Cyclodextrins, Surfactants and Their Inclusion Complexes
Abstract
1. Introduction
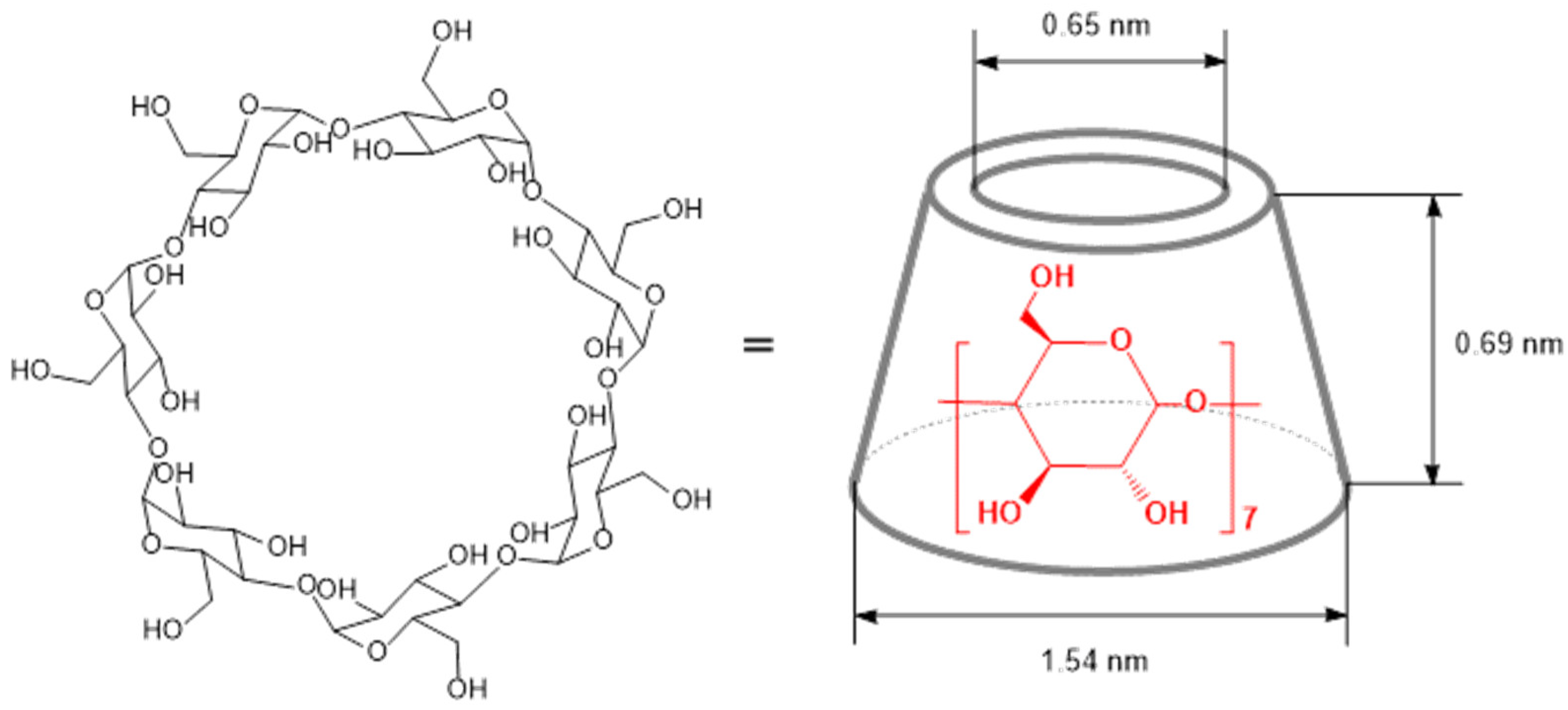
2. Structure, Properties, and Chemical Modifications of Cyclodextrins
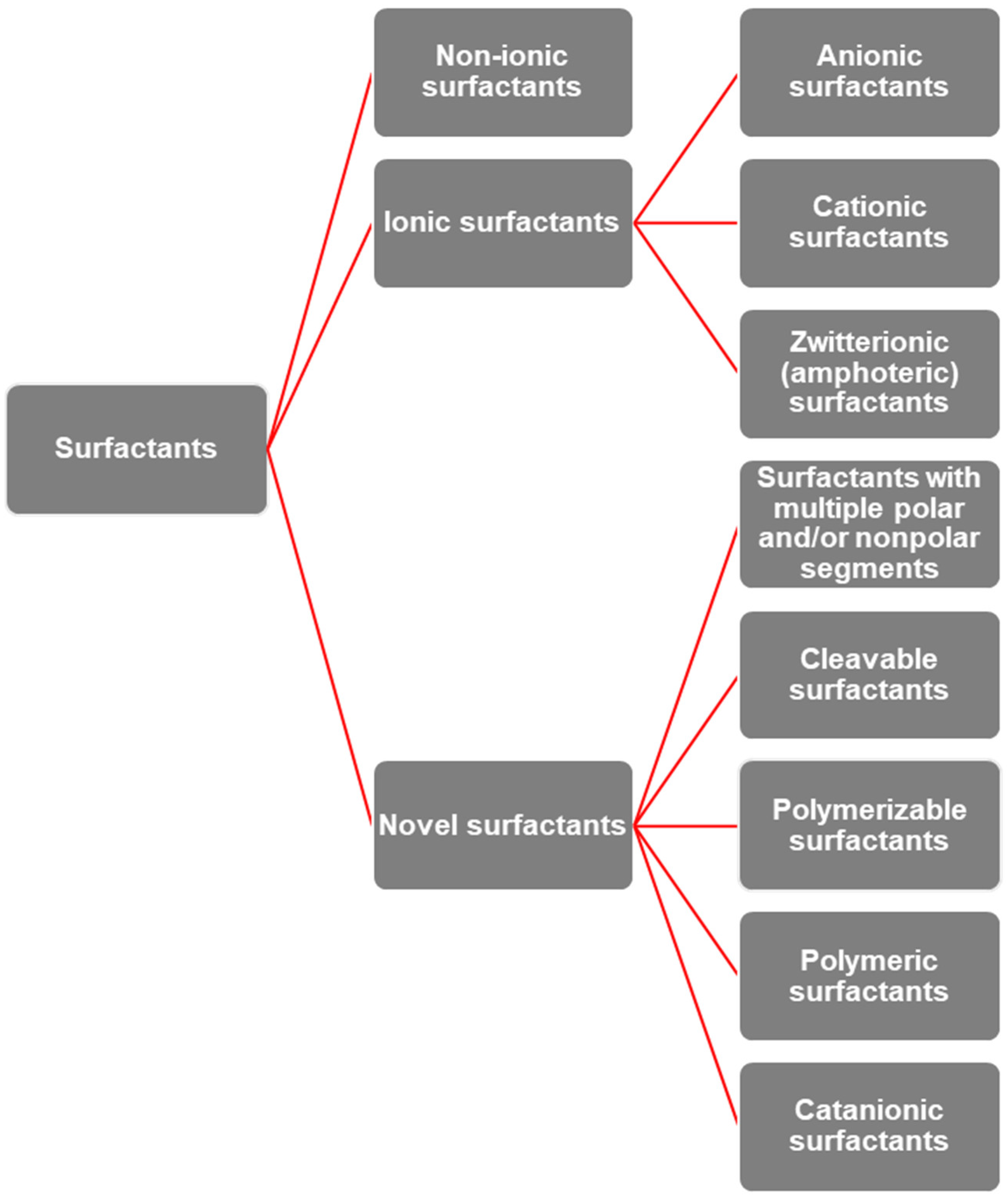
3. Surfactants: Properties, Behavior, and Structural Diversity
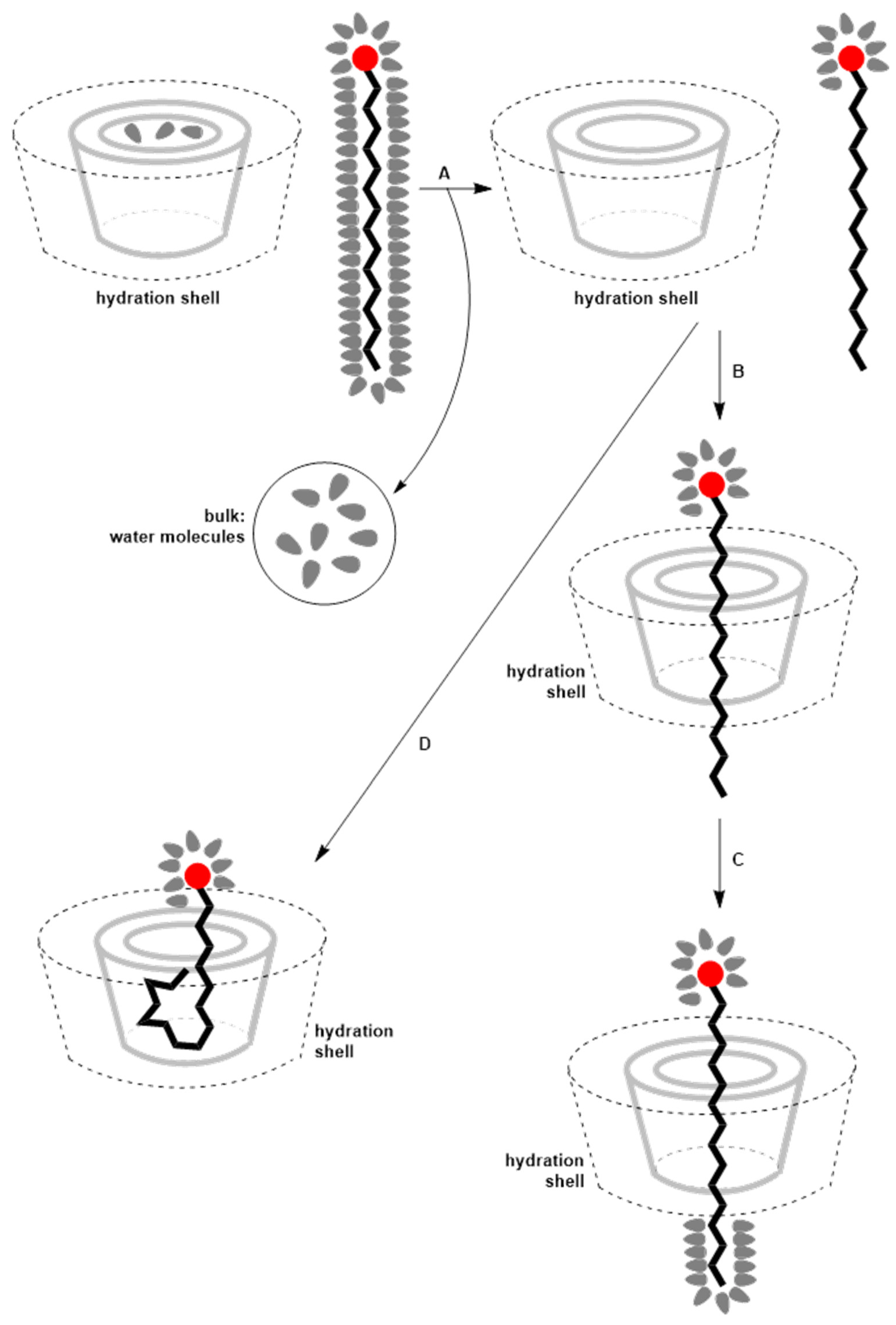
4. Thermodynamics of Inclusion Complex Formation Between Surfactants and Cyclodextrins
5. Cyclodextrins Host–Guest Complexes with Surfactants: Binding Constants and the Stoichiometry
5.1. The Influence of Surfactant Concentration, Cyclodextrin and Temperature on the Formation of the Inclusion Complex and the Self-Association of the Inclusion Complex
5.2. Methods Applied to the Study of Cyclodextrin Surfactant Systems
5.3. Applications of Cyclodextrin Inclusion Complexes
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CMC | critical micellar concentration |
| CD | cyclodextrin |
References
- Valente, A.J.M.; Söderman, O. The formation of host–guest complexes between surfactants and cyclodextrins. Adv. Colloid Interface Sci. 2014, 205, 156–176. [Google Scholar] [CrossRef]
- Araújo, L.S.S.; Giuseppe Lazzara, G.; Chiappisi, L. Cyclodextrin/surfactant inclusion complexes: An integrated view of their thermodynamic and structural properties. Rev. Adv. Colloid Interface Sci. 2021, 289, 102375. [Google Scholar] [CrossRef]
- Uitdehaag, J.C.M.; Van Der Veen, B.A.; Dijkhuizen, L.; Dijkstra, B.W. Catalytic mechanism and product specificity of cyclodextrin glycosyltransferase, a prototypical transglycosylase from the α-amylase family. Enzyme Microb. Technol. 2002, 30, 295–304. [Google Scholar] [CrossRef]
- Biwer, A.A.G.; Antranikian, G.; Heinzle, E. Enzymatic production of cyclodextrins. Appl. Microbiol. Biotechnol. 2002, 59, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Terada, Y.; Yanase, M.; Takata, H.; Takaha, T.; Okada, S. Cyclodextrins are not the major cyclic α-1,4-glucans produced by the initial action of cyclodextrin glucanotransferase on amylose. J. Biol. Chem. 1997, 272, 15729–15733. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Tanaka, N.; Kobayashi, S. Structure of δ-Cyclodextrin·13.75H2O. Chem. Lett. 1990, 19, 739–742. [Google Scholar] [CrossRef]
- Miyazawa, I.; Ueda, H.; Nagase, H.; Endo, T.; Kobayashi, S.; Nagai, T. Physicochemical properties and inclusion complex formation of δ-cyclodextrin. Eur. J. Pharm. Sci. 1995, 3, 153–162. [Google Scholar] [CrossRef]
- Endo, T.; Ueda, H.; Kobayashi, S.; Nagai, T. Isolation, purification, and characterization of cyclomaltododecaose (ν-cyclodextrin). Carbohydr. Res. 1995, 269, 369–373. [Google Scholar] [CrossRef]
- Wakamiya, A.; Endo, T.; Nagase, H.; Ueda, H.; Kobayashi, S.; Nagai, T. Isolation and purification of cyclomaltononaose (δ-CD) from CELDEX SG-30. Yakuzaigaku 1997, 57, 220–223. [Google Scholar] [CrossRef]
- Endo, T.; Nagase, H.; Ueda, H.; Kobayashi, S.; Nagai, T. Isolation, purification, and characterization of cyclomaltodecaose (ε-cyclodextrin), cyclomaltoundecaose (ζ-cyclodextrin), and cyclomaltotridecaose (θ-cyclodextrin). Chem. Pharm. Bull. 1997, 45, 532–536. [Google Scholar] [CrossRef]
- Koizumi, K.; Sanbe, H.; Kubota, Y.; Terada, Y.; Takaha, T. Isolation and characterization of cyclic α-(1→4)-glucans having degrees of polymerization 9–31 and their quantitative analysis by high-performance anion-exchange chromatography with pulsed amperometric detection. J. Chromatogr. A 1999, 852, 407–416. [Google Scholar] [CrossRef]
- Sonnendecker, C.; Zimmermann, W. Change of the product specificity of a cyclodextrin glucanotransferase by semi-rational mutagenesis to synthesize large-ring cyclodextrins. Catalysts 2019, 9, 242. [Google Scholar] [CrossRef]
- Sonnendecker, C.; Melzer, S.; Zimmermann, W. Engineered cyclodextrin glucanotransferases from Bacillus sp. G-825−6 produce large-ring cyclodextrins with high specificity. Microbiologyopen 2019, 8, e00757. [Google Scholar] [CrossRef]
- Hansen, K.H.; Erichsen, A.; Larsen, D.; Beeren, S.R. Enzyme-mediated dynamic combinatorial chemistry enables large-scale synthesis of δ-cyclodextrin. J. Am. Chem. Soc. 2015, 137, 9101–9104. [Google Scholar] [CrossRef] [PubMed]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Esteso, M.A.; Romero, C.M. Cyclodextrins: Properties and Applications. Int. J. Mol. Sci. 2024, 25, 4547. [Google Scholar] [CrossRef] [PubMed]
- Larsen, K.L. Large cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2002, 43, 1–13. [Google Scholar] [CrossRef]
- Nakagawa, T.; Ueno, K.; Kashiwa, M.; Watanabe, J. Synthesis of large-ring cyclodextrins by enzymatic reactions. Tetrahedron Lett. 1994, 35, 1921–1924. [Google Scholar] [CrossRef]
- Ikuta, D.; Hirata, Y.; Wakamori, S.; Shimada, H.; Tomabechi, Y.; Kawasaki, Y.; Ikeuchi, K.; Hagimori, T.; Matsumoto, S.; Yamada, H. Conformationally supple glucose monomers enable synthesis of the smallest cyclodextrins. Science 2019, 364, 674–677. [Google Scholar] [CrossRef]
- Wakamori, S. Chemical synthesis of small cyclodextrins utilizing bridged pyranose ring. Glycoforum 2021, 24, A5. [Google Scholar] [CrossRef]
- Samuelsen, L.; Holm, R.; Schönbeck, C. Simultaneous determination of cyclodextrin stability constants as a function of pH and temperature—A tool for drug formulation and process design. J. Drug Deliv. Sci. Technol. 2021, 65, 102675. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins: Basic science and product development. J. Pharm. Pharmacol. 2010, 62, 1607–1621. [Google Scholar] [CrossRef]
- BeMiller, J.N.; Whistler, R.L. Starch: Chemistry and Technology, 3rd ed.; BeMiller, J.N., Whistler, R.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 833–848. [Google Scholar]
- Manor, P.; Saenger, W. Water molecule in hydrophobic surroundings: Structure of α-cyclodextrin-hexahydrate (C6H10O5)6·6H2O. Nature 1972, 237, 392–393. [Google Scholar] [CrossRef]
- Manor, P.; Saenger, W. Topography of cyclodextrin inclusion complexes. III. Crystal and molecular structure of cyclohexaamylose hexahydrate, the water dimer inclusion complex. J. Am. Chem. Soc. 1974, 96, 3630–3636. [Google Scholar] [CrossRef]
- Köse, K.; Tüysüz, M.; Aksüt, D.; Uzun, L. Modification of cyclodextrin and use in environmental applications. Environ. Sci. Pollut. Res. 2022, 29, 182–209. [Google Scholar] [CrossRef] [PubMed]
- Wenz, G.; Keller, B. Speed control for cyclodextrin rings on polymer chains. Macromol. Symp. 1994, 87, 11–16. [Google Scholar] [CrossRef]
- Fejős, I.; Kalydi, E.; Malanga, M.; Benkovics, G.; Béni, S. Cyclodextrin-based chiral stationary phases for enantioseparation of β-adrenergic blockers by capillary electrochromatography. J. Chromatogr. A 2020, 1627, 461375. [Google Scholar] [CrossRef]
- Scriba, G.K.E.; Konjaria, M.-L.; Krait, S. Cyclodextrins. In Chiral Separations and Stereochemical Elucidation; Cass, Q.B., Tiritan, M.E., Batista Junior, J.M., Barreiro, J.C., Eds.; Wiley: Hoboken, NJ, USA, 2023; pp. 273–323. [Google Scholar] [CrossRef]
- Kasal, P.; Jindřich, J. Mono-6-Substituted Cyclodextrins—Synthesis and Applications. Molecules 2021, 26, 5065. [Google Scholar] [CrossRef]
- Liu, J.Y.; Zhang, X.; Tian, B. Selective modifications at the different positions of cyclodextrins: A review of strategies. Turk. J. Chem. 2020, 44, 229–255. [Google Scholar] [CrossRef]
- Řezanka, M. Monosubstituted cyclodextrins as precursors for further use. Eur. J. Org. Chem. 2016, 2016, 4531–4541. [Google Scholar] [CrossRef]
- Řezanka, M. Synthesis of substituted cyclodextrins. Environ. Chem. Lett. 2019, 17, 49–63. [Google Scholar] [CrossRef]
- García-Pérez, P.; Losada-Barreiro, S.; Gallego, P.P. Cyclodextrin-elicited Bryophyllum suspension cultured cells: Enhancement of the production of bioactive compounds. Int. J. Mol. Sci. 2019, 20, 5180. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Forgo, P.; Stine, K.J.; D’Souza, V.T. Methods for selective modifications of cyclodextrins. Chem. Rev. 1998, 98, 1977–1996. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Ng, S.C. Monosubstituted positively charged cyclodextrins: Synthesis and applications in chiral separation. J. Sep. Sci. 2008, 31, 2973–2982. [Google Scholar] [CrossRef]
- Kumprecht, L.; Budesinsky, M.; Bouř, P.; Kraus, T. α-Cyclodextrins reversibly capped with disulfide bonds. New J. Chem. 2010, 34, 2115–2120. [Google Scholar] [CrossRef]
- Asim, M.H.; Nazir, I.; Jalil, A.; Matuszczak, B.; Bernkop-Schnürch, A. Tetradeca-thiolated cyclodextrins: Highly mucoadhesive and in-situ gelling oligomers with prolonged mucosal adhesion. Int. J. Pharm. 2020, 577, 119040. [Google Scholar] [CrossRef]
- Zultanski, S.L.; Kuhl, N.; Zhong, W.; Cohen, R.D.; Davies, I.W. Mechanistic understanding of a robust and scalable synthesis of per(6-deoxy-6-halo)cyclodextrins, versatile intermediates for cyclodextrin modification. Org. Process Res. Dev. 2020, 24, 2671–2679. [Google Scholar] [CrossRef]
- Grassiri, B.; Knoll, P.; Fabiano, A.; Piras, A.M.; Zambito, Y.; Bernkop-Schnürch, A. Thiolated hydroxypropyl-β-cyclodextrin: A potential multifunctional excipient for ocular drug delivery. Int. J. Pharm. 2023, 635, 122746. [Google Scholar] [CrossRef]
- Kali, G.; Haddadzadegan, S.; Laffleur, F.; Bernkop-Schnürch, A. Per-thiolated cyclodextrins: Nanosized drug carriers providing a prolonged gastrointestinal residence time. Carbohydr. Polym. 2023, 300, 120275. [Google Scholar] [CrossRef]
- Bálint, M.; Darcsi, A.; Benkovics, G.; Varga, E.; Szabó, E.; Béni, S. Synthesis of the chiral selector heptakis(6-O-methyl)-β-cyclodextrin by phase-transfer catalysis and hydrazine-mediated transfer-hydrogenation. J. Sep. Sci. 2019, 42, 2983–2990. [Google Scholar] [CrossRef]
- Bucur, S.; Niculaua, M.; Ciobanu, C.I.; Lungu, N.C.; Mangalagiu, I. A simple synthesis route for selectively methylated β-cyclodextrin using a copper complex sandwich protecting strategy. Molecules 2021, 26, 5669. [Google Scholar] [CrossRef]
- D’Aria, F.; Pagano, B.; Giancola, C. Thermodynamic properties of hydroxypropyl-β-cyclodextrin/guest interaction: A survey of recent studies. J. Incl. Phenom. Macrocycl. Chem. 2022, 147, 4889–4897. [Google Scholar] [CrossRef]
- Gould, S.; Scott, R.C. 2-Hydroxypropyl-β-cyclodextrin (HP-β-CD): A toxicology review. Food Chem. Toxicol. 2005, 43, 1451–1459. [Google Scholar] [CrossRef]
- Pitha, J.; Milecki, J.; Fales, H.; Pannell, L.; Uekama, K. Hydroxypropyl-β-cyclodextrin: Preparation and characterization; effects on solubility of drugs. Int. J. Pharm. 1986, 29, 73–82. [Google Scholar] [CrossRef]
- Gidwani, B.; Vyas, A. Synthesis, characterization and application of epichlorohydrin-β-cyclodextrin polymer. Colloids Surf. B Biointerfaces 2014, 114, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Renard, E.; Sebille, B.; Barnathan, G.; Deratani, A. Polycondensation of cyclodextrins with epichlorohydrin. Influence of reaction conditions on the polymer structure. Macromol. Symp. 1997, 120, 35–43. [Google Scholar] [CrossRef]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of cyclodextrins and drug/cyclodextrin complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef] [PubMed]
- Schlüter, F.; Bela, M.M.; Glikman, D.; Braunschweig, B.; Ravoo, B.J. A cyclodextrin surfactant for stable emulsions with an accessible cavity for host–guest complexation. Chem. Commun. 2020, 56, 15438–15441. [Google Scholar] [CrossRef]
- Kato, K.; Hori, A.; Ito, K. An efficient synthesis of low-covered polyrotaxanes grafted with poly(ε-caprolactone) and the mechanical properties of its cross-linked elastomers. Polymer 2018, 147, 67–73. [Google Scholar] [CrossRef]
- Mayumi, K.; Liu, C.; Yasuda, Y.; Ito, K. Softness, elasticity, and toughness of polymer networks with slide-ring cross-links. Gels 2021, 7, 26. [Google Scholar] [CrossRef]
- Añibarro, M.; Gessler, K.; Usón, I.; Sheldrick, G.M.; Harata, K.; Uekama, K.; Hirayama, F.; Abe, Y.; Saenger, W. Effect of peracylation of β-cyclodextrin on the molecular structure and on the formation of inclusion complexes: An X-ray study. J. Incl. Phenom. Macrocycl. Chem. 2001, 123, 11854–11862. [Google Scholar] [CrossRef]
- Filardo, G.; Di Blasi, M.; Galia, A.; Ponchel, A.; Bricout, H.; Sayede, A.D.; Monflier, E. Peracetylated β-cyclodextrin as solubilizer of arylphosphines in supercritical carbon dioxide. J. Supercrit. Fluids 2006, 36, 173–181. [Google Scholar] [CrossRef]
- Jicsinszky, L.; Caporaso, M.; Tuza, K.; Martina, K.; Calcio Gaudino, E.; Cravotto, G. Nucleophilic substitutions of 6I-O-monotosyl-β-cyclodextrin in a planetary ball mill. ACS Sustainable Chem. Eng. 2016, 4, 919–929. [Google Scholar] [CrossRef]
- Pedersen, N.R.; Kristensen, J.B.; Bauw, G.; Ravoo, B.J.; Darcy, R.; Larsen, K.L.; Pedersen, L.H. Thermolysin catalyses the synthesis of cyclodextrin esters in DMSO. Tetrahedron 2005, 61, 4797–4803. [Google Scholar] [CrossRef]
- Putaux, J.L.; Lancelon-Pin, C.; Legrand, F.X.; Pastrello, M.; Choisnard, L.; Gèze, A.; Rochas, C.; Wouessidjewe, D. Self-assembly of amphiphilic biotransesterified β-cyclodextrins: Supramolecular structure of nanoparticles and surface properties. Langmuir 2017, 33, 10387–10395. [Google Scholar] [CrossRef] [PubMed]
- Putaux, J.L.; Lancelon-Pin, C.; Choisnard, L.; Gèze, A.; Wouessidjewe, D. Topological defects in polycrystalline hexosomes from β-cyclodextrin fatty esters. Soft Matter 2022, 18, 5267–5276. [Google Scholar] [CrossRef]
- Blaj, D.A.; Kowalczuk, M.; Peptu, C.A. Mass spectrometry of esterified cyclodextrins. Molecules 2023, 28, 2001. [Google Scholar] [CrossRef]
- Shahiwala, A. Cyclodextrin conjugates for colon drug delivery. J. Drug Deliv. Sci. Technol. 2020, 55, 101448. [Google Scholar] [CrossRef]
- Blaj, D.A.; Balan-Porcarasu, M.; Petre, B.A.; Harabagiu, V.; Peptu, C. MALDI mass spectrometry monitoring of cyclodextrin-oligolactide derivatives synthesis. Polymers 2021, 233, 124186. [Google Scholar] [CrossRef]
- Meimoun, J.; Phuphuak, Y.; Miyamachi, R.; Miao, Y.; Bria, M. Cyclodextrins initiated ring-opening polymerization of lactide using 4-dimethylaminopyridine (DMAP) as catalyst: Study of DMAP/β-CD inclusion complex and access. Molecules 2022, 27, 1083. [Google Scholar] [CrossRef]
- Peptu, C.; Blaj, D.A.; Balan-Porcarasu, M.; Rydz, J. Cyclodextrin-Oligocaprolactone Derivatives—Synthesis and Advanced Structural Characterization by MALDI Mass Spectrometry. Polymers 2022, 14, 1436. [Google Scholar] [CrossRef]
- Caldera, F.; Tannous, M.; Cavalli, R.; Zanetti, M.; Trotta, F. Evolution of Cyclodextrin Nanosponges. Int. J. Pharm. 2017, 531, 470–479. [Google Scholar] [CrossRef]
- Escobar, K.; Garrido-Miranda, K.A.; Pulido, R.; Naveas, N.; López-Castillo, A.; Sánchez-Aké, C.; Palma-Rodríguez, H.M. Coatings of Cyclodextrin/Citric-Acid Biopolymer as Drug Delivery Systems: A Review. Pharmaceutics 2023, 15, 296. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fernandez, M.J.; Tabary, N.; Chai, F.; Nicolas, V.; Grohens, Y.; Fessi, H.; Elaissari, A. New Multifunctional Pharmaceutical Excipient in Tablet Formulation Based on Citric Acid-Cyclodextrin Polymer. Int. J. Pharm. 2016, 511, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Martel, B.; Ruffin, D.; Weltrowski, M.; Lekchiri, Y.; Morcellet, M. Water-Soluble Polymers and Gels from the Polycondensation between Cyclodextrins and Poly(Carboxylic Acids): A Study of the Preparation Parameters. J. Appl. Polym. Sci. 2005, 96, 1979–1987. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Crini, G. Environmental applications of water-insoluble β-cyclodextrin–epichlorohydrin polymers. Prog. Polym. Sci. 2013, 38, 344–368. [Google Scholar] [CrossRef]
- Thi, T.T.H.; Lee, Y.; Ryu, S.B.; Sung, H.J.; Park, K.D. Oxidized cyclodextrin-functionalized injectable gelatin hydrogels as a new platform for tissue-adhesive hydrophobic drug delivery. RSC Adv. 2017, 7, 34053–34062. [Google Scholar] [CrossRef]
- Ye, Y.; Ren, H.; Zhu, S.; Tan, H.; Li, X.; Li, D.; Mu, C. Synthesis of oxidized β-cyclodextrin with high aqueous solubility and broad-spectrum antimicrobial activity. Carbohydr. Polym. 2017, 174, 823–831. [Google Scholar] [CrossRef]
- Ijaz, M.; Ahmad, M.; Akhtar, N.; Laffleur, F.; Matuszczak, B.; Bernkop-Schnürch, A. Thiolated α-cyclodextrin: The invisible choice to prolong ocular drug residence time. J. Pharm. Sci. 2016, 105, 2296–2302. [Google Scholar] [CrossRef]
- Ijaz, M.; Rösch, A.C.; Bernkop-Schnürch, A. Thiolated cyclodextrins: New perspectives for old excipients. Coord. Chem. Rev. 2020, 416, 213433. [Google Scholar] [CrossRef]
- Wenz, G.; Han, B.H.; Müller, A. Cyclodextrin rotaxanes and polyrotaxanes. Chem. Rev. 2006, 106, 782–817. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, T.M.; Purro, M.; Xiong, M.P. Enzymatically biodegradable polyrotaxane–deferoxamine conjugates for iron chelation. ACS Appl. Mater. Interfaces 2016, 8, 29879–29890. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, Y.; Tamura, A.; Tsuda, S.; Domae, E.; Zhang, S.; Yui, N.; Ikeo, T.; Yoshizawa, T. Calcium phosphate-adsorbable and acid-degradable carboxylated polyrotaxane consisting of β-cyclodextrins suppresses osteoclast resorptive activity. Dent. Mater. J. 2022, 41, 2021–2331. [Google Scholar] [CrossRef] [PubMed]
- Tamura, A.; Yui, N. Polyrotaxane-based systemic delivery of β-cyclodextrins for potentiating therapeutic efficacy in a mouse model of Niemann–Pick type C disease. J. Control. Release 2018, 269, 148–158. [Google Scholar] [CrossRef]
- Higashi, T.; Iohara, D.; Motoyama, K.; Arima, H. Supramolecular pharmaceutical sciences: A novel concept combining pharmaceutical sciences and supramolecular chemistry with a focus on cyclodextrin-based systems. Chem. Pharm. Bull. 2018, 66, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Soe, H.M.S.H.; Kerdpol, K.; Rungrotmongkol, T.; Pruksakorn, P.; Autthateinchai, R.; Wet-osot, S.; Loftsson, T.; Jansook, P. Voriconazole eye drops: Enhanced solubility and stability through ternary voriconazole/sulfobutyl ether β–cyclodextrin/polyvinyl alcohol complexes. Int. J. Mol. Sci. 2023, 24, 2343. [Google Scholar] [CrossRef]
- Galindres, D.M.; Espitia-Galindo, N.; Valente, A.J.M.; Sofio, S.P.C.; Rodrigo, M.M.; Cabral, A.M.T.D.P.V.; Esteso, M.A.; Zapata-Rivera, J.; Vargas, E.F.; Ribeiro, A.C.F. Interactions of sodium salicylate with β–cyclodextrin and an anionic resorcin[4]arene: Mutual diffusion coefficients and computational study. Int. J. Mol. Sci. 2023, 24, 3921. [Google Scholar] [CrossRef]
- Sierpe, R.; Donoso-González, O.; Lang, E.; Noyong, M.; Simon, U.; Kogan, M.J.; Yutronic, N. Solid-state formation of a potential melphalan delivery nanosystem based on β–cyclodextrin and silver nanoparticles. Int. J. Mol. Sci. 2023, 24, 3990. [Google Scholar] [CrossRef]
- Sangkhawasi, M.; Kerdpol, K.; Ismail, A.; Nutho, B.; Hanpiboon, C.; Wolschann, P.; Krusong, K.; Rungrotmongkol, T.; Hannongbua, S. In vitro and in silico study on the molecular encapsulation of α–tocopherol in a large-ring cyclodextrin. Int. J. Mol. Sci. 2023, 24, 4425. [Google Scholar] [CrossRef]
- Gao, S.; Yang, G.; Zhang, X.; Shi, R.; Chen, R.; Zhang, X.; Peng, Y.; Yang, H.; Lu, Y.; Song, C. β–Cyclodextrin polymer-based fluorescence enhancement strategy via host–guest interaction for sensitive assay of SARS-CoV-2. Int. J. Mol. Sci. 2023, 24, 7174. [Google Scholar] [CrossRef]
- Poša, M. The Gibbs-Helmholtz equation and the enthalpy-entropy compensation (EEC) phenomenon in the formation of micelles in an aqueous solution of surfactants and the cloud point effect. J. Mol. Liq. 2024, 396, 124109. [Google Scholar] [CrossRef]
- Kumar, D.; Poša, M. Thermodynamics of micelle formation of selected homologous 7-alkyl derivatives of Na-cholate in aqueous solution: Steroid skeleton and the alkyl chain conformation. Int. J. Mol. Sci. 2024, 25, 13055. [Google Scholar] [CrossRef]
- Kumar, D.; Khan, J.M.; Poša, M. Analysis of MixedMicellization of Promethazine Hydrochloride and 1-Hexadecyl-3-Methylimidazolium Chloride Using Different Techniques: Influence ofAdditives. ChemistrySelect 2025, 10, e202405789. [Google Scholar] [CrossRef]
- Mikelić, A. Površinski Aktivni Tvari. Bachelor’s Thesis, University of Zagreb, Zagreb, Croatia, 2016. Available online: https://urn.nsk.hr/urn:nbn:hr:217:274967 (accessed on 20 May 2025).
- Myers, D. Surfaces, Interfaces, and Colloids: Principles and Applications, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1999. [Google Scholar] [CrossRef]
- Surfactant Chemistry and General Phase Behaviour. Available online: https://www.chm.bris.ac.uk/eastoe/Surf_Chem/1%20Surfactant%20chemistry%20and%20general%20phase%20behaviour.pdf (accessed on 30 May 2025).
- Hidalgo, A.A.; Caetano, W.; Tabak, M.; Oliveira, O.N., Jr. Interaction of two phenothiazine derivatives with phospholipid monolayers. Biophys. Chem. 2004, 109, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, K.; Jönsson, B.; Kronberg, B.; Lindman, B. Surfactants and Polymers in Aqueous Solution, 2nd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2003. [Google Scholar] [CrossRef]
- Lomax, E.G. Amphoteric Surfactants, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- van Os, N.M.; Haak, J.R.; Rupert, L.A.M. Physico-Chemical Properties of Selected Anionic, Cationic and Nonionic Surfactants; Elsevier Science: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Rouchotas, C.; Cassidy, O.E.; Rowley, G. Comparison of surface modification and solid dispersion techniques for drug dissolution. Int. J. Pharm. 2000, 195, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wattebled, L. Oligomeric Surfactants as Novel Type of Amphiphiles: Structure–Property Relationships and Behaviour with Additives. Ph.D. Thesis, Potsdam University, Potsdam, Germany, 2006. [Google Scholar]
- Ćirin, D.M.; Poša, M.M.; Krstonošić, V.S. Interactions between selected bile salts and Triton X-100 or sodium lauryl ether sulfate. Chem. Cent. J. 2011, 5, 89. [Google Scholar] [CrossRef]
- Salager, J.L. Surfactants, Types and Uses; FIRP Booklet No. E300A; Universidad de Los Andes: Mérida, Venezuela, 2002; Available online: https://es.firp-ula.org/wp-content/uploads/2019/07/E300A (accessed on 30 May 2025).
- Jaeger, D.A.; Mendoza, A.; Jose, R.; Apkarian, R.P. Shamrock surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2007, 302, 506–516. [Google Scholar] [CrossRef]
- Deshpande, S.; Wesson, L.; Wade, D.; Sabatini, D.A.; Harwell, J.H. DOWFAX surfactant components for enhancing contaminant solubilization. Water Res. 2000, 34, 1030–1036. [Google Scholar] [CrossRef]
- Liu, L.; Guo, Q.X. The driving forces in the inclusion complexation of cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2002, 42, 1–14. [Google Scholar] [CrossRef]
- Connors, K.A. The stability of cyclodextrin complexes in solution. Chem. Rev. 1997, 97, 1325–1358. [Google Scholar] [CrossRef]
- Rekharsky, M.V.; Mori, T.; Inoue, Y. Host–guest chemistry of cyclodextrins. J. Phys. Chem. B 1999, 103, 5113–5119. [Google Scholar]
- Dill, K.A. Dominant forces in protein folding. Biochemistry 1990, 29, 7133–7155. [Google Scholar] [CrossRef]
- Chandler, D. Interfaces and the driving force of hydrophobic assembly. Nature 2005, 437, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Pereva, S.; Nikolova, V.; Angelova, S.; Spassov, T.; Dudev, T. Water inside β-cyclodextrin cavity: Amount, stability and mechanism of binding. Beilstein J. Org. Chem. 2019, 15, 1592–1600. [Google Scholar] [CrossRef] [PubMed]
- Sandilya, A.A.; Natarajan, U.; Priya, M.H. Molecular view into the cyclodextrin cavity: Structure and hydration. ACS Omega 2020, 5, 25655–25667. [Google Scholar] [CrossRef] [PubMed]
- Chavelas, E.A.; García-Hernández, E. Heat capacity changes in carbohydrates and protein–carbohydrate complexes. Biochem. J. 2009, 420, 239–247. [Google Scholar] [CrossRef]
- Bouchemal, K.; Mazzaferro, S. How to conduct and interpret ITC experiments accurately for cyclodextrin–guest interactions. Drug Discov. Today 2012, 17, 623–629. [Google Scholar] [CrossRef]
- Iza, N.; Guerrero-Martínez, A.; Tardajos, G.; Ortiz, M.J.; Palao, E.; Montoro, T.; Radulescu, A.; Dreiss, C.A.; González-Gaitano, G. Using inclusion complexes with cyclodextrins to explore the aggregation behavior of a ruthenium metallosurfactant. Langmuir 2015, 31, 2677–2688. [Google Scholar] [CrossRef]
- Nagaraj, K.; Arunachalam, S. Synthesis, CMC determination and influence of the micelles, β-cyclodextrin, ionic liquids and liposome (dipalmitoylphosphatidylcholine) vesicles on the kinetics of an outer-sphere electron transfer reaction. J. Incl. Phenom. Macrocycl. Chem. 2014, 79, 425–435. [Google Scholar] [CrossRef]
- García-Río, L.; Méndez, M.; Paleo, M.R.; Sardina, F.J. New insights in cyclodextrin: Surfactant mixed systems from the use of neutral and anionic cyclodextrin derivatives. J. Phys. Chem. B 2007, 111, 12756–12764. [Google Scholar] [CrossRef]
- Vargas, C.; Schönbeck, C.; Heimann, I.; Keller, S. Extracavity effect in cyclodextrin/surfactant complexation. Langmuir 2018, 34, 5781–5787. [Google Scholar] [CrossRef]
- Rekharsky, M.V.; Inoue, Y. Complexation thermodynamics of cyclodextrins. Chem. Rev. 1998, 98, 1875–1917. [Google Scholar] [CrossRef]
- Jiang, L.; Yan, Y.; Huang, J. Versatility of cyclodextrins in self-assembly systems of amphiphiles. Adv. Colloid Interface Sci. 2011, 169, 13–25. [Google Scholar] [CrossRef]
- Funasaki, N.; Ishikawa, S.; Neya, S. Proton NMR study of α-cyclodextrin inclusion of short-chain surfactants. J. Phys. Chem. B 2003, 107, 10336–10340. [Google Scholar] [CrossRef]
- Funasaki, N.; Ishikawa, S.; Neya, S. 1:1 and 1:2 Complexes between Long-Chain Surfactant and α-Cyclodextrin Studied by NMR. J. Phys. Chem. B 2004, 108, 9593–9598. [Google Scholar] [CrossRef]
- De Lisi, R.; Lazzara, G.; Milioto, S.; Muratore, N. Characterization of the Cyclodextrin−Surfactant Interactions by Volume and Enthalpy. J. Phys. Chem. B 2003, 107, 13150–13157. [Google Scholar] [CrossRef]
- Poša, M.; Farkaš Agatić, Z.; Popović, K. Influence of cations of the first group of the Periodic Table of Elements on the thermodynamic stabilization of cholic and deoxycholic acid anion micelles. J. Mol. Liq. 2019, 296, 111840. [Google Scholar] [CrossRef]
- Poša, M. Parallel (competitive) reactions of micelle formation and cyclodextrin–surfactant inclusion complex formation in aqueous solution: A consequence of the Gibbs–Duhem equation—Invariance of the mass action law and the possibility of determining the equilibrium constant of the inclusion complex. J. Mol. Liq. 2024, 415, 126409. [Google Scholar] [CrossRef]
- Tepavčević, V.; Farkaš Agatić, Z.; Pilipović, A.; Puača, G.; Poša, M. Effect of β-Cyclodextrin on the Aggregation Behavior of Sodium Deoxycholate and Sodium Cholate in Aqueous Solution. Molecules 2025, 30, 2197. [Google Scholar] [CrossRef]
- He, Y.; Chen, Q.-D.; Xu, C.; Shen, X. Interaction between Ionic Liquids and β-Cyclodextrin: A Discussion of Association Pattern. J. Phys. Chem. B 2009, 113, 231–238. [Google Scholar] [CrossRef]
- Yunus, W.M.Z.W.; Taylor, J.; Bloor, D.M.; Hall, D.G.; Wyn-Jones, E. Electrochemical measurements on the binding of sodium dodecyl sulfate and dodecyltrimethylammonium bromide with .alpha.- and. beta.-cyclodextrins. J. Phys. Chem. 1992, 96, 8979–8982. [Google Scholar]
- Mwakibete, H.; Cristantino, R.; Bloor, D.M.; Wyn-Jones, E.; Holzwarth, J.F. Reliability of the Experimental Methods to Determine Equilibrium Constants for Surfactant–Cyclodextrin Inclusion Complexes. Langmuir 1995, 11, 57–60. [Google Scholar] [CrossRef]
- Tutaj, B.; Kasprzyk, A.; Czapkiewicz, J. The Spectral Displacement Technique for Determining the Binding Constants of β-Cyclodextrin–Alkyltrimethylammonium Inclusion Complexes. J. Incl. Phenom. 2003, 47, 133–136. [Google Scholar] [CrossRef]
- Spildo, K.; Høiland, H. Complex Formation Between Alkane-α,ω-Diols and Cyclodextrins Studied by Partial Molar Volume and Compressibility Measurements. J. Solution Chem. 2002, 31, 149–164. [Google Scholar] [CrossRef]
- Xing, H.; Lin, S.-S.; Yan, P.; Xiao, J.-X.; Chen, Y.-M. NMR Studies on Selectivity of β-Cyclodextrin to Fluorinated/Hydrogenated Surfactant Mixtures. J. Phys. Chem. B 2007, 111, 8089–8095. [Google Scholar] [CrossRef] [PubMed]
- Eli, W.; Chen, W.; Xue, Q. The Association of Triton X Surfactants with β-Cyclodextrin. An Isothermal Titration Calorimetry Study. J. Incl. Phenom. 2000, 36, 439–445. [Google Scholar] [CrossRef]
- Nilsson, M.; Cabaleiro-Lago, C.; Valente, A.J.M.; Söderman, O. Interactions between Gemini Surfactants, 12-s-12, and β-Cyclodextrin as Investigated by NMR Diffusometry and Electric Conductometry. Langmuir 2006, 22, 8663–8669. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guerrero-Martínez, A.; González-Gaitano, G.; Viñas, M.H.; Tardajos, G. Inclusion Complexes between β-Cyclodextrin and a Gemini Surfactant in Aqueous Solution: An NMR Study. J. Phys. Chem. B 2006, 110, 13819–13828. [Google Scholar] [CrossRef]
- Ondo, D.; Costas, M. Complexation thermodynamics of α-cyclodextrin with ionic surfactants in water. J. Colloid Interface Sci. 2017, 505, 445–453. [Google Scholar] [CrossRef]
- Popova, E.I.; Topchieva, I.N. Complex formation between polyethylene oxide-containing nonionic surfactants and α- and β-cyclodextrins. Russ. Chem. Bull. 2001, 50, 620–625. [Google Scholar] [CrossRef]
- Saito, Y.; Ueda, H.; Abe, M.; Sato, T.; Christian, S.D. Inclusion complexation of Triton X-100 with α-, β- and γ-cyclodextrins. Colloids Surf. A Physicochem. Eng. Asp. 1998, 135, 103–108. [Google Scholar] [CrossRef]
- Mahata, A.; Bose, D.; Ghosh, D.; Jana, B.; Bhattacharya, B.; Sarkar, D.; Chattopadhyay, N. Studies of Triton X-165–β-cyclodextrin interactions using both extrinsic and intrinsic fluorescence. J. Colloid Interface Sci. 2010, 347, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.D.; Verrall, R.E. A volumetric study of β-cyclodextrin/hydrocarbon and β-cyclodextrin/fluorocarbon surfactant inclusion complexes in aqueous solutions. J. Phys. Chem. B 1997, 101, 9270–9279. [Google Scholar] [CrossRef]
- Valente, M.; Tabajdi, R.; Király, Z. Thermodynamics of formation of β-cyclodextrin inclusion complexes with four series of surfactant homologs. J. Therm. Anal. Calorim. 2013, 112, 969–976. [Google Scholar] [CrossRef]
- Petek, A.; Krajnc, M.; Petek, A. Study of host–guest interaction between β-cyclodextrin and alkyltrimethylammonium bromides in water. J. Incl. Phenom. Macrocycl. Chem. 2016, 86, 221–229. [Google Scholar] [CrossRef]
- Rafati, A.A.; Bagheri, A.; Iloukhani, H.; Zarinehzad, M. Study of inclusion complex formation between a homologous series of n-alkyltrimethylammonium bromides and β-cyclodextrin, using conductometric technique. J. Mol. Liq. 2005, 116, 37–41. [Google Scholar] [CrossRef]
- Sammalkorpi, M.; Karttunen, M.; Haataja, M. Structural properties of ionic detergent aggregates: A large-scale molecular dynamics study of sodium dodecyl sulfate. J. Phys. Chem. B 2007, 111, 11722–11733. [Google Scholar] [CrossRef]
- MacPherson, Y.; Palepu, R.; Reinsborough, V. Counterion binding by surfactant/β-cyclodextrin inclusion complexes. J. Incl. Phenom. Mol. Recognit. Chem. 1990, 9, 137–143. [Google Scholar] [CrossRef]
- Junquera, E.; Peña, L.; Aicart, E. A conductimetric study of the interaction of β-cyclodextrin or hydroxypropyl-β-cyclodextrin with dodecyltrimethylammonium bromide in water solution. Langmuir 1995, 11, 4685–4690. [Google Scholar] [CrossRef]
- Liu, K.; Ma, C.; Wu, T.; Qi, W.; Yan, Y.; Huang, J. Recent advances in assemblies of cyclodextrins and amphiphiles: Construction and regulation. Curr. Opin. Colloid Interface Sci. 2020, 45, 44–56. [Google Scholar] [CrossRef]
- Wu, C.; Xie, Q.; Xu, W.; Tu, M.; Jiang, L. Lattice self-assembly of cyclodextrin complexes and beyond. Curr. Opin. Colloid Interface Sci. 2019, 39, 76–85. [Google Scholar] [CrossRef]
- Ouhajji, S.; Landman, J.; Prévost, S.; Jiang, L.; Philipse, A.P.; Petukhov, A.V. In situ observation of self-assembly of sugars and surfactants from nanometres to microns. Soft Matter 2017, 13, 2421–2425. [Google Scholar] [CrossRef]
- Yang, S.; Yan, Y.; Huang, J.; Petukhov, A.V.; Kroon-Batenburg, L.M.; Drechsler, M.; Zhou, C.; Tu, M.; Granick, S.; Jiang, L. Giant capsids from lattice self-assembly of cyclodextrin complexes. Nat. Commun. 2017, 8, 15751. [Google Scholar] [CrossRef]
- Wang, J.; Yao, M.; Li, Q.; Yi, S.; Chen, X. β-Cyclodextrin induced hierarchical self-assembly of a cationic surfactant bearing an adamantane end group in aqueous solution. Soft Matter 2016, 12, 9641–9648. [Google Scholar] [CrossRef] [PubMed]
- Makedonopoulou, S.; Mavridis, I.M. Structure of the inclusion complex of β-cyclodextrin with 1,12-dodecanedioic acid using synchrotron radiation data; a detailed dimeric β-cyclodextrin structure. Acta Crystallogr. Sect. B Struct. Sci. 2000, 56, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Peng, Y.; Yan, Y.; Huang, J.; Landman, J.; Philipse, A.P. “Annular ring” microtubes formed by SDS@ 2β-CD complexes in aqueous solution. Soft Matter 2010, 6, 1749–1753. [Google Scholar] [CrossRef]
- Fajalia, A.I.; Antoniou, E.; Alexandridis, P.; Tsianou, M. Self-assembly of sodium bis(2-ethylhexyl) sulfosuccinate in aqueous solutions: Modulation of micelle structure and interactions by cyclodextrins investigated by small-angle neutron scattering. J. Mol. Liq. 2015, 210, 125–135. [Google Scholar] [CrossRef]
- Carlstedt, J.; Bilalov, A.; Krivtsova, E.; Olsson, U.; Lindman, B. Cyclodextrin-surfactant coassembly depends on the cyclodextrin ability to crystallize. Langmuir 2012, 28, 2387–2394. [Google Scholar] [CrossRef]
- Astray, G.; Cid, A.; García-Río, L.; Lodeiro, C.; Mejuto, J.C.; Moldes, O.; Morales, J. Cyclodextrin–surfactant mixed systems as reaction media. Prog. React. Kinet. Mech. 2010, 35, 105–129. [Google Scholar] [CrossRef]
- dos Santos Silva Araújo, L.; Watson, L.; Traore, D.A.K.; Lazzara, G.; Chiappisi, L. Hierarchical assembly of pH-responsive surfactant–cyclodextrin complexes. Soft Matter 2022, 18, 6529–6537. [Google Scholar] [CrossRef]
- Meng, Q.; Xu, W.; Xiao, Z.; Ke, Q.; Kou, X. Morphological evolution of self-assembled sodium dodecyl sulfate/dodecyltrimethylammonium bromide@epoxy-β-cyclodextrin supramolecular aggregates induced by temperature. J. Renew. Mater. 2024, 12, 629–641. [Google Scholar] [CrossRef]
- Musuc, A.M. Cyclodextrins: Advances in Chemistry, Toxicology, and Multifaceted Applications. Molecules 2024, 29, 5319. [Google Scholar] [CrossRef] [PubMed]
- Benkő, M.; Király, L.A.; Puskás, S.; Király, Z. Complexation of β-cyclodextrin with a gemini surfactant studied by isothermal titration microcalorimetry and surface tensiometry. Langmuir 2014, 30, 6756–6762. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gaitano, G.; Crespo, A.; Tardajos, G. Thermodynamic investigation (volume and compressibility) of the systems β-cyclodextrin + n-alkyltrimethylammonium bromides + water. J. Phys. Chem. B 2000, 104, 1869–1879. [Google Scholar] [CrossRef]
- Nilsson, M.; Valente, A.J.M.; Olofsson, G.; Söderman, O.; Bonini, M. Thermodynamic and kinetic characterization of host-guest association between bolaform surfactants and α- and β-cyclodextrins. J. Phys. Chem. B 2008, 112, 11310–11316. [Google Scholar] [CrossRef]
- Xing, H.; Lin, S.-S.; Xiao, J.-X. A Study of the behavior of α-cyclodextrin with single solutions of hydrogenated and fluorinated surfactants and their mixtures. J. Chem. Eng. Data 2010, 55, 1940–1944. [Google Scholar] [CrossRef]
- Alami, E.; Abrahmsen-Alami, S.; Eastoe, J.; Grillo, I.; Heenan, R.K. Interactions between a nonionic gemini surfactant and cyclodextrins investigated by small-angle neutron scattering. J. Colloid Interface Sci. 2002, 255, 403–409. [Google Scholar] [CrossRef]
- Mura, P. Analytical techniques for characterization of cyclodextrin complexes in aqueous solution: A review. J. Pharm. Biomed. Anal. 2014, 101, 238–250. [Google Scholar] [CrossRef]
- Mazurek, A.H.; Szeleszczuk, Ł. Current Status of Quantum Chemical Studies of Cyclodextrin Host–Guest Complexes. Molecules 2022, 27, 3874. [Google Scholar] [CrossRef]
- Jitapunkul, K.; Toochinda, P.; Lawtrakul, L. Molecular Dynamic Simulation Analysis on the Inclusion Complexation of Plumbagin with β-Cyclodextrin Derivatives in Aqueous Solution. Molecules 2021, 26, 6784. [Google Scholar] [CrossRef]
- Kaushik, R.; Verma, R.; Budhwar, V.; Kaushik, D. An Overview of Recent Patents and Future Perspective Based on Cyclodextrin Complexation. Recent Adv. Drug Deliv. Formul. 2023, 17, 31–46. [Google Scholar] [CrossRef]
- Wang, N.; Ma, Z.; Wang, X.; Zhang, X. Photocontrolled reversible supramolecular assemblies of an azobenzene-containing surfactant with α-cyclodextrin. Angew. Chem. Int. Ed. 2007, 46, 2823–2826. [Google Scholar] [CrossRef]
- Zhang, H.; An, W.; Liu, Z.; Hao, A.; Hao, J.; Shen, J.; Zhao, X.; Sun, H.; Sun, L. Redox-responsive vesicles prepared from supramolecular cyclodextrin amphiphiles. Carbohydr. Res. 2010, 345, 87–96. [Google Scholar] [CrossRef]
- Naik, T.; Berdnikova, D.V.; Dutta Choudhury, S. Detecting emerging surfactant pollutants with a highly fluorescent host–guest ensemble. J. Mol. Liq. 2025, 428, 127546. [Google Scholar] [CrossRef]


| Type of Reaction | Reaction Details | Resulting Products |
|---|---|---|
| Substitution | Monosubstitution at OH groups (positions 6, 2, or 3) | Mono-substituted CDs |
| O-substitution | Methylated CDs, Hydroxypropylated CDs, Halogenated CDs | |
| Disubstitution | Di-substituted CDs (e.g., Di-thiolated α-CDs) | |
| Per-substitution | Per-substituted CDs (e.g., Per-halogenated CDs, Per-thiolated CDs) | |
| Random substitution | Randomly methylated β-CDs | |
| Condensation | Condensation with propylene oxide | 2-Hydroxypropylated CDs |
| Polycondensation with epichlorohydrin or polyepoxides | Branched polymeric CD materials | |
| Formation of polymeric CD derivatives | PEG–βCD, Dextran–βCD, CD-based polyrotaxanes | |
| Esterification | Acetylation | Peracetylated CDs |
| Oxidation | Introduction of aldehyde groups | Aldehyde-functionalized CDs |
| Cyclodextrin | Surfactant | Parameter/Condition | Result | Ref. |
|---|---|---|---|---|
| α-CD | Alkyl sulfates (C6–C12) | Binding constant (logK) | logK increases with alkyl chain length; α-CD forms stronger complexes than β-CD (e.g., C8: logK α-CD ≈ 2.6 vs. β-CD ≈ 1.9) | [113,116] |
| β-CD | SDS | Binding constant (logK) | logK ≈ 2.0 (calculated from CMC increase from 8.2 mM → 15 mM) | [140,144] |
| β-CD | Cationic surfactants (DTAB, CTAB) | Binding constant (logK) | β-CD–DTAB: logK ≈ 2.3; β-CD–CTAB: logK ≈ 3.1 | [141,145] |
| Hydroxypropyl-β-CD | SDS + DTAB mixture | Temperature-dependent self-assembly | Worm-like micelles at 25 °C; vesicles at 50 °C | [151] |
| γ-CD | Alkyl ether carboxylates | Charge variation (pH) | Complexation and supramolecular aggregates form spontaneously; multilayer periodicity ≈ 10 nm | [149] |
| β-CD | C12E5, C12E10, C14E5, C16E5 | Complexation behavior/Free CD fraction | β-CD forms complexes with all studied surfactants; fraction of free β-CD ranges from ~5% (C12E5) to ~95% (C16E5) depending on surfactant chain length and ethoxylation; vesicular structures are observed in some cases | [150] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilipović, A.; Tepavčević, V.; Kumar, D.; Poša, M. Cyclodextrins, Surfactants and Their Inclusion Complexes. Molecules 2025, 30, 3944. https://doi.org/10.3390/molecules30193944
Pilipović A, Tepavčević V, Kumar D, Poša M. Cyclodextrins, Surfactants and Their Inclusion Complexes. Molecules. 2025; 30(19):3944. https://doi.org/10.3390/molecules30193944
Chicago/Turabian StylePilipović, Ana, Vesna Tepavčević, Dileep Kumar, and Mihalj Poša. 2025. "Cyclodextrins, Surfactants and Their Inclusion Complexes" Molecules 30, no. 19: 3944. https://doi.org/10.3390/molecules30193944
APA StylePilipović, A., Tepavčević, V., Kumar, D., & Poša, M. (2025). Cyclodextrins, Surfactants and Their Inclusion Complexes. Molecules, 30(19), 3944. https://doi.org/10.3390/molecules30193944







