Solvent-Responsive Luminescence of an 8-Hydroxyquinoline-Modified 1H-Imidazo[4,5-f][1,10]phenanthroline Ligand and Its Cu(I) Complexes: Excited-State Mechanisms and Structural Effects
Abstract
1. Introduction
2. Results and Discussion
2.1. Syntheses
2.2. X-Ray Structure Analysis
2.3. FTIR Spectra
2.4. 1H and 31P{1H} NMR Spectra
2.5. Photophysical Properties
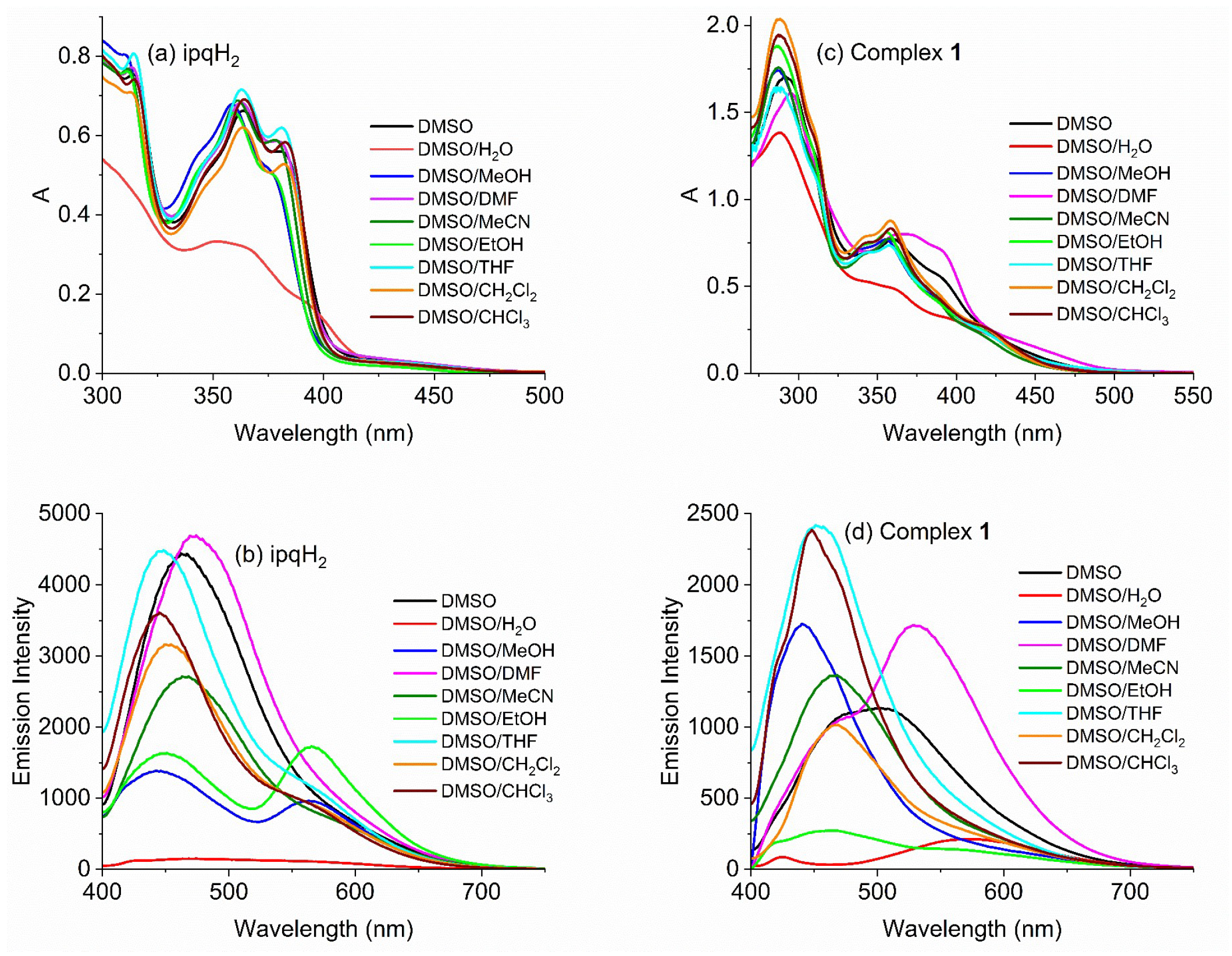
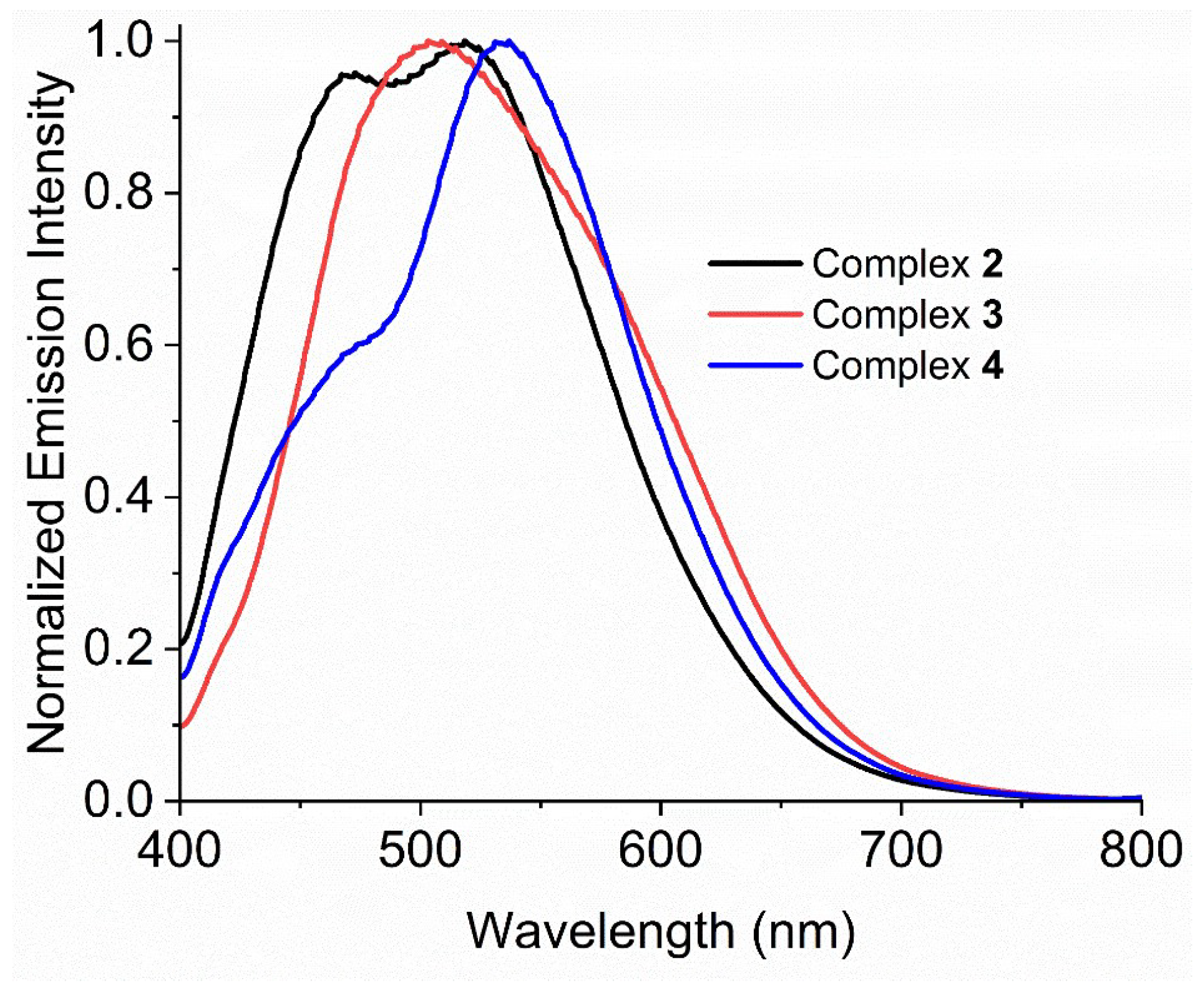
2.5.1. Electron Absorption and Emission Spectra in DMSO
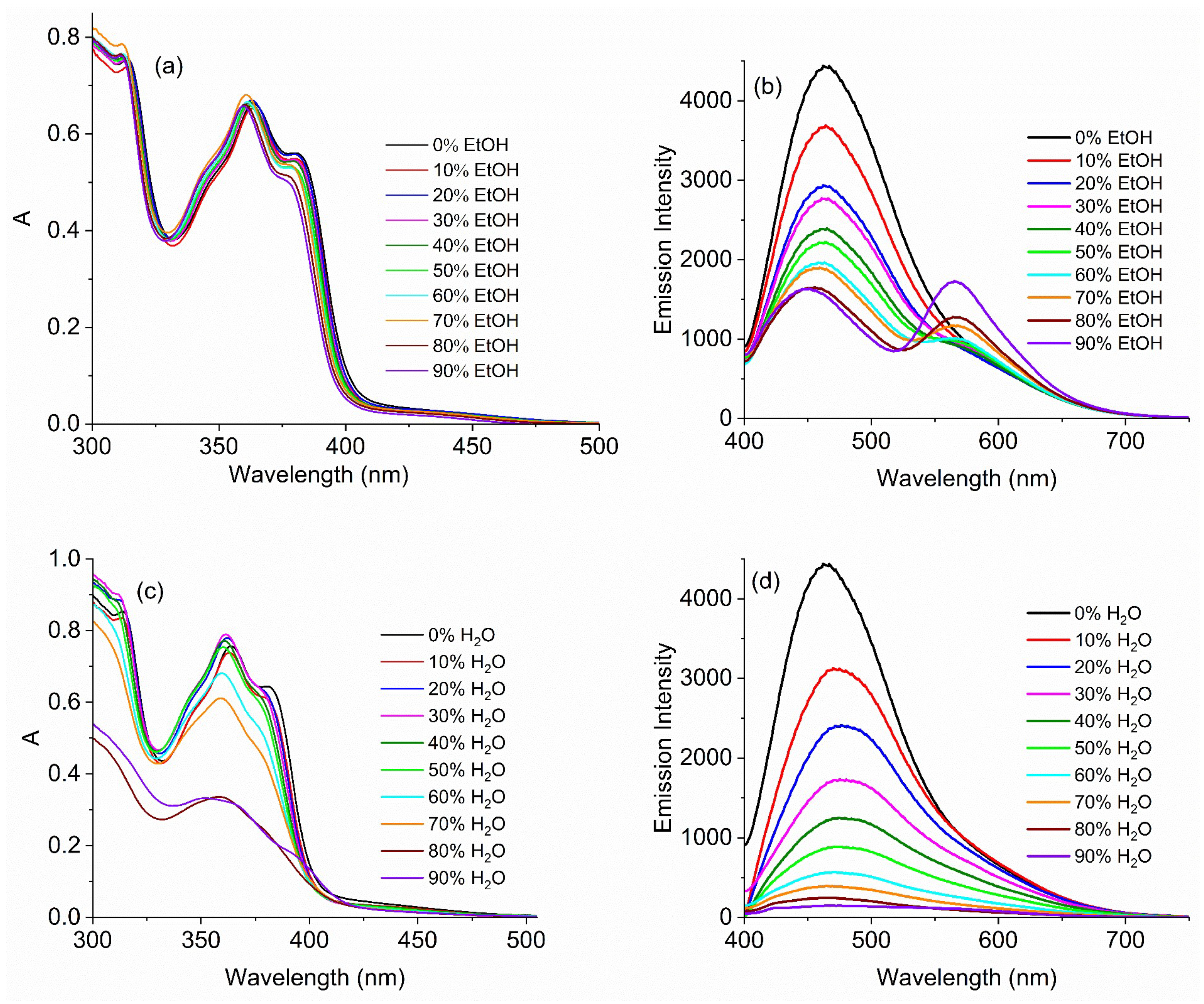
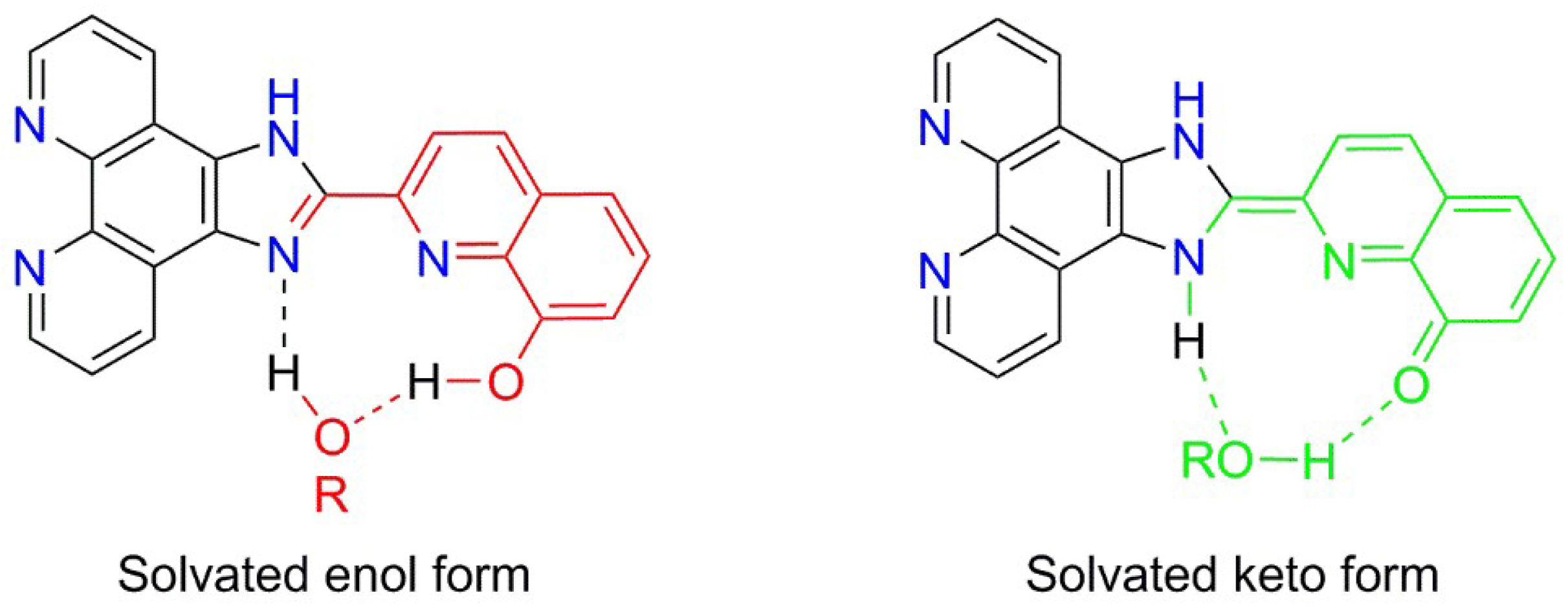
2.5.2. Solvent Effects on Photoluminescence
3. Materials and Methods
3.1. Chemicals and Instrumentation
3.2. Synthesis
3.2.1. IpqH2
3.2.2. [Cu(ipqH2)(POP)]PF6 (1)
3.2.3. [Cu(ipqH2)(xantphos)]PF6 (2)
3.2.4. [Cu2(ipqH2)(POP)2](PF6)2 (3)
3.2.5. [Cu2(ipqH2)(xantphos)2](PF6)2 (4)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexander, C.; Guo, Z.; Glover, P.B.; Faulkner, S.; Pikramenou, Z. Luminescent Lanthanides in Biorelated Applications: From Molecules to Nanoparticles and Diagnostic Probes to Therapeutics. Chem. Rev. 2025, 125, 2269–2370. [Google Scholar] [CrossRef]
- Lee, L.C.; Lo, K.K. Shining New Light on Biological Systems: Luminescent Transition Metal Complexes for Bioimaging and Biosensing Applications. Chem. Rev. 2024, 124, 8825–9014. [Google Scholar] [CrossRef]
- Chang, C.J. Introduction: Fluorescent Probes in Biology. Chem. Rev. 2024, 124, 11639–11640. [Google Scholar] [CrossRef]
- Yang, J.; Feng, X.; Xie, G.; Li, N.; Li, J.; Song, X.; Li, M.; Zhang, J.; Chang, X.; Li, K. A Modular Approach to Efficient Thermally Activated Delayed Fluorescence from Metal-Perturbed Intraligand Charge-Transfer Excited State of Au(I) Complexes. Sci. China Chem. 2024, 67, 4149–4157. [Google Scholar] [CrossRef]
- Tzeng, B.C.; Chen, Y.T.; Chang, J.H.; Sun, B.J.; Chang, A.H.H.; Chien, S.Y. Supramolecular Assembly, Solvent-Induced, and Mechanochromic Luminescence of Au(I) Quinoline-8-thiolates. Inorg. Chem. 2024, 63, 24532–24541. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Zhang, Y.; Liu, S.; Zhang, J. Synthesis, Structure and Tri-stimuli-responsive Luminescent Switching Properties of a Bis(σ-acetylide) Platinum(II) Complex. J. Organomet. Chem. 2024, 1004, 122948. [Google Scholar] [CrossRef]
- Ni, J.; Guo, Z.; Zhu, Q.; Liu, S.; Zhang, J. The Two-stepwise Luminescent Switching Properties of Triple-stimuli-responsive Platinum(II) Complexes Bearing 4,4′-Bis (2-phenylethynyl)-2,2′-bipyridine Ligand. Dyes Pigments 2023, 217, 111406. [Google Scholar] [CrossRef]
- Joshi, B.; Shivashankar, M. Recent Advancement in the Synthesis of Ir-Based Complexes. ACS Omega 2023, 8, 43408–43432. [Google Scholar] [CrossRef]
- Gautam, A.; Sasmal, P.K. Eradication of Antibiotic-Resistant Gram-Positive Bacteria and Biofilms by Rationally Designed AIE-Active Iridium(III) Complexes Derived from Cyclometalating 2-Phenylquinoline and Ancillary Bipyridyl Ligands. Inorg. Chem. 2025, 64, 2905–2918. [Google Scholar] [CrossRef]
- Beaudelot, J.; Oger, S.; Perusko, s.; Phan, T.; Teunens, T.; Moucheron, C.; Evano, G. Photoactive Copper Complexes: Properties and Applications. Chem. Rev. 2022, 122, 16365–16609. [Google Scholar] [CrossRef]
- Cui, S.; Zhao, Z.; Wu, Z.; Chen, J.; Liu, S.; Hu, Z.; Zhou, T.; Li, X.L. A 1H-imidazo[4,5-f][1,10]phenanthroline Based Ligand and its Binuclear Cu(I) Complexes: Syntheses, Structures and Luminescence Sensing for Cr2O72− and MnO4−. Polyhedron 2024, 263, 117210. [Google Scholar] [CrossRef]
- Liu, X.; Li, R.; Ma, L.; Feng, X.; Ding, Y. Influences of the Protonic State of an Imidazole-phenanthroline Ligand on the Luminescence Properties of Copper(I) Complexes: Experimental and Theoretical Research. New J. Chem. 2016, 40, 619–625. [Google Scholar] [CrossRef]
- Wu, Z.; Cui, S.; Zhao, Z.; He, B.; Li, X.L. Photophysical Properties of Homobimetallic Cu(I)–Cu(I) and Heterobimetallic Cu(I)–Ag(I) complexes of 2-(6-Bromo-2-pyridyl)-1H-imidazo[4,5-f][1,10]phenanthroline. New J. Chem. 2022, 46, 8881–8891. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, X.; Shan, Y.; Zhang, X.; Kong, W.; Lu, Y.; Tan, Z.; Li, X.L. Naked-eye Repeatable Off–on–off and On–off–on Switching Luminescence of Copper(I)-1H-imidazo[4,5-f][1,10]phenanthroline Complexes with Reversible Acid–base Responses. Dalton Trans. 2019, 48, 2430–2441. [Google Scholar] [CrossRef]
- Li, G.F.; Zhang, X.Y.; Li, R.F.; Liu, X.F. Synthesis and Properties of Two New Cu(I) Complexes Based on 5,6-Substituted Imidazole-2,9-dimethyl-1,10-phenanthroline and Triphenylphosphine. Russ. J. Gen. Chem. 2016, 86, 387–390. [Google Scholar] [CrossRef]
- Rozhkov, A.V.; Eliseeva, S.N.; Baykov, S.V.; Zelenkov, L.E.; Goriachiy, D.O.; Taydakov, I.V. Copper(I) Ionic Complexes Based on Imidazo[4,5-f][1,10]phenanthrolin Diimine Chelating Ligands: Crystal Structures, and Photo- and Electroluminescence Properties. New J. Chem. 2020, 44, 110–120. [Google Scholar] [CrossRef]
- Xu, S.; Wang, J.; Zhao, F.; Xia, H.; Wang, Y. Copper(I) Complexes of Phenanthrolineimidazole Ligands: Structures, Photophysical Properties, and Quantum Chemical Studies. Transit. Met. Chem. 2015, 40, 723–732. [Google Scholar] [CrossRef]
- Hori, A.; Matsumoto, A.; Ikenouchi, J.; Konishi, G. D−π−A Fluorophores with Strong Solvatochromism for Single-Molecule Ratiometric Thermometers. J. Am. Chem. Soc. 2025, 147, 9953–9961. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mu, H.; Sun, Y.; Gao, J.; Zhu, X.; Li, H. Modulating the ESIPT Mechanism and Luminescence Characteristics of Two Reversible Fluorescent Probes by Solvent Polarity: A Novel Perspective. Molecules 2024, 29, 1629. [Google Scholar] [CrossRef]
- Pylova, E.K.; Sukhikh, T.S.; Prieto, A.; Jaroschik, F.; Konchenko, S.N. Chemistry of 2-(2′-Aminophenyl)benzothiazole Derivatives: Syntheses, Photophysical Properties and Applications. Molecules 2024, 30, 1659. [Google Scholar] [CrossRef]
- Nakashima, K.; Georgiev, A.; Yordanov, D.; Matsushima, Y.; Hirashima, S.; Miura, T.; Antonov, L. Solvent-Triggered Long-Range Proton Transport in 7-Hydroxyquinoline Using a Sulfonamide Transporter Group. J. Org. Chem. 2022, 87, 6794–6806. [Google Scholar] [CrossRef]
- Yi, S.; Li, B.; Fu, P.; Pan, M.; Su, C.Y. Interplay of Dual-Proton Transfer Relay to Achieve Full-Color Panel Luminescence in Excited-State Intramolecular Proton Transfer (ESIPT) Fluorophores. ACS Appl. Mater. Interfaces 2023, 15, 3172–3181. [Google Scholar] [CrossRef]
- Das, M.; Brahma, M.; Krishnamoorthy, G. Light-Driven Switching Between Intramolecular Proton-Transfer and Charge-Transfer States. J. Phys. Chem. B 2021, 125, 2339–2350. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Li, S.; Zhang, F.Q.; Zhang, X.M. Simultaneous Luminescent Thermochromism, Vapochromism, Solvatochromism, and Mechanochromism in a C3-Symmetric Cubane [Cu4I4P4] Cluster Without Cu−Cu Interaction. Inorg. Chem. 2016, 55, 7323–7325. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hu, G.; Mo, L.; Li, Y.; Wei, H.; Lei, B.; Zhang, X.; Hu, C.; Zhuang, J.; Liu, Y. Red, Orange, Yellow and Green Luminescence by Carbon Dots: Hydrogen-Bond-Induced Solvation Effects. Nanoscale 2021, 13, 6846–6855. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, S.; Mi, Y.; Zhao, H.; Zheng, Z.; Zhao, X. A Hydroxyquinoline-Appended Ruthenium(II)-polypyridyl Complex That Induces and Stabilizes G-quadruplex DNA. J. Coord. Chem. 2019, 72, 201–217. [Google Scholar] [CrossRef]
- Sun, Z.; Zhu, N.; Pan, X.; Wang, G.; Li, Z.; Xin, X.; Han, H.; Feng, Y.; Jin, Q.; Yang, Y.; et al. A New Application of Terahertz Time-domain Absorption Spectra in Luminescent Complexes: Characterization of the C–H⋯π Weak Interactions in Cu(I) Complexes. Dalton Trans. 2021, 50, 10214–10224. [Google Scholar] [CrossRef]
- Carosati, E.; Sciabola, S.; Cruciani, G. Hydrogen Bonding Interactions of Covalently Bonded Fluorine Atoms: From Crystallographic Data to a New Angular Function in the GRID Force Field. J. Med. Chem. 2004, 47, 5114–5125. [Google Scholar] [CrossRef]
- Jin, L.; Lei, Y.; Dodd, A.; Nockemann, P.; Gunaratne, H.Q.N. A Bis-Imidazolium Receptor for the Selective Crystallization of Fluoride through Hydrogen Bonding. Cryst. Growth Des. 2025, 25, 1725–1730. [Google Scholar] [CrossRef]
- Ma, X.; Lu, J.; Yang, P.; Zhang, Z.; Huang, B.; Li, R.; Ye, R. 8-Hydroxyquinoline-Modified Ruthenium(II) Polypyridyl Complexes for JMJD Inhibition and Photodynamic Antitumor Therapy. Dalton Trans. 2022, 51, 13902–13909. [Google Scholar] [CrossRef]
- Wang, J.; Lin, W.; Li, W. Single Fluorescent Probe Displays a Distinct Response to Zn2+ and Cd2+. Chem. Eur. J. 2012, 18, 13629–13632. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.; Pertegás, A.; Longo, G.; Martínez, L.; Cerdá, J.; Junquera-Hernández, J.M.; Prescimone, A.; Constable, E.C.; Housecroft, C.E.; Ortí, E.; et al. Shine Bright or Live Long: Substituent Effects in [Cu(N^N)(P^P)]+-based Light-emitting Electrochemical Cells Where N^N is a 6-Substituted 2,2′-Bipyridine. J. Mater. Chem. C 2016, 4, 3857–3871. [Google Scholar] [CrossRef]
- Crestani, M.G.; Manbeck, G.F.; Brennessel, W.W.; McCormick, T.M.; Eisenberg, R. Synthesis and Characterization of Neutral Luminescent Diphosphine Pyrrole- and Indole-Aldimine Copper(I) Complexes. Inorg. Chem. 2011, 50, 7172–7188. [Google Scholar] [CrossRef] [PubMed]
- Shee, M.; Schleisiek, J.; Maity, N.; Das, G.; Montesdeoca, N.; Ha-Thi, M.; Gore, K.R.; Karges, J.; Singh, N.D.P. Exploring Excited-State Intramolecular Proton-Coupled Electron Transfer in Dinuclear Ir(III)-Complex via Covalently Tagged Hydroquinone: Phototherapy Through Futile Redox Cycling. Small 2025, 21, 2408437. [Google Scholar] [CrossRef]
- Zhao, N.; Wu, Y.; Wen, H.; Zhang, X.; Chen, Z.N. Conversion from ILCT to LLCT/MLCT Excited State by Heavy Metal Ion Binding in Iridium(III) Complexes with Functionalized 2,2′-Bipyridyl Ligands. Organometallics 2009, 28, 5603–5611. [Google Scholar] [CrossRef]
- Favarin, L.R.V.; Rosa, P.P.; Pizzuti, L.; Machulek, A., Jr.; Caires, A.R.L.; Bezerra, L.S.; Pinto, L.M.C.; Maia, G.; Gatto, C.C.; Back, D.F.; et al. Synthesis and Structural Characterization of New Heteroleptic Copper(I) Complexes Based on Mixed Phosphine/Thiocarbamoyl-pyrazoline ligands. Polyhedron 2017, 121, 185–190. [Google Scholar] [CrossRef]
- Shahida, M.; Misra, A. Photoenolization via Excited State Proton Transfer and Ion Sensing Studies of Hydroxy Imidazole Derivatives. J. Photochem. Photobiol. A Chem. 2017, 335, 190–199. [Google Scholar] [CrossRef]
- Alarcos, N.; Gutierrez, M.; Liras, M.; Sánchez, F.; Douhal, A. An Abnormally Slow Proton Transfer Reaction in a Simple HBO Derivative Due to Ultrafast Intramolecular-Charge Transfer Events. Phys. Chem. Chem. Phys. 2015, 17, 16257–16269. [Google Scholar] [CrossRef]
- Kang, B.; Ko, K.C.; Park, S.-Y.; Jang, D.J.; Lee, J.Y. Solvent Effect on the Excited-State Proton Transfer of 7-Hydroxyquinoline Along a Hydrogen-Bonded Ethanol Dimer. Phys. Chem. Chem. Phys. 2011, 13, 6332–6339. [Google Scholar] [CrossRef]
- Kwon, O.-H.; Lee, Y.-S.; Yoo, B.K.; Jang, D.J. Excited-State Triple Proton Transfer of 7-Hydroxyquinoline Along a Hydrogen-Bonded Alcohol Chain: Vibrationally Assisted Proton Tunneling. Angew. Chem. Int. Ed. 2006, 45, 415–419. [Google Scholar] [CrossRef]
- Chou, P.-T.; Wei, C.-Y.; Wang, C.-R.C.; Hung, F.-T.; Chang, C.-P. Proton-Transfer Tautomerism of 7-Hydroxyquinolines Mediated by Hydrogen-Bonded Complexes. J. Phys. Chem. A 1999, 103, 1939–1949. [Google Scholar] [CrossRef]
- Zhang, L.; Chai, F.; Dong, H.; Bao, Y.; Yan, K.; Min, S.; Yao, Y.; Li, S.; Liu, Y.; Gao, T.; et al. Coumarin-Based ACQ-AIE Conversion Photosensitizer for Mitochondrial Imaging and Synergistic Cancer Therapy. J. Phys. Chem. Lett. 2024, 15, 10866–10872. [Google Scholar] [CrossRef]
- Hu, Y.; Khoo, R.S.H.; Lu, J.; Zhang, X.; Zhang, J. Robust Carbazole-Based Rare-Earth MOFs: Tunable White-Light Emission for Temperature and DMF Sensing. ACS Appl. Mater. Interfaces 2022, 14, 41178–41185. [Google Scholar] [CrossRef]
- Bruker. APEX2, SAINT and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2009. [Google Scholar]
- Sheldrick, G.M. SHELXT-Integrated Space-group and Crystal-structure Determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A Tool for the Calculation of the Disordered Solvent Contribution to the Calculated Structure Factors. Acta Crystallogr. Sect. C Struct. Commun. 2015, 71, 9–18. [Google Scholar] [CrossRef]

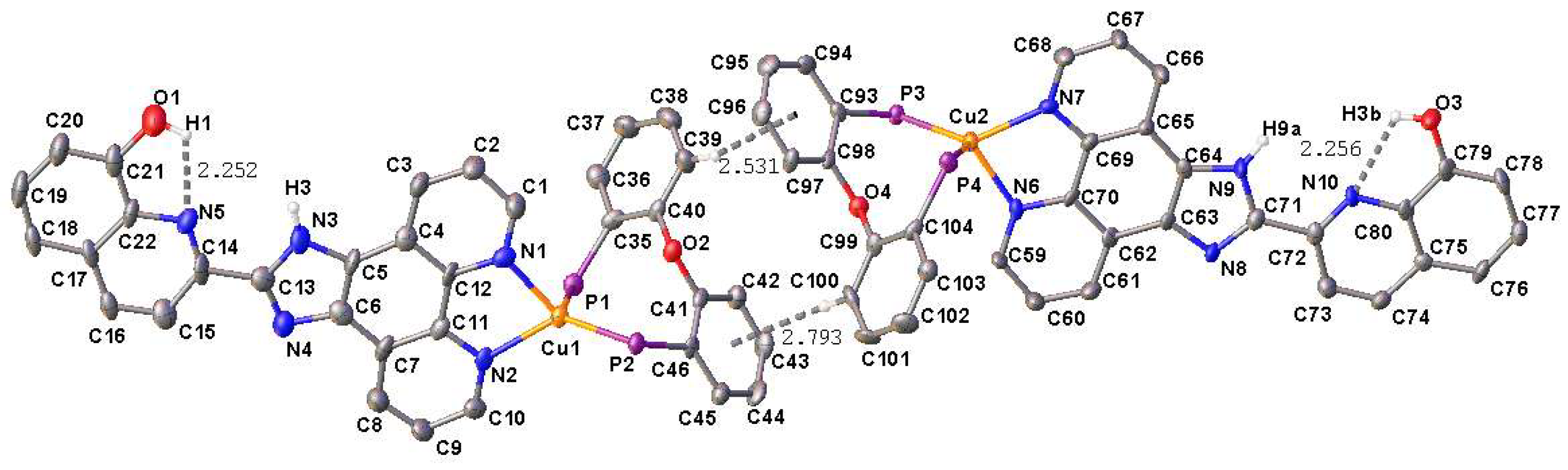
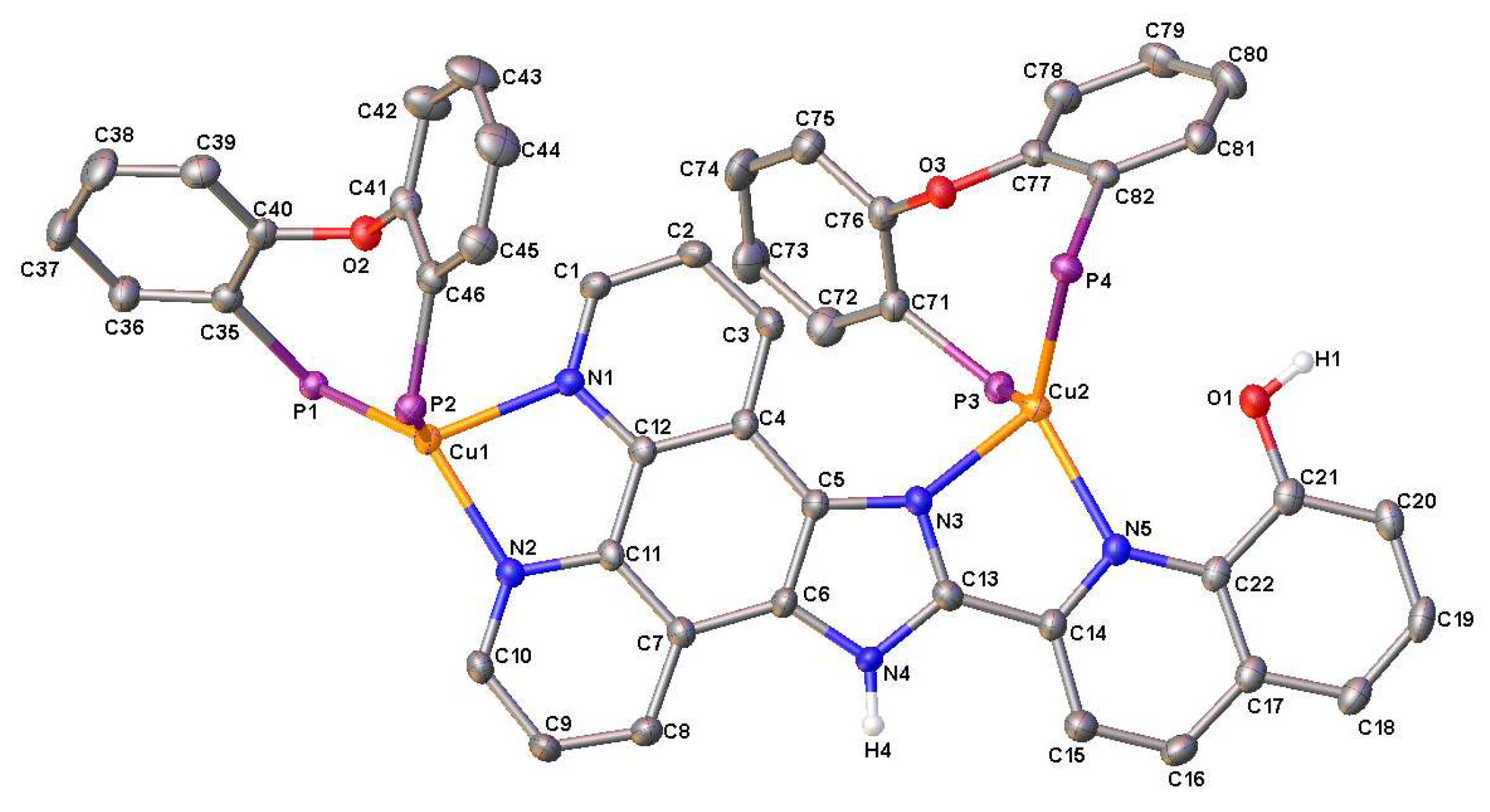
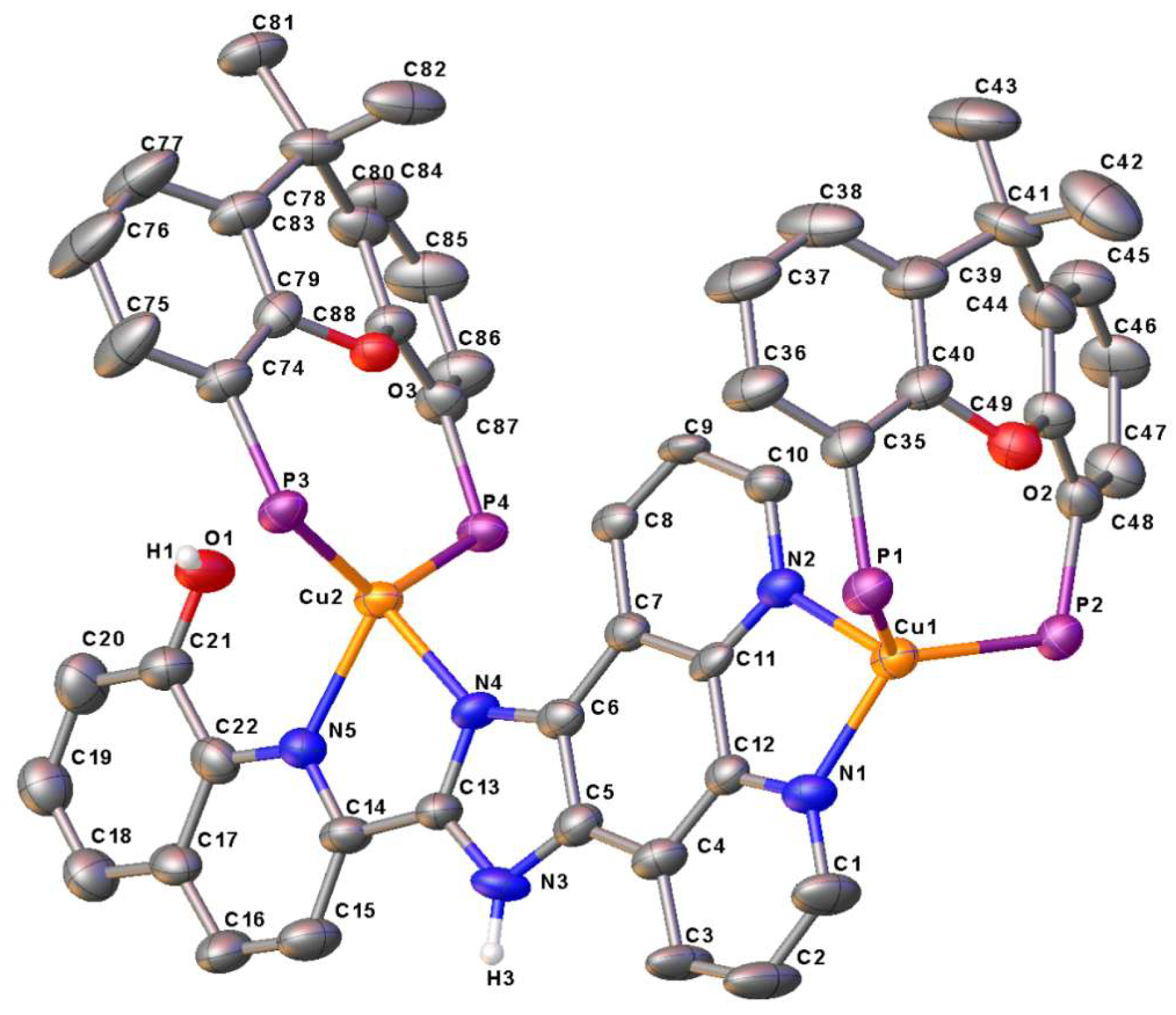




| 1·CH2Cl2 | 3 | 4 | |||
|---|---|---|---|---|---|
| Cu1–N1 | 2.067 (5) | Cu1–N1 | 2.063 (2) | Cu1–N1 | 2.047 (5) |
| Cu1–N2 | 2.092 (5) | Cu1–N2 | 2.098 (2) | Cu1–N2 | 2.083 (5) |
| Cu2–N6 | 2.068 (5) | Cu2–N3 | 2.098 (2) | Cu2–N4 | 2.099 (5) |
| Cu2–N7 | 2.069 (5) | Cu2–N5 | 2.204 (2) | Cu2–N5 | 2.123 (5) |
| Cu1–P1 | 2.2990 (16) | Cu1–P1 | 2.2281 (7) | Cu1–P1 | 2.239 (2) |
| Cu1–P2 | 2.2306 (16) | Cu1–P2 | 2.2740 (7) | Cu1–P2 | 2.2334 (19) |
| Cu2–P3 | 2.2395 (16) | Cu2–P3 | 2.3142 (7) | Cu2–P3 | 2.2816 (19) |
| Cu2–P4 | 2.2948 (16) | Cu2–P4 | 2.2516 (7) | Cu2–P4 | 2.2765 (18) |
| C13–N3 | 1.351 (8) | C13–N3 | 1.322 (3) | C13–N3 | 1.394 (9) |
| C13–N4 | 1.321 (8) | C13–N4 | 1.360 (3) | C13–N4 | 1.330 (8) |
| C71–N8 | 1.323 (7) | C21–O1 | 1.355 (3) | C21–O1 | 1.337 (8) |
| C71–N9 | 1.363 (7) | ||||
| C21–O1 | 1.389 (8) | ||||
| C79–O3 | 1.361 (7) | ||||
| N1–Cu1–N2 | 80.0 (2) | N1–Cu1–N2 | 80.35 (8) | N1–Cu1–N2 | 80.6 (2) |
| N6–Cu2–N7 | 80.78 (19) | N3–Cu2–N5 | 79.36 (8) | N4–Cu2–N5 | 79.2 (2) |
| P1–Cu1–P2 | 114.74 (6) | P1–Cu1–P2 | 120.80 (3) | P1–Cu1–P2 | 116.75 (7) |
| P3–Cu2–P4 | 112.99 (6) | P3–Cu2–P4 | 117.20 (3) | P3–Cu2–P4 | 129.85 (7) |
| N1–Cu1–P1 | 99.82 (12) | N1–Cu1–P1 | 115.00 (6) | N1–Cu1–P1 | 117.69 (16) |
| N1–Cu1–P2 | 124.61 (15) | N1–Cu1–P2 | 98.62 (6) | N1–Cu1–P2 | 117.66 (17) |
| N2–Cu1–P1 | 99.82 (12) | N2–Cu1–P1 | 130.13 (6) | N2–Cu1–P1 | 107.73 (15) |
| N2–Cu1–P2 | 128.96 (14) | N2–Cu1–P2 | 101.31 (6) | N2–Cu1–P2 | 109.16 (15) |
| N6–Cu2–P3 | 118.40 (13) | N3–Cu2–P3 | 101.51 (6) | N4–Cu2–P3 | 104.70 (16) |
| N6–Cu2–P4 | 105.34 (13) | N3–Cu2–P4 | 118.24 (6) | N4–Cu2–P4 | 106.90 (16) |
| N7–Cu2–P3 | 127.80 (13) | N5–Cu2–P3 | 109.26 (6) | N5–Cu2–P3 | 110.80 (16) |
| N7–Cu2–P4 | 106.14 (13) | N5–Cu2–P4 | 123.61 (6) | N5–Cu2–P4 | 112.65 (16) |
| Compound | λabs/nm (ε/M−1cm−1) (DMSO) | λem/nm (Solid) | λem/nm (DMSO) |
|---|---|---|---|
| ipqH2 | 283 (43,370), 315 (30,030), 364 (26,480), 381 (22,400) | 473 | 463 |
| 1 | 291 (67,880), 360 (30,830), 400~500 | 477, 551 | 501 |
| 2 | 291 (80,220), 360 (31,900), 400~500 | 475, 550 | 484 |
| 3 | 290 (63,530), 360 (23,550), 400~500 | 472, 609 | 494 |
| 4 | 287 (68,770), 360 (21,160), 400~500 | 472, 543 | 490 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Liu, S.; Cui, S.; Zhang, Y.; Jiang, Z.; Li, X. Solvent-Responsive Luminescence of an 8-Hydroxyquinoline-Modified 1H-Imidazo[4,5-f][1,10]phenanthroline Ligand and Its Cu(I) Complexes: Excited-State Mechanisms and Structural Effects. Molecules 2025, 30, 3973. https://doi.org/10.3390/molecules30193973
Zhao Z, Liu S, Cui S, Zhang Y, Jiang Z, Li X. Solvent-Responsive Luminescence of an 8-Hydroxyquinoline-Modified 1H-Imidazo[4,5-f][1,10]phenanthroline Ligand and Its Cu(I) Complexes: Excited-State Mechanisms and Structural Effects. Molecules. 2025; 30(19):3973. https://doi.org/10.3390/molecules30193973
Chicago/Turabian StyleZhao, Zhenqin, Siyuan Liu, Shu Cui, Yichi Zhang, Ziqi Jiang, and Xiuling Li. 2025. "Solvent-Responsive Luminescence of an 8-Hydroxyquinoline-Modified 1H-Imidazo[4,5-f][1,10]phenanthroline Ligand and Its Cu(I) Complexes: Excited-State Mechanisms and Structural Effects" Molecules 30, no. 19: 3973. https://doi.org/10.3390/molecules30193973
APA StyleZhao, Z., Liu, S., Cui, S., Zhang, Y., Jiang, Z., & Li, X. (2025). Solvent-Responsive Luminescence of an 8-Hydroxyquinoline-Modified 1H-Imidazo[4,5-f][1,10]phenanthroline Ligand and Its Cu(I) Complexes: Excited-State Mechanisms and Structural Effects. Molecules, 30(19), 3973. https://doi.org/10.3390/molecules30193973







