Development of a Validated High-Performance Thin-Layer Chromatography (HPTLC) Analysis Protocol for Salivary Caffeine Used as a Probe Drug
Abstract
1. Introduction
2. Results and Discussion
2.1. HPTLC Method Optimisation
2.2. Method Validation
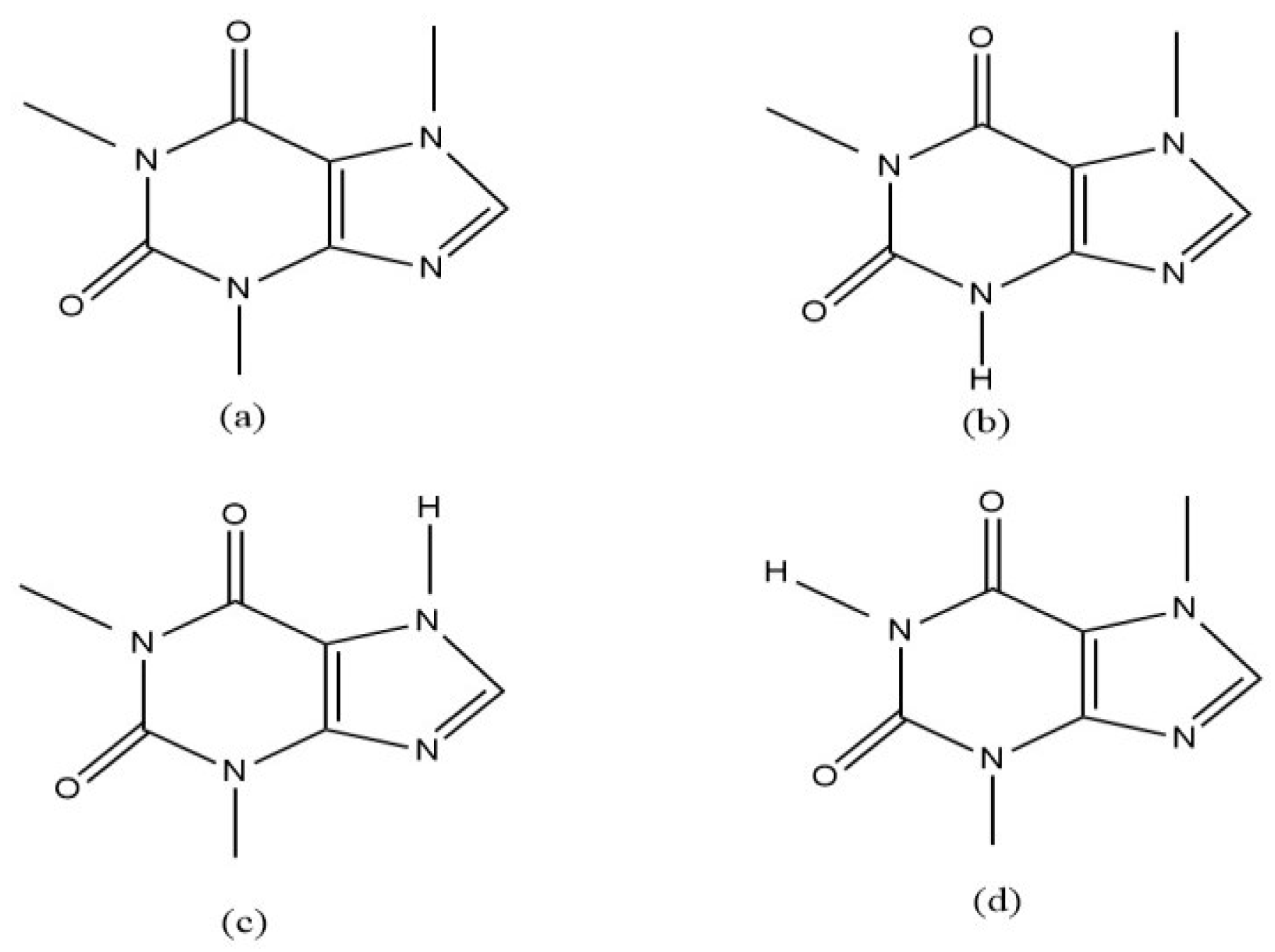
2.2.1. Specificity
2.2.2. Linearity
2.2.3. Sensitivity
2.2.4. Accuracy and Precision
2.2.5. Repeatability
2.2.6. Robustness
2.3. Optimisation of Saliva Sample Preparation
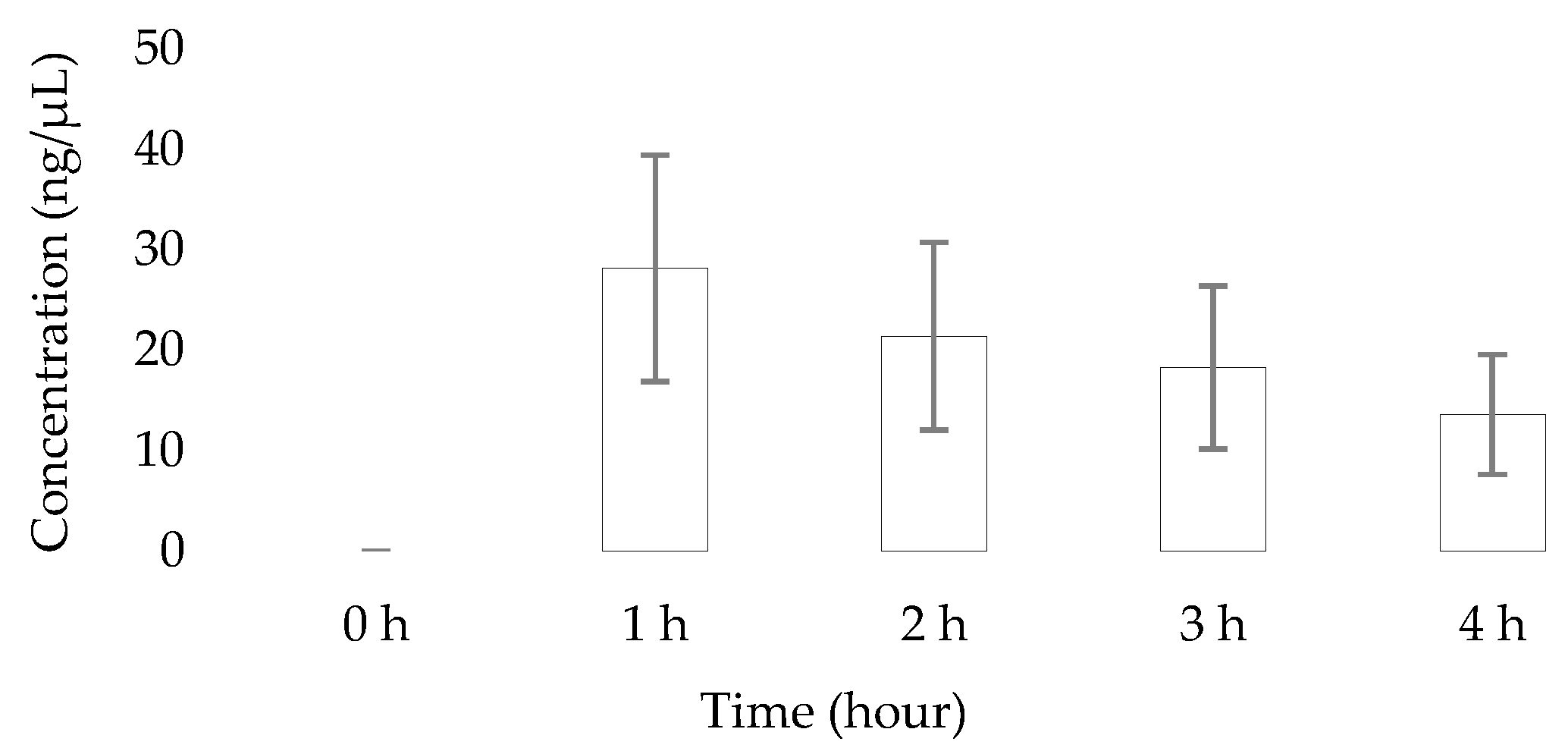
2.4. Application of the Developed HPTLC Method to the Analysis of Caffeine in Saliva Samples
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Standard Solution and Mobile Phase Preparation
3.3. HPTLC Instruments and Method Development
3.3.1. Standard and Sample Application
3.3.2. Sample Development
3.4. Method Validation
3.4.1. Specificity
3.4.2. Linearity
3.4.3. Sensitivity
3.4.4. Accuracy
3.4.5. Precision
3.4.6. Repeatability (System Precision)
3.4.7. Robustness
3.5. Method Optimisation for Saliva Sample Preparation
3.6. Human Ethics Approval and Clinical Saliva Sample Collection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roden, D.M.; McLeod, H.L.; Relling, M.V.; Williams, M.S.; Mensah, G.A.; Peterson, J.F.; Van Driest, S.L. Pharmacogenomics. Lancet 2019, 394, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhu, X.; Badawy, S.; Ihsan, A.; Liu, Z.; Xie, C.; Wang, X. Metabolism and Mechanism of Human Cytochrome P450 Enzyme 1A2. Curr. Drug Metab. 2021, 22, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.F.; Wang, B.; Yang, L.P.; Liu, J.P. Structure, function, regulation and polymorphism and the clinical significance of human cytochrome P450 1A2. Drug Metab. Rev. 2010, 42, 268–354. [Google Scholar] [CrossRef] [PubMed]
- Magliocco, G.; Desmeules, J.; Samer, C.F.; Thomas, A.; Daali, Y. Evaluation of CYP1A2 activity: Relationship between the endogenous urinary 6-hydroxymelatonin to melatonin ratio and paraxanthine to caffeine ratio in dried blood spots. Clin. Transl. Sci. 2022, 15, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
- Oliphant, E.A.; Purohit, T.J.; Alsweiler, J.M.; McKinlay, C.J.; Hanning, S.M. Validation and application of a simple and rapid stability-indicating liquid chromatographic assay for the quantification of caffeine from human saliva. J. Liq. Chromatogr. Relat. Technol. 2022, 22, 10–17. [Google Scholar] [CrossRef]
- Grzegorzewski, J.; Bartsch, F.; Koller, A.; Konig, M. Pharmacokinetics of Caffeine: A Systematic Analysis of Reported Data for Application in Metabolic Phenotyping and Liver Function Testing. Front. Pharmacol. 2021, 12, 752826. [Google Scholar] [CrossRef] [PubMed]
- Bonati, M.; Latini, R.; Galletti, F.; Young, J.F.; Tognoni, G.; Garattini, S. Caffeine Disposition after Oral Doses. Clin. Pharmacol. Ther. 1982, 32, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Liguori, A.; Hughes, J.R.; Grass, J.A. Absorption and subjective effects of caffeine from coffee, cola and capsules. Pharmacol. Biochem. Behav. 1997, 58, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Committee on Military Nutrition Research. Caffeine for the Sustainment of Mental Task Performance: Formulations for Military Operations. In Pharmacology of Caffeine; National Academies Press: Washington, DC, USA, 2001; Volume 2. [Google Scholar]
- Perera, V.; Gross, A.S.; McLachlan, A.J. Caffeine and paraxanthine HPLC assay for CYP1A2 phenotype assessment using saliva and plasma. Biomed. Chromatogr. 2010, 24, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Tiwari, B.; Patil, R.; Khanna, V.; Singh, V. The role of salivary caffeine clearance in the diagnosis of chronic liver disease. J. Oral Biol. Craniofac. Res. 2015, 5, 28–33. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Loos, W.J. Pharmacokinetic Concepts and Analytical Methodologies. In AACR Education Book; Case Western Reserve University: Cleveland, OH, USA, 2008; pp. 695–699. [Google Scholar][Green Version]
- Urry, E.; Jetter, A.; Landolt, H.P. Assessment of CYP1A2 enzyme activity in relation to type-2 diabetes and habitual caffeine intake. Nutr. Metab. 2016, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Perera, V.; Gross, A.S.; McLachlan, A.J. Influence of Environmental and Genetic Factors on CYP1A2 Activity in Individuals of South Asian and European Ancestry. Clin. Pharmacol. Ther. 2012, 92, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Begas, E.; Kouvaras, E.; Tsakalof, A.K.; Bounitsi, M.; Asprodini, E.K. Development and validation of a reversed-phase HPLC method for CYP1A2 phenotyping by use of a caffeine metabolite ratio in saliva. Biomed. Chromatogr. 2015, 29, 1657–1663. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadi, R.-M.; Berti, F.; Colomban, S.; Tavagnacco, C.; Navarini, L.; Resmini, M. Simultaneous Quantification of Antioxidants Paraxanthine and Caffeine in Human Saliva by Electrochemical Sensing for CYP1A2 Phenotyping. Antioxidants 2021, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.K.; Ghuge, A.; Parab, M.; Al-Refaei, Y.; Khandare, A.; Dand, N.; Waghmare, N. A comparative review on High-Performance Liquid Chromatography (HPLC), Ultra Performance Liquid Chromatography (UPLC) & High-Performance Thin Layer Chromatography (HPTLC) with current updates. Curr. Iss. Pharm. Med. Sci. 2022, 35, 224–228. [Google Scholar] [CrossRef]
- Faiyazuddin, M.; Rauf, A.; Ahmad, N.; Ahmad, S.; Iqbal, Z.; Talegaonkar, S.; Bhatnagar, A.; Khar, R.K.; Ahmad, F.J. A validated HPTLC method for determination of terbutaline sulfate in biological samples: Application to pharmacokinetic study. Saudi Pharm. J. 2011, 19, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Fenske, M. Caffeine determination in human saliva and urine by TLC and ultraviolet absorption densitometry. Chromatographia 2007, 65, 233–238. [Google Scholar] [CrossRef]
- Ruddy, D.; Sherma, J. Analysis of the caffeine in alertness tablets and caplets by high-performance thin-layer chromatography with ultraviolet absorption densitometry of fluorescence-quenched zones. Acta Chromatogr. 2002, 12, 143–150. [Google Scholar]
- Arage, A.; Layloff, T.; Hymete, A.; Ashenef, A. High Performance Thin Layer Chromatography (HPTLC) method development and validation for the simultaneous determination of paracetamol, caffeine, chlorpheniramine and phenylepherine in tablet formulation. Acta Chromatogr. 2023, 35, 170–178. [Google Scholar] [CrossRef]
- Oellig, C.; Schunck, J.; Schwack, W. Determination of caffeine, theobromine and theophylline in Mate beer and Mate soft drinks by high-performance thin-layer chromatography. J. Chromatogr. A 2018, 1533, 208–212. [Google Scholar] [CrossRef] [PubMed]
- International Council for Harmonisation (ICH). Validation of Analytical Procedures Q2 (R2); International Council for Harmonisation (ICH): Geneva, Switzerland, 2022; pp. 5–7. [Google Scholar]

| RF | Run | Linearity Range (ng/Band) | Regression Equation | Correlation Coefficient (R2) | Slope (m) | Slope (Average) | y-Intercept | SD of y-Intercept | LOD (ng/Band) | LOQ (ng/Band) | 95% CI (ng/Band) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.25 | Run 1 | 20–100 | y = 1 × 10−3x + 3.9 × 10−3 | 0.9990 | 9.57 × 10−4 | 1.01 × 10−3 | 5.04 × 10−3 | 7.41 × 10−4 | 2.42 | 7.34 | LOD: 0.91–6.44 LOQ: 2.75–19.5 |
| Run 2 | 20–100 | y = 1 × 10−3x + 4.9 × 10−3 | 0.9970 | 1.042 × 10−3 | 4.92 × 10−3 | ||||||
| Run 3 | 20–100 | y = 1 × 10−3x + 6.3 × 10−3 | 0.9964 | 1.032 × 10−3 | 6.26 × 10−3 |
| Theoretical Amount (ng/Band) | Run 1 | Run 2 | Run 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Amount Recovered (ng/Band) | % Recovery | % Mean Recovery | Amount Recovered (ng/Band) | % Recovery | % Mean Recovery | Amount Recovered (ng/Band) | % Recovery | % Mean Recovery | |
| 24 | 24.81 | 103.38 | 24.04 | 100.17 | 24.42 | 101.75 | |||
| 30 | 31.68 | 105.60 | 102.50 | 30.94 | 103.13 | 101.06 | 31.48 | 104.93 | 101.99 |
| 36 | 35.47 | 98.53 | 35.96 | 99.89 | 35.74 | 99.28 | |||
| Theoretical Amount (ng/Band) | Inter-Day | Intra-Day | ||||
|---|---|---|---|---|---|---|
| Amount Recovered (ng/Band) (Mean ± SD) | %RSD | % Recovery | Amount Recovered (ng/Band) (Mean ± SD) | %RSD | % Recovery | |
| 24 | 23.19 ± 0.15 | 0.65 | 96.63 | 24.43 ± 0.54 | 2.23 | 101.77 |
| 30 | 29.77 ± 0.92 | 3.08 | 99.23 | 31.31 ± 0.52 | 1.67 | 104.37 |
| 36 | 35.80 ± 0.98 | 2.74 | 99.43 | 35.72 ± 0.35 | 0.97 | 99.21 |
| Caffeine Theoretical Amount (ng/Band) | Caffeine Measured Amount (ng/Band) |
|---|---|
| 30 | 29.73 |
| 30 | 30.89 |
| 30 | 31.09 |
| 30 | 30.86 |
| 30 | 31.01 |
| Average | 30.72 |
| SD | 0.56 |
| %RSD | 1.82 |
| Parameters | Theoretical Amount (ng/Band) | % Recovery | RF Value (Mean ± SD) |
|---|---|---|---|
| Mobile Phase Development Amount (mL) | |||
| 8 | 50 | 104.42 | 0.25 ± 0.01 |
| 12 | 50 | 102.18 | 0.25 ± 0.03 |
| Saturation Time (minutes) | |||
| 15 | 50 | 100.60 | 0.25 ± 0.01 |
| 25 | 50 | 98.88 | 0.25 ± 0.02 |
| Mobile Phase Composition | |||
| Acetone/Toluene/Chloroform (4:4:3) | 50 | 98.66 | 0.25 ± 0.01 |
| Acetone/Toluene/Chloroform (4:3:4) | 50 | 99.62 | 0.25 ± 0.03 |
| Parameters | Theoretical Amount (ng/Band) | Recovered Amount (ng/Band) | Mean | SD | %RSD | ||
|---|---|---|---|---|---|---|---|
| Run 1 | Run 2 | Run 3 | |||||
| Non-centrifuged (1:1) | 20 | 19.75 | 20.61 | 20.21 | 20.19 | 0.43 | 2.15 |
| Centrifuged (1:1) | 20 | 19.13 | 21.04 | 19.97 | 20.05 | 0.96 | 4.78 |
| Non-centrifuged (1:0.5) | 20 | 18.93 | 19.89 | 20.23 | 19.68 | 0.67 | 3.43 |
| Centrifuged (1:0.5) | 20 | 19.49 | 19.44 | 20.25 | 19.73 | 0.45 | 2.30 |
| Non-centrifuged (1:1) | 40 | 40.14 | 41.39 | 41.99 | 41.17 | 0.94 | 2.29 |
| Centrifuged (1:1) | 40 | 41.67 | 42.64 | 41.82 | 42.04 | 0.52 | 1.24 |
| Non-centrifuged (1:0.5) | 40 | 37.68 | 41.60 | 40.62 | 39.97 | 2.04 | 5.10 |
| Centrifuged (1:0.5) | 40 | 40.42 | 39.10 | 37.40 | 38.97 | 1.51 | 3.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikdar, K.M.Y.K.; Shalan, A.; Castejon, V.; Chambers, C.; Coverley, S.R.; Yoo, O.; Islam, M.K.; Sostaric, T.; Lim, L.Y.; Burcham, P.; et al. Development of a Validated High-Performance Thin-Layer Chromatography (HPTLC) Analysis Protocol for Salivary Caffeine Used as a Probe Drug. Molecules 2025, 30, 3859. https://doi.org/10.3390/molecules30193859
Sikdar KMYK, Shalan A, Castejon V, Chambers C, Coverley SR, Yoo O, Islam MK, Sostaric T, Lim LY, Burcham P, et al. Development of a Validated High-Performance Thin-Layer Chromatography (HPTLC) Analysis Protocol for Salivary Caffeine Used as a Probe Drug. Molecules. 2025; 30(19):3859. https://doi.org/10.3390/molecules30193859
Chicago/Turabian StyleSikdar, K. M. Yasif Kayes, Ahmed Shalan, Vincent Castejon, Carly Chambers, Samara Renae Coverley, Okhee Yoo, Md Khairul Islam, Tomislav Sostaric, Lee Yong Lim, Philip Burcham, and et al. 2025. "Development of a Validated High-Performance Thin-Layer Chromatography (HPTLC) Analysis Protocol for Salivary Caffeine Used as a Probe Drug" Molecules 30, no. 19: 3859. https://doi.org/10.3390/molecules30193859
APA StyleSikdar, K. M. Y. K., Shalan, A., Castejon, V., Chambers, C., Coverley, S. R., Yoo, O., Islam, M. K., Sostaric, T., Lim, L. Y., Burcham, P., & Locher, C. (2025). Development of a Validated High-Performance Thin-Layer Chromatography (HPTLC) Analysis Protocol for Salivary Caffeine Used as a Probe Drug. Molecules, 30(19), 3859. https://doi.org/10.3390/molecules30193859










