Calorimetric Studies of the Silver-Titanium System
Abstract
1. Introduction
2. Results and Discussion
2.1. Experimental Study
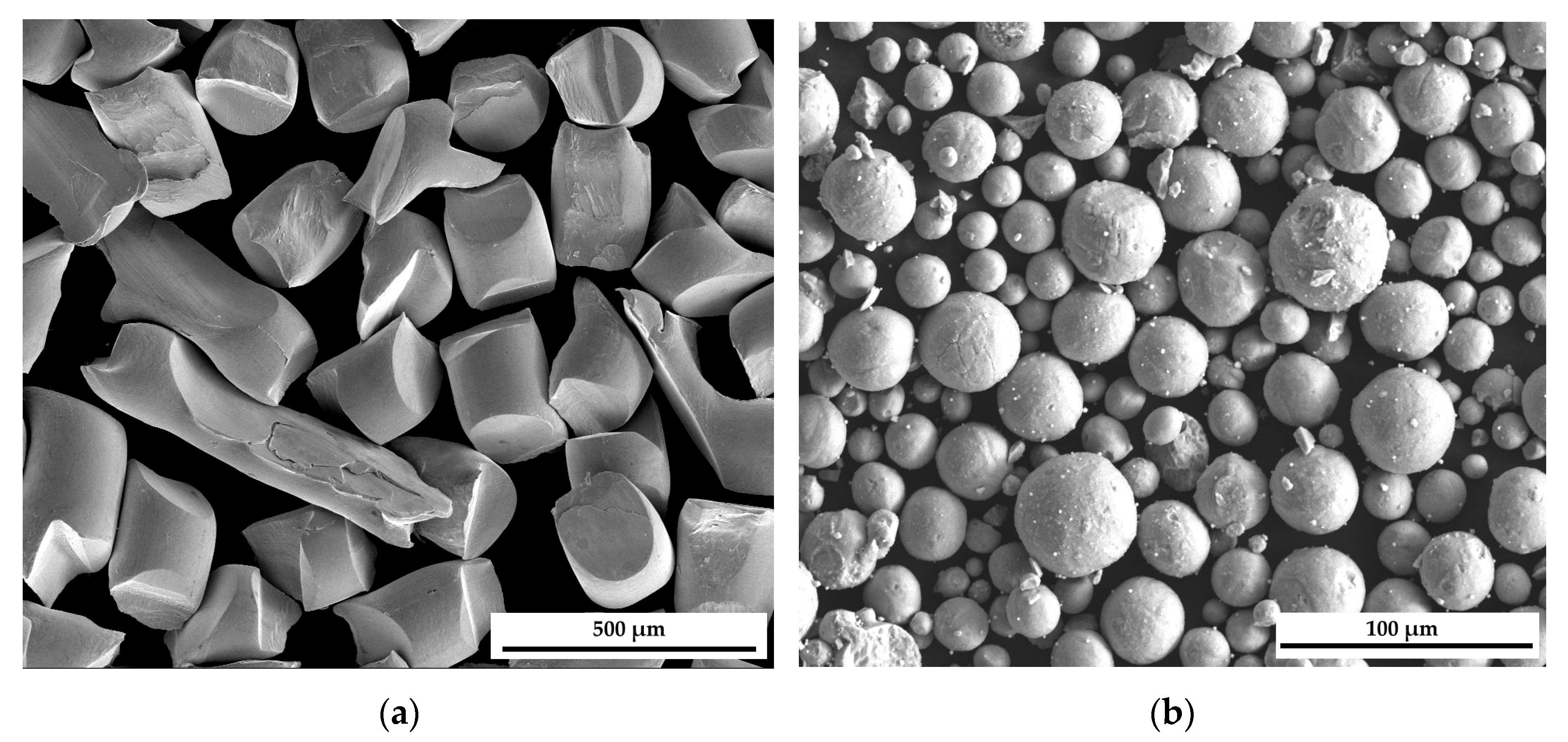
2.1.1. Phase Analysis and Microstructural Characterization
2.1.2. Differential Scanning Calorimetry (DSC)
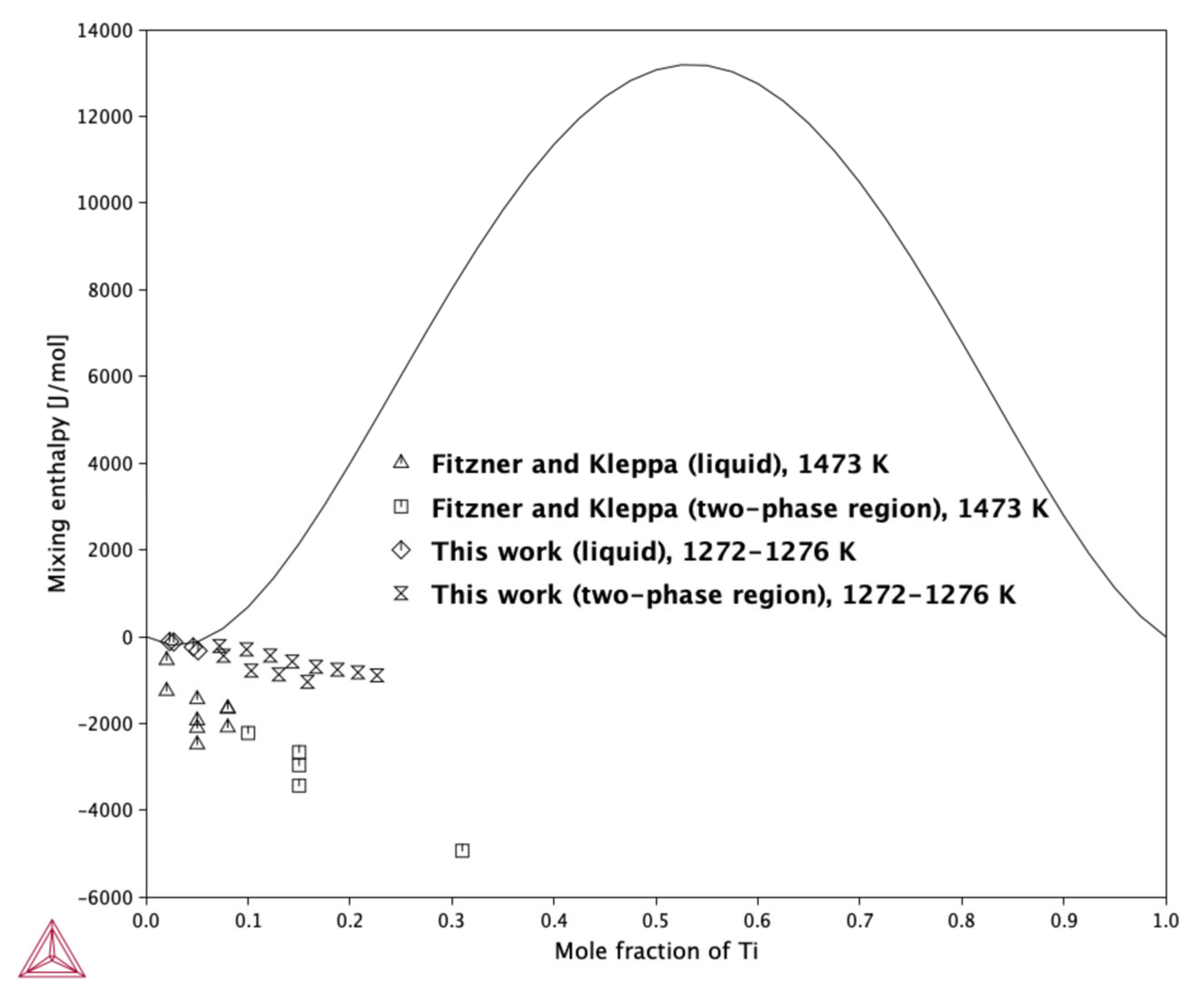
2.1.3. High-Temperature Drop Calorimetry—Enthalpy of Mixing
2.1.4. High-Temperature Drop Calorimetry—Enthalpy of Formation
2.2. Theoretical Study
2.2.1. Thermodynamic Optimization—CALPHAD
2.2.2. Enthalpy of Formation Calculation
2.2.3. Enthalpy of Mixing Calculations
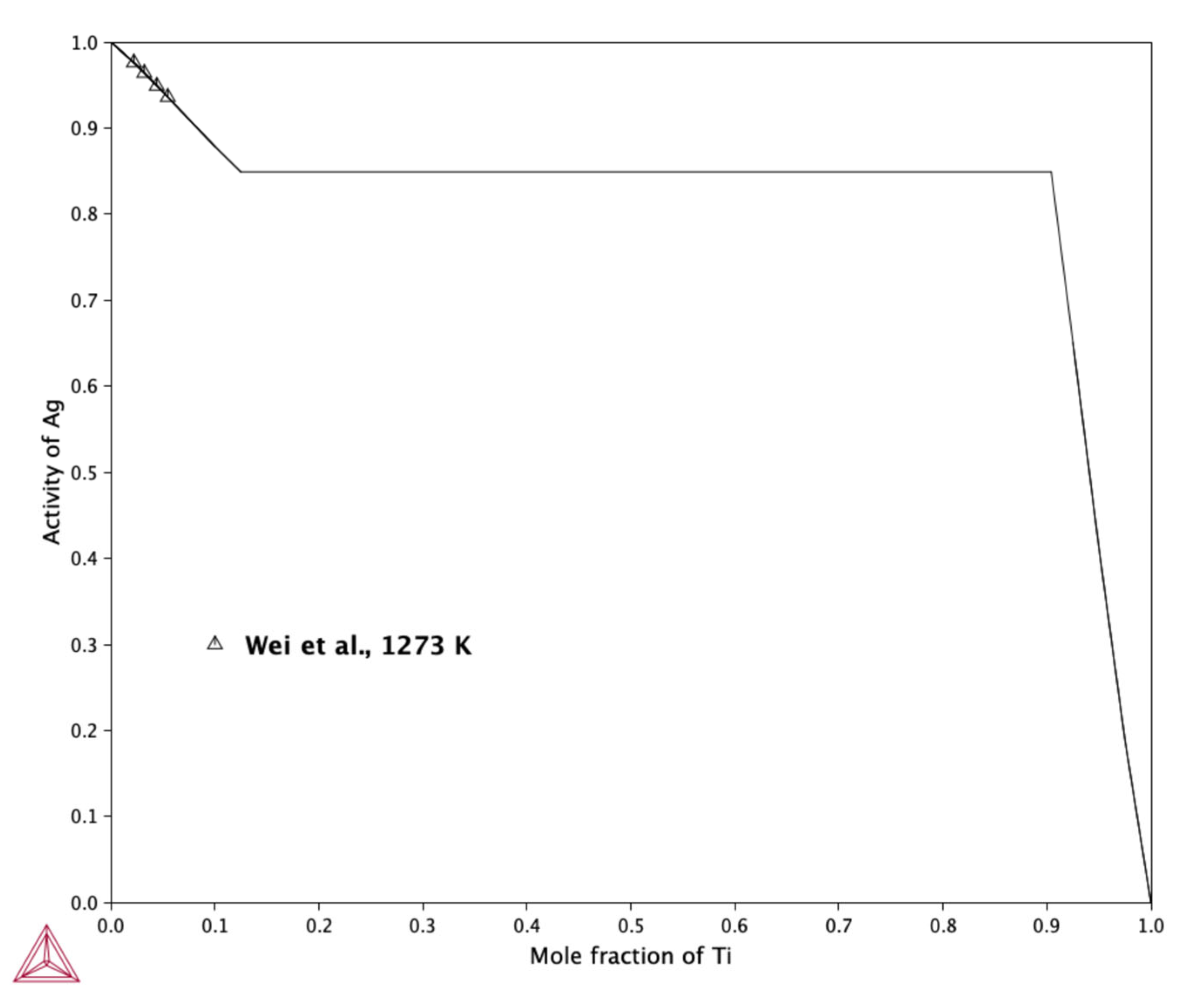
2.2.4. Silver Activity in the Liquid Phase Calculation
3. Materials and Methods
3.1. Experimental Study
3.1.1. Sample Preparation
3.1.2. X-Ray Diffraction (XRD)
3.1.3. Scanning Electron Microscopy (SEM), Energy Dispersive X-Ray Spectroscopy (EDS)
3.1.4. Differential Scanning Calorimetry (DSC)
3.1.5. High-Temperature Drop Calorimetry—Mixing Enthalpy Change
| Series A | Series B | |
|---|---|---|
| Crucible material | ZrO2 + Y2O3 | |
| Calibrant material | Silver | |
| Protective atmosphere | Argon at pressure p = 0.1 MPa | |
| Starting amount of silver—nAg [mol] | nAg = 0.0473 | nAg = 0.0557 |
| Calibration constant —K [kJ∙μVs] | K = 8.376 × 10−6 | K = 8.340 × 10−6 |
| Temperature —T [K] | TD= 298, TM = 1401 | TD= 298, TM = 1400 |
| [kJ/mol] | = 43.3110 = 46.8315 | = 43.2775 = 46.7941 |
| Standard uncertainties of the number of moles —u(ni) [mol] | u(nTi) = 2.09 × 10−6, u(nAg) = 9.27 × 10−7 | |
| Standard uncertainties of temperatures —u(T) [K] | u(TD) = 1, u(TM) = 1 | |
| Standard uncertainties of argon pressure —u(p) [kPa] | u(p) = 10 | |
| Standard uncertainties calibration constant —u(K) [kJ∙μVs] | u(K) = 3.27 × 10−7 | u(K) = 5.26 × 10−8 |
3.1.6. High-Temperature Drop Calorimetry—Enthalpy of Formation
3.2. Theoretical Investigation
4. Conclusions
- Concerning the results of the mixing enthalpy measurements of alloys in the range of xTi = 0.02–0.226, the integral molar mixing enthalpy change and partial molar enthalpy of Ti were determined based on two measurement series. The results showed negative values across the entire investigated concentration range.
- Regarding the enthalpy of formation values for the AgTi intermetallic phase ascertained calorimetrically via the direct synthesis method, the mean value was −2.4 (±0.3) kJ/mol∙at. The results reported in this study are consistent with the values calculated in [11] and fall within the range of the experimental data reported in [20]. However, the theoretically determined enthalpy of formation shows more negative values than those obtained by experiment.
- The formation enthalpies of the intermetallic compounds AgTi and AgTi2 show good agreement with both theoretical predictions and experimental measurements.
- The results of calculated silver activity in liquid Ti at 1273 K show good agreement with the experimental measurements reported by Wei et al. [17], indicating that the thermodynamic model accurately captures the behavior of the Ag-Ti liquid solution at this temperature.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, M.; Zhang, E.; Zhang, L. Microstructure, mechanical properties, bio-corrosion properties and antibacterial properties of Ti-Ag sintered alloys. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 62, 350–360. [Google Scholar] [CrossRef]
- Paulasto, M.; van Loo, F.J.J.; Kivilahti, J.K. Thermodynamic and experimental study of Ti-Ag-Cu alloys. J. Alloys Compd. 1995, 220, 136–141. [Google Scholar] [CrossRef]
- Maeshima, T.; Nishida, M. Shape Memory Properties of Biomedical Ti-Mo-Ag and Ti-Mo-Sn Alloys. Mater. Trans. 2004, 45, 1096–1100. [Google Scholar] [CrossRef]
- Dziedzic, A.; Augustowski, D.; Kwaśnicki, P.; Adamiak, S.; Bochnowski, W.; Żaczek, A.; Skała, P.; Cieniek, B.; Potera, P.; Dziedzic, J.; et al. Structure and Properties of Silver-Platinum-Titanium Dioxide Nanocomposite Coating. Coatings 2025, 15, 587. [Google Scholar] [CrossRef]
- Dudek, K.; Dulski, M.; Podwórny, J.; Kujawa, M.; Gerle, A.; Rawicka, P. Functionalization of the NiTi Shape Memory Alloy Surface through Innovative Hydroxyapatite/Ag-TiO2 Hybrid Coatings. Materials 2024, 17, 604. [Google Scholar] [CrossRef]
- Adenstedt, H.K.; Freeman, W.R. The Tentative Titanium–Silver Binary System; Technical Report; WADC: Dayton, OH, USA, 1953; pp. 53–109. [Google Scholar]
- Worner, H.W. The Structure of Titanium–Silver Alloys in the Range 0–30 at.% Silver. J. Inst. Met. 1953, 82, 222–226. [Google Scholar]
- McQuillan, M.K. A Study of the Titanium–Silver System. J. Inst. Met. 1960, 88, 235–239. [Google Scholar]
- Eremenko, V.N.; Buyanov, Y.I.; Panchenko, N.M. Constitution diagram of the system titanium-silver. Sov. Powder Metall. Metal. Ceram. 1969, 8, 562–566. [Google Scholar] [CrossRef]
- Plichta, M.R.; Williams, J.C.; Aaronson, H.I. On the existence of the β→αm transformation in the alloy systems Ti-Ag, Ti-Au, and Ti-Si. Metall. Trans. A 1977, 8, 1885–1892. [Google Scholar] [CrossRef]
- Murray, J.L.; Bhansali, K.J. The Ag–Ti (Silver-Titanium) system. Bull. Alloy Phase Diagr. 1983, 4, 178–183. [Google Scholar] [CrossRef]
- Arróyave, R. Thermodynamics and Kinetics of Ceramic/Metal Interfacial Interactions. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2004. [Google Scholar]
- Li, M.; Li, C.; Wang, F.; Zhang, W. Experimental Study and Thermodynamic Assessment of the Ag–Ti System. Calphad 2005, 29, 269–275. [Google Scholar] [CrossRef]
- Gierlotka, W.; Dębski, A.; Gąsior, W.; Polański, M. Computational Approach to the Ag–Ti System. Comput. Mater. Sci. 2023, 230, 112519. [Google Scholar] [CrossRef]
- Dezellus, O.; Arroyave, R.; Fries, S.G. Thermodynamic modelling of the Ag–Cu–Ti ternary system. Int. J. Mater. Res. 2011, 102, 286–297. [Google Scholar] [CrossRef]
- Nagase, T.; Matsumoto, M.; Fujii, Y. Microstructure of Ti–Ag Immiscible Alloys with Liquid Phase Separation. J. Alloys Compd. 2018, 738, 440–447. [Google Scholar] [CrossRef]
- Dececco, N.A.; Parks, J.M. The Brazing of Titanium. Welding J. 1953, 32, 1071–1081. [Google Scholar]
- Fitzner, K.; Kleppa, O.J. Thermochemistry of binary alloys of transition metals: The Me–Ti, Me–Zr, and Me–Hf (Me = Ag, Au) systems. Metall. Trans. A 1992, 23, 997–1003. [Google Scholar] [CrossRef]
- Wei, P.; Rongti, L.; Jian, C.; Ruifeng, S.; Jie, L. Thermodynamic Properties of Ti in Ag–Ti Alloys. Mater. Sci. Eng. A 2000, 287, 72–77. [Google Scholar] [CrossRef]
- Meschel, S.V.; Kleppa, O.J. Thermochemistry of Some Binary Alloys of Noble Metals (Cu, Ag, Au) and Transition Metals by High Temperature Direct Synthesis Calorimetry. J. Alloys Compd. 2003, 350, 205–212. [Google Scholar] [CrossRef]
- Carrullo, J.C.Z.; Borrás, A.D.; Borrás, V.A.; Navarro-Laboulais, J.; Falcón, J.C.P. Electrochemical Corrosion Behavior and Mechanical Properties of Ti–Ag Biomedical Alloys Obtained by Two Powder Metallurgy Processing Routes. J. Mech. Behav. Biomed. Mater. 2020, 112, 104063. [Google Scholar] [CrossRef]
- Adamek, G.; Kozlowski, M.; Junka, A.; Siwak, P.; Jakubowicz, J. Preparation and Properties of Bulk and Porous Ti-Ta-Ag Biomedical Alloys. Materials 2022, 15, 4332. [Google Scholar] [CrossRef]
- Dębski, A.; Dębski, R.; Gąsior, W. New Features of Entall Database: Comparison of Experimental and Model Formation Enthalpies. Arch. Metall. Mater. 2014, 59, 1337–1343. [Google Scholar] [CrossRef]
- Reinbach, R.; Fischmann, D. Diffusion in the Titanium–Silver System. Z. Metallkd. 1963, 56, 197–199. [Google Scholar]
- Dinsdale, A.T. SGTE Data for Pure Elements. Calphad 1991, 15, 317–425. [Google Scholar] [CrossRef]
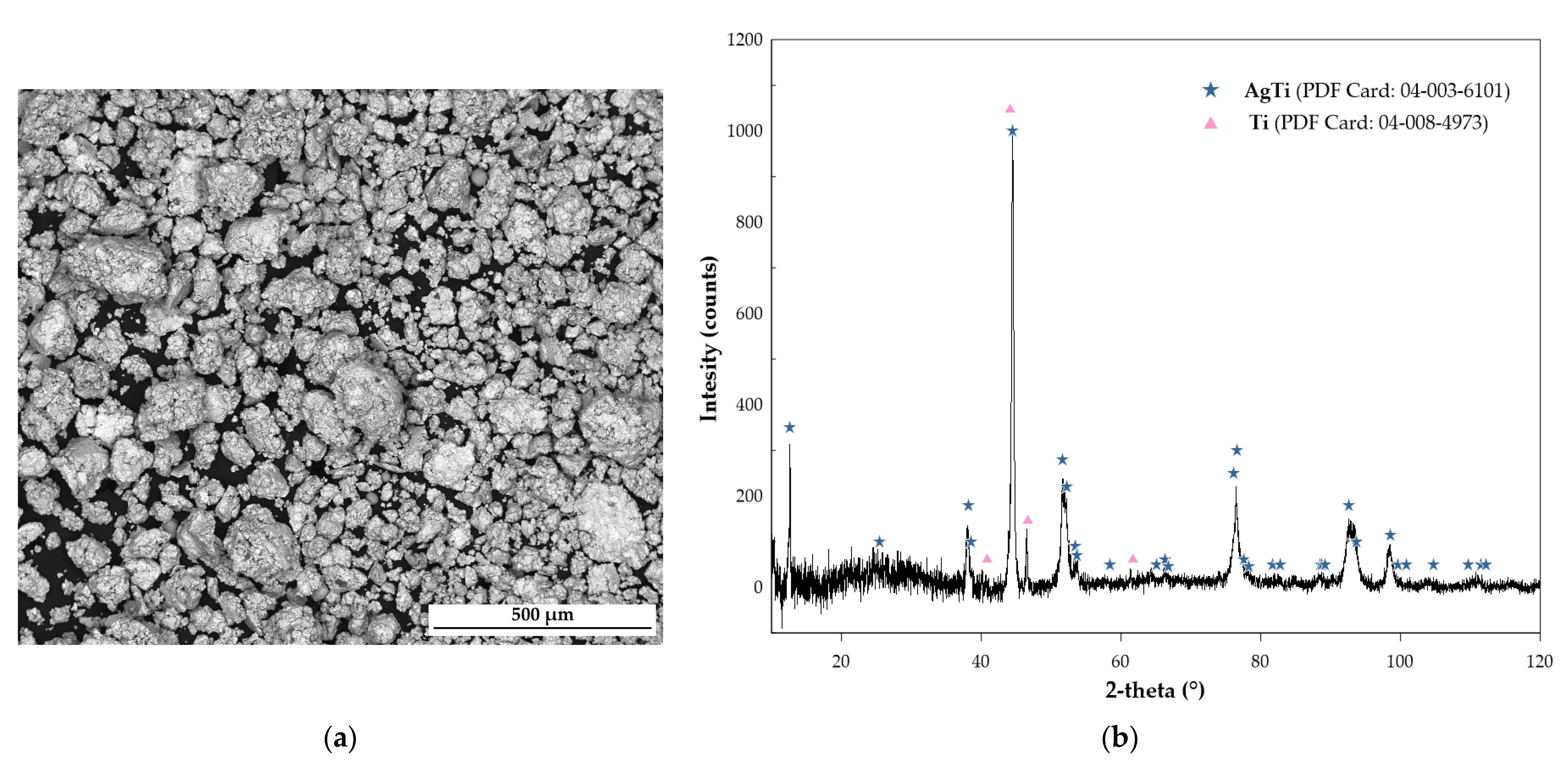
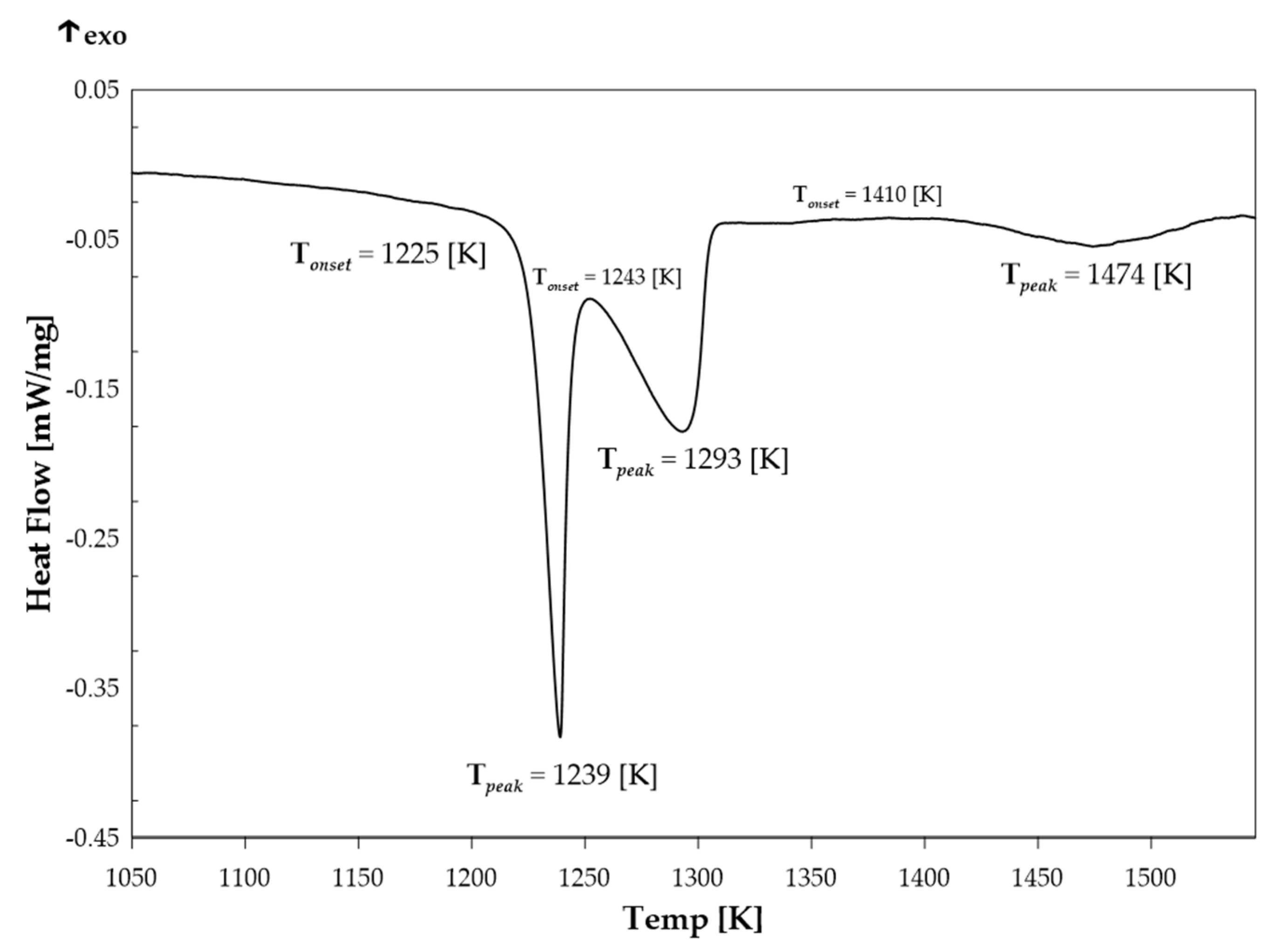
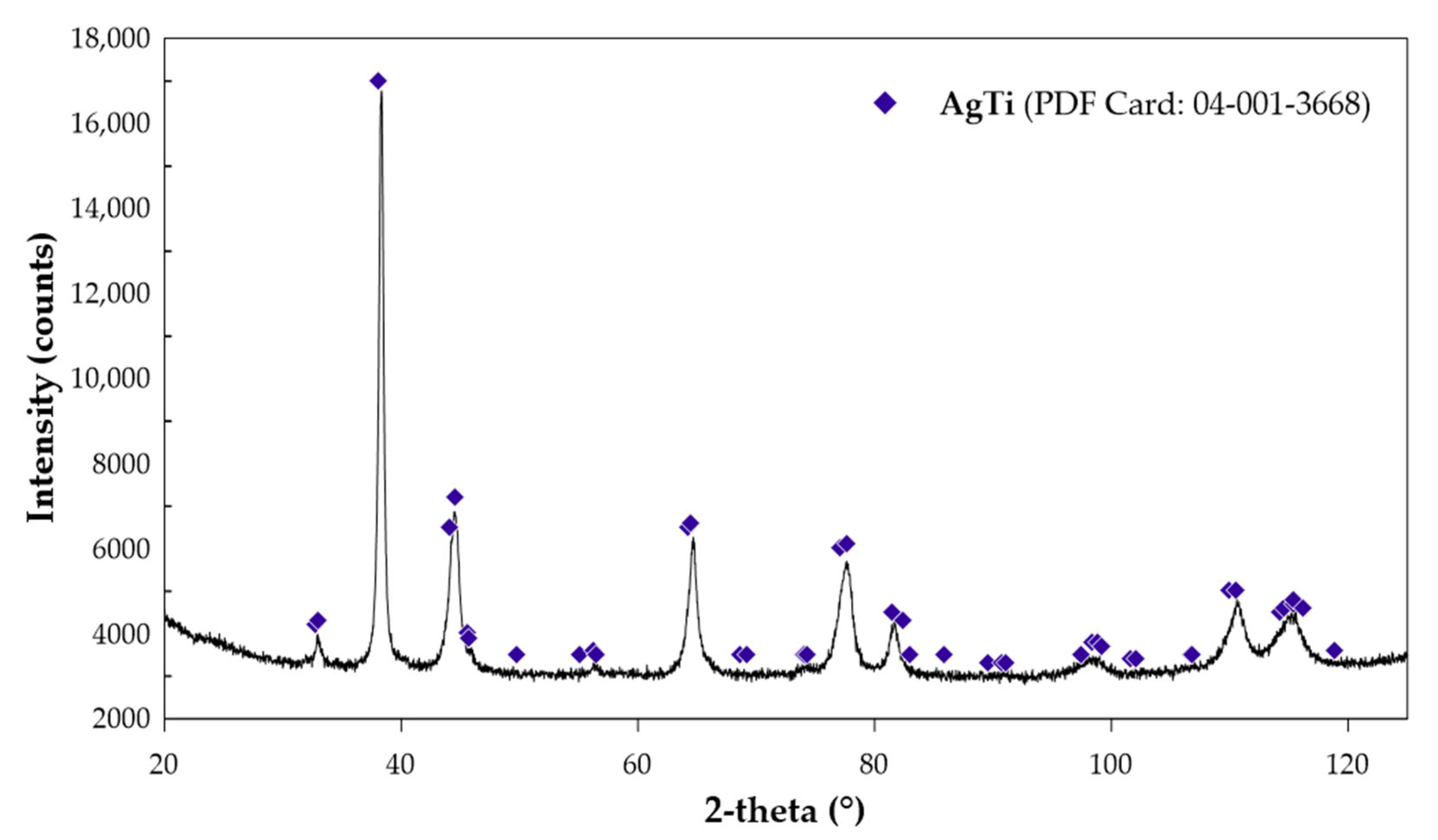
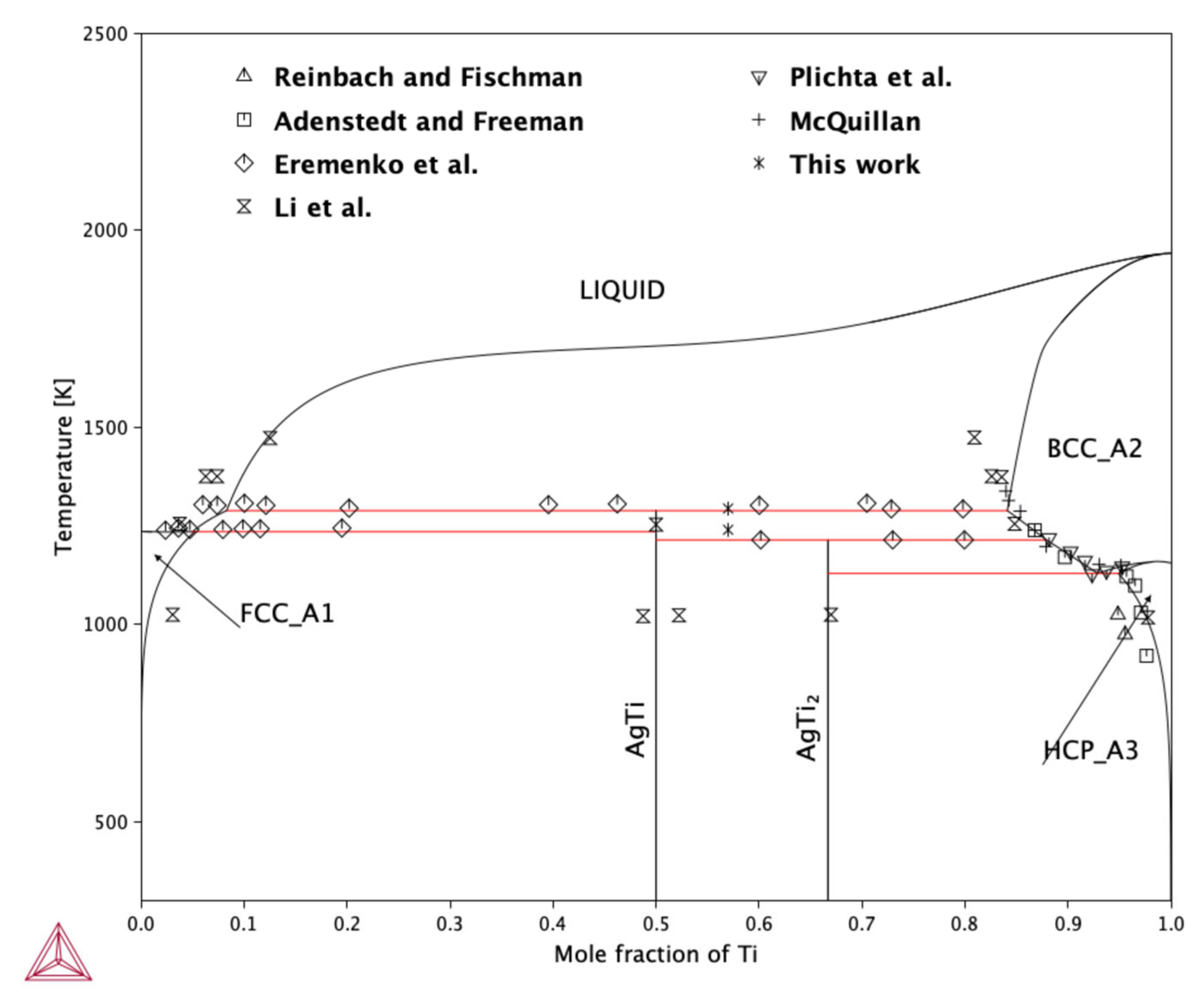
| Phase | Pearson Symbol | Strukturbericht Designation | Space Group | Prototype |
|---|---|---|---|---|
| (αTi) | hP2 | A3 | P63/mmc | Mg |
| (βTi) | cI2 | A2 | Im3m | W |
| AgTi2 | tI6 | C11 | I4/mmm | MoSi2 |
| AgTi | tP4 | B11 | P4/mmm | γCuTi |
| (Ag) | cF4 | A1 | Fm3m | Cu |
| Reaction | Composition xTi | Temperature [K] | Method | Reference | ||
|---|---|---|---|---|---|---|
| I | II | III | ||||
| L + (βTi) →AgTi | 0.083 | 0.841 | 0.500 | 1288 | CALPHAD | This work |
| — | — | 0.500 | 1273 | Melting of AgTi | [17] | |
| 0.163 | — | 0.500 | 1311 | Metallography | [6] | |
| 0.150 | 0.900 | 0.500 | 1290–1303 | Metallography | [8] | |
| — | 0.940 | 0.500 | 1293 | Thermal analysis | [9] | |
| 0.058 | 0.845 | 0.500 | 1294.8 | CALPHAD | [14] | |
| 0.059 | 0.835 | 0.500 | 1289 | CALPHAD | [13] | |
| 0.085 | 0.836 | 0.500 | 1297 | CALPHAD | [12] | |
| AgTi + (βTi) → AgTi2 | 0.500 | 0.879 | 0.667 | 1213 | CALPHAD | This work |
| 0.500 | 0.883 | 0.667 | 1213 | Microprobe, metallography | [10] | |
| 0.500 | 0.900 | 0.667 | 1173 | Metallography | [6] | |
| 0.500 | 0.880 | 0.667 | 1203 | Metallography | [8] | |
| 0.500 | — | 0.667 | 1218 | Thermal analysis | [9] | |
| 0.500 | 0.889 | 0.667 | 1212 | CALPHAD | [14] | |
| 0.500 | 0.887 | 0.667 | 1206 | CALPHAD | [13] | |
| 0.500 | 0.885 | 0.667 | 1216 | CALPHAD | [12] | |
| (βTi) → (αTi) + AgTi2 | 0.928 | 0.948 | 0.667 | 1129 | CALPHAD | This work |
| 0.924 | 0.953 | 0.667 | 1128 | Assessed | [11] | |
| 0.934 | 0.947 | 0.667 | 1126 | CALPHAD | [14] | |
| 0.924 | 0.931 | 0.667 | 1129 | CALPHAD | [13] | |
| 0.933 | 0.958 | 0.667 | 1126 | CALPHAD | [12] | |
| L → (Ag) + AgTi | 0.049 | 0.051 | 0.500 | 1234 | CALPHAD | This work |
| 0.050 | 0.050 | 0.500 | 1232 | Assessed | [11] | |
| 0.061 | 0.057 | 0.500 | 1232 | CALPHAD | [12] | |
| L + AgTi → (Ag) | 0.039 | 0.500 | 0.043 | 1235 | CALPHAD | [14] |
| 0.042 | 0.500 | 0.047 | 1237 | CALPHAD | [13] | |
| Element | Chemical Composition | |
|---|---|---|
| [atomic%] | [mass%] | |
| Ag | 42.9 (±0.6) | 62.8 (±0.6) |
| Ti | 57.1 (±0.6) | 37.2 (±0.6) |
| Number of Dropped Moles | Heat Effect | Drop Enthalpy | Mole Fraction | Integral Molar Mixing Enthalpy | Partial Molar Enthalpy | Standard Uncertainties |
|---|---|---|---|---|---|---|
| [mol] | [kJ] | [kJ/mol] | [kJ/mol] | [kJ/mol] | [kJ/mol] | |
| 0.00131 | 0.05544 | −0.006 | 0.0270 | −0.124 | −4.6 | 0.045 |
| 0.00122 | 0.04704 | −0.010 | 0.0509 | −0.327 | −8.4 | 0.081 |
| 0.00135 | 0.05697 | −0.006 | 0.0759 | −0.439 | −4.6 | 0.125 |
| 0.00155 | 0.05379 | −0.019 | 0.1031 | −0.783 | −12.1 | 0.165 |
| 0.00164 | 0.07057 | −0.006 | 0.1301 | −0.870 | −3.7 | 0.215 |
| 0.00181 | 0.07369 | −0.011 | 0.1582 | −1.040 | −6.1 | 0.266 |
| Number of Dropped Moles | Heat Effect | Drop Enthalpy | Mole Fraction | Integral Molar Mixing Enthalpy | Partial Molar Enthalpy | Standard Uncertainties |
|---|---|---|---|---|---|---|
| [mol] | [kJ] | [kJ/mol] | [kJ/mol] | [kJ/mol] | [kJ/mol] | |
| 0.00131 | 0.05499 | −0.006 | 0.0230 | −0.112 | −4.9 | 0.006 |
| 0.00138 | 0.05716 | −0.007 | 0.0461 | −0.237 | −5.4 | 0.013 |
| 0.00162 | 0.07693 | 0.001 | 0.0719 | −0.215 | 0.6 | 0.022 |
| 0.00177 | 0.07768 | −0.005 | 0.0985 | −0.293 | −2.9 | 0.030 |
| 0.00164 | 0.06712 | −0.009 | 0.1217 | −0.434 | −5.8 | 0.037 |
| 0.00160 | 0.06512 | −0.010 | 0.1433 | −0.572 | −6.0 | 0.044 |
| 0.00182 | 0.07586 | −0.009 | 0.1666 | −0.695 | −5.1 | 0.052 |
| 0.00173 | 0.07542 | −0.006 | 0.1876 | −0.759 | −3.2 | 0.059 |
| 0.00174 | 0.07556 | −0.006 | 0.2077 | −0.823 | −3.3 | 0.066 |
| 0.00170 | 0.07299 | −0.006 | 0.2264 | −0.892 | −3.8 | 0.073 |
| No. | Temperature | Enthalpy Change | Heat Effect | Enthalpy of Formation | Phases in the Alloy According to XRD 1 |
|---|---|---|---|---|---|
| ΔHef | ΔfH | ||||
| [K] | [kJ/mol] | [kJ/mol∙at] | [kJ/mol∙at] | ||
| 1. | TD = 298 TM = 1276 | ΔHAg = 39.1270 ΔHTi = 28.4007 | 17.38 | −2.18 | #PDF 04-001-3668—AgTi |
| 2. | TD = 298 TM = 1272 | ΔHAg = 38.9931 ΔHTi = 28.2837 | 16.71 | −2.78 | #PDF 04-004-3668—AgTi #PDF 04-004-9042—Ti |
| 3. | TD = 299 TM = 1272 | ΔHAg = 39.9677 ΔHTi = 28.2585 | 17.23 | −2.25 | #PDF 04-004-3668—AgTi #PDF 04-002-2539—Ti |
| Enthalpy of Formation 1 | Method | Reference | |
|---|---|---|---|
| AgTi | AgTi2 | ||
| −2.4 2 (±0.3) | — | Experimental—high-temperature direct synthesis calorimetry | This work |
| −1.6 (±2.4) | −2.3 (±1.1) | Experimental—high-temperature direct synthesis calorimetry | [20] |
| −5.9 | −4.6 | Calculated—CALPHAD | This work |
| −6.0 | −5.0 | Calculated—ab initio (0 K) | This work |
| −2.37 | −1.83 | Calculated—Miedema Model | [23] |
| −2 | −2 | Calculated—CALPHAD | [13] |
| Phase | Function [J/mol at.] |
|---|---|
| Liquid | |
| FCC_A1 (Ag) | |
| AgTi | |
| AgTi2 | |
| HCP_A3 (αTi) | |
| BCC_A2 (βTi) | |
| No. 1 | No. 2 | No. 3 | |
|---|---|---|---|
| Crucible material | ZrO2 + Y2O3 | ||
| Calibrant material | Titanium | ||
| Protective atmosphere | Argon at pressure p = 0.1 MPa | ||
| Calibration constant —K [kJ∙μVs] | K = 7.055 × 10−6 | K = 7.024 × 10−6 | K = 7.340 × 10−6 |
| Temperatures —T [K] | TD= 298, TM = 1276 | TD= 298, TM = 1272 | TD= 299, TM = 1272 |
| = 39.1270 = 28.4007 | = 38.9931 = 28.2837 | = 38.9677 = 28.2585 | |
| Standard uncertainties of the number of moles —u(ni) [mol] | u(nTi) = 2.09 × 10−6, u(nAg) = 9.27 × 10−7 | ||
| Standard uncertainties of temperatures —u(T) [K] | u(TD) = 1, u(TM) = 1 | ||
| Standard uncertainties of argon pressure —u(p) [kPa] | u(p) = 10 | ||
| Standard uncertainties of the calibration constant —u(K) [kJ∙μVs] | u(K) = 1.47 × 10−7 | u(K) = 1.38 × 10−8 | u(K) = 2.47 × 10−7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gozdur, W.; Gierlotka, W.; Gąsior, W.; Wierzbicka-Miernik, A.; Czeppe, T.; Budziak, A.; Radziwonko, A.; Pęska, M.; Dębski, A. Calorimetric Studies of the Silver-Titanium System. Molecules 2025, 30, 3898. https://doi.org/10.3390/molecules30193898
Gozdur W, Gierlotka W, Gąsior W, Wierzbicka-Miernik A, Czeppe T, Budziak A, Radziwonko A, Pęska M, Dębski A. Calorimetric Studies of the Silver-Titanium System. Molecules. 2025; 30(19):3898. https://doi.org/10.3390/molecules30193898
Chicago/Turabian StyleGozdur, Weronika, Wojciech Gierlotka, Władysław Gąsior, Anna Wierzbicka-Miernik, Tomasz Czeppe, Andrzej Budziak, Agata Radziwonko, Magda Pęska, and Adam Dębski. 2025. "Calorimetric Studies of the Silver-Titanium System" Molecules 30, no. 19: 3898. https://doi.org/10.3390/molecules30193898
APA StyleGozdur, W., Gierlotka, W., Gąsior, W., Wierzbicka-Miernik, A., Czeppe, T., Budziak, A., Radziwonko, A., Pęska, M., & Dębski, A. (2025). Calorimetric Studies of the Silver-Titanium System. Molecules, 30(19), 3898. https://doi.org/10.3390/molecules30193898








