Preclinical Theranostic Profiling of [64Cu]Cu-Acetate in Prostate Cancer
Abstract
1. Introduction
2. Results
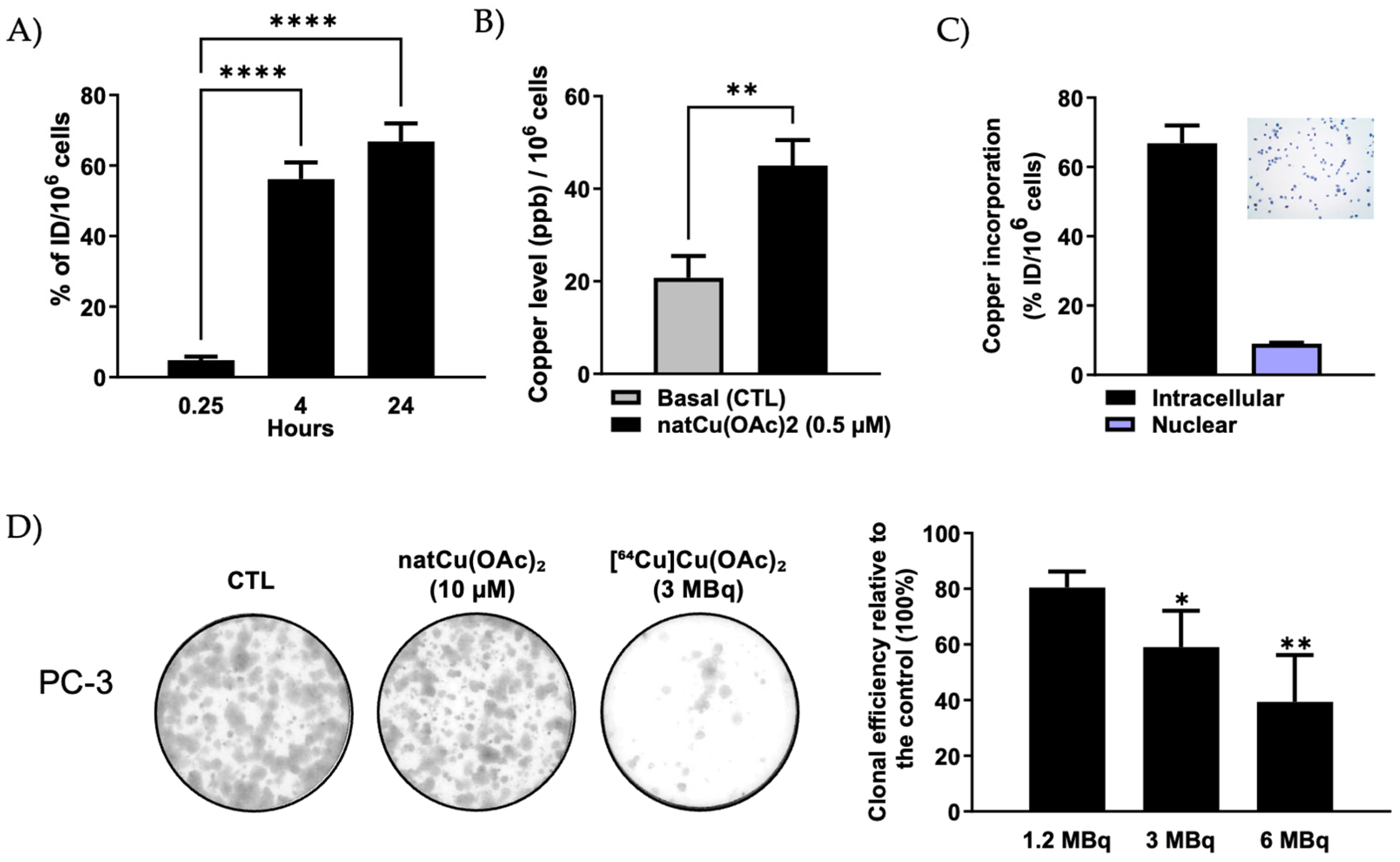
2.1. Cellular Accumulation and Subcellular Localization of [64Cu]Cu-Acetate in PC-3 Cells
2.2. In Vitro Radiocytotoxicity of [64Cu]Cu-Acetate in PC-3 Cells
2.3. Ex Vivo Biodistribution of [64Cu]Cu-Acetate and [64Cu]CuCl2 in PC-3 Tumor Xenograft-Bearing Nude Mice
2.4. In Vivo PET/CT Imaging of [64Cu]Cu-Acetate in PC-3 Xenograft-Bearing Nude Mice
2.5. Therapeutic Effect of [64Cu]Cu-Acetate on Tumor Growth in PC-3 Tumor-Bearing Mice
3. Discussion
4. Materials and Methods
4.1. Production of [64Cu]Cu
4.2. Cell Cultures
4.3. In Vitro Cellular Uptake and Nuclear Localization of [64Cu]Cu-Acetate
4.4. Cell Uptake Analysis of NatCu-Acetate Measured by ICP-MS
4.5. Clonogenic Assay
4.6. Subcutaneous Tumor Xenograft Model
4.7. Biodistribution and PET Imaging in PC-3 Xenograft Mice
4.8. Radiotherapy with [64Cu]Cu-Acetate in PC-3 Xenograft Mice
4.9. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CTR1 | Copper transporter 1 |
| FBS | Fetal bovine serum |
| PBS | Phosphate-buffered saline |
| HBSS | Hank’s balanced salt solution |
| ICP-MS | Inductively coupled plasma–mass spectrometry |
| PCa | Prostate cancer |
| Pen/Strep | Penicillin/Streptomycin |
| PET | Positron emission tomography |
References
- Capriotti, G.; Piccardo, A.; Giovannelli, E.; Signore, A. Targeting Copper in Cancer Imaging and Therapy: A New Theragnostic Agent. J. Clin. Med. 2022, 12, 223. [Google Scholar] [CrossRef] [PubMed]
- Brady, D.C.; Crowe, M.S.; Turski, M.L.; Hobbs, G.A.; Yao, X.; Chaikuad, A.; Knapp, S.; Xiao, K.; Campbell, S.L.; Thiele, D.J.; et al. Copper Is Required for Oncogenic BRAF Signalling and Tumorigenesis. Nature 2014, 509, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.I.; Jang, S.J.; Park, J.H.; Lee, Y.J.; Lee, T.S.; Woo, K.S.; Park, H.; Choe, J.G.; An, G.I.; Kang, J.H. Detection of Increased 64Cu Uptake by Human Copper Transporter 1 Gene Overexpression Using PET with 64CuCl2 in Human Breast Cancer Xenograft Model. J. Nucl. Med. 2014, 55, 1692–1698. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Liu, H.; Chen, K.; Hu, X.; Ma, X.; Lan, X.; Zhang, Y.; Cheng, Z. Theranostics of Malignant Melanoma with 64CuCl2. J. Nucl. Med. 2014, 55, 812–817. [Google Scholar] [CrossRef]
- Ferrari, C.; Asabella, A.N.; Villano, C.; Giacobbi, B.; Coccetti, D.; Panichelli, P.; Rubini, G. Copper-64 Dichloride as Theranostic Agent for Glioblastoma Multiforme: A Preclinical Study. Biomed. Res. Int. 2015, 2015, 129764. [Google Scholar] [CrossRef]
- Kong, F.-S.; Ren, C.-Y.; Jia, R.; Zhou, Y.; Chen, J.-H.; Ma, Y. Systematic Pan-Cancer Analysis Identifies SLC31A1 as a Biomarker in Multiple Tumor Types. BMC Med. Genomics 2023, 16, 61. [Google Scholar] [CrossRef]
- Chakravarty, R.; Chakraborty, S.; Dash, A. 64Cu2+ Ions as PET Probe: An Emerging Paradigm in Molecular Imaging of Cancer. Mol. Pharm. 2016, 13, 3601–3612. [Google Scholar] [CrossRef]
- Speltri, G.; Porto, F.; Boschi, A.; Uccelli, L.; Martini, P. Recent Advances in Preclinical Studies of the Theranostic Agent [64Cu]CuCl2. Molecules 2024, 29, 4085. [Google Scholar] [CrossRef]
- Cai, H.; Wu, J.; Muzik, O.; Hsieh, J.-T.; Lee, R.J.; Peng, F. Reduced 64Cu Uptake and Tumor Growth Inhibition by Knockdown of Human Copper Transporter 1 in Xenograft Mouse Model of Prostate Cancer. J. Nucl. Med. 2014, 55, 622–628. [Google Scholar] [CrossRef]
- Peng, F.; Lu, X.; Janisse, J.; Muzik, O.; Shields, A.F. PET of Human Prostate Cancer Xenografts in Mice with Increased Uptake of 64CuCl2. J. Nucl. Med. 2006, 47, 1649–1652. [Google Scholar]
- Guerreiro, J.F.; Alves, V.; Abrunhosa, A.J.; Paulo, A.; Gil, O.M.; Mendes, F. Radiobiological Characterization of 64CuCl2 as a Simple Tool for Prostate Cancer Theranostics. Molecules 2018, 23, 2944. [Google Scholar] [CrossRef]
- Paparo, F.; Peirano, A.; Matos, J.; Bacigalupo, L.; Rossi, U.; Mussetto, I.; Bottoni, G.; Ugolini, M.; Introini, C.; Grillo Ruggieri, F.; et al. Diagnostic Value of Retrospectively Fused 64CuCl2 PET/MRI in Biochemical Relapse of Prostate Cancer: Comparison with Fused 18F-Choline PET/MRI, 64CuCl2 PET/CT, 18F-Choline PET/CT, and mpMRI. Abdom. Radiol. 2020, 45, 3896–3906. [Google Scholar] [CrossRef]
- Piccardo, A.; Paparo, F.; Puntoni, M.; Righi, S.; Bottoni, G.; Bacigalupo, L.; Zanardi, S.; DeCensi, A.; Ferrarazzo, G.; Gambaro, M.; et al. 64CuCl2 PET/CT in Prostate Cancer Relapse. J. Nucl. Med. 2018, 59, 444–451. [Google Scholar] [CrossRef]
- Capasso, E.; Durzu, S.; Piras, S.; Zandieh, S.; Knoll, P.; Haug, A.; Hacker, M.; Meleddu, C.; Mirzaei, S. Role of 64CuCl2 PET/CT in Staging of Prostate Cancer. Ann. Nucl. Med. 2015, 29, 482–488. [Google Scholar] [CrossRef]
- Wang, Q.; Song, D.; Ma, X.; Wu, X.; Jiang, L. Preclinical PET Imaging Study of Lung Cancer with 64CuCl2. Ann. Nucl. Med. 2020, 34, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Wachsmann, J.; Peng, F. Molecular Imaging and Therapy Targeting Copper Metabolism in Hepatocellular Carcinoma. World J. Gastroenterol. 2016, 22, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Fiz, F.; Bottoni, G.; Ugolini, M.; Righi, S.; Cirone, A.; Garganese, M.C.; Verrico, A.; Rossi, A.; Milanaccio, C.; Ramaglia, A.; et al. Diagnostic and Dosimetry Features of [64Cu]CuCl2 in High-Grade Paediatric Infiltrative Gliomas. Mol. Imaging Biol. 2023, 25, 391–400. [Google Scholar] [CrossRef]
- Ahmedova, A.; Todorov, B.; Burdzhiev, N.; Goze, C. Copper Radiopharmaceuticals for Theranostic Applications. Eur. J. Med. Chem. 2018, 157, 1406–1425. [Google Scholar] [CrossRef]
- Peng, F. Recent Advances in Cancer Imaging with 64CuCl2 PET/CT. Nucl. Med. Mol. Imaging 2022, 56, 80–85. [Google Scholar] [CrossRef]
- Gutfilen, B.; Souza, S.A.; Valentini, G. Copper-64: A Real Theranostic Agent. Drug Des. Devel. Ther. 2018, 12, 3235–3245. [Google Scholar] [CrossRef]
- Wang, H.; Chen, X. Visualization of Copper Metabolism by 64CuCl2-PET. Mol. Imaging Biol. 2012, 14, 14–16. [Google Scholar] [CrossRef]
- Gutfilen, B.; Duatti, A. Editorial: In-Vivo Targeting of Nuclear DNA with Radioactive Copper-64 Ions. Front. Med. 2023, 10, 1334294. [Google Scholar] [CrossRef]
- Buchegger, F.; Perillo-Adamer, F.; Dupertuis, Y.M.; Delaloye, A.B. Auger radiation targeted into DNA: A therapy perspective. Eur. J. Nucl. Med. Mol. Imaging 2023, 33, 1352–1363. [Google Scholar] [CrossRef] [PubMed]
- Hatori, Y.; Lutsenko, S. The Role of Copper Chaperone Atox1 in Coupling Redox Homeostasis to Intracellular Copper Distribution. Antioxidants 2016, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Beaino, W.; Guo, Y.; Chang, A.J.; Anderson, C.J. Roles of Atox1 and P53 in the Trafficking of Copper-64 to Tumor Cell Nuclei: Implications for Cancer Therapy. J. Biol. Inorg. Chem. 2014, 19, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Righi, S.; Ugolini, M.; Bottoni, G.; Puntoni, M.; Iacozzi, M.; Paparo, F.; Cabria, M.; Ceriani, L.; Gambaro, M.; Giovanella, L.; et al. Biokinetic and Dosimetric Aspects of 64CuCl2 in Human Prostate Cancer: Possible Theranostic Implications. EJNMMI Res. 2018, 8, 18. [Google Scholar] [CrossRef]
- Wadas, T.J.; Wong, E.H.; Weisman, G.R.; Anderson, C.J. Copper Chelation Chemistry and Its Role in Copper Radiopharmaceuticals. Curr. Pharm. Des. 2007, 13, 3–16. [Google Scholar] [CrossRef]
- Yang, N.; Guo, X.-Y.; Ding, J.; Wang, F.; Liu, T.-L.; Zhu, H.; Yang, Z. Copper-64 Based PET-Radiopharmaceuticals: Ways to Clinical Translational. Semin. Nucl. Med. 2024, 54, 792–800. [Google Scholar] [CrossRef]
- Fu, X.; Zhang, Y.; Jiang, W.; Monnot, A.D.; Bates, C.A.; Zheng, W. Regulation of Copper Transport Crossing Brain Barrier Systems by Cu-ATPases: Effect of Manganese Exposure. Toxicol. Sci. 2014, 139, 432–451. [Google Scholar] [CrossRef]
- Avila-Rodriguez, M.A.; Rios, C.; Carrasco-Hernandez, J.; Manrique-Arias, J.C.; Martinez-Hernandez, R.; García-Pérez, F.O.; Jalilian, A.R.; Martinez-Rodriguez, E.; Romero-Piña, M.E.; Diaz-Ruiz, A. Biodistribution and Radiation Dosimetry of [64Cu]Copper Dichloride: First-in-Human Study in Healthy Volunteers. EJNMMI Res. 2017, 7, 98. [Google Scholar] [CrossRef]
- Ghanaatgar Kasbi, S.; Savard, M.; Couture, F.; Dubuc, C.; Dumulon-Perreault, V.; Nepveu-Traversy, M.-E.; Ait-Mohand, S.; Sabbagh, R.; Geha, S.; Guérin, B.; et al. Theranostic Potential of a New 64Cu-Labeled NOTA-R954 Peptide Conjugate for Kinin B1R Expressing Prostate Cancer. Pharmaceutics 2025, 17, 1215. [Google Scholar] [CrossRef]
- Lutsenko, S. Dynamic and Cell-Specific Transport Networks for Intracellular Copper Ions. J. Cell Sci. 2021, 134, jcs240523. [Google Scholar] [CrossRef]
- Kirsipuu, T.; Zadorožnaja, A.; Smirnova, J.; Friedemann, M.; Plitz, T.; Tõugu, V.; Palumaa, P. Copper(II)-Binding Equilibria in Human Blood. Sci. Rep. 2020, 10, 5686. [Google Scholar] [CrossRef]
- Linder, M.C. Ceruloplasmin and Other Copper Binding Components of Blood Plasma and Their Functions: An Update. Metallomics 2016, 8, 887–905. [Google Scholar] [CrossRef]
- International Atomic Energy Agency (IAEA). Copper-64 Radiopharmaceuticals: Production, Quality Control and Clinical Applications; IAEA Radioisotopes and Radiopharmaceuticals Series No. 7; IAEA: Vienna, Austria, 2022. [Google Scholar]
- Chaudhary, P.K.; Kim, S. An Insight into GPCR and G-Proteins as Cancer Drivers. Cells 2021, 10, 3288. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cai, H.; Wu, X.; Li, L.; Wu, H.; Tian, R. New Frontiers in Molecular Imaging Using Peptide-Based Radiopharmaceuticals for Prostate Cancer. Front. Chem. 2020, 8, 583309. [Google Scholar] [CrossRef] [PubMed]
- Cantiello, F.; Crocerossa, F.; Cascini, G.L.; Russo, G.I.; Ferro, M.; Cimino, S.; Lucarelli, G.; Damiano, R. 64CuCl2 PET/CT as a Potential New Imaging Method in Prostate Cancer: Illusion or Reality? Minerva Urol. Nephrol. 2021, 73, 668–671. [Google Scholar] [CrossRef]
- Pouliot, F.; Saad, F.; Rousseau, E.; Richard, P.O.; Zamanian, A.; Probst, S.; Lévesque, É.; Castonguay, V.; Marcoux, N.; Lodde, M.; et al. Intrapatient Intermetastatic Heterogeneity Determined by Triple-Tracer PET Imaging in mCRPC Patients and Correlation to Survival: The 3TMPO Cohort Study. J. Nucl. Med. 2024, 65, 1710–1717. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, T.; Wang, Y.; Zhu, F.; Shi, H.; Zhang, J.; Wang, Z.; Qu, M.; Zhang, H.; Wang, T.; et al. Intratumor Heterogeneity and Clonal Evolution Revealed in Castration-Resistant Prostate Cancer by Longitudinal Genomic Analysis. Transl. Oncol. 2022, 16, 101311. [Google Scholar] [CrossRef]
- Haffner, M.C.; Zwart, W.; Roudier, M.P.; True, L.D.; Nelson, W.G.; Epstein, J.I.; De Marzo, A.M.; Nelson, P.S.; Yegnasubramanian, S. Genomic and Phenotypic Heterogeneity in Prostate Cancer. Nat. Rev. Urol. 2021, 18, 79–92. [Google Scholar] [CrossRef]
- Zeisler, S.K.; Pavan, R.A.; Orzechowski, J.; Langlois, R.; Rodrigue, S.; van Lier, J.E. Production of 64Cu on the Sherbrooke TR-PET Cyclotron. J. Radioanal. Nucl. Chem. 2003, 257, 175–177. [Google Scholar] [CrossRef]
- McCarthy, D.W.; Shefer, R.E.; Klinkowstein, R.E.; Bass, L.A.; Margeneau, W.H.; Cutler, C.S.; Anderson, C.J.; Welch, M.J. Efficient production of high specific activity 64Cu using a biomedical cyclotron. Nucl. Med. Biol. 1997, 24, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Caruano, A.L.; Lewis, M.R.; Meyer, L.A.; VanderWaal, R.P.; Anderson, C.J. Subcellular Localization of Radio-labeled Somatostatin Analogues: Implications for Targeted Radiotherapy of Cancer. Cancer Res. 2003, 63, 6864–6869. [Google Scholar] [PubMed]
- Dubuc, C.; Savard, M.; Bovenzi, V.; Lessard, A.; Côté, J.; Neugebauer, W.; Geha, S.; Chemtob, S.; Gobeil, F. Antitumor Activity of Cell-Penetrant Kinin B1 Receptor Antagonists in Human Triple-Negative Breast Cancer Cells. J. Cell. Physiol. 2019, 234, 2851–2865. [Google Scholar] [CrossRef]
- Bergeron, M.; Cadorette, J.; Tetrault, M.-A.; Beaudoin, J.-F.; Leroux, J.-D.; Fontaine, R.; Lecomte, R. Imaging Performance of LabPET APD-Based Digital PET Scanners for Pre-Clinical Research. Phys. Med. Biol. 2014, 59, 661–678. [Google Scholar] [CrossRef]
- Khosravifarsani, M.; Ait-Mohand, S.; Paquette, B.; Sanche, L.; Guérin, B. In Vivo Behavior of [64Cu]NOTA-Terpyridine Platinum, a Novel Chemo-Radio-Theranostic Agent for Imaging and Therapy of Colorectal Cancer. Front. Med. 2022, 9, 975213. [Google Scholar] [CrossRef]
- Yoshii, Y.; Furukawa, T.; Kiyono, Y.; Watanabe, R.; Mori, T.; Yoshii, H.; Asai, T.; Okazawa, H.; Welch, M.J.; Fujibayashi, Y. Internal Radiotherapy with Copper-64-Diacetyl-Bis(N4-Methylthiosemicarbazone) Reduces CD133+ Highly Tumorigenic Cells and Metastatic Ability of Mouse Colon Carcinoma. Nucl. Med. Biol. 2011, 38, 151–157. [Google Scholar] [CrossRef]
- Métivier, C.; Le Saëc, P.; Gaschet, J.; Chauvet, C.; Marionneau-Lambot, S.; Hofgaard, P.O.; Bogen, B.; Pineau, J.; Le Bris, N.; Tripier, R.; et al. Preclinical Evaluation of a 64Cu-Based Theranostic Approach in a Murine Model of Multiple Myeloma. Pharmaceutics 2023, 15, 1817. [Google Scholar] [CrossRef]


| Organ | Blood | Plasma | Kidney | Pancreas | Liver | Heart | Lung | Tumor | Muscle | Bone | Brain |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [64Cu]Cu(OAc)2 | 1.95 ± 0.65 | 2.96 ± 0.86 | 9.26 ± 2.52 | 3.15 ± 1.18 | 31.56 ± 11.33 | 2.84 ± 0.43 | 4.19 ± 1.49 | 1.96 ± 0.21 | 0.61 ± 0.13 | 0.81 ± 0.08 | 0.36 ± 0.10 |
| [64Cu]CuCl2 | 3.23 ± 1.97 | 2.28 ± 0.44 | 9.66 ± 1.65 | 2.35 ± 0.41 | 29.98 ± 4.69 | 3.52 ± 1.35 | 5.64 ± 3.78 | 5.02 ± 5.18 | 0.64 ± 0.16 | 1.11 ± 0.59 | 0.34 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghanaatgar Kasbi, S.; Savard, M.; Dubuc, C.; Dory, Y.; Guérin, B.; Gobeil, F. Preclinical Theranostic Profiling of [64Cu]Cu-Acetate in Prostate Cancer. Molecules 2025, 30, 3957. https://doi.org/10.3390/molecules30193957
Ghanaatgar Kasbi S, Savard M, Dubuc C, Dory Y, Guérin B, Gobeil F. Preclinical Theranostic Profiling of [64Cu]Cu-Acetate in Prostate Cancer. Molecules. 2025; 30(19):3957. https://doi.org/10.3390/molecules30193957
Chicago/Turabian StyleGhanaatgar Kasbi, Sadaf, Martin Savard, Céléna Dubuc, Yves Dory, Brigitte Guérin, and Fernand Gobeil. 2025. "Preclinical Theranostic Profiling of [64Cu]Cu-Acetate in Prostate Cancer" Molecules 30, no. 19: 3957. https://doi.org/10.3390/molecules30193957
APA StyleGhanaatgar Kasbi, S., Savard, M., Dubuc, C., Dory, Y., Guérin, B., & Gobeil, F. (2025). Preclinical Theranostic Profiling of [64Cu]Cu-Acetate in Prostate Cancer. Molecules, 30(19), 3957. https://doi.org/10.3390/molecules30193957







