Design and Color Prediction of Anthracene-Based Dyes Based on Quantum Chemical Calculations
Abstract
1. Introduction
2. Results and Discussion
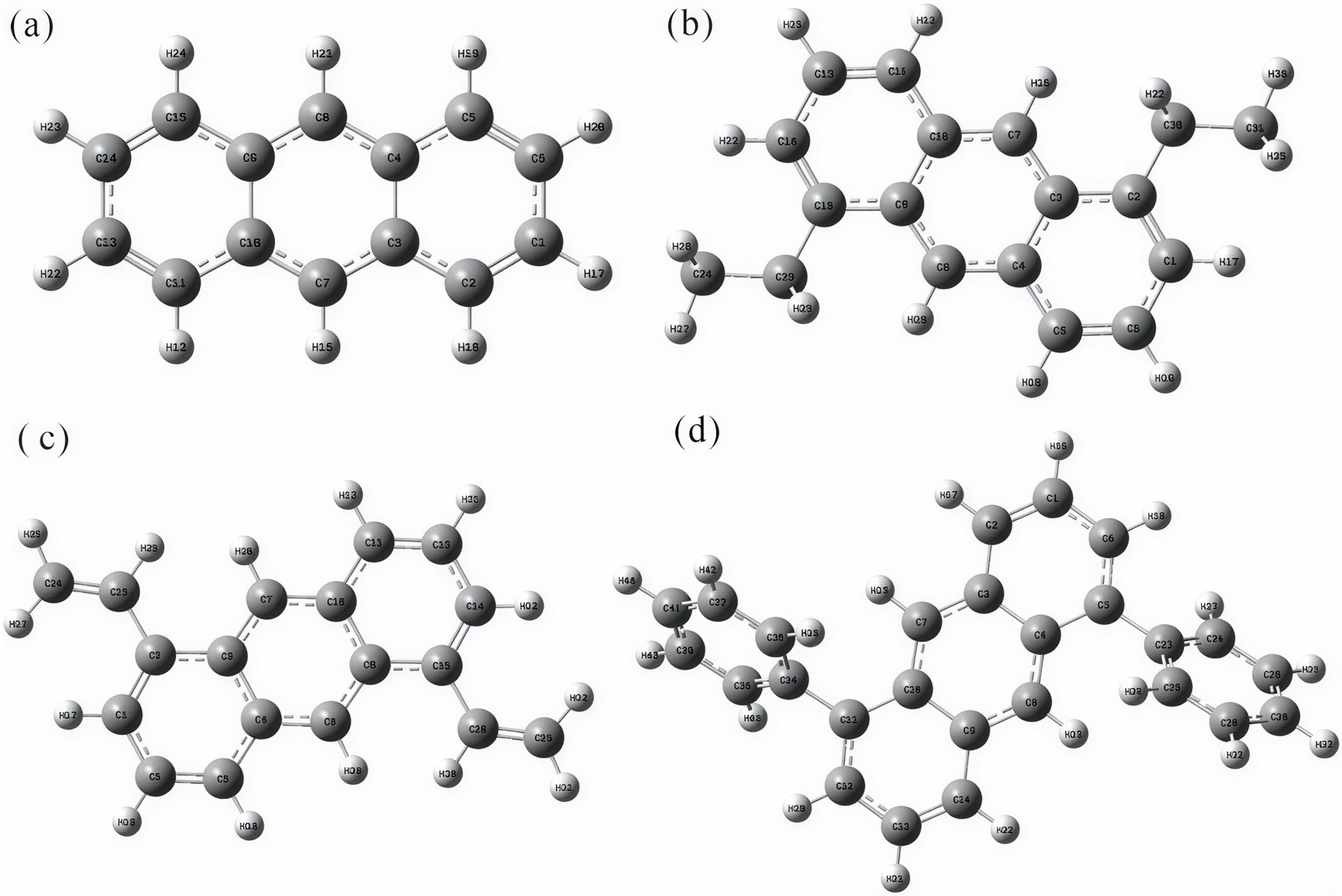
2.1. Structural Optimization and Frequency Calculation
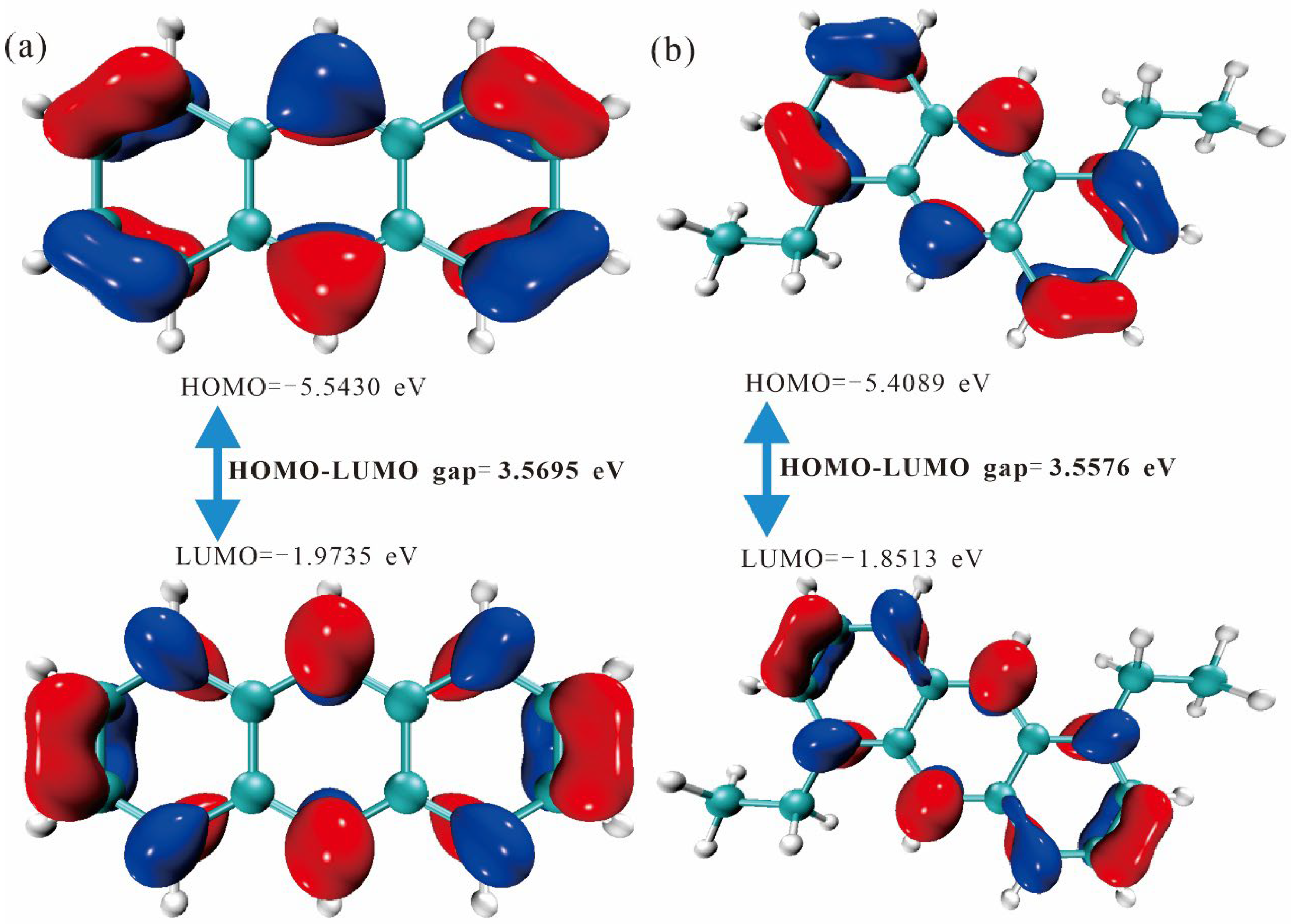
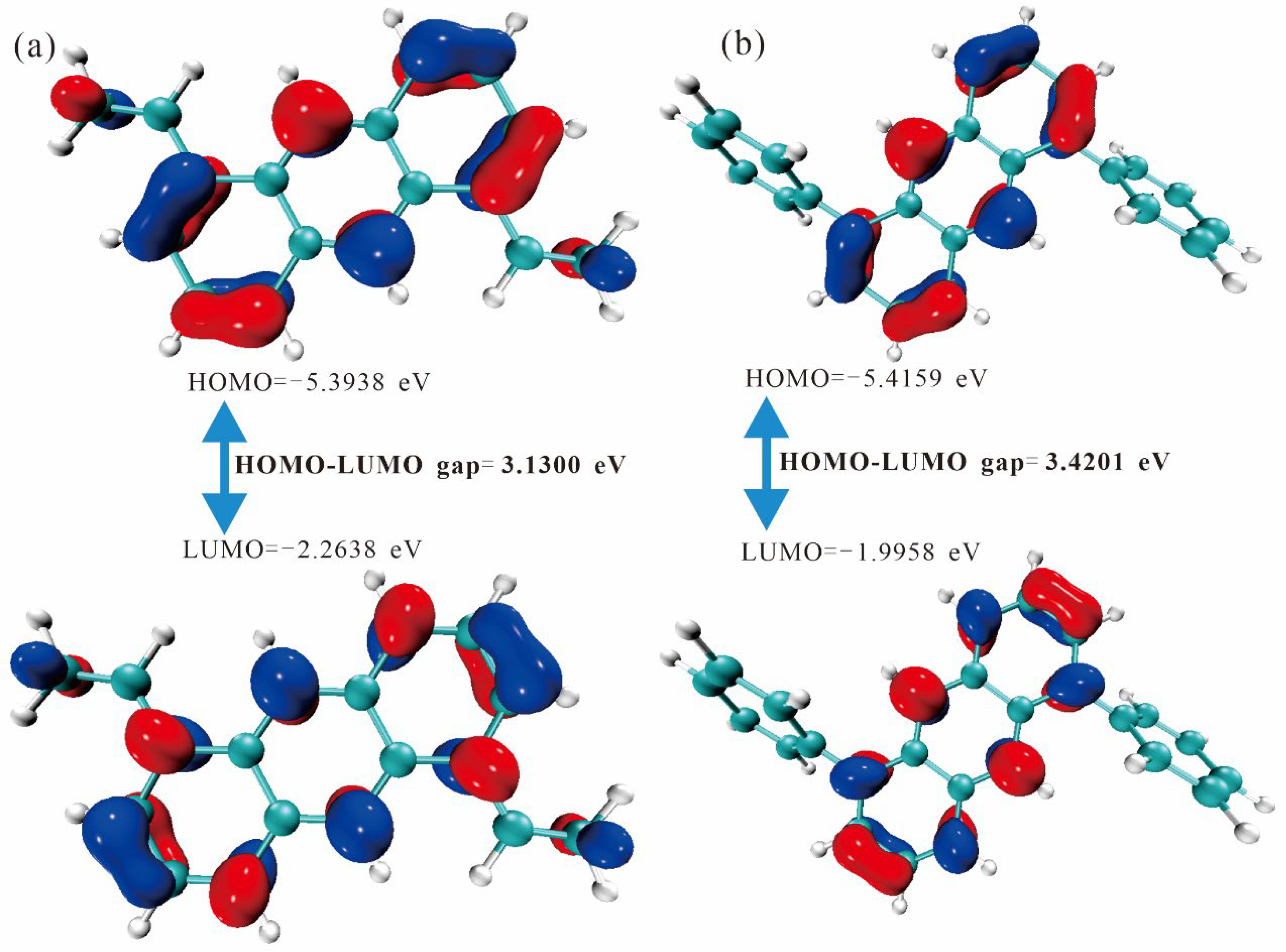
2.2. Frontier Molecular Orbitals
2.3. Electrostatic Potential
2.4. Excited State Color Prediction
2.5. Electron Density Difference
3. Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, Y.; Hwang, K.M.; Lee, S.; Park, B.B.; Kim, T.; Han, W.-S. Substituent effect on anthracene-benzophenone triad system: Photophysical properties and application to OLEDs with “hot-exciton” characteristics. Dye. Pigment. 2023, 213, 111171. [Google Scholar] [CrossRef]
- Kastrati, A.; Oswald, F.; Scalabre, A.; Fromm, K.M. Photophysical Properties of Anthracene Derivatives. Photochem 2023, 3, 227–273. [Google Scholar] [CrossRef]
- Wang, R.; Zhu, Y.; Hu, D.; Hu, J.; Chen, S.-W.; Lin, J.; Xing, L.; Huo, Y.; Ji, S. Phosphorus-oxygen modified anthracene-based emitters for high-efficiency deep-blue OLEDs approaching the BT.2020 blue standard. Chem. Eng. J. 2025, 506, 160269. [Google Scholar] [CrossRef]
- Liu, B.; Yu, Z.W.; He, D.; Li, M.D.; Xie, W.F.; Tong, Q.X. Productive harvesting of triplet excitons in anthracene-based emitters toward high-performance deep-blue nondoped organic light-emitting diodes. Mater. Today Chem. 2022, 23, 100630. [Google Scholar] [CrossRef]
- San, L.K.; Balser, S.; Reeves, B.J.; Clikeman, T.T.; Chen, Y.-S.; Strauss, S.H.; Boltalina, O.V. Deep-blue emitting 9,10-bis(perfluorobenzyl)anthracene. Beilstein J. Org. Chem. 2025, 21, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.-Y.; Sun, X.-Q.; Lin, P.-P.; Wei, X.; Guo, J.-F.; Cui, W.-B.; Fan, J.-X.; Li, H.; Zou, L.-Y.; Ren, A.-M. The effect of heteroatoms at end groups of anthracene derivatives on the photoelectric properties and crystal/film morphology: A theoretical perspective. J. Mater. Chem. C 2023, 11, 13018–13029. [Google Scholar] [CrossRef]
- Al-Shamiri, H.A.S. Design and construction of tunable solid-state dye laser pumped by flashlamp. Int. J. Mod. Phys. B 2023, 38, 2450404. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Song, J.; Li, M.; Yang, Y.; Gu, W.; Xu, X.; Xu, H.; Wang, S. A highly specific and sensitive turn-on fluorescence probe for hypochlorite detection based on anthracene fluorophore and its bioimaging applications. Dye. Pigment. 2019, 161, 172–181. [Google Scholar] [CrossRef]
- Sahar, H.; Asif, M.; Ahsan, A.; Aetizaz, M.; Ayub, K. Band gap engineering of 9,10-(bis-4-phenylazenyl)anthracene for application in dye sensitized solar cells. Sol. Energy 2024, 277, 112713. [Google Scholar] [CrossRef]
- Zainuri, D.A.; Abdullah, M.; Bakar, M.A.A.; Arshad, S.; Razak, I.A. An experimental and DFT investigations of fused ring anthracenyl chalcones with effective modification in acceptor moiety: Nonlinear optical and optical limiting response. Int. J. Quantum Chem. 2023, 123, e27178. [Google Scholar] [CrossRef]
- Hütter, M.; Kröger, M. Phoretic forces on convex particles from kinetic theory and nonequilibrium thermodynamics. J. Chem. Phys. 2006, 124, 044511. [Google Scholar] [CrossRef]
- De Melo, J.S.S.; Rondão, R.; Burrows, H.D.; Melo, M.J.; Navaratnam, S.; Edge, R.; Voss, G. Spectral and Photophysical Studies of Substituted Indigo Derivatives in Their Keto Forms. ChemPhysChem 2006, 7, 2303–2311. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Yoo, D.; Nishikawa, R.; Kawamoto, T.; Mori, T. Charge injected proton transfer in indigo derivatives. Phys. Chem. Chem. Phys. 2021, 23, 21972–21980. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.F.; Oliveira, M.S.; Aguiar, A.S.N.; Custodio, J.M.F.; Di Mascio, P.; Sabino, J.R.; Verde, G.V.; Souza, J.C.; Santin, L.G.; Camargo, A.J.; et al. Synthesis and Structural Studies of Two New Anthracene Derivatives. Crystals 2021, 11, 934–953. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, H.; Geng, T.; Duan, R.; Li, X.; Liu, Y.; Liu, W.; He, B.-G.; Sui, L.; Wang, K.; et al. Piezochromic fluorescence of anthracene derivative crystals with different stacking patterns designed around excimers. J. Mater. Chem. C 2023, 11, 4892–4898. [Google Scholar] [CrossRef]
- Lu, Z.; Li, C.; Li, S.; Yu, Q.; Zhang, J. DFT Study on Fused N-Heteroaromatic Frameworks: Stability, Aromaticity, and Energetic Insights from Five-Membered Fused Six-Membered N-Heteroaromatic Skeletons. Molecules 2025, 30, 1101–1112. [Google Scholar] [CrossRef]
- Wen, Y.; Yu, Y.; Gu, H.; Kang, Y.; Zhao, G.; Li, Y.; Huang, Q. First-Principles Study of Rh Segregation in the Au–Rh(111) Alloy with Adsorbed NO, CO, or O2. Molecules 2025, 30, 2389–2398. [Google Scholar] [CrossRef]
- Piękoś, P.; Lipkowski, P.; Dehaen, W.; Wieczorek, R.; Filarowski, A. Theoretical Insights into the Impact of Pyrrole and Imidazole Substituents on the BODIPY Chromophore. Molecules 2025, 30, 2209. [Google Scholar] [CrossRef]
- De Paul Zoua, V.; Atangana, A.F.; Asi, A.Q.; Pagoré, I.F.; Tatsimo, S.J.N.; Ntieche, R.A. Structural, electronic, and NLO properties of two acridone alkaloîds: DFT and TD-DFT studies. J. Mol. Model. 2024, 30, 315. [Google Scholar] [CrossRef]
- Kushwaha, P.K.; Srivastava, S.K. Tuning optoelectronic properties of indandione-based D-A materials by malononitrile group acceptors: A DFT and TD-DFT approach. J. Mol. Model. 2024, 30, 356. [Google Scholar] [CrossRef]
- Li, X.; Lu, H.; He, D.; Luo, C.; Huang, J. Synthesis, Fluorescence Properties and Theoretical Calculations of Novel Stilbene Derivatives Based on 1,3,4-Oxadiazole Bearing Anthracene Core. J. Fluoresc. 2013, 23, 1039–1044. [Google Scholar] [CrossRef]
- Luo, Y.; Tao, S.; Wu, Y.; Feng, W.; Jiang, W.; Xia, Y.; Xiao, W.; Li, Y.; Liu, Z.; Ou, Y.-P.; et al. Donor-acceptor type dithienylethenes derivatives based on anthraquinone and anthracene moieties: Synthesis, photochromic properties and theoretical calculations. Dye. Pigment. 2024, 224, 112000. [Google Scholar] [CrossRef]
- Hleli, E.; Mbarek, M.; Gouid, Z.; Ulbricsht, C.; Romdhane, S.; Ben Said, R.; Guesmi, M.; Egbe, D.A.M.; Bouchriha, H. DFT study of optical and electronic properties of anthracene containing PPE-PPVs. J. Phys. Chem. Solids 2020, 136, 109157. [Google Scholar] [CrossRef]
- Alrub, S.A.; Shah, Y.; Umar, M.; Mansha, A.; Ali, A.I.; Hussein, R.K. Theoretical investigations of the auxochromic effect on novel thermally activated delayed fluorescence (TADF) anthracene derivatives. BMC Chem. 2025, 19, 41. [Google Scholar] [CrossRef] [PubMed]
- Cysewski, P.; Jeliński, T.; Przybyłek, M.; Shyichuk, A. Color prediction from first principle quantum chemistry computations: A case of alizarin dissolved in methanol. New J. Chem. 2012, 36, 1836–1843. [Google Scholar] [CrossRef]
- Jeon, B.K.; Jang, S.H.; Kim, S.H.; Lee, H.; Choi, I.; Lee, B.-S.; Kang, K.; Choi, J. Development of electron-flow-strengthened azo dyes with a wide color reproduction range for application in the color conversion layers of microdisplays. Dye. Pigment. 2025, 238, 112732. [Google Scholar] [CrossRef]
- Trussell, H.J. DSP solutions run the gamut for color systems. IEEE Signal Process. Mag. 1993, 10, 8–23. [Google Scholar] [CrossRef]
- Aroca-Trujillo, J.L.; Perez-Ruiz, A. Colombian coffee tree leaves multispectral images dataset. Data Brief. 2025, 59, 111421. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Rev. B.01. Gaussian: Wallingford, CT, USA, 2016.
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, Version 6; Gaussian: Wallingford, CT, USA, 2016.
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Gulde, R.; Pollak, P.; Weigend, F. Error-Balanced Segmented Contracted Basis Sets of Double-ζ to Quadruple-ζ Valence Quality for the Lanthanides. J. Chem. Theory Comput. 2012, 8, 4062–4068. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-F.; Su, M.-D. Insights into the Factors Controlling the Origin of Activation Barriers in the [2 + 2] Cycloaddition Reactions of Heavy Imine-like Molecules Featuring a Ge=Group 15 Double Bond with Heterocumulenes. Molecules 2025, 30, 1905–1923. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Inoue, K.; Namiguchi, R.; Morita, S.; Hayakawa, S.; Yokota, M.; Sakai, K.; Matsumoto, K.; Aoki, S. Identification of Novel Compounds That Bind to the HGF β-Chain In Silico, Verification by Molecular Mechanics and Quantum Mechanics, and Validation of Their HGF Inhibitory Activity In Vitro. Molecules 2025, 30, 1801–1813. [Google Scholar] [CrossRef]
- Paciotti, R.; Re, N.; Storchi, L. Combining the Fragment Molecular Orbital and GRID Approaches for the Prediction of Ligand–Metalloenzyme Binding Affinity: The Case Study of hCA II Inhibitors. Molecules 2024, 29, 3600–3618. [Google Scholar] [CrossRef]
- Manzetti, S.; Lu, T. The geometry and electronic structure of Aristolochic acid: Possible implications for a frozen resonance. J. Phys. Org. Chem. 2013, 26, 473–483. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Quantitative analysis of molecular surface based on improved Marching Tetrahedra algorithm. J. Mol. Graph. Model. 2012, 38, 314–323. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, T. Efficient evaluation of electrostatic potential with computerized optimized code. Phys. Chem. Chem. Phys. 2021, 23, 20323–20328. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Lu, T. A comprehensive electron wavefunction analysis toolbox for chemists, Multiwfn. J. Chem. Phys. 2024, 161, 082503. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Li, M.; Reimers, J.R.; Ford, M.J.; Kobayashi, R.; Amos, R.D. Accurate prediction of the properties of materials using the CAM-B3LYP density functional. J. Comput. Chem. 2021, 42, 1486–1497. [Google Scholar] [CrossRef]
- Wahl, A.C. Molecular Orbital Densities: Pictorial Studies. Science 1966, 151, 961–967. [Google Scholar] [CrossRef]
- Schwarz, W.H.E.; Mensching, L.; Valtaznos, P.; Von Niessen, W. A chemically useful definition of electron difference densities. Int. J. Quantum Chem. 1986, 29, 909–914. [Google Scholar] [CrossRef]
- Nagy, M.; Fiser, B.; Szőri, M.; Vanyorek, L.; Viskolcz, B. Optical Study of Solvatochromic Isocyanoaminoanthracene Dyes and 1,5-Diaminoanthracene. Int. J. Mol. Sci. 2022, 23, 1315–1331. [Google Scholar] [CrossRef]

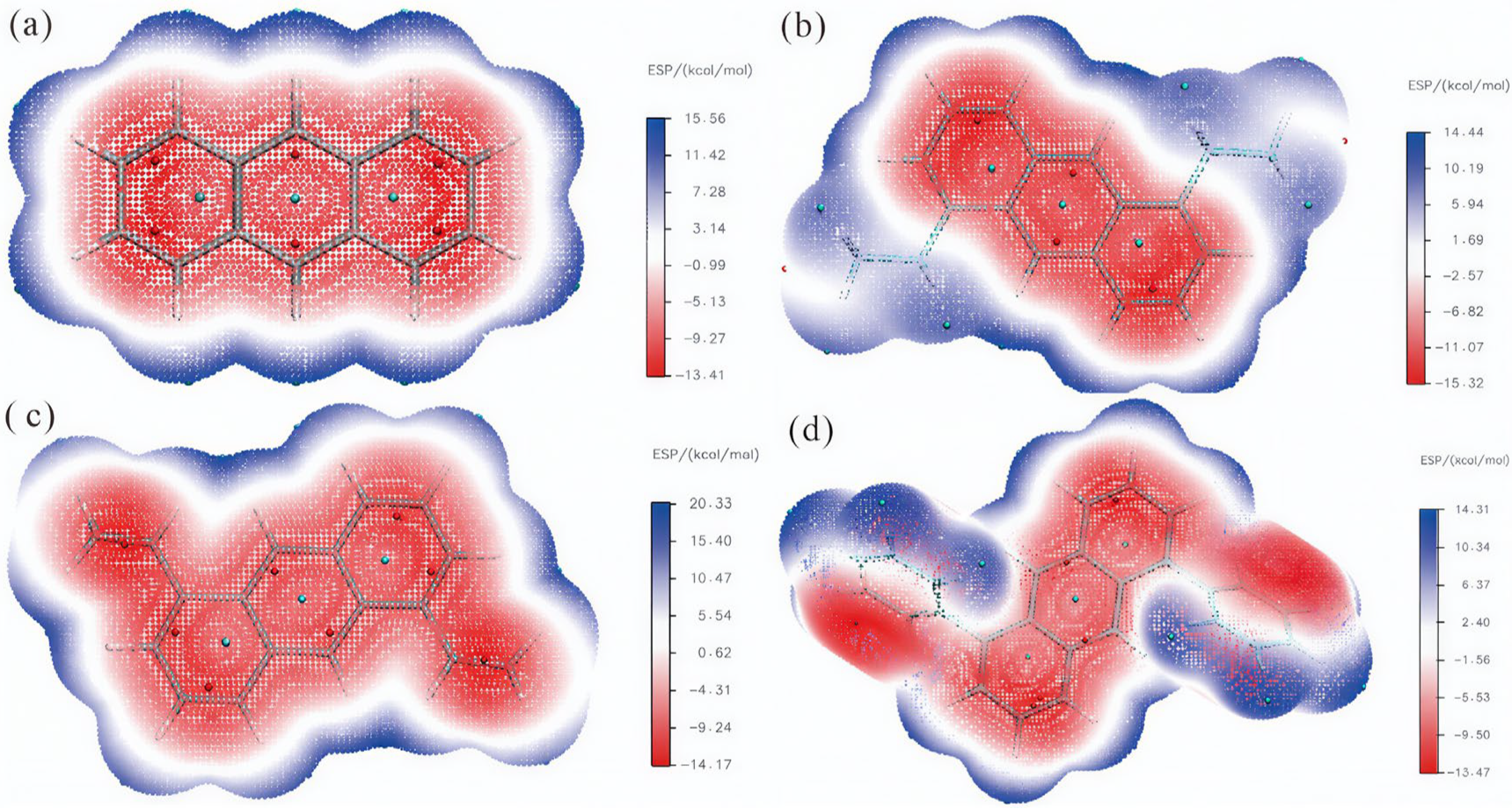
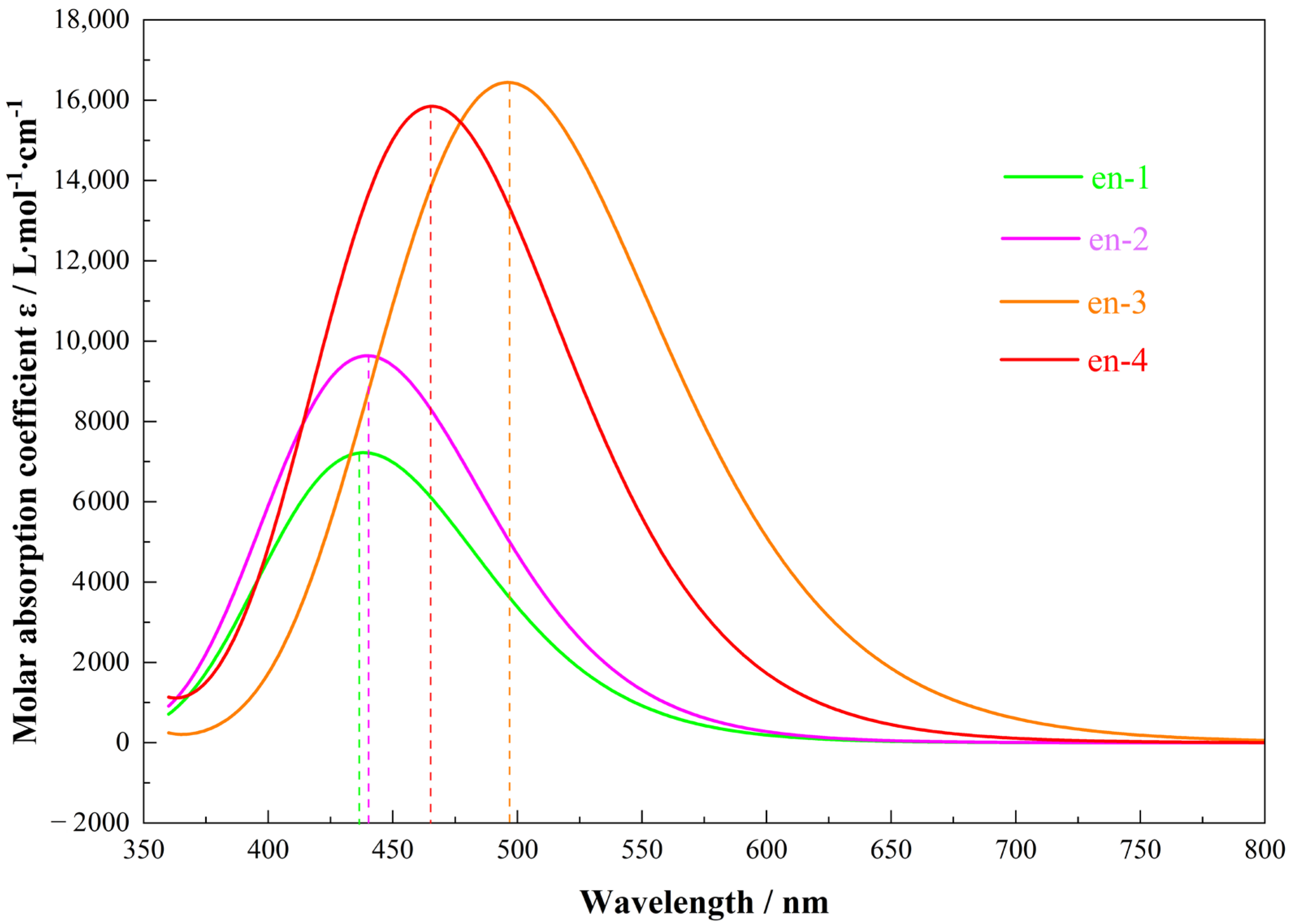
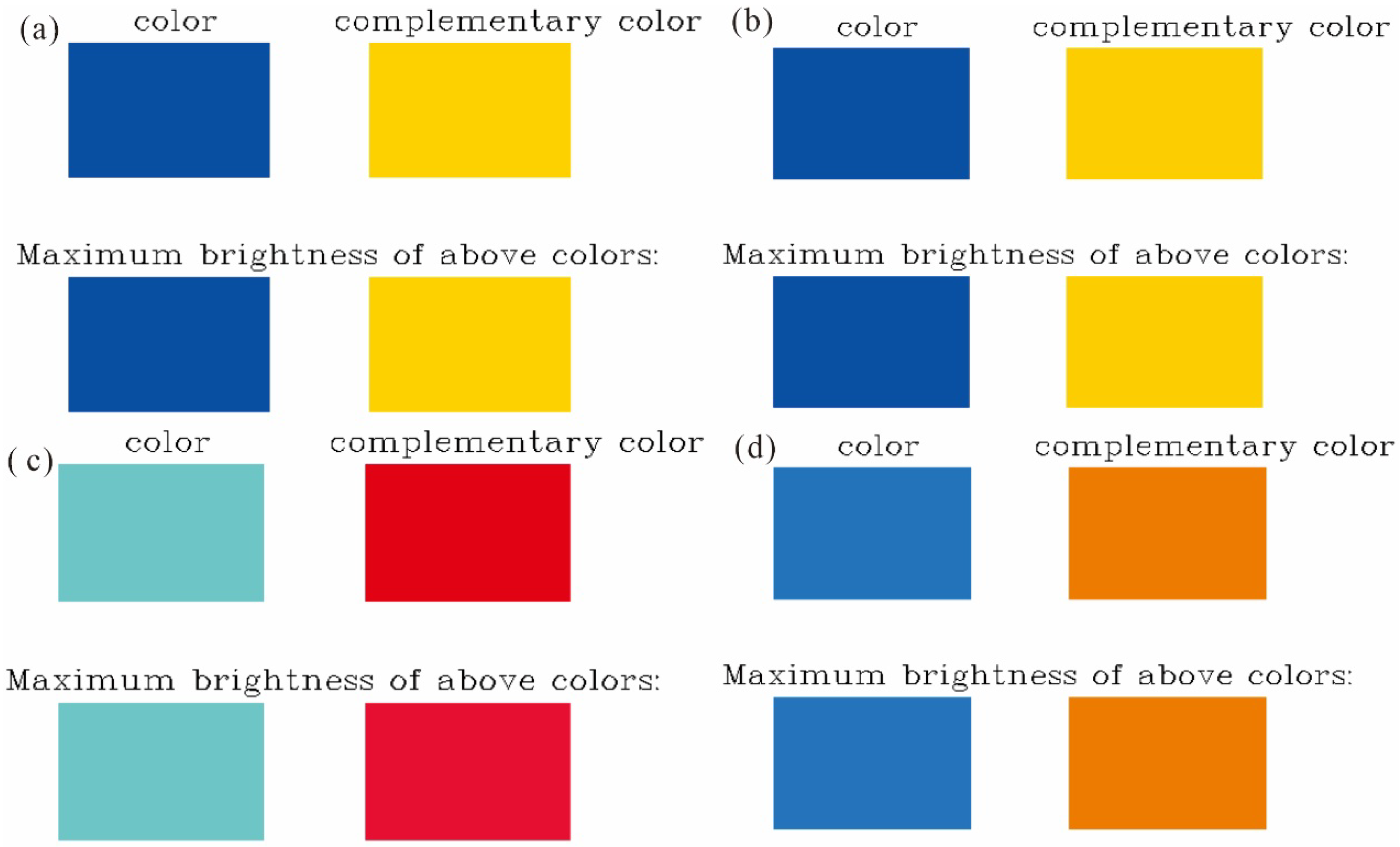


| Parameter | en-1 | en-2 | en-3 | en-4 |
|---|---|---|---|---|
| Overall Average/kcal·mol−1 | 0.41745 | 0.64212 | 0.64160 | −0.00172 |
| Positive Average/kcal mol−1 | 8.49458 | 6.04357 | 8.78666 | 7.24483 |
| Negative Average/kcal·mol−1 | –7.91073 | –8.70221 | –7.52192 | –7.24501 |
| Overall Variance/(kcal·mol−1)2 | 31.20947 | 28.70497 | 36.28215 | 27.18365 |
| Positive Variance/(kcal·mol−1)2 | 17.60668 | 10.93180 | 23.84217 | 14.07184 |
| Negative Variance/(kcal·mol−1)2 | 13.60279 | 17.77318 | 12.43997 | 13.11180 |
| Charge Balance (ν) | 0.24589 | 0.23580 | 0.22531 | 0.24969 |
| Internal Charge Separation/kcal·mol−1 | 8.20431 | 6.85638 | 8.16419 | 7.24492 |
| Molecular Polarity Index/kcal·mol−1 | 8.20712 | 7.01744 | 8.15501 | 7.24492 |
| Nonpolar surface area (|ESP| ≤ 10 kcal/mol) | 59.02% | 74.23% | 64.84% | 72.63% |
| Polar surface area (|ESP| > 10 kcal/mol) | 40.98% | 25.77% | 35.16% | 27.37% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, J.; Bai, M.; Li, H.; Ke, Z.; Zhou, C. Design and Color Prediction of Anthracene-Based Dyes Based on Quantum Chemical Calculations. Molecules 2025, 30, 3975. https://doi.org/10.3390/molecules30193975
Li Y, Zhang J, Bai M, Li H, Ke Z, Zhou C. Design and Color Prediction of Anthracene-Based Dyes Based on Quantum Chemical Calculations. Molecules. 2025; 30(19):3975. https://doi.org/10.3390/molecules30193975
Chicago/Turabian StyleLi, Yanyi, Jiahao Zhang, Mei Bai, Hao Li, Zengbo Ke, and Chunsheng Zhou. 2025. "Design and Color Prediction of Anthracene-Based Dyes Based on Quantum Chemical Calculations" Molecules 30, no. 19: 3975. https://doi.org/10.3390/molecules30193975
APA StyleLi, Y., Zhang, J., Bai, M., Li, H., Ke, Z., & Zhou, C. (2025). Design and Color Prediction of Anthracene-Based Dyes Based on Quantum Chemical Calculations. Molecules, 30(19), 3975. https://doi.org/10.3390/molecules30193975






