Mycotoxin Contamination: Occurrence, Biotransformation, Pathogenic Mechanisms, and Strategies for Nutritional Intervention
Abstract
1. Introduction
2. Biosynthetic and In Vivo Metabolic Pathways
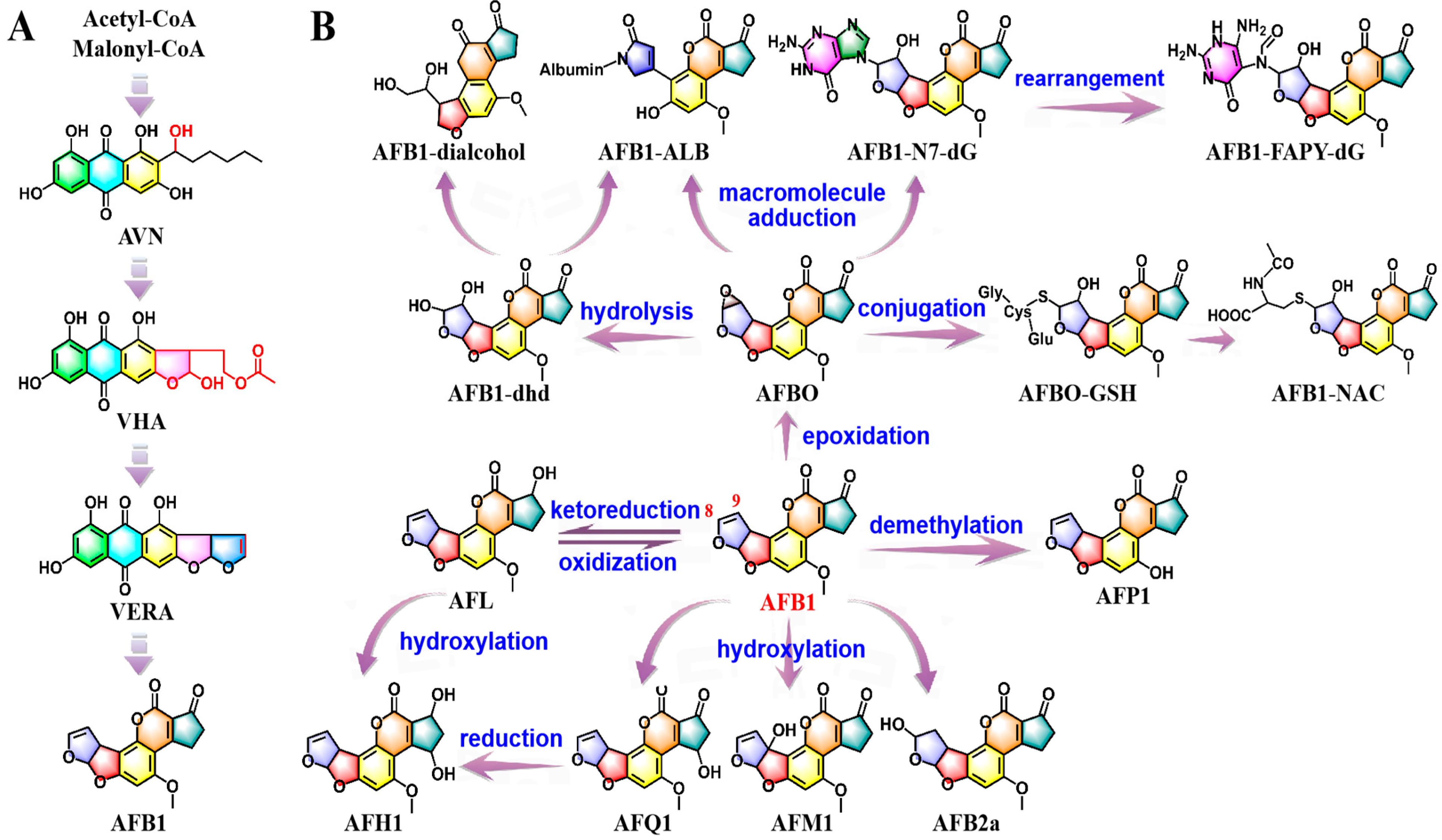
2.1. Biosynthesis and In Vivo Metabolic Pathways of AFB1
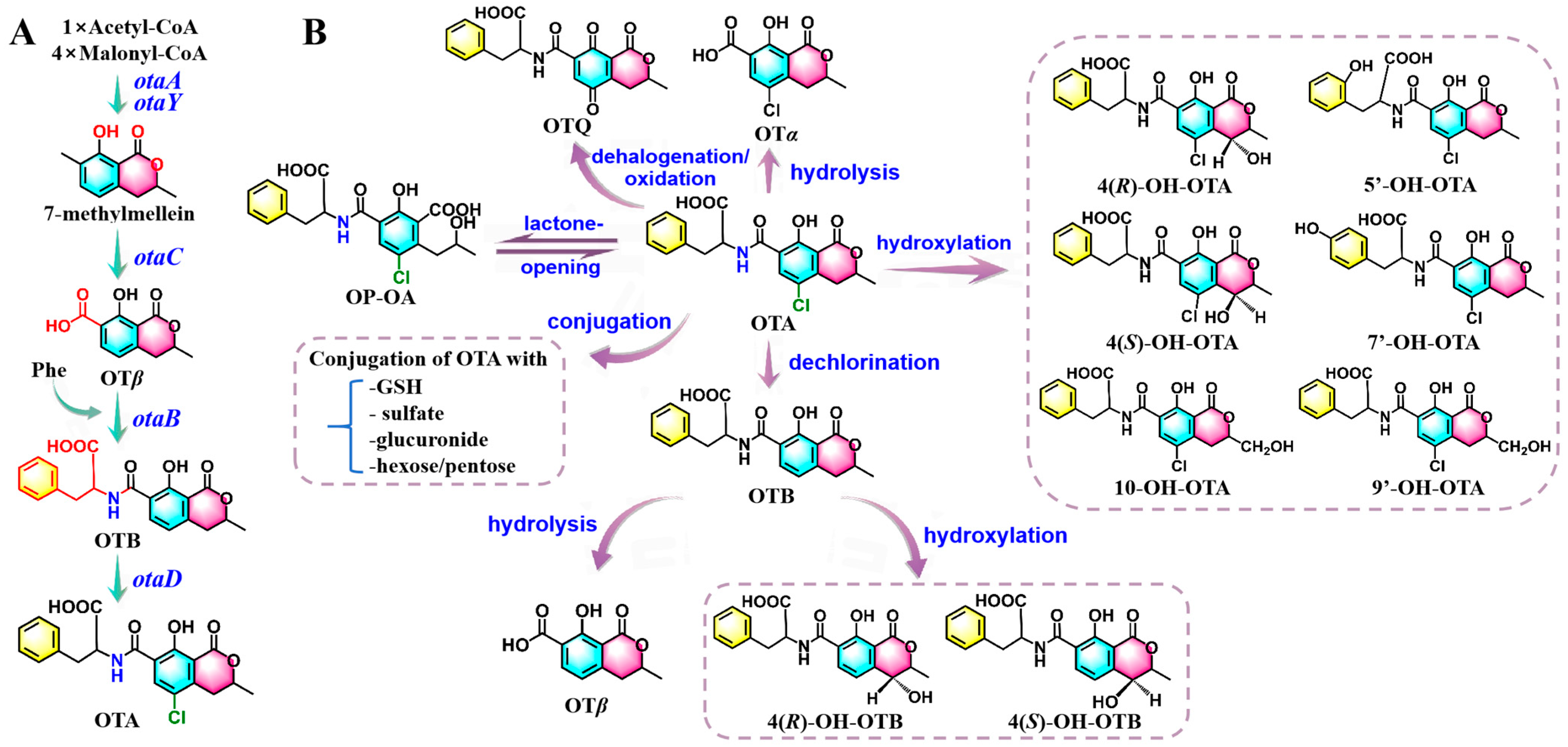
2.2. Biosynthesis and In Vivo Metabolic Pathways of OTA
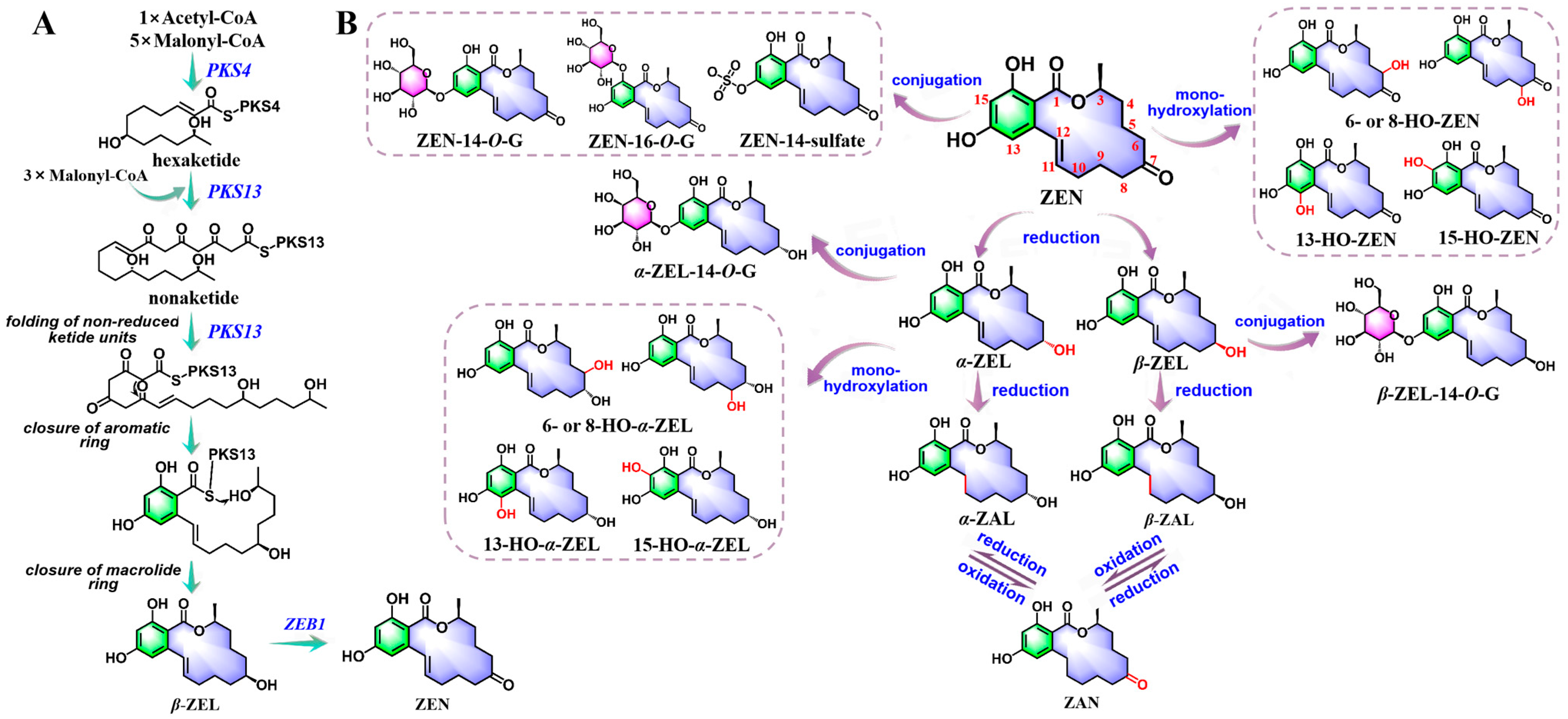
2.3. Biosynthesis and In Vivo Metabolic Pathways of ZEN
3. Mechanisms of Mycotoxin Pathogenesis
3.1. Structure-Related Toxicopathological Mechanisms of Mycotoxins
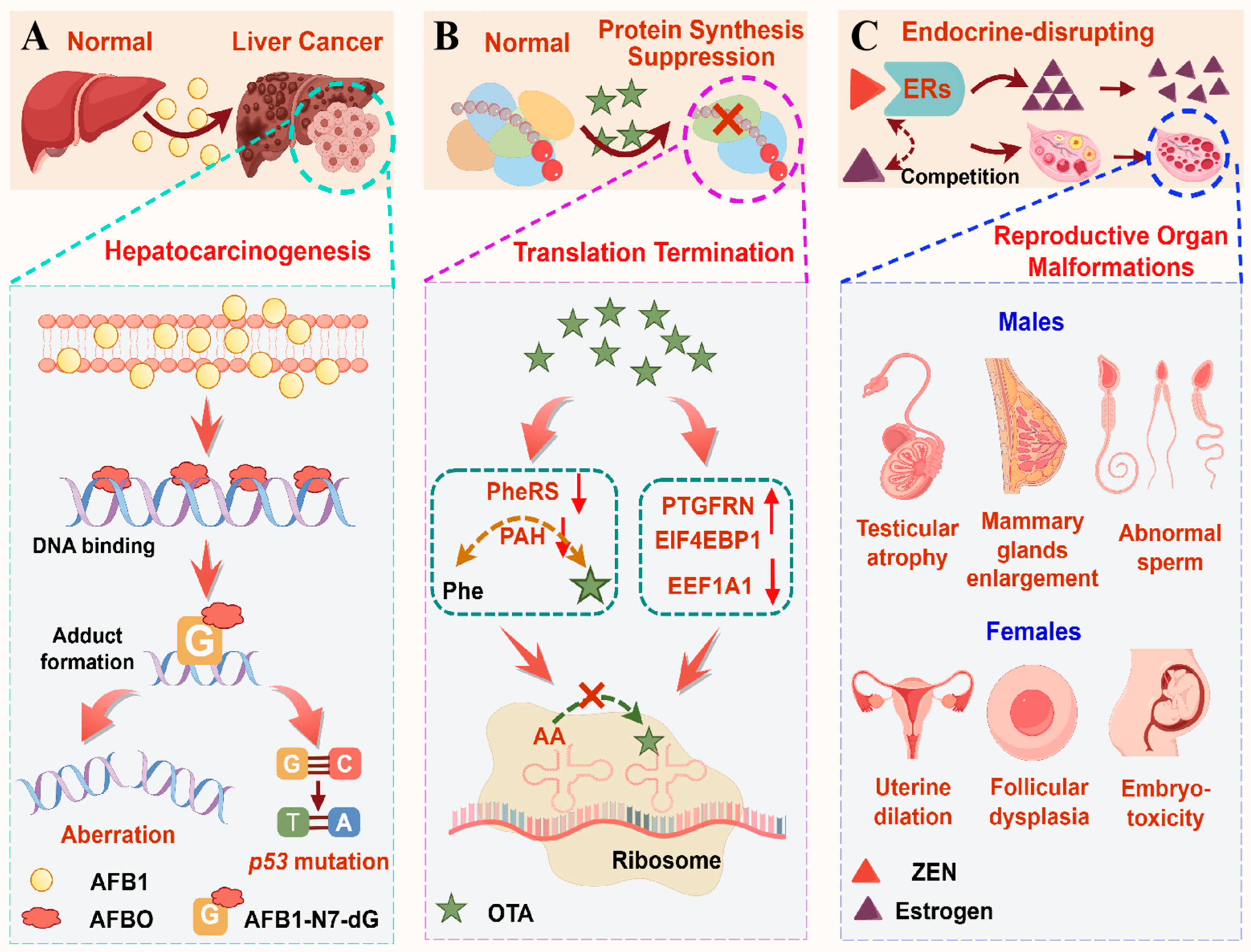
3.1.1. AFB1-Induced Hepatocarcinogenesis by Genotoxic Adduct Formation, Oxidative Mutagenesis, and Epigenetic Dysregulation
3.1.2. OTA-Induced Protein Synthesis Suppression by Phe/Isocoumarin-Mediated Dual Enzyme Inhibition Coupled with Ribosomal Translational Arrest
3.1.3. ZEN-Mediated Estrogenic Disruption by Endocrine Homeostasis Impairment and Pathological Alterations
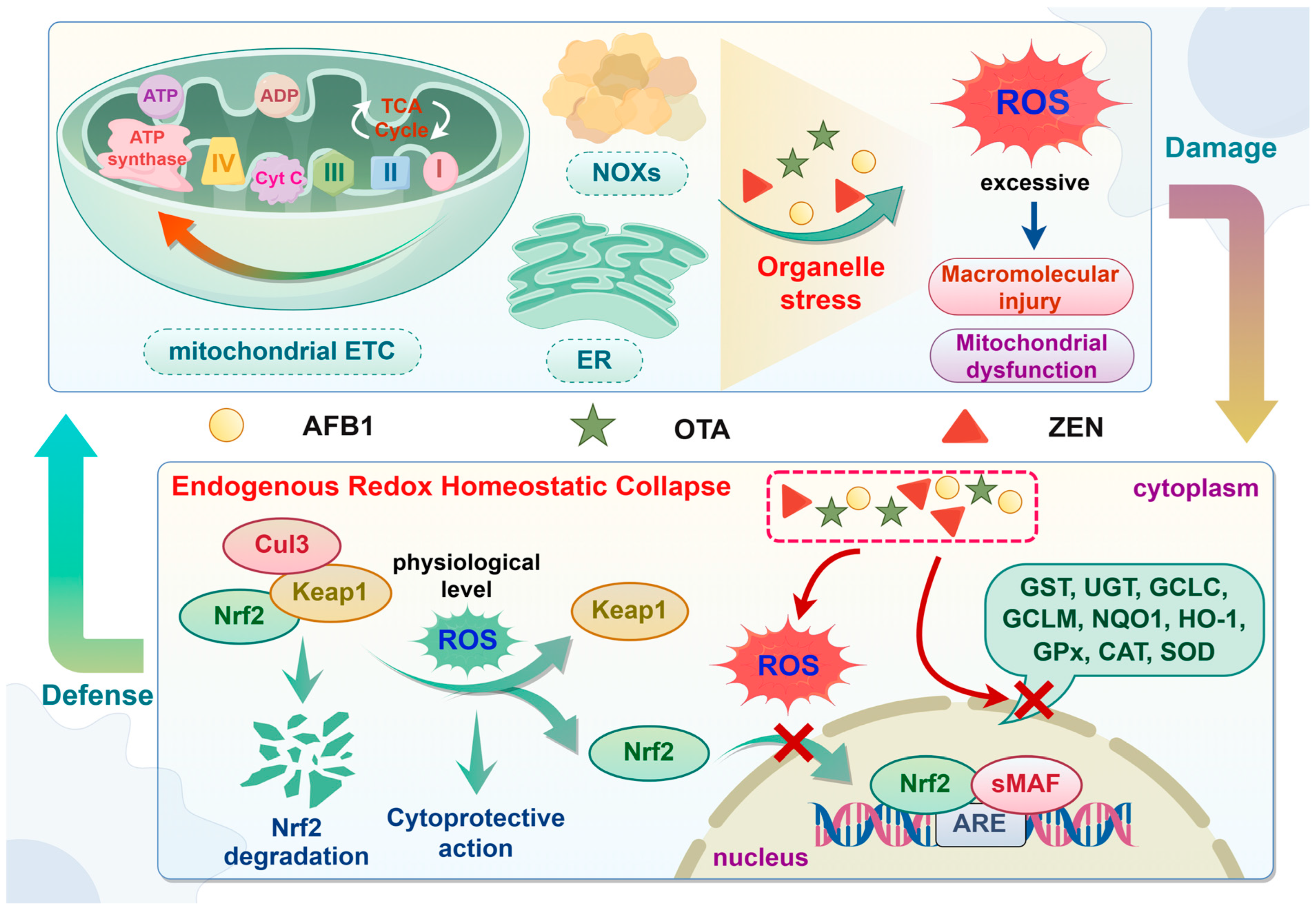
3.2. Oxidative Stress
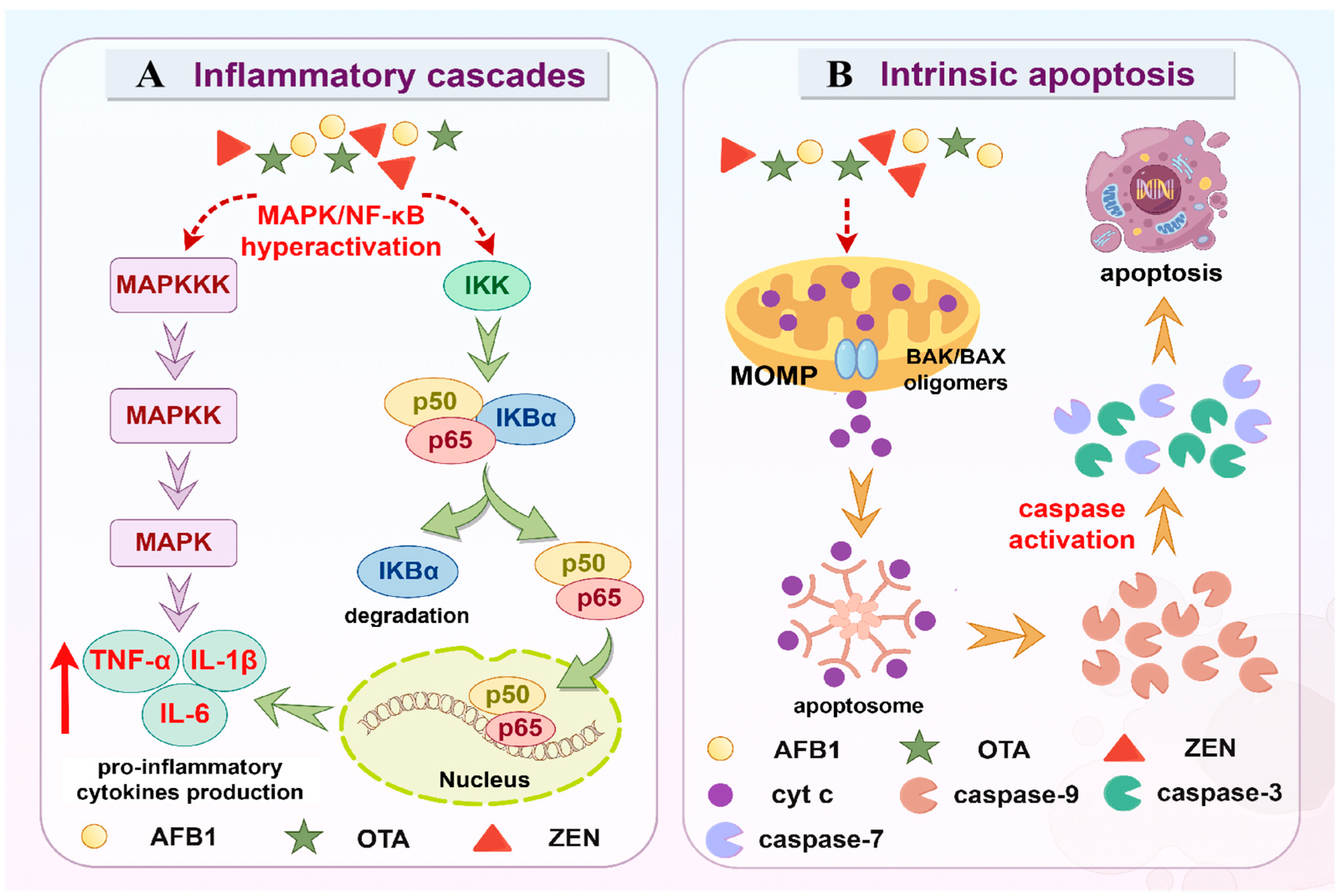
3.3. Inflammation
3.4. Apoptosis
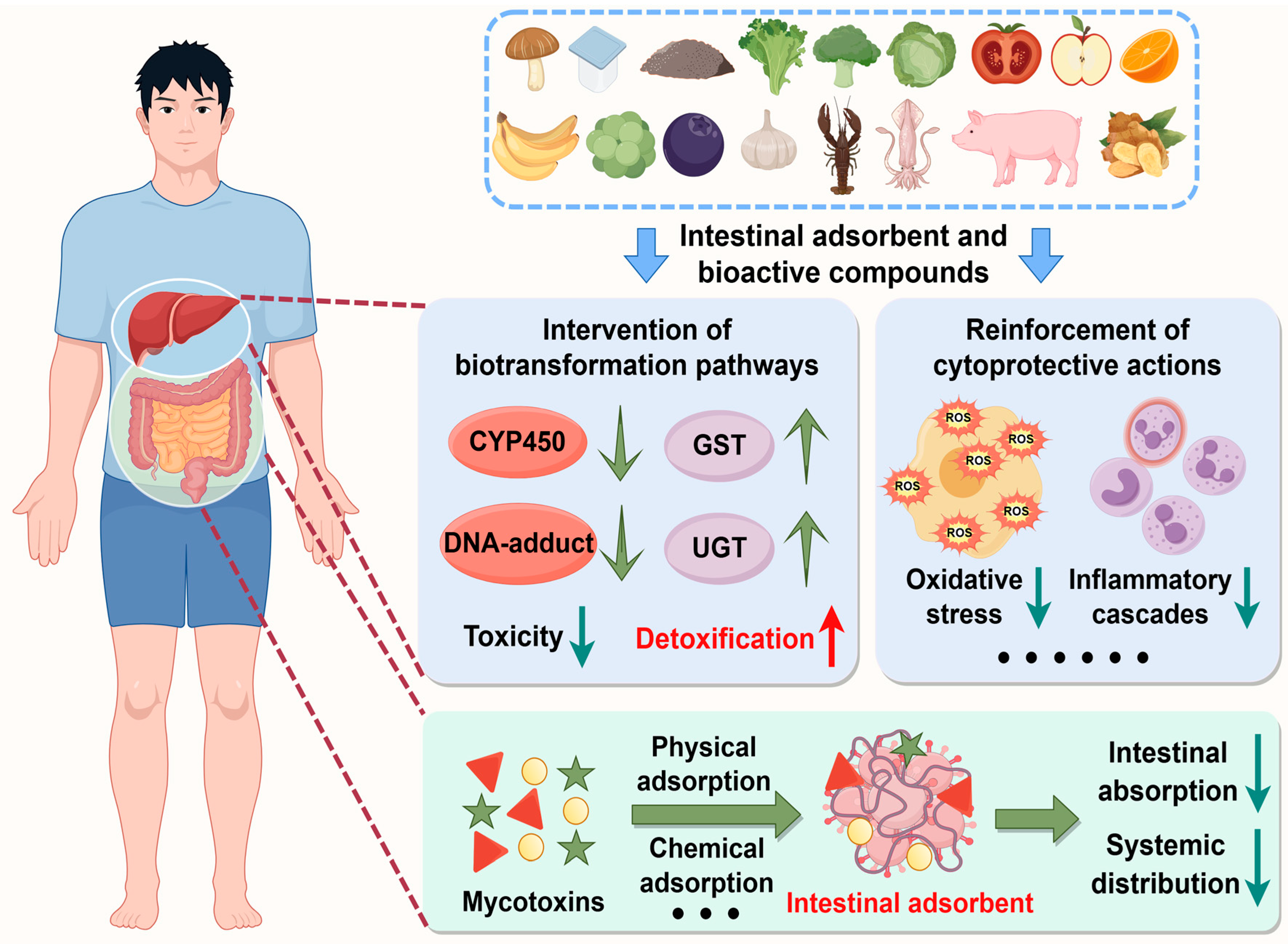
4. Nutritional Intervention
4.1. Natural and Engineered Enterosorbents
4.2. Dietary Small-Molecule Bioactive Compounds
4.2.1. Flavonoids and Their Derivates
4.2.2. Phenolic Acids and Their Derivates
4.2.3. Sulfur-Containing Organic Compounds
4.2.4. Other Promising Natural Agents
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFs | aflatoxins |
| OTs | ochratoxins |
| ZEN | zearalenone |
| TCMs | traditional Chinese medicines |
| FAO | Food and Agriculture Organization of the United Nations |
| AFB1 | aflatoxin B1 |
| OTA | ochratoxin A |
| IARC | International Agency for Research on Cancer |
| ERs | estrogen receptors |
| NAA | norsolorinic acid anthrone |
| NOR | norsolorinic acid |
| AVN | averantin |
| HAVN | 5′-hydroxyl-averantin |
| OAVN | 5′-oxoaverantin |
| AVF | averufin |
| HVN | hydroxyversicolorone |
| VHA | versiconal hemiacetal acetate |
| VAL | versiconal |
| VOAc | versiconol acetate |
| VOH | versiconol |
| VERB | versicolorin B |
| VERA | versicolorin A |
| DMST | demethylsterigmatocystin |
| ST | sterigmatocystin |
| OMST | O-methylsterigmatocystin |
| CYP450 | cytochrome P450 |
| NR | nuclear receptor |
| AHR | aryl hydrocarbon receptor |
| CAR | constitutive androstane receptor |
| PXR | pregnane X receptor |
| GSH | glutathione |
| GSTs | glutathione-S-transferases |
| mEH | microsomal epoxide hydrolase |
| AFAR | aflatoxin–aldehyde reductase |
| AFBO | AFB1-exo-8, 9-epoxide |
| AFL | aflatoxicol |
| AFM1 | aflatoxin M1 |
| AFP1 | aflatoxin P1 |
| AFQ1 | aflatoxin Q1 |
| AFH1 | aflatoxicol H1 |
| AFB2a | aflatoxin B2a |
| GGT | γ-glutamyltranspeptidase |
| DPEP | dipeptidase |
| NAT | N-acetyltransferase |
| AFB1-NAC | AFB1-mercapturic acid |
| dG | deoxyguanosine |
| AFB1-FAPY-dG | AFB1-formamidopyrimidine-dG |
| AP | abasic site |
| AFB1-ALB | AFB1-albumin |
| AFB1-dhd | AFB1-8,9-dihydrodiol |
| FAD | flavin adenine dinucleotide |
| PKS | polyketide synthase |
| OTβ | ochratoxin β |
| NRPS | nonribosomal peptide synthase |
| Phe | L-β-phenylalanine |
| OTα | Ochratoxin α |
| OP-OA | lactone-opened OTA |
| OTQ | OTA-quinone |
| OTHQ | OTA-hydroquinone |
| β-ZEL | β-zearalenol |
| HSDs | 3α-/3β-hydroxysteroid dehydrogenases |
| α-ZEL | α-zearalenol |
| α-ZAL | α-zearalanol |
| β-ZAL | β-zearalanol |
| ZAN | zearalanone |
| UGT | UDP glucuronosyltransferases |
| SULTs | sulfotransferases |
| ZEN-14-O-G | ZEN-14-O-glucuronide |
| 8-OHdG | 8-hydroxydeoxyguanosine |
| HAT | histone acetyl transferase |
| HDAC | histone deacetylase |
| PheRS | phenylalanine tRNA synthase |
| PAH | phenylalanine hydroxylase |
| EREs | estrogen response elements |
| ROS | reactive oxygen species |
| ETC | electron transport chain |
| ER | endoplasmic reticulum |
| NOXs | NADPH oxidases |
| mtDNA | mitochondrial DNA |
| MMP | mitochondrial membrane potential |
| mPTP | mitochondrial permeability transition pore |
| Keap1 | Kelch-like ECH-associated protein 1 |
| Nrf2 | nuclear factor-E2-related factor 2 |
| Cul3 | Cullin 3 |
| sMAF | small musculo-aponeurotic fibrosarcoma |
| AREs | antioxidant responsive elements |
| GCL | glutamate-cysteine ligase |
| NQO1 | NAD(P)H oxidoreductase 1 |
| HO-1 | heme oxygenase-1 |
| GPx | glutathione peroxidase |
| CAT | catalase |
| SOD | superoxide dismutase |
| MDA | malondialdehyde |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MAPK | mitogen-activated protein kinase |
| NF-κB | nuclear factor kappa-B |
| ERK | extracellular signal-regulated protein kinase |
| JNK | c-Jun amino (N)-terminal kinase |
| IKK | IκB kinase |
| TNF | tumor necrosis factor |
| IL | interleukin |
| COX-2 | cyclooxygenase-2 |
| iNOS | inducible nitric oxide synthase |
| NLRP3 | NOD-like receptor thermal protein domain associated protein 3 |
| TLR4 | Toll-like receptor 4 |
| TXNIP | Thioredoxin-interacting protein |
| LPS | lipopolysaccharides |
| PRRs | pattern-recognizing receptors |
| MyD88 | myeloid differentiation factor 88 |
| MOM | mitochondrial outer membrane |
| cyt c | cytochrome c |
| SMAC | second mitochondria-derived activator of caspase |
| APAF1 | apoptotic peptidase activating factor 1 |
| IAPs | inhibitors of apoptosis |
| LRB | Lacticaseibacillus rhamnosus |
| SCB | Saccharomyces cerevisiae |
| QUE | quercetin |
| QUN | quercitrin |
| API | apigenin |
| PAs | proanthocyanidins |
| CATE | catechin |
| TSH | total sulfhydryl group |
| PI3K | phosphatidylinositol-3-kinase |
| mTOR | mammalian target of rapamycin |
| JAK | Janus Kinases |
| STAT | Signal Transducer and Activator of Transcription |
| FA | ferulic acid |
| CA | p-coumaric acid |
| GA | gallic acid |
| CGA | chlorogenic acid |
| CCGA | cryptochlorogenic acid |
| ICAM-1 | intercellular adhesion molecule 1 |
| NO | nitric oxide |
| SFN | sulforaphane |
| PEITC | phenylethyl isothiocyanate |
| GSHS | glutathione synthase |
| DNMTs | DNA methyltransferases |
| LYC | lycopene |
| ASTA | astaxanthin |
| CUR | curcumin |
| Res | resveratrol |
| JECFA | Joint Expert Committee on Food Additives |
| WHO | World Health Organization |
References
- Wang, R.; Zhang, Q.; Chen, G.; Kou, R.; Zhang, C.; Wang, Y.; Wang, J.; Huang, Y.; Chen, C. Mechanistic insights into ferroptosis and apoptosis pathways: Synergistic effects of multi-organ toxicity and transgenerational effects induced by co-exposure of epoxiconazole and aflatoxin B1 in zebrafish. J. Adv. Res. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Awuchi, C.G.; Ondari, E.N.; Nwozo, S.; Odongo, G.A.; Eseoghene, I.J.; Twinomuhwezi, H.; Ogbonna, C.U.; Upadhyay, A.K.; Adeleye, A.O.; Okpala, C.O.R. Mycotoxins’ Toxicological Mechanisms Involving Humans, Livestock and Their Associated Health Concerns: A Review. Toxins 2022, 14, 167. [Google Scholar] [CrossRef]
- Yue, J.; Guo, D.; Gao, X.; Wang, J.; Nepovimova, E.; Wu, W.; Kuca, K. Deoxynivalenol (Vomitoxin)-Induced Anorexia Is Induced by the Release of Intestinal Hormones in Mice. Toxins 2021, 13, 512. [Google Scholar] [CrossRef]
- Chen, J.; Wen, J.; Tang, Y.; Shi, J.; Mu, G.; Yan, R.; Cai, J.; Long, M. Research Progress on Fumonisin B1 Contamination and Toxicity: A Review. Molecules 2021, 26, 5238. [Google Scholar] [CrossRef]
- Alsulami, T.; Nath, N.; Flemming, R.; Wang, H.; Zhou, W.; Yu, J.H. Development of a novel homogeneous immunoassay using the engineered luminescent enzyme NanoLuc for the quantification of the mycotoxin fumonisin B1. Biosens Bioelectron 2021, 177, 112939. [Google Scholar] [CrossRef] [PubMed]
- Twarużek, M.; Ałtyn, I.; Kosicki, R. Dietary Supplements Based on Red Yeast Rice—A Source of Citrinin? Toxins 2021, 13, 497. [Google Scholar] [CrossRef]
- Khodaei, D.; Javanmardi, F.; Khaneghah, A.M. The global overview of the occurrence of mycotoxins in cereals: A three-year survey. Curr. Opin. Food Sci. 2021, 39, 36–42. [Google Scholar] [CrossRef]
- Wei, G.; Guo, X.; Liang, Y.; Liu, C.; Zhang, G.; Liang, C.; Huang, Z.; Zheng, Y.; Chen, S.; Dong, L. Occurrence of fungi and mycotoxins in herbal medicines and rapid detection of toxin-producing fungi. Environ. Pollut. 2023, 333, 122082. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajslova, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25. Crit. Rev. Food Sci. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Lu, P.S.; Sun, S.C. Mycotoxin toxicity and its alleviation strategy on female mammalian reproduction and fertility. J. Adv. Res. 2025, in press. [Google Scholar] [CrossRef]
- Wang, X.; Wang, T.; Nepovimova, E.; Long, M.; Wu, W.; Kuca, K. Progress on the detoxification of aflatoxin B1 using natural anti-oxidants. Food Chem. Toxicol. 2022, 169, 113417. [Google Scholar] [CrossRef]
- Santos, A.R.; Carreiro, F.; Freitas, A.; Barros, S.; Brites, C.; Ramos, F.; Sanches Silva, A. Mycotoxins Contamination in Rice: Analytical Methods, Occurrence and Detoxification Strategies. Toxins 2022, 14, 647. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, D.; Wang, C.; Yu, H.; Zhong, P.; Dang, W.; Yang, Y.; Wang, Y.; Yan, X. Developing a Ni-grafted magnetic nanoparticle for direct CotA capture in rapid detoxification of aflatoxin B1. J. Hazard. Mater. 2025, 485, 136829. [Google Scholar] [CrossRef]
- Wu, Y.; Adeel, M.M.; Xia, D.; Sancar, A.; Li, W. Nucleotide excision repair of aflatoxin-induced DNA damage within the 3D human genome organization. Nucleic Acids Res. 2024, 52, 11704–11719. [Google Scholar] [CrossRef]
- Chen, X.; Abdallah, M.F.; Grootaert, C.; Van Nieuwerburgh, F.; Rajkovic, A. New insights into the combined toxicity of aflatoxin B1 and fumonisin B1 in HepG2 cells using Seahorse respirometry analysis and RNA transcriptome sequencing. Environ. Int. 2023, 175, 107945. [Google Scholar] [CrossRef]
- Wieckowska, M.; Szelenberger, R.; Niemcewicz, M.; Harmata, P.; Poplawski, T.; Bijak, M. Ochratoxin A-The Current Knowledge Concerning Hepatotoxicity, Mode of Action and Possible Prevention. Molecules 2023, 28, 6617. [Google Scholar] [CrossRef]
- Gu, K.; Ryu, D.; Lee, H.J. Ochratoxin A and its reaction products affected by sugars during heat processing. Food Chem. 2021, 348, 129038. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Han, M.; Wang, X.; Guo, Y. Ochratoxin A: Overview of Prevention, Removal, and Detoxification Methods. Toxins 2023, 15, 565. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Applegate, T. Zearalenone (ZEN) in Livestock and Poultry: Dose, Toxicokinetics, Toxicity and Estrogenicity. Toxins 2020, 12, 377. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, J.; Chen, Y.; Zhu, J. Zearalenone and Its Masked Forms in Cereals and Cereal-Derived Products: A Review of the Characteristics, Incidence, and Fate in Food Processing. J. Fungi 2022, 8, 976. [Google Scholar] [CrossRef]
- Han, X.; Huangfu, B.; Xu, T.; Xu, W.; Asakiya, C.; Huang, K.; He, X. Research Progress of Safety of Zearalenone: A Review. Toxins 2022, 14, 386. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lei, X.; Wang, Q.; Liu, H.; Liu, J. Zearalenone degradation by peptide-based enzyme mimics attached membrane reactor: Performance and mechanism. Food Chem. 2025, 463, 141399. [Google Scholar] [CrossRef] [PubMed]
- Caceres, I.; Khoury, A.A.; Khoury, R.E.; Lorber, S.; Oswald, I.P.; Khoury, A.E.; Atoui, A.; Puel, O.; Bailly, J.D. Aflatoxin Biosynthesis and Genetic Regulation: A Review. Toxins 2020, 12, 150. [Google Scholar] [CrossRef]
- Ehrlich, K.C. Predicted roles of the uncharacterized clustered genes in aflatoxin biosynthesis. Toxins 2009, 1, 37–58. [Google Scholar] [CrossRef] [PubMed]
- Georgianna, D.R.; Payne, G.A. Genetic regulation of aflatoxin biosynthesis: From gene to genome. Fungal Genet. Biol. 2009, 46, 113–125. [Google Scholar] [CrossRef]
- Flaherty, J.E.; Payne, G.A. Overexpression of aflR Leads to Upregulation of Pathway Gene Transcription and Increased Aflatoxin Production in Aspergillus flavus. Appl. Environ. Microb. 1997, 63, 3995–4000. [Google Scholar] [CrossRef]
- Du, W.; Obrian, G.R.; Payne, G.A. Function and regulation of aflJ in the accumulation of aflatoxin early pathway intermediate in Aspergillus flavus. Food Addit. Contam. 2007, 24, 1043–1050. [Google Scholar] [CrossRef]
- Kolawole, O.; Meneely, J.; Petchkongkaew, A.; Elliott, C. A review of mycotoxin biosynthetic pathways: Associated genes and their expressions under the influence of climatic factors. Fungal Biol. Rev. 2021, 37, 8–26. [Google Scholar] [CrossRef]
- Yu, J.; Bhatnagar, D.; Cleveland, T.E. Completed sequence of aflatoxin pathway gene cluster in Aspergillus parasiticus. FEBS Lett. 2004, 564, 126–130. [Google Scholar] [CrossRef]
- Sakuno, E.; Wen, Y.; Hatabayashi, H.; Arai, H.; Aoki, C.; Yabe, K.; Nakajima, H. Aspergillus parasiticus cyclase catalyzes two dehydration steps in aflatoxin biosynthesis. Appl. Environ. Microb. 2005, 71, 2999–3006. [Google Scholar] [CrossRef]
- Wen, Y.; Hatabayashi, H.; Arai, H.; Kitamoto, H.K.; Yabe, K. Function of the cypX and moxY genes in aflatoxin biosynthesis in Aspergillus parasiticus. Appl. Environ. Microb. 2005, 71, 3192–3198. [Google Scholar] [CrossRef] [PubMed]
- Kelkar, H.S.; Skloss, T.W.; Haw, J.F.; Keller, N.P.; Adams, T.H. Aspergillus nidulans stcL encodes a putative cytochrome P-450 monooxygenase required for bisfuran desaturation during aflatoxin/sterigmatocystin biosynthesis. J. Biol. Chem. 1997, 272, 1589–1594. [Google Scholar] [CrossRef]
- Henry, K.M.; Townsend, C.A. Ordering the reductive and cytochrome P450 oxidative steps in demethylsterigmatocystin formation yields general insights into the biosynthesis of aflatoxin and related fungal metabolites. J. Am. Chem. Soc. 2005, 127, 3724–3733. [Google Scholar] [CrossRef]
- Ehrlich, K.C.; Montalbano, B.; Boue, S.M.; Bhatnagar, D. An aflatoxin biosynthesis cluster gene encodes a novel oxidase required for conversion of versicolorin a to sterigmatocystin. Appl. Environ. Microb. 2005, 71, 8963–8965. [Google Scholar] [CrossRef]
- Cary, J.W.; Ehrlich, K.C.; Bland, J.M.; Montalbano, B.G. The aflatoxin biosynthesis cluster gene, aflX, encodes an oxidoreductase involved in conversion of versicolorin A to demethylsterigmatocystin. Appl. Environ. Microb. 2006, 72, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Woloshuk, C.P.; Bhatnagar, D.; Cleveland, T.E. Cloning and characterization of avfA and omtB genes involved in aflatoxin biosynthesis in three Aspergillus species. Gene 2000, 248, 157–167. [Google Scholar] [CrossRef]
- Yabe, K.; Ando, Y.; Hashimoto, J.; Hamasaki, T. Two distinct O-methyltransferases in aflatoxin biosynthesis. Appl. Environ. Microb. 1989, 55, 2172–2177. [Google Scholar] [CrossRef]
- Deng, J.; Zhao, L.; Zhang, N.Y.; Karrow, N.A.; Krumm, C.S.; Qi, D.S.; Sun, L.H. Aflatoxin B(1) metabolism: Regulation by phase I and II metabolizing enzymes and chemoprotective agents. Mutat. Res. Rev. Mutat. 2018, 778, 79–89. [Google Scholar] [CrossRef]
- Zhu, Q.; Ma, Y.; Liang, J.; Wei, Z.; Li, M.; Zhang, Y.; Liu, M.; He, H.; Qu, C.; Cai, J.; et al. AHR mediates the aflatoxin B1 toxicity associated with hepatocellular carcinoma. Signal Transduct. Target. Ther. 2021, 6, 299. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, S.; Yang, J.; Qin, C.; Jin, B.; Liang, Z.; Yang, S.; Li, L.; Long, M. Protective Effects of Astaxanthin on Ochratoxin A-Induced Liver Injury: Effects of Endoplasmic Reticulum Stress and Mitochondrial Fission-Fusion Balance. Toxins 2024, 16, 68. [Google Scholar] [CrossRef]
- Hua, Z.; Liu, R.; Chen, Y.; Liu, G.; Li, C.; Song, Y.; Cao, Z.; Li, W.; Li, W.; Lu, C.; et al. Contamination of Aflatoxins Induces Severe Hepatotoxicity Through Multiple Mechanisms. Front. Pharmacol. 2020, 11, 605823. [Google Scholar] [CrossRef]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef]
- Wang, L.; Huang, Q.; Wu, J.; Wu, W.; Jiang, J.; Yan, H.; Huang, J.; Sun, Y.; Deng, Y. The metabolism and biotransformation of AFB(1): Key enzymes and pathways. Biochem. Pharmacol. 2022, 199, 115005. [Google Scholar] [CrossRef]
- Lin, Y.C.; Li, L.; Makarova, A.V.; Burgers, P.M.; Stone, M.P.; Lloyd, R.S. Error-prone replication bypass of the primary aflatoxin B1 DNA adduct, AFB1-N7-Gua. J. Biol. Chem. 2014, 289, 18497–18506. [Google Scholar] [CrossRef]
- Wang, X.; He, Y.; Tian, J.; Muhammad, I.; Liu, M.; Wu, C.; Xu, C.; Zhang, X. Ferulic acid prevents aflatoxin B1-induced liver injury in rats via inhibiting cytochrome P450 enzyme, activating Nrf2/GST pathway and regulating mitochondrial pathway. Ecotoxicol. Environ. Saf. 2021, 224, 112624. [Google Scholar] [CrossRef]
- Geisen, R.; Schmidt-Heydt, M.; Karolewiez, A. A gene cluster of the ochratoxin A biosynthetic genes in Penicillium. Mycotoxin Res. 2006, 22, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Bruno, K.S.; Solfrizzo, M.; Perrone, G.; Mulè, G.; Visconti, A.; Baker, S.E. New Insight into the Ochratoxin A Biosynthetic Pathway through Deletion of a Nonribosomal Peptide Synthetase Gene in Aspergillus carbonarius. Appl. Environ. Microb. 2012, 78, 8208–8218. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Wu, F.; Liu, F.; Wang, Q.; Zhang, X.; Selvaraj, J.N.; Zhao, Y.; Xing, F.; Yin, W.B.; et al. A Consensus Ochratoxin A Biosynthetic Pathway: Insights from the Genome Sequence of Aspergillus ochraceus and a Comparative Genomic Analysis. Appl. Environ. Microb. 2018, 84, e01009–e01018. [Google Scholar] [CrossRef]
- Wang, G.; Wu, W.; Keller, N.P.; Guo, X.; Li, E.; Ma, J.; Xing, F. Metarhizium spp. encode an ochratoxin cluster and a high efficiency ochratoxin-degrading amidohydrolase revealed by genomic analysis. J. Adv. Res. 2024, 72, 85–95. [Google Scholar] [CrossRef]
- Ferrara, M.; Gallo, A.; Perrone, G.; Magistà, D.; Baker, S.E. Comparative Genomic Analysis of Ochratoxin A Biosynthetic Cluster in Producing Fungi: New Evidence of a Cyclase Gene Involvement. Front. Microbiol. 2020, 11, 581309. [Google Scholar] [CrossRef]
- Ferrara, M.; Perrone, G.; Gambacorta, L.; Epifani, F.; Solfrizzo, M.; Gallo, A.; Brakhage, A.A. Identification of a Halogenase Involved in the Biosynthesis of Ochratoxin A in Aspergillus carbonarius. Appl. Environ. Microb. 2016, 82, 5631–5641. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Dohnal, V.; Huang, L.; Kuča, K.; Wang, X.; Chen, G.; Yuan, Z. Metabolic pathways of ochratoxin A. Curr. Drug Metab. 2011, 12, 1–10. [Google Scholar] [CrossRef]
- Kőszegi, T.; Poór, M. Ochratoxin A: Molecular Interactions, Mechanisms of Toxicity and Prevention at the Molecular Level. Toxins 2016, 8, 111. [Google Scholar] [CrossRef]
- Liu, W.C.; Pushparaj, K.; Meyyazhagan, A.; Arumugam, V.A.; Pappuswamy, M.; Bhotla, H.K.; Baskaran, R.; Issara, U.; Balasubramanian, B.; Mousavi Khaneghah, A. Ochratoxin A as an alarming health threat for livestock and human: A review on molecular interactions, mechanism of toxicity, detection, detoxification, and dietary prophylaxis. Toxicon 2022, 213, 59–75. [Google Scholar] [CrossRef]
- Yu, J.; Smith, I.N.; Mikiashvili, N. Reducing Ochratoxin A Content in Grape Pomace by Different Methods. Toxins 2020, 12, 424. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, H.; De Saeger, S.; De Boevre, M.; Sun, F.; Zhang, S.; Cao, X.; Wang, Z. In vitro and in vivo metabolism of ochratoxin A: A comparative study using ultra-performance liquid chromatography-quadrupole/time-of-flight hybrid mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 3579–3589. [Google Scholar] [CrossRef] [PubMed]
- Heussner, A.; Bingle, L. Comparative Ochratoxin Toxicity: A Review of the Available Data. Toxins 2015, 7, 4253–4282. [Google Scholar] [CrossRef]
- Tao, Y.; Xie, S.; Xu, F.; Liu, A.; Wang, Y.; Chen, D.; Pan, Y.; Huang, L.; Peng, D.; Wang, X.; et al. Ochratoxin A: Toxicity, oxidative stress and metabolism. Food Chem. Toxicol. 2018, 112, 320–331. [Google Scholar] [CrossRef]
- Mahato, D.K.; Devi, S.; Pandhi, S.; Sharma, B.; Maurya, K.K.; Mishra, S.; Dhawan, K.; Selvakumar, R.; Kamle, M.; Mishra, A.K.; et al. Occurrence, Impact on Agriculture, Human Health, and Management Strategies of Zearalenone in Food and Feed: A Review. Toxins 2021, 13, 92. [Google Scholar] [CrossRef]
- Stakheev, A.A.; Erokhin, D.V.; Meleshchuk, E.A.; Mikityuk, O.D.; Statsyuk, N.V. Effect of Compactin on the Mycotoxin Production and Expression of Related Biosynthetic and Regulatory Genes in Toxigenic Fusarium culmorum. Microorganisms 2022, 10, 1347. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.T.; Lee, Y.R.; Jin, J.; Han, K.H.; Kim, H.; Kim, J.C.; Lee, T.; Yun, S.H.; Lee, Y.W. Two different polyketide synthase genes are required for synthesis of zearalenone in Gibberella zeae. Mol. Microbiol. 2005, 58, 1102–1113. [Google Scholar] [CrossRef]
- Kim, J.E.; Son, H.; Lee, Y.W. Biosynthetic mechanism and regulation of zearalenone in Fusarium graminearum. JSM Mycotoxins 2018, 68, 1–6. [Google Scholar] [CrossRef]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a fusarium mycotoxin, zearalenone. Crit. Rev. Food Sci. 2019, 60, 2710–2729. [Google Scholar] [CrossRef]
- Ropejko, K.; Twarużek, M. Zearalenone and Its Metabolites—General Overview, Occurrence, and Toxicity. Toxins 2021, 13, 35. [Google Scholar] [CrossRef]
- Flasch, M.; Bueschl, C.; Del Favero, G.; Adam, G.; Schuhmacher, R.; Marko, D.; Warth, B. Elucidation of xenoestrogen metabolism by non-targeted, stable isotope-assisted mass spectrometry in breast cancer cells. Environ. Int. 2022, 158, 106940. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, E.; Hildebrand, A.; Damm, G.; Rapp, A.; Cramer, B.; Humpf, H.U.; Metzler, M. Aromatic hydroxylation is a major metabolic pathway of the mycotoxin zearalenone in vitro. Mol. Nutr. Food Res. 2009, 53, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, E.; Hildebrand, A.; Mikula, H.; Metzler, M. Glucuronidation of zearalenone, zeranol and four metabolitesin vitro: Formation of glucuronides by various microsomes and human UDP-glucuronosyltransferase isoforms. Mol. Nutr. Food Res. 2010, 54, 1468–1476. [Google Scholar] [CrossRef]
- Rogowska, A.; Pomastowski, P.; Sagandykova, G.; Buszewski, B. Zearalenone and its metabolites: Effect on human health, metabolism and neutralisation methods. Toxicon 2019, 162, 46–56. [Google Scholar] [CrossRef]
- Cao, W.; Yu, P.; Yang, K.; Cao, D. Aflatoxin B1: Metabolism, toxicology, and its involvement in oxidative stress and cancer development. Toxicol. Mech. Method. 2022, 32, 395–419. [Google Scholar] [CrossRef]
- Soni, P.; Ghufran, M.S.; Kanade, S.R. Aflatoxin B1 induced multiple epigenetic modulators in human epithelial cell lines. Toxicon 2018, 151, 119–128. [Google Scholar] [CrossRef]
- Creppy, E.E.; Chakor, K.; Fisher, M.J.; Dirheimer, G. The myocotoxin ochratoxin A is a substrate for phenylalanine hydroxylase in isolated rat hepatocytes and in vivo. Arch. Toxicol. 1990, 64, 279–284. [Google Scholar] [CrossRef]
- Niaz, K.; Shah, S.Z.A.; Khan, F.; Bule, M. Ochratoxin A–induced genotoxic and epigenetic mechanisms lead to Alzheimer disease: Its modulation with strategies. Environ. Sci. Pollut. Res. Int. 2020, 27, 44673–44700. [Google Scholar] [CrossRef]
- Cai, P.; Liu, S.; Tu, Y.; Shan, T. Toxicity, biodegradation, and nutritional intervention mechanism of zearalenone. Sci. Total Environ. 2024, 911, 168648. [Google Scholar] [CrossRef]
- Balló, A.; Busznyákné Székvári, K.; Czétány, P.; Márk, L.; Török, A.; Szántó, Á.; Máté, G. Estrogenic and Non-Estrogenic Disruptor Effect of Zearalenone on Male Reproduction: A Review. Int. J. Mol. Sci. 2023, 24, 1578. [Google Scholar] [CrossRef]
- Zhang, G.L.; Feng, Y.L.; Song, J.L.; Zhou, X.S. Zearalenone: A Mycotoxin with Different Toxic Effect in Domestic and Laboratory Animals’ Granulosa Cells. Front. Genet. 2018, 9, 667. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, C.H.; Chen, S.; Sun, S.C. Zearalenone exposure impairs organelle function during porcine oocyte meiotic maturation. Theriogenology 2022, 177, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Jing, S.; Liu, C.; Zheng, J.; Dong, Z.; Guo, N. Toxicity of zearalenone and its nutritional intervention by natural products. Food Funct. 2022, 13, 10374–10400. [Google Scholar] [CrossRef]
- Bai, R.; Guo, J.; Ye, X.Y.; Xie, Y.; Xie, T. Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res. Rev. 2022, 77, 101619. [Google Scholar] [CrossRef]
- Li, B.; Ming, H.; Qin, S.; Nice, E.C.; Dong, J.; Du, Z.; Huang, C. Redox regulation: Mechanisms, biology and therapeutic targets in diseases. Signal Transduct. Target. Ther. 2025, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Bio. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, Y.; Xu, C.; An, P.; Luo, Y.; Jiao, L.; Luo, J.; Li, Y. Mitochondrial Dysfunction and Therapeutic Perspectives in Cardiovascular Diseases. Int. J. Mol. Sci. 2022, 23, 16053. [Google Scholar] [CrossRef]
- Dash, U.C.; Bhol, N.K.; Swain, S.K.; Samal, R.R.; Nayak, P.K.; Raina, V.; Panda, S.K.; Kerry, R.G.; Duttaroy, A.K.; Jena, A.B. Oxidative stress and inflammation in the pathogenesis of neurological disorders: Mechanisms and implications. Acta Pharm. Sin. B 2025, 15, 15–34. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 System: A Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, L.F.M.F.; Pedruzzi, L.M.; Stenvinkel, P.; Stockler-Pinto, M.B.; Daleprane, J.B.; Leite, M.; Mafra, D. Nutritional strategies to modulate inflammation and oxidative stress pathways via activation of the master antioxidant switch Nrf2. Biochimie 2013, 95, 1525–1533. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Liu, Y.; Guo, Y.; Ma, Q.; Ji, C.; Zhao, L. Transcriptional Profiling of Aflatoxin B1-Induced Oxidative Stress and Inflammatory Response in Macrophages. Toxins 2021, 13, 401. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, X.; Chai, X.; Jiao, Y.; Sun, J.; Wang, S.; Yu, H.; Feng, X. Curcumin Mitigates Oxidative Damage in Broiler Liver and Ileum Caused by Aflatoxin B1-Contaminated Feed through Nrf2 Signaling Pathway. Animals 2024, 14, 409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.L.; Ma, H.H.; Dong, P.Y.; Yan, Y.M.C.; Chen, Y.; Yang, G.M.; Shen, W.; Zhang, X.F. Bacillus licheniformis ameliorates Aflatoxin B1-induced testicular damage by improving the gut-metabolism-testis axis. J. Hazard. Mater. 2024, 468, 133836. [Google Scholar] [CrossRef]
- Feng, C.; Bai, H.; Chang, X.; Wu, Z.; Dong, W.; Ma, Q.; Yang, J. Aflatoxin B1-induced early developmental hepatotoxicity in larvae zebrafish. Chemosphere 2023, 340, 139940. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Zhou, X.; Liu, M.; Zang, H.; Liu, X.; Shan, A.; Feng, X. Alleviation of Oral Exposure to Aflatoxin B1-Induced Renal Dysfunction, Oxidative Stress, and Cell Apoptosis in Mice Kidney by Curcumin. Antioxidants 2022, 11, 1082. [Google Scholar] [CrossRef]
- Lee, H.J.; Pyo, M.C.; Shin, H.S.; Ryu, D.; Lee, K.W. Renal toxicity through AhR, PXR, and Nrf2 signaling pathway activation of ochratoxin A-induced oxidative stress in kidney cells. Food Chem. Toxicol. 2018, 122, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Jin, R.; Pan, F.; Guo, R.; Li, M.; Zhang, S.; Shi, J.; Zheng, J.; Wang, H.; Yang, X.; et al. Integration analysis of miRNA-mRNA uncovers the mechanisms of ochratoxin A-induced hepatotoxicity. Ecotoxicol. Environ. Saf. 2025, 293, 118039. [Google Scholar] [CrossRef]
- Damiano, S.; Longobardi, C.; Andretta, E.; Prisco, F.; Piegari, G.; Squillacioti, C.; Montagnaro, S.; Pagnini, F.; Badino, P.; Florio, S.; et al. Antioxidative Effects of Curcumin on the Hepatotoxicity Induced by Ochratoxin A in Rats. Antioxidants 2021, 10, 125. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Jiao, D.; Yang, S.; Li, L.; Li, P. Protective Effect of Astaxanthin on Ochratoxin A-Induced Kidney Injury to Mice by Regulating Oxidative Stress-Related NRF2/KEAP1 Pathway. Molecules 2020, 25, 1386. [Google Scholar] [CrossRef]
- Muthulakshmi, S.; Maharajan, K.; Habibi, H.R.; Kadirvelu, K.; Venkataramana, M. Zearalenone induced embryo and neurotoxicity in zebrafish model (Danio rerio): Role of oxidative stress revealed by a multi biomarker study. Chemosphere 2018, 198, 111–121. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhou, X.Q.; Jiang, W.D.; Wu, P.; Liu, Y.; Jiang, J.; Wang, S.W.; Kuang, S.Y.; Tang, L.; Feng, L. Effects of Dietary Zearalenone on Oxidative Stress, Cell Apoptosis, and Tight Junction in the Intestine of Juvenile Grass Carp (Ctenopharyngodon idella). Toxins 2019, 11, 333. [Google Scholar] [CrossRef]
- Rajendran, P.; Ammar, R.B.; Al-Saeedi, F.J.; Mohamed, M.E.; ElNaggar, M.A.; Al-Ramadan, S.Y.; Bekhet, G.M.; Soliman, A.M. Kaempferol Inhibits Zearalenone-Induced Oxidative Stress and Apoptosis via the PI3K/Akt-Mediated Nrf2 Signaling Pathway: In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2020, 22, 217. [Google Scholar] [CrossRef]
- Solier, S.; Müller, S.; Cañeque, T.; Versini, A.; Mansart, A.; Sindikubwabo, F.; Baron, L.; Emam, L.; Gestraud, P.; Pantoș, G.D.; et al. A druggable copper-signalling pathway that drives inflammation. Nature 2023, 617, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Luo, X.; Huang, L.; Zhang, Y.; Li, F.; Li, S.; Zhang, Z.; Yang, X.; Wang, X.; OuYang, X.; et al. A viral protein activates the MAPK pathway to promote viral infection by downregulating callose deposition in plants. Nat. Commun. 2024, 15, 10548. [Google Scholar] [CrossRef] [PubMed]
- García-Flores, N.; Jiménez-Suárez, J.; Garnés-García, C.; Fernández-Aroca, D.M.; Sabater, S.; Andrés, I.; Fernández-Aramburo, A.; Ruiz-Hidalgo, M.J.; Belandia, B.; Sanchez-Prieto, R.; et al. P38 MAPK and Radiotherapy: Foes or Friends? Cancers 2023, 15, 861. [Google Scholar] [CrossRef]
- Morrison, D.K. MAP Kinase Pathways. CSH. Perspect. Biol. 2012, 4, a011254. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cheng, D.; Zhang, J.; Tang, H.; Li, F.; Peng, Y.; Duan, X.; Meng, E.; Zhang, C.; Zeng, T.; et al. Role of macrophage AHR/TLR4/STAT3 signaling axis in the colitis induced by non-canonical AHR ligand aflatoxin B1. J. Hazard. Mater. 2023, 452, 131262. [Google Scholar] [CrossRef]
- Li, J.; Shi, M.; Wang, Y.; Liu, J.; Liu, S.; Kang, W.; Liu, X.; Chen, X.; Huang, K.; Liu, Y. Probiotic-derived extracellular vesicles alleviate AFB1-induced intestinal injury by modulating the gut microbiota and AHR activation. J. Nanobiotechnol. 2024, 22, 697. [Google Scholar] [CrossRef] [PubMed]
- Taranu, I.; Hermenean, A.; Bulgaru, C.; Pistol, G.C.; Ciceu, A.; Grosu, I.A.; Marin, D.E. Diet containing grape seed meal by-product counteracts AFB1 toxicity in liver of pig after weaning. Ecotoxicol. Environ. Saf. 2020, 203, 110899. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Yang, H.; Jiao, Y.; Pang, Q.; Wang, Y.; Wang, M.; Shan, A.; Feng, X. Dietary Curcumin Alleviated Acute Ileum Damage of Ducks (Anas platyrhynchos) Induced by AFB1 through Regulating Nrf2-ARE and NF-κB Signaling Pathways. Foods 2021, 10, 1370. [Google Scholar] [CrossRef]
- Longobardi, C.; Damiano, S.; Andretta, E.; Prisco, F.; Russo, V.; Pagnini, F.; Florio, S.; Ciarcia, R. Curcumin Modulates Nitrosative Stress, Inflammation, and DNA Damage and Protects against Ochratoxin A-Induced Hepatotoxicity and Nephrotoxicity in Rats. Antioxidants 2021, 10, 1239. [Google Scholar] [CrossRef]
- Yang, Y.; Lv, L.; Shi, S.; Cai, G.; Yu, L.; Xu, S.; Zhu, T.; Su, X.; Mao, N.; Zhang, Y.; et al. Polysaccharide from walnut green husk alleviates liver inflammation and gluconeogenesis dysfunction by altering gut microbiota in ochratoxin A-induced mice. Carbohyd. Polym. 2023, 322, 121362. [Google Scholar] [CrossRef]
- Wang, W.; Zhai, S.; Xia, Y.; Wang, H.; Ruan, D.; Zhou, T.; Zhu, Y.; Zhang, H.; Zhang, M.; Ye, H.; et al. Ochratoxin A induces liver inflammation: Involvement of intestinal microbiota. Microbiome 2019, 7, 151. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, A.; Liu, X.; Ma, Y.; Zhao, F.; Wang, M.; Loor, J.J.; Wang, H. Melatonin ameliorates ochratoxin A induced liver inflammation, oxidative stress and mitophagy in mice involving in intestinal microbiota and restoring the intestinal barrier function. J. Hazard. Mater. 2021, 407, 124489. [Google Scholar] [CrossRef]
- AbuZahra, H.M.; Rajendran, P.; Ismail, M.B. Zerumbone Exhibit Protective Effect against Zearalenone Induced Toxicity via Ameliorating Inflammation and Oxidative Stress Induced Apoptosis. Antioxidants 2021, 10, 1593. [Google Scholar] [CrossRef]
- Lin, X.; Zhu, L.; Gao, X.; Kong, L.; Huang, Y.; Zhao, H.; Chen, Y.; Wen, L.; Li, R.; Wu, J.; et al. Ameliorative effect of betulinic acid against zearalenone exposure triggers testicular dysfunction and oxidative stress in mice via p38/ERK MAPK inhibition and Nrf2-mediated antioxidant defense activation. Ecotoxicol. Environ. Saf. 2022, 238, 113561. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Lv, Y.; Ren, S.; Shao, M.; Shen, T.; Huang, K.; Zhou, J.; Yan, L.; Song, S. Zearalenone (ZEA)-induced intestinal inflammation is mediated by the NLRP3 inflammasome. Chemosphere 2018, 190, 272–279. [Google Scholar] [CrossRef]
- Wang, S.; Fu, W.; Zhao, X.; Chang, X.; Liu, H.; Zhou, L.; Li, J.; Cheng, R.; Wu, X.; Li, X.; et al. Zearalenone disturbs the reproductive-immune axis in pigs: The role of gut microbial metabolites. Microbiome 2022, 10, 234. [Google Scholar] [CrossRef]
- Yuan, J.; Ofengeim, D. A guide to cell death pathways. Nat. Rev. Mol. Cell Biol. 2024, 25, 379–395. [Google Scholar] [CrossRef]
- Harrington, J.S.; Ryter, S.W.; Plataki, M.; Price, D.R.; Choi, A.M.K. Mitochondria in health, disease, and aging. Physiol. Rev. 2023, 103, 2349–2422. [Google Scholar] [CrossRef]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell. Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef] [PubMed]
- Kesavardhana, S.; Malireddi, R.K.S.; Kanneganti, T.D. Caspases in Cell Death, Inflammation, and Pyroptosis. Annu. Rev. Immunol. 2020, 38, 567–595. [Google Scholar] [CrossRef]
- Czabotar, P.E.; Garcia-Saez, A.J. Mechanisms of BCL-2 family proteins in mitochondrial apoptosis. Nat. Rev. Mol. Cell Bio. 2023, 24, 732–748. [Google Scholar] [CrossRef] [PubMed]
- Bahuguna, A.; Kumar, V.; Ramalingam, S.; Yadav, A.K.; Kim, M. Zingiber officinale mediated aflatoxins cleanup in aqueous medium: Kinetics, toxicity and cytoprotective effect. J. Clean. Prod. 2022, 381, 135155. [Google Scholar] [CrossRef]
- Guo, Y.; Qin, X.; Tang, Y.; Ma, Q.; Zhang, J.; Zhao, L. CotA laccase, a novel aflatoxin oxidase from Bacillus licheniformis, transforms aflatoxin B1 to aflatoxin Q1 and epi-aflatoxin Q1. Food Chem. 2020, 325, 126877. [Google Scholar] [CrossRef]
- Dey, D.K.; Kang, S.C. Aflatoxin B1 induces reactive oxygen species-dependent caspase-mediated apoptosis in normal human cells, inhibits Allium cepa root cell division, and triggers inflammatory response in zebrafish larvae. Sci. Total Environ. 2020, 737, 139704. [Google Scholar] [CrossRef]
- Li, M.; Kong, Y.; Guo, W.; Wu, X.; Zhang, J.; Lai, Y.; Kong, Y.; Niu, X.; Wang, G. Dietary aflatoxin B1 caused the growth inhibition, and activated oxidative stress and endoplasmic reticulum stress pathway, inducing apoptosis and inflammation in the liver of northern snakehead (Channa argus). Sci. Total Environ. 2022, 850, 157997. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.Z.; Jiang, W.D.; Wu, P.; Liu, Y.; Zeng, Y.Y.; Jiang, J.; Kuang, S.Y.; Tang, L.; Zhou, X.Q.; Feng, L. Dietary aflatoxin B1 decreases growth performance and damages the structural integrity of immune organs in juvenile grass carp (Ctenopharyngodon idella). Aquaculture 2019, 500, 1–17. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, W.; Feng, S.; Liu, S.; Chen, C.; Yao, B.; Shen, X.L. Ochratoxin A-induced mitochondrial pathway apoptosis and ferroptosis by promoting glycolysis. Apoptosis 2025, 30, 1440–1452. [Google Scholar] [CrossRef]
- Li, H.; He, W.; Yue, D.; Wang, M.; Yuan, X.; Huang, K. Low doses of fumonisin B1 exacerbate ochratoxin A-induced renal injury in mice and the protective roles of heat shock protein 70. Chem. Biol. Interact. 2023, 369, 110240. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lim, W.; Ryu, S.; Kim, J.; Song, G. Ochratoxin A mediates cytotoxicity through the MAPK signaling pathway and alters intracellular homeostasis in bovine mammary epithelial cells. Environ. Pollut. 2019, 246, 366–373. [Google Scholar] [CrossRef]
- Zhang, F.L.; Zhu, K.X.; Wang, J.Y.; Zhang, M.; Yan, J.M.; Liu, Q.C.; Zhang, X.Y.; Guo, J.C.; Liu, X.; Sun, Q.C.; et al. Cross-species analysis of transcriptome emphasizes a critical role of TNF-α in mediating MAP2K7/AKT2 signaling in zearalenone-induced apoptosis. J. Hazard. Mater. 2023, 459, 132226. [Google Scholar] [CrossRef]
- Lee, R.; Kim, D.W.; Lee, W.Y.; Park, H.J. Zearalenone Induces Apoptosis and Autophagy in a Spermatogonia Cell Line. Toxins 2022, 14, 148. [Google Scholar] [CrossRef]
- Bai, J.; Deng, S.; Zhang, X.; Dai, Z.; Ji, Y.; Zeng, S.; Ren, F.; Yang, Y.; Wu, Z. Cinnamaldehyde alleviates zearalenone-induced LS174T cell apoptosis, barrier dysfunction and mucin reduction through JNK/NF-κB signaling pathway. Ecotoxicol. Environ. Saf. 2023, 263, 115276. [Google Scholar] [CrossRef]
- Kamle, M.; Mahato, D.K.; Devi, S.; Lee, K.E.; Kang, S.G.; Kumar, P. Fumonisins: Impact on Agriculture, Food, and Human Health and their Management Strategies. Toxins 2019, 11, 328. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Huang, M.; Lei, J.; Song, H.; Hu, L.; Mo, H. Auricularia auricular Adsorbs Aflatoxin B1 and Ameliorates Aflatoxin B1-Induced Liver Damage in Sprague Dawley Rats. Foods 2023, 12, 2644. [Google Scholar] [CrossRef]
- Taheur, F.B.; Fedhila, K.; Chaieb, K.; Kouidhi, B.; Bakhrouf, A.; Abrunhosa, L. Adsorption of aflatoxin B1, zearalenone and ochratoxin A by microorganisms isolated from Kefir grains. Int. J. Food Microbiol. 2017, 251, 1–7. [Google Scholar] [CrossRef]
- Pires, R.C.; da Costa Calumby, J.; Rosim, R.E.; Pires, R.D.A.; Borowsky, A.M.; Ali, S.; de Paiva, E.L.; Silva, R.; Pimentel, T.C.; da Cruz, A.G.; et al. Evaluation of Ability of Inactivated Biomasses of Lacticaseibacillus rhamnosus and Saccharomyces cerevisiae to Adsorb Aflatoxin B1 In Vitro. Foods 2024, 13, 3299. [Google Scholar] [CrossRef]
- Miljanić, J.; Krstović, S.; Perović, L.; Kojić, J.; Travičić, V.; Bajac, B. Assessment of the Nutritional Benefits and Aflatoxin B1 Adsorption Properties of Blackberry Seed Cold-Pressed Oil By-Product. Foods 2024, 13, 3140. [Google Scholar] [CrossRef]
- Karmanov, A.P.; Kanarsky, A.V.; Kanarskaya, Z.A.; Kocheva, L.S.; Semenov, E.I.; Bogdanovich, N.I.; Belyy, V.A. In vitro adsorption-desorption of aflatoxin B1 on Pepper’s lignins isolated from grassy plants. Int. J. Biol. Macromol. 2020, 144, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Solís-Cruz, B.; Hernández-Patlán, D.; Beyssac, E.; Latorre, J.; Hernandez-Velasco, X.; Merino-Guzman, R.; Tellez, G.; López-Arellano, R. Evaluation of Chitosan and Cellulosic Polymers as Binding Adsorbent Materials to Prevent Aflatoxin B1, Fumonisin B1, Ochratoxin, Trichothecene, Deoxynivalenol, and Zearalenone Mycotoxicoses Through an In Vitro Gastrointestinal Model for Poultry. Polymers 2017, 9, 529. [Google Scholar] [CrossRef]
- Vasiljević, M.; Marinković, D.; Milićević, D.; Pleadin, J.; Stefanović, S.; Trialović, S.; Raj, J.; Petrujkić, B.; Trialović, J.N. Efficacy of a Modified Clinoptilolite Based Adsorbent in Reducing Detrimental Effects of Ochratoxin A in Laying Hens. Toxins 2021, 13, 469. [Google Scholar] [CrossRef]
- Wang, G.; Lian, C.; Xi, Y.; Sun, Z.; Zheng, S. Evaluation of nonionic surfactant modified montmorillonite as mycotoxins adsorbent for aflatoxin B1 and zearalenone. J. Colloid Interf. Sci. 2018, 518, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Luo, J.; Sheng, Z.; Fang, Z.; Fu, Y.; Wang, N.; Yang, B.; Xu, B. Latest research progress on anti-microbial effects, mechanisms of action, and product developments of dietary flavonoids: A systematic literature review. Trends Food Sci. Technol. 2025, 156, 104839. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, L.; Ran, W.; Zhong, K.; Zhao, Y.; Gao, H. Antimicrobial activities of natural flavonoids against foodborne pathogens and their application in food industry. Food Chem. 2024, 460, 140476. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Sharma, G.; Liu, G.; Shen, J.; Shao, B.; Hao, Z. Therapeutic detoxification of quercetin for aflatoxin B1-related toxicity: Roles of oxidative stress, inflammation, and metabolic enzymes. Environ. Pollut. 2024, 345, 123474. [Google Scholar] [CrossRef] [PubMed]
- Owumi, S.E.; Ajakaiye, B.; Akinwunmi, A.O.; Nwozo, S.O.; Oyelere, A.K. The Hydrophobic Extract of Sorghum bicolor (L. Moench) Enriched in Apigenin-Protected Rats against Aflatoxin B1-Associated Hepatorenal Derangement. Molecules 2023, 28, 3013. [Google Scholar] [CrossRef]
- Tsega, S.A.; Manoj, V.R.; Gebretsadik, M.H.; Lumsangkul, C.; Chen, Y.P. Catechin and quercitrin mitigate the cytotoxic effects of aflatoxin-B1 on liver and colon cells by inhibiting cytochrome P450 1A2 and 3A4, in silico. Food Biosci. 2025, 64, 105989. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Huang, C.; He, C.; Yang, T.; Ren, R.; Xin, Z.; Wang, X. Quercetin-Driven Akkermansia Muciniphila Alleviates Obesity by Modulating Bile Acid Metabolism via an ILA/m(6)A/CYP8B1 Signaling. Adv. Sci. 2025, 12, e2412865. [Google Scholar] [CrossRef]
- Erichsen, T.J.; Aehlen, A.; Ehmer, U.; Kalthoff, S.; Manns, M.P.; Strassburg, C.P. Regulation of the human bile acid UDP-glucuronosyltransferase 1A3 by the farnesoid X receptor and bile acids. J. Hepatol. 2010, 52, 570–578. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Zhang, N.; Zhou, Q.; Fan, D.; Wang, M. Lipophilized apigenin derivatives produced during the frying process as novel antioxidants. Food Chem. 2022, 379, 132178. [Google Scholar] [CrossRef]
- Lv, J.M.; Gouda, M.; Ye, X.Q.; Shao, Z.P.; Chen, J.C. Evaluation of Proanthocyanidins from Kiwi Leaves (Actinidia chinensis) against Caco-2 Cells Oxidative Stress through Nrf2-ARE Signaling Pathway. Antioxidants 2022, 11, 1367. [Google Scholar] [CrossRef]
- Li, T.; Li, F.; Liu, X.; Liu, J.; Li, D. Synergistic anti-inflammatory effects of quercetin and catechin via inhibiting activation of TLR4–MyD88-mediated NF-κB and MAPK signaling pathways. Phytother. Res. 2019, 33, 756–767. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, Q.; Shen, L.; Sharma, G.; Jiang, H.; Wang, Z.; Shen, J. Quercetin Attenuates Quinocetone-Induced Cell Apoptosis In Vitro by Activating the P38/Nrf2/HO-1 Pathway and Inhibiting the ROS/Mitochondrial Apoptotic Pathway. Antioxidants 2022, 11, 1498. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Parama, D.; Daimari, E.; Girisa, S.; Banik, K.; Harsha, C.; Dutta, U.; Kunnumakkara, A.B. Rationalizing the therapeutic potential of apigenin against cancer. Life Sci. 2021, 267, 118814. [Google Scholar] [CrossRef]
- Rashmi, H.B.; Negi, P.S. Phenolic acids from vegetables: A review on processing stability and health benefits. Food Res. Int. 2020, 136, 109298. [Google Scholar] [CrossRef]
- Wang, X.; Yang, F.; Na, L.; Jia, M.; Ishfaq, M.; Zhang, Y.; Liu, M.; Wu, C. Ferulic acid alleviates AFB1-induced duodenal barrier damage in rats via up-regulating tight junction proteins, down-regulating ROCK, competing CYP450 enzyme and activating GST. Ecotoxicol. Environ. Saf. 2022, 241, 113805. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Y.; Xu, Y.; Zhang, Z.; Tang, R.; Liu, J.; Jiang, H.; Zhao, R. Procyanidin B1 and p-coumaric acid from whole highland barley ameliorated HFD-induced impaired glucose tolerance via small intestinal barrier and hepatic glucose metabolism. Food Funct. 2024, 15, 9272–9283. [Google Scholar] [CrossRef]
- Owumi, S.E.; Bello, S.A.; Najophe, S.E.; Nwozo, S.O.; Esan, I.O. Coadministration of gallic acid abates zearalenone-mediated defects in male rat’s reproductive function. J. Biochem. Mol. Toxic. 2021, 36, e22940. [Google Scholar] [CrossRef]
- Cheng, K.; Niu, J.; Zhang, J.; Qiao, Y.; Dong, G.; Guo, R.; Zheng, X.; Song, Z.; Huang, J.; Wang, J.; et al. Hepatoprotective effects of chlorogenic acid on mice exposed to aflatoxin B1: Modulation of oxidative stress and inflammation. Toxicon 2023, 231, 107177. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.L.; Yu, L.; Zhang, S.D.; Ping, K.; Ni, H.Y.; Qin, X.Y.; Zhao, C.J.; Wang, W.; Efferth, T.; Fu, Y.J. Cryptochlorogenic acid attenuates LPS-induced inflammatory response and oxidative stress via upregulation of the Nrf2/HO-1 signaling pathway in RAW 264.7 macrophages. Int. Immunopharmacol. 2020, 83, 106436. [Google Scholar] [CrossRef] [PubMed]
- Kose, T.; Sharp, P.A.; Latunde-Dada, G.O. Upregulation of Nrf2 Signalling and the Inhibition of Erastin-Induced Ferroptosis by Ferulic Acid in MIN6 Cells. Int. J. Mol. Sci. 2022, 23, 15886. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wu, X.; Chen, D.; Yu, B.; He, J. Dietary ferulic acid supplementation enhances antioxidant capacity and alleviates hepatocyte pyroptosis in diquat challenged piglets. J. Anim. Sci. Biotechnol. 2024, 15, 134. [Google Scholar] [CrossRef]
- Li, D.; Wan, M.; Xue, L.; Zhang, Z.; Qiu, Y.; Mei, F.; Tang, N.; Yu, C.; Yu, Y.; Chen, T.; et al. Zinc promotes microbial p-coumaric acid production that protects against cholestatic liver injury. Cell Host Microbe 2024, 32, 2195–2211.e9. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Atanasov, A.G.; Li, Y.; Kumar, N.; Bishayee, A. Chlorogenic acid for cancer prevention and therapy: Current status on efficacy and mechanisms of action. Pharmacol. Res. 2022, 186, 106505. [Google Scholar] [CrossRef]
- Wang, Q.; Du, T.; Zhang, Z.; Zhang, Q.; Zhang, J.; Li, W.; Jiang, J.D.; Chen, X.; Hu, H.Y. Target fishing and mechanistic insights of the natural anticancer drug candidate chlorogenic acid. Acta. Pharm. Sin. B 2024, 14, 4431–4442. [Google Scholar] [CrossRef]
- Sundaram, M.K.; Preetha, R.; Haque, S.; Akhter, N.; Khan, S.; Ahmad, S.; Hussain, A. Dietary isothiocyanates inhibit cancer progression by modulation of epigenome. Semin. Cancer Biol. 2022, 83, 353–376. [Google Scholar] [CrossRef]
- Salehi, B.; Zucca, P.; Orhan, I.E.; Azzini, E.; Adetunji, C.O.; Mohammed, S.A.; Banerjee, S.K.; Sharopov, F.; Rigano, D.; Sharifi-Rad, J.; et al. Allicin and health: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 502–516. [Google Scholar] [CrossRef]
- Duan, H.; Song, W.; Guo, J.; Yan, W. Taurine: A Source and Application for the Relief of Visual Fatigue. Nutrients 2023, 15, 1843. [Google Scholar] [CrossRef]
- Al-Temimi, A.A.; Al-Mossawi, A.E.B.; Al-Hilifi, S.A.; Korma, S.A.; Esatbeyoglu, T.; Rocha, J.M.; Agarwal, V. Glutathione for Food and Health Applications with Emphasis on Extraction, Identification, and Quantification Methods: A Review. Metabolites 2023, 13, 465. [Google Scholar] [CrossRef] [PubMed]
- Gross-Steinmeyer, K.; Stapleton, P.L.; Tracy, J.H.; Bammler, T.K.; Strom, S.C.; Eaton, D.L. Sulforaphane- and Phenethyl Isothiocyanate–Induced Inhibition of Aflatoxin B1–Mediated Genotoxicity in Human Hepatocytes: Role of GSTM1 Genotype and CYP3A4 Gene Expression. Toxicol. Sci. 2010, 116, 422–432. [Google Scholar] [CrossRef]
- Fiala, J.L.A.; Egner, P.A.; Wiriyachan, N.; Ruchirawat, M.; Kensler, K.H.; Wogan, G.N.; Groopman, J.D.; Croy, R.G.; Essigmann, J.M. Sulforaphane-Mediated Reduction of Aflatoxin B1-N7-Guanine in Rat Liver DNA: Impacts of Strain and Sex. Toxicol. Sci. 2011, 121, 57–62. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Gao, M.; Zhang, X.; Lei, P.; Yang, H.; Qing, Y.; Zhang, L. The Protective Effect of Sulforaphane on Dextran Sulfate Sodium-Induced Colitis Depends on Gut Microbial and Nrf2-Related Mechanism. Front. Nutr. 2022, 9, 893344. [Google Scholar] [CrossRef] [PubMed]
- Nan, B.; Yang, C.; Li, L.; Ye, H.; Yan, H.; Wang, M.; Yuan, Y. Allicin alleviated acrylamide-induced NLRP3 inflammasome activation via oxidative stress and endoplasmic reticulum stress in Kupffer cells and SD rats liver. Food Chem. Toxicol. 2021, 148, 111937. [Google Scholar] [CrossRef]
- Berges, R. Comparison of the chemopreventive efficacies of garlic powders with different alliin contents against aflatoxin B1 carcinogenicity in rats. Carcinogenesis 2004, 25, 1953–1959. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, L.; Chu, Y.; Feng, F.; Tang, W.; Chen, C.; Qiu, Y.; Hu, Z.; Diao, H.; Tang, Z. Dietary Taurine Improves Growth Performance and Intestine Health via the GSH/GSSG Antioxidant System and Nrf2/ARE Signaling Pathway in Weaned Piglets. Antioxidants 2023, 12, 1852. [Google Scholar] [CrossRef]
- Kaiser, A.E.; Baniasadi, M.; Giansiracusa, D.; Giansiracusa, M.; Garcia, M.; Fryda, Z.; Wong, T.L.; Bishayee, A. Sulforaphane: A Broccoli Bioactive Phytocompound with Cancer Preventive Potential. Cancers 2021, 13, 4796. [Google Scholar] [CrossRef]
- Qin, S.; Yang, C.; Huang, W.; Du, S.; Mai, H.; Xiao, J.; Lü, T. Sulforaphane attenuates microglia-mediated neuronal necroptosis through down-regulation of MAPK/NF-κB signaling pathways in LPS-activated BV-2 microglia. Pharmacol. Res. 2018, 133, 218–235. [Google Scholar] [CrossRef]
- Ji, X.; Ding, H.; Zhou, F.; Zhang, F.; Wu, D. Taurine ameliorates deoxynivalenol-induced intestinal injury in piglets: Restoration of mitochondrial function linked to the PGC1α-NRF1/2 axis. Ecotoxicol. Environ. Saf. 2025, 292, 117938. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, E.; Canistro, D.; Pellicioni, V.; Vivarelli, F.; Fimognari, C. Anticancer potential of allicin: A review. Pharmacol. Res. 2022, 177, 106118. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, W.; Latif, A.; Lianfu, Z.; Jian, Z.; Chenqiang, W.; Rehman, A.; Hussain, A.; Siddiquy, M.; Karim, A. Technological Advancement in the Processing of Lycopene: A Review. Food Rev. Int. 2020, 38, 857–883. [Google Scholar] [CrossRef]
- Wei, R.R.; Lin, Q.Y.; Adu, M.; Huang, H.L.; Yan, Z.H.; Shao, F.; Zhong, G.Y.; Zhang, Z.L.; Sang, Z.P.; Cao, L.; et al. The sources, properties, extraction, biosynthesis, pharmacology, and application of lycopene. Food Funct. 2023, 14, 9974–9998. [Google Scholar] [CrossRef]
- Chen, Y.; Su, W.; Tie, S.; Zhang, L.; Tan, M. Advances of astaxanthin-based delivery systems for precision nutrition. Trends Food Sci. Technol. 2022, 127, 63–73. [Google Scholar] [CrossRef]
- Hasanzadeh, S.; Read, M.I.; Bland, A.R.; Majeed, M.; Jamialahmadi, T.; Sahebkar, A. Curcumin: An inflammasome silencer. Pharmacol. Res. 2020, 159, 104921. [Google Scholar] [CrossRef]
- Silva, L.N.; Zimmer, K.R.; Macedo, A.J.; Trentin, D.S. Plant Natural Products Targeting Bacterial Virulence Factors. Chem. Rev. 2016, 116, 9162–9236. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Fang, M.; Li, X.; Wang, D.; Yu, L.; Ma, F.; Jiang, J.; Zhang, L.; Li, P. Contributions of Common Foods to Resveratrol Intake in the Chinese Diet. Foods 2024, 13, 1267. [Google Scholar] [CrossRef]
- Li, M.; Tang, S.; Peng, X.; Sharma, G.; Yin, S.; Hao, Z.; Li, J.; Shen, J.; Dai, C. Lycopene as a Therapeutic Agent against Aflatoxin B1-Related Toxicity: Mechanistic Insights and Future Directions. Antioxidants 2024, 13, 452. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Ding, Y.; Li, W.; Li, S.; Li, X.; Zhao, D.; Zhang, Y.; Wei, G.; Zhang, X. Protective role of curcumin on broiler liver by modulating aflatoxin B1-induced DNA methylation and CYPs expression. Ecotoxicol. Environ. Saf. 2023, 260, 115086. [Google Scholar] [CrossRef]
- Muhammad, I.; Wang, H.; Sun, X.; Wang, X.; Han, M.; Lu, Z.; Cheng, P.; Hussain, M.A.; Zhang, X. Dual Role of Dietary Curcumin Through Attenuating AFB1-Induced Oxidative Stress and Liver Injury via Modulating Liver Phase-I and Phase-II Enzymes Involved in AFB1 Bioactivation and Detoxification. Front. Pharmacol. 2018, 9, 554. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Y.; Zhou, X.; Liu, M.; Jin, S.; Shan, A.; Feng, X. Resveratrol Relieved Acute Liver Damage in Ducks (Anas platyrhynchos) Induced by AFB1 via Modulation of Apoptosis and Nrf2 Signaling Pathways. Animals 2021, 11, 3516. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, Y.; Wang, J.; Wang, X.; Chen, C.; Yin, D.; Zhao, F.; Yin, J.; Guo, M.; Zhang, L.; et al. Resveratrol represses estrogen-induced mammary carcinogenesis through NRF2-UGT1A8-estrogen metabolic axis activation. Biochem. Pharmacol. 2018, 155, 252–263. [Google Scholar] [CrossRef]
- Imran, M.; Ghorat, F.; Ul-Haq, I.; Ur-Rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M.; et al. Lycopene as a Natural Antioxidant Used to Prevent Human Health Disorders. Antioxidants 2020, 9, 706. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Ma, J.; Xv, Q.; Gao, H.; Yin, H.; Yan, G.; Jiang, X.; Yu, W. Lycopene attenuates the inflammation and apoptosis in aristolochic acid nephropathy by targeting the Nrf2 antioxidant system. Redox Biol. 2022, 57, 102494. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yan, X.; Zhang, Y.; Yang, M.; Ma, Y.; Zhang, Y.; Xu, Q.; Tu, K.; Zhang, M. Oral administration of turmeric-derived exosome-like nanovesicles with anti-inflammatory and pro-resolving bioactions for murine colitis therapy. J. Nanobiotechnol. 2022, 20, 206. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Jia, W.; Zhang, R.; Wang, X.; Zhang, L. Improving curcumin bioavailability: Targeted delivery of curcumin and loading systems in intestinal inflammation. Food Res. Int. 2024, 196, 115079. [Google Scholar] [CrossRef]
- Yang, G.; Chang, C.C.; Yang, Y.; Yuan, L.; Xu, L.; Ho, C.T.; Li, S. Resveratrol Alleviates Rheumatoid Arthritis via Reducing ROS and Inflammation, Inhibiting MAPK Signaling Pathways, and Suppressing Angiogenesis. J. Agric. Food Chem. 2018, 66, 12953–12960. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Yosifova Aneva, I.; Farzaei, M.H.; Sobarzo-Sánchez, E. The Neuroprotective Effects of Astaxanthin: Therapeutic Targets and Clinical Perspective. Molecules 2019, 24, 2640. [Google Scholar] [CrossRef]
- Ozkan, G.; Günal-Köroğlu, D.; Karadag, A.; Capanoglu, E.; Cardoso, S.M.; Al-Omari, B.; Calina, D.; Sharifi-Rad, J.; Cho, W.C. A mechanistic updated overview on lycopene as potential anticancer agent. Biomed. Pharmacother. 2023, 161, 114428. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, C.; Ye, M.; Wang, C.; Zou, L.; Zhang, X.; Chai, X.; Yu, H.; Zhang, C.; Wang, Y. Mycotoxin Contamination: Occurrence, Biotransformation, Pathogenic Mechanisms, and Strategies for Nutritional Intervention. Molecules 2025, 30, 3860. https://doi.org/10.3390/molecules30193860
Yao C, Ye M, Wang C, Zou L, Zhang X, Chai X, Yu H, Zhang C, Wang Y. Mycotoxin Contamination: Occurrence, Biotransformation, Pathogenic Mechanisms, and Strategies for Nutritional Intervention. Molecules. 2025; 30(19):3860. https://doi.org/10.3390/molecules30193860
Chicago/Turabian StyleYao, Chenyu, Mengyu Ye, Cong Wang, Lin Zou, Ximeng Zhang, Xin Chai, Huijuan Yu, Chengyu Zhang, and Yuefei Wang. 2025. "Mycotoxin Contamination: Occurrence, Biotransformation, Pathogenic Mechanisms, and Strategies for Nutritional Intervention" Molecules 30, no. 19: 3860. https://doi.org/10.3390/molecules30193860
APA StyleYao, C., Ye, M., Wang, C., Zou, L., Zhang, X., Chai, X., Yu, H., Zhang, C., & Wang, Y. (2025). Mycotoxin Contamination: Occurrence, Biotransformation, Pathogenic Mechanisms, and Strategies for Nutritional Intervention. Molecules, 30(19), 3860. https://doi.org/10.3390/molecules30193860






