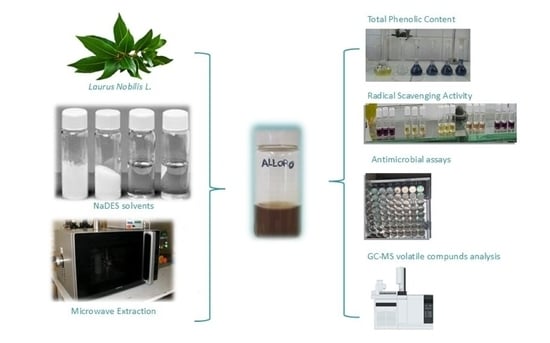
NaDES-Based Extracts by Microwave Activation from Laurus nobilis L. Leaves: Sustainable Multifunctional Ingredients for Potential Cosmetic and Pharmaceutical Applications
Abstract
1. Introduction
2. Results
2.1. MW NaDES Extraction
2.2. Total Phenolic Content (TPC) and Radical Scavenging Activity (RSA%)
2.3. Minimal Inhibitory Concentrations (MICs)
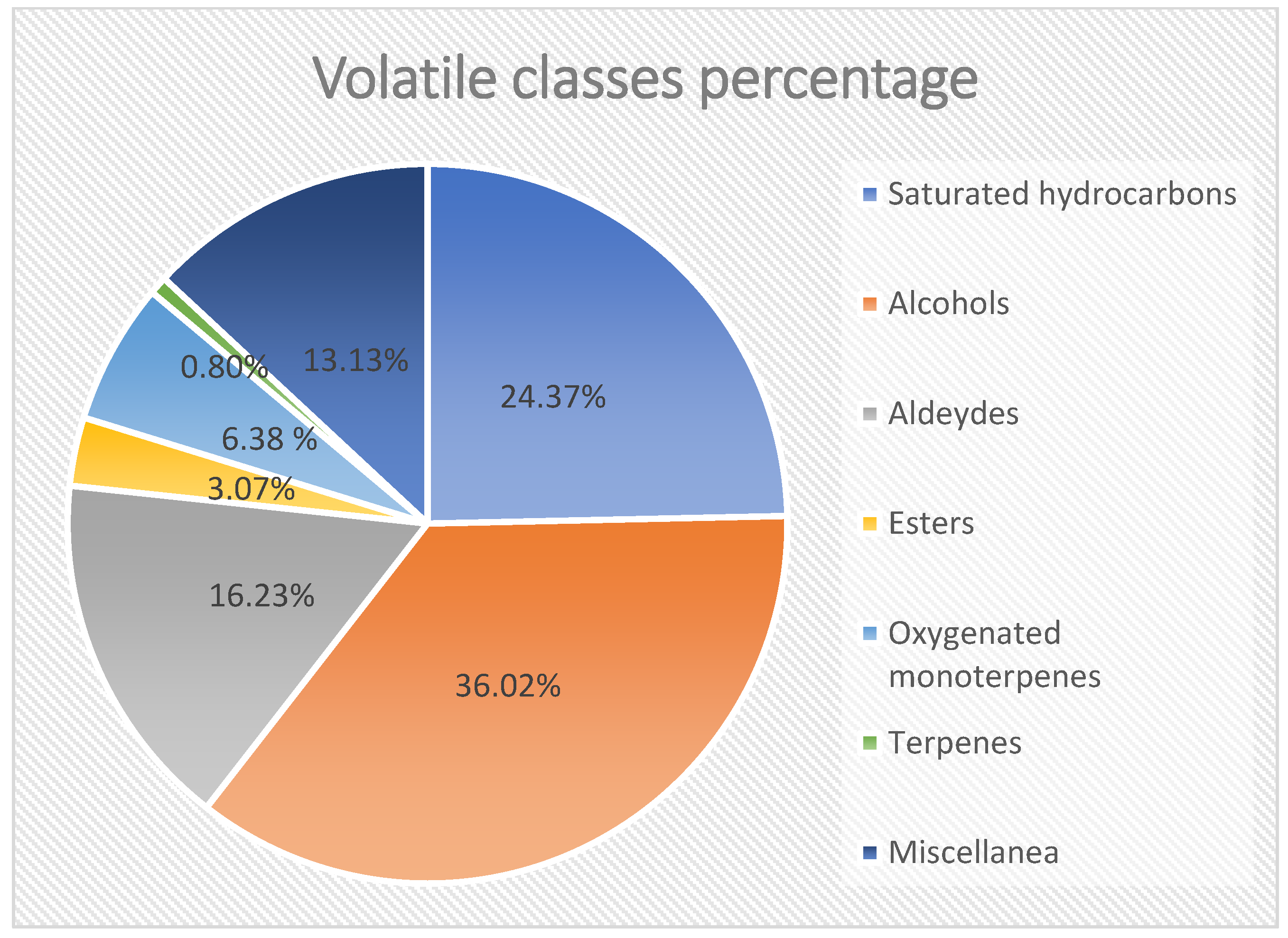
2.4. GC-MS Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Chemicals
4.3. Apparatus
4.4. Bacterial Strains
4.5. MW NaDES Extraction
4.6. Total Phenolic Content (TPC)
4.7. Radical Scavenging Activity (RSA%)
4.8. Minimal Inhibitory Concentrations (MICs)
4.9. GC/MS Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NaDES | Natural deep eutectic solvent |
| BG | Betaine/glycerol NaDES |
| BGA | Betaine/glycerol/lactic acid NaDES |
| CG | Choline/glycerol NaDES |
| CGA | Choline/glycerol/lactic acid NaDES |
| BGs | Betaine/glycerol NaDES-based extract sample |
| BGAs | Betaine/glycerol/lactic acid NaDES-based extract sample |
| CGs | Choline/glycerol NaDES-based extract sample |
| CGAs | Choline/glycerol/lactic acid NaDES-based extract sample |
| MWs | Microwaves |
| MICs | Minimal inhibitory concentrations |
| NIST | National Institute of Standards and Technology |
| RIs | Retention indices |
| STD | GC-MS standard |
| GAEs | Gallic acid equivalents |
References
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Saini, A.; Panesar, P.S.; Bera, M.B. Valorization of Fruits and Vegetables Waste through Green Extraction of Bioactive Compounds and Their Nanoemulsions-Based Delivery System. Bioresour. Bioprocess. 2019, 6, 26. [Google Scholar] [CrossRef]
- Chawla, P.; Rana, R.; Sridhar, K.; Stephen Inbaraj, B. Editorial: Green and Sustainable Extraction Techniques for Bioactives in Food, Plants, Pharmaceuticals, and Cosmetics. Front. Chem. 2024, 12, 1388362. [Google Scholar] [CrossRef] [PubMed]
- Cannavacciuolo, C.; Pagliari, S.; Celano, R.; Campone, L.; Rastrelli, L. Critical Analysis of Green Extraction Techniques Used for Botanicals: Trends, Priorities, and Optimization Strategies-A Review. TrAC Trends Anal. Chem. 2024, 173, 117627. [Google Scholar] [CrossRef]
- Gonçalves, S.; Romano, A. Chapter 13—Green Approaches for the Extraction of Bioactives from Natural Sources for Pharmaceutical Applications. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Inamuddin, Boddula, R., Ahamed, M.I., Asiri, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 249–267. ISBN 978-0-12-821885-3. [Google Scholar]
- Dini, I.; Laneri, S. The New Challenge of Green Cosmetics: Natural Food Ingredients for Cosmetic Formulations. Molecules 2021, 26, 3921. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.; Barbosa, A.; Advinha, B.; Sales, H.; Pontes, R.; Nunes, J. Green Extraction Techniques of Bioactive Compounds: A State-of-the-Art Review. Processes 2023, 11, 2255. [Google Scholar] [CrossRef]
- Batool, S.; Khera, R.A.; Hanif, M.A.; Ayub, M.A. Chapter 5—Bay Leaf. In Medicinal Plants of South Asia; Hanif, M.A., Nawaz, H., Khan, M.M., Byrne, H.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 63–74. ISBN 978-0-08-102659-5. [Google Scholar]
- Fidan, H.; Stefanova, G.; Kostova, I.; Stankov, S.; Damyanova, S.; Stoyanova, A.; Zheljazkov, V.D. Chemical Composition and Antimicrobial Activity of Laurus nobilis L. Essential Oils from Bulgaria. Molecules 2019, 24, 804. [Google Scholar] [CrossRef] [PubMed]
- Caputo, L.; Nazzaro, F.; Souza, L.F.; Aliberti, L.; De Martino, L.; Fratianni, F.; Coppola, R.; De Feo, V. Laurus nobilis: Composition of Essential Oil and Its Biological Activities. Molecules 2017, 22, 930. [Google Scholar] [CrossRef]
- Dobroslavić, E.; Repajić, M.; Dragović-Uzelac, V.; Elez Garofulić, I. Isolation of Laurus nobilis Leaf Polyphenols: A Review on Current Techniques and Future Perspectives. Foods 2022, 11, 235. [Google Scholar] [CrossRef] [PubMed]
- Shehata, A.I.; Taha, S.A.; Elmaghraby, A.M.; Elhetawy, A.I.G.; Mohamed Srour, T.; El Basuini, M.F.; Shahin, S.A. Effects of Dietary Bay Leaf (Laurus nobilis) Aqueous Extract on Growth Performance, Feed Utilization, Antioxidant Activity, Immunity, and Gene Expression in Nile tilapia (Oreochromis Niloticus). Aquaculture 2025, 599, 742155. [Google Scholar] [CrossRef]
- Awada, F.; Hamade, K.; Kassir, M.; Hammoud, Z.; Mesnard, F.; Rammal, H.; Fliniaux, O. Laurus nobilis Leaves and Fruits: A Review of Metabolite Composition and Interest in Human Health. Appl. Sci. 2023, 13, 4606. [Google Scholar] [CrossRef]
- Karadağ, O.G.M.; Başar, Y.; Alma, M.K. Phytochemical Content of Laurus nobilis Leaves and Extract; Potential For Use in Value-Added Cosmetics, Health and Food Products. In Proceedings of the International Congress on Sustainable Agriculture-II, Iğdır, Türkiye, 4–5 March 2025. [Google Scholar]
- Sahithi, A.; Mohan Chinala, K.; Chaithanya, A.; Achyuth Reddy, C.; Sawrov, M.; Al Amin, M. Phytochemical and Antimicrobial Evaluation of Laurus nobilis Leaves Against Acne and Dandruff-Causing Microorganisms. J. Pharm. Sci. Comput. Chem. 2025, 1, 50–57. [Google Scholar] [CrossRef]
- Muñiz-Márquez, D.B.; Wong-Paz, J.E.; Contreras-Esquivel, J.C.; Rodríguez-Herrera, R.; Aguilar, C.N. Bioactive Compounds from Bay Leaves (Laurus nobilis) Extracted by Microwave Technology. Z. Naturforsch. C J. Biosci. 2018, 73, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Chatzimitakos, T.; Athanasiadis, V.; Kalompatsios, D.; Kotsou, K.; Mantiniotou, M.; Bozinou, E.; Lalas, S.I. Optimizing Extract Preparation from Laurel (Laurus nobilis L.) Leaves Using a Pulsed Electric Field. ChemEngineering 2024, 8, 26. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as New Potential Media for Green Technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.-J.; Verpoorte, R. Are Natural Deep Eutectic Solvents the Missing Link in Understanding Cellular Metabolism and Physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Abert Vian, M.; Fabiano-Tixier, A.-S.; Nutrizio, M.; Rezek Jambrak, A.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A Review of Sustainable and Intensified Techniques for Extraction of Food and Natural Products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green Extraction of Natural Products. Origins, Current Status, and Future Challenges. TrAC Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Cannavacciuolo, C.; Pagliari, S.; Frigerio, J.; Giustra, C.M.; Labra, M.; Campone, L. Natural Deep Eutectic Solvents (NADESs) Combined with Sustainable Extraction Techniques: A Review of the Green Chemistry Approach in Food Analysis. Foods 2023, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- GRAS Substances (SCOGS) Database, U.S. Food and Drug Administration. Available online: https://www.fda.gov/food/generally-recognized-safe-gras/gras-substances-scogs-database (accessed on 4 July 2025).
- Hayyan, M.; Hashim, M.A.; Hayyan, A.; Al-Saadi, M.A.; AlNashef, I.M.; Mirghani, M.E.S.; Saheed, O.K. Are Deep Eutectic Solvents Benign or Toxic? Chemosphere 2013, 90, 2193–2195. [Google Scholar] [CrossRef] [PubMed]
- Shikov, A.N.; Obluchinskaya, E.D.; Flisyuk, E.V.; Terninko, I.I.; Generalova, Y.E.; Pozharitskaya, O.N. The Impact of Natural Deep Eutectic Solvents and Extraction Method on the Co-Extraction of Trace Metals from Fucus vesiculosus. Mar. Drugs 2022, 20, 324. [Google Scholar] [CrossRef] [PubMed]
- Shikov, A.N.; Shikova, V.A.; Whaley, A.O.; Burakova, M.A.; Flisyuk, E.V.; Whaley, A.K.; Terninko, I.I.; Generalova, Y.E.; Gravel, I.V.; Pozharitskaya, O.N. The Ability of Acid-Based Natural Deep Eutectic Solvents to Co-Extract Elements from the Roots of Glycyrrhiza glabra L. and Associated Health Risks. Molecules 2022, 27, 7690. [Google Scholar] [CrossRef] [PubMed]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Zakharova, L.V.; Daurtseva, A.V.; Flisyuk, E.V.; Shikov, A.N. Efficacy of Natural Deep Eutectic Solvents for Extraction of Hydrophilic and Lipophilic Compounds from Fucus vesiculosus. Molecules 2021, 26, 4198. [Google Scholar] [CrossRef] [PubMed]
- Caviglia, D.; Russo, E.; Preda, S.; Robustelli della Cuna, F.S.; Villa, C. In Situ NADES Microwave-Mediated Extraction of Bioactive Compounds from Beta vulgaris L. Var. Rubra Waste. Int. J. Food Sci. Technol. 2024, 59, 3271–3282. [Google Scholar] [CrossRef]
- Popovic, B.M.; Micic, N.; Potkonjak, A.; Blagojevic, B.; Pavlovic, K.; Milanov, D.; Juric, T. Novel Extraction of Polyphenols from Sour Cherry Pomace Using Natural Deep Eutectic Solvents—Ultrafast Microwave-Assisted NADES Preparation and Extraction. Food Chem. 2022, 366, 130562. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.; Caviglia, D.; Robustelli della Cuna, F.S.; Zuccari, G.; Russo, E. NaDES Application in Cosmetic and Pharmaceutical Fields: An Overview. Gels 2024, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.T.; Smaniotto, F.A.; Costa, I.F.; Baranzelli, J.; Muller, A.; Somacal, S.; Monteiro, C.S.; Vizzotto, M.; Rodrigues, E.; Barcia, M.T.; et al. Natural deep eutectic solvent (NADES): A strategy to improve the bioavailability of blueberry phenolic compounds in a ready-to-use extract. Food Chem. 2021, 364, 130370. [Google Scholar] [CrossRef] [PubMed]
- Panić, M.; Radić Stojković, M.; Kraljić, K.; Škevin, D.; Radojčić Redovniković, I.; Gaurina Srček, V.; Radošević, K. Ready-to-use green polyphenolic extracts from food by-products. Food Chem. 2019, 283, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Grillo, G.; Calcio Gaudino, E.; Rosa, R.; Leonelli, C.; Timonina, A.; Grygiškis, S.; Tabasso, S.; Cravotto, G. Green Deep Eutectic Solvents for Microwave-Assisted Biomass Delignification and Valorisation. Molecules 2021, 26, 798. [Google Scholar] [CrossRef] [PubMed]
- Souza, O.A.; da Ramalhão, V.G.S.; de Trentin, L.M.; Funari, C.S.; Carneiro, R.L.; da Bolzani, V.S.; Rinaldo, D. Combining Natural Deep Eutectic Solvent and Microwave Irradiation towards the Eco-Friendly and Optimized Extraction of Bioactive Phenolics from Eugenia uniflora L. Sustain. Chem. Pharm. 2022, 26, 100618. [Google Scholar] [CrossRef]
- Kilic, A.; Hafizoglu, H.; Kollmannsberger, H.; Nitz, S. Volatile Constituents and Key Odorants in Leaves, Buds, Flowers, and Fruits of Laurus nobilis L. J. Agric. Food Chem. 2004, 52, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing—EUCAST. Available online: https://www.eucast.org/ (accessed on 5 May 2025).
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 978-1-932633-21-4. [Google Scholar]
- Flavornet. Available online: http://www.flavornet.org/ (accessed on 15 May 2025).
- Cavalloro, V.; Robustelli della Cuna, F.S.; Malovini, A.; Villa, C.; Sottani, C.; Balestra, M.; Bracco, F.; Martino, E.; Collina, S. Essential Oils from Papaver Rhoeas and Their Metabolomic Profiling. Metabolites 2024, 14, 664. [Google Scholar] [CrossRef] [PubMed]
- NIST/EPA/NIH Mass Spectral Library 2023. Available online: https://sciencesolutions.wiley.com/solutions/technique/gc-ms/nist-epa-nih-mass-spectral-library/ (accessed on 5 May 2025).
- Pozharitskaya, O.N.; Obluchinskaya, E.D.; Shikova, V.A.; Flisyuk, E.V.; Vishnyakov, E.V.; Makarevich, E.V.; Shikov, A.N. Physicochemical and Antimicrobial Properties of Lactic Acid-Based Natural Deep Eutectic Solvents as a Function of Water Content. Appl. Sci. 2024, 14, 10409. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Oxidants and Antioxidants Part A; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Herbello-Hermelo, P.; Lamas, J.P.; Lores, M.; Domínguez-González, R.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Polyphenol Bioavailability in Nuts and Seeds by an in Vitro Dialyzability Approach. Food Chem. 2018, 254, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Spallarossa, A.; Comite, A.; Pagliero, M.; Guida, P.; Belotti, V.; Caviglia, D.; Schito, A.M. Valorization and Potential Antimicrobial Use of Olive Mill Wastewater (OMW) from Italian Olive Oil Production. Antioxidants 2022, 11, 903. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Palermiti, A.; Cafaro, A.; Barco, S.; Bucchioni, P.; Franceschini, P.; Cusato, J.; De Nicolò, A.; Manca, A.; De Vivo, E.D.; Russo, E.; et al. Analysis of Cannabinoids Concentration in Cannabis Oil Galenic Preparations: Harmonization between Three Laboratories in Northern Italy. Pharmaceuticals 2021, 14, 462. [Google Scholar] [CrossRef] [PubMed]

| NaDES Sample * | TPC (mg GAE/g ± SD) | RSA (% ± SD) |
|---|---|---|
| BGs | 11.72 ± 0.02 a | 88.1 ± 0.1 a |
| BGAs | 11.57 ± 0.02 a | 80.9 ± 0.3 b |
| CGs | 10.38 ± 0.07 b | 77.8 ± 0.4 b |
| CGAs | 10.12 ± 0.03 b | 62.2 ± 1.0 c |
| MICs (mg/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Bacterial Species | Strain | BG * | BGs | BGA | BGAs | CG * | CGs | CGA | CGAs |
| S. aureus (MRSA) | 17 | na | 7.0 | 27.8 | 7.1 | na | 55.7 | 27.1 | 7.0 |
| 18 | na | 3.5 | 27.8 | 3.5 | na | 55.7 | 27.1 | 3.5 | |
| S. epidermidis (MRSE) | 2 | na | 3.5 | 27.8 | 3.5 | na | 55.7 | 27.1 | 3.5 |
| 22 | na | 3.5 | 27.8 | 3.5 | na | 27.9 | 27.1 | 3.5 | |
| E. coli (CR) | 477 | na | na | 13.9 | 3.5 | na | na | 13.5 | 3.5 |
| P. aeruginosa (CR, K) | 259 | na | na | 13.9 | 3.5 | na | na | 13.5 | 3.5 |
| Compound a | RI b | RI c | Relative % | Mean % (± SD) | Identif. Method d | Olfactive Description | |||
|---|---|---|---|---|---|---|---|---|---|
| BGAs1 | BGAs2 | BGAs3 | |||||||
| 1 | Decane | 1000 | 1000 | 0.48 | - | 0.58 | 0.53 ± 0.08 | STD | alkane |
| 2 | 1.8-cineole | 1042 | 1043 | 1.34 | 0.50 | 1.06 | 0.96 ± 0.43 | NIST, RI | mint, sweety |
| 3 | Linalool | 1106 | 1109 | 5.60 | 3.72 | 3.67 | 4.33 ± 1.10 | NIST, RI | flower, lavender |
| 4 | 2,4-dimethyldecane | 1115 | 1113 | 0.90 | 0.42 | 0.62 | 0.65 ± 0.24 | NIST, RI | alkane |
| 5 | Borneol | 1188 | 1186 | 0.73 | 0.90 | - | 0.81 ± 0.12 | NIST, RI | camphor |
| 6 | Terpinen-4-ol | 1192 | 1194 | 1.32 | 1.31 | 0.64 | 1.09 ± 0.39 | NIST, RI | turpentine, nutmeg |
| 7 | Dodecane | 1200 | 1200 | 8.28 | 5.70 | 7.47 | 7.15 ± 1.32 | STD | alkane |
| 8 | 4,8-dimethyl-undecane | 1212 | 1221 | 2.30 | 1.43 | 2.49 | 2.08 ± 0.57 | NIST, RI | alkane |
| 9 | 2,4-dimethylundecane | 1223 | 1230 | 1.60 | 0.66 | 1.31 | 1.19 ± 0.48 | NIST, RI | alkane |
| 10 | 1,3-di-tert-butylbenzene | 1249 | 1259 | 15.77 | 9.17 | 14.44 | 13.13 ± 3.49 | NIST, RI | - |
| 11 | Tridecane | 1300 | 1330 | 4.06 | 3.13 | 5.02 | 4.07 ± 0.95 | STD | alkane |
| 12 | α-terpinyl acetate | 1356 | 1359 | 2.37 | 4.28 | 2.64 | 3.1 ± 1.03 | NIST, RI | wax |
| 13 | Eugenol | 1363 | 1365 | 6.44 | 9.04 | 3.38 | 6.29 ± 2.83 | NIST, RI | clove, honey |
| 14 | 2-methyl-tridecane | 1366 | 1373 | 1.24 | 1.17 | 1.55 | 1.32 ± 0.20 | NIST, RI | alkane |
| 15 | Tetradecane | 1400 | 1400 | 0.41 | 0.48 | 0.69 | 0.53 ± 0.14 | NIST, RI | alkane |
| 16 | Methyl eugenol | 1408 | 1410 | 15.70 | 19.56 | 14.13 | 16.46 ± 2.80 | NIST, RI | clove, spice |
| 17 | 2,4-di-tert-butyl-phenol | 1513 | 1517 | 10.97 | 13.70 | 15.14 | 13.27 ± 2.12 | NIST, RI | - |
| 18 | Hexadecane | 1600 | 1600 | 1.08 | 0.92 | 1.32 | 1.11 ± 0.20 | STD | alkane |
| 19 | Heptadecane | 1700 | 1700 | 5.31 | 5.86 | 7.31 | 6.16 ± 1.04 | STD | alkane |
| 20 | Hexadecanal | 1831 | 1830 | 4.57 | 6.18 | 5.36 | 5.37 ± 0.80 | NIST, RI | alkane |
| 21 | Octadecanal | 2037 | 2036 | 9.53 | 11.89 | 11.16 | 10.86 ± 1.21 | NIST, RI | oil |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caviglia, D.; Russo, E.; Schito, A.M.; Robustelli della Cuna, F.S.; Grignani, E.; Lionetti, N.; Villa, C. NaDES-Based Extracts by Microwave Activation from Laurus nobilis L. Leaves: Sustainable Multifunctional Ingredients for Potential Cosmetic and Pharmaceutical Applications. Molecules 2025, 30, 3006. https://doi.org/10.3390/molecules30143006
Caviglia D, Russo E, Schito AM, Robustelli della Cuna FS, Grignani E, Lionetti N, Villa C. NaDES-Based Extracts by Microwave Activation from Laurus nobilis L. Leaves: Sustainable Multifunctional Ingredients for Potential Cosmetic and Pharmaceutical Applications. Molecules. 2025; 30(14):3006. https://doi.org/10.3390/molecules30143006
Chicago/Turabian StyleCaviglia, Debora, Eleonora Russo, Anna Maria Schito, Francesco Saverio Robustelli della Cuna, Elena Grignani, Nicola Lionetti, and Carla Villa. 2025. "NaDES-Based Extracts by Microwave Activation from Laurus nobilis L. Leaves: Sustainable Multifunctional Ingredients for Potential Cosmetic and Pharmaceutical Applications" Molecules 30, no. 14: 3006. https://doi.org/10.3390/molecules30143006
APA StyleCaviglia, D., Russo, E., Schito, A. M., Robustelli della Cuna, F. S., Grignani, E., Lionetti, N., & Villa, C. (2025). NaDES-Based Extracts by Microwave Activation from Laurus nobilis L. Leaves: Sustainable Multifunctional Ingredients for Potential Cosmetic and Pharmaceutical Applications. Molecules, 30(14), 3006. https://doi.org/10.3390/molecules30143006