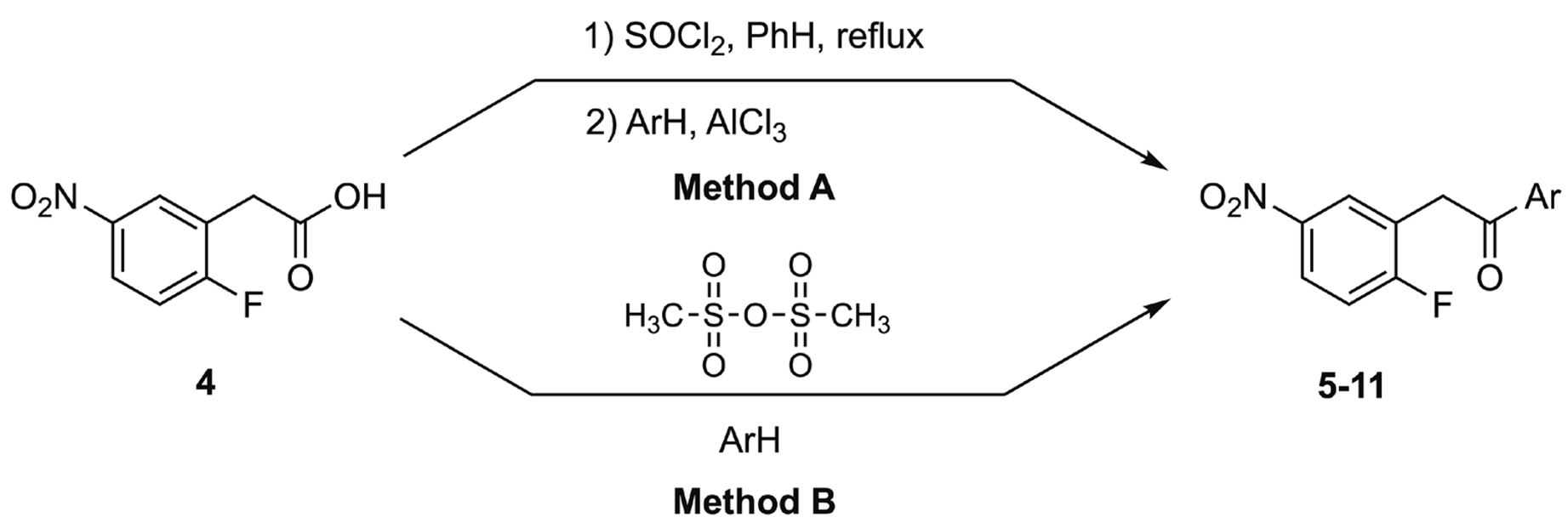
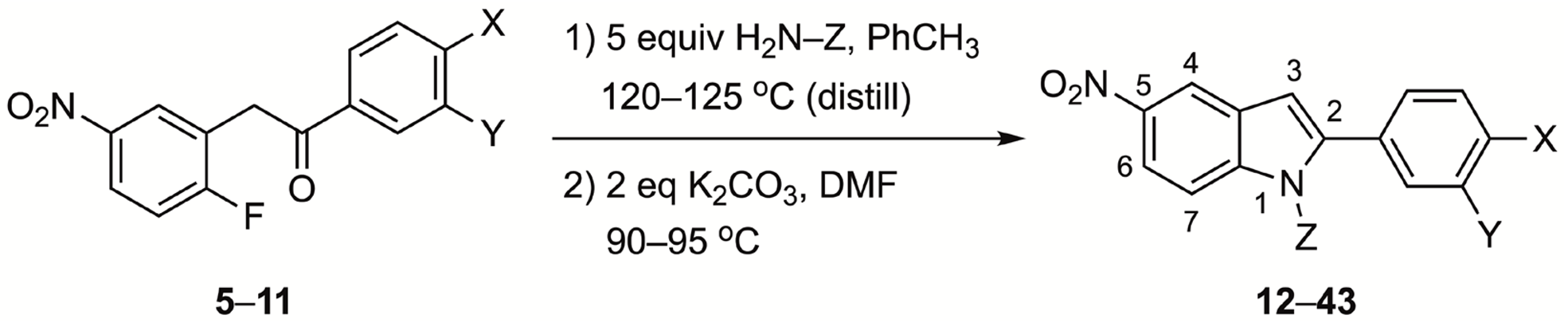3.3. Representative Procedure to Prepare 1H-Indoles
Method C: A solution of the deoxybenzoin (0.1 g, 1.0 equiv) and the benzyl- or phenethylamine, the high-boiling aliphatic amine or the aniline derivative (5 equiv) in toluene (8 mL) was heated in a silicone oil bath at 120–125 °C for 18–24 h with slow distillation of the toluene. [Note: In addition to TLC indicating reaction was complete, droplets of water were observed in the condenser after all the toluene was removed.] The last traces of toluene and excess amine were carefully removed under high vacuum. The resulting residue, in each case, was dissolved in DMF (4 mL), anhydrous K2CO3 (2 equiv) was added, and the mixture was stirred at 90–95 °C for 18–24 h. The crude reaction mixture was added to saturated aqueous NaCl (25 mL) and extracted with EtOAc (2 × 20 mL). The combined organic extracts were washed with saturated aqueous NaCl (3 × 20 mL) and then dried (MgSO4) and concentrated onto silica gel (2–3 g) under vacuum. The silica was added to the top of a 40 cm × 2 cm silica gel column and eluted with 2–5% EtOAc in hexane to remove unreacted amine and any benzofuran product. This was followed by elution with 10–25% EtOAc in hexane to give the 1H-indole product. Concentration of the eluent under vacuum gave a light yellow solid or oil which crystallized upon addition of 5% ether in hexane. Filtration of the solid gave the following 1H-indoles.
Method D: For volatile amines and two of the anilines, a mixture of the deoxybenzoin and the amine (1:10 equiv, respectively) were heated at the indicated temperature. Workup and purification were as described for Method C.
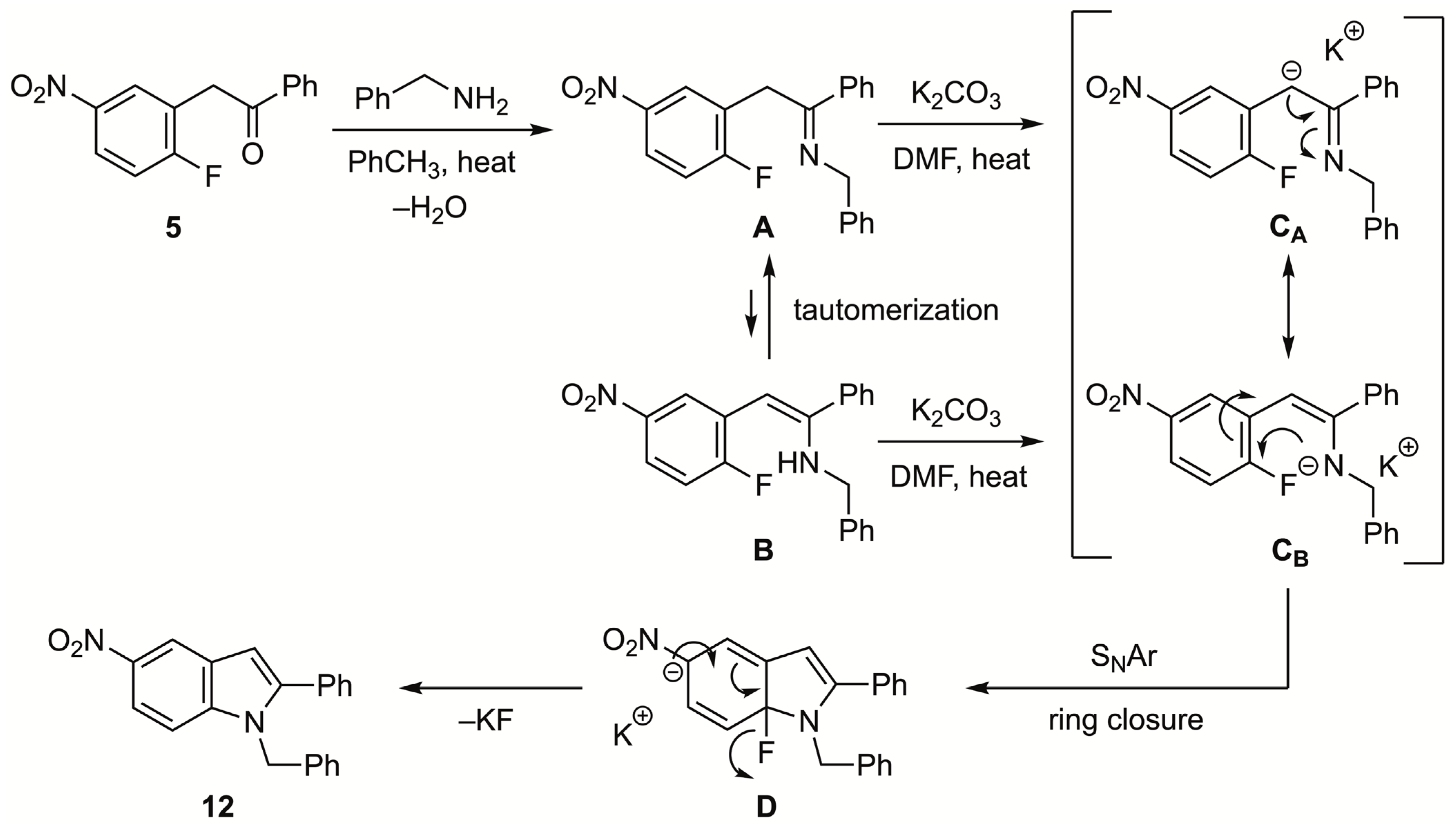
3.3.1. 1-Benzyl-5-nitro-2-phenyl-1H-indole (12)
Method C: Deoxybenzoin
5 (1 equiv) with benzylamine (5 equiv) gave indole
12 (101 mg, 80%) as a yellow solid, mp 123–124 °C; IR: 1523, 1344 cm
−1;
1H NMR (400 MHz, CDCl
3): δ 8.62 (d,
J = 2.2 Hz, 1H), 8.05 (dd,
J = 9.0, 2.2 Hz, 1H), 7.43 (s, 5H), 7.32–7.24 (complex, 3H), 7.20 (d,
J = 9.0 Hz, 1H), 6.97 (m, 2H), 6.80 (d,
J = 0.8 Hz, 1H), 5.41 (s, 2H);
13C {
1H} NMR (101 MHz, CDCl
3): δ 145.0, 142.1, 140.6, 136.9, 131.4, 129.3, 129.0, 128.9, 128.8, 127.7, 127.5, 125.8, 117.7, 117.5, 109.4, 104.4, 48.1; these NMR data matched those reported in the literature [
27]; MS (
m/
z): 328; Anal. Calcd for C
21H
16N
2O
2: C, 76.81; H, 4.91; N, 8.53; Found: C, 76.57; H, 4.89; N, 8.48.
3.3.2. 1-(3-Methoxybenzyl)-5-nitro-2-phenyl-1H-indole (13)
Method C: Deoxybenzoin 5 (1 equiv) with 3-methoxybenzylamine (5 equiv) gave indole 13 (114 mg, 83%) as a yellow solid, mp 126–127 °C; IR: 2838, 1520, 1336 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.62 (d, J = 2.2 Hz, 1H), 8.06 (dd, J = 9.0, 2.2 Hz, 1H), 7.46–7.42 (complex, 5H), 7.21 (m, 2H), 6.80 (coincident m, 1H and d, J = 0.8 Hz, 1H), 6.57 (d, J = 7.6 Hz, 1H), 6.51 (s, 1H) 5.37 (s, 2H), 3.71 (s, 3H); 13C {1H} NMR (101 MHz, CDCl3): δ 160.1, 145.0, 142.2, 140.7, 138.6, 132.4, 130.1, 129.3, 129.0, 128.8, 127.5, 118.1, 117.7, 117.6, 112.7, 111.9, 110.4, 104.4, 55.2, 48.0; MS (m/z): 358; Anal. Calcd for C22H18N2O3: C, 73.73; H, 5.06; N, 7.82; Found: C, 73.61; H, 5.02; N, 7.76.
3.3.3. 5-Nitro-2-phenyl-1-(3-(trifluoromethyl)phenyl)-1H-indole (14)
Method C: Deoxybenzoin 5 (1 equiv) with 3-(trifluoromethyl)benzylamine (5 equiv) gave indole 14 (103 mg, 67%) as a yellow solid, mp 128–129 °C; IR: 1516, 1328, 1111, 1064 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.64 (d, J = 2.2 Hz, 1H), 8.09 (dd, J = 9.0, 2.2 Hz, 1H), 7.53 (d, J = 7.8 Hz, 1H), 7.47–7.38 (complex, 6H), 7.25 (s, 1H), 7.20 (d, J = 9.0 Hz, 1H), 7.07 (d, J = 7.8 Hz, 1H), 6.82 (d, J = 0.8 Hz, 1H), 5.45 (s, 2H); 13C {1H} NMR (101 MHz, CDCl3): δ 144.9, 142.4, 140.4, 137.9, 131.6, 131.23, 131.15, 129.6, 129.23, 129.15, 128.9, 127.7, 125.1, 123.8 (q, J = 272.5 Hz), 124.7 (q, J = 3.8 Hz), 122.9 (q, J = 3.8 Hz), 117.84, 117.80, 110.1, 47.6; 19F {1H} NMR (376 MHz, CDCl3 referenced to C6H5F): δ -62.8; MS (m/z): 396; Anal. Calcd for C22H15F3N2O2: C, 66.67; H, 3.81; N, 7.07; Found: C, 66.55; H, 3.88; N, 7.08.
3.3.4. 1-(2-Fluorophenethyl)-5-nitro-2-phenyl-1H-indole (15)
Method C: Deoxybenzoin 5 (1 equiv) with 2-fluorophenethylamine (5 equiv) gave indole 15 (95 mg, 69%) as a yellow solid, mp 121–123 °C; IR: 1518, 1344 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.58 (d, J = 2.2 Hz, 1H), 8.12 (dd, J = 9.0, 2.2 Hz, 1H), 7.49–7.44 (complex, 3H), 7.38 (d, J = 9.0 Hz, 1H), 7.38–7.34 (complex, 2H), 7.15 (m, 1H), 6.90 (m, 2H), 6.72 (td, J = 7.5, 1.7 Hz, 1H), 6.65 (d, J = 0.8 Hz, 1H), 4.44 (t, J = 7.3 Hz, 2H), 2.96 (t, J = 7.3 Hz, 2H); 13C {1H} NMR (101 MHz, CDCl3): δ 159.7 (d, J = 246.2 Hz), 144.7, 144.5, 143.7, 142.2, 140.4, 129.4 (d, J = 8.1 Hz), 127.5, 127.4 (d, J = 3.8 Hz), 124.6 (d, J = 3.6 Hz), 124.4, 124.1, 124.0, 122.4, 118.2, 117.8, 117.5 (d, J = 5.4 Hz), 115.5 (d, J = 20.9 Hz), 110.1, 104.2, 64.2 (d, J = 11.6 Hz), 42.0 (d, J = 5.6 Hz); 19F {1H} NMR (376 MHz, CDCl3 referenced to C6H5F): δ -118.9; MS (m/z): 360; Anal. Calcd for C22H17FN2O2: C, 73.32; H, 4.75; N, 7.77; Found: C, 73.14; H, 4.76; N, 7.75.
3.3.5. 1-(3-Isopropoxypropyl)-5-nitro-2-phenyl-1H-indole (16)
Method C: Deoxybenzoin 5 (1 equiv) with 3-isopropoxypropylamine (5 equiv) gave indole 16 (101 mg, 77%) as a yellow solid, mp 44–45 °C; IR: 1512, 1328 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.58 (d, J = 2.3 Hz, 1H), 8.13 (dd, J = 9.1, 2.3 Hz, 1H), 7.52–7.46 (complex, 6H), 6.69 (d, J = 0.8 Hz, 1H), 4.35 (t, J = 7.1 Hz, 2H), 3.75 (septet, J = 6.1 Hz, 1H), 3.20 (t, J = 5.7 Hz, 2H), 1.87 (pentet, J = 6.0 Hz, 2H), 1.06 (d, J = 6.1 Hz, 6H); 13C {1H} NMR (101 MHz, CDCl3): δ 144.4, 141.8, 140.5, 131.9, 129.3, 128.79, 128.77, 127.2, 117.6, 117.1, 110.1, 104.5, 71.6, 64.2, 41.4, 30.7, 22.0; MS (m/z): 338; Anal. Calcd for C20H22N2O3: C, 70.99; H, 6.55; N, 8.28; Found: C, 71.13; H, 6.51; N, 8.12.
3.3.6. 1-Cyclohexyl-5-nitro-2-phenyl-1H-indole (17)
Method D: Deoxybenzoin 5 (1 equiv) with cyclohexylamine (10 equiv) at a ring closing temperature of 90–95 °C gave indole 17 (72 mg, 58%) as a yellow solid, mp 120–121 °C; IR: 1516, 1328 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.56 (d, J = 2.3 Hz, 1H), 8.07 (dd, J = 9.2, 2.3 Hz, 1H), 7.66 (d, J = 9.2 Hz, 1H), 7.53–7.46 (complex, 3H), 7.45–7.41 (complex, 2H), 6.62 (s, 1H), 4.23 (tt, J = 12.5, 3.9 Hz, 1H), 2.37–2.23 (complex, 2H), 1.98–1.87 (complex, 4H), 1.74 (m, 1H), 1.35–1.24 (complex, 3H); 13C {1H} NMR (101 MHz, CDCl3): δ 144.8, 141.3, 138.7, 132.4, 129.5, 128.8, 128.7, 128.1, 117.7, 116.4, 112.2, 104.5, 56.9, 31.5, 26.1, 25.1; MS (m/z): 320; Anal. Calcd for C20H20N2O2: C, 74.98; H, 6.29; N, 8.74; Found: C, 74.75; H, 6.24; N, 8.71.
3.3.7. 1-(4-Methoxyphenyl)-5-nitro-2-phenyl-1H-indole (18)
Method C: Deoxybenzoin 5 (1 equiv) with 4-methoxyaniline (5 equiv) gave indole 18 (111 mg, 84%) as a yellow solid, mp 169–171 °C; IR: 2837, 1516, 1333 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.63 (d, J = 2.2 Hz, 1H), 8.06 (dd, J = 9.1, 2.2 Hz, 1H), 7.28 (m, 5H), 7.22 (d, J = 9.1 Hz, 1H), 7.17 (d, J = 8.9 Hz, 2H), 6.93 (d, J = 8.9 Hz, 2H), 6.92 (d, J = 0.8 Hz, 1H), 3.86 (s, 3H); 13C {1H} NMR (101 MHz, CDCl3): δ 159.3, 144.2, 142.3, 142.0, 131.3, 130.0, 129.01, 128.97, 128.4, 128.2, 127.3, 117.7, 117.6, 114.8, 110.6, 104.6, 55.5; MS (m/z): 344; Anal. Calcd for C21H16N2O3: C, 73.24; H, 4.68; N: 8.13; Found: C, 73.32; H, 4.74; N, 8.09.
3.3.8. 5-Nitro-1,2-diphenyl-1H-indole (19)
Method D: Deoxybenzoin
5 (1 equiv) with aniline (10 equiv) at a ring closure temperature of 120–125 °C gave indole
19 (51 mg, 42%) as a yellow solid, mp 187–188 °C (lit [
28] mp 182–185 °C); using aniline (5 equiv) and a ring closure temperature of 90–95 °C produced 28 mg (23%) of
19; IR: 1516, 1328 cm
−1;
1H NMR (400 MHz, CDCl
3): δ 8.64 (d,
J = 2.2 Hz, 1H), 8.08 (dd,
J = 9.1, 2.2 Hz, 1H), 7.49–7.40 (complex, 4H), 7.29–7.24 (complex 7H), 6.94 (d,
J = 0.8 Hz, 1H);
13C {
1H} NMR (101 MHz, CDCl
3): δ 144.1, 142.5, 141.6, 137.3, 131.2, 129.7, 129.0, 128.4, 128.3, 128.2, 127.9, 127.5, 117.8, 117.6, 110.6, 105.0; MS (
m/
z): 314; Anal. Calcd for C
20H
14N
2O
2: C, 76.42; H, 4.49; N: 8.91; Found: C, 76.13; H, 4.44; N, 8.87.
3.3.9. 1-(4-Fluorophenyl)-5-nitro-2-phenyl-1H-indole (20)
Method D: Deoxybenzoin 5 (1 equiv) with 4-fluoroaniline (10 equiv) at a ring closure temperature of 120–125 °C gave indole 20 (105 mg, 82%) as a yellow solid, mp 149–150 °C; using 4-fluoroaniline (5 equiv) and a ring closure temperature of 90–95 °C produced 12 mg (9%) of 20; IR: 1516, 1325 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.64 (d, J = 2.2 Hz, 1H), 8.09 (dd, J = 9.1, 2.2 Hz, 1H), 7.32–7.28 (complex, 3H), 7.28–7.22 (complex, 5H), 7.16 (t, J = 8.3 Hz, 2H), 6.94 (d, J = 0.9 Hz, 1H); 13C {1H} NMR (101 MHz, CDCl3): δ 162.0 (d, J = 249.2 Hz), 144.1, 142.6, 141.7, 133.3 (d, J = 3.1 Hz), 131.0, 129.6 (d, J = 8.7 Hz), 129.0, 128.5, 128.4, 127.5, 118.0, 117.6, 116.7 (d, J = 22.8 Hz), 110.4, 105.1; 19F {1H} NMR (376 MHz, CDCl3 referenced to C6H5F): δ -112.3; MS (m/z): 332; Anal. Calcd for C20H13FN2O2: C, 72.28; H, 3.94; N, 8.43; Found: C, 71.99; H, 3.91; N, 8.38.
In one case, a mixture of deoxybenzoin
5 (1 equiv) and 4-fluoroaniline (5 equiv) was slowly heated from 80 to 125 °C to give 5-nitro-2-phenylbenzofuran (
20a, 32 mg, 25%) as the major product; light yellow solid, mp 156–158 °C (lit [
29] mp 158–159 °C); IR: 1524, 1351 cm
−1;
1H NMR (400 MHz, CDCl
3): δ 8.51 (d,
J = 2.4 Hz, 1H), 8.22 (dd,
J = 9.0, 2.4 Hz, 1H), 7.88 (m, 2H), 7.59 (d,
J = 9.0 Hz, 1H), 7.49 (m, 2H), 7.43 (m, 1H), 7.13 (d,
J = 0.9 Hz, 1H);
13C {
1H} NMR (101 MHz, CDCl
3): δ 159.3, 157.6, 144.4, 129.7, 129.6, 129.2, 129.0, 125.3, 120.1, 117.3, 111.4, 101.6; MS (
m/
z): 239.
3.3.10. 5-Nitro-2-phenyl-1-(4-(trifluoromethyl)phenyl)-1H-indole (21)
Methods C or D: No 1H-indole product was isolated from reaction of 4-(trifluoromethyl)aniline with deoxybenzoin 5.
3.3.11. 1-Benzyl-2-(4-methylphenyl)-5-nitro-1H-indole (22)
Method C: Deoxybenzoin 6 (1 equiv) with benzylamine (5 equiv) gave indole 22 (98 mg, 78%) as a yellow solid, mp 127–128 °C; IR: 1523, 1321 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.60 (d, J = 2.2 Hz, 1H), 8.03 (dd, J = 9.0, 2.2 Hz, 1H), 7.34–7.27 (complex, 5H), 7.23 (d, J = 8.1 Hz, 2H), 7.18 (d, J = 9.0 Hz, 1H), 6.97 (d, J = 7.8 Hz, 2H), 6.76 (s, 1H), 5.39 (s, 2H), 2.40 (s, 3H); 13C {1H} NMR (101 MHz, CDCl3): δ 145.2, 142.1, 140.6, 139.0, 137.0, 129.5, 129.1, 129.0, 128.4, 127.64, 127.55, 125.8, 117.5, 117.4, 110.4, 104.1, 48.0, 21.3; MS (m/z): 342; Anal. Calcd for C22H18N2O2: C, 77.17; H, 5.30; N, 8.18; Found: C, 77.02; H, 5.29; N, 8.14.
3.3.12. 1-(3-Methoxybenzyl)-2-(4-methylphenyl)-5-nitro-1H-indole (23)
Method C: Deoxybenzoin 6 (1 equiv) with 3-methoxybenzylamine (5 equiv) gave indole 23 (105 mg, 77%) as a yellow solid, mp 93–94 °C; IR: 2844, 1602, 1523, 1321 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.60 (d, J = 2.2 Hz, 1H), 8.04 (dd, J = 9.1, 2.2 Hz, 1H), 7.33 (d, J = 8.3 Hz, 2H), 7.24–7.18 (complex, 4H), 6.80 (dd, J = 8.1, 2.5 Hz, 1H), 6.76 (d, J = 0.7 Hz, 1H), 6.57 (ddd, J = 7.8, 1.8, 0.9 Hz, 1H), 6.51 (t, J = 1.8 Hz, 1H), 5.36 (s, 2H), 3.71 (s, 3H), 2.40 (s, 3H); 13C {1H} NMR (101 MHz, CDCl3): δ 160.1, 145.2, 142.1, 140.6, 139.0, 138.7, 130.1, 129.5, 129.2, 128.4, 127.6, 118.1, 117.5, 117.4, 112.6, 111.9, 110.4, 104.0, 55.2, 47.9, 22.3; MS (m/z): 372; Anal. Calcd for C23H20N2O3: C, 74.18; H, 5.41; N, 7.52; Found: C, 74.38; H, 5.45; N, 7.50.
3.3.13. 2-(4-Methylphenyl)-5-nitro-1-phenethyl-1H-indole (24)
Method C: Deoxybenzoin 6 (1 equiv) with phenethylamine (5 equiv) gave indole 24 (81 mg, 62%) as a yellow solid, mp 120–122 °C; IR: 1523, 1321 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.57 (d, J = 2.2 Hz, 1H), 8.10 (dd, J = 9.1, 2.2 Hz, 1H), 7.32 (d, J = 9.1 Hz, 1H), 7.27 (d, J = 8.2 Hz, 2H), 7.22 (d, J = 8.2 Hz, 2H), 7.19–7.15 (complex, 3H), 6.87–6.85 (complex, 2H), 6.62 (d, J = 0.8 Hz, 1H), 4.40 (t, J = 7.5 Hz, 2H), 2.92 (t, J = 7.5 Hz, 2H), 2.44 (s, 3H); 13C {1H} NMR (101 MHz, CDCl3): δ 144.7, 141.8, 140.0, 138.8, 137.5, 129.4, 129.3, 128.7, 128.63, 128.58, 127.4, 126.8, 117.6, 117.1, 109.7, 104.2, 45.9, 36.2, 21.4; MS (m/z): 356; Anal. Calcd for C23H20N2O2: C, 77.51; H, 5.66; N, 7.86; Found: C, 77.40; H, 5.67; N, 7.89.
3.3.14. 1-Benzyl-2-(4-methoxyphenyl)-5-nitro-1H-indole (25)
Method C: Deoxybenzoin 7 (1 equiv) with benzylamine (5 equiv) gave indole 25 (106 mg, 86%) as a yellow solid, mp 143–144 °C; IR: 2837, 1516, 1328 cm−1; 1H NMR (400 MHz, CDCl3: δ 8.60 (d, J = 2.2 Hz, 1H), 8.04 (dd, J = 9.1, 2.2 Hz, 1H), 7.35 (d, J = 8.7 Hz, 2H), 7.31–7.24 (complex, 3H), 7.18 (d, J = 9.0 Hz, 1H), 6.98 (d, J = 9.0 Hz, 2H), 6.95 (d, J = 8.7 Hz, 2H), 6.74 (s, 1H), 5.39 (s, 2H), 3.84 (s, 3H); 13C {1H} NMR (101 MHz, CDCl3): δ 160.2, 145.0, 142.1, 140.5, 137.0, 130.6, 129.0, 127.7, 127.6, 125.8, 123.7, 117.4, 117.3, 114.3, 110.3, 103.8, 55.4, 48.0; MS (m/z): 358; Anal. Calcd for C22H18N2O3: C, 73.73; H, 5.06; N, 7.82; Found: C, 73.66; H, 5.05; N, 7.79.
3.3.15. 1-(3-Methoxybenzyl)-2-(4-methoxyphenyl)-5-nitro-1H-indole (26)
Method C: Deoxybenzoin 7 (1 equiv) with 3-methoxybenzylamine (5 equiv) gave indole 26 (107 mg, 80%) was isolated as a yellow solid, mp 118–119 °C; IR: 2842, 1516, 1325 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.58 (d, J = 2.2 Hz, 1H), 8.03 (dd, J = 9.0, 2.2 Hz, 1H), 7.36 (d, J = 8.8 Hz, 2H), 7.22 (d, J = 8.0 Hz, 1H), 7.18 (d, J = 8.7 Hz, 1H), 6.94 (d, J = 8.8 Hz, 2H), 6.79 (ddd, J = 8.3, 2.5, 0.9 Hz, 1H), 6.72 (d, J = 0.9 Hz, 1H), 6.56 (ddd, J = 7.6, 1.7, 0.9 Hz, 1H), 6.51 (t, J = 2.2 Hz, 1H), 5.35 (s, 2H), 3.84 (s, 3H), 3.71 (s, 3H); 13C {1H} NMR (101 MHz, CDCl3): δ 160.2, 160.1, 144.9, 142.1, 140.6, 138.7, 130.6, 130.1, 127.5, 123.6, 118.0, 117.4, 117.3, 114.3, 112.6, 111.8, 110.3, 103.7, 55.4, 55.2, 47.9; MS (m/z): 388; Anal. Calcd for C23H20N2O4: C, 71.12; H, 5.19; N, 7.21; Found: C, 70.98; H, 5.16; N, 7.15.
3.3.16. 1-(2-Chlorobenzyl)-2-(4-methoxyphenyl)-5-nitro-1H-indole (27)
Method C: Deoxybenzoin 7 (1 equiv) with 2-chlorobenzylamine (5 equiv) gave indole 27 (104 mg, 77%) was isolated as a yellow solid, mp 147–149 °C; IR: 2841, 1516, 1328 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.62 (d, J = 2.2 Hz, 1H), 8.05 (dd, J = 9.0, 2.2 Hz, 1H), 7.44 (dd, J = 8.0, 1.3 Hz, 1H), 7.31 (d, J = 8.8 Hz, 2H), 7.23 (td, J = 7.9, 1.6 Hz, 1H), 7.14–7.08 (complex, 2H), 6.96 (d, J = 8.8 Hz, 2H), 6.78 (d, J = 0.9 Hz, 1H), 6.52 (dd, J = 7.5, 1.6 Hz, 1H), 5.43 (s, 2H), 3.83 (s, 3H); 13C {1H} NMR (101 MHz, CDCl3): δ 160.2, 145.0, 142.3, 140.4, 134.4, 131.9, 130.3, 129.8, 128.9, 127.6, 127.4, 126.8, 123.3, 117.50, 117.48, 114.4, 110.1, 103.9, 55.4, 46.0; MS (m/z): 392, 394 (ca 3:1); Anal. Calcd for C22H17ClN2O3: C, 67.26; H, 4.36; N, 7.13; Found: C, 67.25; H, 4.39; N, 7.18.
3.3.17. 2-(4-Methoxyphenyl)-5-nitro-1-(3-(trifluoromethyl)benzyl)-1H-indole (28)
Method C: Deoxybenzoin 7 (1 equiv) with 3-(trifluoromethyl)benzylamine (5 equiv) gave indole 28 (119 mg, 81%) as a yellow solid, mp 115–117 °C; IR: 2844, 1516, 1328, 1105, 1071 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.62 (d, J = 2.2 Hz, 1H), 8.08 (dd, J = 9.0, 2.2 Hz, 1H), 7.53 (d, J = 7.8 Hz, 1H), 7.40 (t, J = 7.8 Hz, 1H), 7.30 (d, J = 8.7 Hz, 2H), 7.26 (obscured, 1H), 7.18 (d, J = 9.0 Hz, 1H), 7.08 (d, J = 7.8 Hz, 1H), 6.95 (d, J = 8.7 Hz, 2H), 6.76 (d, J = 0.8 Hz, 1H), 5.43 (s, 2H), 3.85 (s, 3H); 13C {1H} NMR (101 MHz, CDCl3): δ 160.3, 144.8, 142.3, 140.3, 138.1, 131.5, 131.4 (q, J = 32.5 Hz), 130.6, 129.6, 129.2, 127.7, 124.7 (q, J = 3.7 Hz), 123.8 (q, J = 272.5 Hz), 122.9 (q, J = 3.8 Hz), 117.62, 117.58, 114.4, 109.9, 104.3, 55.4, 47.6; 19F {1H} NMR (376 MHz, CDCl3 referenced to C6H5F): δ -62.8; MS (m/z): 426; Anal. Calcd for C23H17F3N2O3: C, 64.79; H, 4.02; N, 6.57; Found: C, 64.89; H, 3.91; N, 6.59.
3.3.18. 2-(4-Methoxyphenyl)-5-nitro-1-phenethyl-1H-indole (29)
Method C: Deoxybenzoin 7 (1 equiv) with phenethylamine (5 equiv) gave indole 29 (97 mg, 75%) as a yellow solid, mp 141–143 °C; IR: 2838, 1516, 1334 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.56 (d, J = 2.2 Hz, 1H), 8.10 (dd, J = 9.1, 2.2 Hz, 1H), 7.32 (d, J = 9.1 Hz, 1H), 7.24 (d, J = 8.8 Hz, 2H), 7.19–7.15 (complex, 3H), 6.98 (d, J = 8.8 Hz, 2H), 6.87–6.82 (complex, 2H), 6.59 (d, J = 0.7 Hz, 1H), 4.38 (t, J = 7.5 Hz, 2H), 3.89 (s, 3H), 2.92 (t, J = 7.5 Hz, 2H); 13C {1H} NMR (101 MHz, CDCl3): δ 160.0, 144.5, 141.7, 139.9, 137.5, 130.7, 128.63, 128.59, 127.4, 126.8, 123.9, 117.5, 117.0, 114.1, 109.6, 104.0, 55.4, 45.8, 36.2; MS (m/z): 372; Anal. Calcd for C23H20N2O3: C, 74.18; H, 5.41; N, 7.52; Found: C, 74.07; H, 5.38; N, 7.51.
3.3.19. 1-Hexyl-2-(4-methoxyphenyl)-5-nitro-1H-indole (30)
Method D: Deoxybenzoin 7 (1 equiv) with hexylamine (10 equiv) at a ring closing temperature of 90–95 °C gave indole 30 (44 mg, 36%) was isolated as a yellow solid, mp 69–70 °C; IR: 2835, 1516, 1330 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.56 (d, J = 2.2 Hz, 1H), 8.11 (dd, J = 9.0, 2.2 Hz, 1H), 7.40 (d, J = 8.7 Hz, 2H), 7.36 (d, J = 9.0 Hz, 1H), 7.03 (d, J = 8.7 Hz, 2H), 6.61 (d, J = 0.8 Hz, 1H), 4.15 (t, J = 7.6 Hz, 2H), 3.89 (s, 3H), 1.67 (pentet, J = 7.1 Hz, 2H), 1.25–1.12 (complex, 6H), 0.81 (t, J = 6.6 Hz, 3H); 13C {1H} NMR (101 MHz, CDCl3): δ 160.0, 144.5, 141.6, 140.0, 130.7, 127.4, 124.2, 117.5, 116.9, 114.2, 109.7, 103.8, 55.4, 44.3, 31.2, 29.9, 26.3, 22.4, 13.9; MS (m/z): 352; Anal. Calcd for C21H24N2O3: C, 71.57; H, 6.86; N, 7.95; Found: C, 71.64; H, 6.91; N, 7.93.
3.3.20. 1-Benzyl-2-(4-fluorophenyl)-5-nitro-1H-indole (31)
Method C: Deoxybenzoin 8 (1 equiv) with benzylamine (5 equiv) gave indole 31 (102 mg, 85%) as a yellow solid, mp 129–130 °C; IR: 1510, 1335 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.62 (d, J = 2.2 Hz, 1H), 8.07 (dd, J = 9.0, 2.2 Hz, 1H), 7.38 (dd, J = 8.8, 5.2 Hz, 2H), 7.35–7.25 (complex, 3H), 7.22 (d, J = 9.0 Hz, 1H), 7.11 (t, J = 8.7 Hz, 2H), 6.95 (m, 2H), 6.78 (d, J = 0.7 Hz, 1H), 5.37 (s, 2H); 13C {1H} NMR (101 MHz, CDCl3): δ 163.1 (d, J = 249.5 Hz,), 143.9, 142.2, 140.6, 136.7, 131.1 (d, J = 8.2 Hz), 129.1, 127.8, 127.5 (d, J = 3.5 Hz), 127.4, 125.7, 117.7, 116.9 (d, J = 21.8 Hz), 110.4, 104.5, 48.0 (1 aromatic carbon unresolved); 19F {1H} NMR (376 MHz, CDCl3 referenced to C6H5F): δ -111.9; MS (m/z): 346; Anal. Calcd for C21H15FN2O2: C, 72.82; H, 4.37; N, 8.09; Found: C, 72.73; H, 4.33; N, 8.06.
3.3.21. 2-(4-Fluorophenyl)-5-nitro-1-(3-(trifluoromethyl)benzyl)-1H-indole (32)
Method C: Deoxybenzoin 8 (1 equiv) with 3-(trifluoromethyl)benzylamine (5 equiv) gave indole 32 (81 mg, 55%) as a yellow solid, mp 129–130 °C; IR: 1516, 1327 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.64 (d, J = 2.2 Hz, 1H), 8.10 (dd, J = 9.0, 2.2 Hz, 1H), 7.54 (d, J = 7.8 Hz, 1H), 7.40 (t, J = 7.8 Hz, 1H), 7.35 (dd, J = 8.8, 5.2 Hz, 2H), 7.24 (br s, 1H), 7.21 (d, J = 9.0 Hz, 1H), 7.13 (t, J = 8.7 Hz, 2H), 7.05 (br d, J = 7.8 Hz, 1H), 6.80 (d, J = 0.8 Hz, 1H), 5.42 (s, 2H); 13C {1H} NMR (101 MHz, CDCl3): δ 163.2 (d, J = 250.1 Hz), 143.7, 142.4, 140.4, 137.8, 131.5 (q, J = 32.6 Hz), 131.1 (d, J = 8.2 Hz), 129.7, 129.1, 127.5, 127.2 (d, J = 3.6 Hz), 124.8 (q, J = 3.8 Hz), 123.7 (q, J = 272.5 Hz), 122.8 (q, J = 3.8 Hz), 117.94, 117.86, 116.1 (d, J = 21.7 Hz), 110.1, 105.0, 47.6; 19F {1H} NMR (376 MHz, CDCl3 referenced to C6H5F): δ -62.9, -111.5; MS (m/z): 414: Anal. Calcd for C22H14F4N2O2: C, 63.77; H, 3.41; N, 6.76; Found: C, 63.49; H, 3.40; N, 6.70.
3.3.22. 2-(4-Fluorophenyl)-5-nitro-1-phenethyl-1H-indole (33)
Method C: Deoxybenzoin 8 (1 equiv) with phenethylamine (5 equiv) gave indole 33 (103 mg, 79%) as a yellow solid, mp 129–130 °C; IR: 1516, 1338 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.58 (d, J = 2.2 Hz, 1H), 8.13 (dd, J = 9.0, 2.2 Hz, 1H), 7.37 (d, J = 9.0 Hz, 1H), 7.23–7.09 (complex, 7H), 6.79 (m, 2H), 6.60 (s, 1H), 4.39 (t, J = 7.2 Hz, 2H), 2.92 (t, J = 7.2 Hz, 2H); 13C {1H} NMR (101 MHz, CDCl3): δ 163.0 (d, J = 249.3 Hz), 143.5, 141.9, 139.8, 137.4, 131.2 (d, J = 8.4 Hz), 128.7, 128.6, 127.7 (d, J = 3.4 Hz), 127.3, 126.9, 117.8, 117.4, 115.7 (d, J = 21.7 Hz), 109.8, 104.5, 45.8, 36.1; 19F {1H} NMR (376 MHz, CDCl3 referenced to C6H5F): δ -112.3; MS (m/z): 360; Anal. Calcd for C22H17FN2O2: C, 73.32; H, 4.75; N, 7.77; Found: C, 73.19; H, 4.77; N, 7.73.
3.3.23. 1-Isobutyl-2-(4-fluorophenyl)-5-nitro-1H-indole (34)
Method D: Deoxybenzoin 8 (1 equiv) with isobutylamine (10 equiv) at a ring closing temperature of 90–95 °C gave indole 34 (36 mg, 32%) as a yellow solid, mp 93–94 °C; IR: 1516, 1320 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.57 (d, J = 2.2 Hz, 1H), 8.12 (dd, J = 9.1, 2,2 Hz, 1H), 7.45 (dd, J = 8.7, 5.3 Hz, 2H), 7.38 (d, J = 9.1 Hz, 1H), 7.19 (t, J = 8.7 Hz, 2H), 6.65 (d, J = 0.8 Hz, 1H), 4.02 (d, J = 7.6 Hz, 2H), 1.96 (nonet, J = 6.9 Hz, 1H), 0.68 (d, J = 6.7 Hz, 6H); 13C {1H} NMR (101 MHz, CDCl3): δ 161.9 (d, J = 249.1 Hz), 142.7, 140.7, 139.4, 130.5 (d, J = 8.3 Hz), 127.2 (d, J = 3.4 Hz), 126.1, 116.7, 116.2, 114.8 (d, J = 21.7 Hz), 109.2, 103.7, 50.6, 28.1, 19.0; 19F {1H} NMR (376 MHz, CDCl3 referenced to C6H5F): δ -112.3; MS (m/z): 312; Anal. Calcd for C18H17FN2O2: C, 69.22; H, 5.49; N, 8.97; Found: C, 69.10; H, 5.49; N, 8.94.
3.3.24. 1-Benzyl-2-(4-fluoro-3-methylphenyl)-5-nitro-1H-indole (35)
Method C: Deoxybenzoin 9 (1 equiv) with benzylamine (5 equiv) gave indole 35 (103 mg, 83%) as a yellow solid, mp 124–125 °C; IR: 1516, 1325 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.61 (d, J = 2.2 Hz, 1H), 8.06 (dd, J = 9.0, 2.2 Hz, 1H), 7.32–7.25 (complex, 3H), 7.24–7.17 (complex, 3H), 7.04 (d, J = 9.0 Hz, 1H), 6.95 (m, 2H), 6.75 (d, J = 0.8 Hz, 1H), 5.37 (s, 2H), 2.26 (d, J = 1.9 Hz, 3H); 13C {1H} NMR (101 MHz, CDCl3): δ 161.7 (d, J = 248.1 Hz), 144.2, 142.2, 140.5, 136.9, 132.7 (d, J = 5.5 Hz), 129.0, 128.3 (d, J = 8.4 Hz), 127.8, 127.4, 127.1 (d, J = 3.9 Hz), 125.8, 125.5 (d, J = 17.7 Hz), 117.62, 117.57, 115.4 (d, J = 22.7 Hz), 110.3, 104.3, 48.0, 14.7 (d, J = 3.5 Hz); 19F {1H} NMR (376 MHz, CDCl3 referenced to C6H5F): δ -116.3; MS (m/z): 360; Anal. Calcd for C22H17FN2O2: C, 73.32; H, 4.75; N, 7.77; Found: C, 73.16; H, 4.76; N, 7.79.
3.3.25. 2-(4-Fluoro-3-methylphenyl)-5-nitro-1-phenethyl-1H-indole (36)
Method C: Deoxybenzoin 9 (1 equiv) with phenethylamine (5 equiv) gave indole 36 (102 mg, 80%) as a yellow solid, mp 126–128 °C; IR: 1516, 1329 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.57 (d, J = 2.2 Hz, 1H), 8.06 (dd, J = 9.0, 2.2 Hz, 1H), 7.34 (d, J = 9.0 Hz, 1H), 7.21–7.13 (complex, 3H), 7.08–7.01 (complex, 2H), 6.98 (d, J = 7.1 Hz, 1H), 6.78 (m, 2H), 6.57 (s, 1H), 4.39 (t, J = 7.2 Hz, 2H), 2.93 (t, J = 7.2 Hz, 2H), 2.31 (d, J = 2.0 Hz, 3H); 13C {1H} NMR (101 MHz, CDCl3): δ 161.6 (d, J = 247.9 Hz), 143.9, 141.9, 139.8, 137.5, 132.7 (d, J = 5.4 Hz), 128.6, 128.4 (d, J = 8.2 Hz), 127.4, 127.3, 126.8, 125.3 (d, J = 17.6 Hz), 117.7, 117.3, 115.2 (d, J = 22.7 Hz), 109.8, 104.3, 45.1, 36.1, 14.6 (d, J = 3.5 Hz), (1 aromatic carbon unresolved); 19F {1H} NMR (376 MHz, CDCl3 referenced to C6H5F): δ -116.7; MS (m/z): 374; Anal. Calcd for C23H19FN2O2: C, 73.78; H, 5.12; N, 7.48; Found: C, 73.58; H, 5.07; N, 7.46.
3.3.26. 2-(4–Fluoro-3-methylphenyl)-1-(2-fluorophenethyl)-5-nitro-1H-indole (37)
Method C: Deoxybenzoin 9 (1 equiv) with 2-fluorophenethylamine (5 equiv) gave indole 37 (101 mg, 75%) as a yellow solid, mp 129–130 °C; IR: 1516, 1328 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.57 (d, J = 2.2 Hz, 1H), 8.13 (dd, J = 9.0, 2.2 Hz, 1H), 7.39 (d, J = 9.0 Hz, 1H), 7.20–7.05 (complex, 4H), 6.91 (m, 2H), 6.71 (td, J = 7.8, 1.9 Hz, 1H), 6.58 (d, J = 0.8 Hz, 1H), 4.41 (t, J = 7.2 Hz, 2H), 2.97 (t, J = 7.2 Hz, 2H), 2.33 (d, J = 2.0 Hz, 3H); 13C {1H} NMR (101 MHz, CDCl3): δ 161.6 (d, J = 247.7 Hz), 161.2 (d, J = 245.5 Hz), 143.7, 141.9, 139.9, 132.6 (d, J = 5.5 Hz), 130.9 (d, J = 4.7 Hz), 128.8 (d, J = 8.2 Hz), 128.3 (d, J = 8.3 Hz), 127.3, 127.2 (d, J = 3.8 Hz), 125.4 (d, J = 17.7 Hz), 124.3 (d, J = 13.1 Hz), 124.2, 117.7, 117.3, 115.33 (d, J = 21.7 Hz), 115.28 (d, J = 22.8 Hz), 109.7, 104.3, 44.2, 30.0 (d, J = 1.9 Hz), 14.6 (d, J = 3.5 Hz); 19F {1H} NMR (376 MHz, CDCl3 referenced to C6H5F): δ -116.6, -118.9; MS (m/z): 392; Anal. Calcd for C23H18F2N2O2: C, 70.40; H, 4.62; N, 7.14; Found: C, 70.29; H, 4.54; N, 7.16.
3.3.27. 1-Benzyl-2-(4-chlorophenyl)-5-nitro-1H-indole (38)
Method C: Deoxybenzoin 10 (1 equiv) with benzylamine (5 equiv) gave indole 38 (95 mg, 77%) as a yellow solid, mp 129–130 °C; IR: 1516, 1331 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.62 (d, J = 2.2 Hz, 1H), 8.07 (dd, J = 9.1, 2.2 Hz, 1H), 7.40 (d, J = 8.6 Hz, 2H), 7.35 (d, J = 8.6 Hz, 2H), 7.33–7.27 (complex, 3H), 7.22 (d, J = 9.1 Hz, 1H), 6.95 (m, 2H), 6.80 (d, J = 0.8 Hz, 1H), 5.38 (s, 2H); 13C {1H} NMR (101 MHz, CDCl3): δ 143.7, 142.3, 140.7, 136.7, 135.2, 130.5, 129.8, 129.1, 127.8, 127.4, 125.7, 117.80, 117.76, 110.5, 104.7, 48.1 (1 aromatic carbon unresolved); MS (m/z) 362, 364 (ca 3:1); Anal. Calcd for C21H15ClN2O2: C, 69.52; H, 4.17; N, 7.72; Found: C, 69.23; H, 4.10; N, 7.71.
3.3.28. 2-(4-Chlorophenyl)-5-nitro-1-(3-(trifluoromethyl)benzyl)-1H-indole (39)
Method C: Deoxybenzoin 10 (1 equiv) with 3-(trifluoromethyl)benzylamine (5 equiv) gave indole 39 (91 mg, 62%) as a yellow solid, mp 150–151 °C; IR: 1516, 1333, 1111, 1071 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.63 (d, J = 2.2 Hz, 1H), 8.10 (dd, J = 9.1, 2.2 Hz, 1H), 7.54 (d, J = 7.8 Hz, 1H), 7.42 (d, J = 8.5 Hz, 2H), 7.40 (obscured, 1H), 7.32 (d, J = 8.5 Hz, 2H), 7.30 (br s, 1H), 7.21 (d, J = 9.1 Hz, 1H), 7.05 (d, J = 7.8 Hz, 1H), 6.82 (d, J = 0.8 Hz, 1H), 5.43 (s, 2H); 13C {1H} NMR (101 MHz, CDCl3): δ 143.5, 142.5, 140.5, 137.7, 135.5, 131.5 (q, J = 32.5 Hz), 130.4, 129.7, 129.6, 129.2, 129.0, 127.5, 124.8 (q, J = 3.6 Hz), 123.7 (q, J = 272.5 Hz), 122.7 (q, J = 3.7 Hz), 118.1, 117.9, 110.1, 105.2, 47.6; 19F {1H} NMR (376 MHz, CDCl3 referenced to C6H5F): δ -62.9; MS (m/z): 430, 432 (ca 3:1); Anal. Calcd for C22H14ClF3N2O2: C, 61.34; H, 3.28; N, 6.50; Found: C, 61.07; H, 3.33; N, 6.45.
3.3.29. 2-(4-Chlorophenyl)-5-nitro-1-phenethyl-1H-indole (40)
Method C: Deoxybenzoin 10 (1 equiv) with phenethylamine (5 equiv) gave indole 40 (93 mg, 73%) as a yellow solid, mp 153–154 °C; IR: 1516, 1333 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.58 (d, J = 2.2 Hz, 1H), 8.13 (d, J = 9.1, 2.2 Hz, 1H), 7.40 d, J = 8.5 Hz, 2H), 7.36 (d, J = 9.1 Hz, 1H), 7.21–7.12 (obscured, 3H), 7.16 (d, J = 8.6 Hz, 2H), 6.78 (m, 2H), 6.61 (s, 1H), 4.40 (t, J = 7.2 Hz, 2H), 2.93 (t, J = 7.2 Hz, 2H); 13C {1H} NMR (101 MHz, CDCl3): δ 143.3, 142.0, 140.0, 137.3, 134.9, 130.6, 130.1, 128.9, 128.7, 128.6, 127.3, 126.9, 117.8, 117.5, 109.9, 104.7, 45.8, 36.1; MS (m/z): 376, 378 (ca 3:1); Anal. Calcd for C22H17ClN2O2: C, 70.12; H, 4.55; N, 7.43; Found: C, 69.98; H, 4.49; N, 7.49.
3.3.30. 1-Benzyl-2-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl-)-5-nitro-1H-indole (41)
Method C: Deoxybenzoin 11 (1 equiv) with benzylamine (5 equiv) gave indole 41 (103 mg, 85%) as a yellow solid, mp 124–125 °C; IR: 1518, 1336 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.59 (d, J = 2.2 Hz, 1H), 8.03 (dd, J = 9.1, 2.2 Hz, 1H), 7.31–7.24 (complex, 3H), 7.16 (d, J = 9.1 Hz, 1H), 6.96 (m, 3H), 6.89 (m, 2H), 6.73 (d, J = 0.8 Hz, 1H), 5.41 (s, 2H), 4.29 (m, 4H); 13C {1H} NMR (101 MHz, CDCl3): δ 144.7, 144.4, 143.7, 142.1, 140.5, 136.9, 129.0, 127.7, 127.5, 125.8, 124.6, 122.5, 118.3, 117.7, 117.5, 117.4, 110.4, 103.9, 64.5, 64.3, 48.0; MS (m/z): 386; Anal. Calcd for C23H18N2O4: C, 71.49; H, 4.70; N, 7.25; Found: C, 71.43; H, 4.73; N, 7.29.
3.3.31. 2-(2,3-Dihydobenzo[b][1,4]dioxin-6-yl)-1-(2-fluorobenzyl)-5-nitro-1H-indole (42)
Method C: Deoxybenzoin 11 (1 equiv) with 2-fluorobenzylamine (5 equiv) gave indole 42 (109 mg, 86%) as a yellow solid, mp 153–155 °C; IR: 1520, 1334 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.60 (d, J = 2.2 Hz, 1H), 8.05 (dd, J = 9.0, 2.2 Hz, 1H), 7.25 (m, 1H), 7.18 (d, J = 9.0 Hz, 1H), 7.09 (ddd, J = 10.1, 8.3, 1.2 Hz, 1H), 6.98 (td, J = 7.6, 1.2 Hz, 1H), 6.95 (d, J = 2.0 Hz, 1H), 6.91 (d, J = 8.3 Hz, 1H), 6.88 (dd, J = 8.3, 2.0 Hz, 1H), 6.74 (d, J = 0.8 Hz, 1H), 6.56 (td, J = 7.6, 1.7 Hz, 1H), 5.45 (s, 2H), 4.30 (m, 4H); 13C {1H} NMR (101 MHz, CDCl3): δ 159.7 (d, J = 246.2 Hz), 144.7, 144.5, 143.7, 142.2, 140.4, 129.4 (d, J = 8.1 Hz), 127.5, 127.4 (d, J = 3.9 Hz), 124.6 (d, J = 3.6 Hz), 124.4, 124.0 (d, J = 14.2 Hz), 122.4, 118.2, 117.7, 117.52, 117.47, 115.5 (d, J = 20.8 Hz), 110.1, 104.2, 64.5, 64.3, 42.0 (d, J = 5.5 Hz); 19F {1H} NMR (376 MHz, CDCl3 referenced to C6H5F): δ -118.9; MS (m/z): 404; Anal. Calcd for C23H17FN2O4: C, 68.31; H, 4.24; N, 6.93; Found: C, 68.19; H, 4.27; N, 6.89.
3.3.32. 2-(2,3-Dihydobenzo[b][1,4]dioxin-6-yl)-5-nitro-1-phenethyl-1H-indole (43)
Method C: Deoxybenzoin 11 (1 equiv) with phenethylamine (5 equiv) gave indole 43 (109 mg, 86%) as a yellow solid, mp 104–105 °C; IR: 1516, 1334 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.55 (d, J = 2.2 Hz, 1H), 8.09 (dd, J = 9.1, 2.2 Hz, 1H), 7.30 (d, J = 9.1 Hz, 1H), 7.16 (m, 3H), 6.94 (d, J = 8.3 Hz, 1H), 6.87 (m, 2H), 6.84 (dd, J = 2.1 Hz, 1H), 6.80 (dd, J = 8.3, 2.1 Hz, 1H), 6.53 (d, J = 0.8 Hz, 1H), 4.41 (t, J = 7.5 Hz, 2H), 4.33 (m, 4H), 2.92 (t, J = 7.5 Hz, 2H); 13C {1H} NMR (101 MHz, CDCl3): δ 144.22, 144.17, 143.6, 141.8, 139.9, 137.6, 128.6, 127.3, 126.9, 124.9, 122.5, 118.4, 117.6, 117.5, 117.1, 109.7, 104.1, 64.5, 64.4, 45.9, 36.2 (1 aromatic carbon unresolved); MS (m/z): 400; Anal. Calcd for C24H20N2O4: C, 71.99; H, 5.03; N, 7.00; Found: C, 71.95; H, 4.98; N, 6.97.