Characteristics of Novel Fermented Cloudy Fruit Juices Produced Using Lactiplantibacillus plantarum and Lactic Acid-Producing Lachancea spp. Yeasts
Abstract
1. Introduction
2. Results and Discussion
2.1. Fermentation Kinetics and Basic Physicochemical Parameters
2.2. Volatile Compounds
2.3. Polyphenols
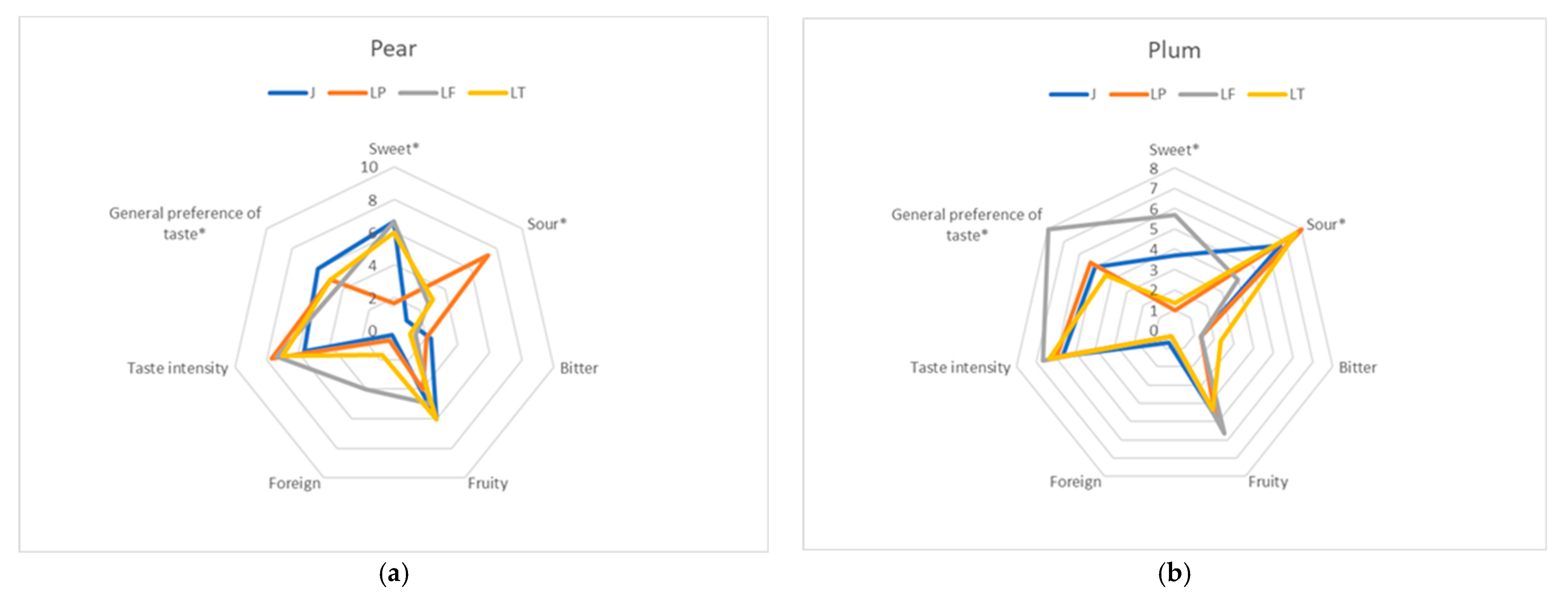
2.4. Sensory Analysis
3. Materials and Methods
3.1. Microbial Strains and Media Used
3.2. Preparation and Fermentation of Juices
3.3. pH and Acidity Measurement
3.4. Organic Acids’ Analysis
3.5. Sugar and Ethanol Analysis
3.6. Free Amino Nitrogen Analysis
3.7. Color Analysis
3.8. Volatile Compounds’ Analysis (HS-SPME-GC-MS)
3.9. Odor-Active Volatile Components (HS-SPME-GC-O)
3.10. Determination of Volatile Organic Acids by Derivatization with Benzyl Bromide
3.11. Polyphenol Analysis
3.12. Sensory Analysis (QDA)
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| J | Raw juice |
| LP | Juice fermented by Lactilantibacillus plantarum K7 |
| LT | Juice fermented by Lachancea thermotolerans PYCC6375 |
| LF | Juice fermented by Lachancea fermentati PYCC5883 |
References
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 24 September 2025).
- Core Team. Available online: https://coreteam.pl/369449-ulubione-owoce-listopada-kantar-polska (accessed on 25 September 2025).
- Francini, A.; Pintado, M.; Manganaris, G.A.; Ferrante, A. Bioactive compounds biosynthesis and metabolism in fruit and vegetables. Front. Plant Sci. 2020, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Zhang, N.; Wang, J.; Cheng, Y.; Guan, J. Health Benefits and Bioactive Compounds of Pear and Its Products: A Comprehensive Review. Food Sci. Hum. Wellness 2025. [Google Scholar] [CrossRef]
- Ayub, H.; Nadeem, M.; Mohsin, M.; Ambreen, S.; Khan, F.A.; Oranab, S.; Rahim, M.A.; Khalid, M.Z.; Zongo, E.; Zarlasht, M.; et al. A comprehensive review on the availability of bioactive compounds, phytochemicals, and antioxidant potential of plum (Prunus domestica). Int. J. Food Prop. 2023, 26, 2388–2406. [Google Scholar] [CrossRef]
- Hong, S.Y.; Lansky, E.; Kang, S.S.; Yang, M. A review of pears (Pyrus spp.), ancient functional food for modern times. BMC Complement. Med. Ther. 2021, 21, 219. [Google Scholar] [CrossRef]
- Mahboubi, M. Prunus domestica as effective and acceptable treatment for stool softening and relief of constipation symptoms. Songklanakarin J. Sci. Technol. 2021, 43, 1183–1189. [Google Scholar] [CrossRef]
- Mishra, S.; Vyas, S. Therapeutic and pharmacological potential of Prunus domestica: A Comprehensive Review. Int. J. Pharm. Sci. Res. 2021, 12, 3034–3041. [Google Scholar] [CrossRef]
- Saud, S.; Xiaojuan, T.; Fahad, S. The consequences of fermentation metabolism on the qualitative qualities and biological activity of fermented fruit and vegetable juices. Food Chem. X 2024, 21, 101209. [Google Scholar] [CrossRef]
- Hu, Q.-Y.; Tang, X.-X.; Li, Z.; Wei, L.-F.; Wu, X.-P.; Zhao, H. Effects of lactic acid bacteria fermentation on antioxidant activity and sensory quality of Rosa sterilis S D Shi. Food Med. Homol. 2025, 2, 9420026. [Google Scholar] [CrossRef]
- Li, Z.; Teng, J.; Lyu, Y.; Hu, X.; Zhao, Y.; Wang, M. Enhanced antioxidant activity for apple juice fermented with Lactobacillus plantarum ATCC14917. Molecules 2018, 24, 51. [Google Scholar] [CrossRef]
- Rizzi, F.; Juan, B.; Espadaler-Mazo, J.; Capellas, M.; Huedo, P. Lactiplantibacillus plantarum KABP051: Stability in Fruit Juices and Production of Bioactive Compounds During Their Fermentation. Foods 2024, 13, 3851. [Google Scholar] [CrossRef]
- Zeng, H.; Shuai, Y.; Zeng, X.; Xin, B.; Huang, M.; Li, B.; Qiao, J.; Wang, Y.; Qui, X.; Wang, C. Evaluation of health-related composition and bioactivity of five fruit juices following Lactobacillus plantarum fermentation and simulated digestion. Int. J. Food Sci. Technol. 2021, 56, 648–660. [Google Scholar] [CrossRef]
- Yang, J.; Sun, Y.; Gao, T.; Wu, Y.; Sun, H.; Zhu, Q.; Liu, C.; Zhou, C.; Han, Y.; Tao, Y. Fermentation and storage characteristics of “Fuji” apple juice using Lactobacillus acidophilus, Lactobacillus casei and Lactobacillus plantarum: Microbial growth, metabolism of bioactives and in vitro bioactivities. Front. Nutr. 2022, 9, 833906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, W.; Wei, Z.; Yin, B.; Man, C.; Jiang, Y. Enhancement of functional characteristics of blueberry juice fermented by Lactobacillus plantarum. LWT 2021, 139, 110590. [Google Scholar] [CrossRef]
- Cheng, Z.; Wang, S.-H.; Li, L.-Y.; Zhou, Y.C.; Zhang, C.Y.; Liu, H.; Pan, T.M.; Liu, G.-M.; Liu, Q.-M. Fermented Porphyra haitanensis polysaccharides inhibit the degranulation of mast cell and passive cutaneous anaphylaxis. Food Med. Homol. 2025. [Google Scholar] [CrossRef]
- Fairbairn, S.; Engelbrecht, L.; Setati, M.E.; Du Toit, M.; Bauer, F.F.; Divol, B.; Rossouw, D. Combinatorial analysis of population dynamics, metabolite levels and malolactic fermentation in Saccharomyces cerevisiae/Lachancea thermotolerans mixed fermentations. Food Microbiol. 2021, 96, 103712. [Google Scholar] [CrossRef]
- Fernández-Vázquez, D.; Sunyer-Figueres, M.; Vázquez, J.; Puxeu, M.; Nart, E.; De Lamo, S.; Andorrà, I. Selection and Use of Wild Lachancea thermotolerans Strains from Rioja AOC with Bioacidificant Capacity as Strategy to Mitigate Climate Change Effects in Wine Industry. Beverages 2025, 11, 70. [Google Scholar] [CrossRef]
- Izquierdo-Cañas, P.M.; Del Fresno, J.M.; Malfeito-Ferreira, M.; Mena-Morales, A.; García-Romero, E.; Heras, J.M.; Loira, I.; González, C.; Morata, A. Wine bioacidification: Fermenting Airén grape juices with Lachancea thermotolerans and Metschnikovia pulcherrima followed by sequential Saccharomyces cerevisiae inoculation. Int. J. Food Microbiol. 2025, 427, 110977. [Google Scholar] [CrossRef]
- Sizzano, F.; Bianconi, V.; Blackford, M.; Bieri, S.; Vuichard, F.; Monnard, C.; Amiet, L.; Spring, J.-L.; Dorsaz, E.; Pfenninger-Bridy, N.; et al. Use of Lachancea thermotolerans for the Bioacidification of White Grape Musts: Assays from the Bench to the Cellar Scale. Fermentation 2024, 10, 458. [Google Scholar] [CrossRef]
- Bellut, K.; Michel, M.; Hutzler, M.; Zarnkow, M.; Jacob, F.; De Schutter, D.P.; Daenen, L.; Lynch, K.M.; Zannini, E.; Arendt, E.K. Investigation into the potential of Lachancea fermentati strain KBI 12.1 for low alcohol beer brewing. J. Am. Soc. Brew. Chem. 2019, 77, 157–169. [Google Scholar] [CrossRef]
- Bellut, K.; Krogerus, K.; Arendt, E.K. Lachancea fermentati strains isolated from kombucha: Fundamental insights, and practical application in low alcohol beer brewing. Front. Microbiol. 2020, 11, 764. [Google Scholar] [CrossRef]
- Domizio, P.; House, J.F.; Joseph, C.L.; Bisson, L.F.; Bamforth, C.W. Lachancea thermotolerans as an alternative yeast for the production of beer. J. Inst. Brew. 2016, 122, 599–604. [Google Scholar] [CrossRef]
- Fu, X.; Guo, L.; Li, Y.; Chen, X.; Song, Y.; Li, S. Transcriptional Analysis of Mixed-Culture Fermentation of Lachancea thermotolerans and Saccharomyces cerevisiae for Natural Fruity Sour Beer. Fermentation 2024, 10, 180. [Google Scholar] [CrossRef]
- Postigo, V.; Esteban, S.; Arroyo, T. Lachancea thermotolerans, an innovative alternative for sour beer production. Beverages 2023, 9, 20. [Google Scholar] [CrossRef]
- Zdaniewicz, M.; Satora, P.; Kania, P.; Florkiewicz, A. The Impact of Selected Lachancea Yeast Strains on the Production Process, Chemical Composition and Aroma Profiles of Beers. Molecules 2024, 29, 5674. [Google Scholar] [CrossRef] [PubMed]
- Satora, P.; Skotniczny, M.; Strnad, S.; Piechowicz, W. Chemical composition and sensory quality of sauerkraut produced from different cabbage varieties. LWT 2021, 136, 110325. [Google Scholar] [CrossRef]
- Willems, J.L.; Low, N.H. Authenticity analysis of pear juice employing chromatographic fingerprinting. J. Agric. Food Chem. 2014, 62, 11737–11747. [Google Scholar] [CrossRef]
- Cupic, T.; Josipovic, M.; Viljevac, M.; Sudar, R.; Dugalic, K. Sorbitol and sugar composition in plum fruits influenced by climatic conditions. J. Agr. Sci. Technol. 2014, 16, 1145–1155. [Google Scholar]
- Martínez-Miranda, J.G.; Chairez, I.; Durán-Páramo, E. Mannitol production by heterofermentative lactic acid bacteria: A review. Appl. Biochem. Biotechnol. 2022, 194, 2762–2795. [Google Scholar] [CrossRef]
- Ma, S.; Neilson, A.P.; Lahne, J.; Peck, G.M.; O’Keefe, S.F.; Stewart, A.C. Free amino acid composition of apple juices with potential for cider making as determined by UPLC-PDA. J. Inst. Brew. 2018, 124, 467–476. [Google Scholar] [CrossRef]
- Moon, K.M.; Kwon, E.B.; Lee, B.; Kim, C.Y. Recent trends in controlling the enzymatic browning of fruit and vegetable products. Molecules 2020, 25, 2754. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Zhao, J.; Wang, Y.; Shi, Z.; Xie, K.; Liao, X.; Tan, J. Factors affecting the stability of anthocyanins and strategies for improving their stability: A review. Food Chem. X 2024, 24, 101883. [Google Scholar] [CrossRef] [PubMed]
- Gänzle, M.G. Lactic metabolism revisited: Metabolism of lactic acid bacteria in food fermentations and food spoilage. Curr. Opin. Food Sci. 2015, 2, 106–117. [Google Scholar] [CrossRef]
- Yang, X.; Teng, K.; Su, R.; Li, L.; Zhang, T.; Fan, K.; Zhang, J.; Zhong, J. AcrR and Rex Control Mannitol and Sorbitol Utilization through Their Cross-Regulation of Aldehyde-Alcohol Dehydrogenase (AdhE) in Lactobacillus plantarum. Appl. Environ. Microbiol. 2019, 85, e02035-18. [Google Scholar] [CrossRef]
- Ijoma, G.N.; Adegbenro, G.; Rashama, C.; Matambo, T.S. Peculiar response in the co-culture fermentation of Leuconostoc mesenteroides and Lactobacillus plantarum for the production of ABE solvents. Fermentation 2021, 7, 212. [Google Scholar] [CrossRef]
- von Weymarn, N.; Hujanen, M.; Leisola, M. Production of D-mannitol by heterofermentative lactic acid bacteria. Process Biochem. 2002, 37, 1207–1213. [Google Scholar] [CrossRef]
- Chen, W.; Xie, C.; He, Q.; Sun, J.; Bai, W. Improvement in color expression and antioxidant activity of strawberry juice fer-mented with lactic acid bacteria: A phenolic-based research. Food Chem. X 2023, 17, 100535. [Google Scholar] [CrossRef]
- Charoenchongsuk, N.; Ikeda, K.; Itai, A.; Oikawa, A.; Murayama, H. Comparison of the expression of chlorophyll-degrada-tion-related genes during ripening between stay-green and yellow-pear cultivars. Sci. Hortic. 2015, 181, 89–94. [Google Scholar] [CrossRef]
- Usenik, V.; Štampar, F.; Veberič, R. Anthocyanins and fruit colour in plums (Prunus domestica L.) during ripening. Food Chem. 2009, 114, 529–534. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Lei, H. Phenolics profile, antioxidant activity and flavor volatiles of pear juice: Influence of lactic acid fermentation using three Lactobacillus strains in monoculture and binary mixture. Foods 2021, 11, 11. [Google Scholar] [CrossRef]
- Srinivasan, L.V.; Rana, S.S. Anthocyanins: A promising source of natural colorants and nutraceuticals. Discov. Appl. Sci. 2025, 7, 694. [Google Scholar] [CrossRef]
- Sun, H.; Lu, X.; Wang, Y.; Li, J.; Liu, S. Study on Evaluation of Fruit Aroma of Plum Variety Resources Based on Headspace Solid-Phase Microextraction Combined with Gas Chromatography–Mass Spectrometry. Foods 2024, 13, 3515. [Google Scholar] [CrossRef]
- Ismail, H.; Williams, A.; Tucknott, O. The flavour of plums (Prunus domestica L.). An examination of the aroma components of plum juice from the cultivar victoria. J. Sci. Food and Agri. 1981, 32, 613–619. [Google Scholar] [CrossRef]
- Niimi, J.; Guixer, B.; Splivallo, R. Odour active compounds determined in the headspace of yellow and black plum wines (Prunus domestica L.). LWT 2020, 130, 109702. [Google Scholar] [CrossRef]
- Taiti, C.; Pandolfi, C.; Caparrotta, S.; Dei, M.; Giordani, E.; Mancuso, S.; Nencetti, V. Fruit aroma and sensorial characteristics of traditional and innovative Japanese plum (Prunus salicina Lindl.) cultivars grown in Italy. Eur. Food Res. Technol. 2019, 245, 2655–2668. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, H.X.; Xia, N.; Duan, C.; Yang, W.; Pan, Q. Leaching and evolution of anthocyanins and aroma compounds during Cabernet Sauvignon wine fermentation with whole-process skin-seed contact. Food Chem. 2023, 436, 137727. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, T.; Herraiz, M.; Reglero, G.; Martín-Alvarez, P.; Cabezudo, M. Changes in the composition of alcohols and aldehydes of C6 chain length during the alcoholic fermentation of grape must. J. Agric. Food Chem. 1990, 38, 969–972. [Google Scholar] [CrossRef]
- Zoecklein, B.; Marcy, J.; Williams, J.; Jasinski, Y. Effect of Native Yeasts and Selected Strains of Saccharomyces cerevisiae on Glycosyl Glucose, Potential Volatile Terpenes, and Selected Aglycones of White Riesling (Vitis vinifera L.) Wines. J. Food Compos. Anal. 1997, 10, 55–65. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Song, H.; Tao, Y.; Russo, N. Phenolic matrix effect on aroma formation of terpenes during simulated wine fermentation—Part I: Phenolic acids. Food Chem. 2021, 341, 128288. [Google Scholar] [CrossRef]
- Yin, H.; Liu, L.; Yang, M.; Ding, X.; Jia, S.; Dong, J.; Zhong, C. Enhancing Medium-Chain Fatty Acid Ethyl Ester Production During Beer Fermentation Through EEB1 and ETR1 Overexpression in Saccharomyces pastorianus. J. Agric. Food Chem. 2019, 67, 5607–5613. [Google Scholar] [CrossRef]
- Mukdsi, M.; Medina, R.; Alvarez, M.; González, S. Ester synthesis by lactic acid bacteria isolated from goat’s and ewe’s milk and cheeses. Food Chem. 2009, 117, 241–247. [Google Scholar] [CrossRef]
- De Godoy Alves Filho, E.; Rodrigues, T.; Fernandes, F.; Pereira, A.; Narain, N.; De Brito, E.; Rodrigues, S. Chemometric evaluation of the volatile profile of probiotic melon and probiotic cashew juice. Food Res. Int. 2017, 99, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Filannino, P.; Cardinali, G.; Rizzello, C.; Buchin, S.; De Angelis, M.; Gobbetti, M.; Di Cagno, R. Metabolic Responses of Lactobacillus plantarum Strains during Fermentation and Storage of Vegetable and Fruit Juices. Appl. Environ. Microbiol. 2014, 80, 2206–2215. [Google Scholar] [CrossRef] [PubMed]
- Braga, C.; Zielinski, A.; Da Silva, K.; De Souza, F.; De Arruda Moura Pietrowski, G.; Couto, M.; Granato, D.; Wosiacki, G.; Nogueira, A. Classification of juices and fermented beverages made from unripe, ripe and senescent apples based on the aromatic profile using chemometrics. Food Chem. 2013, 141, 967–974. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Zhang, J.; Wang, Z.; Qi, K.; Li, H.; Tian, R.; Wu, X.; Qiao, X.; Zhang, S.; et al. Comparative analysis of volatile aromatic compounds from a wide range of pear (Pyrus L.) germplasm resources based on HS-SPME with GC-MS. Food Chem. 2023, 418, 135963. [Google Scholar] [CrossRef]
- Soledad, M.; Moya, P.; Leahu, A.; Ghinea, C.; Zhang, W.; Yan, M.; Zheng, X.; Chen, Z.; Li, H.; Mao, J.; et al. Exploring the Aroma Fingerprint of Various Chinese Pear Cultivars through Qualitative and Quantitative Analysis of Volatile Compounds Using HS-SPME and GC×GC-TOFMS. Molecules 2023, 28, 4794. [Google Scholar] [CrossRef]
- Zierer, B.; Schieberle, P.; Granvogl, M. Aroma-Active Compounds in Bartlett Pears and Their Changes during the Manufacturing Process of Bartlett Pear Brandy. J. Agric. Food Chem. 2016, 64, 9515–9522. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, W.; Li, H.; Mao, J.; Guo, C.; Ding, R.; Wang, Y.; Fang, L.; Chen, Z.; Yang, G. Analysis of Volatile Compounds in Pears by HS-SPME-GC×GC-TOFMS. Molecules 2019, 24, 1795. [Google Scholar] [CrossRef]
- Delcros, L.; Collas, S.; Hervé, M.; Blondin, B.; Roland, A. Evolution of Markers Involved in the Fresh Mushroom Off-Flavor in Wine During Alcoholic Fermentation. J. Agric. Food Chem. 2023, 71, 14687–14696. [Google Scholar] [CrossRef]
- Lan, T.; Lv, X.; Zhao, Q.; Lei, Y.; Gao, C.; Yuan, Q.; Sun, X.; Liu, X. Optimization of strains for fermentation of kiwifruit juice and effects of mono- and mixed culture fermentation on its sensory and aroma profiles. Food Chem. X 2023, 17, 100595. [Google Scholar] [CrossRef]
- Wang, D.; Deng, Y.; Chen, X.; Wang, K.; Zhao, L.; Wang, Z.; Liu, X.; Hu, Z. Elucidating the effects of Lactobacillus plantarum fermentation on the aroma profiles of pasteurized litchi juice using multi-scale molecular sensory science. CRFS 2023, 6, 100481. [Google Scholar] [CrossRef]
- Mandha, J.; Shumoy, H.; Devaere, J.; Schouteten, J.; Gellynck, X.; De Winne, A.; Matemu, A.; Raes, K. Effect of lactic acid fermentation of watermelon juice on its sensory acceptability and volatile compounds. Food Chem. 2021, 358, 129809. [Google Scholar] [CrossRef] [PubMed]
- Willner, B.; Granvogl, M.; Schieberle, P. Characterization of the key aroma compounds in Bartlett pear brandies by means of the sensomics concept. J. Agric. Food Chem. 2013, 61, 9583–9593. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, N.; Capone, D.; Ugliano, M.; Taylor, D.; Skouroumounis, G.; Sefton, M.; Elsey, G. Formation of Damascenone under both commercial and model fermentation conditions. J. Agric. Food Chem. 2011, 59, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Lin, B.; Jiang, W.; Li, Q.; Zhu, L.; Zhang, G.; Chen, Q.; Yang, Q.; Yang, S.; Chen, S. Screening of β-damascenone-producing strains in light-flavor Baijiu and its production optimization via response surface methodology. Front. Microbiol. 2022, 13, 1067671. [Google Scholar] [CrossRef]
- Chang, Y.; Reddy, M. Butyric Acid and Caproic Acid Production Using Single and Mixed Bacterial Cultures. Eng. Proc. 2023, 56, 73. [Google Scholar] [CrossRef]
- Park, Y.; Kim, T.; Ham, J.; Choi, J.; Lee, H.; Yeon, Y.; Choi, S.; Kim, N.; Kim, Y.; Seok, Y. Physiological activity of E. coli engineered to produce butyric acid. Microb. Biotechnol. 2021, 15, 832–843. [Google Scholar] [CrossRef]
- Kim, D.; Chun, O.; Kim, Y.; Moon, H.; Lee, C. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef]
- Cosmulescu, S.; Trandafir, I.; Nour, V.; Botu, M. Total Phenolic, Flavonoid Distribution and Antioxidant Capacity in Skin, Pulp and Fruit Extracts of Plum Cultivars. J. Food Biochem. 2015, 39, 64–69. [Google Scholar] [CrossRef]
- Manzoor, M.; Mahmood, A.; Ajaz, M.; Rasool, W.; Shabbir, M. Juicy Gems of Nutrition: Exploring the Nutrient Profile and Antioxidant Activity of Rosaceae Fruits. DIET FACTOR (J. Nutr. Food Sci.) 2023, 4, 08–19. [Google Scholar] [CrossRef]
- Cabrera-Bañegil, M.; Rodas, N.; Losada, M.; Cipollone, F.; Espino, M.; Peña, A.; Durán-Merás, I. Evolution of polyphenols content in plum fruits (Prunus salicina) with harvesting time by second-order excitation-emission fluorescence multivariate calibration. Microchem J. 2020, 158, 105299. [Google Scholar] [CrossRef]
- DiNardo, A.; Subramanian, J.; Singh, A. Investigation of Antioxidant Content and Capacity in Yellow European Plums. Int. J. Fruit Sci. 2018, 18, 116–199. [Google Scholar] [CrossRef]
- Zhang, H.; Pu, J.; Tang, Y.; Wang, M.; Tian, K.; Wang, Y.; Luo, X.; Deng, Q. Changes in Phenolic Compounds and Antioxidant Activity during Development of ‘Qiangcuili’ and ‘Cuihongli’ Fruit. Foods 2022, 11, 3198. [Google Scholar] [CrossRef] [PubMed]
- Chun, O.; Kim, D. Consideration on equivalent chemicals in total phenolic assay of chlorogenic acid-rich plums. Food Res. Int. 2004, 37, 337–342. [Google Scholar] [CrossRef]
- He, J.; Cheng, Y.; Guan, J.; Ge, W.; Zhao, Z. Changes of chlorogenic acid content and its synthesis-associated genes expression in Xuehua pear fruit during development. J. Integr. Agric. 2017, 16, 471–477. [Google Scholar] [CrossRef]
- Bouayed, J.; Rammal, H.; Dicko, A.; Younos, C.; Soulimani, R. Chlorogenic acid, a polyphenol from Prunus domestica (Mirabelle), with coupled anxiolytic and antioxidant effects. J. Neurol. Sci. 2007, 262, 77–84. [Google Scholar] [CrossRef]
- Zagoskina, N.; Zubova, M.; Nechaeva, T.; Kazantseva, V.; Goncharuk, E.; Katanskaya, V.; Baranova, E.; Aksenova, M. Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications (Review). Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Fan, J.; Li, Q.; Jia, L.; Xu, L.; Wu, X.; Wang, Z.; Li, H.; Qi, K.; Qiao, X.; et al. Variation of Organic Acids in Mature Fruits of 193 Pear (Pyrus spp.) Cultivars. J. Food Compos. Anal. 2022, 109, 104483. [Google Scholar] [CrossRef]
- Bae, H.; Yun, S.; Yoon, I.; Nam, E.; Kwon, J.; Jun, J. Assessment of organic acid and sugar composition in apricot, plumcot, plum, and peach during fruit development. J. Appl. Bot. Food Qual. 2014, 87, 24–29. [Google Scholar] [CrossRef]
- Kim, M.; Sim, D.; Lee, H.; Lee, H.; Kim, S. Hypolipogenic Effect of Shikimic Acid Via Inhibition of MID1IP1 and Phosphorylation of AMPK/ACC. Int. J. Mol. Sci. 2019, 20, 582. [Google Scholar] [CrossRef]
- Liu, S.; He, Y.; He, W.; Song, X.; Peng, Y.; Hu, X.; Bian, S.; Li, Y.; Nie, S.; Yin, J.; et al. Exploring the Biogenic Transformation Mechanism of Polyphenols by Lactobacillus plantarum NCU137 Fermentation and Its Enhancement of Antioxidant Properties in Wolfberry Juice. J. Agric. Food Chem. 2024, 72, 12752–12761. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Yue, T.; Yuan, Y. Evolution of polyphenols and organic acids during the fermentation of apple cider. J. Sci. Food Agric. 2014, 94, 2951–2957. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, S.; Tao, Y.; Li, D.; Han, Y.; Show, P.; Wen, G.; Zhou, J. Fermentation of blueberry and blackberry juices using Lactobacillus plantarum, Streptococcus thermophilus and Bifidobacterium bifidum: Growth of probiotics, metabolism of phenolics, antioxidant capacity in vitro and sensory evaluation. Food Chem. 2021, 348, 129083. [Google Scholar] [CrossRef]
- Mantzourani, I.; Nikolaou, A.; Kourkoutas, Y.; Alexopoulos, A.; Dasenaki, M.; Mastrotheodoraki, A.; Proestos, C.; Thomaidis, N.; Plessas, S. Chemical Profile Characterization of Fruit and Vegetable Juices after Fermentation with Probiotic Strains. Foods 2024, 13, 1136. [Google Scholar] [CrossRef] [PubMed]
- Akpabli-Tsigbe, N.; Ma, Y.; Ekumah, J.; Osabutey, J.; Hu, J.; Xu, M.; Johnson, N.; Quaisie, J. Two-step optimization of solid-state fermentation conditions of heilong48 soybean variety for maximum chlorogenic acid extraction yield with improved antioxidant activity. Ind. Crops Prod. 2021, 168, 113565. [Google Scholar] [CrossRef]
- Junge, J.Y.; Bertelsen, A.S.; Mielby, L.A.; Zeng, Y.; Sun, Y.-X.; Byrne, D.V.; Kidmose, U. Taste Interactions between Sweetness of Sucrose and Sourness of Citric and Tartaric Acid among Chinese and Danish Consumers. Foods 2020, 9, 1425. [Google Scholar] [CrossRef]


| Fruit Juice | Sample | Glucose | Fructose | Sucrose | Glycerol | Sorbitol + Mannitol | FAN |
|---|---|---|---|---|---|---|---|
| [g/L] | |||||||
| Pear | J | 29.7 d ± 0.5 | 98.4 e ± 1.6 | 3.7 b ± 1.2 | 0.00 a ± 0.00 | 31.4 b ± 2.0 | 65.4 ± 1.8 |
| LP | 4.5 b ± 0.4 | 45.6 cd ± 5.4 | 0.0 a ± 0.0 | 4.26 b ± 0.68 | 39.8 c ± 3.3 | 24.6 ± 8.9 | |
| LT | 1.2 a ± 0.3 | 37.5 bcd ± 5.0 | 0.0 a ± 0.0 | 4.82 bc ± 1.70 | 43.4 c ± 1.1 | 15.1 ± 2.2 | |
| LF | 4.0 b ± 0.2 | 48.3 d ± 4.7 | 0.0 a ± 0.0 | 5.99 bc ± 0.74 | 39.1 c ± 0.4 | 36.9 ± 10.4 | |
| Plum | J | 69.9 e ± 0.1 | 27.6 ab ± 0.8 | 11.7 c ± 0.8 | 0.00 a ± 0.00 | 30.7 ab ± 0.3 | 165 ± 2.9 |
| LP | 9.9 c ± 0.8 | 36.5 bc ± 3.1 | 0.0 a ± 0.0 | 9.05 d ± 1.12 | 26.9 ab ± 0.4 | 129 ± 20.3 | |
| LT | 4.4 b ± 0.7 | 18.1 a ± 4.8 | 0.0 a ± 0.0 | 7.62 cd ± 1.56 | 25.1 a ± 1.6 | 134 ± 25.0 | |
| LF | 4.8 b ± 1.5 | 19.6 a ± 2.1 | 0.0 a ± 0.0 | 6.21 bcd ± 0.78 | 27.8 ab ± 3.9 | 120 ± 21.0 | |
| Sig. | Fruit juice | *** | *** | *** | *** | *** | *** |
| Fermentation | *** | *** | *** | *** | ns | *** | |
| Fruit juice × fermentation | *** | *** | *** | ** | *** | ns | |
| Fruit Juice | Sample | pH | Total Acidity | Citric Acid | Malic Acid | Succinic Acid | Lactic Acid | Acetic Acid | Ethanol |
|---|---|---|---|---|---|---|---|---|---|
| [g/L] | |||||||||
| Pear | J | 4.50 a ± 0.02 | 6.17 ± 0.29 | 1.91 a ± 0.21 | 3.64 ± 0.19 | 0.23 a ± 0.16 | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 |
| LP | 3.21 c ± 0.02 | 12.9 ± 2.36 | 1.54 b ± 0.25 | 3.49 ± 0.37 | 1.18 b ± 0.75 | 0.29 ab ± 0.02 | 1.50 c ± 0.21 | 1.32 e ± 0.29 | |
| LT | 3.92 b ± 0.06 | 6.84 ± 2.00 | 0.80 b ± 0.87 | 2.79 ± 0.27 | 0.88 b ± 0.96 | 0.96 b ± 0.33 | 1.32 bc ± 0.06 | 1.14 de ± 0.20 | |
| LF | 3.81 c ± 0.06 | 7.08 ± 1.70 | 1.30 b ± 0.66 | 3.01 ± 0.47 | 1.82 c ± 0.30 | 0.45 ab ± 0.12 | 1.02 b ± 0.29 | 0.31 ab ± 0.06 | |
| Plum | J | 3.30 c ± 0.03 | 15.25 ± 0.43 | 3.13 c ± 0.11 | 2.35 ± 0.12 | 0.02 a ± 0.01 | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 |
| LP | 3.07 d ± 0.01 | 24.12 ± 0.62 | 3.14 c ± 0.21 | 2.32 ±0.66 | 0.31 a ± 0.03 | 2.71 c ± 0.37 | 0.07 a ±0.04 | 0.86 cd ± 0.21 | |
| LT | 3.11 d ± 0.00 | 18.17 ± 0.29 | 3.27 c ± 0.09 | 2.30 ± 0.68 | 0.27 a ± 0.05 | 2.79 c ± 0.57 | 0.14 a ± 0.04 | 0.36 ab ± 0.09 | |
| LF | 3.10 d ± 0.02 | 20.52 ± 4.38 | 3.21 c ± 0.32 | 2.36 ± 0.20 | 0.29 a ± 0.02 | 2.25 c ± 0.39 | 0.13 a ± 0.07 | 0.45 bc ± 0.04 | |
| Sig. | Fruit juice | *** | *** | *** | *** | *** | *** | *** | *** |
| Fermentation | *** | *** | ** | ns | *** | *** | *** | *** | |
| Fruit juice × fermentation | *** | ns | *** | ns | *** | *** | *** | *** | |
| Fruit Juice | Sample | L- | a- | b- | C- | h* | ΔE |
|---|---|---|---|---|---|---|---|
| Pear | J | 70.9 d ± 1.9 | 11.6 ± 0.8 | 55.5 bc ± 1.7 | 56.7 a ± 1.8 | 78.2 d ± 0.5 | |
| LP | 63.8 cd ± 4.0 | 24.0 ± 2.1 | 64.6 d ± 1.5 | 69.0 cd ± 0.7 | 69.6 c ± 2.1 | 15.7 ± 2.4 | |
| LT | 68.9 d ± 5.0 | 17.4 ± 2.5 | 58.8 cd ± 6.7 | 61.4 ab ± 6.8 | 73.5 cd ± 2.1 | 9.4 ± 2.1 | |
| LF | 64.6 cd ± 2.5 | 19.7 ± 2.2 | 62.5 d ± 1.4 | 65.5 bc ± 1.8 | 72.6 c ± 1.6 | 10.8 ± 3.1 | |
| Plum | J | 55.8 b ± 2.6 | 35.0 ± 3.5 | 62.4 d ± 1.5 | 71.7 de ± 0.7 | 60.7 b ± 3.0 | |
| LP | 39.8 a ± 1.5 | 46.5 ± 2.2 | 47.4 a ± 1.6 | 66.4 bcd ± 2.0 | 45.5 a ± 1.7 | 22.3 ± 2.2 | |
| LT | 43.6 a ± 2.9 | 46.2 ± 2.6 | 52.0 ab ± 3.4 | 69.6 cd ± 1.9 | 48.4 a ± 3.2 | 17.0 ± 4.9 | |
| LF | 57.4 bc ± 4.5 | 41.7 ± 6.0 | 64.5 d ± 3.1 | 77.0 e ± 1.1 | 57.1 b ± 5.0 | 9.4 ± 2.8 | |
| Sig. | Fruit juice | *** | *** | *** | *** | *** | |
| Fermentation | *** | *** | *** | *** | *** | ||
| Fruit juice × fermentation | *** | ns | *** | *** | *** |
| Compound [μg/L] | m/z | LRI | THR | Pear Juice | Plum Juice | Sig. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| J | LP | LT | LF | J | LP | LT | LF | Fruit Juice | Fermentation | Fruit Juice x Fermentation | ||||
| Alcohols | ||||||||||||||
| 3-Methyl-1-butanol | 42, 55, 70 | 724 | 71 | 2 a | 111 ab | 358 cd | 256 bc | 2 a | 341 cd | 410 cd | 422 d | *** | *** | * |
| (Z)-3-Heksen-1-ol | 41, 55, 67 | 837 | 13.0 | 1.5 a | 0.1 a | 0.2 a | 0.1 a | 30.1 b | 3.3 a | 3.3 a | 3.2 a | *** | *** | *** |
| Heksan-1-ol | 41, 43, 56 | 852 | 100 | 297 c | 45 a | 50 a | 41 a | 185 b | 29 a | 28 a | 27 a | ns | *** | *** |
| 1-Heptanol | 41, 56, 70 | 953 | 5.4 | 0.0 a | 4.4 bc | 7.8 c | 7.7 c | 0.0 a | 3.5 ab | 2.3 ab | 0.8 ab | *** | *** | *** |
| 1-Octen-3-ol | 43, 57, 72 | 961 | 0.01 1 0.5 2,3 | 5.0 bc | 7.1 cde | 9.4 e | 8.4 de | 0.2 a | 5.9 bcd | 3.9 bc | 3.0 ab | *** | *** | *** |
| Carbonyl compounds | ||||||||||||||
| 2-Methylbutanal | 42, 55, 86 | 640 | 1.0 | 0.0 a | 0.0 a | 0.0 a | 0.0 a | 2.3 b | 0.0 a | 0.0 a | 0.0 a | *** | *** | *** |
| 3-Hexenal | 41, 55, 69 | 773 | 0.25 | 0.0 a | 0.0 a | 0.0 a | 0.0 a | 9.4 b | 0.0 a | 0.0 a | 0.0 a | *** | *** | *** |
| Hexanal | 41, 44, 56 | 776 | 10.0 | 287 c | 0.0 a | 1.0 a | 1.0 a | 160 b | 0.0 a | 0.0 a | 0.0 a | *** | *** | *** |
| 2-Hexenal | 41, 55, 69 | 825 | 17.0 | 44.5 c | 0.0 a | 0.0 a | 0.0 a | 25.1 b | 0.0 a | 0.0 a | 0.0 a | *** | *** | *** |
| 1-Octen-3-one | 55, 70, 97 | 954 | 0.01 1 0.5 2,3 | 2.2 b | 2.6 b | 3.6 c | 2.1 b | 0.0 a | 0.2 a | 0.3 a | 0.5 a | *** | *** | *** |
| Octanal | 44, 56, 81 | 978 | 0.7 | 0.0 a | 1.1 d | 0.9 c | 0.2 b | 0.3 b | 0.0 a | 0.0 a | 0.0 a | *** | *** | *** |
| Phenylacetaldehyde | 65, 91, 120 | 1005 | 2.0 | 0.3 a | 0.1 a | 0.1 a | 0.1 a | 12.0 b | 0.1 a | 0.1 a | 0.2 a | *** | *** | *** |
| (E)-2-Octenal | 41, 55, 70 | 1036 | 0.2 | 6.7 c | 0.9 b | 1.7 b | 0.9 b | 0.0 a | 0.2 a | 0.2 a | 0.3 a | *** | *** | *** |
| Nonanal | 41, 55, 70 | 1084 | 1.0 | 3.8 b | 0.4 a | 0.5 a | 0.3 a | 3.4 b | 0.3 a | 0.2 a | 0.2 a | ns | *** | ns |
| (E)-2-Nonenal | 41, 55, 70 | 1134 | 0.1 | 3.0 b | 0.1 a | 0.2 a | 0.1 a | 0.0 a | 0.0 a | 0.0 a | 0.0 a | *** | *** | *** |
| Decanal | 41, 55, 70 | 1181 | 0.1 | 1.0 b | 0.2 a | 0.2 a | 0.1 a | 1.5 c | 0.0 a | 0.1 a | 0.0 a | *** | *** | ns |
| (Z,Z)-2,4-Decadienal | 41, 67, 81 | 1264 | 0.2 | 4.5 b | 0.0 a | 0.0 a | 0.0 a | 0.0 a | 0.0 a | 0.0 a | 0.0 a | *** | *** | *** |
| (E,E)-2,4-Decadienal | 41, 67, 81 | 1284 | 0.4 | 3.2 b | 0.0 a | 0.0 a | 0.0 a | 0.0 a | 0.0 a | 0.0 a | 0.0 a | *** | *** | *** |
| (E)-2-Undecenal | 41, 57, 70 | 1332 | 0.8 | 0.0 a | 0.4 b | 1.0 d | 0.7 c | 0.0 a | 0.0 a | 0.0 a | 0.0 a | *** | *** | *** |
| Esters | ||||||||||||||
| Ethyl butyrate | 43, 71, 88 | 785 | 1.0 | 0.0 a | 0.6 ab | 0.6 ab | 0.8 bc | 0.0 a | 1.4 c | 0.8 bc | 0.7 abc | ns | *** | ns |
| Ethyl L-lactate | 43, 45, 75 | 795 | 50.0 | 0.0 a | 63.3 c | 65.0 c | 59.2 c | 0.0 a | 56.3 bc | 52.6 bc | 32.2 b | ** | *** | ns |
| Butyl acetate | 43, 56, 73 | 798 | 10.0 | 2.6 ab | 16.5 d | 3.7 b | 12.4 c | 1.4 ab | 0.0 a | 0.0 a | 0.0 a | *** | *** | *** |
| Ethyl 2-methylbutyrate | 57, 85, 102 | 836 | 0.1 | 0.5 a | 0.1 a | 0.3 a | 0.1 a | 1.4 b | 0.3 a | 0.4 a | 0.2 a | ** | *** | *** |
| Ethyl isovalerate | 41, 57, 102 | 838 | 0.1 | 0.0 a | 0.0 a | 0.1 b | 0.0 a | 0.0 a | 0.4 c | 0.4 c | 0.4 c | *** | *** | *** |
| Isoamyl acetate | 55, 43, 70 | 860 | 12.0 | 0.0 a | 27.2 c | 12.4 b | 15.3 b | 0.0 a | 18.5 bc | 16.3 b | 10.0 b | ns | *** | * |
| 2-Methylbutyl acetate | 43, 55, 70 | 862 | 5.0 | 0.0 a | 1.4 abc | 2.5 abc | 1.2 ab | 0.0 a | 5.2 c | 4.7 bc | 3.2 abc | *** | *** | ns |
| Ethyl hexanoate | 43, 88, 99 | 979 | 1.0 | 0.3 a | 3.5 b | 5.4 b | 3.6 b | 0.3 a | 5.5 b | 5.1 b | 3.3 ab | ns | *** | ns |
| Hexyl acetate | 43, 56, 61 | 992 | 2.0 | 5.7 e | 2.2 d | 1.1 bc | 1.3 c | 0.5 ab | 0.0 a | 0.0 a | 0.0 a | ns | *** | *** |
| Methyl salicylate | 92, 120, 152 | 1170 | 35 | 31.3 b | 0.1 a | 0.1 a | 0.1 a | 83.2 c | 2.7 a | 2.4 a | 5.0 a | *** | *** | *** |
| Methyl (E,Z)-2,4-decadienoate | 67, 81, 111 | 1367 | 0.1 | 0.8 c | 0.1 ab | 0.2 b | 0.1 ab | 0.0 a | 0.0 a | 0.0 a | 0.0 a | *** | *** | *** |
| Ethyl decanoate | 70, 88, 101 | 1370 | 5.0 | 3.1 ab | 4.3 bc | 3.6 abc | 3.2 ab | 0.9 a | 6.1 cd | 7.6 d | 3.6 abc | *** | *** | * |
| Ethyl (E,Z)-2,4- decadienoate | 67, 81, 125 | 1434 | 0.1 | 0.6 b | 0.1 a | 0.0 a | 0.0 a | 0.0 a | 0.0 a | 0.0 a | 0.0 a | ** | *** | *** |
| Terpenes and others | ||||||||||||||
| Acetal | 45, 47, 73 | 721 | 4.9 | 0.0 a | 1.8 a | 6.5 ab | 9.4 b | 0.0 a | 20.6 c | 9.3 b | 4.6 ab | *** | *** | *** |
| Linalool | 43, 71, 93 | 1086 | 1.0 | 4.4 c | 0.7 ab | 1.2 ab | 0.4 a | 4.1 c | 1.4 b | 1.3 ab | 1.1 ab | ** | *** | ns |
| (Z)-Rose oxide | 55, 69, 139 | 1095 | 0.1 | 77.4 c | 0.1 a | 0.1 a | 0.1 a | 45.8 b | 0.2 a | 0.1 a | 0.1 a | ** | *** | *** |
| (E)-Rose oxide | 55, 69, 139 | 1113 | 0.1 | 34.6 c | 0.1 a | 0.1 a | 0.1 a | 20.4 b | 0.1 a | 0.1 a | 0.1 a | ** | *** | *** |
| (E)-β-Damascenone | 69, 105, 121 | 1365 | 0.002 1 0.1 2,3 | 0.0 a | 1.5 bc | 0.4 a | 0.6 ab | 2.6 cd | 2.7 d | 2.6 d | 2.3 cd | *** | * | *** |
| α-Ionone | 43, 93, 121 | 1411 | 0.4 | 0.5 b | 0.0 a | 0.0 a | 0.0 a | 0.3 ab | 0.0 a | 0.0 a | 0.0 a | ns | *** | ns |
| β-Ionone | 43, 73, 177 | 1475 | 0.007 | 1.9 b | 0.1 a | 0.1 a | 0.1 a | 1.4 b | 0.1 a | 0.1 a | 0.1 a | ns | *** | ns |
| Volatile organic acids [μg/L] | ||||||||||||||
| Butyric acid | 41, 60, 73 | 780 | 10.0 | 0.0 a | 7.3 b | 15.7 c | 32.7 d | 0.0 a | 0.0 a | 0.0 a | 3.4 ab | *** | *** | *** |
| Hexanoic acid | 41, 60, 73 | 962 | 36.0 | 6.5 c | 1.7 a | 3.2 a | 6.8 c | 2.1 a | 5.5 bc | 4.5 bc | 6.6 c | ns | * | *** |
| Octanoic acid | 43, 60, 73 | 1162 | 910 | 17.5 b | 4.4 a | 5.9 a | 7.4 a | 26.9 c | 4.5 a | 6.7 a | 8.8 a | * | *** | * |
| Decanoic acid | 41, 60, 73 | 1339 | 130 | 6.8 b | 1.6 ab | 1.4 a | 30.8 d | 13.1 c | 2.0 ab | 2.6 ab | 2.2 ab | *** | *** | *** |
| Dodecanoic acid | 43, 60, 73 | 1530 | 7200 | 1.9 bc | 0.0 a | 5.2 d | 4.4 d | 2.5 c | 1.8 bc | 1.3 b | 1.2 ab | *** | *** | *** |
| Compound | LRI | Pear Juice | Plum Juice | Aroma | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| J | LP | LT | LF | J | LP | LT | LF | |||
| 2-Methylbutanal | 640 | 0 | 0 | 0 | 0 | 1.5 | 0 | 0 | 0 | malty fermented |
| Acetal | 721 | 0 | 0 | 0.5 | 1 | 0 | 2 | 1 | 0.5 | ether green |
| 3-Methyl-1-butanol | 724 | 0 | 1 | 2 | 2 | 0 | 2 | 2 | 2 | fusel alcoholic |
| 3-Hexenal | 773 | 0 | 0 | 0 | 0 | 2.5 | 0 | 0 | 0 | leafy green |
| Hexanal | 776 | 2.5 | 0 | 0 | 0 | 2.5 | 0 | 0 | 0 | green aldehydic |
| Butyric acid | 780 | 0 | 0 | 0.5 | 1.5 | 0 | 0 | 0 | 0.5 | buttery cheesy |
| Ethyl butyrate | 785 | 0 | 0 | 0 | 0 | 0 | 0.5 | 0 | 0 | fruity pineapple |
| Ethyl L-lactate | 795 | 0 | 0.5 | 0.5 | 0.5 | 0 | 0.5 | 0.5 | 0 | fruity buttery |
| Butyl acetate | 798 | 0 | 1 | 0 | 0.5 | 0 | 0 | 0 | 0 | fruity banana |
| 2-Hexenal | 825 | 1.5 | 0 | 0 | 0 | 0.5 | 0 | 0 | 0 | almond green |
| Ethyl 2-methylbutyrate | 836 | 2 | 0.5 | 1.5 | 0.5 | 2.5 | 1.5 | 2 | 1 | apple fruity |
| (Z)-3-Heksen-1-ol | 837 | 0 | 0 | 0 | 0 | 1.5 | 0 | 0 | 0 | green grassy |
| Ethyl isovalerate | 838 | 0 | 0 | 0.5 | 0 | 0 | 2 | 2 | 2 | fruity apple |
| Heksan-1-ol | 852 | 1.5 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | pungent green |
| Isoamyl acetate | 860 | 0 | 1.5 | 0.5 | 0.5 | 0 | 1 | 0.5 | 0 | fruity banana |
| 2-Methylbutyl acetate | 862 | 0 | 0 | 0 | 0 | 0 | 0.5 | 0.5 | 0 | sweet banana |
| 1-Heptanol | 953 | 0 | 0 | 0.5 | 0.5 | 0 | 0 | 0 | 0 | herbal green |
| 1-Octen-3-one | 954 | 3 | 2 | 2.5 | 2 | 0 | 0 | 0 | 0.5 | herbal mushroom |
| 1-Octen-3-ol | 960 | 3 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2 | mushroom earthy |
| Octanal | 978 | 0 | 1 | 0.5 | 0 | 0 | 0 | 0 | 0 | aldehydic citrus |
| Ethyl hexanoate | 979 | 0 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | fruity apple |
| Hexyl acetate | 992 | 1.5 | 0.5 | 0 | 0 | 0 | 0 | 0 | 0 | fruity green apple |
| Phenylacetaldehyde | 1005 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | green floral |
| (E)-2-Octenal | 1035 | 2.5 | 2 | 2.5 | 2 | 0 | 0.5 | 0.5 | 0.5 | cucumber green |
| Nonanal | 1084 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | aldehydic rose |
| Linalool | 1086 | 2 | 0 | 0.5 | 0 | 2 | 0.5 | 0.5 | 0.5 | citrus floral |
| (Z)-Rose oxide | 1095 | 3 | 0.5 | 0.5 | 0.5 | 3 | 1 | 0.5 | 0.5 | red rose |
| (E)-Rose oxide | 1113 | 3 | 0.5 | 0.5 | 0.5 | 3 | 0.5 | 0.5 | 0.5 | herbal |
| (E)-2-Nonenal | 1134 | 2.5 | 0.5 | 1 | 0.5 | 0 | 0 | 0 | 0 | green cucumber |
| Methyl salicylate | 1170 | 0 | 0 | 0 | 0 | 1.5 | 0 | 0 | 0 | wintergreen mint |
| Decanal | 1181 | 2.5 | 1 | 1 | 0.5 | 2.5 | 0 | 0.5 | 0 | aldehydic orange peel |
| (Z,Z)-2,4-Decadienal | 1264 | 2.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | seaweed |
| (E,E)-2,4-Decadienal | 1284 | 2.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | oily cucumber |
| (E)-2-Undecenal | 1332 | 0 | 0 | 0.5 | 0 | 0 | 0 | 0 | 0 | fruity citrus |
| (E)-β-Damascenone | 1365 | 0 | 2.5 | 2 | 2 | 3 | 2.5 | 2.5 | 2.5 | sweet plum |
| Methyl (E,Z)-2,4- decadienoate | 1367 | 2.5 | 0.5 | 1 | 0.5 | 0 | 0 | 0 | 0 | fruity pear |
| Ethyl decanoate | 1370 | 0 | 0 | 0 | 0 | 0 | 0.5 | 1 | 0 | fruity apple brandy |
| α-Ionone | 1411 | 0.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | woody floral |
| Ethyl (E,Z)-2,4- decadienoate | 1434 | 2 | 0.5 | 0 | 0 | 0 | 0 | 0 | 0 | green pear |
| β-Ionone | 1475 | 3 | 2.5 | 2.5 | 2.5 | 3 | 2.5 | 2.5 | 2.5 | floral woody |
| Compound [mg/L] | Pear Juice | Plum Juice | Sig. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| J | LP | LF | LT | J | LP | LF | LT | Fruit Juice | Fermentation | Fruit Juice × Fermentation | |
| Phenolic acids | |||||||||||
| Neochlorogenic acid | 0.00 a | 0.00 a | 0.00 a | 0.00 a | 129 b | 371 c | 946 d | 458 c | *** | *** | *** |
| Chlorogenic acid | 3.09 a | 1.90 a | 5.02 a | 1.50 a | 13.37 a | 36.09 b | 91.25 c | 45.10 b | *** | *** | *** |
| Caffeic acid | 2.22 bc | 0.88 a | 1.14 ab | 0.72 a | 0.06 a | 0.37 a | 2.76 c | 0.82 a | ns | *** | *** |
| Vanillic acid | 0.25 a | 1.46 c | 2.93 d | 1.59 c | 0.34 a | 0.55 ab | 4.23 e | 1.08 bc | ns | *** | *** |
| Salicylic acid | 1.72 ab | 0.99 a | 0.98 a | 1.39 ab | 2.43 b | 1.63 ab | 4.74 c | 4.24 c | *** | *** | *** |
| Gentisic acid | 0.05 ab | 0.01 a | 0.05 ab | 0.02 ab | 0.07 bc | 0.06 bc | 0.22 d | 0.11 c | *** | *** | *** |
| Gallic acid | 0.96 ab | 0.20 ab | 0.25 ab | 0.00 a | 8.94 d | 2.45 bc | 12.18 e | 3.96 c | *** | *** | *** |
| Ferulic acid | 0.02 a | 0.00 a | 0.00 a | 0.00 a | 0.00 a | 0.39 b | 0.34 b | 1.39 c | *** | *** | *** |
| p-Coumaric acid | 0.49 b | 0.00 a | 0.00 a | 0.00 a | 0.17 a | 0.24 ab | 0.97 c | 1.07 c | *** | *** | *** |
| Ellagic acid | 0.00 a | 0.00 a | 0.00 a | 0.00 a | 1.26 e | 0.31 b | 1.00 d | 0.67 c | *** | *** | *** |
| Flavonoids | |||||||||||
| Catechin | 0.31 a | 0.46 a | 0.61 a | 0.41 a | 6.87 a | 22.52 b | 69.16 c | 27.34 b | *** | *** | *** |
| Quercetin | 1.40 bc | 1.31 abc | 1.46 bc | 0.97 ab | 1.70 c | 1.64 c | 1.46 bc | 0.73 a | ns | ** | *** |
| Anthocyanins | |||||||||||
| Cyanidin-3-O-rutinoside | 0.71 c | 0.00 a | 0.00 a | 0.00 a | 0.65 bc | 0.13 a | 0.75 c | 0.45 b | *** | *** | *** |
| Cyanidin-3-O-glucoside | 1.08 ab | 0.97 ab | 1.56 c | 1.00 ab | 0.74 a | 1.12 ab | 1.26 bc | 1.22 bc | ns | *** | *** |
| Others | |||||||||||
| Shikimic acid | 361 d | 141 b | 201 c | 247 c | 48 a | 7 a | 10 a | 9 a | *** | *** | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satora, P.; Skotniczny, M.; Maziarek, M. Characteristics of Novel Fermented Cloudy Fruit Juices Produced Using Lactiplantibacillus plantarum and Lactic Acid-Producing Lachancea spp. Yeasts. Molecules 2025, 30, 3928. https://doi.org/10.3390/molecules30193928
Satora P, Skotniczny M, Maziarek M. Characteristics of Novel Fermented Cloudy Fruit Juices Produced Using Lactiplantibacillus plantarum and Lactic Acid-Producing Lachancea spp. Yeasts. Molecules. 2025; 30(19):3928. https://doi.org/10.3390/molecules30193928
Chicago/Turabian StyleSatora, Paweł, Magdalena Skotniczny, and Martyna Maziarek. 2025. "Characteristics of Novel Fermented Cloudy Fruit Juices Produced Using Lactiplantibacillus plantarum and Lactic Acid-Producing Lachancea spp. Yeasts" Molecules 30, no. 19: 3928. https://doi.org/10.3390/molecules30193928
APA StyleSatora, P., Skotniczny, M., & Maziarek, M. (2025). Characteristics of Novel Fermented Cloudy Fruit Juices Produced Using Lactiplantibacillus plantarum and Lactic Acid-Producing Lachancea spp. Yeasts. Molecules, 30(19), 3928. https://doi.org/10.3390/molecules30193928







