Highly Stable Supramolecular Donor–Acceptor Complexes Involving (Z)-, (E)-di(3-pyridyl)ethylene Derivatives as Weak Acceptors: Structure—Property Relationships
Abstract
1. Introduction
2. Results and Discussion
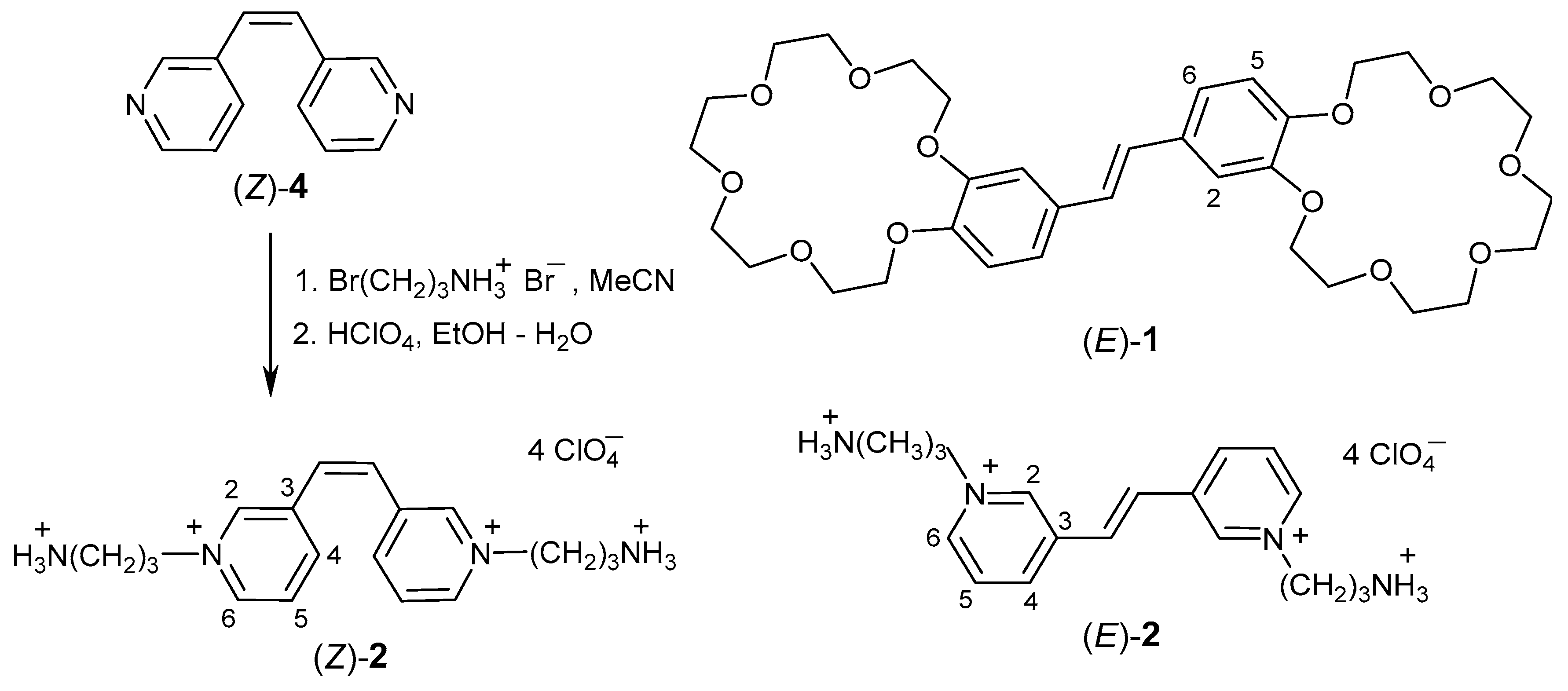
2.1. Synthesis
2.2. Absorption and NMR Spectroscopy
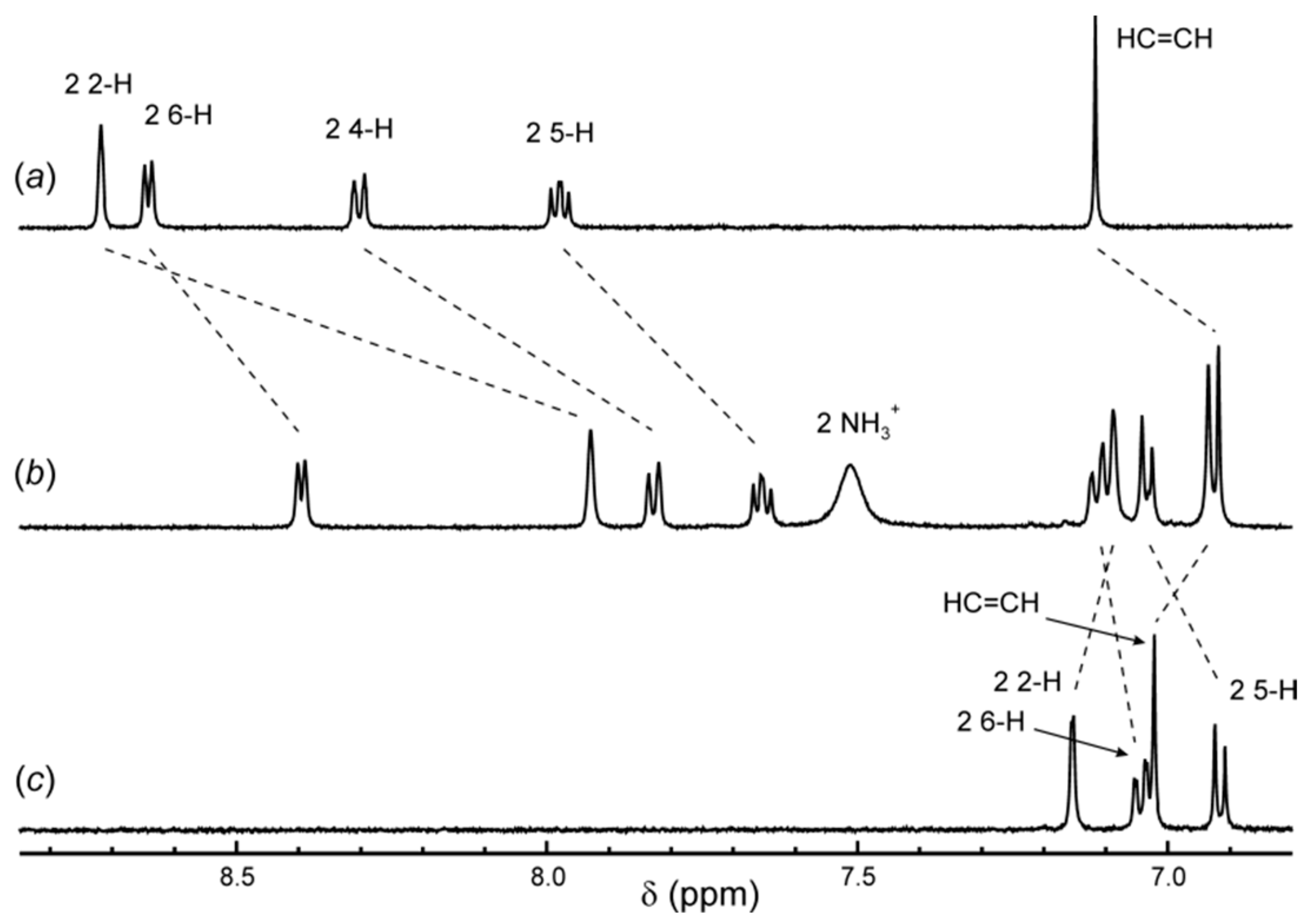
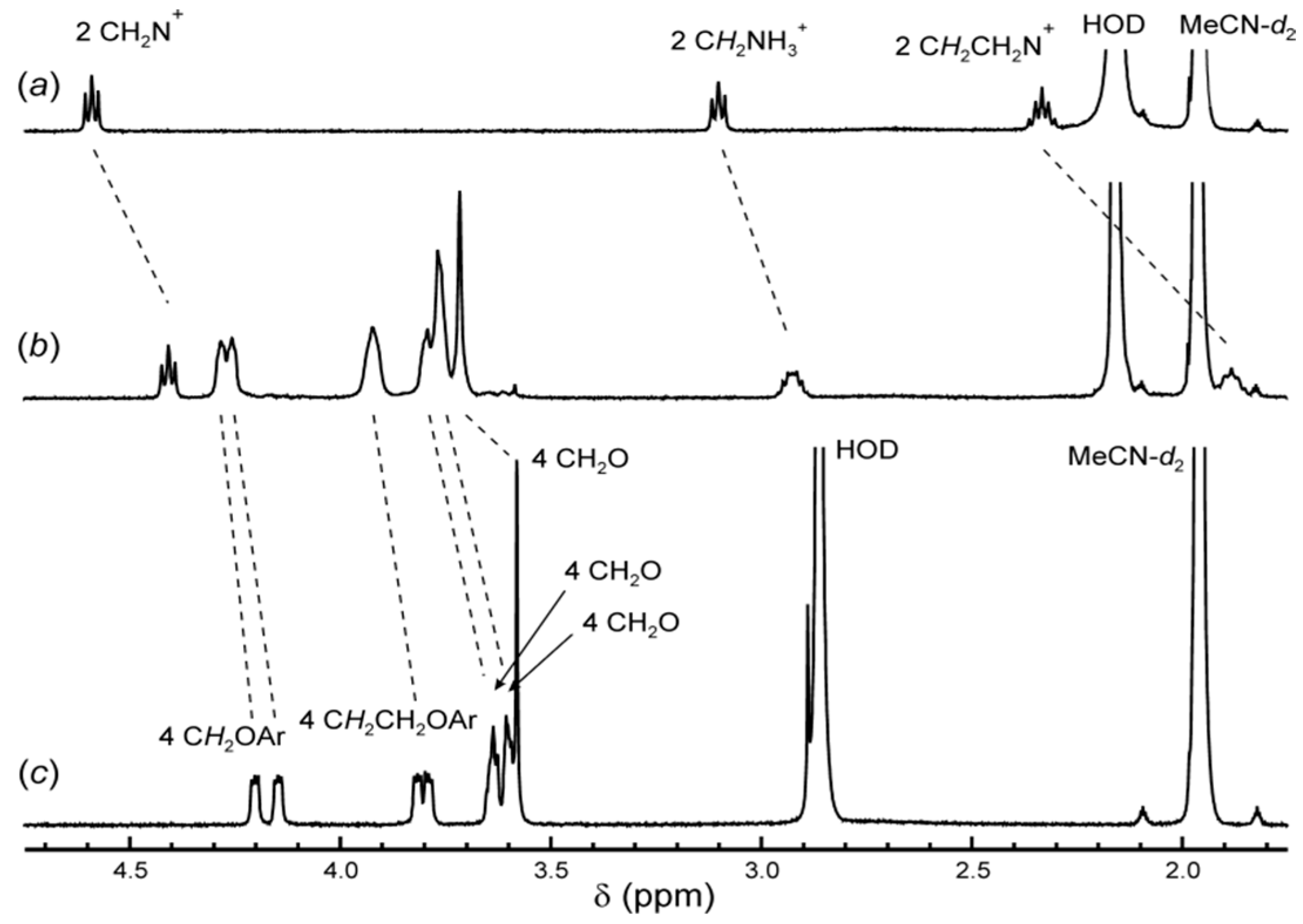
2.3. 1H NMR Spectroscopy
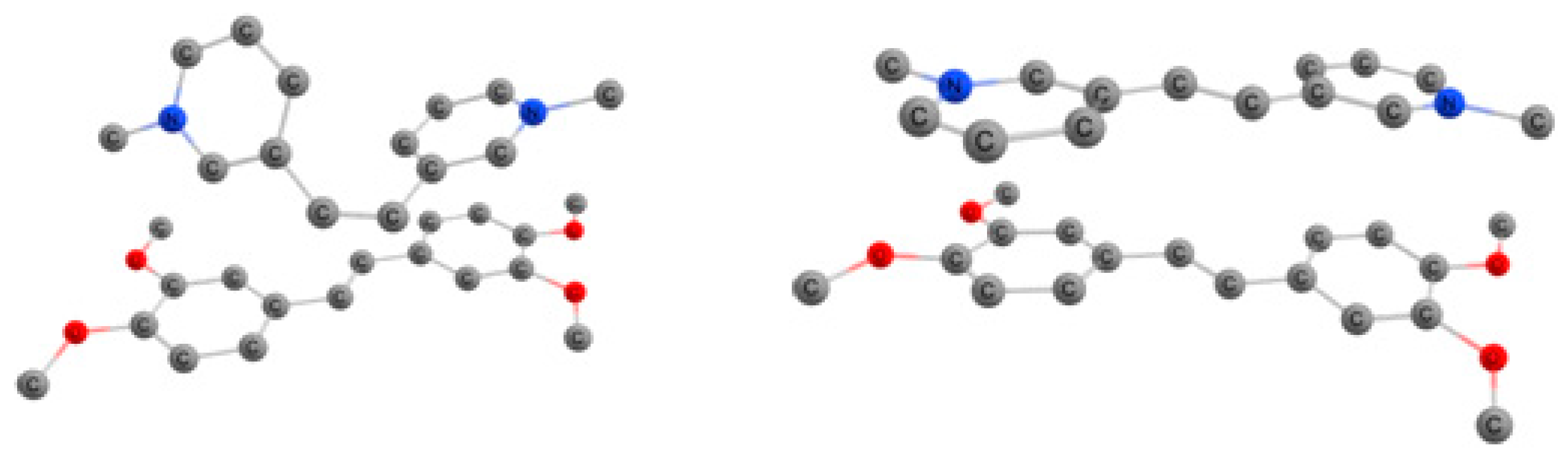
2.4. X-Ray Crystallography
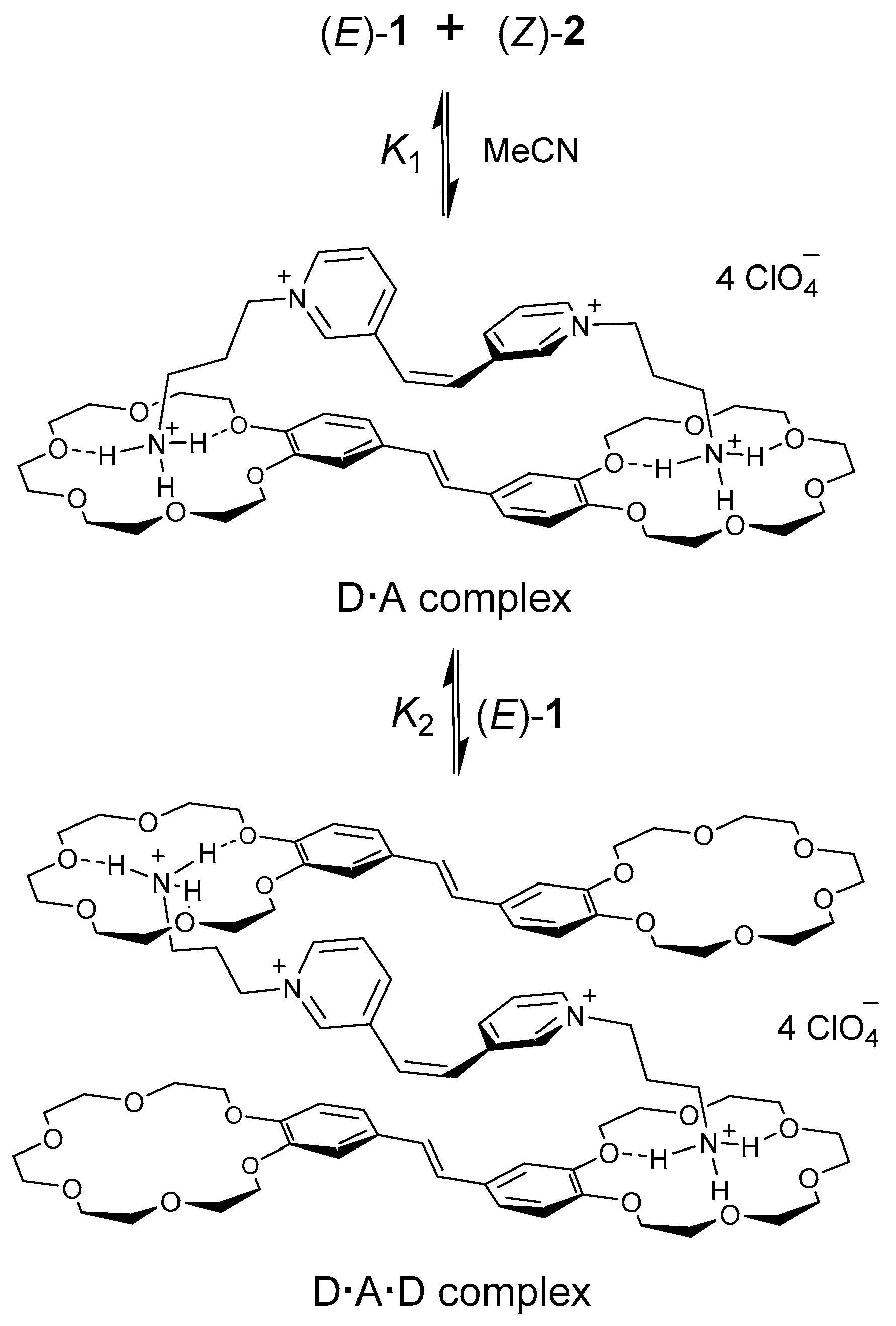
2.5. Intrasupramolecular Photo-Driven Electron Transfer in Complexes (E)-1·(Z)-2, (E)-1·(E)-2
2.5.1. Steady-State Spectroscopy
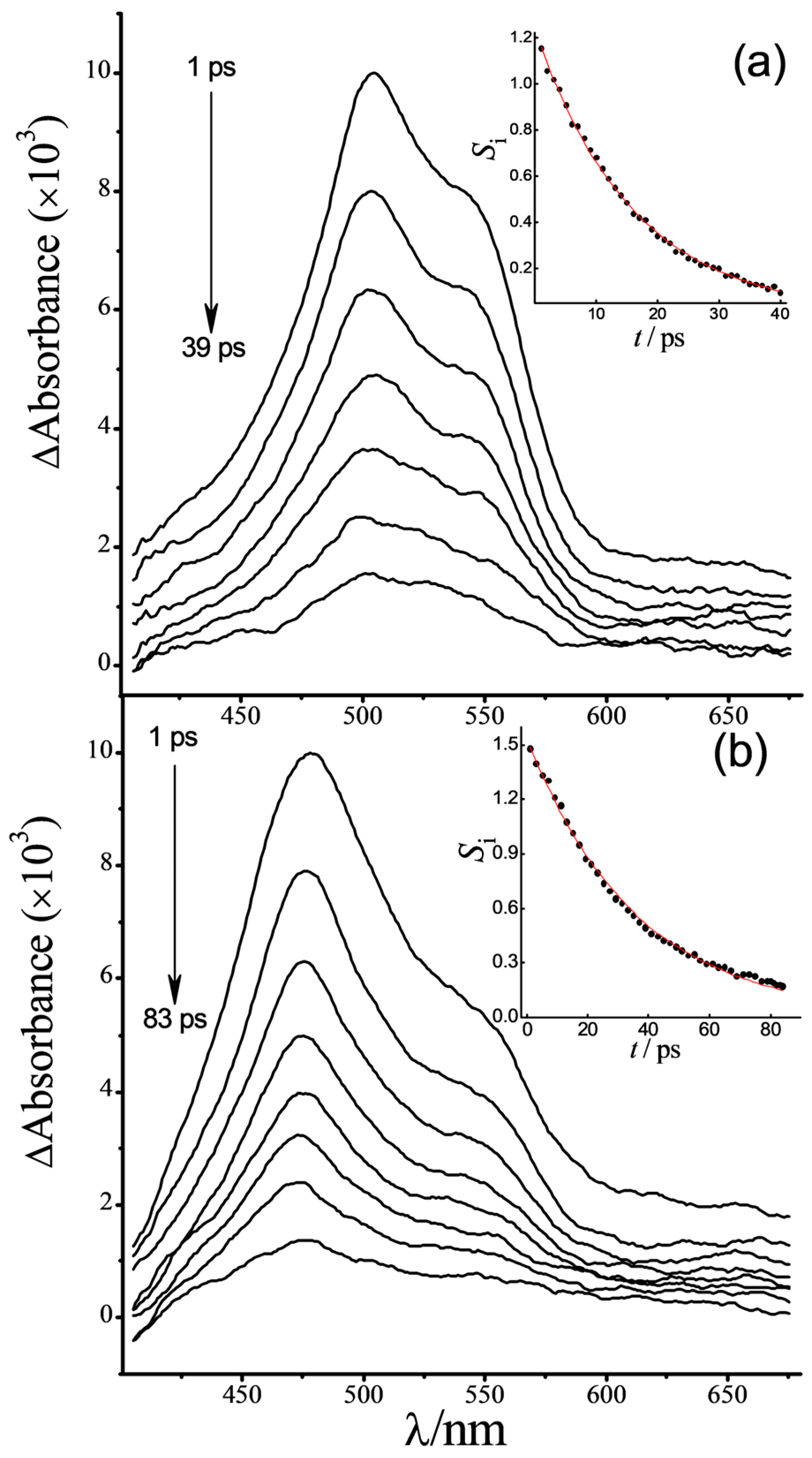
2.5.2. Transient Absorption
2.6. Quantum Chemical Calculations
3. Materials and Methods
3.1. Materials
3.2. Synthesis of 3,3′-(Z)-Ethene-1,2-diylbis[1-(3-ammoniopropyl)pyridinium] Tetraperchlorate ((Z)-2)
3.2.1. Synthesis of Complex (E)-1·(Z)-2
3.2.2. 3,3′-(E)-Ethene-1,2-diylbis[1-(3-ammoniopropyl)pyridinium] Tetraperchlorate
3.3. Methods
3.3.1. Absorption and Fluorescence Spectroscopy
3.3.2. Titrations
3.3.3. X-Ray Crystallography
3.3.4. Femtosecond Transient Absorption Spectroscopy
3.3.5. Quantum Chemical Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gunes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated polymer-based organic solar cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef]
- Huo, Y.; Zhang, H.-L.; Zhan, X. Nonfullerene all-small-molecule organic solar cells. ACS Energy Lett. 2019, 4, 1241–1250. [Google Scholar] [CrossRef]
- Al Khalyfeh, K.; Afaneh, A.T.; Marashdeh, A.; Almatarneh, M.H.; Al-Mazaideh, G.M.; Mizyed, S.; Ashram, M. Thiacrown ethers engaged C60 through charge transfer: Experimental and theoretical study. ACS Omega 2020, 5, 25049–25058. [Google Scholar] [CrossRef]
- Wu, L.; Wu, F.; Sun, Q.; Shi, J.; Xie, A.; Zhu, X.; Dong, W. A TTF–TCNQ complex: An organic charge-transfer system with extraordinary electromagnetic response behavior. J. Mater. Chem. C 2021, 9, 3316–3323. [Google Scholar] [CrossRef]
- Morita, Y.; Murata, T.; Nakasuji, K. Cooperation of hydrogen-bond and charge-transfer interactions in molecular complexes in the solid state. Bull. Chem. Soc. Jpn. 2013, 86, 183–197. [Google Scholar] [CrossRef]
- Yuan, Y.-Q.; Majumder, S.; Yang, M.-H.; Guo, S.-R. Recent advances in catalyst-free photochemical reactions via electron-donor-acceptor (EDA) complex process. Tetrahedron Lett. 2020, 61, 151506. [Google Scholar] [CrossRef]
- Yao, Q.; Lu, B.; Ji, C.; Cai, Y.; Yin, M. Supramolecular host–guest system as ratiometric Fe3+ ion sensor based on water-soluble pillar[5]arene. ACS Appl. Mater. Interfaces 2017, 9, 36320–36326. [Google Scholar] [CrossRef]
- Shakya, S.; Khan, I.M. Charge transfer complexes: Emerging and promising colormetric real-time chemosensors for hazardous materials. J. Hazard Mater. 2021, 403, 123537. [Google Scholar] [CrossRef]
- Chen, L.; Peng, Z.; Liu, S.; Li, X.; Chen, R.; Ren, Y.; Feng, W.; Yuan, L. Cyclo[6]aramide-tropylium charge transfer complex as a colorimetric chemosensor for differentiation of intimate and loose ion pairs. Org. Lett. 2015, 17, 5950–5953. [Google Scholar] [CrossRef]
- Ke, X.-S.; Kim, T.; Lynch, V.M.; Kim, D.; Sessler, J.L. Flattened calixarene-like cyclic BODIPY array: A new photosynthetic antenna model. J. Am. Chem. Soc. 2017, 139, 13950–13956. [Google Scholar] [CrossRef]
- Lian, Z.; Jiang, M.; Qiao, F.; Chen, M.-N.; Wang, R.-Z.; Zhuo, S.; Xing, L.-B. Artificial light-harvesting supramolecular assemblies with different morphology formed by cucurbit[n]urils-based host–guest complexation. J. Photochem. Photobiol. A 2020, 386, 112135. [Google Scholar] [CrossRef]
- Schröder, H.V.; Hupatz, H.; Achazi, A.J.; Sobottka, S.; Sarkar, B.; Paulus, B.; Schalley, C.A. A divalent pentastable redox-switchable donor–acceptor rotaxane. Chem. Eur. J. 2017, 23, 2960–2967. [Google Scholar] [CrossRef]
- Gromov, S.P. Molecular constructor for light-sensitive and light-emitting nanosized systems based on unsaturated and macrocyclic compounds. Russ. Chem. Bull. 2008, 57, 1325–1350. [Google Scholar] [CrossRef]
- Gromov, S.P.; Vedernikov, A.I.; Ushakov, E.N.; Alfimov, M.V. Unusial supramolecular donor–acceptor complexes of bis(crown)stilbenes and bis(crown)azobenzene with viologen analogs. Russ. Chem. Bull. 2008, 57, 793–801. [Google Scholar] [CrossRef]
- Foster, R. Organic Charge-Transfer Complexes; Academic Press: New York, NY, USA, 1969; 472p. [Google Scholar]
- Ushakov, E.N.; Nadtochenko, V.A.; Gromov, S.P.; Vedernikov, A.I.; Lobova, N.A.; Alfimov, M.V.; Gostev, F.E.; Petrukhin, A.N.; Sarkisov, O.M. Ultrafast excited state dynamics of the bi- and termolecular stilbene–viologen charge-transfer complexes assembled via host–guest interactions. Chem. Phys. 2004, 298, 251–261. [Google Scholar] [CrossRef]
- Rusalov, M.V.; Volchkov, V.V.; Ivanov, V.L.; Mel’nikov, M.Y.; Shelaev, I.V.; Gostev, F.E.; Nadtochenko, V.A.; Vedernikov, A.I.; Gromov, S.P.; Alfimov, M.V. Femtosecond excited state dynamics of a stilbene–viologen charge transfer complex assembled via host–guest interaction. Photochem. Photobiol. Sci. 2017, 16, 1801–1811. [Google Scholar] [CrossRef]
- Gromov, S.P.; Vedernikov, A.I.; Ushakov, E.N.; Lobova, N.A.; Botsmanova, A.A.; Kuz’mIna, L.G.; Churakov, A.V.; Strelenko, Y.A.; Alfimov, M.V.; Howard, J.A.K.; et al. Novel supramolecular charge-transfer systems based on bis(18-crown-6)stilbene and viologen analogues bearing two ammonioalkyl groups. New. J. Chem. 2005, 29, 881–894. [Google Scholar] [CrossRef]
- Volchkov, V.V.; Rusalov, M.V.; Gostev, F.E.; Shelaev, I.V.; Nadtochenko, V.A.; Vedernikov, A.I.; Efremova, A.A.; Kuz’MIna, L.G.; Gromov, S.P.; Alfimov, M.V.; et al. Complexation of bis-crown stilbene with alkali and alkaline earth metal cations. Ultrafast excited state dynamics of the stilbene–viologen charge transfer complex. J. Phys. Org. Chem. 2018, 31, e3759. [Google Scholar] [CrossRef]
- Volchkov, V.V.; Khimich, M.N.; Rusalov, M.V.; Gostev, F.E.; Shelaev, I.V.; Nadtochenko, V.A.; Vedernikov, A.I.; Gromov, S.P.; Freidzon, A.Y.; Alfimov, M.V.; et al. Formation of supramolecular charge-transfer complex. Ultrafast excited state dynamics and quantum-chemical calculations. Photochem. Photobiol. Sci. 2019, 18, 232–241. [Google Scholar] [CrossRef]
- Rusalov, M.V.; Volchkov, V.V.; Ivanov, V.L.; Mel’nikov, M.Y.; Gostev, F.E.; Nadtochenko, V.A.; Vedernikov, A.I.; Gromov, S.P.; Alfimov, M.V. Ultrafast excited state dynamics of a stilbene–viologen charge transfer complex and its interaction with alkanediammonium salts. J. Photochem. Photobiol. A Chem. 2019, 372, 89–98. [Google Scholar] [CrossRef]
- Volchkov, V.V.; Martyanov, T.P.; Khimich, M.N.; Rusalov, M.V.; Neznaeva, D.A.; Gostev, F.E.; Shelaev, I.V.; Nadtochenko, V.A.; Melnikov, M.Y.; Moiseeva, A.A.; et al. Ultrafast excited state dynamics, direct and back [2 + 2]-cross-photocycloaddition of a styryl dye–stilbene charge transfer complex. Dyes Pigments 2021, 185, 108952. [Google Scholar] [CrossRef]
- Vedernikov, A.I.; Ushakov, E.N.; Efremova, A.A.; Kuz’mina, L.G.; Moiseeva, A.A.; Lobova, N.A.; Churakov, A.V.; Strelenko, Y.A.; Alfimov, M.V.; Howard, J.A.K.; et al. Synthesis, structure, and properties of supramolecular charge-transfer complexes between bis(18-crown-6)stilbene and ammonioalkyl derivatives of 4,4’-bipyridine and 2,7-diazapyrene. J. Org. Chem. 2011, 76, 6768–6779. [Google Scholar] [CrossRef] [PubMed]
- Rusalov, M.V.; Volchkov, V.V.; Ivanov, V.L.; Melnikov, M.Y.; Gostev, F.E.; Shelaev, I.V.; Nadtochenko, V.A.; Vedernikov, A.I.; Gromov, S.P.; Alfimov, M.V. Femtosecond excited state dynamics of stilbene–viologen complexes with a weakly pronounced charge transfer. Photochem. Photobiol. Sci. 2020, 19, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Ushakov, E.N.; Gromov, S.P.; Vedernikov, A.I.; Malysheva, E.V.; Botsmanova, A.A.; Alfimov, M.V.; Eliasson, B.; Edlund, U.G.; Whitesell, J.K.; Fox, M.A. Self-organization of highly stable electron donor–acceptor complexes via host–guest interactions. J. Phys. Chem. A 2002, 106, 2020–2023. [Google Scholar] [CrossRef]
- Vedernikov, A.I.; Basok, S.S.; Gromov, S.P.; Kuz’mIna, L.G.; Avakyan, V.G.; Lobova, N.A.; Kulygina, E.Y.; Titkov, T.V.; Strelenko, Y.A.; Ivanov, E.I.; et al. Synthesis and structure of bis-crown-containing stilbenes. Russ. J. Org. Chem. 2005, 41, 843–854. [Google Scholar] [CrossRef]
- Gromov, S.P.; Vedernikov, A.I.; Lobova, N.A.; Kuz’mina, L.G.; Basok, S.S.; Strelenko, Y.A.; Alfimov, M.V.; Howard, J.A.K. Controlled self-assembly of bis(crown)stilbenes into unusual bis-sandwich complexes: Structure and stereoselective [2 + 2] photocycloaddition. New J. Chem. 2011, 35, 724–737. [Google Scholar] [CrossRef]
- Ushakov, E.N.; Martyanov, T.P.; Vedernikov, A.I.; Pikalov, O.V.; Efremova, A.A.; Kuz’mina, L.G.; Howard, J.A.K.; Alfimov, M.V.; Gromov, S.P. Self-assembly through hydrogen bonding and photochemical properties of supramolecular complexes of bis(18-crown-6)stilbene with alkanediammonium ions. J. Photochem. Photobiol. A Chem. 2017, 340, 80–87. [Google Scholar] [CrossRef]
- Vedernikov, A.I.; Kuz’mina, L.G.; Botsmanova, A.A.; Strelenko, Y.A.; Howard, J.A.K.; Alfimov, M.V.; Gromov, S.P. Stacking structure of complexes between bis(crown)azobenzene and dipyridylethylene derivative in crystal and solution. Mendeleev Commun. 2007, 17, 148–150. [Google Scholar] [CrossRef]
- Demas, J.N.; Crosby, G.A. The measurement of photoluminescence quantum yields. A review. J. Phys. Chem. 1971, 75, 991–1024. [Google Scholar] [CrossRef]
- Frassineti, C.; Ghelli, S.; Gans, P.; Sabatini, A.; Moruzzi, M.S.; Vacca, A. Nuclear magnetic resonance as a tool for determining protonation constants of natural polyprotic bases in solution. Anal. Biochem. 1995, 231, 374–382. [Google Scholar] [CrossRef]
- SAINT, version 6.02A; Bruker AXS, Inc.: Madison, WI, USA, 2001.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Volchkov, V.V.; Gostev, F.E.; Shelaev, I.V.; Nadtochenko, V.A.; Dmitrieva, S.N.; Gromov, S.P.; Alfimov, M.V.; Melnikov, M.Y. Complexation of donor–acceptor substituted aza-crowns and alkali and alkaline earth metal cations. Charge transfer and recoordination in excited state. J. Fluoresc. 2016, 26, 585–592. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Baldridge, K.K.; Boatz, J.A.; Elbert, S.T.; Gordon, M.S.; Jensen, J.H.; Koseki, S.; Matsunaga, N.; Nguyen, K.A.; Su, S.; et al. General atomic and molecular electronic structure system. J. Comput. Chem. 1993, 14, 1347–1363. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]

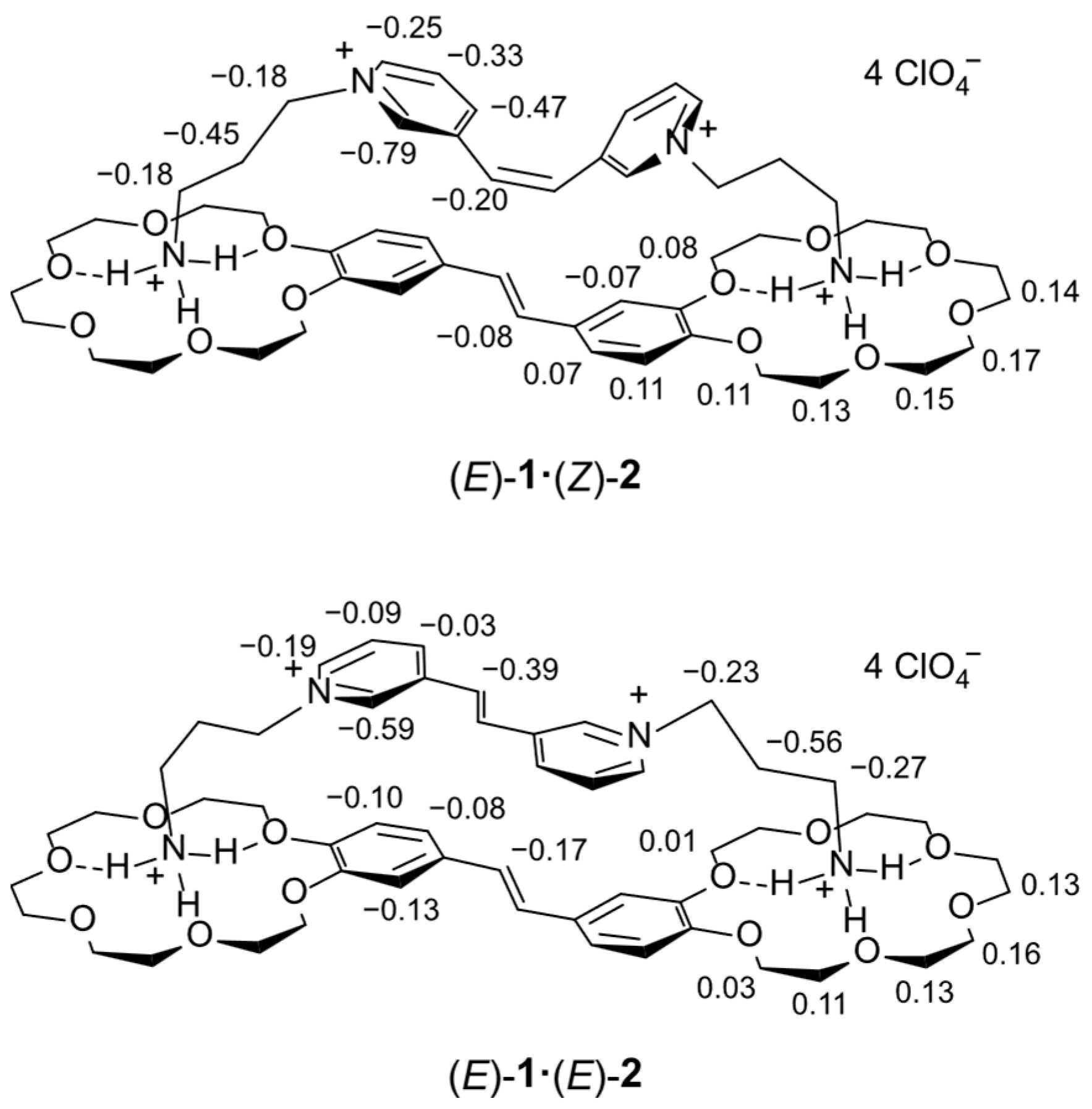
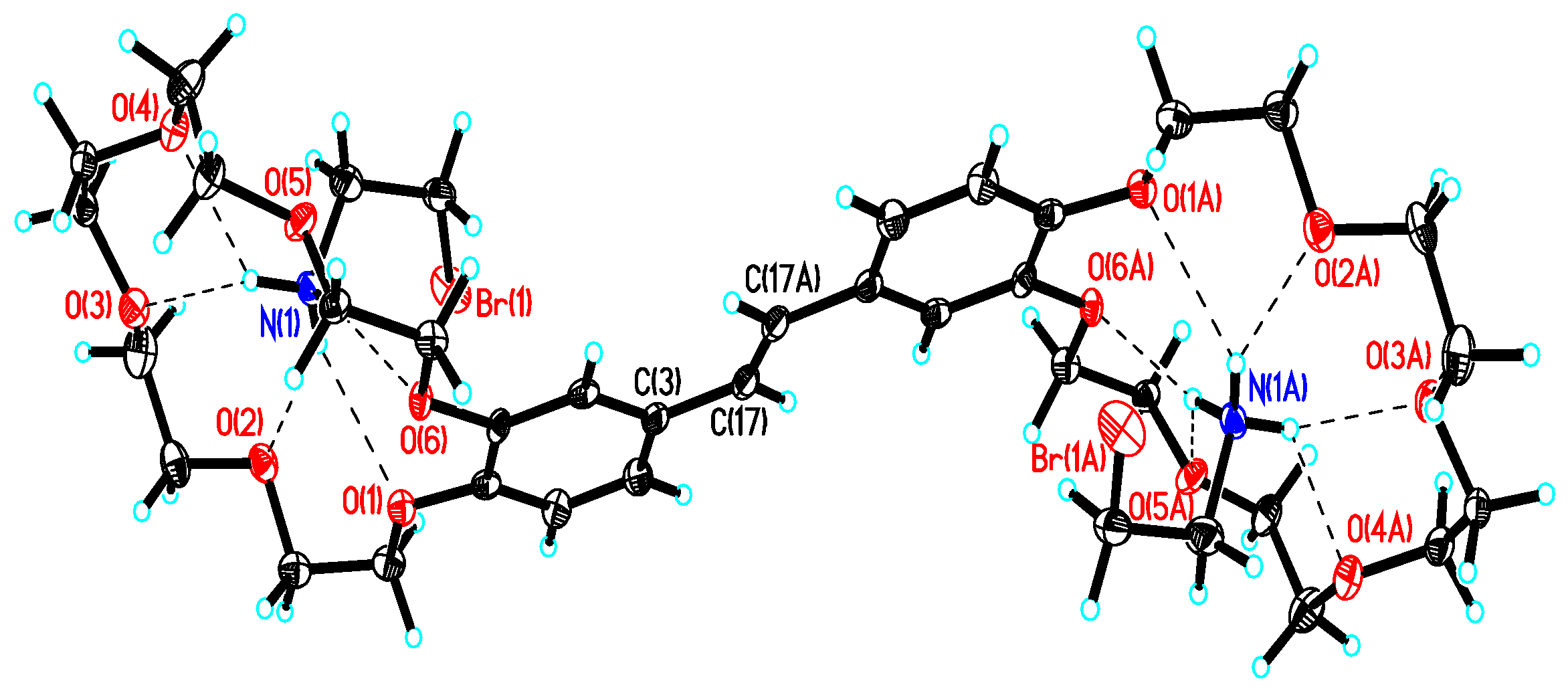
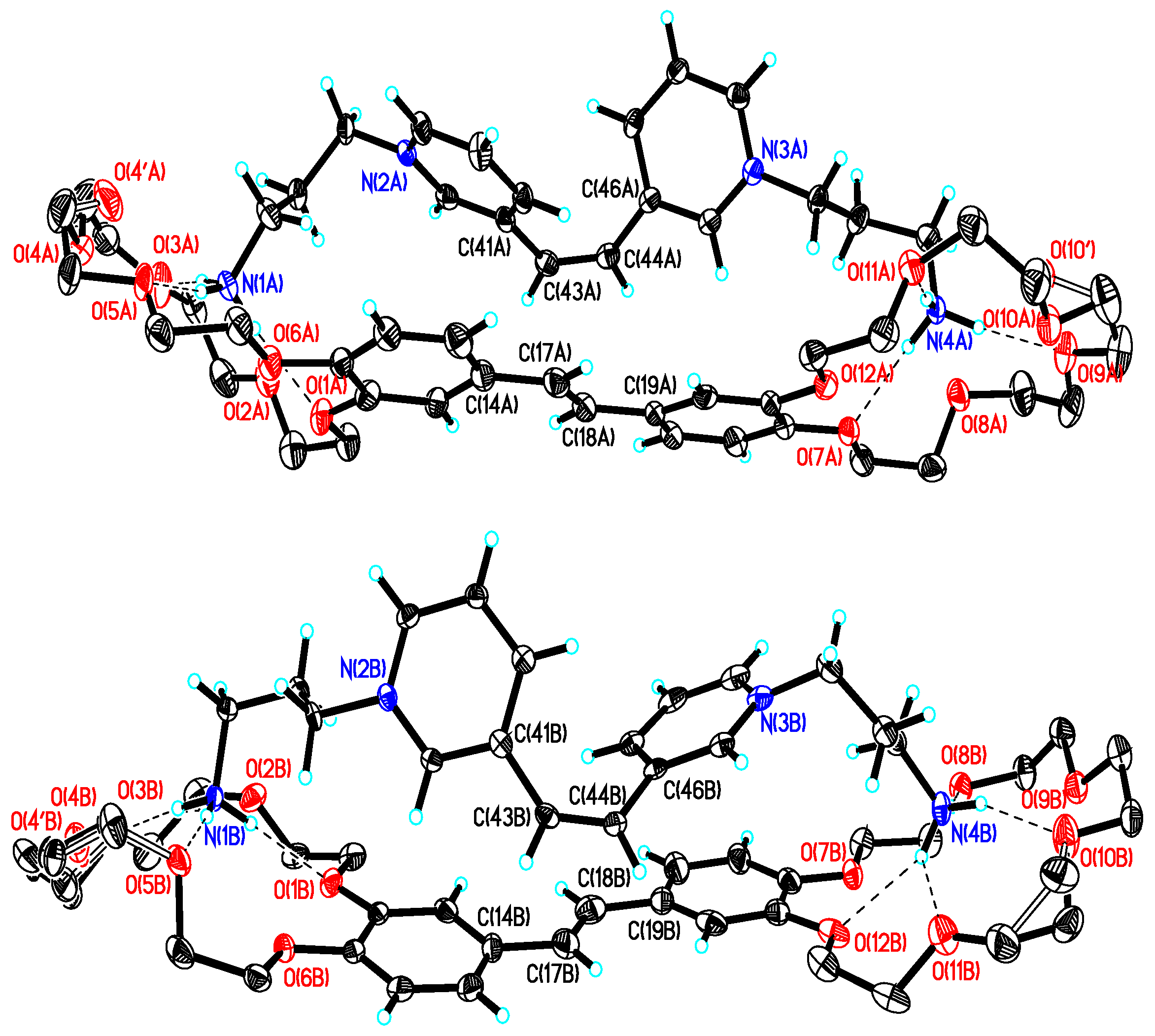


| Complex | logK1 (logK2) | λamax/nm | εamax/ M−1 cm−1 | |
|---|---|---|---|---|
| Spectrophoto-Metric Titration | 1H NMR Titration | |||
| (E)-1·(Z)-2 | 8.48 b | 8.2 | <400 | – |
| (E)-1·(E)-2 | 8.67 c | 8.5 | 405 c,d | 430 c |
| (E)-1·(E)-3 | 9.08 c | 8.9 | 502 c | 390 c |
| (E)-1·(Z)-2·(E)-1 | – | (0.6) | <400 | – |
| (E)-1·(E)-2·(E)-1 | (2.18) c | (2.02) c | 416 c,d | 790 c |
| (E)-1·(E)-3·(E)-1 | (3.20) c | (3.27) c | 519 c | 1020 c |
| Compound | λamax/nm | εamax/M−1cm−1 | λfmax/nm | Stokes Shift/nm | Δλfmax/nm 293–77 K (in n-PrCN) | ϕf |
|---|---|---|---|---|---|---|
| (E)-1 b | 336 | 37,500 | 386 | 50 | 4 | 0.3 |
| (E)-1·C10 c | 334 | 36,900 | 383 | 49 | 4 | 0.27 |
| (Z)-2 | 278 | 16,080 | 400 | 112 | – | 0.067 |
| (E)-2 | 284 | 27,350 | 393 | 109 | – | 0.218 |
| (E)-3 d | 321 | 44,000 | 369 | 48 | – | 0.02 |
| (E)-1·(Z)-2 | 332 | 32,180 | 407 | 75 | 1.5 | 0.006 |
| (E)-1·(E)-2 | 318 | 44,160 | 388 | 70 | 2 | 0.011 |
| (E)-1·(E)-3 d | 321 | 65,500 | – | – | – | <10−4 |
| Compound | λTAmax/nm | τ1/fs b | τ2/ps b | τ3/ns b |
|---|---|---|---|---|
| (E)-1 a | 561 | 69 | – | ~1.1 |
| (Z)-2 | – | 250 ± 70 | – | – |
| (E)-2 | 483 → 466 | 130 ± 20 | – | 2.9 ± 0.1 |
| (E)-1·C10 | 563 → 573 | 45 ± 1 | – | 0.69 ± 0.04 |
| (E)-1·(Z)-2 | 504 | 95 ± 10 | 16 ± 0.1 | – |
| (E)-1·(E)-2 | 477 | 71 ± 3 | 36 ± 0.4 | – |
| (E)-1·(E)-3 a | 500 | 150 | 0.295 | 5.36 × 10−4 |
| Complex | lC/Å | lmin(D–A)/Å | θAA/° | θDD/° | θAD/° | θAD2/° | ∆ES0/S1(ET) ∆ES1r(ET)/S0 /eV | |
|---|---|---|---|---|---|---|---|---|
| (E)-1·(Z)-2 | S0 | 4.1 | 3.4–4.3 | 54 | 3 | 65 | 22 | 3.2 |
| S1 | 3.9 | 3.2–4.1 | 41 | 10 | 52 | 21 | 2.1 | |
| (E)-1·(E)-2 | S0 | 3.6 | 3.5–4.0 | 2 | 14 | 7 | 7 | 2.7 |
| S1 | 3.3 | 3.3–3.8 | 10 | 5 | 7 | 7 | 1.9 | |
| (E)-1·(Z)-3 | S0 | 4.6 | 3.4–4.7 | 52 | 11 | 25 | 38 | 2.6 |
| S1 | 4.2 | 3.4–4.4 | 38 | 14 | 24 | 28 | 1.4 | |
| (E)-1·(E)-3 | S0 | 3.8 | 3.4–4.2 | 7 | 8 | 1 | 8 | 2.3 |
| S1 | 3.9 | 3.2–4.0 | 7 | 7 | 4 | 7 | 1.3 | |
| Structure | (E)-1·8C2H7Br2N | [(E)-1·(Z)-2] 0.15 MeCN·1.275 H2O |
|---|---|---|
| Formula | C50H104Br16N8O12 | C52.3H79Cl4N4.15O29.275 |
| Formula weight, g·mol−1 | 2287.97 | 1376.09 |
| Crystal system | monoclinic | triclinic |
| Space group | P21/n | |
| a, Å | 16.7010 (3) | 12.8857 (6) |
| b, Å | 8.1317 (2) | 15.8062 (7) |
| c, Å | 28.8097 (6) | 34.0819 (15) |
| α, ° | 90 | 78.271 (1) |
| β, ° | 91.738 (1) | 79.560 (2) |
| γ, ° | 90 | 68.616 (1) |
| Volume, Å3 | 3910.77 (14) | 6272.6 (5) |
| Z | 2 | 4 |
| ρcalc, g cm−3 | 1.943 | 1.457 |
| F (000) | 2232 | 2896 |
| μ (Mo-Kα), mm−1 | 8.243 | 0.280 |
| Crystal size, mm | 0.22 × 0.12 × 0.04 | 0.12 × 0.02 × 0.02 |
| Temperature, K | 120 (2) | 100 (2) |
| 2θ range for data collection, ° | 2.83 to 58.00 | 4.53 to 52.87 |
| Index ranges | −22 ≤ h ≤ 19, −10 ≤ k ≤ 11, −39 ≤ l ≤ 39 | −16 ≤ h ≤ 16, −19 ≤ k ≤ 19, −42 ≤ l ≤ 42 |
| Collected reflections | 32037 | 79617 |
| Independent reflections | 10,358 [Rint = 0.0674] | 25,715 [Rint = 0.0903] |
| Reflections with I > 2σ (I) | 6766 | 14,088 |
| Data/restraints/parameters | 10,358/7/402 | 25,715/1552/1904 |
| Final R indices [I > 2σ (I)] | R1 = 0.0400, wR2 = 0.0693 | R1 = 0.0736, wR2 = 0.1502 |
| Final R indices [all data] | R1 = 0.0833, wR2 = 0.0769 | R1 = 0.1507, wR2 = 0.1812 |
| Goodness-of-fit on F2 | 0.942 | 1.025 |
| Largest diff. peak/hole (max/min), ē Å−3 | 1.463/−1.060 | 0.763/−0.478 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vedernikov, A.I.; Volchkov, V.V.; Khimich, M.N.; Mel’nikov, M.Y.; Gostev, F.E.; Shelaev, I.V.; Nadtochenko, V.A.; Kuz’mina, L.G.; Howard, J.A.K.; Efremova, A.A.; et al. Highly Stable Supramolecular Donor–Acceptor Complexes Involving (Z)-, (E)-di(3-pyridyl)ethylene Derivatives as Weak Acceptors: Structure—Property Relationships. Molecules 2025, 30, 3920. https://doi.org/10.3390/molecules30193920
Vedernikov AI, Volchkov VV, Khimich MN, Mel’nikov MY, Gostev FE, Shelaev IV, Nadtochenko VA, Kuz’mina LG, Howard JAK, Efremova AA, et al. Highly Stable Supramolecular Donor–Acceptor Complexes Involving (Z)-, (E)-di(3-pyridyl)ethylene Derivatives as Weak Acceptors: Structure—Property Relationships. Molecules. 2025; 30(19):3920. https://doi.org/10.3390/molecules30193920
Chicago/Turabian StyleVedernikov, Artem I., Valeriy V. Volchkov, Mikhail N. Khimich, Mikhail Y. Mel’nikov, Fedor E. Gostev, Ivan V. Shelaev, Victor A. Nadtochenko, Lyudmila G. Kuz’mina, Judith A. K. Howard, Asya A. Efremova, and et al. 2025. "Highly Stable Supramolecular Donor–Acceptor Complexes Involving (Z)-, (E)-di(3-pyridyl)ethylene Derivatives as Weak Acceptors: Structure—Property Relationships" Molecules 30, no. 19: 3920. https://doi.org/10.3390/molecules30193920
APA StyleVedernikov, A. I., Volchkov, V. V., Khimich, M. N., Mel’nikov, M. Y., Gostev, F. E., Shelaev, I. V., Nadtochenko, V. A., Kuz’mina, L. G., Howard, J. A. K., Efremova, A. A., Rusalov, M. V., & Gromov, S. P. (2025). Highly Stable Supramolecular Donor–Acceptor Complexes Involving (Z)-, (E)-di(3-pyridyl)ethylene Derivatives as Weak Acceptors: Structure—Property Relationships. Molecules, 30(19), 3920. https://doi.org/10.3390/molecules30193920








