Diastereoselective Reformatsky Reaction Mediated by Dichlorocyclopentadienyltitanium(III)
Abstract
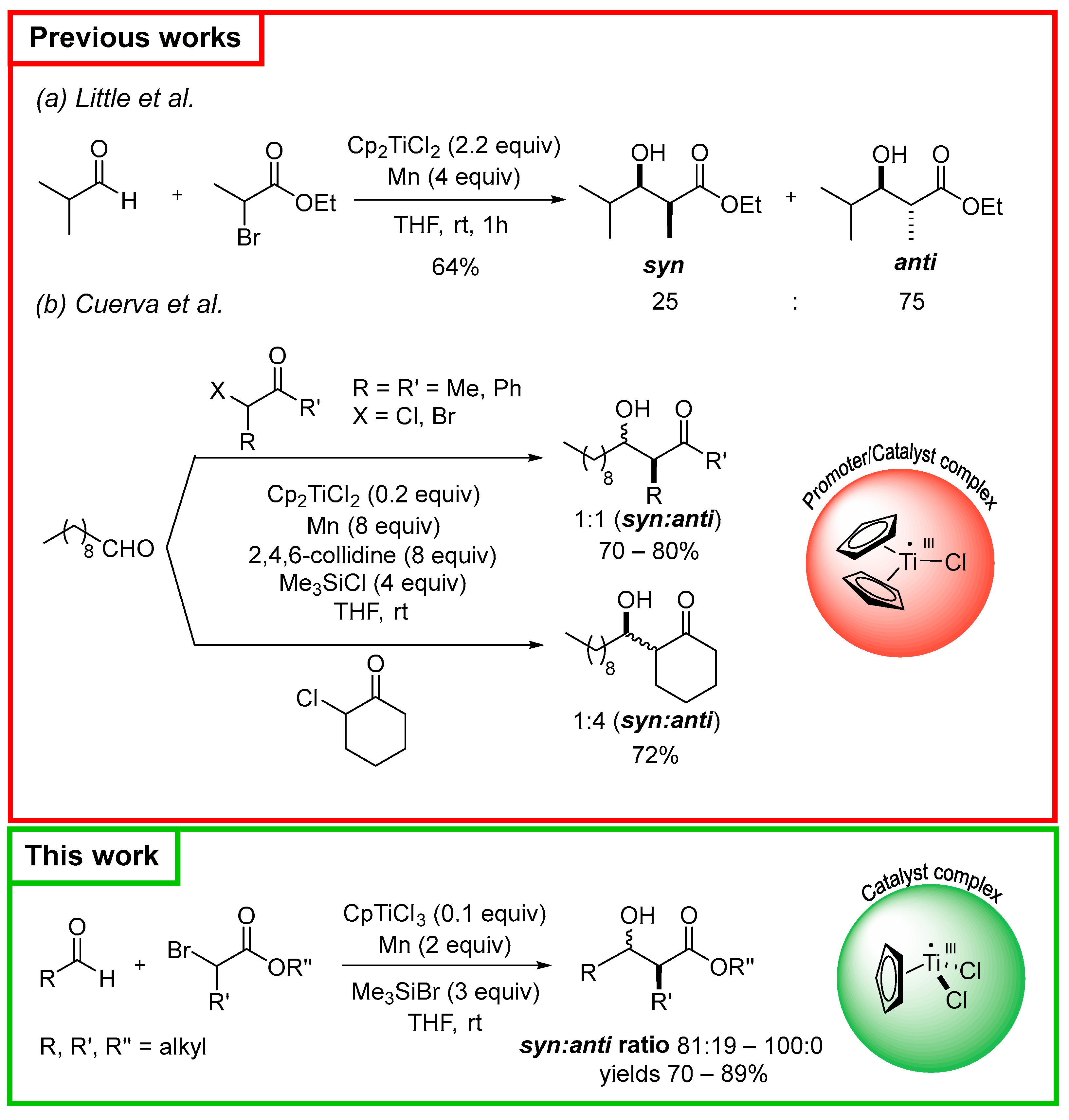
1. Introduction
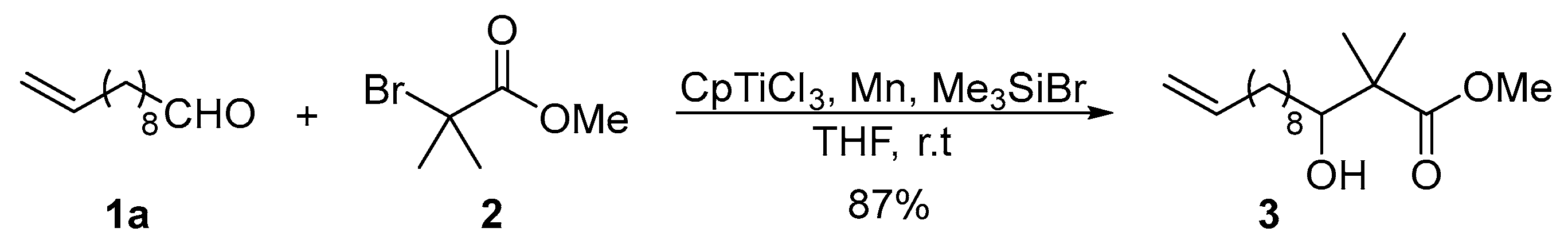
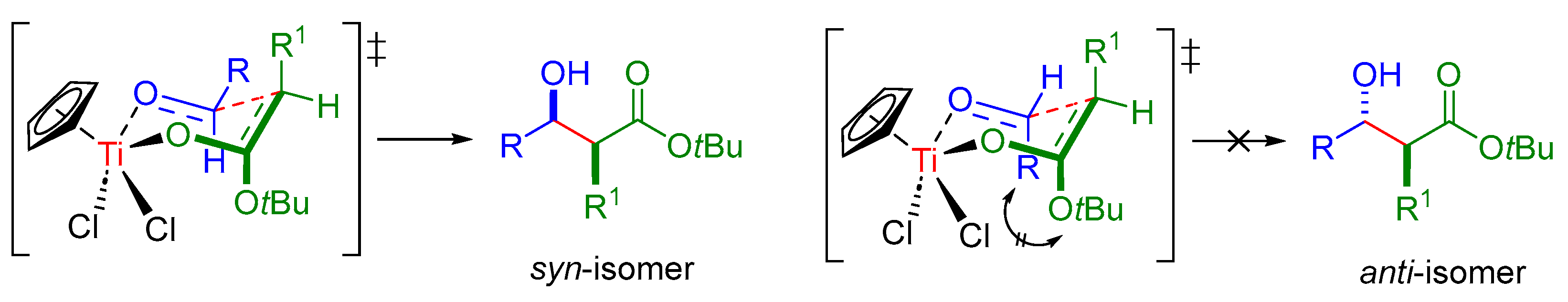
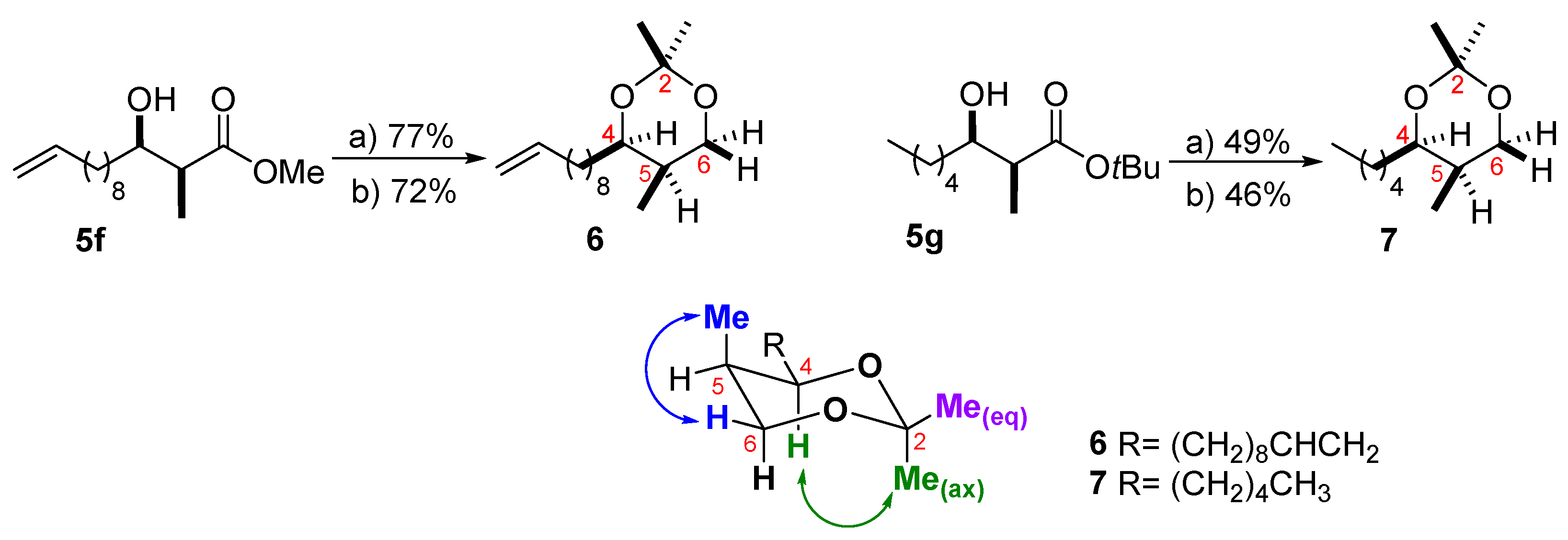
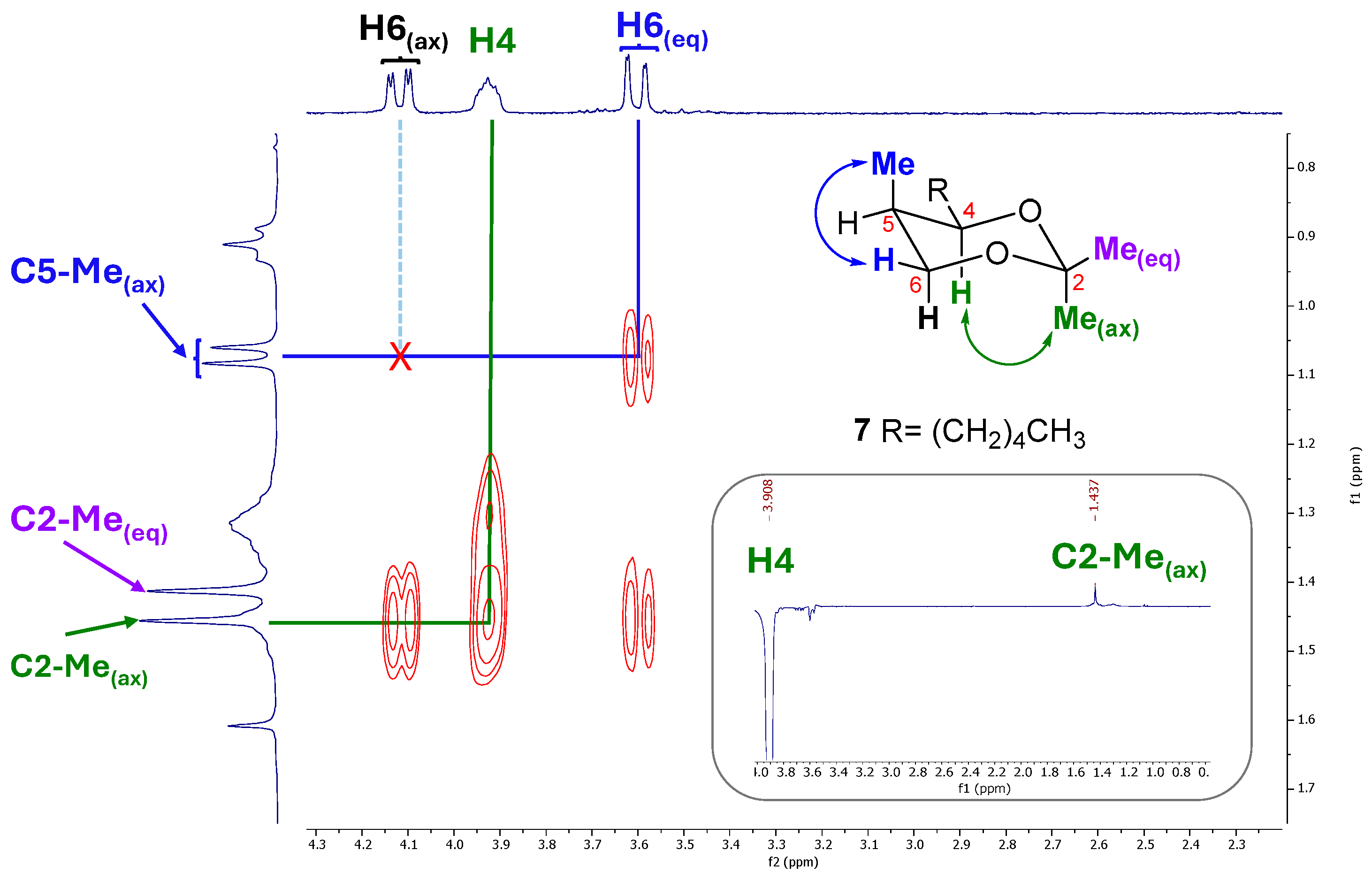
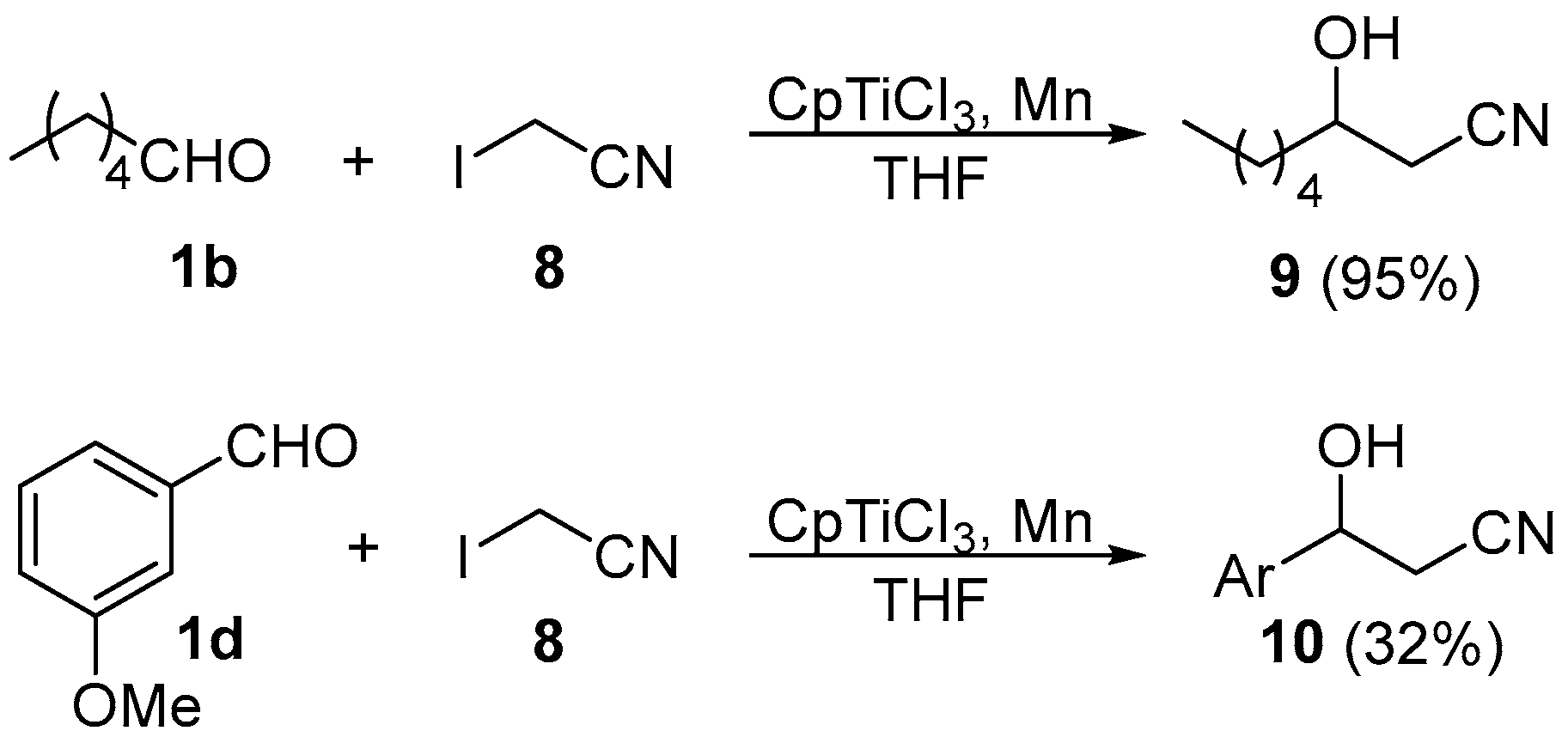
2. Results and Discussion
3. Materials and Methods
3.1. General Remarks
3.2. General Procedure A for Ti-Catalyzed Reformatsky Reaction
3.3. General Procedure B for Ti-Induced Reformatsky Reaction
3.4. Reduction of the β-Hydroxy Esters
3.5. Synthesis of the Ketal Derivatives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reformatsky, S. Neue Synthese zweiatomiger einbasischer Säuren aus den Ketonen. Berichte Dtsch. Chem. Ges. 1887, 20, 1210–1211. [Google Scholar] [CrossRef]
- Lewis, D.E. Early Organic Chemistry in Kyiv: Serhii Mykolayovych Reformatskyi (1860–1934) and his Name Reaction*. ChemPlusChem 2023, 88, e202300224. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Li, Z.; Zhang, T.; Xu, Y.; Li, Y.; Wu, B.; Shen, Q. Revitalizing reformatsky reagent for catalyst-free direct alkylation with unactivated alkyl halides. Nat. Commun. 2025, 16, 7627. [Google Scholar] [CrossRef]
- Choppin, S.; Ferreiro-Medeiros, L.; Barbarotto, M.; Colobert, F. Recent advances in the diastereoselective Reformatsky-type reaction. Chem. Soc. Rev. 2013, 42, 937–949. [Google Scholar] [CrossRef]
- Pellissier, H. Recent developments in the asymmetric Reformatsky-type reaction. Beilstein J. Org. Chem. 2018, 14, 325–344. [Google Scholar] [CrossRef]
- Cozzi, P.G. Reformatsky reactions meet catalysis and stereoselectivity. Angew. Chem. Int. Ed. 2007, 46, 2568–2571. [Google Scholar] [CrossRef]
- Cozzi, P.G.; Mignogna, A.; Zoli, L. Catalytic enantioselective Reformatsky reactions. Pure Appl. Chem. 2008, 80, 891–901. [Google Scholar] [CrossRef]
- Lv, Y.-F.; Liu, G.; Shi, Z.; Wang, Z. Chromium Catalyzed Asymmetric Reformatsky Reaction. Angew. Chem. Int. Ed. 2024, 63, e202406109. [Google Scholar] [CrossRef] [PubMed]
- Biallas, P.; Yamazaki, K.; Dixon, D.J. Difluoroalkylation of Tertiary Amides and Lactams by an Iridium-Catalyzed Reductive Reformatsky Reaction. Org. Lett. 2022, 24, 2002–2007. [Google Scholar] [CrossRef]
- Pal, D.; Veeranna, K.D.; Wong, Y.F.; Evans, P.A. Enantioselective iridium-catalyzed allylic substitution with a Reformatsky reagent: Direct construction of β-stereogenic homoallylic esters. Sci. China Chem. 2024, 67, 3791–3797. [Google Scholar] [CrossRef]
- Carny, T.; Mravcova, D.; Steinhublova, B.; Sebesta, R. Mechanochemical Pd-Catalyzed Cross-Coupling of In-Situ Generated Reformatsky Zn-Enolates. Adv. Synth. Catal. 2025, 367, e202401403. [Google Scholar] [CrossRef]
- Li, Z.; Huang, J.; Ni, Z.; Sun, R.; Nie, H.; Tang, H.; Song, L.; Wu, F. Indium-Mediated Reformatsky Reaction of Iododifluoro Ketones with Aldehydes: Preparation of α,α-Difluoro-β-hydroxyketone Derivatives in Water. SynOpen 2022, 6, 19–30. [Google Scholar] [CrossRef]
- Kagoshima, H.; Hashimoto, Y.; Oguro, D.; Saigo, K. An Activated Germanium Metal-Promoted, Highly Diastereoselective Reformatsky Reaction. J. Org. Chem. 1998, 63, 691–697. [Google Scholar] [CrossRef]
- Guo, G.; Zhou, J.; He, X.; Li, N.; Lin, N.; Zhang, X.; Lian, Z. Elemental Germanium Activation and Catalysis Enabled by Mechanical Force. Angew. Chem. Int. Ed. 2025, 64, e202421446. [Google Scholar] [CrossRef]
- Mahasneh, A.S. Tin(II) chloride-mediated addition reaction of bromonitromethane to aldehydes. Z. Naturforsch. B Chem. Sci. 2005, 60, 416–418. [Google Scholar] [CrossRef]
- Shibata, I.; Suwa, T.; Sakakibara, H.; Baba, A. Highly diastereoselective Reformatskii-type reaction promoted by tin iodide ate complex. Org. Lett. 2002, 4, 301–303. [Google Scholar] [CrossRef]
- Ribeiro, C.M.R.; Cordeiro de Farias, F.M. Chiral ligands in the asymmetric Reformatsky reaction. Mini-Rev. Org. Chem. 2006, 3, 1–10. [Google Scholar] [CrossRef]
- Barik, R.; Das, J.; Nanda, S. Synthetic studies towards naturally occurring sesquiterpene capillosanane V: Construction of a fully functionalized cycloheptane core through an intramolecular Reformatsky reaction. Org. Biomol. Chem. 2025, 23, 1696–1707. [Google Scholar] [CrossRef]
- Chen, Q.-L.; Mao, L.; Pan, Y.-F.; Cai, H.; Zhang, X.-M.; Zhang, F.-M.; Ma, A.-J.; Peng, J.-B.; Tu, Y.-Q. Photoinduced reductive Reformatsky reaction of α-haloesters and aldehydes or ketones by cooperative dual-metal catalysis. Chem. Commun. 2023, 59, 14427–14430. [Google Scholar] [CrossRef]
- Saeed, S.; Zahoor, A.F.; Ahmad, S.; Akhtar, R.; Sikandar, S. Reformatsky reaction as a key step in the synthesis of natural products: A review. Synth. Commun. 2022, 52, 317–345. [Google Scholar] [CrossRef]
- Rosales Martínez, A.; Pozo Morales, L.; Díaz Ojeda, E.; Castrp Rodriguez, M.C.; Rodríguez-García, I. The Proven Versatility of Cp2TiCl. J. Org. Chem. 2021, 86, 1311–1329. [Google Scholar] [CrossRef]
- Castro Rodriguez, M.; Rodriguez Garcia, I.; Rodriguez Maecker, R.N.; Pozo Morales, L.; Oltra, J.E.; Rosales Martinez, A. Cp2TiCl: An Ideal Reagent for Green Chemistry? Org. Process Res. Dev. 2017, 21, 911–923. [Google Scholar] [CrossRef]
- Saito, T.; Nishiyama, H.; Tanahashi, H.; Kawakita, K.; Tsurugi, H.; Mashima, K. 1,4-Bis(trimethylsilyl)-1,4-diaza-2,5-cyclohexadienes as Strong Salt-Free Reductants for Generating Low-Valent Early Transition Metals with Electron-Donating Ligands. J. Am. Chem. Soc. 2014, 136, 5161–5170. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Leng, D.; Wang, Z.; Wang, X.; Gao, Z. Titanocene Complexes Applied in Organic Transformations. Catalysts 2025, 15, 779. [Google Scholar] [CrossRef]
- Streuff, J. A titanium(III)-catalyzed redox umpolung reaction for the reductive cross-coupling of enones with acrylonitriles. Chem. Eur. J. 2011, 17, 5507–5510. [Google Scholar] [CrossRef] [PubMed]
- Streuff, J. Reductive umpolung reactions with low-valent titanium catalysts. Chem. Rec. 2014, 14, 1100–1113. [Google Scholar] [CrossRef]
- Meloche, J.L.; Vednor, P.T.; Gianino, J.B.; Oliver, A.G.; Ashfeld, B.L. Titanocene-catalyzed metallation of propargylic acetates in homopropargyl alcohol synthesis. Tetrahedron Lett. 2014, 55, 5025–5028. [Google Scholar] [CrossRef]
- Fleury, L.M.; Kosal, A.D.; Masters, J.T.; Ashfeld, B.L. Cooperative Titanocene and Phosphine Catalysis: Accelerated C-X Activation for the Generation of Reactive Organometallics. J. Org. Chem. 2013, 78, 253–269. [Google Scholar] [CrossRef]
- Gianino, J.B.; Campos, C.A.; Lepore, A.J.; Pinkerton, D.M.; Ashfeld, B.L. Redox and Lewis Acid Relay Catalysis: A Titanocene/Zinc Catalytic Platform in the Development of Multicomponent Coupling Reactions. J. Org. Chem. 2014, 79, 12083–12095. [Google Scholar] [CrossRef]
- Rosales Martínez, A.; Rodríguez-García, I. Photochemical water splitting via transition metal complexes. J. Catal. 2024, 432, 115415. [Google Scholar] [CrossRef]
- Parrish, J.D.; Shelton, D.R.; Little, R.D. Titanocene(III)-Promoted Reformatsky Additions. Org. Lett. 2003, 5, 3615–3617. [Google Scholar] [CrossRef]
- Sgreccia, L.; Bandini, M.; Morganti, S.; Quintavalla, A.; Umani-Ronchi, A.; Cozzi, P.G. Titanium-catalyzed Reformatsky-type reaction. J. Organomet. Chem. 2007, 692, 3191–3197. [Google Scholar] [CrossRef]
- Estevez, R.E.; Paradas, M.; Millan, A.; Jimenez, T.; Robles, R.; Cuerva, J.M.; Oltra, J.E. Ti-Catalyzed Reformatsky-Type Coupling between α-Halo Ketones and Aldehydes. J. Org. Chem. 2008, 73, 1616–1619. [Google Scholar] [CrossRef]
- Roldan-Molina, E.; Padial, N.M.; Lezama, L.; Oltra, J.E. CpTiCl2, an Improved Titanocene(III) Catalyst in Organic Synthesis. Eur. J. Org. Chem. 2018, 2018, 5997–6001. [Google Scholar] [CrossRef]
- López-Martínez, J.L.; Torres-García, I.; Rodríguez-García, I.; Muñoz-Dorado, M.; Álvarez-Corral, M. Stereoselective Barbier-Type Allylations and Propargylations Mediated by CpTiCl3. J. Org. Chem. 2019, 84, 806–816. [Google Scholar] [CrossRef]
- Torres-García, I.; López-Martínez, J.L.; Martínez-Martínez, R.; Oltra, J.E.; Muñoz-Dorado, M.; Rodríguez-García, I.; Álvarez-Corral, M. The half-sandwich titanocene CpTiIIICl2 as efficient system for the preparation of 2,5-dihydrofurans via α-allenols. Appl. Organomet. Chem. 2020, 34, e5244. [Google Scholar] [CrossRef]
- Gansaeuer, A. Pinacol coupling of aromatic aldehydes catalyzed by a titanocene complex: A transition metal catalyzed radical reaction. Chem. Commun. 1997, 457–458. [Google Scholar] [CrossRef]
- Mulzer, M.; Whiting, B.T.; Coates, G.W. Regioselective Carbonylation of trans-Disubstituted Epoxides to β-Lactones: A Viable Entry into syn-Aldol-Type Products. J. Am. Chem. Soc. 2013, 135, 10930–10933. [Google Scholar] [CrossRef]
- Sone, H.; Kondo, T.; Kiryu, M.; Ishiwata, H.; Ojika, M.; Yamada, K. Dolabellin, a Cytotoxic Bisthiazole Metabolite from the Sea Hare Dolabella auricularia: Structural Determination and Synthesis. J. Org. Chem. 1995, 60, 4774–4781. [Google Scholar] [CrossRef]
- Nagai, H.; Morita, Y.; Shimizu, Y.; Kanai, M. Ligand-Promoted, Boron-Mediated Chemoselective Carboxylic Acid Aldol Reaction. Org. Lett. 2016, 18, 2276–2279. [Google Scholar] [CrossRef]
- Nishiyama, H.; Shiomi, T.; Tsuchiya, Y.; Matsuda, I. High Performance of Rh(Phebox) Catalysts in Asymmetric Reductive Aldol Reaction: High Anti-Selectivity. J. Am. Chem. Soc. 2005, 127, 6972–6973. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, S.; Kanai, M.; Shibasaki, M. Novel Multiaction of Zr Catalyst: One-Pot Synthesis of β-Cyanohydrins from Olefins. J. Am. Chem. Soc. 2001, 123, 1256–1257. [Google Scholar] [CrossRef]
- Kamila, S.; Zhu, D.; Biehl, E.R.; Hua, L. Unexpected Stereorecognition in Nitrilase-Catalyzed Hydrolysis of β-Hydroxy Nitriles. Org. Lett. 2006, 8, 4429–4431. [Google Scholar] [CrossRef]
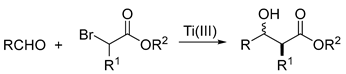 | |||||
|---|---|---|---|---|---|
| Entry | Aldehyde | Bromoester | Hydroxyester | Syn:Anti Ratio | Yield (c) |
| 1 (a) | 1a | 4a R1 = R2 = Me |  | 82:18 | 89% |
| 2 (a) | 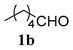 | 4a R1 = R2 = Me |  | 84:16 | 72% |
| 3 (a) |  | 4a R1 = R2 = Me |  | 81:19 | 70% |
| 4 (a) | 1a | 4b R1 = iPr R2 = Et |  | 83:17 | 71% |
| 5 (a) | 1b | 4b R1 = iPr R2 = Et |  | 82:18 | 79% |
| 6 (a) | 1a | 4c R1 = Me R2 = tBu |  | 100:0 | 78% |
| 7 (a) | 1b | 4c R1 = Me R2 = tBu |  | 100:0 | 80% |
| 8 (b) | 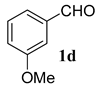 | 4c R1 = Me R2 = tBu | 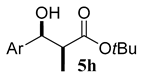 | 100:0 | 78% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Martínez, J.L.; Torres-García, I.; Muñoz-Dorado, M.; Álvarez-Corral, M.; Rodríguez-García, I. Diastereoselective Reformatsky Reaction Mediated by Dichlorocyclopentadienyltitanium(III). Molecules 2025, 30, 3893. https://doi.org/10.3390/molecules30193893
López-Martínez JL, Torres-García I, Muñoz-Dorado M, Álvarez-Corral M, Rodríguez-García I. Diastereoselective Reformatsky Reaction Mediated by Dichlorocyclopentadienyltitanium(III). Molecules. 2025; 30(19):3893. https://doi.org/10.3390/molecules30193893
Chicago/Turabian StyleLópez-Martínez, Josefa L., Irene Torres-García, Manuel Muñoz-Dorado, Miriam Álvarez-Corral, and Ignacio Rodríguez-García. 2025. "Diastereoselective Reformatsky Reaction Mediated by Dichlorocyclopentadienyltitanium(III)" Molecules 30, no. 19: 3893. https://doi.org/10.3390/molecules30193893
APA StyleLópez-Martínez, J. L., Torres-García, I., Muñoz-Dorado, M., Álvarez-Corral, M., & Rodríguez-García, I. (2025). Diastereoselective Reformatsky Reaction Mediated by Dichlorocyclopentadienyltitanium(III). Molecules, 30(19), 3893. https://doi.org/10.3390/molecules30193893







