Detection of Biomarker Clusterin in SERS Immunoassays on Al Foil After Substrate Selection and Assay Optimization with Fluorescently Labeled Antibodies
Abstract
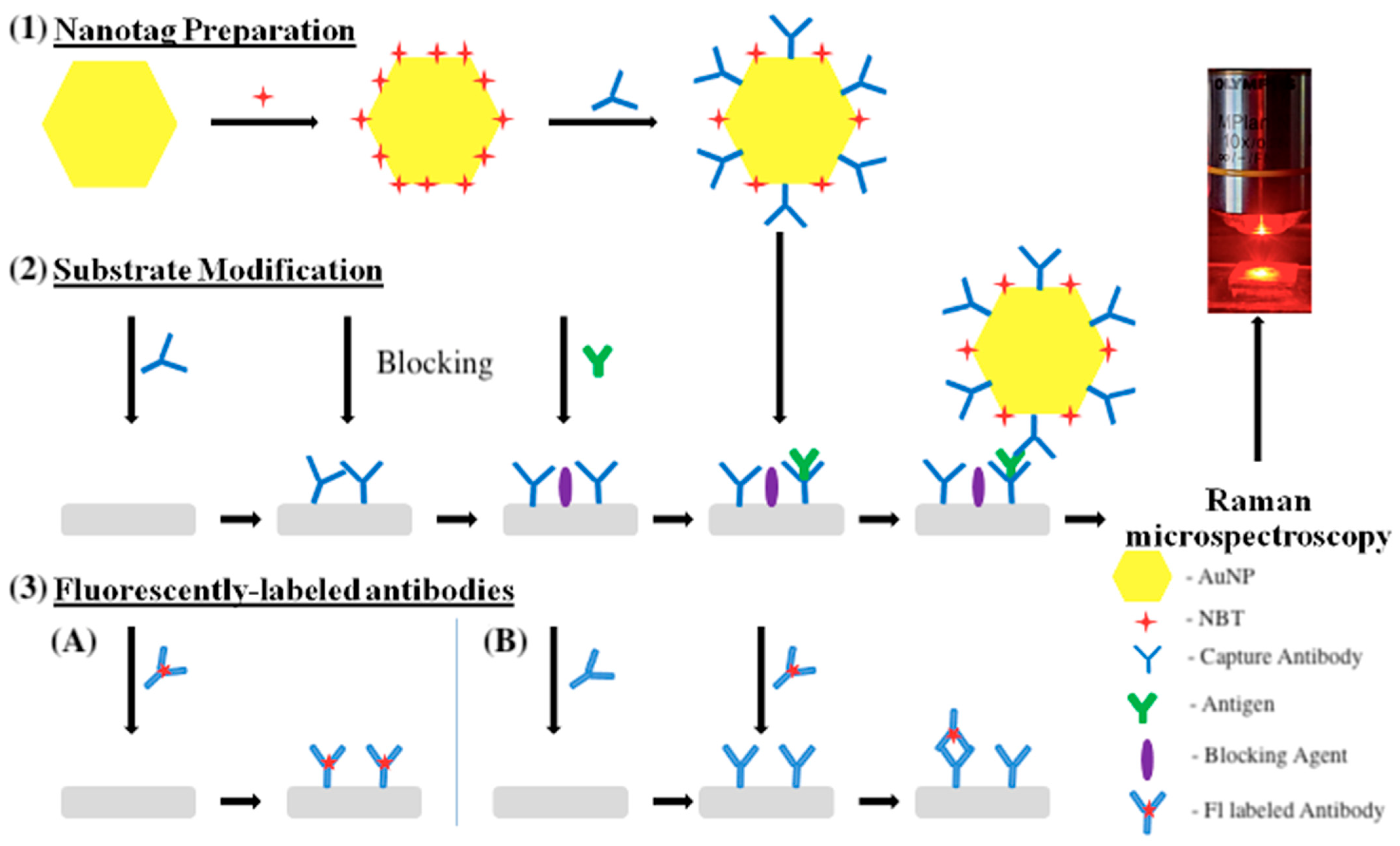
1. Introduction
2. Results and Discussion
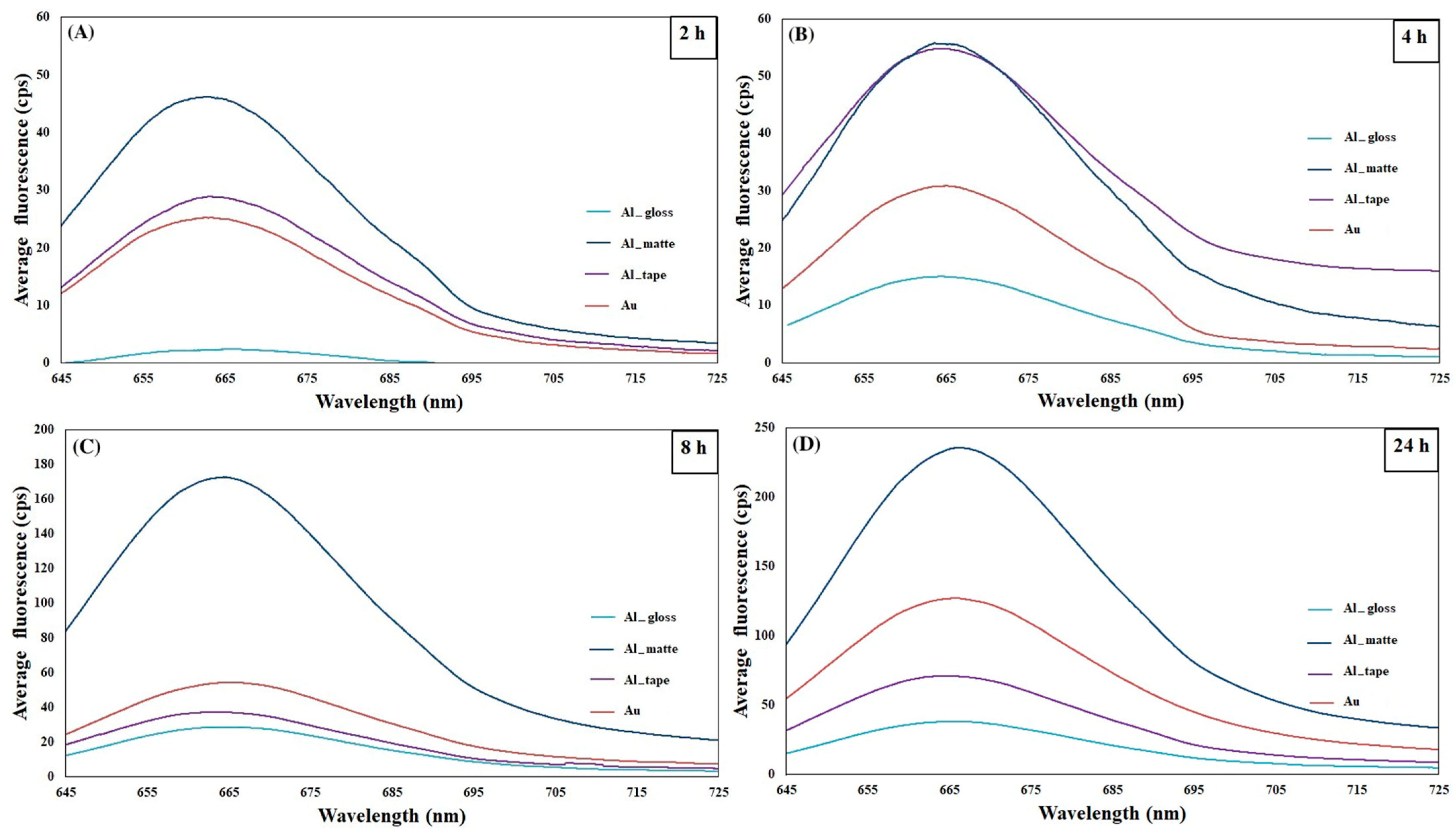
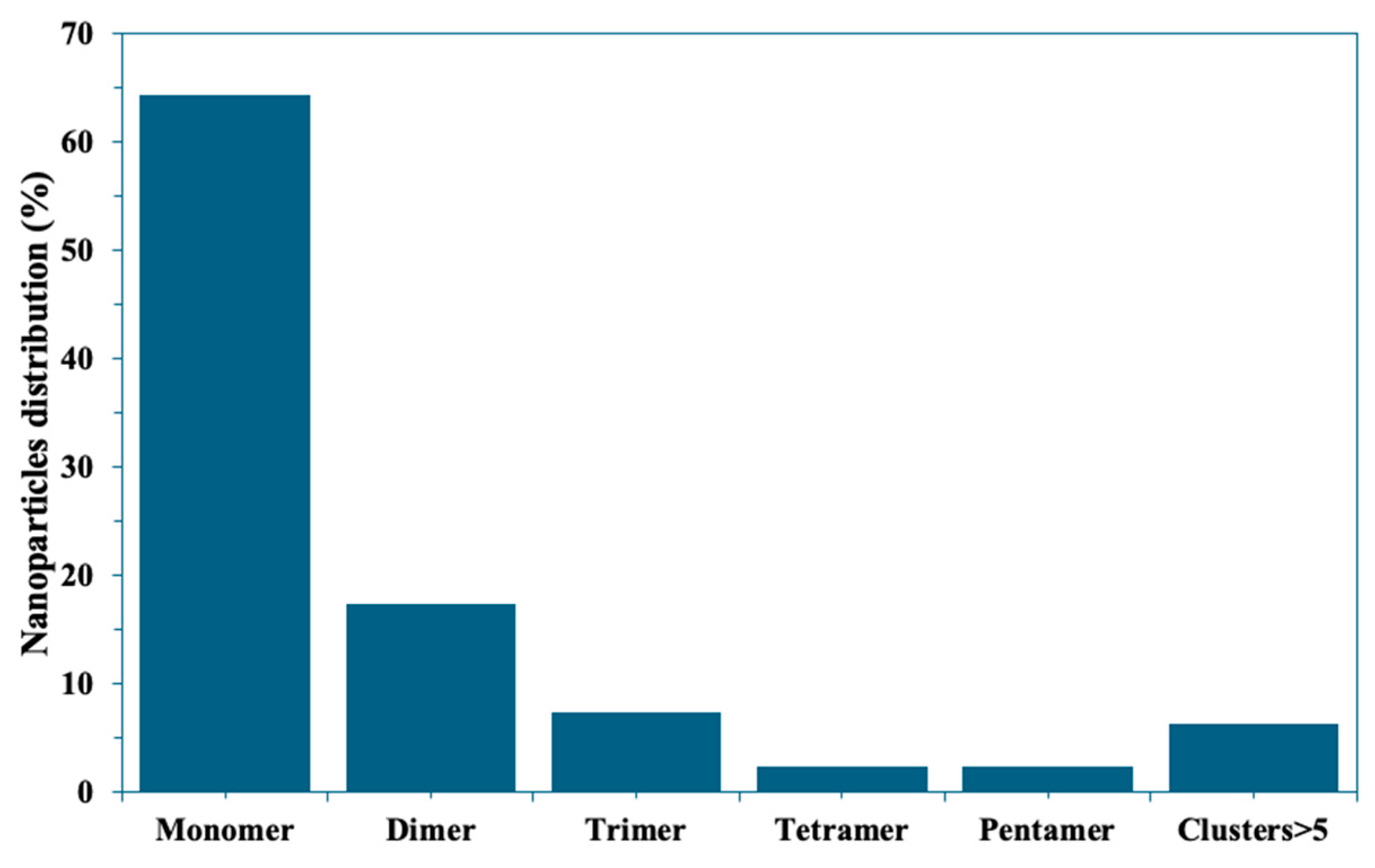
2.1. The Optimization of Assay Parameters
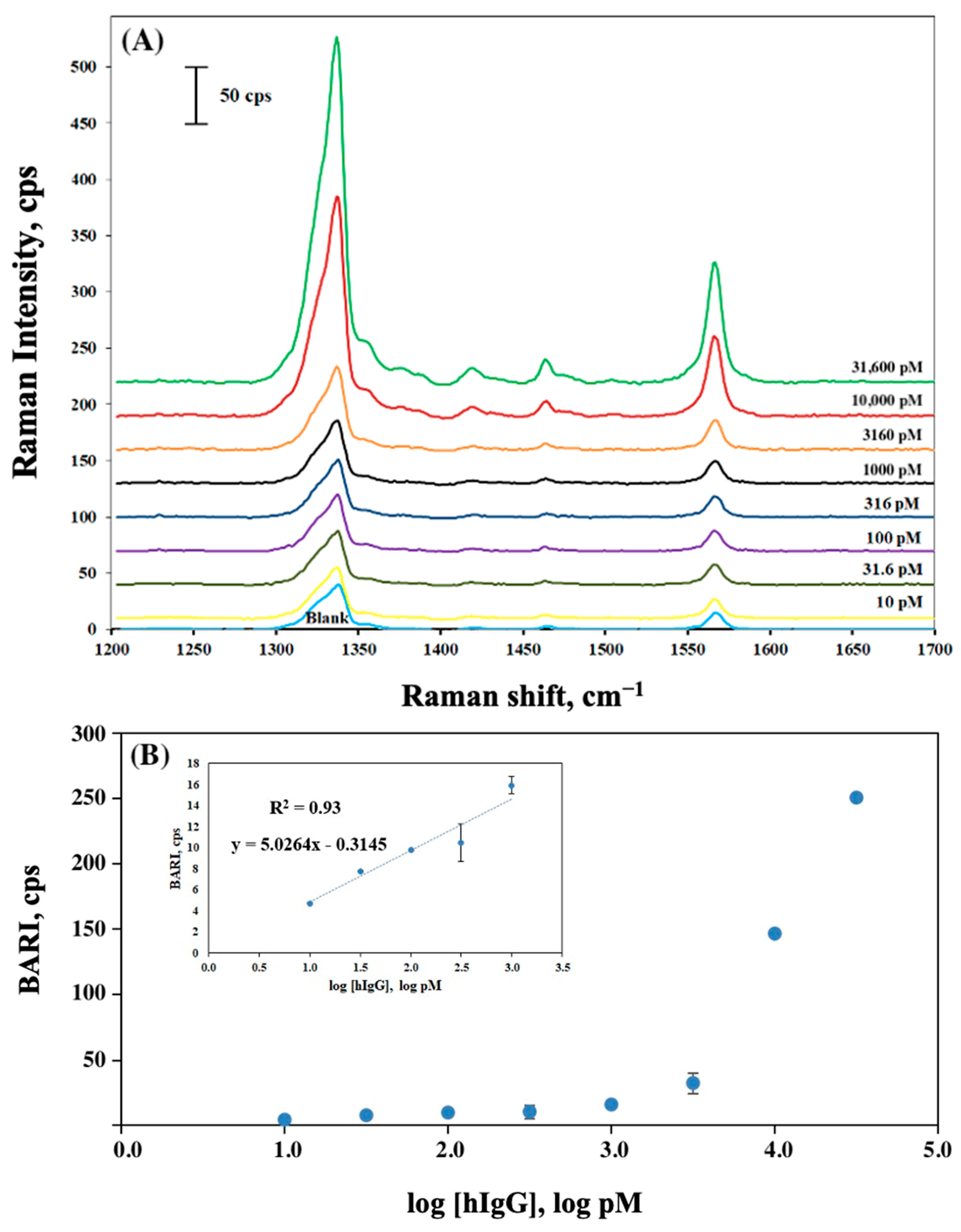
2.2. The Detection of hIgG
2.3. The Detection of Clusterin
3. Materials and Methods
3.1. Materials
3.2. Measurements
3.3. Preparation of SERS-Active Substrates
3.4. Assay Protocol
3.4.1. Assay Protocol of Optimization Experiments
3.4.2. Assay Protocol for hIgG
3.4.3. Assay Protocol for Clusterin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strohkamp, S.; Gemoll, T.; Habermann, J.K. Possibilities and Limitations of 2DE-Based Analyses for Identifying Low-Abundant Tumor Markers in Human Serum and Plasma. Proteomics 2016, 16, 2519–2532. [Google Scholar] [CrossRef] [PubMed]
- Visconti, V.V.; Greggi, C.; Cariati, I.; Gasperini, B.; Mastrogregori, A.; Botta, A.; Tarantino, U. Deregulated Clusterin as a Marker of Bone Fragility: New Insights into the Pathophysiology of Osteoporosis. Genes 2022, 13, 652. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Halberg, R.B.; Ehrhardt, W.M.; Torrealba, J.; Dove, W.F. Clusterin as a Biomarker in Murine and Human Intestinal Neoplasia. Proc. Natl. Acad. Sci. USA 2003, 100, 9530–9535. [Google Scholar] [CrossRef] [PubMed]
- Shabayek, M.I.; Sayed, O.M.; Attaia, H.A.; Awida, H.A.; Abozeed, H. Diagnostic Evaluation of Urinary Angiogenin (ANG) and Clusterin (CLU) as Biomarker for Bladder Cancer. Pathol. Oncol. Res. 2014, 20, 859–866. [Google Scholar] [CrossRef]
- Yang, P.; Yang, Z.; Dong, Y.; Yang, L.; Peng, S.; Yuan, L.; Hu, X.; Chen, S.; Tang, H.; Yang, X.; et al. Clusterin Is a Biomarker of Breast Cancer Prognosis and Correlated with Immune Microenvironment. Transl. Cancer Res. 2023, 12, 31. [Google Scholar] [CrossRef]
- Qiu, H.; Wang, M.; Jiang, S.; Zhang, L.; Yang, Z.; Li, L.; Li, J.; Cao, M.; Huang, J. Reliable Molecular Trace-Detection Based on Flexible SERS Substrate of Graphene/Ag-Nanoflowers/PMMA. Sens. Actuators B Chem. 2017, 249, 439–450. [Google Scholar] [CrossRef]
- Pucci, S.; Bonanno, E.; Sesti, F.; Mazzarelli, P.; Mauriello, A.; Ricci, F.; Zoccai, G.B.; Rulli, F.; Galatà, G.; Spagnoli, L.G. Clusterin in Stool: A New Biomarker for Colon Cancer Screening? Off. J. Am. Coll. Gastroenterol. 2009, 104, 2807. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Yang, H.-Y.; Chien, H.-P.; Tseng, M.-H.; Huang, J.-L. Urinary Clusterin—A Novel Urinary Biomarker Associated with Pediatric Lupus Renal Histopathologic Features and Renal Survival. Pediatr. Nephrol. 2018, 33, 1189–1198. [Google Scholar] [CrossRef]
- Yu, W.; Chen, D.; Wang, Z.; Zhou, C.; Luo, J.; Xu, Y.; Shen, L.; Yin, H.; Tao, S.; Xiao, Z.; et al. Time-Dependent Decrease of Clusterin as a Potential Cerebrospinal Fluid Biomarker for Drug-Resistant Epilepsy. J. Mol. Neurosci. 2014, 54, 1–9. [Google Scholar] [CrossRef]
- Cunin, P.; Beauvillain, C.; Miot, C.; Augusto, J.-F.; Preisser, L.; Blanchard, S.; Pignon, P.; Scotet, M.; Garo, E.; Fremaux, I.; et al. Clusterin Facilitates Apoptotic Cell Clearance and Prevents Apoptotic Cell-Induced Autoimmune Responses. Cell Death Dis. 2016, 7, e2215. [Google Scholar] [CrossRef]
- Bungon, T.; Haslam, C.; Damiati, S.; O’Driscoll, B.; Whitley, T.; Davey, P.; Siligardi, G.; Charmet, J.; Awan, S.A. Graphene FET Sensors for Alzheimer’s Disease Protein Biomarker Clusterin Detection. Front. Mol. Biosci. 2021, 8, 651232. [Google Scholar] [CrossRef] [PubMed]
- Sciacca, B.; François, A.; Hoffmann, P.; Monro, T.M. Multiplexing of Radiative-Surface Plasmon Resonance for the Detection of Gastric Cancer Biomarkers in a Single Optical Fiber. Sens. Actuators B Chem. 2013, 183, 454–458. [Google Scholar] [CrossRef]
- Rodríguez-Piñeiro, A.M.; García-Lorenzo, A.; Blanco-Prieto, S.; Álvarez-Chaver, P.; Rodríguez-Berrocal, F.J.; de la Cadena, M.P.; Martínez-Zorzano, V.S. Secreted Clusterin in Colon Tumor Cell Models and Its Potentialas Diagnostic Marker for Colorectal Cancer. Cancer Investig. 2012, 30, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Jongbloed, W.; Herrebout, M.A.; Blankenstein, M.A.; Veerhuis, R. Quantification of Clusterin in Paired Cerebrospinal Fluid and Plasma Samples. Ann. Clin. Biochem. 2014, 51, 557–567. [Google Scholar] [CrossRef]
- Ritter, S.Y.; Collins, J.; Krastins, B.; Sarracino, D.; Lopez, M.; Losina, E.; Aliprantis, A.O. Mass Spectrometry Assays of Plasma Biomarkers to Predict Radiographic Progression of Knee Osteoarthritis. Arthritis Res. Ther. 2014, 16, 456. [Google Scholar] [CrossRef]
- Aguilar-Mahecha, A.; Cantin, C.; O’Connor-McCourt, M.; Nantel, A.; Basik, M. Development of Reverse Phase Protein Microarrays for the Validation of Clusterin, a Mid-Abundant Blood Biomarker. Proteome Sci. 2009, 7, 15. [Google Scholar] [CrossRef]
- Brazaca, L.C.; Moreto, J.R.; Martín, A.; Tehrani, F.; Wang, J.; Zucolotto, V. Colorimetric Paper-Based Immunosensor for Simultaneous Determination of Fetuin B and Clusterin toward Early Alzheimer’s Diagnosis. ACS Nano 2019, 13, 13325–13332. [Google Scholar] [CrossRef]
- Grubisha, D.S.; Lipert, R.J.; Park, H.-Y.; Driskell, J.; Porter, M.D. Femtomolar Detection of Prostate-Specific Antigen: An Immunoassay Based on Surface-Enhanced Raman Scattering and Immunogold Labels. Anal. Chem. 2003, 75, 5936–5943. [Google Scholar] [CrossRef]
- Terzapulo, X.; Kassenova, A.; Bukasov, R. Immunoassays: Analytical and Clinical Performance, Challenges, and Perspectives of SERS Detection in Comparison with Fluorescent Spectroscopic Detection. Int. J. Mol. Sci. 2024, 25, 2080. [Google Scholar] [CrossRef]
- Wang, G.; Park, H.-Y.; Lipert, R.J.; Porter, M.D. Mixed Monolayers on Gold Nanoparticle Labels for Multiplexed Surface-Enhanced Raman Scattering Based Immunoassays. Anal. Chem. 2009, 81, 9643–9650. [Google Scholar] [CrossRef]
- Smolsky, J.; Kaur, S.; Hayashi, C.; Batra, S.K.; Krasnoslobodtsev, A.V. Surface-Enhanced Raman Scattering-Based Immunoassay Technologies for Detection of Disease Biomarkers. Biosensors 2017, 7, 7. [Google Scholar] [CrossRef]
- Pollap, A.; Świt, P. Recent Advances in Sandwich SERS Immunosensors for Cancer Detection. Int. J. Mol. Sci. 2022, 23, 4740. [Google Scholar] [CrossRef]
- Ilyas, A.; Dyussupova, A.; Sultangaziyev, A.; Shevchenko, Y.; Filchakova, O.; Bukasov, R. SERS Immuno- and Apta-Assays in Biosensing/Bio-Detection: Performance Comparison, Clinical Applications, Challenges. Talanta 2023, 265, 124818. [Google Scholar] [CrossRef] [PubMed]
- Nanomaterials|Free Full-Text|Solid Plasmonic Substrates for Breast Cancer Detection by Means of SERS Analysis of Blood Plasma. Available online: https://www.mdpi.com/2079-4991/10/6/1212 (accessed on 12 March 2024).
- Lu, Y.; Lei, B.; Zhao, Q.; Yang, X.; Wei, Y.; Xiao, T.; Zhu, S.; Ouyang, Y.; Zhang, H.; Cai, W. Solid-State Au Nanocone Arrays Substrate for Reliable SERS Profiling of Serum for Disease Diagnosis. ACS Omega 2023, 8, 29836–29846. [Google Scholar] [CrossRef] [PubMed]
- Bukasov, R.; Sultangaziyev, A.; Kunushpayeva, Z.; Rapikov, A.; Dossym, D. Aluminum Foil vs. Gold Film: Cost-Effective Substrate in Sandwich SERS Immunoassays of Biomarkers Reveals Potential for Selectivity Improvement. Int. J. Mol. Sci. 2023, 24, 5578. [Google Scholar] [CrossRef] [PubMed]
- Kunushpayeva, Z.; Rapikov, A.; Akhmetova, A.; Sultangaziyev, A.; Dossym, D.; Bukasov, R. Sandwich SERS Immunoassay of Human Immunoglobulin on Silicon Wafer Compared to Traditional SERS Substrate, Gold Film. Sens. Bio-Sens. Res. 2020, 29, 100355. [Google Scholar] [CrossRef]
- Palaghias, G.; Eliades, G.; Vougiouklakis, G. In Vivo Corrosion Behavior of Gold-Plated versus Titanium Dental Retention Pins. J. Prosthet. Dent. 1992, 67, 194–198. [Google Scholar] [CrossRef]
- Hendrik, K.; Hermann, S.; Tino, J.; Ursula, V.; Gerhard, F.; Ludwig, J. Biodegradation of Gold and Platinum Implants in Rats Studied by Electron Microscopy. Int. J. Phys. Res. Appl. 2019, 2, 41–48. [Google Scholar] [CrossRef]
- Gudun, K.; Elemessova, Z.; Khamkhash, L.; Ralchenko, E.; Bukasov, R. Commercial Gold Nanoparticles on Untreated Aluminum Foil: Versatile, Sensitive, and Cost-Effective SERS Substrate. J. Nanomater. 2017, 2017, e9182025. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.; Yu, J.; Li, Z.; Zhao, X.; Liu, A.; Jiang, S.; Yang, C.; Zhang, C.; Man, B. Aluminum Nanoparticle Films with an Enhanced Hot-Spot Intensity for High-Efficiency SERS. Opt. Express OE 2020, 28, 9174–9185. [Google Scholar] [CrossRef]
- Betz, J.F.; Yu, W.W.; Cheng, Y.; White, I.M.; Rubloff, G.W. Simple SERS Substrates: Powerful, Portable, and Full of Potential. Phys. Chem. Chem. Phys. 2014, 16, 2224–2239. [Google Scholar] [CrossRef]
- Nguyen, M.-K.; Su, W.-N.; Hwang, B.-J. A Plasmonic Coupling Substrate Based on Sandwich Structure of Ultrathin Silica-Coated Silver Nanocubes and Flower-Like Alumina-Coated Etched Aluminum for Sensitive Detection of Biomarkers in Urine. Adv. Healthc. Mater. 2017, 6, 1601290. [Google Scholar] [CrossRef]
- Treerattrakoon, K.; Roeksrungruang, P.; Dharakul, T.; Japrung, D.; Faulds, K.; Graham, D.; Bamrungsap, S. Detection of a miRNA Biomarker for Cancer Diagnosis Using SERS Tags and Magnetic Separation. Anal. Methods 2022, 14, 1938–1945. [Google Scholar] [CrossRef]
- Ni, J.; Lipert, R.J.; Dawson, G.B.; Porter, M.D. Immunoassay Readout Method Using Extrinsic Raman Labels Adsorbed on Immunogold Colloids. Anal. Chem. 1999, 71, 4903–4908. [Google Scholar] [CrossRef] [PubMed]
- Porter, M.D.; Lipert, R.J.; Siperko, L.M.; Wang, G.; Narayanan, R. SERS as a Bioassay Platform: Fundamentals, Design, and Applications. Chem. Soc. Rev. 2008, 37, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.Y.; Owens, N.A.; Wampler, R.D.; Ying, Y.; Granger, J.H.; Porter, M.D.; Takahashi, M.; Shimazu, K. Succinimidyl Ester Surface Chemistry: Implications of the Competition between Aminolysis and Hydrolysis on Covalent Protein Immobilization. Langmuir 2014, 30, 12868–12878. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ren, K.; Zhou, J. Aluminum-Based Localized Surface Plasmon Resonance for Biosensing. TrAC Trends Anal. Chem. 2016, 80, 486–494. [Google Scholar] [CrossRef]
- Atanasov, P.A.; Nedyalkov, N.N.; Dikovska, A.O.; Fukata, N.; Jevasuwan, W. Aluminum Nanostructures for 355 Nm Surface-Enhanced Raman Spectroscopy of Fluorescing Chemicals. J. Raman Spectrosc. 2023, 54, 1383–1391. [Google Scholar] [CrossRef]
- O’Connell, M.A.; Belanger, B.A.; Haaland, P.D. Calibration and Assay Development Using the Four-Parameter Logistic Model. Chemom. Intell. Lab. Syst. 1993, 20, 97–114. [Google Scholar] [CrossRef]
- Arbuz, A.; Sultangaziyev, A.; Rapikov, A.; Kunushpayeva, Z.; Bukasov, R. How Gap Distance between Gold Nanoparticles in Dimers and Trimers on Metallic and Non-Metallic SERS Substrates Can Impact Signal Enhancement. Nanoscale Adv. 2022, 4, 268–280. [Google Scholar] [CrossRef]
- Sergiienko, S.; Moor, K.; Gudun, K.; Yelemessova, Z.; Bukasov, R. Nanoparticle–Nanoparticle vs. Nanoparticle–Substrate Hot Spot Contributions to the SERS Signal: Studying Raman Labelled Monomers, Dimers and Trimers. Phys. Chem. Chem. Phys. 2017, 19, 4478–4487. [Google Scholar] [CrossRef]
- Willets, K.A. Super-Resolution Imaging of SERS Hot Spots. Chem. Soc. Rev. 2014, 43, 3854–3864. [Google Scholar] [CrossRef]
- Shah, J.; Purohit, R.; Singh, R.; Karakoti, A.S.; Singh, S. ATP-Enhanced Peroxidase-like Activity of Gold Nanoparticles. J. Colloid Interface Sci. 2015, 456, 100–107. [Google Scholar] [CrossRef]
- Zhou, Z.; Xiao, R.; Cheng, S.; Wang, S.; Shi, L.; Wang, C.; Qi, K.; Wang, S. A Universal SERS-Label Immunoassay for Pathogen Bacteria Detection Based on Fe3O4@Au-Aptamer Separation and Antibody-Protein A Orientation Recognition. Anal. Chim. Acta 2021, 1160, 338421. [Google Scholar] [CrossRef]
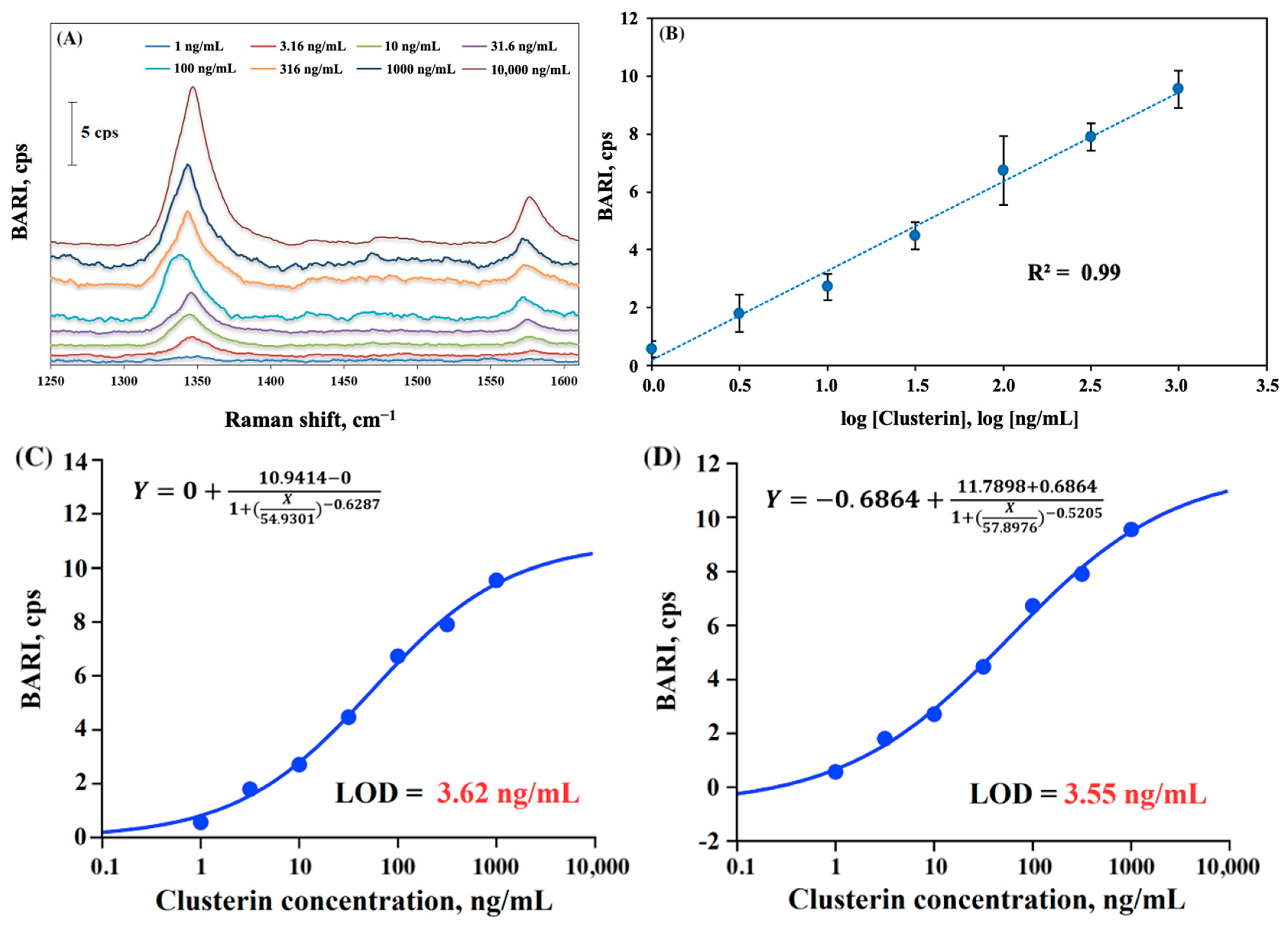
| Concentration, ng/mL | BARI, cps |
|---|---|
| 1 | 0.57 |
| 3.16 | 1.8 |
| 10 | 2.71 |
| 31.6 | 4.47 |
| 100 | 6.73 |
| 316 | 7.91 |
| 1000 | 9.55 |
| St. Dev for blank | 0.55927 |
| 3xSt. Dev. | 1.67781 |
| LOD, ng/mL | 3.03 |
| LOD, pM | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mergenbayeva, S.; Terzapulo, X.; Bukasov, R. Detection of Biomarker Clusterin in SERS Immunoassays on Al Foil After Substrate Selection and Assay Optimization with Fluorescently Labeled Antibodies. Molecules 2025, 30, 3974. https://doi.org/10.3390/molecules30193974
Mergenbayeva S, Terzapulo X, Bukasov R. Detection of Biomarker Clusterin in SERS Immunoassays on Al Foil After Substrate Selection and Assay Optimization with Fluorescently Labeled Antibodies. Molecules. 2025; 30(19):3974. https://doi.org/10.3390/molecules30193974
Chicago/Turabian StyleMergenbayeva, Saule, Xeniya Terzapulo, and Rostislav Bukasov. 2025. "Detection of Biomarker Clusterin in SERS Immunoassays on Al Foil After Substrate Selection and Assay Optimization with Fluorescently Labeled Antibodies" Molecules 30, no. 19: 3974. https://doi.org/10.3390/molecules30193974
APA StyleMergenbayeva, S., Terzapulo, X., & Bukasov, R. (2025). Detection of Biomarker Clusterin in SERS Immunoassays on Al Foil After Substrate Selection and Assay Optimization with Fluorescently Labeled Antibodies. Molecules, 30(19), 3974. https://doi.org/10.3390/molecules30193974







