HX-Linear and Nonlinear Optical Responsiveness of Rationally Designed Heteroleptic d8-Metallo-dithiolene Complexes
Abstract
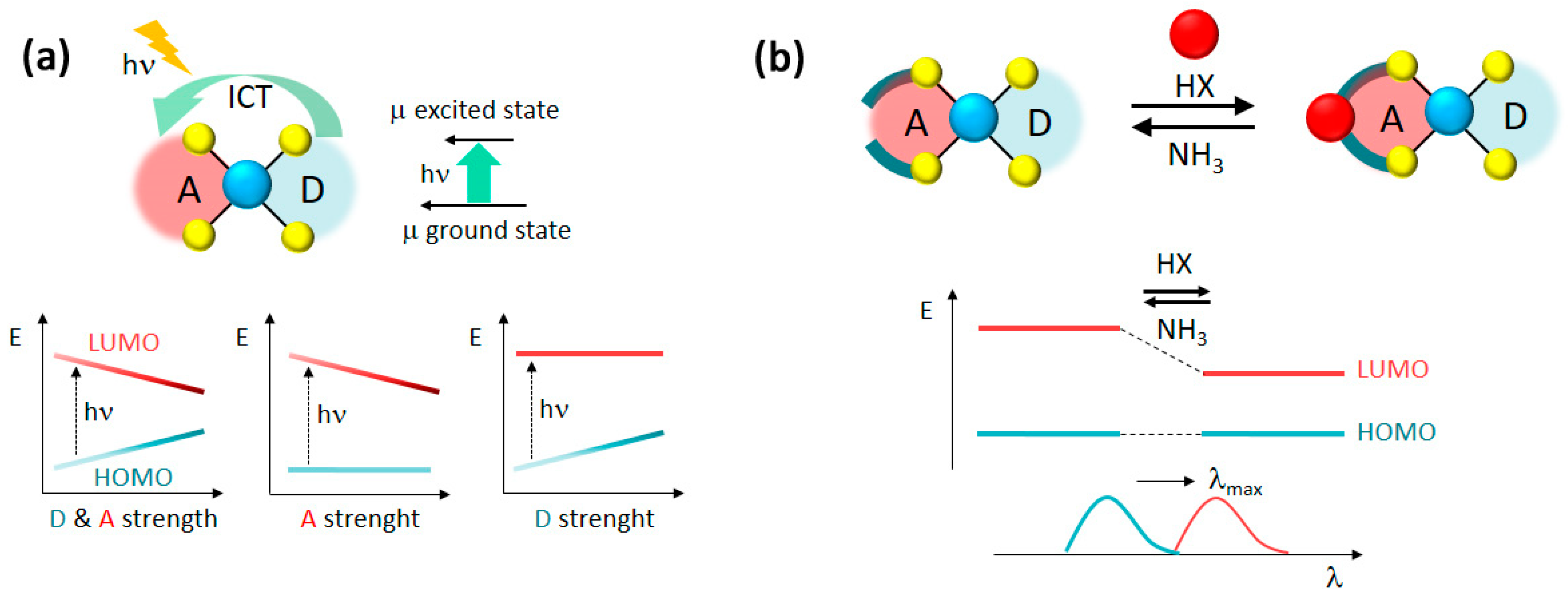
1. Introduction
2. Results and Discussion
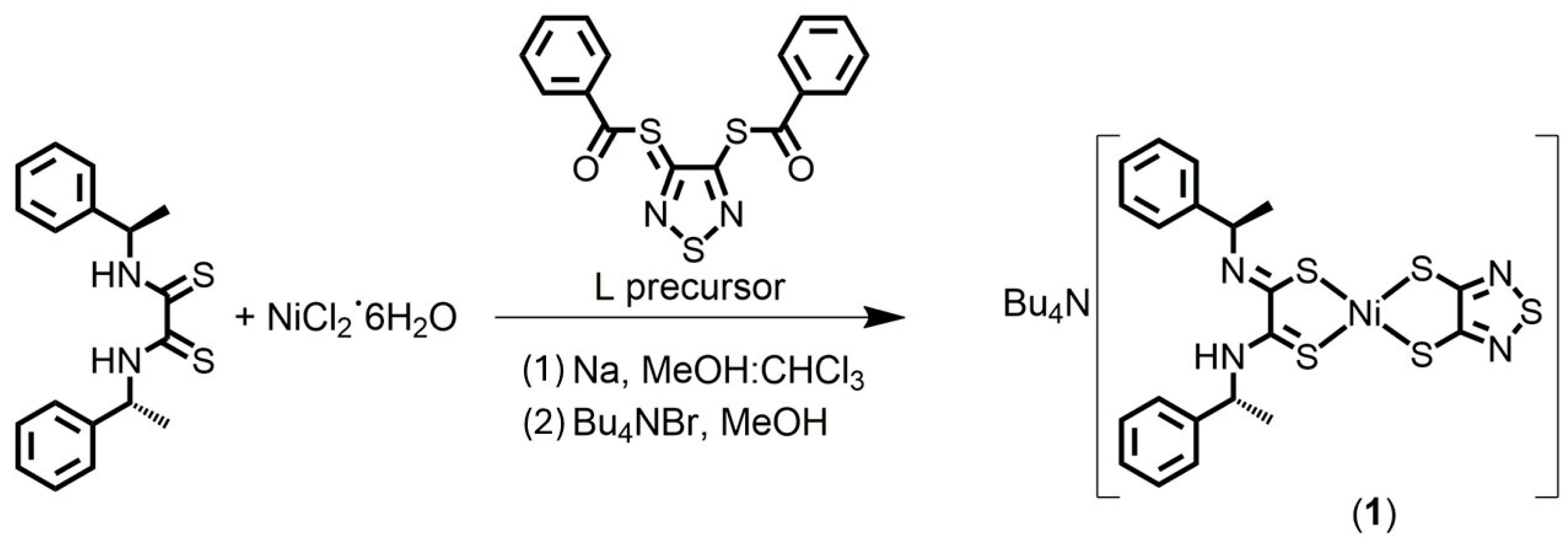
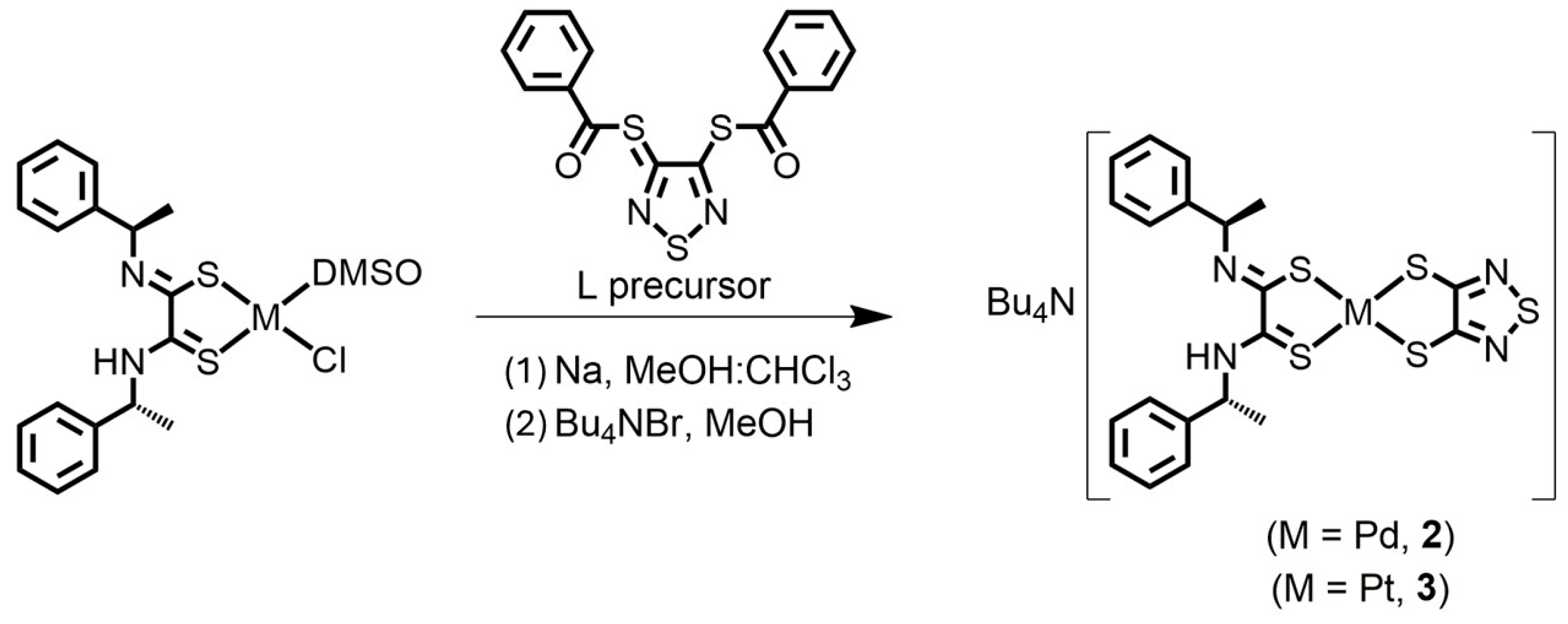
2.1. Preparation of Complexes
2.2. Linear Optical Properties and DFT Calculations
2.3. Linear Optical Sensing
2.4. Nonlinear Optical Properties
3. Materials and Methods
3.1. Synthesis and Characterization of Products
3.1.1. Bu4N[Ni((R)-α-MBAdt)(tdas)] (1)
3.1.2. [Pd((R)-α-MBAdt)(DMSO)Cl]
3.1.3. Bu4N[Pd((R)-α-MBAdt)(tdas)] (2)
3.1.4. Bu4N[Pt((R)-α-MBAdt)(tdas)] (3)
3.2. Computational Studies
3.3. Cellulose Film Sensor Preparation and Characterization
3.4. Polymer Film: Preparation and Characterization
3.5. Corona-Wire Poling Setup
3.6. Maker Fringe Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hong, T.; Choi, Y.K.; Lim, T.; Seo, K.; Jeong, S.-M.; Ju, S. Elastic Halochromic Fiber as a Reversible pH Sensor. Adv. Mater. Technol. 2021, 6, 2001058. [Google Scholar] [CrossRef]
- Picci, G.; Montis, R.; Gilchrist, A.M.; Gale, P.A.; Caltagirone, C. Fluorescent and colorimetric sensors for anions: Highlights from 2020 to 2022. Coord. Chem. Rev. 2024, 501, 215561. [Google Scholar] [CrossRef]
- Mansha, M.; Khan, S.A.; Aziz, M.A.; Khan, A.Z.; Ali, S.; Khan, M. Optical Chemical Sensing of Iodide Ions: A Comprehensive Review for the Synthetic Strategies of Iodide Sensing Probes, Challenges, and Future Aspects. Chem. Rec. 2022, 22, e202200059. [Google Scholar] [CrossRef]
- Li, Z.; Hou, S.; Zhang, H.; Song, Q.; Wang, S.; Guo, H. Recent advances in fluorescent and colorimetric sensing for volatile organic amines and biogenic amines in food. Adv. Agrochem 2023, 2, 79–87. [Google Scholar] [CrossRef]
- Maity, A.; Ghosh, B. Fast response paper based visual color change gas sensor for efficient ammonia detection at room temperature. Sci. Rep. 2018, 8, 16851. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.D.; Norton, A.E.; Hart, R.T., Jr.; Abdolmaleki, M.K.; Krause, J.A.; Connick, W.B. Between red and yellow: Evidence of intermediates ina vapochromic Pt(II) salt. Chem. Commun. 2013, 49, 9161. [Google Scholar] [CrossRef]
- Wadas, T.J.; Wang, Q.-M.; Kim, Y.-J.; Flaschenreim, C.; Blanton, T.N.; Eisenberg, R. Vapochromism and Its Structural Basis in a Luminescent Pt(II) Terpyridine−Nicotinamide Complex. J. Am. Chem. Soc. 2004, 126, 16841–16849. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Schneider, J.; Brennessel, W.W.; Eisenberg, R. Synthesis and Structural Characterization of a New Vapochromic Pt(II) Complex Based on the 1-Terpyridyl-2,3,4,5,6-pentaphenylbenzene (TPPPB) Ligand. Inorg. Chem. 2008, 47, 69–77. [Google Scholar] [CrossRef]
- Kumpfer, J.R.; Taylor, S.D.; Connick, W.B.; Rowan, S.J. Vapochromic and mechanochromic films from square-planar platinum complexes in polymethacrylates. J. Mater. Chem. 2012, 22, 14196. [Google Scholar] [CrossRef]
- Norton, A.E.; Abdolmaleki, M.K.; Andriot, L.; Cashen, C.; Krause, J.A.; Connick, W.B.; Chatterjee, S. Optical sensing of aqueous nitrate anion by a platinum(II) triimine salt based solid state material. Chem. Commun. 2022, 58, 12160. [Google Scholar] [CrossRef]
- Kundu, S.; Egboluche, T.K.; Hossain, M.A. Urea- and Thiourea-Based Receptors for Anion Binding. Acc. Chem. Res. 2023, 56, 1320–1329. [Google Scholar] [CrossRef]
- Fabbrizzi, L.; Poggi, A. Anion recognition by coordinative interactions: Metal–amine complexes as receptors. Chem. Soc. Rev. 2013, 42, 1681. [Google Scholar] [CrossRef] [PubMed]
- Rosace, G.; Giuffrida, G.; Saitta, M.; Guglielmo, G.; Campagna, S.; Lanza, S. Luminescence Properties of Platinum(II) Dithiooxamide Compounds. Inorg. Chem. 1996, 35, 6816–6822. [Google Scholar] [CrossRef] [PubMed]
- Nastasi, F.; Puntoriero, F.; Palmeri, N.; Cavallaro, S.; Campagna, S.; Lanza, S. Solid-state luminescence switching of platinum(II) dithiooxamide complexes in the presence of hydrogen halide and amine gases. Chem. Commun. 2007, 45, 4740–4742. [Google Scholar] [CrossRef]
- Giannetto, A.; Puntoriero, F.; Barattucci, A.; Lanza, S.; Campagna, S. Tight-contact ion pairs involving Pt(II) dithiooxamide complexes: The acid-base reactions between hydrohalogenated ion-paired complexes and pyridine. Inorg. Chem. 2009, 48, 10397–10404. [Google Scholar] [CrossRef] [PubMed]
- Giannetto, A.; Cordaro, M.; Campagna, S.; Lanza, S. Metal Complexes as Self-Indicating Titrants for Acid−Base Reactions in Chloroform. Inorg. Chem. 2018, 57, 2175–2183. [Google Scholar] [CrossRef]
- Artizzu, F.; Espa, D.; Marchiò, L.; Pilia, L.; Serpe, A.; Deplano, P. Progress and Perspectives on strategies to control photochemical properties in Metallo-Dithiolene Donor-Acceptor systems. Inorg. Chim. Acta 2022, 531, 120731. [Google Scholar] [CrossRef]
- Attar, S.; Espa, D.; Artizzu, F.; Pilia, L.; Serpe, A.; Pizzotti, M.; Di Carlo, G.; Marchiò, L.; Deplano, P. Optically Multiresponsive Heteroleptic Platinum-dithiolene Complex with Proton Switchable Properties. Inorg. Chem. 2017, 56, 6763–6767. [Google Scholar] [CrossRef]
- Attar, S.S.; Artizzu, F.; Marchiò, L.; Espa, D.; Pilia, L.; Casula, M.F.; Serpe, A.; Pizzotti, M.; Orbelli-Biroli, A.; Deplano, P. Uncommon Optical Properties and Silver-Responsive Turn-Off/On Luminescence in a PtII Heteroleptic Dithiolene Complex. Chem. Eur. J. 2018, 24, 10503–10512. [Google Scholar] [CrossRef]
- Attar, S.S.; Marchiò, L.; Pilia, L.; Casula, M.F.; Espa, D.; Serpe, A.; Pizzotti, M.; Marinotto, D.; Deplano, P. Design of nickel donor–acceptor dithiolenes for 2nd order nonlinear optics: An experimental and computational study. New J. Chem. 2019, 43, 12570–12579. [Google Scholar] [CrossRef]
- Attar, S.S.; Pilia, L.; Espa, D.; Artizzu, F.; Serpe, A.; Pizzotti, M.; Marinotto, D.; Marchiò, L.; Deplano, P. An insight into the properties of heteroleptic metal dithiolenes: Multi-Stimuli Responsive Luminescence, Chromism and Nonlinear optics. Inorg. Chem. 2021, 60, 9332–9344. [Google Scholar] [CrossRef]
- Zyss, J. Molecular Nonlinear Optics: Materials, Physics and Devices; Academic Press: Boston, MA, USA, 1994. [Google Scholar]
- Coe, B.J. Molecular Materials Possessing Switchable Quadratic Nonlinear Optical Properties. Chem. Eur. J. 1999, 5, 2464–2471. [Google Scholar] [CrossRef]
- Delaire, J.A.; Nakatani, K. Linear and Nonlinear Optical Properties of Photochromic Molecules and Materials. Chem. Rev. 2000, 100, 1817–1846. [Google Scholar] [CrossRef]
- Berkovic, G.; Krongauz, V.; Weiss, V. Spiropyrans and Spirooxazines for Memories and Switches. Chem. Rev. 2000, 100, 1741–1753. [Google Scholar] [CrossRef]
- Guerchais, V.; Ordronneau, L.; Le Bozec, H. Recent developments in the field of metal complexes containing photochromic ligands: Modulation of linear and nonlinear optical properties. Coord. Chem. Rev. 2010, 254, 2533–2545. [Google Scholar] [CrossRef]
- Castet, F.; Rodriguez, V.; Pozzo, J.-L.; Ducasse, L.; Plaquet, A.; Champagne, B. Design and Characterization of Molecular Nonlinear Optical Switches. Acc. Chem. Res. 2013, 46, 2656–2665. [Google Scholar] [CrossRef]
- Pielak, K.; Bondu, F.; Sanguinet, L.; Rodriguez, V.; Castet, F.; Champagne, B. Acido-triggered switching of the second-order nonlinear optical properties of a ferrocenyl-containing indolino-oxazolidine derivative. Dye. Pigment. 2019, 160, 641–646. [Google Scholar] [CrossRef]
- Bibi, T.; Jadoon, T.; Muhammad, S.; Ayub, K. Second-order NLO properties and two-state switching effects of transition metal redox complexes of iron and cobalt: A DFT study. J. Mol. Graph. Model. 2021, 107, 107975. [Google Scholar] [CrossRef] [PubMed]
- Bibia, T.; Jadoonab, T.; Ayub, K. Two state “ON–OFF” NLO switch based on coordination complexes of iron and cobalt containing isomeric ligand: A DFT study. RSC Adv. 2022, 12, 23204–23214. [Google Scholar] [CrossRef] [PubMed]
- Chavan, S.S.; Pawal, S.B.; Lolage, S.R.; Garadkar, K.M. Synthesis, spectroscopic characterization, luminescence and NLO properties of heterometallic M(II)-Ru(II) (M=Ni and Zn) hybrid complexes composed of coordination and organometallic sites. J. Organomet. Chem. 2017, 853, 18. [Google Scholar] [CrossRef]
- Chakrabarty, R.; Dutta, A.; Roy, S.; Das, G.; Ledoux-Rak, I.; Mondal, P.; Prasad, S.K.; Rao, D.S.S.; Bhattacharjee, C.R. Multifunctional Lanthanide Complexes: Mesomorphism, Photoluminescence and Second Order NLO Property. Chem. Select 2018, 3, 8245. [Google Scholar] [CrossRef]
- Colombo, A.; De Soricellis, G.; Dragonetti, C.; Fagnani, F.; Roberto, D.; Carboni, B.; Guerchais, V.; Roisnel, T.; Cocchi, M.; Fantacci, S.; et al. Introduction of a mesityl substituent on pyridyl rings as a facile strategy for improving the performance of luminescent 1,3-bis-(2-pyridyl)benzene platinum(ii) complexes: A springboard for blue OLEDs. J. Mater. Chem. C 2024, 12, 9702–9715. [Google Scholar] [CrossRef]
- Roberto, D.; Colombo, A.; Dragonetti, C.; Fagnani, F.; Cocchi, M.; Marinotto, D. A Novel Class of Cyclometalated Platinum(II) Complexes for Solution-Processable OLEDs. Molecules 2022, 27, 5171. [Google Scholar] [CrossRef] [PubMed]
- Msalmi, R.; Dammak, K.; Elleuch, S.; Hamdi, B.; Tozri, A.; Na, H. Optoelectronic, luminescence, and nonlinear properties of a non-centrosymmetric Cd(II)-based complex. J. Phys. Chem. Solids 2022, 163, 110567. [Google Scholar] [CrossRef]
- Tahmasbi, A.; Jafari, A.; Nikoo, A. Synthesis, characterization, and nonlinear optical properties of copper (II) ligand Schiff base complexes derived from 3-Nitrobenzohydrazide and benzyl. Sci. Rep. 2023, 13, 10988. [Google Scholar] [CrossRef] [PubMed]
- Becke, D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Hariharan, P.C.; Pople, J.A. The influence of polarization functions on molecular orbital hydrogenation energies. Theoret. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Xu, K.; Tang, Q.; Zhao, W.; Yu, X.; Yang, Y.; Yu, T.; Yuan, C. In situ growth of Co3O4@NiMoO4 composite arrays on alumina substrate with improved triethylamine sensing performance. Sens. Actuators B Chem. 2020, 302, 127154. [Google Scholar] [CrossRef]
- Espa, D.; Pilia, L.; Attar, S.; Serpe, A.; Deplano, P. Molecular engineering of heteroleptic metal-dithiolene complexes with optimized second-order NLO response. Inorg. Chim. Acta 2018, 470, 295–302. [Google Scholar] [CrossRef]
- Page, R.H.; Jurich, M.C.; Reck, B.; Sen, A.; Twieg, R.J.; Swalen, J.D.; Bjorklund, G.C.; Wilson, C.G. Electrochromic and optical waveguide studies of corona-poled electro-optic polymer films. J. Opt. Soc. Am. B 1990, 7, 1239–1250. [Google Scholar] [CrossRef]
- Mortazavi, M.A.; Knoesen, A.; Kowel, S.T.; Higgins, B.G.; Dienes, A. Second-harmonic generation and absorption studies of polymer−dye films oriented by corona-onset poling at elevated temperatures. J. Opt. Soc. Am. B 1989, 6, 733–741. [Google Scholar] [CrossRef]
- Colombo, A.; Dragonetti, C.; Marinotto, D.; Righetto, S.; Roberto, D.; Tavazzi, S.; Escadeillas, M.; Guerchais, V.; Le Bozec, H.; Boucekkine, A.; et al. Cyclometalated 4-Styryl-2-phenylpyridine Platinum(II) Acetylacetonate Complexes as Second-Order NLO Building Blocks for SHG Active Polymeric Films. Organometallics 2013, 32, 3890–3894. [Google Scholar] [CrossRef]
- D’Amato, R.; Furlani, A.; Colapietro, M.; Portalone, G.; Casalboni, M.; Falconieri, M.; Russo, M.V. Synthesis, characterisation and optical properties of symmetrical and unsymmetrical Pt(II) and Pd(II) bis-acetylides. Crystal structure of trans-[Pt(PPh3)2(CC–C6H5)(CC–C6H4NO2)]. J. Organomet. Chem. 2001, 627, 13–22. [Google Scholar] [CrossRef]
- Dragonetti, C.; Colombo, A.; Marinotto, D.; Righetto, S.; Roberto, D.; Valore, A.; Escadeillas, M.; Guerchais, V.; Le Bozec, H.; Boucekkine, A.; et al. Functionalized styryl Iridium(III) complexes as active second-order NLO chromophores and building blocks for SHG polymeric films. J. Organomet. Chem. 2014, 751, 568–572. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Thirumoorthy, K.; Dragonetti, C.; Marinotto, D.; Righetto, S.; Colombo, A.; Haukka, M.; Palanisami, N. Ferrocene–quinoxaline Y-shaped chromophores as fascinating second-order NLO building blocks for long lasting highly active SHG polymeric films. Dalton Trans. 2016, 45, 11939–11943. [Google Scholar] [CrossRef]
- Espa, D.; Pilia, L.; Marchiò, L.; Artizzu, F.; Di Carlo, G.; Marinotto, D.; Serpe, A.; Tessore, F.; Deplano, P. A nonlinear optical active polymer film based on Pd(II) dithione/dithiolate second-order NLO chromophores. Dalton Trans. 2016, 45, 17431–17438. [Google Scholar] [CrossRef]
- Singer, K.D.; Sohn, E.; King, L.A.; Gordon, K.M.; Katz, H.E.; Dirk, P.W. Second-order nonlinear-optical properties of donor-and acceptor-substituted aromatic compounds. J. Opt. Soc. Am. B 1989, 6, 1339–1350. [Google Scholar] [CrossRef]
- Hurd, R.N.; De La Mater, G.; McElheny, G.C.; Turner, R.J.; Wallingford, V.H. Preparation of Dithiooxamide Derivatives. J. Org. Chem. 1961, 26, 3980–3987. [Google Scholar] [CrossRef]
- Tomura, M.; Yamashita, Y. Synthesis, structure, and physical properties of novel component molecules with fused heterocycles for organic conductor. Synth. Met. 1997, 86, 1871–1872. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density Functional Theory of Atoms and Molecules; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian, version 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Miertuš, S.; Scrocco, E.; Tomasi, J. Electrostatic Interaction of a Solute with a Continuum. A Direct Utilization of ab initio Molecular Potentials for the Prevision of Solvent Effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Scalmani, G.; Frisch, M.J.; Mennucci, B.; Tomasi, J.; Cammi, R.; Barone, V. Geometries and properties of excited states in the gas phase and in solution: Theory and application of a time-dependent density functional theory polarizable continuum model. J. Chem. Phys. 2006, 124, 094107. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comp. Chem. 2003, 24, 669. [Google Scholar] [CrossRef]
- Thompson, M.A. ArgusLab, version 4.0.1; Planaria Software LLC: Seattle, WA, USA, 2021; Available online: http://www.arguslab.com/arguslab.com/ArgusLab.html (accessed on 6 March 2020).
- O’Boyle, N.M.; Tenderholt, A.L.; Langner, K.M. cclib: A library for package-independent computational chemistry algorithms. J. Comput. Chem. 2008, 29, 839–845. [Google Scholar] [CrossRef]
- Herman, W.N.; Hayden, L.M. Maker fringes revisited: Second harmonic generation from birefringent or absorbing materials. J. Opt. Soc. Am. B 1995, 12, 416–427. [Google Scholar] [CrossRef]

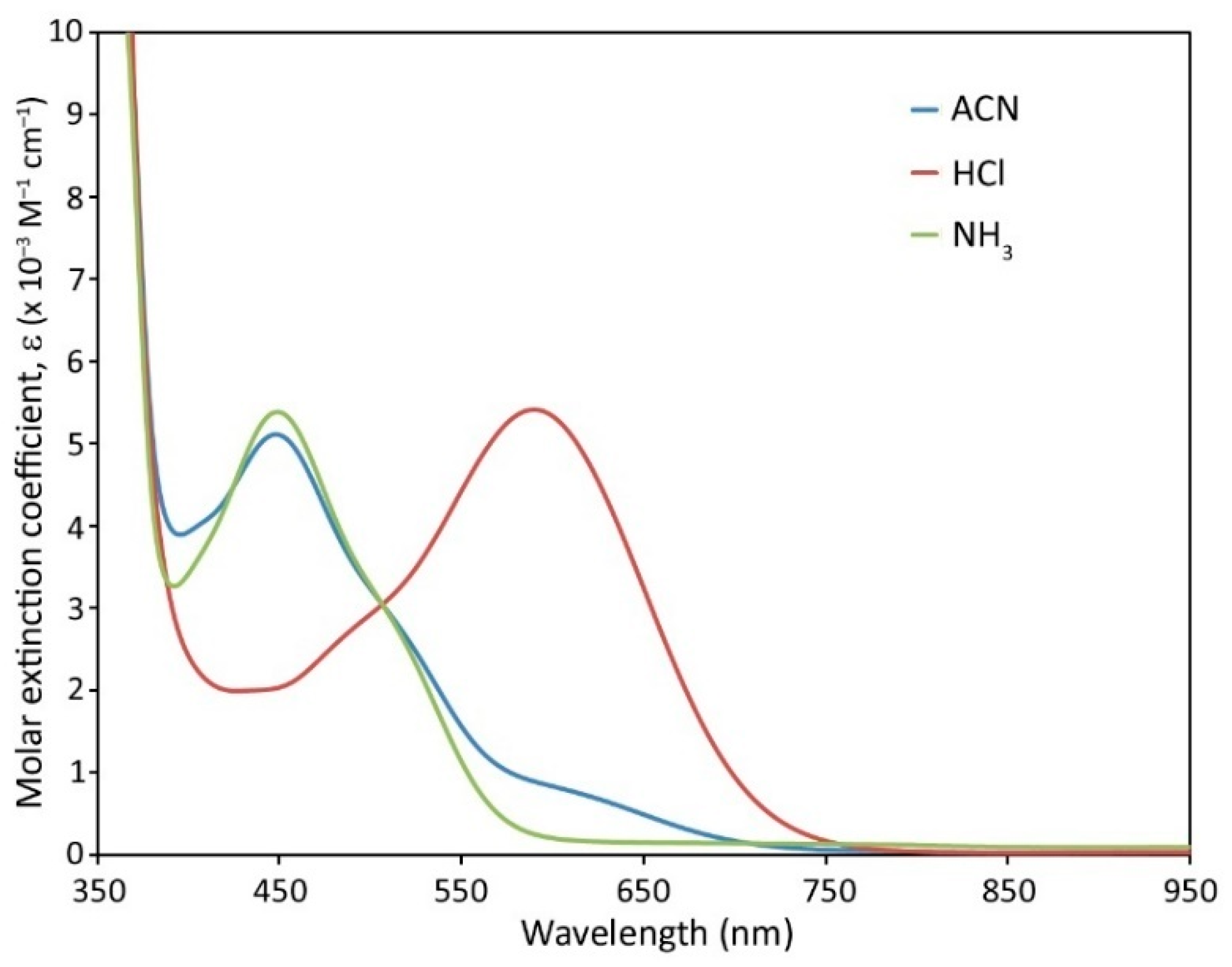



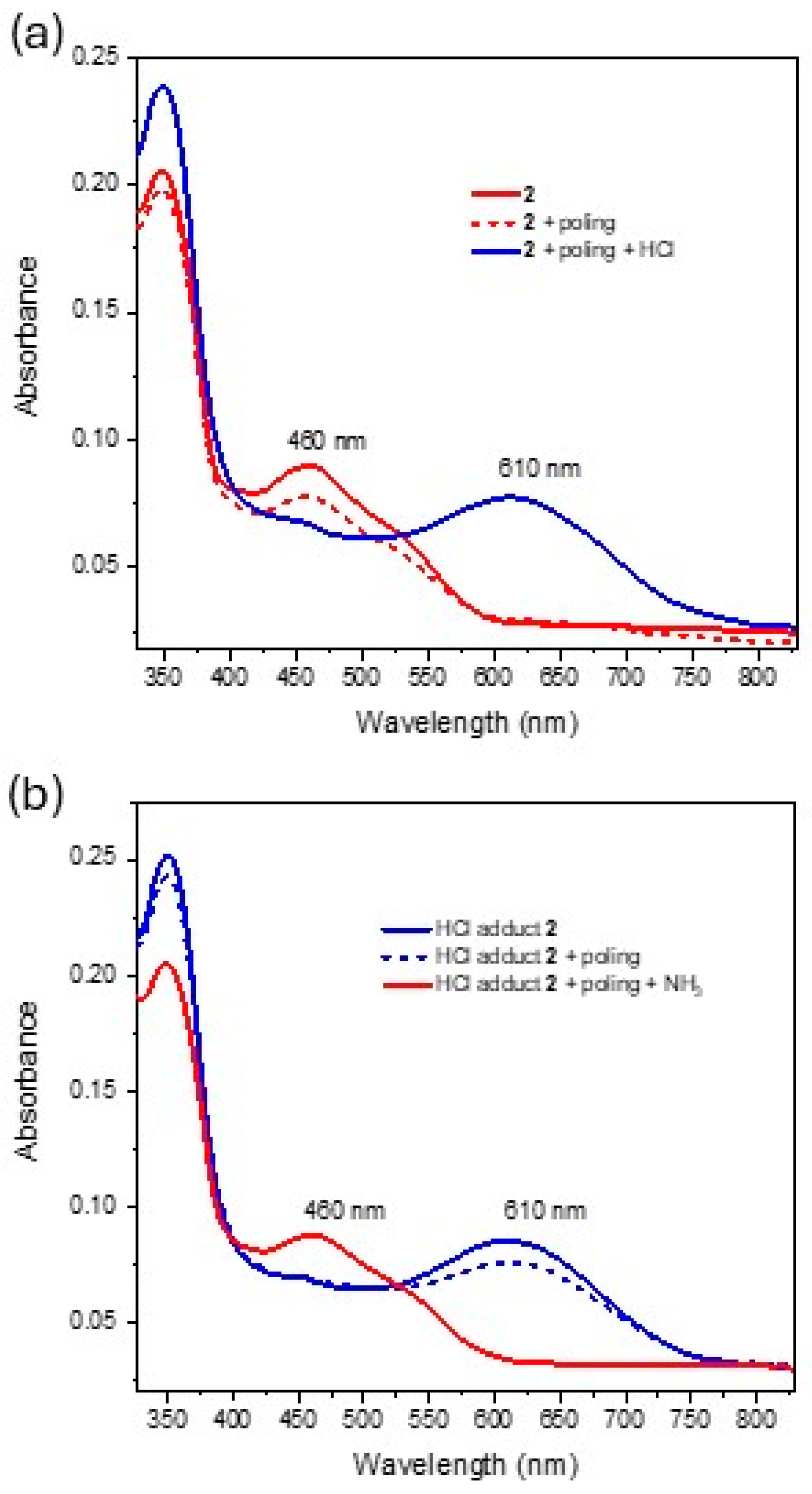
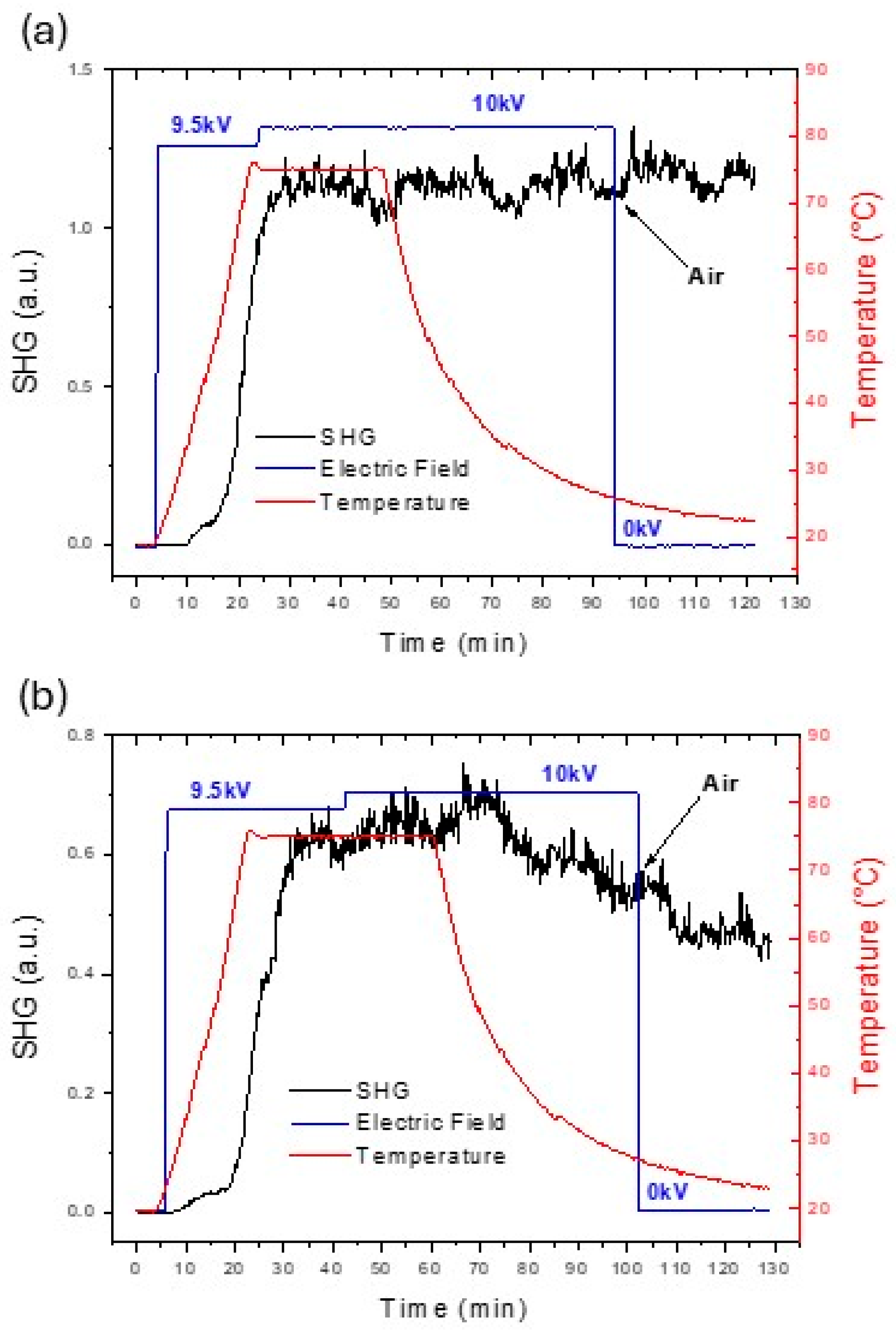
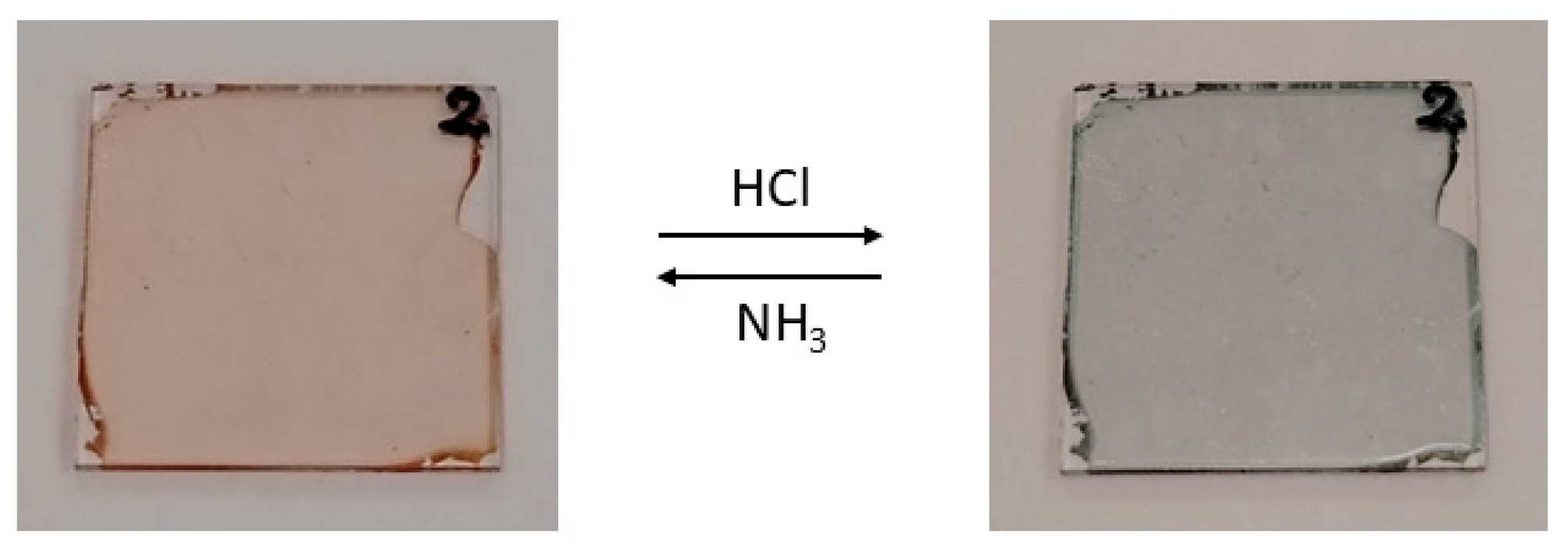
| Complex | λmax (nm) a [ε] b | λmax (nm) c [ε] b | |
|---|---|---|---|
| Bu4N[Pd((R)-α-MBAdt)(tdas)] (2) | 454 [4.9] | 601 [4.9] | This work |
| Bu4N[Pt((R)-α-MBAdt)(tdas)] (3) | 513 [7.6] | 651 [10.9] | This work |
| Bu4N[Pd((R)-α-MBAdt)(dmit)] (4) | 525 [15.7] | 747 [6.7] | Ref. [21] |
| Bu4N[Pt((R)-α-MBAdt)(dmit)] (5) | 543 [9.6] | 781 [9.6] | Ref. [21] |
| Bu4N[Pd((R)-α-MBAdt)(ddmedt)] (6) | 525 [4.0] | 715 [5.7] | Ref. [21] |
| Bu4N[Pt((R)-α-MBAdt)(ddmedt)] (7) | 558 [5.5] | 764 [11.1] | Ref. [21] |
| Bu4N[Pd((R)-α-MBAdt)(quinoxdt)] (8) | 550 [5.5] | 773 [5.8] | Ref. [21] |
| Bu4N[Pt((R)-α-MBAdt)(quinoxdt)] (9) | 606 [7.8] d | 814 [12.4] | Ref. [21] |
| Sample | χ33 (pm/V) | χ31 (pm/V) | χ15 (pm/V) |
|---|---|---|---|
| 1 film | 1.8 | 0.64 | 0.41 |
| 1 film + HCl | 2.78 | 0.84 | 0.66 |
| HCl–adduct 1 film | 3.20 | 0.66 | 0.55 |
| HCl–adduct 1 film + NH3 | 2.08 | 0.34 | 0.16 |
| 2 film | 3.24 | 0.78 | 0.32 |
| 2 film + HCl | 5.94 | 1.26 | 0.74 |
| HCl–adduct 2 film | 4.22 | 1.04 | 0.72 |
| HCl–adduct 2 film + NH3 | 2.82 | 0.82 | 0.42 |
| 3 film | 6.12 | 1.28 | 0.69 |
| 3 film + HCl | 8.06 | 2.06 | 1.02 |
| HCl–adduct 3 film | 8.48 | 2.48 | 2.02 |
| HCl–adduct 3 film + NH3 | 6.40 | 1.92 | 1.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attar, S.S.; Artizzu, F.; Pilia, L.; Serpe, A.; Colombo, A.; Dragonetti, C.; Fagnani, F.; Roberto, D.; Marinotto, D.; Deplano, P. HX-Linear and Nonlinear Optical Responsiveness of Rationally Designed Heteroleptic d8-Metallo-dithiolene Complexes. Molecules 2025, 30, 4004. https://doi.org/10.3390/molecules30194004
Attar SS, Artizzu F, Pilia L, Serpe A, Colombo A, Dragonetti C, Fagnani F, Roberto D, Marinotto D, Deplano P. HX-Linear and Nonlinear Optical Responsiveness of Rationally Designed Heteroleptic d8-Metallo-dithiolene Complexes. Molecules. 2025; 30(19):4004. https://doi.org/10.3390/molecules30194004
Chicago/Turabian StyleAttar, Salahuddin S., Flavia Artizzu, Luca Pilia, Angela Serpe, Alessia Colombo, Claudia Dragonetti, Francesco Fagnani, Dominique Roberto, Daniele Marinotto, and Paola Deplano. 2025. "HX-Linear and Nonlinear Optical Responsiveness of Rationally Designed Heteroleptic d8-Metallo-dithiolene Complexes" Molecules 30, no. 19: 4004. https://doi.org/10.3390/molecules30194004
APA StyleAttar, S. S., Artizzu, F., Pilia, L., Serpe, A., Colombo, A., Dragonetti, C., Fagnani, F., Roberto, D., Marinotto, D., & Deplano, P. (2025). HX-Linear and Nonlinear Optical Responsiveness of Rationally Designed Heteroleptic d8-Metallo-dithiolene Complexes. Molecules, 30(19), 4004. https://doi.org/10.3390/molecules30194004










