Targeting Anti-Apoptotic Bcl-2 Proteins with Triterpene-Heterocyclic Derivatives: A Combined Dual Docking and Molecular Dynamics Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Molecular Docking and Candidate Selection
2.2. Single-Structure MM-GBSA on Docked Poses
2.3. Molecular Dynamics Simulation
3. Materials and Methods
3.1. Ligand Design and Compound Library Construction
3.2. Molecular Docking
3.2.1. Docking with PyRx—Vina
3.2.2. Docking with Schrödinger—Glide
3.3. Candidate Selection Criteria: Scoring and Ranking
3.4. MM-GBSA on Docked Poses
3.5. Molecular Dynamics Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ong, F.; Kim, K.; Konopleva, M.Y. Venetoclax resistance: Mechanistic insights and future strategies. Venetoclax Resist. Mech. Insights Futur. Strateg. 2022, 5, 380–400. [Google Scholar] [CrossRef]
- Bruncko, M.; Oost, T.K.; Belli, B.A.; Ding, H.; Joseph, M.K.; Kunzer, A.; Martineau, D.; McClellan, W.J.; Mitten, M.; Ng, S.-C.; et al. Studies Leading to Potent, Dual Inhibitors of Bcl-2 and Bcl-xL. J. Med. Chem. 2007, 50, 641–662. [Google Scholar] [CrossRef]
- Ritter, V.; Krautter, F.; Klein, D.; Jendrossek, V.; Rudner, J. Bcl-2/Bcl-xL inhibitor ABT-263 overcomes hypoxia-driven radioresistence and improves radiotherapy. Cell Death Dis. 2021, 12, 694. [Google Scholar] [CrossRef]
- Or, C.-H.R.; Huang, C.-W.; Chang, C.-C.; Lai, Y.-C.; Chen, Y.-J.; Chang, C.-C. Obatoclax, a Pan-BCL-2 Inhibitor, Downregulates Survivin to Induce Apoptosis in Human Colorectal Carcinoma Cells Via Suppressing WNT/β-catenin Signaling. Int. J. Mol. Sci. 2020, 21, 1773. [Google Scholar] [CrossRef] [PubMed]
- Balachander, S.B.; Criscione, S.W.; Byth, K.F.; Cidado, J.; Adam, A.; Lewis, P.; Macintyre, T.; Wen, S.; Lawson, D.; Burke, K.; et al. AZD4320, A Dual Inhibitor of Bcl-2 and Bcl-x(L), Induces Tumor Regression in Hematologic Cancer Models without Dose-limiting Thrombocytopenia. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 6535–6549. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.W. Therapeutic development and current uses of BCL-2 inhibition. Hematology 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Wei, A.H.; Roberts, A.W.; Spencer, A.; Rosenberg, A.S.; Siegel, D.; Walter, R.B.; Caenepeel, S.; Hughes, P.; McIver, Z.; Mezzi, K.; et al. Targeting MCL-1 in hematologic malignancies: Rationale and progress. Blood Rev. 2020, 44, 100672. [Google Scholar] [CrossRef]
- Tron, A.E.; Belmonte, M.A.; Adam, A.; Aquila, B.M.; Boise, L.H.; Chiarparin, E.; Cidado, J.; Embrey, K.J.; Gangl, E.; Gibbons, F.D.; et al. Discovery of Mcl-1-specific inhibitor AZD5991 and preclinical activity in multiple myeloma and acute myeloid leukemia. Nat. Commun. 2018, 9, 5341. [Google Scholar] [CrossRef] [PubMed]
- Yim, N.-H.; Jung, Y.P.; Kim, A.; Kim, T.; Ma, J.Y. Induction of apoptotic cell death by betulin in multidrug-resistant human renal carcinoma cells. Oncol. Rep. 2015, 34, 1058–1064. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A. Betulin and betulinic acid in cancer research. J. Pre-Clin. Clin. Res. 2018, 12, 72–75. [Google Scholar] [CrossRef]
- Adewole, K.E.; Ishola, A.A. Phytosterols and triterpenes from Morinda lucida Benth (Rubiaceae) as potential inhibitors of anti-apoptotic BCL-XL, BCL-2, and MCL-1: An in-silico study. J. Recept. Signal Transduct. Res. 2019, 39, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Kazakova, O.; Soica, C.; Babaev, M.; Petrova, A.; Khusnutdinova, E.; Poptsov, A.; Macasoi, I.; Draghici, G.; Avram, S.; Vlaia, L.; et al. 3-Pyridinylidene Derivatives of Chemically Modified Lupane and Ursane Triterpenes as Promising Anticancer Agents by Targeting Apoptosis. Int. J. Mol. Sci. 2021, 22, 10695. [Google Scholar] [CrossRef]
- Nistor, M.; Rugina, D.; Diaconeasa, Z.; Socaciu, C.; Socaciu, M.A. Pentacyclic Triterpenoid Phytochemicals with Anticancer Activity: Updated Studies on Mechanisms and Targeted Delivery. Int. J. Mol. Sci. 2023, 24, 12923. [Google Scholar] [CrossRef]
- Fulda, S. Betulinic Acid for Cancer Treatment and Prevention. Int. J. Mol. Sci. 2008, 9, 1096–1107. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra Precision Glide: Docking and Scoring Incorporating a Model of Hydrophobic Enclosure for Protein−Ligand Complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A New Approach for Rapid, Accurate Docking and Scoring. 1. Method and Assessment of Docking Accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Halgren, T.A.; Murphy, R.B.; Friesner, R.A.; Beard, H.S.; Frye, L.L.; Pollard, W.T.; Banks, J.L. Glide: A New Approach for Rapid, Accurate Docking and Scoring. 2. Enrichment Factors in Database Screening. J. Med. Chem. 2004, 47, 1750–1759. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Concilio, S.; Sessa, L.; Marrafino, F.; Piotto, S. Advancements and novel approaches in modified AutoDock Vina algorithms for enhanced molecular docking. Results Chem. 2024, 7, 101319. [Google Scholar] [CrossRef]
- Garner, T.P.; Lopez, A.; Reyna, D.E.; Spitz, A.Z.; Gavathiotis, E. Progress in targeting the BCL-2 family of proteins. Curr. Opin. Chem. Biol. 2017, 39, 133–142. [Google Scholar] [CrossRef]
- Wolf, E.; Lento, C.; Pu, J.; Dickinson, B.C.; Wilson, D.J. Innate Conformational Dynamics Drive Binding Specificity in Anti-Apoptotic Proteins Mcl-1 and Bcl-2. Biochemistry 2023, 62, 1619–1630. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Milan, A.; Mioc, M.; Mioc, A.; Gogulescu, A.; Mardale, G.; Avram, Ș.; Maksimović, T.; Mara, B.; Șoica, C. Cytotoxic Potential of Betulinic Acid Fatty Esters and Their Liposomal Formulations: Targeting Breast, Colon, and Lung Cancer Cell Lines. Molecules 2024, 29, 3399. [Google Scholar] [CrossRef] [PubMed]
- Anantram, A.; Kundaikar, H.; Degani, M.; Prabhu, A. Molecular dynamic simulations on an inhibitor of anti-apoptotic Bcl-2 proteins for insights into its interaction mechanism for anti-cancer activity. J. Biomol. Struct. Dyn. 2019, 37, 3109–3121. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, A.; Gogulescu, A.; Șoica, C.; Mioc, A.; Mioc, M.; Milan, A.; Lukinich-Gruia, A.T.; Pricop, M.-A.; Jianu, C.; Banciu, C.; et al. In Vitro and In Silico Evaluation of Syzygium aromaticum Essential Oil: Effects on Mitochondrial Function and Cytotoxic Potential Against Cancer Cells. Plants 2024, 13, 3443. [Google Scholar] [CrossRef]
- Mardale, G.; Caruntu, F.; Mioc, A.; Mioc, M.; Lukinich-Gruia, A.T.; Pricop, M.-A.; Jianu, C.; Gogulescu, A.; Maksimovic, T.; Șoica, C. Integrated In Silico and In Vitro Assessment of the Anticancer Potential of Origanum vulgare L. Essential Oil. Processes 2025, 13, 1695. [Google Scholar] [CrossRef]
- Maksimović, T.; Minda, D.; Șoica, C.; Mioc, A.; Mioc, M.; Colibășanu, D.; Lukinich-Gruia, A.T.; Pricop, M.-A.; Jianu, C.; Gogulescu, A. Anticancer Potential of Cymbopogon citratus L. Essential Oil: In Vitro and In Silico Insights into Mitochondrial Dysfunction and Cytotoxicity in Cancer Cells. Plants 2025, 14, 1341. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Dallakyan, S.; Olson, A.J. Small-Molecule Library Screening by Docking with PyRx. Methods Mol. Biol. 2015, 1263, 243–250. [Google Scholar]
- Trott, O.; Olson, A. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Warren, G.L.; Andrews, C.W.; Capelli, A.-M.; Clarke, B.; LaLonde, J.; Lambert, M.H.; Lindvall, M.; Nevins, N.; Semus, S.F.; Senger, S.; et al. A Critical Assessment of Docking Programs and Scoring Functions. J. Med. Chem. 2006, 49, 5912–5931. [Google Scholar] [CrossRef]
- Hevener, K.E.; Zhao, W.; Ball, D.M.; Babaoglu, K.; Qi, J.; White, S.W.; Lee, R.E. Validation of Molecular Docking Programs for Virtual Screening against Dihydropteroate Synthase. J. Chem. Inf. Model. 2009, 49, 444–460. [Google Scholar] [CrossRef] [PubMed]
- Madhavi Sastry, G.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided. Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Plewczynski, D.; Łaźniewski, M.; Augustyniak, R.; Ginalski, K. Can we trust docking results? Evaluation of seven commonly used programs on PDBbind database. J. Comput. Chem. 2011, 32, 742–755. [Google Scholar] [CrossRef] [PubMed]
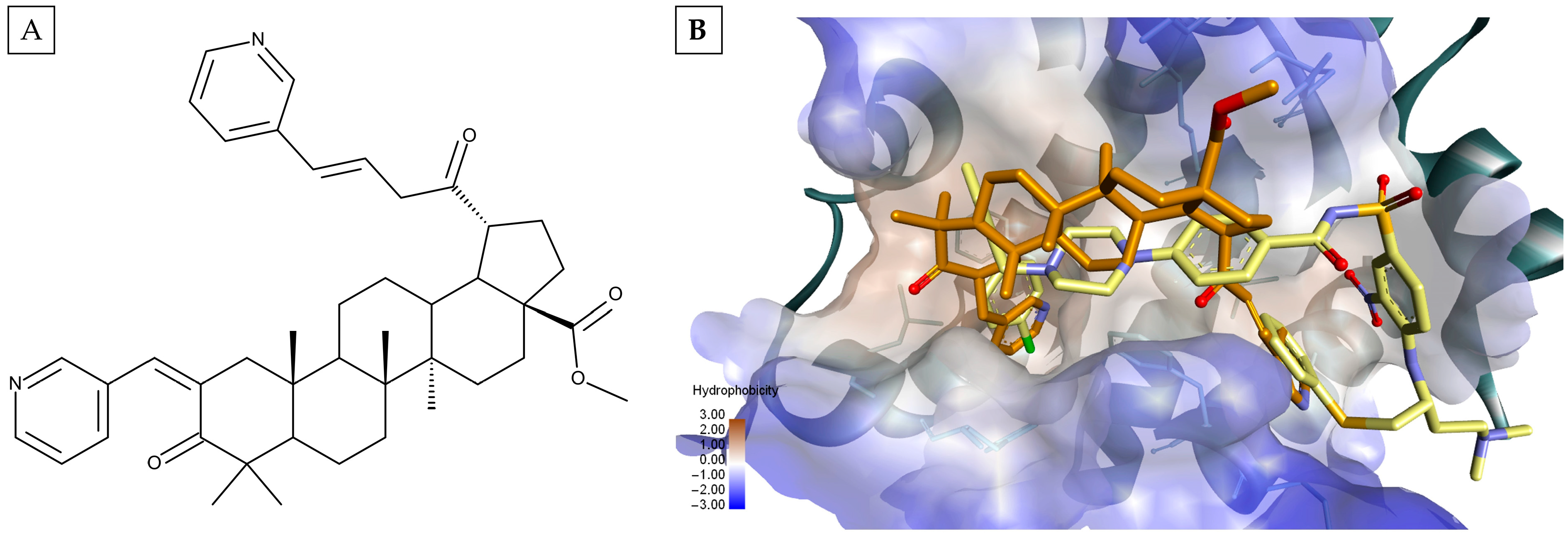
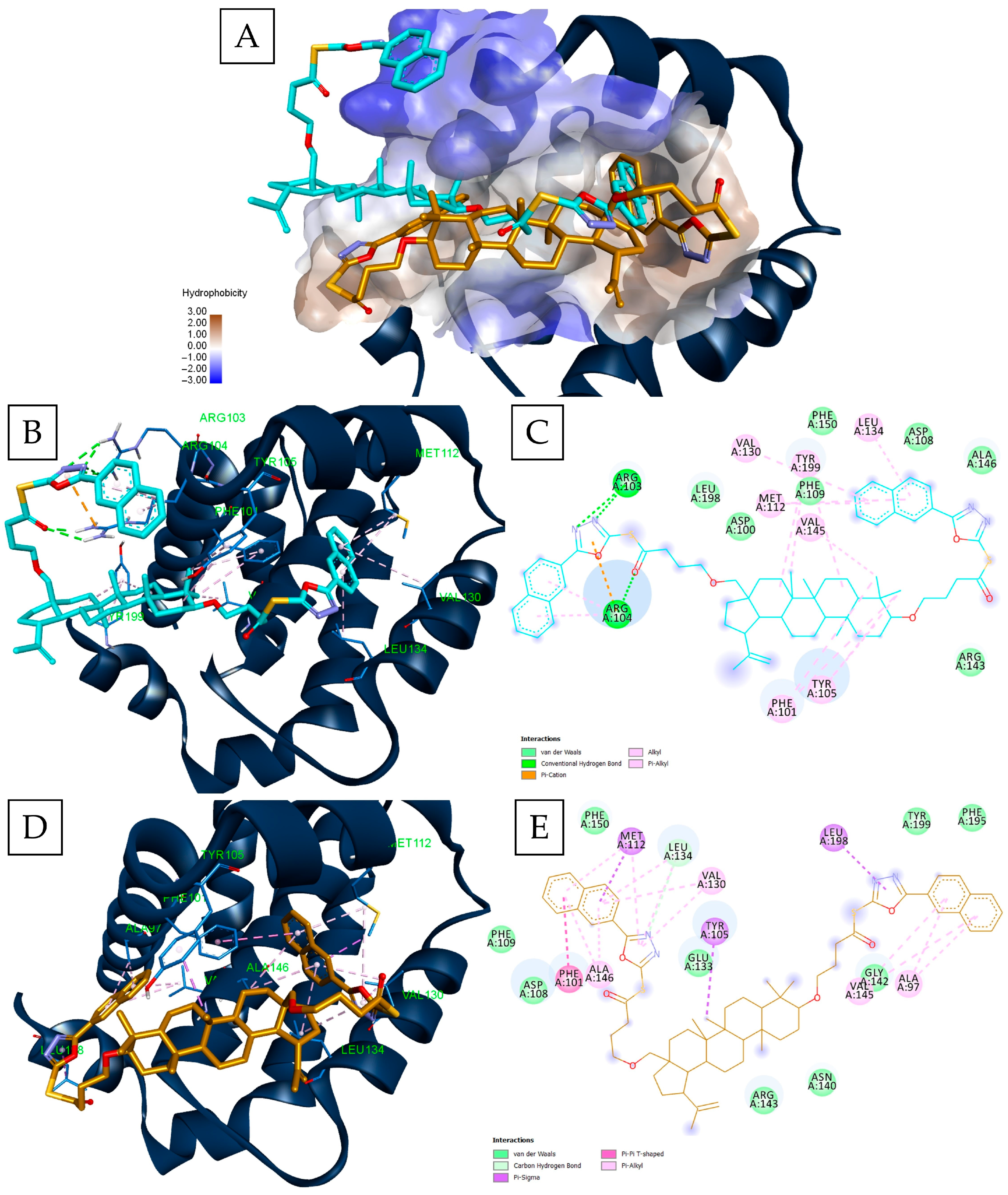
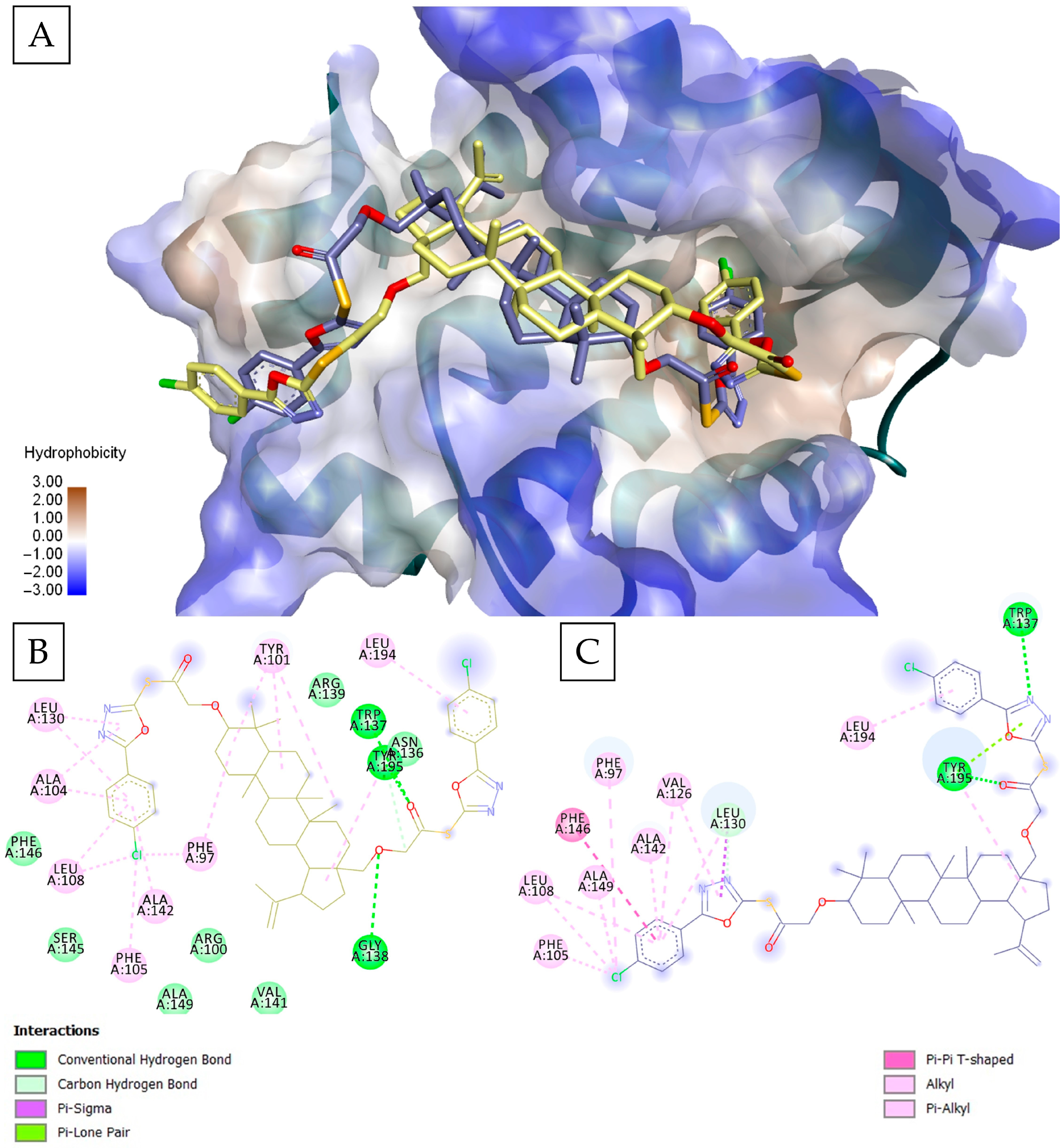





| Rank | GLIDE—Bcl-XL (kcal/mol) | VINA—Bcl-XL (kcal/mol) | GLIDE—Bcl-2 (kcal/mol) | VINA—Bcl-2 (kcal/mol) | GLIDE—MCL1 (kcal/mol) | VINA—Mcl-1 (kcal/mol) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ABT-737 | −9.45 | BT2 | −11.4 | Navitoclax | −12.49 | BTzNaf1 | −11.7 | S63845 | −11.40 | BOxNaf1 | −9.8 |
| 2 | BOxPhCl1 | −8.79 | BT1 | −11.3 | BOxPhCl5 | −8.22 | Navitoclax | −11.4 | BTzPhCl4 | −7.88 | BTzNaf1 | −9.1 |
| 3 | BTzPhCl7 | −8.72 | BOxNaf1 | −10.9 | BOxPhDMN3 | −7.97 | BOxNaf3 | −10.9 | BTzPhDMN1 | −7.56 | BT4 | −8.8 |
| 4 | BT5 | −8.70 | ABT-737 | −10.8 | BOxPhCl3 | −7.91 | BT2 | −10.7 | BTzNaf8 | −7.35 | BTzNaf4 | −8.8 |
| 5 | BOxPhCl8 | −8.56 | BOxNaf2 | −10.7 | BOxPh6 | −7.89 | BOxNaf1 | −10.6 | BTzNaf7 | −7.35 | S63845 | −8.8 |
| 6 | BOxPhCl4 | −8.48 | BOxNaf3 | −10.7 | BOx9 | −7.79 | BT1 | −10.5 | BTzNaf9 | −7.15 | BOxPhCl1 | −8.8 |
| 7 | BTz9 | −8.48 | BOxPhCl1 | −10.7 | BTzPhCl5 | −7.76 | BtzPh1 | −10.3 | BOxPhCl7 | −7.13 | BtzPhCl3 | −8.7 |
| 8 | BOxNaf5 | −8.46 | BtzPhCl1 | −10.4 | BTzPhCl8 | −7.63 | BtzPh2 | −10.3 | BOxPhCl3 | −7.12 | BtzPhOMe1 | −8.6 |
| 9 | BOxPhOMe1 | −8.17 | BOxPh1 | −10.4 | BTz5 | −7.60 | BOxNaf4 | −10.3 | BTzNaf2 | −7.05 | BOxNaf3 | −8.6 |
| 10 | BTzNaf7 | −7.98 | BT3 | −10.3 | BOxPh3 | −7.57 | BTzNaf3 | −10.2 | BOxNaf6 | −7.03 | BOxPh2 | −8.6 |
| 11 | BOxNaf1 | −7.94 | BOxPhCl4 | −10.3 | BTz7 | −7.56 | BOxNaf2 | −10.2 | BTzPhCl5 | −7.02 | BtzPh2 | −8.5 |
| 12 | BOxPhDMN7 | −7.92 | BtzPh2 | −10.2 | BOxPh2 | −7.51 | BOxPh1 | −10.2 | BTzNaf5 | −7.02 | BT2 | −8.4 |
| 13 | BT1 | −7.89 | BtzPhDMN1 | −10.2 | BOxNaf3 | −7.32 | BOxPh2 | −10.2 | BOxPhOMe9 | −7.00 | BTzNaf2 | −8.4 |
| 14 | BTzPhCl3 | −7.87 | BT5 | −10.1 | BOxPhCl7 | −7.31 | BOxPhCl1 | −10.2 | BOxPh4 | −6.96 | BtzPhOMe4 | −8.4 |
| 15 | BOxNaf4 | −7.81 | BtzPhOMe1 | −10.1 | BT6 | −7.30 | BT3 | −10.1 | BOxPh5 | −6.94 | BT3 | −8.3 |
| 16 | BOxPh6 | −7.78 | BOxPhCl2 | −10.1 | BT5 | −7.23 | BT4 | −10 | BTzPhCl3 | −6.90 | BTzNaf6 | −8.3 |
| 17 | BTzPhOMe7 | −7.78 | BOxNaf4 | −10 | BTz4 | −6.96 | BTzNaf2 | −10 | BOxNaf7 | −6.79 | BtzPh1 | −8.3 |
| 18 | BTz7 | −7.77 | BOxNaf5 | −9.9 | BTz3 | −6.95 | BtzPhCl1 | −10 | BOxPhCl6 | −6.76 | BtzPhCl4 | −8.3 |
| 19 | BT3 | −7.73 | BT8 | −9.8 | BTzPh9 | −6.93 | BOxPhCl2 | −9.8 | BTzPhOMe4 | −6.76 | BOxNaf2 | −8.3 |
| 20 | BOxNaf3 | −7.71 | BtzPhCl2 | −9.8 | BOxPhOMe2 | −6.89 | BOxPhOMe1 | −9.8 | BTzNaf3 | −6.74 | BT1 | −8.2 |
| Target | Ligand | G_Bind (kcal/mol) | vdW | Coulomb | Lipo | Solv_GB | Ligand Strain | Receptor Strain | Ligand Efficiency |
|---|---|---|---|---|---|---|---|---|---|
| Bcl-2 (4LVT) | BOxNaf1 | −73.02 | −60.21 | −19.98 | −29.93 | 39.12 | 9.61 | 14.66 | −1.04 |
| Bcl-2 (4LVT) | BOxPhCl1 | −64.58 | −49.68 | −39.74 | −28.82 | 49.66 | 8.77 | 25.50 | −1.01 |
| Bcl-2 (4LVT) | BOxNaf3 | −62.48 | −50.12 | −45.20 | −20.18 | 52.52 | 14.59 | 17.31 | −0.84 |
| Bcl-2 (4LVT) | BT3 | −52.28 | −54.52 | −0.88 | −19.01 | 27.81 | 8.84 | 23.23 | −0.84 |
| Bcl-2 (4LVT) | BT1 | −40.52 | −46.05 | −1.89 | −16.80 | 30.93 | 14.03 | 19.06 | −0.69 |
| Bcl-xL (2YXJ) | BOxNaf3 | −102.26 | −87.38 | 13.60 | −41.26 | 25.50 | 6.19 | 30.22 | −1.38 |
| Bcl-xL (2YXJ) | BOxNaf1 | −100.10 | −83.97 | 1.75 | −40.52 | 17.35 | 15.35 | 24.24 | −1.43 |
| Bcl-xL (2YXJ) | BOxPhCl1 | −85.45 | −67.68 | 4.76 | −30.86 | 5.27 | 5.10 | 15.88 | −1.33 |
| Bcl-xL (2YXJ) | BT1 | −71.00 | −68.80 | 6.23 | −24.51 | 11.65 | 4.82 | 15.22 | −1.22 |
| Bcl-xL (2YXJ) | BT3 | −66.73 | −61.75 | 8.47 | −20.98 | −0.08 | 13.01 | 8.17 | −1.07 |
| Mcl-1 (5LOF) | BOxPhCl1 | −85.94 | −69.04 | −2.14 | −40.39 | 10.26 | 9.84 | 2.41 | −1.34 |
| Mcl-1 (5LOF) | BOxNaf1 | −74.58 | −73.31 | −20.37 | −31.13 | 27.60 | 11.30 | 10.58 | −1.06 |
| Mcl-1 (5LOF) | BOxNaf3 | −72.75 | −47.65 | −53.88 | −23.40 | 48.51 | 26.30 | 13.73 | −0.98 |
| Mcl-1 (5LOF) | BT3 | −70.58 | −64.25 | −4.39 | −29.89 | 9.26 | 4.47 | 11.47 | −1.13 |
| Mcl-1 (5LOF) | BT1 | −67.39 | −58.37 | −18.76 | −28.41 | 32.45 | 8.61 | 22.15 | −1.16 |
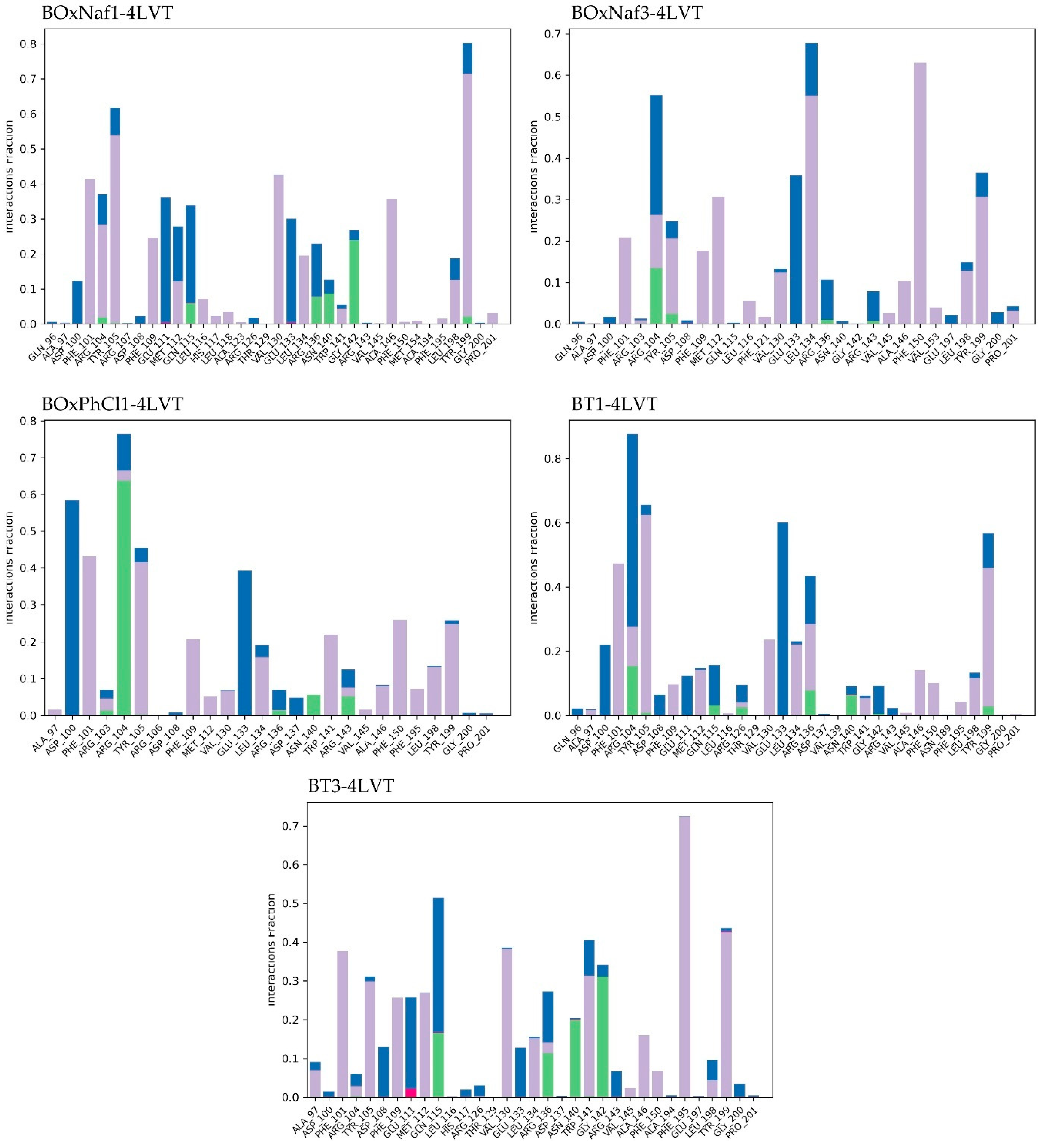
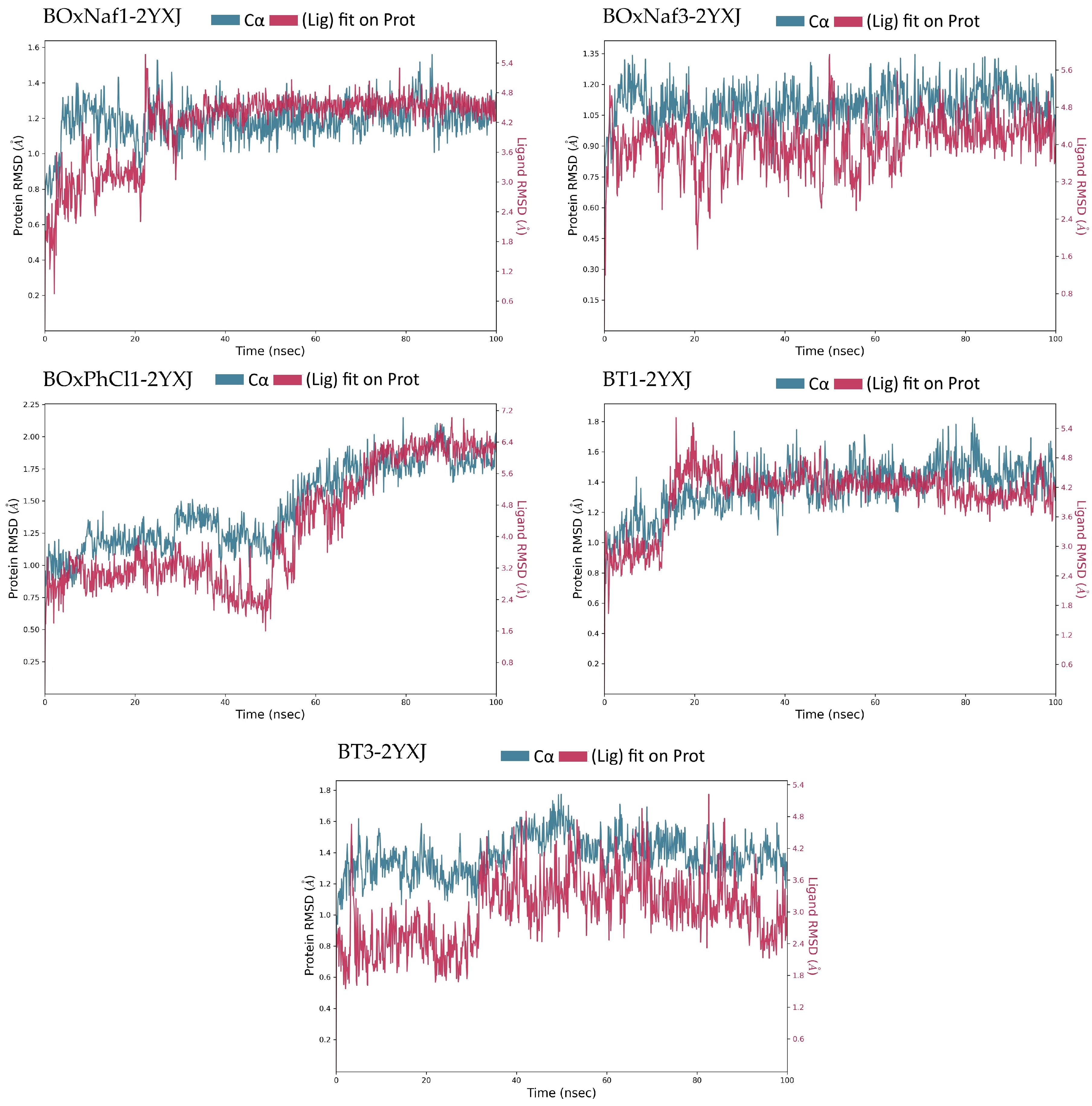
| Ligand | Protein RMSD (Å) | Ligand RMSD (Å) | H-Bonds (≥0.1 frac) | Hydrophobic Contacts (≥0.1 frac) | Water Bridges (≥0.1 frac) |
|---|---|---|---|---|---|
| BoxNaf1 | 0.8–2.0 (stable, minor drift) | 3.0–5.5 (stable) | Asn140 (0.2–0.3), Gly142 (0.2–0.3), Arg143 (0.1–0.2) | ~9 residues | ~5 residues |
| BoxNaf3 | 1.2–1.8 (stable, slight drift) | 4.5–10.5 (fluctuates) | Asn140 (0.1–0.15), Gly142 (0.1–0.15) | ~8 residues | ~4 residues |
| BoxPhCl1 | 1.0–1.7 (stable) | 3.5–8.0 (fluctuates) | Asn140 (0.1–0.15), Gly142 (~0.1) | ~7 residues | ~3 residues |
| BT1 | 1.0–1.8 (stable, post 20 ns drift) | 4.0–6.5 (post 20 ns drift, fluctuates) | Asn140 (0.2–0.3), Gly142 (0.1–0.2), Arg143 (0.1–0.2) | ~10 residues | ~6 residues |
| BT3 | 1.0–2.0 (stable after equilibration) | 2.5–5.5 (mild fluctuation) | Asn140 (0.1–0.2), Gly142 (0.1–0.2) | ~8 residues | ~4 residues |
| Ligand | Protein RMSD (Å) | Ligand RMSD (Å) | H-Bonds (≥0.1 frac) | Hydrophobic Contacts (≥0.1 frac) | Water Bridges (≥0.1 frac) |
|---|---|---|---|---|---|
| BOxNaf1 | ~1.0–1.3 (stable) | ~3.0–5 (stable after 20 ns: 4.2–5) | Arg100 (0.1–0.1) | ~11 residues | ~4 residues |
| BOxNaf3 | ~0.9–1.3 (stable) | ~3.2–5.5 (fluctuates) | Gln111 (0.1–0.2), Asn136 (0.2–0.3), Trp137 (0.3–0.4), Gly138 (0.3–0.4) | ~8 residues | ~6 residues |
| BOxPhCl1 | ~1.0–2.1 (late upward drift) | ~2–6.5 (highly fluctuates) | His113 (0.3–0.4) | ~10 residues | ~3 residues |
| BT1 | ~1.0–1.8 (stable) | ~3.4–5.0 (fluctuates; stabilizes after 20 ns) | Arg100 (0.2–0.3), Gln125 (0.1–0.2) | ~8 residues | ~4 residues |
| BT3 | ~1.1–1.6 (stable) | ~2.0–4.0 (fluctuates) | Gly138 (0.7–0.8) | ~6 residues | ~3 residues |
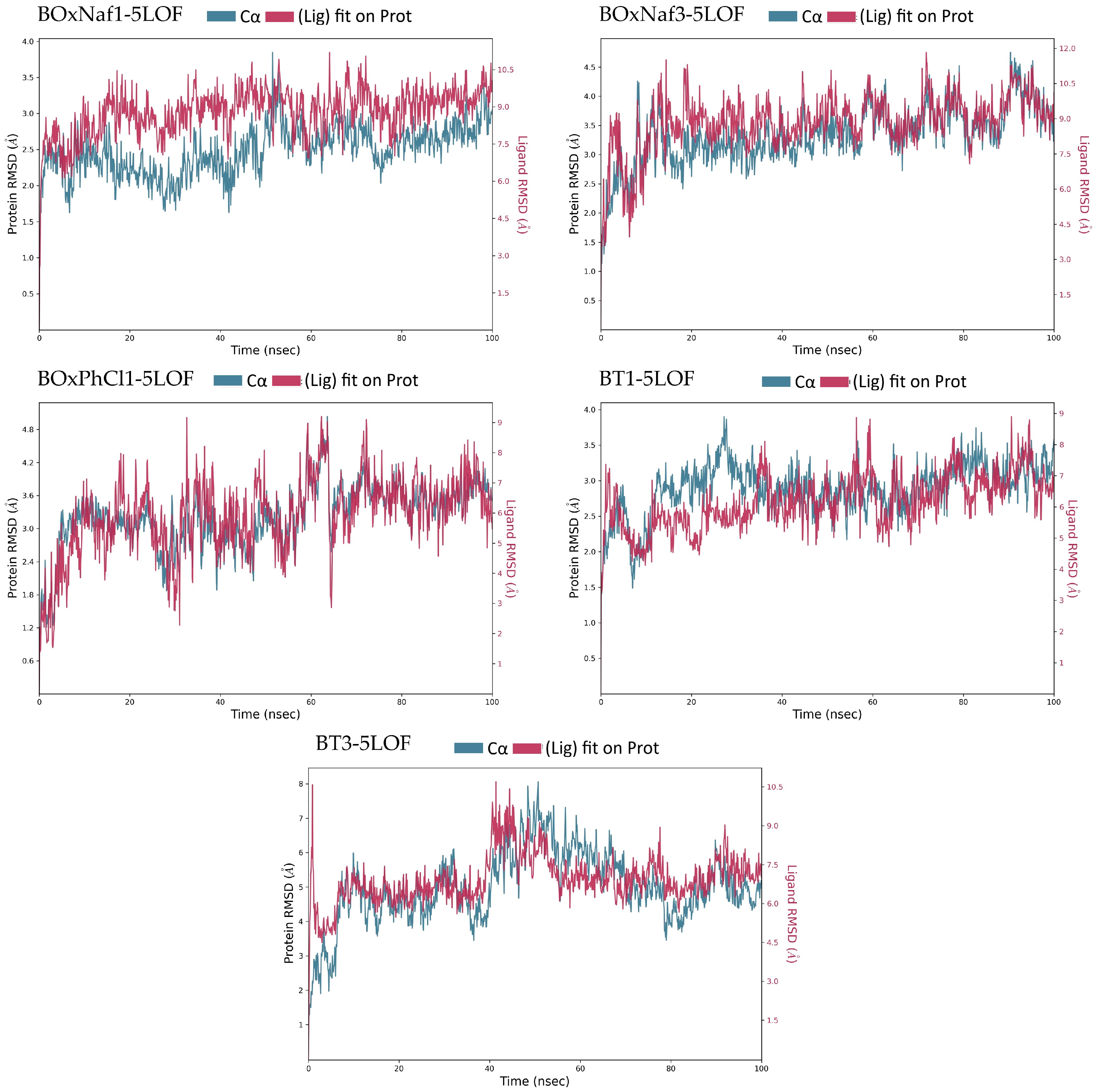
| Ligand | Protein RMSD (Å) | Ligand RMSD (Å) | H-Bonds (≥0.1 frac) | Hydrophobic Contacts (≥0.1 frac) | Water Bridges (≥0.1 frac) |
|---|---|---|---|---|---|
| BOxNaf1 | ~1.5–3.5 (stable) | ~7.5–10.5 (fluctuates) | - | ~7 residues | ~1 residue |
| BOxNaf3 | ~2.5–4.5 (stable) | ~7.5–10.5 (fluctuates) | Arg263 (0.1–0.2) | ~8 residues | ~3 residues |
| BOxPhCl1 | ~2.0–4.0 (minor fluct.) | ~3.0–9.0 (highly fluctuates) | Arg233 (0.1–0.2), Arg263 (0.2–0.3) | ~4 residues | ~6 residues |
| BT1 | ~2.5–3.5 (stable) | ~5.5–8.0 (moderately fluctuates) | Thr226 (0.2–0.3), Arg263 (~0.1) | ~6 residues | ~4 residues |
| BT3 | ~2.0–7.5 (highly fluctuates) | ~6.0–9.5 (highly fluctuates) | - | ~9 residues | ~4 residues |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mioc, M.; Gruin, S.; Gogulescu, A.; Bătrîna, O.; Jorgovan, M.; Mara, B.-I.; Șoica, C. Targeting Anti-Apoptotic Bcl-2 Proteins with Triterpene-Heterocyclic Derivatives: A Combined Dual Docking and Molecular Dynamics Study. Molecules 2025, 30, 3919. https://doi.org/10.3390/molecules30193919
Mioc M, Gruin S, Gogulescu A, Bătrîna O, Jorgovan M, Mara B-I, Șoica C. Targeting Anti-Apoptotic Bcl-2 Proteins with Triterpene-Heterocyclic Derivatives: A Combined Dual Docking and Molecular Dynamics Study. Molecules. 2025; 30(19):3919. https://doi.org/10.3390/molecules30193919
Chicago/Turabian StyleMioc, Marius, Silvia Gruin, Armand Gogulescu, Oana Bătrîna, Mihaela Jorgovan, Bogdan-Ionuț Mara, and Codruța Șoica. 2025. "Targeting Anti-Apoptotic Bcl-2 Proteins with Triterpene-Heterocyclic Derivatives: A Combined Dual Docking and Molecular Dynamics Study" Molecules 30, no. 19: 3919. https://doi.org/10.3390/molecules30193919
APA StyleMioc, M., Gruin, S., Gogulescu, A., Bătrîna, O., Jorgovan, M., Mara, B.-I., & Șoica, C. (2025). Targeting Anti-Apoptotic Bcl-2 Proteins with Triterpene-Heterocyclic Derivatives: A Combined Dual Docking and Molecular Dynamics Study. Molecules, 30(19), 3919. https://doi.org/10.3390/molecules30193919







