Preclinical Evaluation of Puerarin as a Modulator of Metabolic and Inflammatory Parameters in a High-Fat-Diet-Fed Mice Model
Abstract
1. Introduction
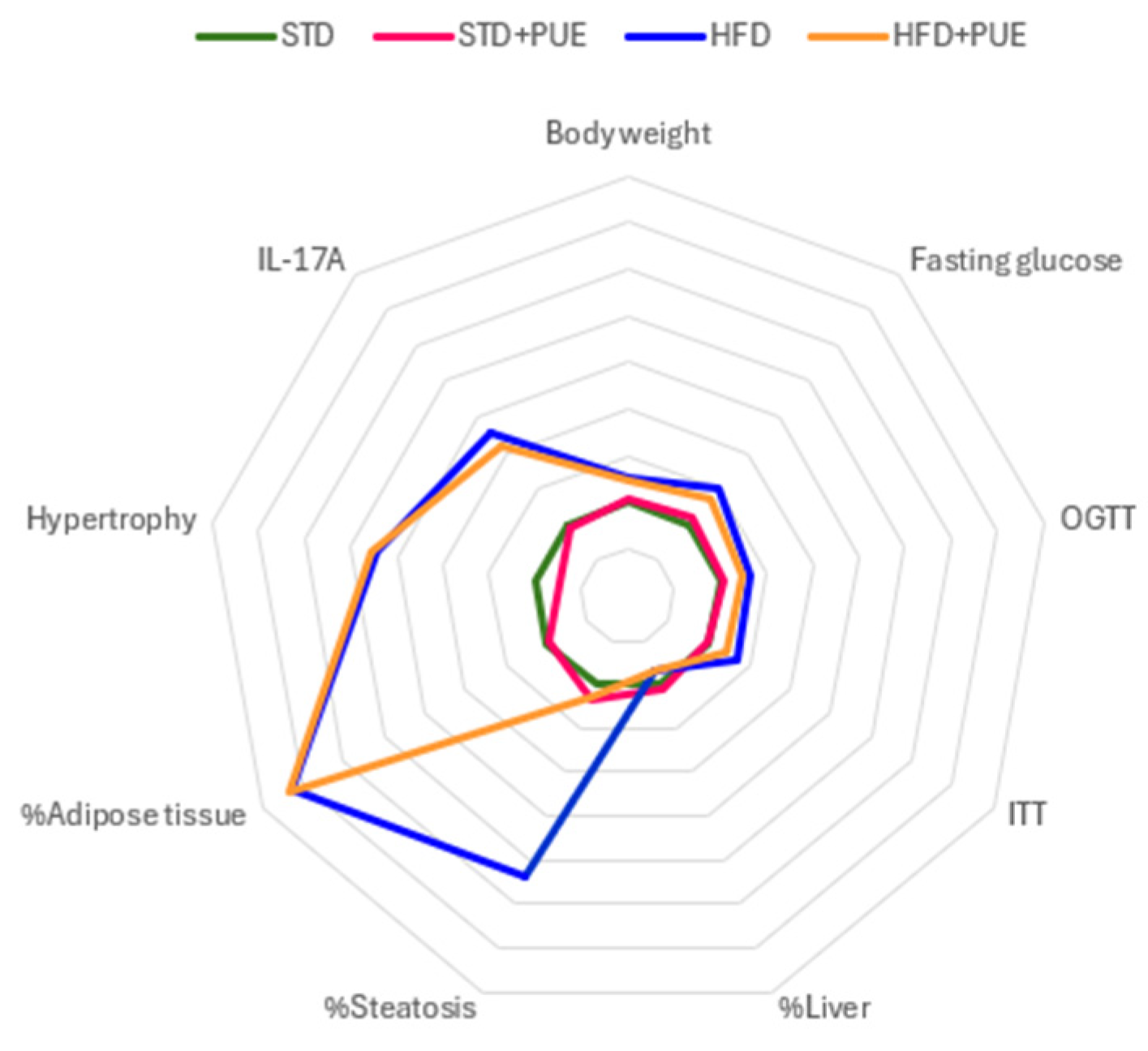
2. Results
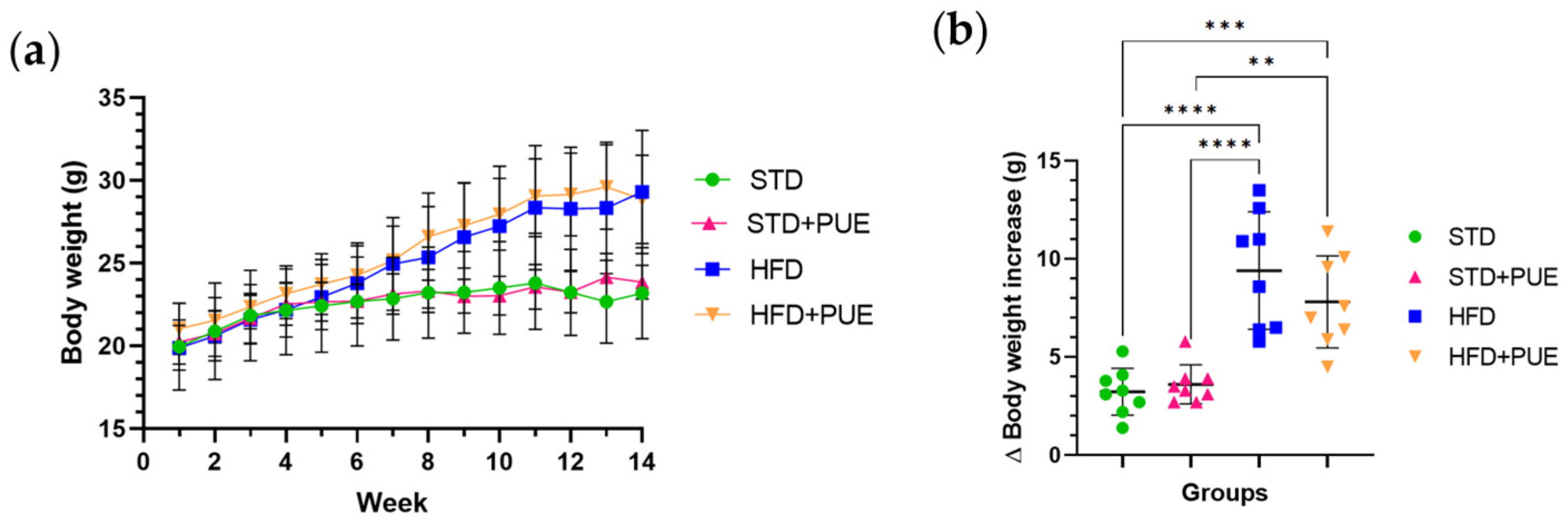
2.1. Effect of Puerarin on Body Weight
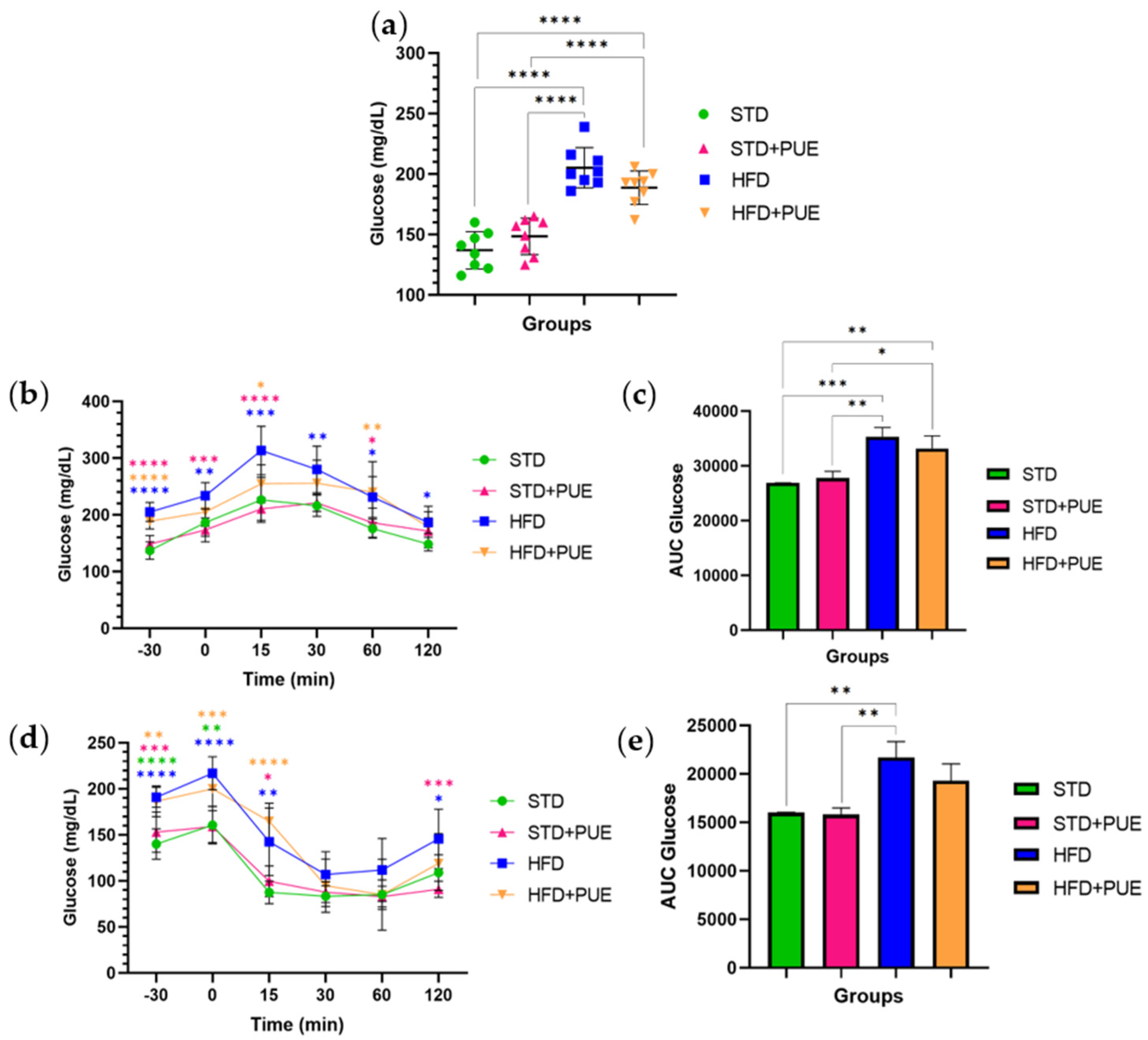
2.2. The Impact of Puerarin on Glucose Metabolism
2.3. Effect of Puerarin on the Liver
2.4. Effect of Puerarin on Adipose Tissue
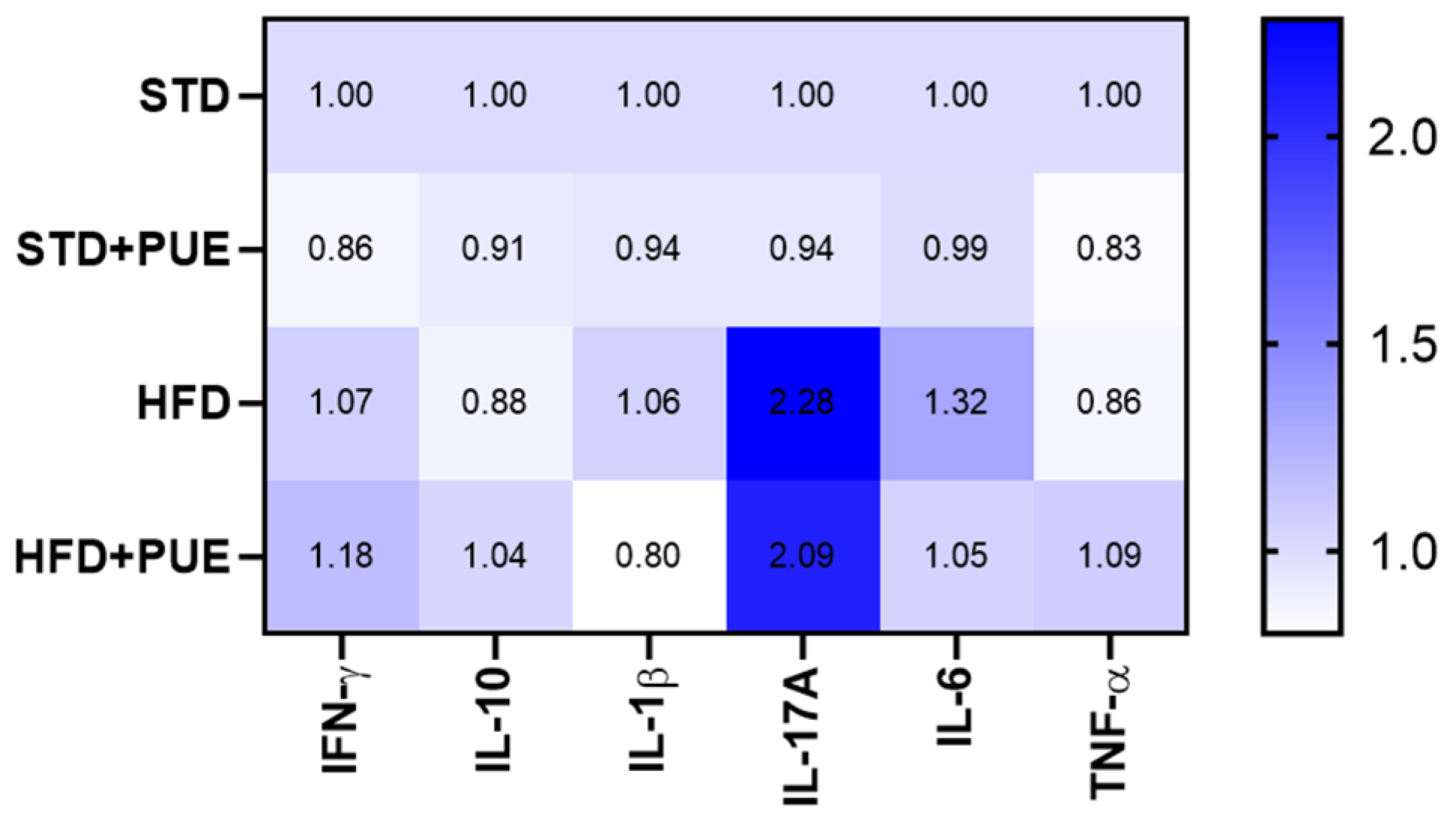
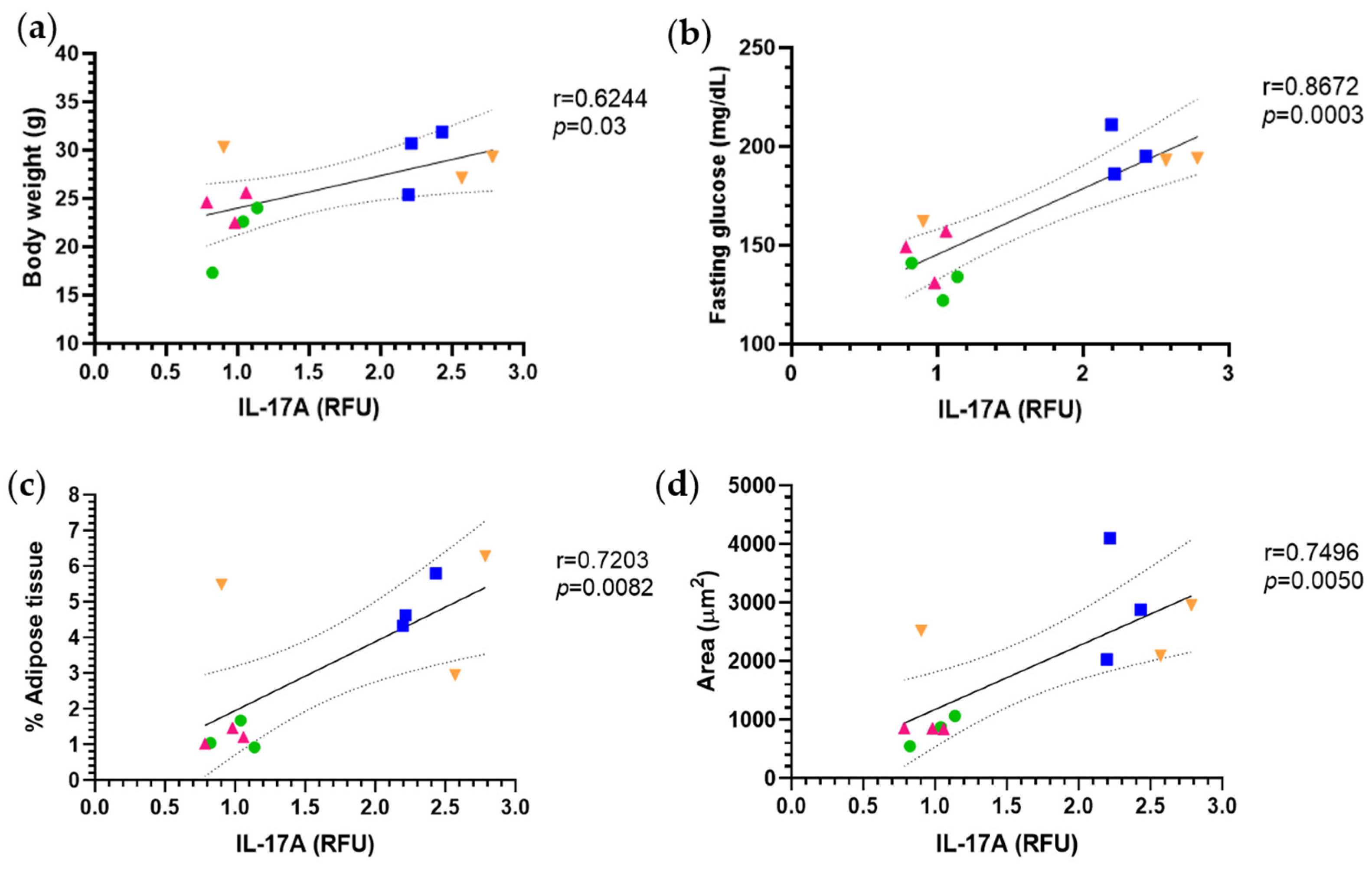
2.5. Anti-Inflammatory Effect of Puerarin
3. Discussion
3.1. Effect of Puerarin on Body Weight and Glucose Metabolism
3.2. Puerarin and the Modulation of Hepatic Lipid Metabolism
3.3. Immunomodulatory Effects and Cytokine Regulation
Limitations and Future Perspectives
4. Materials and Methods
4.1. Puerarin
4.2. Diet
4.3. Animals
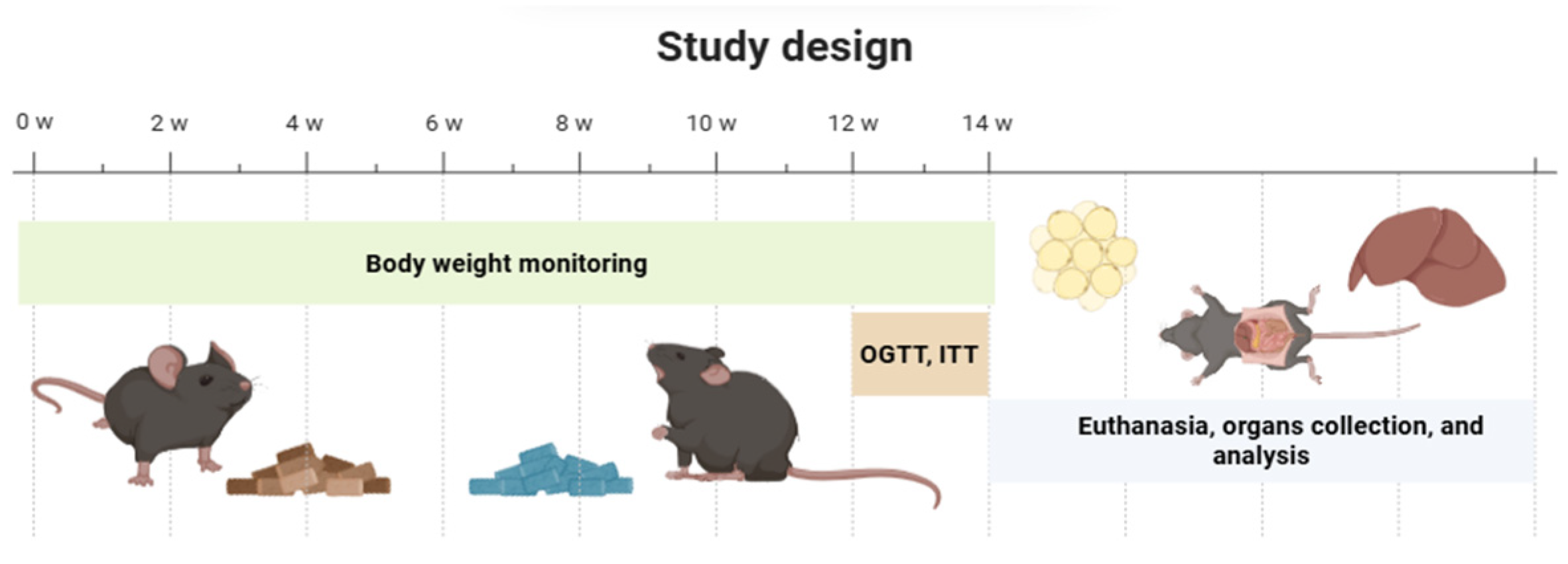
4.4. Experimental Design
4.5. Increased Body Weight and Increased Deposits of Adipose and Liver Tissue
4.6. Fasting Glucose
4.7. Oral Glucose Tolerance Test and Insulin Tolerance Test
4.8. Adipose Tissue and Liver Morphology
4.9. Cytokines
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | Area Under Curve |
| HFD | High-Fat Diet |
| ITT | Insulin Tolerance Test |
| OGTT | Oral Glucose Tolerance Test |
| RFU | Relative Fluorescence Units |
| STD | Standard Diet |
References
- Ruze, R.; Liu, T.; Zou, X.; Song, J.; Chen, Y.; Xu, R.; Yin, X.; Xu, Q. Obesity and Type 2 Diabetes Mellitus: Connections in Epidemiology, Pathogenesis, and Treatments. Front. Endocrinol. 2023, 14, 1161521. [Google Scholar] [CrossRef] [PubMed]
- Villarroya, F.; Cereijo, R.; Gavaldà-Navarro, A.; Villarroya, J.; Giralt, M. Inflammation of Brown/Beige Adipose Tissues in Obesity and Metabolic Disease. J. Intern. Med. 2018, 284, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Chadt, A.; Scherneck, S.; Joost, H.-G.; Al-Hasani, H. Molecular Links Between Obesity and Diabetes: “Diabesity”; MDText.com, Inc.: South Dartmouth, MA, USA, 2018. [Google Scholar]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, A.; Birk, R. Adipose Tissue Hyperplasia and Hypertrophy in Common and Syndromic Obesity—The Case of BBS Obesity. Nutrients 2023, 15, 3445. [Google Scholar] [CrossRef]
- Vega-Robledo, G.B.; Rico-Rosillo, M.G. Tejido Adiposo: Función Inmune y Alteraciones Inducidas Por Obesidad. Rev. Alerg. Mex. 2019, 66, 340–353. [Google Scholar] [CrossRef]
- Engin, A. The Pathogenesis of Obesity-Associated Adipose Tissue Inflammation. In Obesity and Lipotoxicity; Springer: Berlin/Heidelberg, Germany, 2017; pp. 221–245. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose Tissue Inflammation and Metabolic Dysfunction in Obesity. Am. J. Physiol.-Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Rico, D.; Martín Diana, A.B. Nutracéuticos y Alimentos Funcionales Aliados Para La Salud: La Necesidad de Un Diseño “a Medida”. Nutr. Clín. En. Med. 2023, 17, 103–118. [Google Scholar]
- Liu, X.; Huang, R.; Wan, J. Puerarin: A Potential Natural Neuroprotective Agent for Neurological Disorders. Biomed. Pharmacother. 2023, 162, 114581. [Google Scholar] [CrossRef]
- Zhou, Y.-X.; Zhang, H.; Peng, C. Effects of Puerarin on the Prevention and Treatment of Cardiovascular Diseases. Front. Pharmacol. 2021, 12, 771793. [Google Scholar] [CrossRef]
- He, Y.-X.; Liu, M.-N.; Wu, H.; Lan, Q.; Liu, H.; Mazhar, M.; Xue, J.-Y.; Zhou, X.; Chen, H.; Li, Z. Puerarin: A Hepatoprotective Drug from Bench to Bedside. Chin. Med. 2024, 19, 139. [Google Scholar] [CrossRef]
- Liga, S.; Paul, C. Puerarin—A Promising Flavonoid: Biosynthesis, Extraction Methods, Analytical Techniques, and Biological Effects. Int. J. Mol. Sci. 2024, 25, 5222. [Google Scholar] [CrossRef]
- Wan, Q.; Luo, S.; Lu, Q.; Guan, C.; Zhang, H.; Deng, Z. Protective Effects of Puerarin on Metabolic Diseases: Emphasis on the Therapeutical Effects and the Underlying Molecular Mechanisms. Biomed. Pharmacother. 2024, 179, 117319. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, F.; Shang, L.; Zhang, Y.; Zhou, Y.; Shi, X. Puerarin Protects Against High-Fat High-Sucrose Diet-Induced Non-Alcoholic Fatty Liver Disease by Modulating PARP-1/PI3K/AKT Signaling Pathway and Facilitating Mitochondrial Homeostasis. Phytother. Res. 2019, 33, 2347–2359. [Google Scholar] [CrossRef] [PubMed]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef]
- Noh, J.-W.; Yang, H.-K.; Jun, M.-S.; Lee, B.-C. Puerarin Attenuates Obesity-Induced Inflammation and Dyslipidemia by Regulating Macrophages and TNF-Alpha in Obese Mice. Biomedicines 2022, 10, 175. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yao, D.; Yang, H.; Wei, Y.; Peng, Y.; Ding, Y.; Shu, L. Puerarin Protects Pancreatic β-Cells in Obese Diabetic Mice via Activation of GLP-1R Signaling. Mol. Endocrinol. 2016, 30, 361–371. [Google Scholar] [CrossRef]
- Hou, B.-Y.; Zhao, Y.-R.; Ma, P.; Xu, C.-Y.; He, P.; Yang, X.-Y.; Zhang, L.; Qiang, G.-F.; Du, G.-H. Hypoglycemic Activity of Puerarin through Modulation of Oxidative Stress and Mitochondrial Function via AMPK. Chin. J. Nat. Med. 2020, 18, 818–826. [Google Scholar] [CrossRef]
- Jing, X.; Zhou, J.; Zhang, N.; Zhao, L.; Wang, S.; Zhang, L.; Zhou, F. A Review of the Effects of Puerarin on Glucose and Lipid Metabolism in Metabolic Syndrome: Mechanisms and Opportunities. Foods 2022, 11, 3941. [Google Scholar] [CrossRef]
- Rogers, A.B.; Dintzis, R.Z. Hepatobiliary System. In Comparative Anatomy and Histology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 229–239. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, H.; Luo, Y. The Gut-Mediated Function of Polyphenols: Opinions on Functional Food Development for Nonalcoholic Fatty Liver Disease. Curr. Opin. Food Sci. 2023, 49, 100972. [Google Scholar] [CrossRef]
- Jung, H.; Kang, A.; Kang, S.; Park, Y.-K.; Song, M. The Root Extract of Pueraria lobata and Its Main Compound, Puerarin, Prevent Obesity by Increasing the Energy Metabolism in Skeletal Muscle. Nutrients 2017, 9, 33. [Google Scholar] [CrossRef]
- Börgeson, E.; Boucher, J.; Hagberg, C.E. Of Mice and Men: Pinpointing Species Differences in Adipose Tissue Biology. Front. Cell Dev. Biol. 2022, 10, 1003118. [Google Scholar] [CrossRef]
- Li, Z.; Ye, Y.; Lai, S.; Bao, J.; Fu, A. Puerarin Affects Adipose Lipolysis and Ameliorates Obesity by Gut Microbiota. Front. Microbiol. 2025, 16, 1567339. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Xing, X.; Wang, H.; Wan, K.; Fan, R.; Liu, C.; Wang, Y.; Wu, W.; Wang, Y.; Wang, R. SCD1 Is the Critical Signaling Hub to Mediate Metabolic Diseases: Mechanism and the Development of Its Inhibitors. Biomed. Pharmacother. 2024, 170, 115586. [Google Scholar] [CrossRef]
- Lee, O.; Seo, D.; Park, C.; Kim, Y. Puerarin Enhances Adipocyte Differentiation, Adiponectin Expression, and Antioxidant Response in 3T3-L1 Cells. BioFactors 2010, 36, 459–467. [Google Scholar] [CrossRef]
- Williamson, G. Possible Effects of Dietary Polyphenols on Sugar Absorption and Digestion. Mol. Nutr. Food Res. 2013, 57, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, L.; Wu, Y.; Song, S.; Min, H.; Yang, Y.; He, X.; Liang, Q.; Yi, L.; Wang, Y.; et al. Effect of Puerarin in Promoting Fatty Acid Oxidation by Increasing Mitochondrial Oxidative Capacity and Biogenesis in Skeletal Muscle in Diabetic Rats. Nutr. Diabetes 2018, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-W.; Wei, L.-N. Innate Immunity Orchestrates Adipose Tissue Homeostasis. Horm. Mol. Biol. Clin. Investig. 2017, 31, 20170013. [Google Scholar] [CrossRef]
- Huang, L.-Y.; Chiu, C.-J.; Hsing, C.-H.; Hsu, Y.-H. Interferon Family Cytokines in Obesity and Insulin Sensitivity. Cells 2022, 11, 4041. [Google Scholar] [CrossRef]
- Qu, Y.; Zhang, Q.; Ma, S.; Liu, S.; Chen, Z.; Mo, Z.; You, Z. Interleukin-17A Differentially Induces Inflammatory and Metabolic Gene Expression in the Adipose Tissues of Lean and Obese Mice. Int. J. Mol. Sci. 2016, 17, 522. [Google Scholar] [CrossRef]
- Gomes, A.L.; Teijeiro, A.; Burén, S.; Tummala, K.S.; Yilmaz, M.; Waisman, A.; Theurillat, J.-P.; Perna, C.; Djouder, N. Metabolic Inflammation-Associated IL-17A Causes Non-Alcoholic Steatohepatitis and Hepatocellular Carcinoma. Cancer Cell 2016, 30, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, Y.; Zhang, J.; Shi, H.; Jiang, R.; Guo, M.; Liu, Y.; Liu, B.; Wang, N.; Ma, R.; et al. Puerarin Attenuates Insulin Resistance by Inhibiting Endoplasmic Reticulum Stress and Suppresses Inflammation by Modulating the JNK and IKKβ/NF-κB Pathways in Epididymal White Adipose Tissue of Mice on a High-Fat Diet. Mol. Nutr. Food Res. 2024, 68, 2400003. [Google Scholar] [CrossRef]
- de Souza, G.O.; Wasinski, F.; Donato, J. Characterization of the Metabolic Differences between Male and Female C57BL/6 Mice. Life Sci. 2022, 301, 120636. [Google Scholar] [CrossRef]
- NOM-062-ZOO-1999; Especificaciones Técnicas para la Producción, Cuidado y Uso de los Animales de Laboratorio. Gobierno de Mexico: Ciudad de Mexico, Mexico, 1999.
- Liang, T.; Xu, X.; Ye, D.; Chen, W.; Gao, B.; Huang, Y. Caspase/AIF/Apoptosis Pathway: A New Target of Puerarin for Diabetes Mellitus Therapy. Mol. Biol. Rep. 2019, 46, 4787–4797. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shangguan, Z.; Liu, Y.; Wang, J.; Li, X.; Yang, S.; Liu, S. Puerarin Protects Pancreatic β-Cell Survival via PI3K/Akt Signaling Pathway. J. Mol. Endocrinol. 2014, 53, 71–79. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Yu, L.; Chen, J. The Puerarin Improves Renal Function in STZ-Induced Diabetic Rats by Attenuating ENOS Expression. Ren. Fail. 2015, 37, 699–703. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, C.-Q.; Wang, P.-W.; Sun, S.-Y.; Su, W.-J.; Zhang, H.-J.; Li, X.-J.; Yang, S.-Y. Puerarin Improves Insulin Resistance and Modulates Adipokine Expression in Rats Fed a High-Fat Diet. Eur. J. Pharmacol. 2010, 649, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical Power Analyses Using G*Power 3.1: Tests for Correlation and Regression Analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camacho-Padilla, L.G.; Zepeda-Morales, A.S.M.; Arrizon, J.; Flores-Soto, M.E.; Zepeda-Nuño, J.S.; Carrera-Quintanar, L.; Herrera-González, A.; Aparicio-García, G.; López-Roa, R.I. Preclinical Evaluation of Puerarin as a Modulator of Metabolic and Inflammatory Parameters in a High-Fat-Diet-Fed Mice Model. Molecules 2025, 30, 3895. https://doi.org/10.3390/molecules30193895
Camacho-Padilla LG, Zepeda-Morales ASM, Arrizon J, Flores-Soto ME, Zepeda-Nuño JS, Carrera-Quintanar L, Herrera-González A, Aparicio-García G, López-Roa RI. Preclinical Evaluation of Puerarin as a Modulator of Metabolic and Inflammatory Parameters in a High-Fat-Diet-Fed Mice Model. Molecules. 2025; 30(19):3895. https://doi.org/10.3390/molecules30193895
Chicago/Turabian StyleCamacho-Padilla, Luisa Guadalupe, Adelaida Sara Minia Zepeda-Morales, Javier Arrizon, Mario Eduardo Flores-Soto, José Sergio Zepeda-Nuño, Lucrecia Carrera-Quintanar, Azucena Herrera-González, Gerardo Aparicio-García, and Rocío Ivette López-Roa. 2025. "Preclinical Evaluation of Puerarin as a Modulator of Metabolic and Inflammatory Parameters in a High-Fat-Diet-Fed Mice Model" Molecules 30, no. 19: 3895. https://doi.org/10.3390/molecules30193895
APA StyleCamacho-Padilla, L. G., Zepeda-Morales, A. S. M., Arrizon, J., Flores-Soto, M. E., Zepeda-Nuño, J. S., Carrera-Quintanar, L., Herrera-González, A., Aparicio-García, G., & López-Roa, R. I. (2025). Preclinical Evaluation of Puerarin as a Modulator of Metabolic and Inflammatory Parameters in a High-Fat-Diet-Fed Mice Model. Molecules, 30(19), 3895. https://doi.org/10.3390/molecules30193895








