Antibacterial, Antifungal, and Wound-Healing Activities and Chemical Characterization of Propolis from Apis mellifera in Michoacan, Mexico
Abstract
1. Introduction
2. Results
2.1. Organoleptic Characteristics
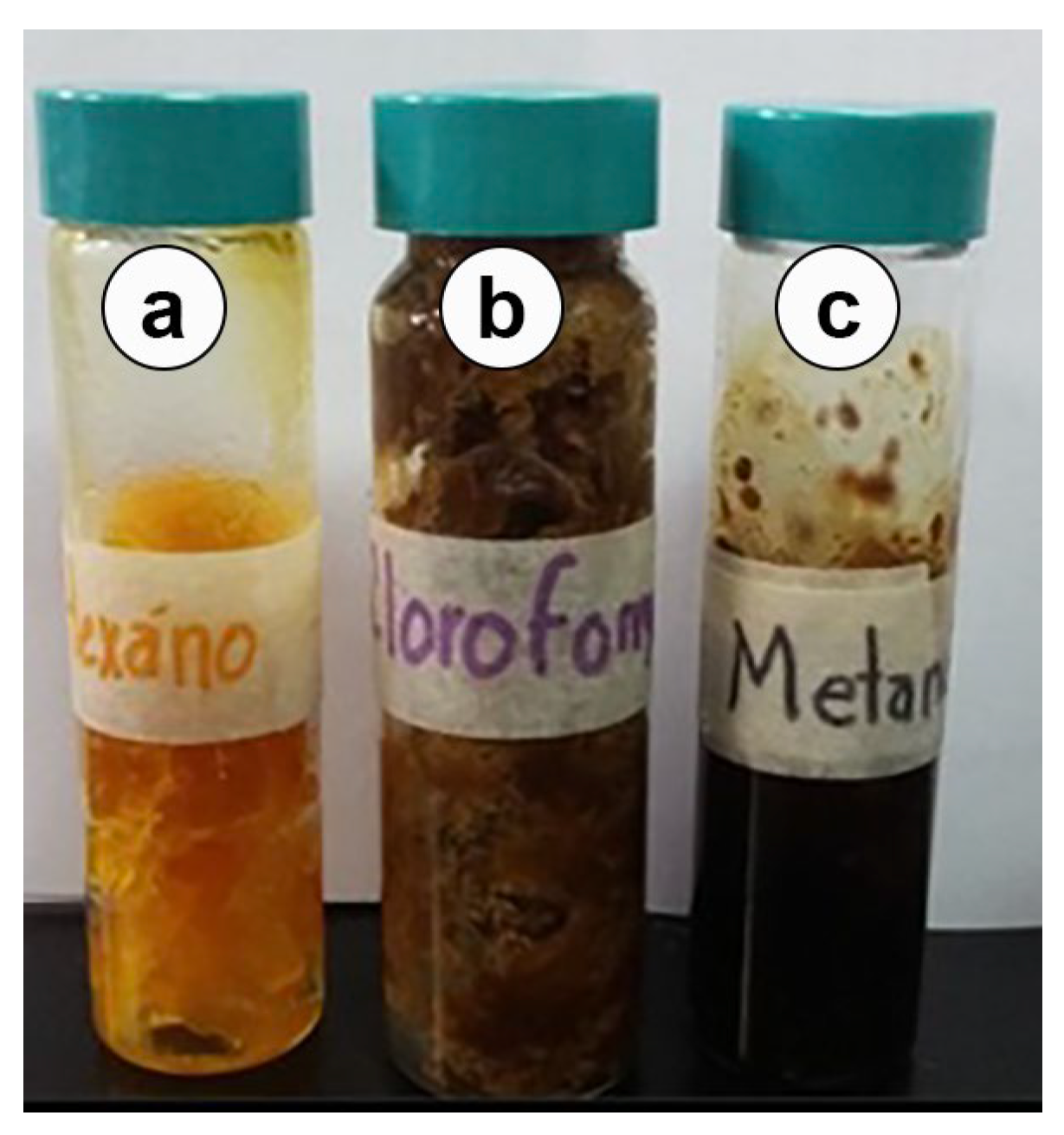
2.2. Propolis Sample
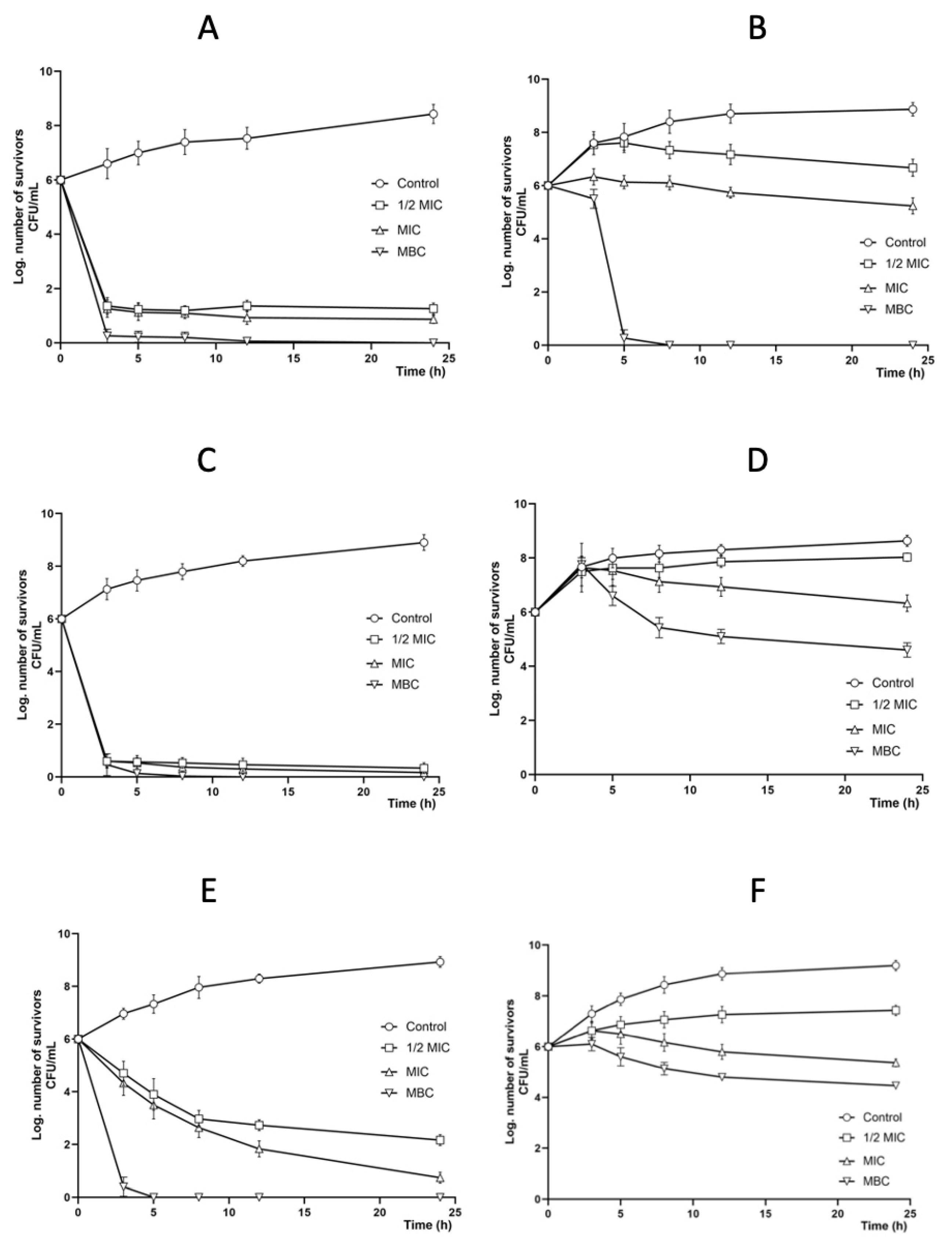
2.3. Antibacterial Activity
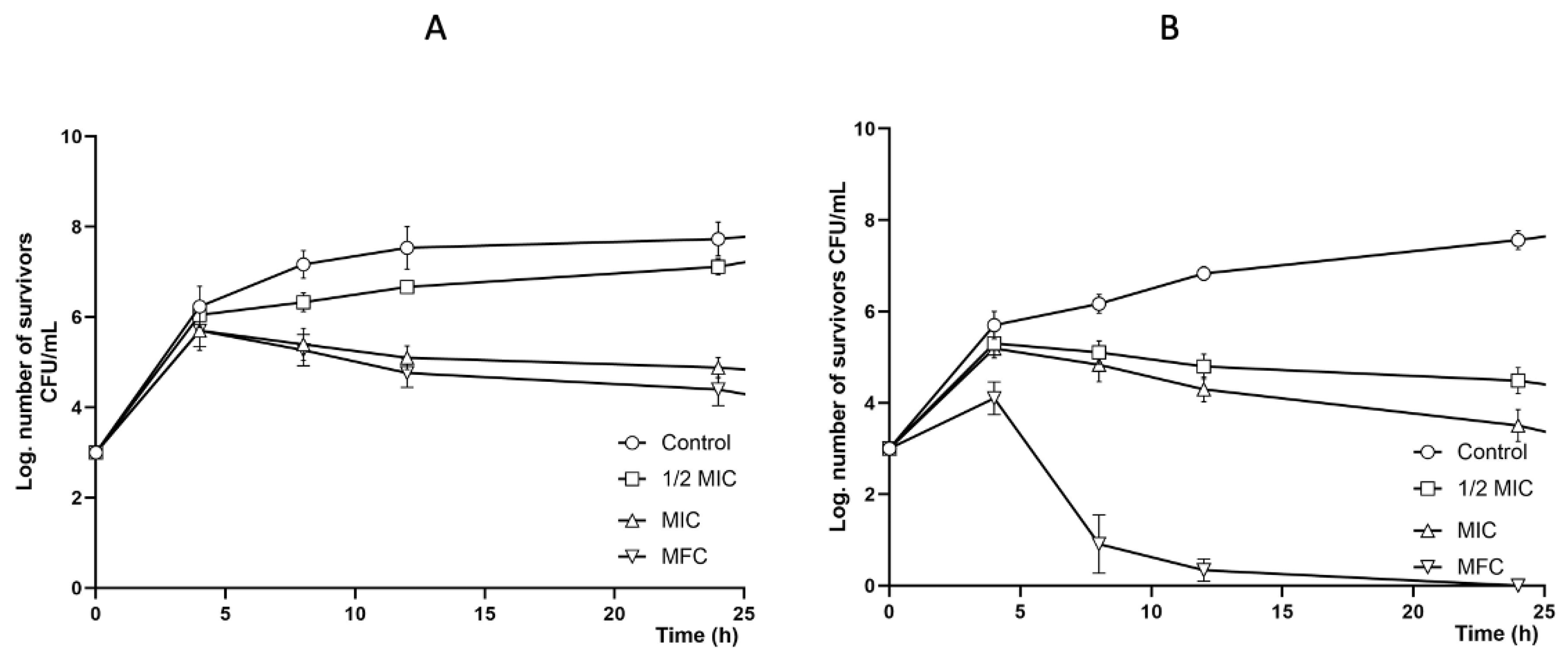
2.4. Antifungal Activity
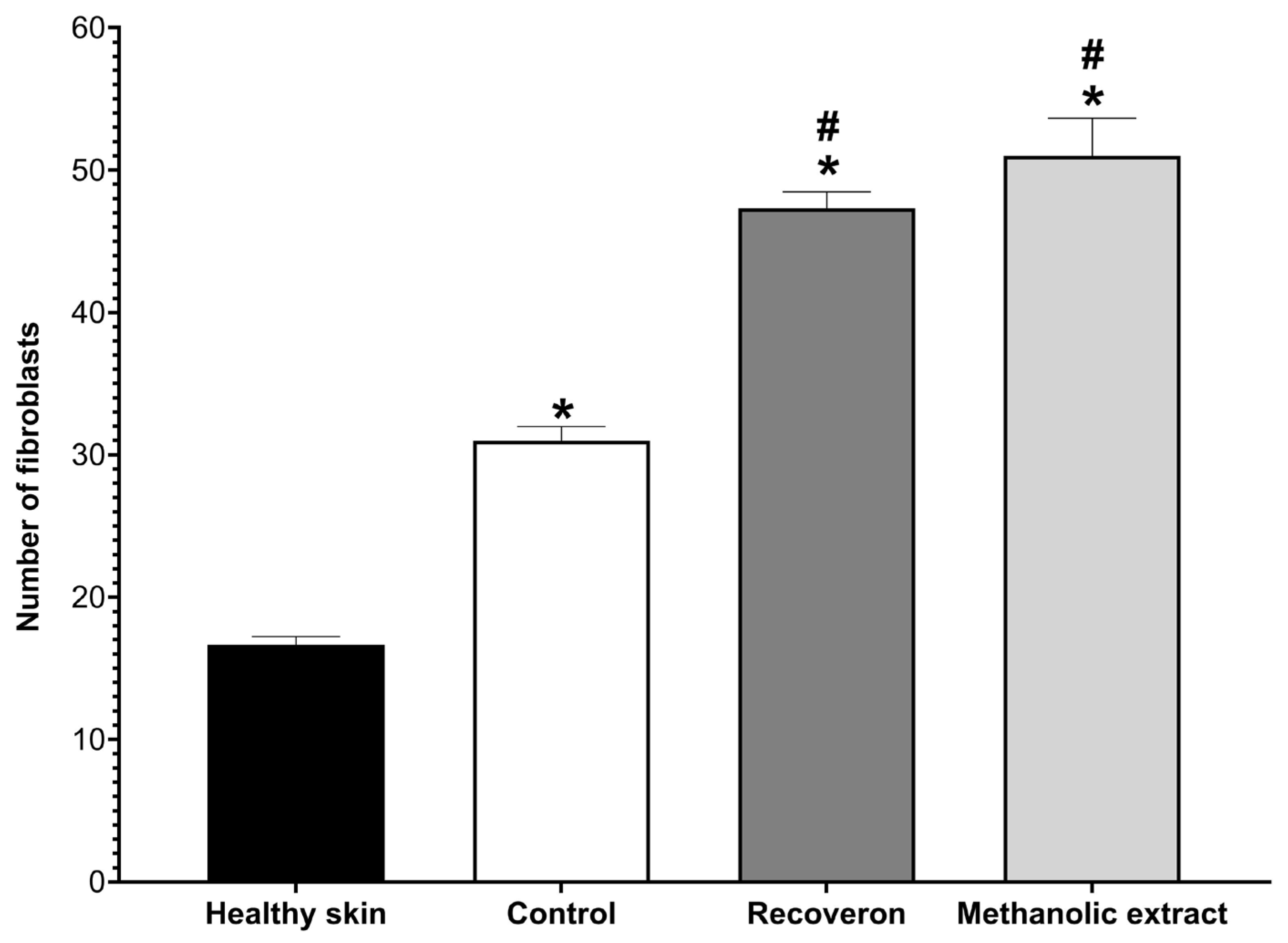
2.5. Wound-Healing Activity
2.5.1. Tensiometric Method
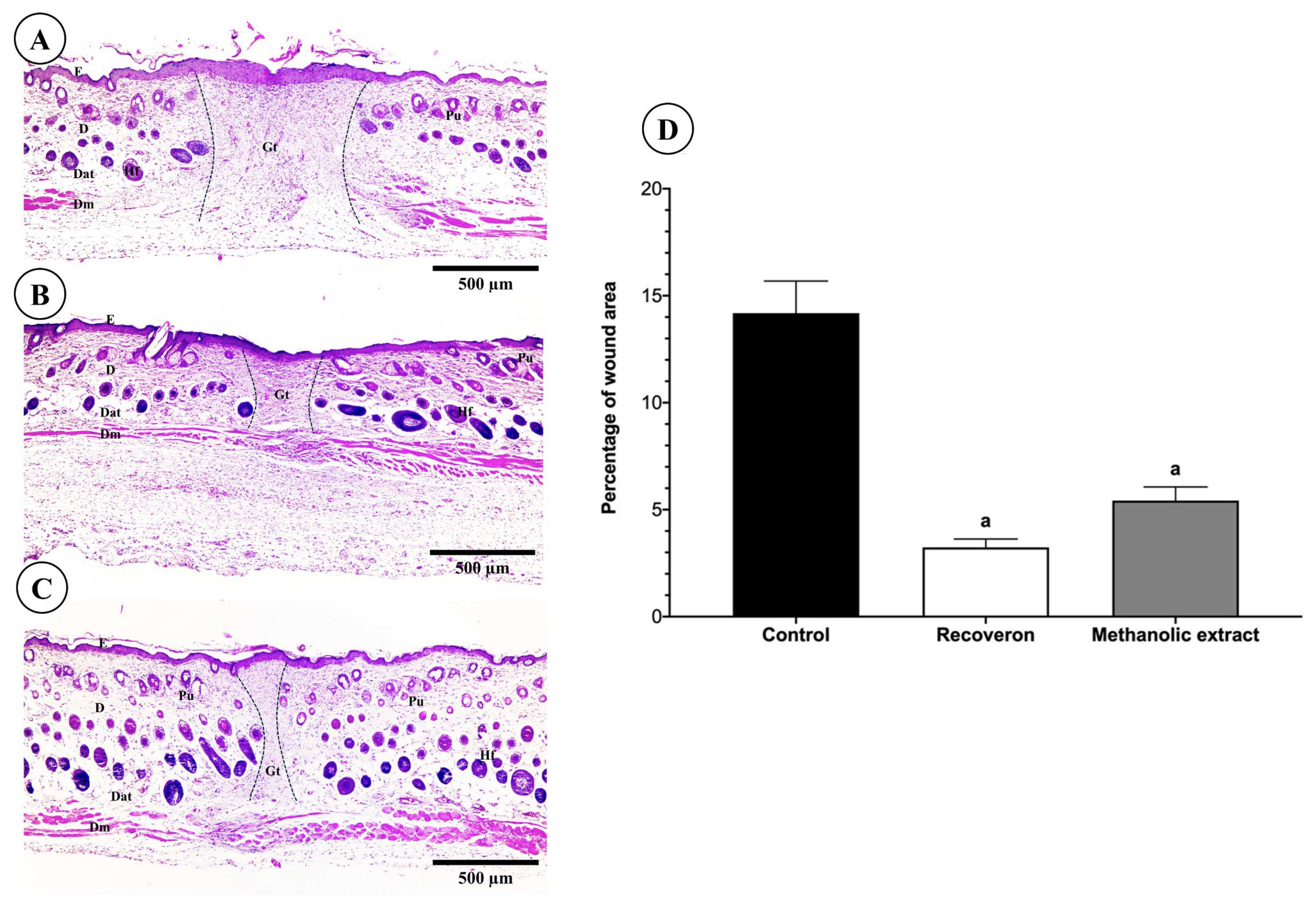
2.5.2. Histological Evaluation
2.6. Antioxidant Activity
2.7. Total Phenol and Flavonoid Contents
2.8. GC-MS, HPLC-DAD, and HPLC-MS Analyses
3. Discussion
4. Materials and Methods
4.1. Organoleptic Characteristics
4.2. Propolis Sample
4.3. Antibacterial Activity Assay
4.4. Antifungal Activity Assay
4.4.1. Yeast
4.4.2. Filamentous Fungi
4.5. Laboratory Animals
4.6. Wound-Healing Activity
4.6.1. Tensiometric Method
4.6.2. Histological Evaluation
4.7. Antioxidant Activity (DPPH Free Radical Scavenging)
4.8. Total Phenolic Content
4.9. Total Flavonoid Content
4.10. Analysis of the Chemical Composition of the Extracts Using GC-MS, HPLC-DAD, and HPLC-TOF-MS
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guzman-Gutierrez, S.; Nieto, A.; Castillo, J.; Huerta, E.; Hernandez-Pasteur, G.; Silva, M.; Argüello-Najera, O.; Sepulveda, O.; Espitia, C.; Reyes-Chilpa, R. Mexican propolis: A source of antioxidants and anti-inflammatory compounds, and isolation of a novel chalcone and epsilon-caprolactone derivative. Molecules 2018, 23, 334. [Google Scholar] [CrossRef]
- Ballouk, M.; Altinawi, M.; Fudalej, P. The multifaceted therapeutic potential of propolis: An integrative report on its pharmacological properties and emerging advances. Discov. Appl. Sci. 2025, 7, 962. [Google Scholar] [CrossRef]
- Zabaiou, N.; Fouache, A.; Trousson, A.; Baron, S.; Zellagui, A.; Lahouel, M.; Lobaccaro, J. Biological properties of propolis extracts: Something new from an ancient product. Chem. Phys. Lipids 2017, 207, 214–222. [Google Scholar] [CrossRef]
- Zulhendri, F.; Chandrasekaran, K.; Kowacz, M.; Ravalia, M.; Kripal, K.; Fearnley, J.; Perera, C. Antiviral, antibacterial, antifungal and antiparasitic properties of propolis: A review. Foods 2021, 10, 1360. [Google Scholar] [CrossRef]
- Galeotti, F.; Maccari, F.; Fachini, A.; Volpi, N. Chemical Composition and Antioxidant Activity of Propolis Prepared in Different Forms and in Different Solvents Useful for Finished Products. Foods 2018, 7, 41. [Google Scholar] [CrossRef]
- Delgado, M.; Ortega, J.; Ramírez, C. Caracterización fisicoquímica de propóleos colectados en el Bosque La Primavera Zapopan, Jalisco. Rev. Mex. Cien. For. 2015, 6, 74–87. [Google Scholar]
- Scherer, R.; Teixeira, H. Antioxidant activity index (AAI) by the 2,2-diphenyl- 1 -picrylhydrazyl method. Food Chem. 2009, 112, 654–658. [Google Scholar] [CrossRef]
- Dezmirean, D.; Paşca, C.; Moise, A.; Bobiş, O. Plant sources responsible for the chemical composition and main bioactive properties of poplar-type proplis. Plants 2020, 10, 22. [Google Scholar] [CrossRef]
- Kuropatnicki, A.; Szliszka, E.; Krol, W. Historical aspects of propolis research in modern times. Evid. Based Complement. Altern. Med. 2013, 2013, 964149. [Google Scholar] [CrossRef]
- NOM-003-SAG/GAN-2017; Propóleos, Producción y Especificaciones Para su Procesamiento. Secretaria de Gobernación: Mexico City, Mexico, 2017; Primera Sección. pp. 27–34.
- Rodriguez, B.; Canales, M.; Penieres, J.; Cruz, T. Composicion química, propiedades antioxidantes y actividad antimicrobiana de propoleos mexicanos. Acta Univ. 2020, 30, 1–29. [Google Scholar] [CrossRef]
- Kaltsas, A. Oxidative stress and male infertility: The protective role of antioxidants. Medicina 2023, 59, 1769. [Google Scholar] [CrossRef] [PubMed]
- Ming, D.; Wang, D.; Cao, F.; Xiang, H.; Mu, D.; Cao, J.; Li, B.; Zhong, L.; Dong, X.; Zhong, X. Kaempferol inhibits the primary attachment phase of biofilm formation in Staphylococcus aureus. Front. Microbiol. 2017, 8, 2263. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; Olomukoro, A.; Emmons, R.; Godage, N.; Gionfriddo, E. Matrix effects demystified: Strategies for resolving challenges in analytical separations of complex samples. J. Sep. Sci. 2023, 46, 2300571. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. The Nrf2/HO-1 axis as targets for flavanones: Neuroprotection by pinocembrin, naringenin, and eriodictyol. Oxid. Med. Cell. Longev. 2019, 13, 4724920. [Google Scholar] [CrossRef]
- Kozyra, M.; Biernasiuk, A.; Wiktor, M.; Kukula-Koch, W.; Malm, A. Comparative HPLC-DAD-ESI-QTOF/MS/MS analysis of bioactive phenolic compounds content in the methanolic extracts from flowering herbs of Monarda species and their free radical scavenging and antimicrobial activities. Pharmaceutics 2023, 15, 964. [Google Scholar] [CrossRef]
- Ramirez, L.; Marin, D. Metodologias para evaluar in vitro la actividad antibacteriana de compuestos de origen vegetal. Sci. Tech. 2009, 42, 263–268. [Google Scholar]
- Kuete, V. Potential of Cameroonian plants and derived products against microbial infections: A review. Planta Medica 2010, 76, 1479–1491. [Google Scholar] [CrossRef]
- Cerqueira, P.; Cunha, A.; Almeida, C. Potential of Propolis antifungal activity for clinical applications. J. Appl. Microbiol. 2022, 133, 1207–1228. [Google Scholar] [CrossRef]
- Granados, J.; Perez, R. Advances in chemical composition and biological activity of Mexican propolis. JPCR 2020, 2. [Google Scholar] [CrossRef]
- Sforcin, J. Biological properties and therapeutic applications of propolis. Phytother. Res. 2016, 30, 894–905. [Google Scholar] [CrossRef]
- Bhatti, N.; Hajam, Y.; Mushtaq, S.; Kaur, L.; Kumar, R.; Rai, S. A review on dynamic pharmacological potency and multifaceted biological activities of propolis. Discov. Sustain. 2024, 5, 185. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, M.; Shang, Z.; Xu, X.; Chen, X.; Zhu, Z.; Wang, W.; Wei, X.; Zhou, X.; Bai, Y. Research progress on the antibacterial activity of natural flavonoids. Antibiotics 2025, 14, 334. [Google Scholar] [CrossRef]
- Wojtyczka, R.; Dziedzic, A.; Idzik, D.; Kepa, M.; Kubina, R.; Kabala-Dzik, A.; Smolen-Dzirba, J.; Stojko, J.; Sajewicz, M.; Wasik, T. Susceptibility of Staphylococcus aureus clinical isolates to propolis extract alone or in combination with antimicrobial drugs. Molecules 2013, 18, 9623–9640. [Google Scholar] [CrossRef]
- Rana, A.; Malik, A.; Chander, R. Anti-bacterial properties of propolis: A comprehensive review. Curr. Microbiol. 2025, 82, 479. [Google Scholar] [CrossRef] [PubMed]
- Klancnik, A.; Simunovic, K.; Kovac, J.; Sahin, O.; Wu, Z.; Vuckovic, D.; Abram, M.; Zhang, Q.; Smole, S. The Anti-Campylobacter activity and mechanisms of pinocembrin action. Microorganism 2019, 7, 675. [Google Scholar] [CrossRef] [PubMed]
- Osonga, F.; Akgul, A.; Miller, R.; Eshun, G.; Yazgan, I.; Akgul, A.; Sadik, O. Antimicrobial activity of a new class of phosphorylated and modified flavonoids. ACS Omega 2019, 4, 12865–12871. [Google Scholar] [CrossRef] [PubMed]
- Soromou, L.; Zhang, Y.; Cui, Y.; Wei, M.; Chen, N.; Yang, X.; Huo, M.; Baldé, A.; Guan, S.; Deng, X. Subinhibitory concentrations of pinocembrin exert anti-Staphylococcus aureus activity by reducing α-toxin expression. J. Appl. Microbiol. 2013, 115, 41–49. [Google Scholar] [CrossRef]
- Kharsany, K.; Viljoen, A.; Leonard, C.; Van Vuuren, S. The new buzz: Investigating the antimicrobial interactions between bioactive compounds found in South African propolis. J. Ethnopharmacol. 2019, 238, 111867. [Google Scholar] [CrossRef]
- Rasul, A.; Martin, F.; Ali, W.; Ali, M.; Li, J.; Li, X. Pinocembrin: A novel natural compound with versatile pharmacological and biological activities. BioMed Res. Int. 2013, 2013, 379850. [Google Scholar] [CrossRef]
- Al-Azzawi, M.; Sami, I.; Mamdooh, I.; Al-Fahham, A.A. The chemical compositions and antimicrobial activity of propolis: A review article. Int. J. Health Res. 2024, 3, 535–539. [Google Scholar] [CrossRef]
- Gucwa, K.; Kusznierewicz, B.; Milewski, S.; Van Dijck, P.; Szweda, P. Antifungal activity and synergism with azoles of Polish propolis. Pathogens 2018, 7, 56. [Google Scholar] [CrossRef]
- Rodriguez, M.; Medina, Y.; Rodriguez, M.; Nava, U.; Bolaños, S.; Mendoza, M.; Campos, J.; Hernandez, A.; Chirino, Y.; Cruz, T.; et al. Activity of Propolis from Mexico on the proliferation and virulence factors of Candida albicans. BMC Microbiol. 2023, 23, 325. [Google Scholar] [CrossRef]
- Iadnut, A.; Mamoon, K.; Thammasit, P.; Pawichai, S.; Tima, S.; Preechasuth, K.; Kaewkod, T.; Tragoolpua, Y.; Tragoolpua, K. In vitro antifungal and antivirulence activities of biologically synthesized ethanolic extract of propolis-loaded PLGA nanoparticles against Candida albicans. Evid. Based Complement. Altern. Med. 2019, 3715481. [Google Scholar] [CrossRef] [PubMed]
- Freires, I.; Queiroz, V.; Furletti, V.; Ikegaki, M.; de Alencar, S.; Duarte, M.; Rosalen, P. Chemical composition and antifungal potential of Brazilian propolis against Candida spp. J. Mycol. Med. 2016, 26, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Calderon, M.; Hernandez-Gonzalez, L.; Gomez-Navia, C.; Blanco-Blanco, M.; Sánchez-Silos, R.; Lucio, L.; Pérez-Giraldo, C. Antifungal and anti-biofilm activity of a new Spanish extract of propolis against Candida glabrata. BMC Complement. Med. Ther. 2021, 21, 147. [Google Scholar] [CrossRef] [PubMed]
- Batac, M.; Sison, M.; Cervancia, C.; Nicolas, M. Honey and propolis have antifungal property against select dermatophytes and candida albicans. Acta Medica Philipp. 2020, 54, 11–16. [Google Scholar] [CrossRef]
- Koç, N.; Silici, S. Comparative study of in vitro methods used to analyse the antifungal activity of propolis against Trichophyton rubrum and Trichophyton mentagrophytes. Ann. Microbiol. 2008, 58, 543–547. [Google Scholar] [CrossRef]
- Gavanji, S.; Asgari, M.; Vaezi, R.; Larki, B. Antifungal effect of the extract of propolis on the growth of three species of Epidermophyton flucosum, Trichophyton violaseum and Trichophyton tonsorans in laboratory environment. Afr. J. Pharm. Pharmacol. 2011, 5, 2642–2646. [Google Scholar] [CrossRef]
- Florez, J.; Armijo, J.; Mediavilla, A. Farmacologia Humana, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Mamun, A.; Shao, C.; Geng, P.; Wang, S.; Xiao, J. Recent advances in molecular mechanisms of skin wound healing and its treatments. Front. Immunol. 2024, 15, 2024. [Google Scholar] [CrossRef]
- Elbatreek, M.; Mahdi, I.; Ouchari, W.; Mahmoud, M.; Sobeh, M. Current advances on the therapeutic potential of pinocembrin: An updated review. Biomed. Pharmacother. 2023, 157, 114032. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, P.; Meena, A.; Luqman, S. Acacetin, a flavone with diverse therapeutic potential in cancer, inflammation, infections and other metabolic disorders. Food Chem. Toxicol. 2020, 145, 111708. [Google Scholar] [CrossRef]
- Yan, B.; Cao, G.; Sun, T.; Zhao, X.; Hu, X.; Yan, J.; Peng, Y.; Shi, A.; Li, Y.; Xue, W.; et al. Determination of pinocembrin in human plasma by solid-phase extraction and LC/MS/MS: Application to pharmacokinetic studies. Biomed. Chromatogr. 2014, 28, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.; Ravikumar, G.; Nagella, P.; Joseph, B.; Alessa, F.; Al-Mssallem, M. Flavonoids as potential anti-inflammatory molecules: A review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Wu, H.; Lin, X.; Yang, X.; Ding, Y.; Wang, J.; Liu, C.; Yu, W. Kaempferol attenuates inflammation in lipopolysaccharide-induced gallbladder epithelial cells by inhibiting the MAPK/NF-κB signaling pathway. Chem. Biol. Drug Des. 2024, 103, 14519. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Deng, Z.; Rong, X.; Li, R.; You, Z.; Guo, X.; Cai, C.; Zhao, Y.; Gao, P.; Cao, G.; et al. Naringenin prevents oxidative stress and inflammation in LPS- induced liver injury through the regulation of lncRNA-mRNA in male mice. Molecules 2023, 28, 198. [Google Scholar] [CrossRef]
- Byun, E.; Song, H.; Kim, W.; Han, J.; Seo, H.; Park, W.; Kim, K.; Byun, E. Chrysin derivative CM1 and exhibited anti-inflammatory action by upregulating toll-interacting protein expression in lipopolysaccharide-stimulated RAW 264.7 macrophage cells. Molecules 2021, 26, 1532. [Google Scholar] [CrossRef] [PubMed]
- Hunt, M.; Torres, M.; Bachar, E.; Wilkstrom, J. Cellular and molecular roles of reactive oxygen species in wound healing. Commun. Biol. 2024, 7, 1534. [Google Scholar] [CrossRef]
- Maleki, S.; Crespo, J.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Da Rosa, C.; Bueno, I.; Martins, A.; Longato, G. Healing potential of propolis in skin wound evidenced by clinical studies. Pharmaceuticals 2022, 15, 1143. [Google Scholar] [CrossRef]
- Hozzein, W.; Badr, G.; Ghamdi, A.; Sayed, A.; Al-Waili, N.; Garraud, O. Topical application of propolis enhances cutaneous wound healing by promoting TGF-Beta/smad-mediated collagen production in a streptozotocin-induced type I diabetic mouse model. Cell. Physiol. Biochem. 2015, 37, 940–954. [Google Scholar] [CrossRef]
- Moscoso, M.; Bonilla, A.; Rivera, D.; Veloz, M. Estrés oxidativo en el envejecimiento y las enfermedades crónicas no transmitibles. RIC 2024, 103, e4807. [Google Scholar] [CrossRef]
- Guanche, D. Evaluación de diferentes extractos de propoleos. CENIC 2022, 53, 243–251. [Google Scholar]
- Aguayo, J.; Mora, S.; Tovar, X.; Navarro, R.; Valdez, M.; Ayala, J. Compuestos fenólicos, capacidad antioxidante y actividad antihipertensiva de membrillo (Cydonia oblonga Miller) cultivado en Zacatecas. Polibotánica 2024, 57, 199–212. [Google Scholar] [CrossRef]
- Lan, X.; Wang, W.; Li, Q.; Wang, J. The natural flavonoid pinocembrin: Molecular targets and potential therapeutic applications. Mol. Neurobiol. 2016, 53, 1794–1801. [Google Scholar] [CrossRef] [PubMed]
- Bastamy, M.; Elhady, M.; Raheel, I.; Elbestawy, A.; Abdel-Latif, M.; Orabi, A. Beneficial effects of pinocembrin and quercetin on the general conditions, immune parameters and gut lactobacilli in broiler chikens. VMJ-G 2025, 71, 40–54. [Google Scholar]
- Elbatreek, M.; Pachado, M.; Cuadrado, A.; Jandeleit-Dahm, K.; Schmidt, H. Reactive oxygen comes of age: Mechanism-based therapy of diabetic end-organ damage. Trends Endocrinol. Metab. 2019, 30, 312–327. [Google Scholar] [CrossRef]
- Cuadrado, A.; Manda, G.; Hassan, A.; Alcaraz, M.; Barbas, C.; Daiber, A.; Ghezzi, P.; Leon, R.; Lopez, M.; Oliva, B.; et al. Transcription factor NRF2 as a therapeutic target for chronic diseases: A systems medicine approach. Pharmacol. Rev. 2018, 70, 348–383. [Google Scholar] [CrossRef]
- Süntar, I.; Cetinkaya, S.; Panieri, E.; Saha, S.; Buttari, B.; Profumo, E.; Saso, L. Regulatory role of Nrf2 signaling pathway in wound healing process. Molecules 2021, 26, 2424. [Google Scholar] [CrossRef]
- Chen, X.; Wan, W.; Guo, Y.; Ye, T.; Fo, Y.; Sun, Y.; Qu, C.; Yang, B.; Zhang, C. Pinocembrin ameliorates post-infarct heart failure through activation of Nrf2/HO-1 signaling pathway. Mol. Med. 2021, 27, 100. [Google Scholar] [CrossRef]
- Said, M.; Azab, S.; Saeed, N.; El-Demerdash, E. Antifibrotic mechanism of pinocembrin: Impact on oxidative stress, inflammation and TGF-β /smad inhibition in rats. Ann. Hepatol. 2018, 17, 307–317. [Google Scholar] [CrossRef]
- Available online: http://ixmati.conabio.gob.mx/conocimiento/regionalizacion/doctos/rhp_060.html (accessed on 27 August 2025).
- CLSI. Performance Standards for Microbial Susceptibility Testing; Twentieth Informational Supplement; Tech. Rep. M100-S22; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2012. [Google Scholar]
- CLSI. Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts; M44; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; M27; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Savickiene, N.; Raudone, L. Trends in plants phytochemistry and bioactivity analysis. Plants 2024, 13, 3173. [Google Scholar] [CrossRef]
- ASTM. Standard Guide for Assessment of Antimicrobial Activity Using a Time-Kill Procedure; American Society for Testing and Materials: West Conshohocken, PA, USA, 2016. [Google Scholar]
- Medina, Y.; Rodriguez, M.; Rodriguez, M.; Hernandez, A.; Delgado, N.; Chirino, Y.; Cruz, T.; Garcia, C.; Canales, M. Effect of the essential oils of Bursera morelensis and Lippia graveolens and five pure compounds of the mycelium, spore production, and germination of species of Fusarium. J. Fungi 2022, 8, 617. [Google Scholar] [CrossRef]
- NOM-062-ZOO-1999; Especificaciones Técnicas Para la Producción, Cuidado y Uso de Los Animales de Laboratorio. Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación (SAGARPA): Mexico City, Mexico, 1999.
- Martinez, K.; Rodriguez, M.; Flores, C.; Hernandez, L.; Barbosa, E.; Canales, M. Comparison of biological properties of two medicinal extracts of the Tehuacan-Cucatlan Valley. Evid. Based Complement. Altern. Med. 2018, 2018, 4918090. [Google Scholar] [CrossRef]
- Hernandez, A.; Alarcon, F.; Almanza, J.; Nieto, O.; Olivares, J.; Duran, A.; Rodriguez, M.; Canales, M. Antimicrobial and anti-inflammatory activities, wound-healing effectiveness and chemical characterization of the latex of Jatropha neopauciflora Pax. J. Ethnopharmacol. 2017, 204, 1–7. [Google Scholar] [CrossRef]
- Chaves, N.; Santiago, A.; Alias, J. Quantification of the antioxidant activity of plant extracts: Analysis of sensitivity and hierarchization based on the method used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Nava, U.; Rodriguez, M.; Hernandez, A.; Velasco, D.; Moreno, B.; Rodriguez, M.; Canales, M. Antimicrobial activity of the methanolic leaf extracto f Prosopis laevigata. Sci. Rep. 2022, 12, 20807. [Google Scholar] [CrossRef]
- Ramamoorthy, K.; Bono, A. Antioxidant activity, total phenolic and flavonoid content of Morinda citrifolia fruit extracts from various extraction processes. JESTEC 2007, 2, 70–80. [Google Scholar]


| Organoleptic Characteristics | |
|---|---|
| Color | Brown |
| Smell | Resinous |
| Taste | Smooth balsamic |
| Consistency | Malleable |
| Extract | Yield (g) | Yield (%) |
|---|---|---|
| Hexanic | 19.20 | 19.55 |
| Chloroformic | 58.90 | 62.14 |
| Methanolic | 15.00 | 15.27 |
| Bacteria | Positive Control IH (mm) | Extract | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hexanic | Chloroformic | Methanolic | ||||||||
| IH (mm) | MIC mg/mL | MBC mg/mL | IH (mm) | MIC mg/mL | MBC mg/mL | IH (mm) | MIC mg/mL | MBC mg/mL | ||
| 1 | 12.0 | 6.6 ± 0.5 | 14.0 | >20.0 | 6.0 ± 0.6 | 10.0 | 20.0 | 18.4 ± 0.2 | 2.0 | 4.0 |
| 2 | 17.0 | 7.2 ± 0.0 | 20.0 | >20.0 | 7.0 ± 0.0 | 14.0 | >20.0 | 8.4 ± 0.5 | 2.0 | 4.0 |
| 3 | 20.0 | 7.2 ± 0.0 | 2.0 | 4.0 | 6.4 ± 0.6 | 12.0 | 20.0 | 13.2 ± 1.1 | 2.0 | 4.0 |
| 4 | 22.5 | 6.4 ± 0.4 | 14.0 | >20.0 | 7.2 ± 0.0 | 10.0 | >20.0 | 16.8 ± 2.7 | 4.0 | 8.0 |
| 5 | 17.2 | 6.7 ± 0.4 | 16.0 | >20.0 | 6.2 ± 0.4 | 8.0 | 20.0 | 18.4 ± 0.5 | 4.0 | 8.0 |
| 6 | 19.5 | 6.8 ± 0.4 | 20.0 | >20.0 | 7.0 ± 0.4 | 10.0 | >20.0 | 8.8 ± 0.8 | 2.0 | 4.0 |
| 7 | 23.2 | 6.7 ± 0.4 | 20.0 | >20.0 | 7.0 ± 0.0 | 20.0 | >20.0 | 16.0 ± 0.8 | 4.0 | 8.0 |
| 8 | 15.2 | 7.2 ± 0.0 | 20.0 | >20.0 | 6.0 ± 0.2 | 15.0 | 20.0 | 17.4 ± 1.1 | 2.0 | 4.0 |
| 9 | 23.5 | 7.0 ± 0.0 | 20.0 | >20.0 | 6.5 ± 0.5 | 10.0 | >20.0 | 11.6 ± 0.5 | 8.0 | 10.0 |
| 10 | 22.4 | 7.4 ± 0.2 | 20.0 | >20.0 | 7.0 ± 0.0 | 20.0 | >20.0 | 16.8 ± 1.7 | 8.0 | 10.0 |
| 11 | 17.5 | 8.3 ± 0.5 | 20.0 | >20.0 | 6.2 ± 0.4 | 15.0 | >20.0 | 16.4 ± 1.5 | 8.0 | 10.0 |
| 12 | 22.0 | 7.2 ± 0.0 | 12.0 | >20.0 | 6.4 ± 0.2 | 20.0 | >20.0 | 14.4 ± 1.0 | 8.0 | 10.0 |
| 13 | 20.6 | 7.2 ± 0.0 | 20.0 | >20.0 | 7.0 ± 0.0 | 10.0 | 20.0 | 16.4 ± 0.8 | 4.0 | 8.0 |
| Inhibition Halos (mm) | ||||
|---|---|---|---|---|
| Yeast | Nystatin | Methanolic Extract | MIC mg/mL | MFC mg/mL |
| C. albicans ATCC 10231 | 7.6 ± 1.1 | 6.3± 0.5 | 2.5 | 5.0 |
| C. albicans ATCC 14065 | 11.3 ± 2.3 | 6.0 ± 0.0 | 1.2 | 10.0 |
| C. albicans ATCC 32354 | 12.6 ± 1.5 | 6.0 ± 0.0 | 2.5 | 15.0 |
| C. albicans CDBB-L-1003 | 11.0 ± 1.7 | 6.0 ± 0.0 | 15.0 | 25.0 |
| C. albicans1 | 10.0 ± 0.0 | 6.6 ± 0.5 | 1.2 | 2.5 |
| C. albicans2 | 12.3 ± 5.6 | 6.0 ± 0.0 | 15.0 | 25.0 |
| C. glabrata CDBB-L-1536 | 7.6 ± 1.1 | 6.3 ± 0.5 | 1.2 | 25.0 |
| C. glabrata CBS 138 | 10.0 ± 1.0 | 6.0 ± 0.0 | 15.0 | 25.0 |
| C. glabrata1 | 10.3 ± 3.0 | 6.6 ± 0.5 | 2.5 | 25.0 |
| C. tropicalis CDBB-L-1098 | 10.3 ± 1.5 | 6.0 ± 0.0 | 2.5 | 25.0 |
| C. tropicalis1 | 11.0 ± 1.0 | 6.3 ± 0.5 | 1.2 | 15.0 |
| C. tropicalis2 | 9.6 ± 1.5 | 6.0 ± 0.0 | 1.2 | 15.0 |
| C. tropicalis3 | 11.0 ± 1.7 | 7.0 ± 1.7 | 2.5 | 15.0 |
| C. neoformans | 8.6 ± 2.3 | 6.0 ± 1.0 | 15.0 | 25.0 |
| Strain | Ketoconazole | Methanolic | Chloroformic | Hexanic |
|---|---|---|---|---|
| F. moniliforme CDBB-H-265 | +++ | ++ | ++ | ++ |
| T. mentagrophytes CDBB-H-1112 | +++ | +++ | ++ | ++ |
| A. niger CDBB-H-179 | ++ | ++ | + | NA |
| R. lilacina CDBB-H-306 | ++ | ++ | NA | ++ |
| Methanolic | Chloroformic | Hexanic | |
|---|---|---|---|
| FC50 (mg/mL) | 1.25 | 2.50 | 2.50 |
| MFC (mg/mL) | 2.50 | 5.00 | 5.00 |
| Extract/Standard | IC50 μg/mL | AAI |
|---|---|---|
| Methanolic | 12.23 | 2.25 |
| Chloroformic | 1210.35 | 0.02 |
| Hexanic | 1354.50 | 0.02 |
| Quercetin | 5.30 | 4.58 |
| Antioxidant Activity Index (AAI) | ||
| Poor | AAI <0.50 | |
| Moderate | AAI 0.50–1.00 | |
| Strong | AAI 1.00–2.00 | |
| Very strong | AAI >2.00 | |
| Biomolecule | Methanolic Extract | Chloroformic Extract |
|---|---|---|
| Phenols (mg AGE/g) | 580.00 | 20.00 |
| Flavonoids (mg QE/g) | 12.35 | 3.65 |
| Compound | Retention Time (min) | Similarity (%) | Abundance in Hexanic Extract (%) |
|---|---|---|---|
| Eicosane | 16.977 | 93 | 21.205 |
| Heptacosane | 23.445 | 91 | 10.692 |
| Hexacosane | 14.216 | 95 | 5.802 |
| Compound | Retention Time (min) | Abundance (%) |
|---|---|---|
| Arabinofuranose | 11.083 | 18.196 |
| beta-D-Lyxopyranose | 11.950 | 0.474 |
| D-Xylose | 13.332 | 1.502 |
| 6-O-methyl-beta-D-Glucopyranose | 13.456 | 9.531 |
| Inositol | 14.507 | 0.867 |
| Compound | Retention Time (min) | λ Max (nm) | ||
|---|---|---|---|---|
| CE | ME | CE | ME | |
| Pinocembrin | 8.273 | 8.636 | 210, 228, 290, 330. | 210, 230, 290, 332. |
| Acacetin | 46.739 | 48.309 | 269, 336. | 210, 268, 324. |
| Kaempferol | ND | 6.984 | - | 266, 294, 366. |
| Compound | Retention Time (min) | Parent Ion (m/z) | ||||
|---|---|---|---|---|---|---|
| HE | CE | ME | HE | CE | ME | |
| Chrysin | 29.649 | 29.732 | 29.817 | 253.0595 | 253.0611 | 253.0621 |
| Pinocembrin | 28.886 | 28.969 | 29.07 | 255.0753 | 255.0769 | 255.0781 |
| Naringenin | 21.884 | 22.018 | 22.086 | 271.0692 | 271.0721 | 271.0729 |
| Acacetin | ND | 31.458 | 31.542 | - | 283.0717 | 283.0725 |
| Parameters | Characteristics |
|---|---|
| Color | Red, yellow-reddish, yellow-dark, chestnut green, brown, or black, varying according to its botanical origin. |
| Smell | Resinous (woody smell) or balsamic (smell of wax), depending on its botanical origin. |
| Taste | Variable, smooth balsamic, strong, or spicy, depending on its botanical origin. |
| Consistency | Malleable or rigid at room temperature, depending on its botanical origin. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez-Hernandez, A.B.; Rodriguez-Canales, M.; Dominguez-Verano, P.; Nava-Solis, U.; Rodriguez-Monroy, M.A.; Canales-Martinez, M.M. Antibacterial, Antifungal, and Wound-Healing Activities and Chemical Characterization of Propolis from Apis mellifera in Michoacan, Mexico. Molecules 2025, 30, 3880. https://doi.org/10.3390/molecules30193880
Hernandez-Hernandez AB, Rodriguez-Canales M, Dominguez-Verano P, Nava-Solis U, Rodriguez-Monroy MA, Canales-Martinez MM. Antibacterial, Antifungal, and Wound-Healing Activities and Chemical Characterization of Propolis from Apis mellifera in Michoacan, Mexico. Molecules. 2025; 30(19):3880. https://doi.org/10.3390/molecules30193880
Chicago/Turabian StyleHernandez-Hernandez, Ana Bertha, Mario Rodriguez-Canales, Pilar Dominguez-Verano, Uriel Nava-Solis, Marco Aurelio Rodriguez-Monroy, and María Margarita Canales-Martinez. 2025. "Antibacterial, Antifungal, and Wound-Healing Activities and Chemical Characterization of Propolis from Apis mellifera in Michoacan, Mexico" Molecules 30, no. 19: 3880. https://doi.org/10.3390/molecules30193880
APA StyleHernandez-Hernandez, A. B., Rodriguez-Canales, M., Dominguez-Verano, P., Nava-Solis, U., Rodriguez-Monroy, M. A., & Canales-Martinez, M. M. (2025). Antibacterial, Antifungal, and Wound-Healing Activities and Chemical Characterization of Propolis from Apis mellifera in Michoacan, Mexico. Molecules, 30(19), 3880. https://doi.org/10.3390/molecules30193880










