleva, A.; Ibrayeva, Z.; Havlicek, D.; Minayeva, Y.; Omasheva, A.; Zhakupbekova, E.; Nurmaganbetova, M. Thermosetting Resins Based on Poly(Ethylene Glycol Fumarate) and Acrylic Acid: Rheological and Thermal Analysis. Molecules 2025, 30, 4020. https://doi.org/10.3390/molecules30194020
Burkeyeva G, Kovaleva A, Ibrayeva Z, Havlicek D, Minayeva Y, Omasheva A, Zhakupbekova E, Nurmaganbetova M. Thermosetting Resins Based on Poly(Ethylene Glycol Fumarate) and Acrylic Acid: Rheological and Thermal Analysis. Molecules. 2025; 30(19):4020. https://doi.org/10.3390/molecules30194020
Chicago/Turabian StyleBurkeyeva, Gulsym, Anna Kovaleva, Zhansaya Ibrayeva, David Havlicek, Yelena Minayeva, Aiman Omasheva, Elmira Zhakupbekova, and Margarita Nurmaganbetova. 2025. "Thermosetting Resins Based on Poly(Ethylene Glycol Fumarate) and Acrylic Acid: Rheological and Thermal Analysis" Molecules 30, no. 19: 4020. https://doi.org/10.3390/molecules30194020
APA StyleBurkeyeva, G., Kovaleva, A., Ibrayeva, Z., Havlicek, D., Minayeva, Y., Omasheva, A., Zhakupbekova, E., & Nurmaganbetova, M. (2025). Thermosetting Resins Based on Poly(Ethylene Glycol Fumarate) and Acrylic Acid: Rheological and Thermal Analysis. Molecules, 30(19), 4020. https://doi.org/10.3390/molecules30194020






