Recent Advances in Metal Nanoclusters: From Novel Synthesis to Emerging Applications
Abstract
1. Introduction
2. Novel Synthesis
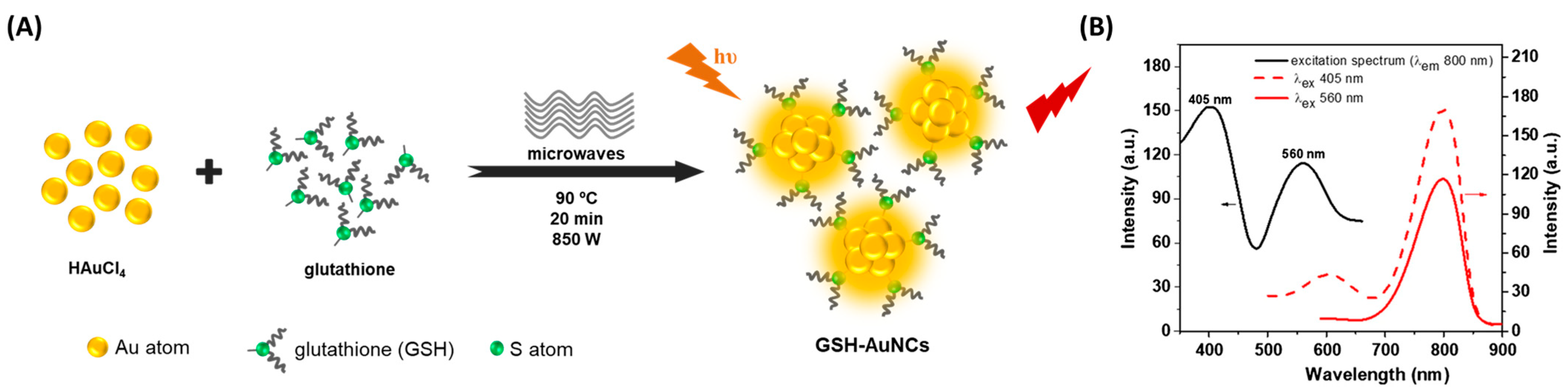
2.1. Microwave-Assisted Synthesis
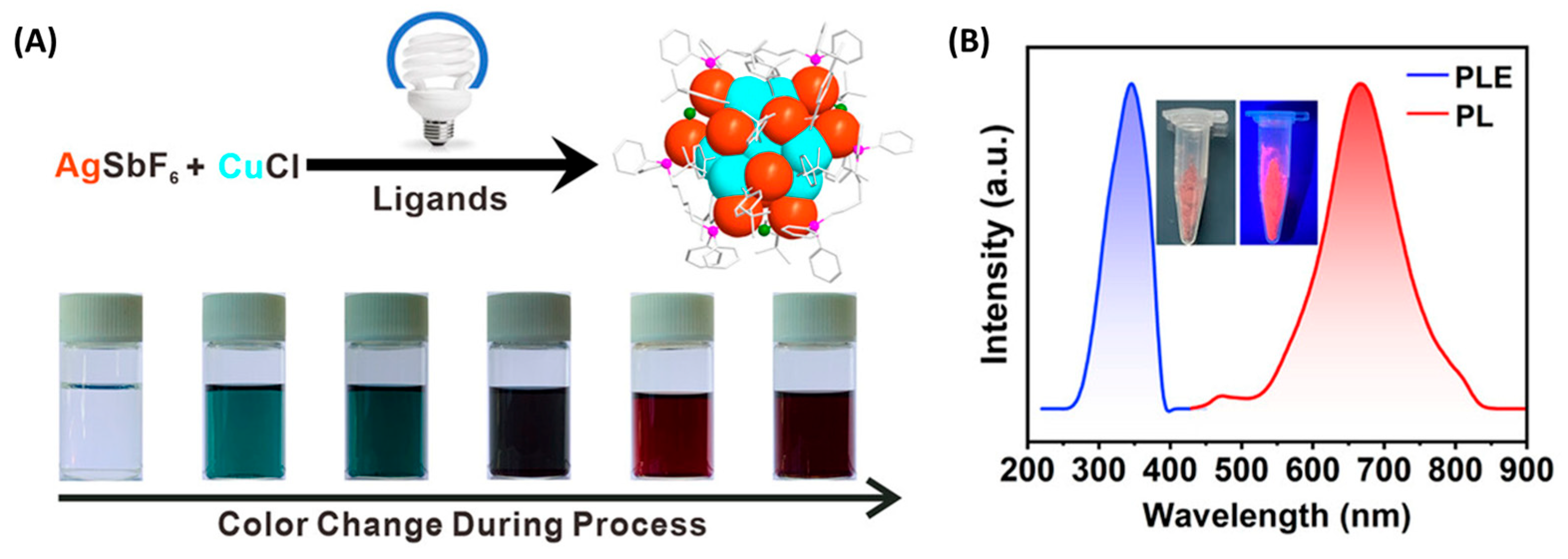
2.2. Photochemical-Assisted Synthesis
2.3. Sonochemical-Assisted Synthesis
2.4. Catalytic-Assisted Synthesis
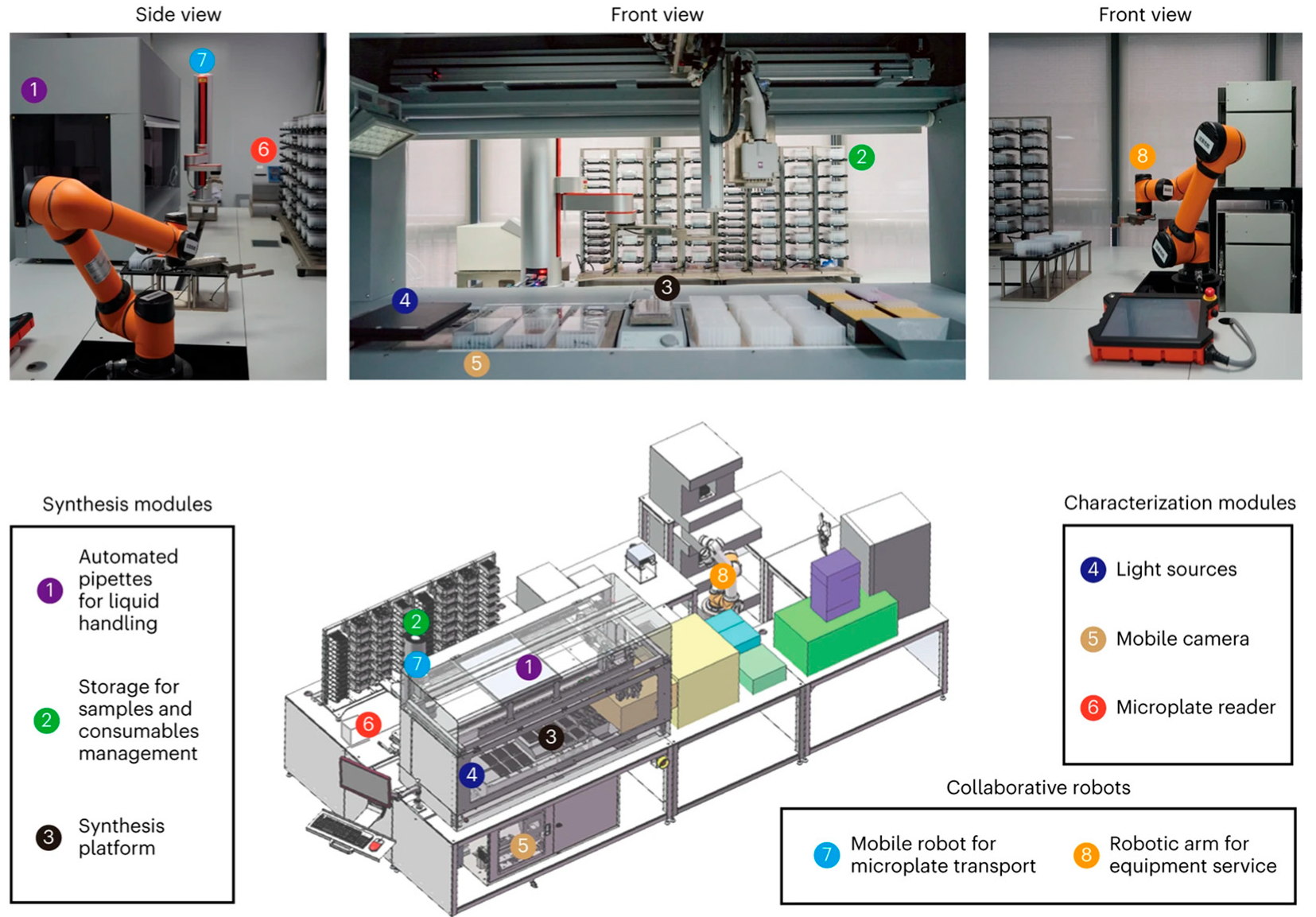
2.5. Smart Synthesis
3. Applications
3.1. Fluorescence Imaging
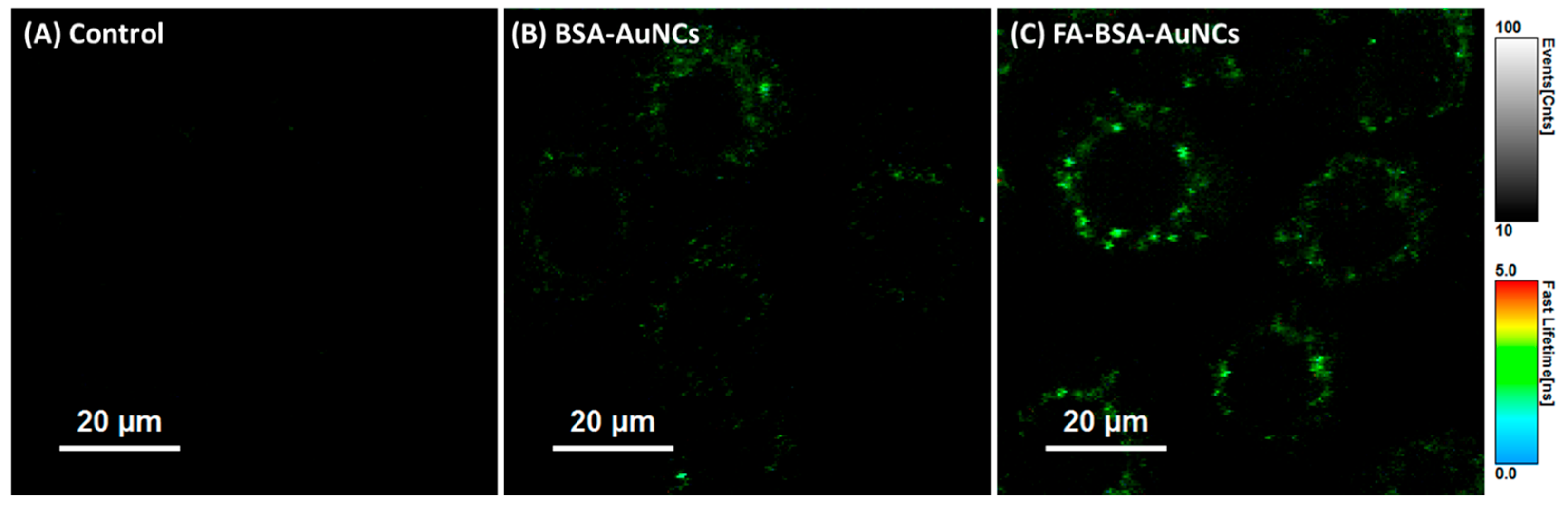
3.1.1. In Vitro Fluorescence Imaging
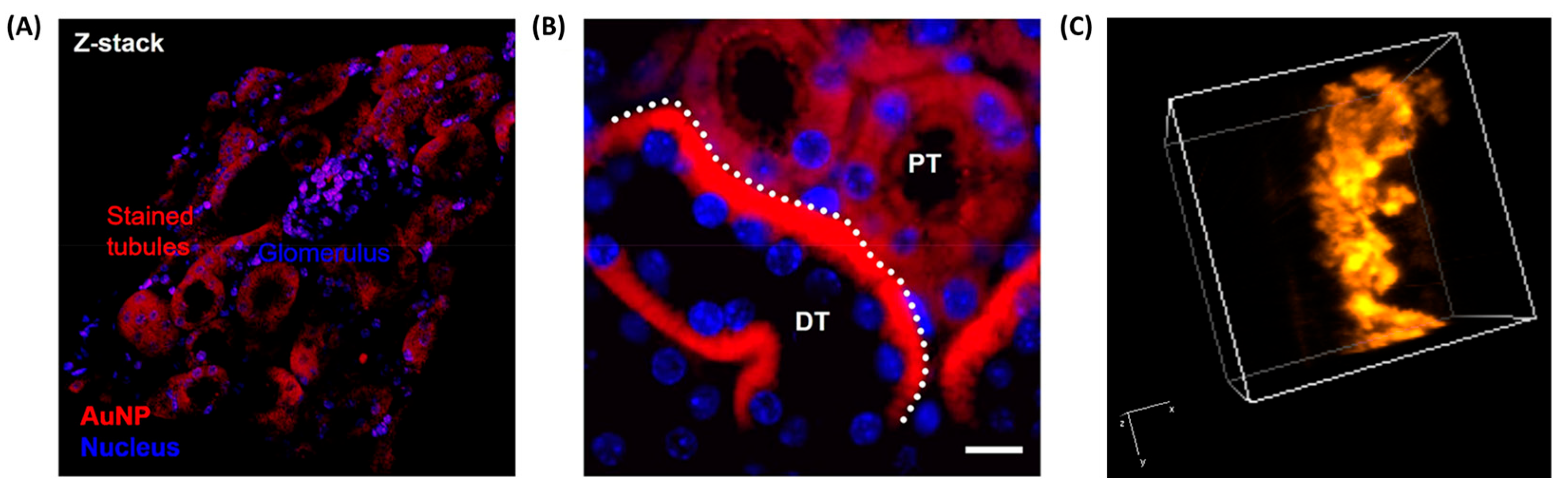
3.1.2. Ex Vivo Fluorescence Imaging
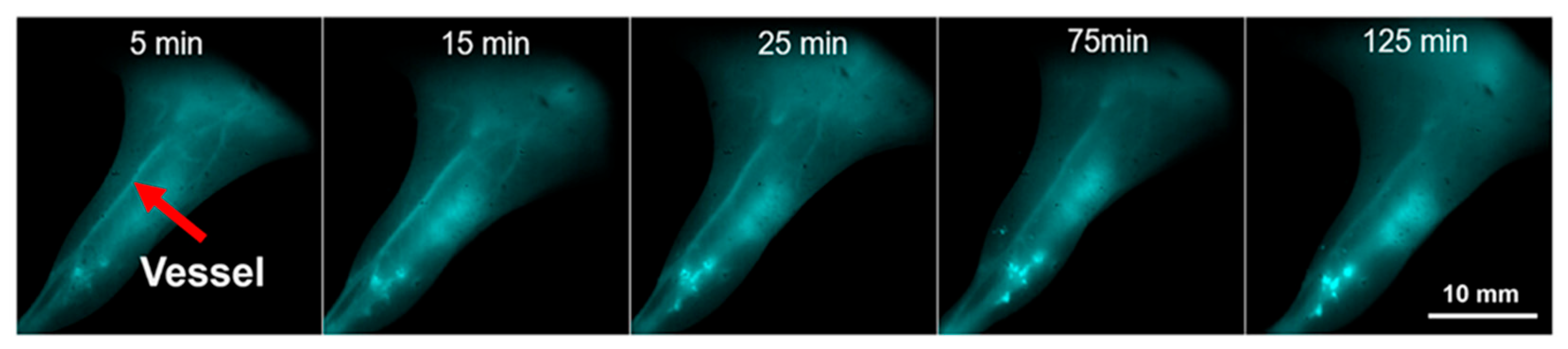
3.1.3. In Vivo Fluorescence Imaging
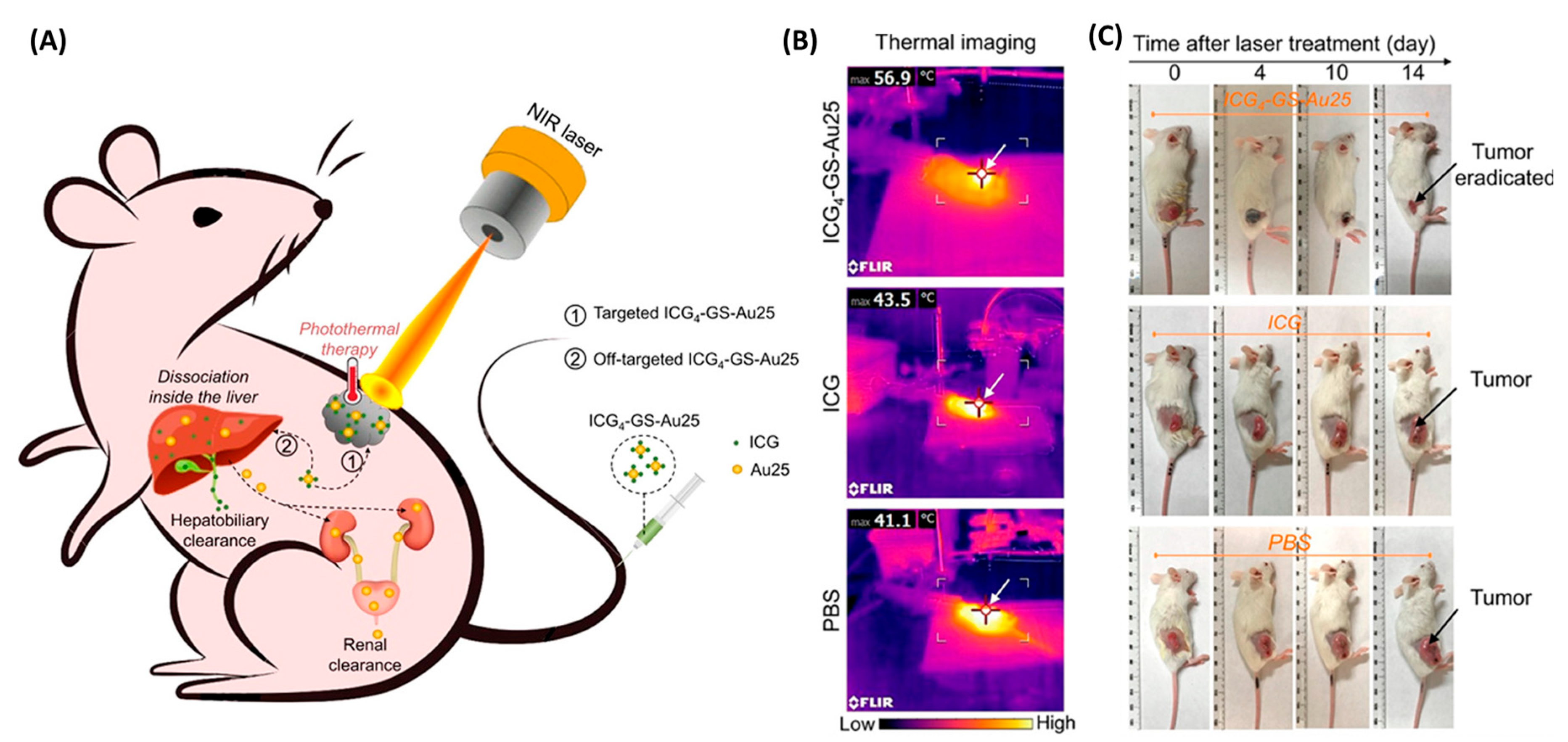
3.2. Theranostics
3.3. Sensing
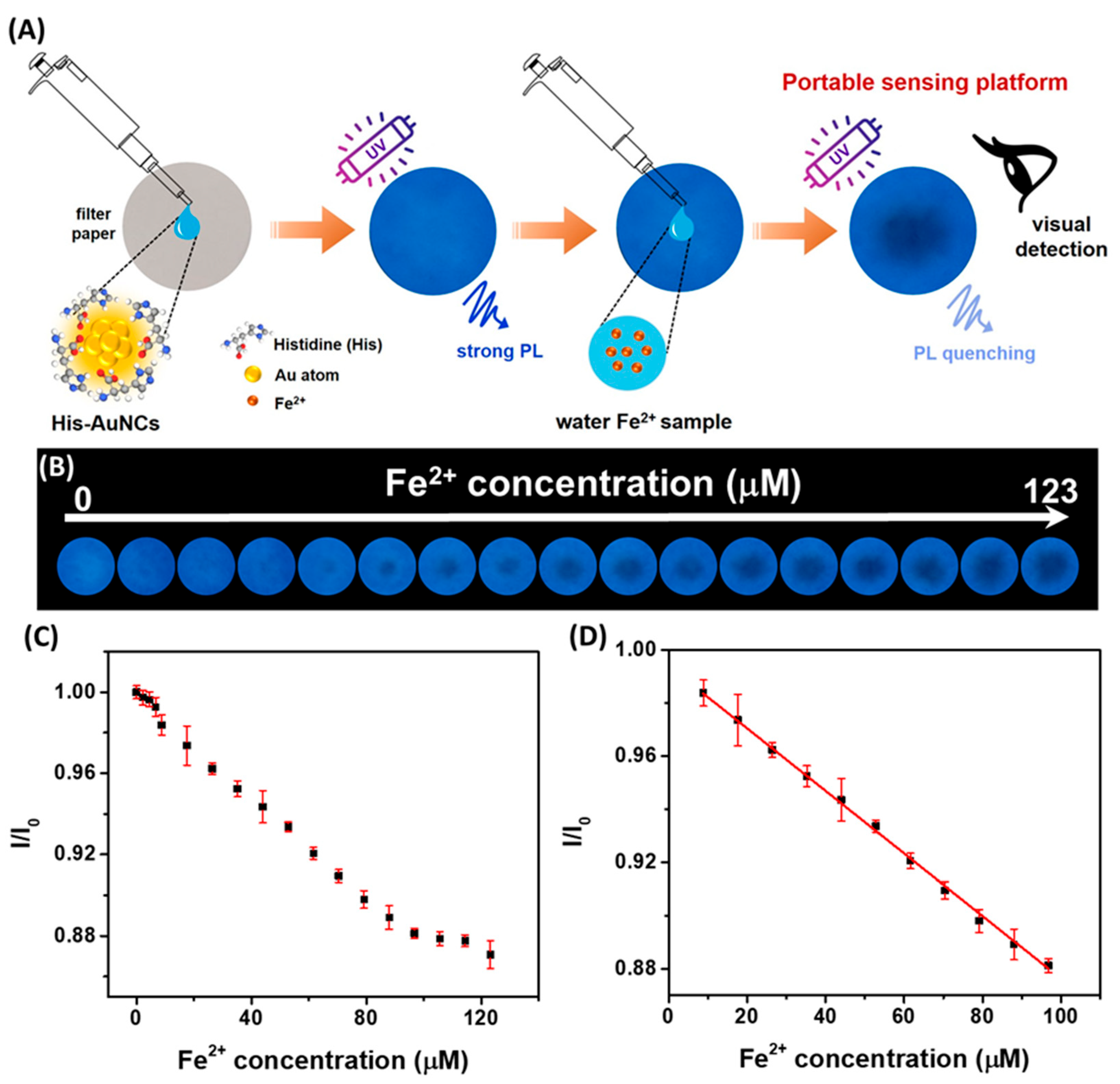
3.3.1. Ion Detection
3.3.2. Small Molecules Detection
3.3.3. Pathogen Detection
3.4. Catalysis
3.4.1. CO2
3.4.2. Water Splitting
3.4.3. Other Reactions
Light-Driven Nitrogen Fixation
Dye Degradation
Selective H2O2 Generation
4. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Garcia, M.A. Surface Plasmons in Metallic Nanoparticles: Fundamentals and Applications. J. Phys. D Appl. Phys. 2011, 44, 283001. [Google Scholar] [CrossRef]
- Tan, S.C.L.; He, Z.; Wang, G.; Yu, Y.; Yang, L. Protein-Templated Metal Nanoclusters: Molecular-like Hybrids for Biosensing, Diagnostics and Pharmaceutics. Molecules 2023, 28, 5531. [Google Scholar] [CrossRef]
- Cui, H.; Shao, Z.-S.; Song, Z.; Wang, Y.-B.; Wang, H.-S. Development of Gold Nanoclusters: From Preparation to Applications in the Field of Biomedicine. J. Mater. Chem. C 2020, 8, 14312–14333. [Google Scholar] [CrossRef]
- Pramanik, G.; Humpolickova, J.; Valenta, J.; Kundu, P.; Bals, S.; Bour, P.; Dracinsky, M.; Cigler, P. Gold Nanoclusters with Bright Near-Infrared Photoluminescence. Nanoscale 2018, 10, 3792–3798. [Google Scholar] [CrossRef]
- Yang, T.-Q.; Peng, B.; Shan, B.-Q.; Zong, Y.-X.; Jiang, J.-G.; Wu, P.; Zhang, K. Origin of the Photoluminescence of Metal Nanoclusters: From Metal-Centered Emission to Ligand-Centered Emission. Nanomaterials 2020, 10, 261. [Google Scholar] [CrossRef]
- Zhou, M.; Zeng, C.; Li, Q.; Higaki, T.; Jin, R. Gold Nanoclusters: Bridging Gold Complexes and Plasmonic Nanoparticles in Photophysical Properties. Nanomaterials 2019, 9, 933. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Shi, W.; Kong, J.; Zhang, W.; Wang, Q.; Luo, Y.; Zhou, M. Triplet Energy Transfer and Photon Upconversion from Metal Nanocluster with Near-Unity NIR Emission Quantum Yield. Adv. Opt. Mater. 2025, 13, 2402991. [Google Scholar] [CrossRef]
- Luo, X.; Han, Y.; Chen, Z.; Li, Y.; Liang, G.; Liu, X.; Ding, T.; Nie, C.; Wang, M.; Castellano, F.N.; et al. Mechanisms of Triplet Energy Transfer across the Inorganic Nanocrystal/Organic Molecule Interface. Nat. Commun. 2020, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Bender, J.A.; Raulerson, E.K.; Li, X.; Goldzak, T.; Xia, P.; Van Voorhis, T.; Tang, M.L.; Roberts, S.T. Surface States Mediate Triplet Energy Transfer in Nanocrystal–Acene Composite Systems. J. Am. Chem. Soc. 2018, 140, 7543–7553. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Yao, Q.; Liu, Z.; Liu, J.; Liu, M.; Long, M.; Xie, J. Aggregation-Induced Emission of Gold Nanoclusters by Ionic Liquids for White Light-Emitting Diode and Multiple-Ion Probe Applications. J. Phys. Chem. Lett. 2022, 13, 7722–7730. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Sui, Y.; Guo, Y.; Zhang, Y. Aggregation-induced emission enhancement of gold nanoclusters in metal–organic frameworks for highly sensitive fluorescent detection of bilirubin. Analyst 2021, 3, 904–910. [Google Scholar] [CrossRef]
- Kang, X.; Wang, S.; Zhu, M. Observation of a New Type of Aggregation-Induced Emission in Nanoclusters. Chem. Sci. 2018, 9, 3062–3068. [Google Scholar] [CrossRef]
- Goswami, N.; Yao, Q.; Luo, Z.; Li, J.; Chen, T.; Xie, J. Luminescent Metal Nanoclusters with Aggregation-Induced Emission. J. Phys. Chem. Lett. 2016, 7, 962–975. [Google Scholar] [CrossRef]
- Shi, Y.; Ma, J.; Feng, A.; Wang, Z.; Rogach, A.L. Aggregation-induced Emission of Copper Nanoclusters. Aggregate 2021, 2, e112. [Google Scholar] [CrossRef]
- Li, H.; Zhu, W.; Wan, A.; Liu, L. The Mechanism and Application of the Protein-Stabilized Gold Nanocluster Sensing System. Analyst 2017, 142, 567–581. [Google Scholar] [CrossRef]
- Mittal, R.; Gupta, N. Towards Green Synthesis of Fluorescent Metal Nanoclusters. J. Fluoresc. 2023, 33, 2161–2180. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Zhu, M.; Yang, Z.; Song, X.; Yuan, X.; Zhang, Z.; Hu, W.; Xie, J. Molecule-like Synthesis of Ligand-Protected Metal Nanoclusters. Nat. Rev. Mater. 2024, 10, 89–108. [Google Scholar] [CrossRef]
- Gabano, E.; Ravera, M. Microwave-Assisted Synthesis: Can Transition Metal Complexes Take Advantage of This “Green” Method? Molecules 2022, 27, 4249. [Google Scholar] [CrossRef]
- Hada, A.-M.; Zetes, M.; Focsan, M.; Astilean, S.; Craciun, A.-M. Photoluminescent Histidine-Stabilized Gold Nanoclusters as Efficient Sensors for Fast and Easy Visual Detection of Fe Ions in Water Using Paper-Based Portable Platform. IJMS 2022, 23, 12410. [Google Scholar] [CrossRef]
- Zetes, M.; Hada, A.-M.; Todea, M.; Gaina, L.I.; Astilean, S.; Craciun, A.-M. Dual-Emissive Solid-State Histidine-Stabilized Gold Nanoclusters for Applications in White-Light Generation. Nanoscale Adv. 2023, 5, 5810–5818. [Google Scholar] [CrossRef]
- Hada, A.-M.; Craciun, A.-M.; Focsan, M.; Vulpoi, A.; Borcan, E.-L.; Astilean, S. Glutathione-Capped Gold Nanoclusters as near-Infrared-Emitting Efficient Contrast Agents for Confocal Fluorescence Imaging of Tissue-Mimicking Phantoms. Microchim. Acta 2022, 189, 337. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Gao, H.; Li, L.; Ma, C.; Gu, J.; Zhu, C.; Yang, Z.; Wang, C.; Zhang, Y.; Chen, G. Green Synthesis of Fluorescent Ag Nanoclusters for Detecting Cu2+ Ions and Its “Switch-On” Sensing Application for GSH. J. Spectrosc. 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Saleh, S.M.; El-Sayed, W.A.; El-Manawaty, M.A.; Gassoumi, M.; Ali, R. An Eco-Friendly Synthetic Approach for Copper Nanoclusters and Their Potential in Lead Ions Sensing and Biological Applications. Biosensors 2022, 12, 197. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Zhang, J.; Su, H.-F.; Cui, X.; Wei, C.-Y.; Li, H.; Zhang, X.-M. Photochemical Synthesis of Atomically Precise Ag Nanoclusters. ACS Nano 2023, 17, 11607–11615. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, F.; Qi, Z.; Zhao, X.; Zhang, N.; Li, H.; Li, H.; Zhang, X. Photochemical Synthesis of Ag12Cu7 Nanocluster with Cuprophilicity-related Long-lived Phosphorescence. Aggregate 2025, 6. [Google Scholar] [CrossRef]
- Fan, J.-Q.; Cen, K.; Xu, H.-J.; Wang, H.-Y.; Yang, Y.; Zhu, Z.-M.; Liu, H.; Chen, D.; Fan, W.; Li, M.-B. Photochemical Synthesis of Group 10 Metal Nanoclusters for Electrocatalysis. Nanoscale 2023, 15, 19079–19084. [Google Scholar] [CrossRef] [PubMed]
- Ferlazzo, A.; Bonforte, S.; Florio, F.; Petralia, S.; Sorace, L.; Muzzi, B.; Caneschi, A.; Gulino, A. Photochemical Eco-Friendly Synthesis of Photothermal and Emissive Copper Nanoclusters in Water: Towards Sustainable Nanomaterials. Mater. Adv. 2024, 5, 8034–8041. [Google Scholar] [CrossRef]
- Xu, H.; Suslick, K.S. Sonochemical Synthesis of Highly Fluorescent Ag Nanoclusters. ACS Nano 2010, 4, 3209–3214. [Google Scholar] [CrossRef]
- Kang, J.; Gao, P.; Zhang, G.; Shi, L.; Zhou, Y.; Wu, J.; Shuang, S.; Zhang, Y. Rapid Sonochemical Synthesis of Copper Nanoclusters with Red Fluorescence for Highly Sensitive Detection of Silver Ions. Microchem. J. 2022, 178, 107370. [Google Scholar] [CrossRef]
- Liu, W.; Guo, F.; Long, J.; Feng, G.; Huang, M.; Lu, Z.-H. Ultrasound-Assisted Synthesis of Highly Dispersed Nickel Nanoclusters on MXene for Efficient Dehydrogenation of Hydrazine Borane. Surf. Interfaces 2025, 56, 105563. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, P.; Li, H.; Li, T.; Wang, P.; Dong, J.; Zhang, F.; Chen, Z.-N.; Li, H. Heterogeneously Catalytic Synthesis of Au 13 Nanocluster. CCS Chem. 2025, 7, 2579–2586. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, A.; Zhang, R.; Ji, Z.; Li, J.; Lyu, J.; Qian, J.; Chen, T.; Wang, X.; You, F.; et al. When Metal Nanoclusters Meet Smart Synthesis. ACS Nano 2024, 18, 27138–27166. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, W.; Huang, H.; Sun, Z.; Chen, Z.; Wu, L.; Zhang, B.; Lai, F.; Wang, Z.; Adam, M.L.; et al. A Robotic Platform for the Synthesis of Colloidal Nanocrystals. Nat. Synth 2023, 2, 505–514. [Google Scholar] [CrossRef]
- Duros, V.; Grizou, J.; Xuan, W.; Hosni, Z.; Long, D.; Miras, H.N.; Cronin, L. Human versus Robots in the Discovery and Crystallization of Gigantic Polyoxometalates. Angew. Chem. Int. Ed. 2017, 56, 10815–10820. [Google Scholar] [CrossRef]
- Jiang, Y.; Salley, D.; Sharma, A.; Keenan, G.; Mullin, M.; Cronin, L. An Artificial Intelligence Enabled Chemical Synthesis Robot for Exploration and Optimization of Nanomaterials. Sci. Adv. 2022, 8, eabo2626. [Google Scholar] [CrossRef]
- Li, J.; Chen, T.; Lim, K.; Chen, L.; Khan, S.A.; Xie, J.; Wang, X. Deep Learning Accelerated Gold Nanocluster Synthesis. Adv. Intell. Syst. 2019, 1, 1900029. [Google Scholar] [CrossRef]
- Ekins, S.; Puhl, A.C.; Zorn, K.M.; Lane, T.R.; Russo, D.P.; Klein, J.J.; Hickey, A.J.; Clark, A.M. Exploiting Machine Learning for End-to-End Drug Discovery and Development. Nat. Mater. 2019, 18, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Baghdasaryan, A.; Dai, H. Molecular Gold Nanoclusters for Advanced NIR-II Bioimaging and Therapy. Chem. Rev. 2025, 125, 5195–5227. [Google Scholar] [CrossRef]
- Sharma, N.; Mohammad, W.; Le Guével, X.; Shanavas, A. Gold Nanoclusters as High Resolution NIR-II Theranostic Agents. Chem. Biomed. Imaging 2024, 2, 462–480. [Google Scholar] [CrossRef] [PubMed]
- Hada, A.-M.; Craciun, A.-M.; Focsan, M.; Borlan, R.; Soritau, O.; Todea, M.; Astilean, S. Folic Acid Functionalized Gold Nanoclusters for Enabling Targeted Fluorescence Imaging of Human Ovarian Cancer Cells. Talanta 2021, 225, 121960. [Google Scholar] [CrossRef]
- Feng, B.; Xing, Y.; Lan, J.; Su, Z.; Wang, F. Synthesis of MUC1 Aptamer-Stabilized Gold Nanoclusters for Cell-Specific Imaging. Talanta 2020, 212, 120796. [Google Scholar] [CrossRef]
- Tan, H.; Liu, S.; He, Y.; Cheng, G.; Zhang, Y.; Wei, X.; Hu, L. Spider Toxin Peptide-Induced NIR Gold Nanocluster Fabrication for GSH-Responsive Cancer Cell Imaging and Nuclei Translocation. Front. Bioeng. Biotechnol. 2021, 9, 780223. [Google Scholar] [CrossRef]
- Peng, C.; Yu, M.; Zheng, J. In Situ Ligand-Directed Growth of Gold Nanoparticles in Biological Tissues. Nano Lett. 2020, 20, 1378–1382. [Google Scholar] [CrossRef] [PubMed]
- Hada, A.-M.; Craciun, A.-M.; Astilean, S. Intrinsic Photoluminescence of Solid-State Gold Nanoclusters: Towards Fluorescence Lifetime Imaging of Tissue-Like Phantoms Under Two-Photon Near-Infrared Excitation. Front. Chem. 2021, 9, 761711. [Google Scholar] [CrossRef] [PubMed]
- Hada, A.-M.; Craciun, A.-M.; Astilean, S. Gold Nanoclusters Performing as Contrast Agents for Non-Invasive Imaging of Tissue-like Phantoms via Two-Photon Excited Fluorescence Lifetime Imaging. Analyst 2021, 146, 7126–7130. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhu, W.; Ge, X.; Li, R.; Li, S.; Chen, X.; Song, J.; Xie, J.; Chen, X.; Yang, H. A New Class of NIR-II Gold Nanocluster-Based Protein Biolabels for In Vivo Tumor-Targeted Imaging. Angew. Chem. 2021, 133, 1326–1332. [Google Scholar] [CrossRef]
- Nie, W.; He, K.; Zhao, Z.; Luo, X.; Liu, J. Luminescent Gold Nanoparticles with Discrete DNA Valences for Precisely Controlled Transport at the Subcellular Level. Angew. Chem. Int. Ed. 2023, 62, e202314896. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, H.; He, K.; Lin, J.; Cai, W.; Sun, Y.; Liu, J. Glutathione-Activated Emission of Ultrasmall Gold Nanoparticles in the Second Near-Infrared Window for Imaging of Early Kidney Injury. Anal. Chem. 2023, 95, 5061–5068. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, G.; Zhao, R.; Ma, H.; Yan, Y.; Yang, S.; Meng, J.; Huang, Y.; Zhang, X.-D.; Wang, H.; et al. Ligand Engineering of Gold Nanoclusters for NIR-II Imaging. ACS Appl. Nano Mater. 2023, 6, 15945–15958. [Google Scholar] [CrossRef]
- Liu, P.; Shi, T.; Li, H.; Chen, H.; Huang, Y.; Ma, H.; Zhu, T.; Zhao, R.; Li, Y.; Xin, Q.; et al. Airy Beam Assisted NIR-II Light-Sheet Microscopy. Nano Today 2022, 47, 101628. [Google Scholar] [CrossRef]
- Jiang, X.; Du, B.; Huang, Y.; Yu, M.; Zheng, J. Cancer Photothermal Therapy with ICG-Conjugated Gold Nanoclusters. Bioconjugate Chem. 2020, 31, 1522–1528. [Google Scholar] [CrossRef]
- Kong, Y.; Santos-Carballal, D.; Martin, D.; Sergeeva, N.N.; Wang, W.; Liu, G.; Johnson, B.; Bhayana, B.; Lin, Z.; Wang, Y.; et al. A NIR-II-Emitting Gold Nanocluster-Based Drug Delivery System for Smartphone-Triggered Photodynamic Theranostics with Rapid Body Clearance. Mater. Today 2021, 51, 96–107. [Google Scholar] [CrossRef]
- Wang, M.; Wang, L.; Feng, H.; Jiang, H.; Zhou, J.; Wang, X. Precise Therapeutic Effect of Self-Assembling Gold Nanocluster–PTEN Complexes on an Orthotropic Model of Liver Cancer. J. Cancer. Res. Clin. Oncol. 2020, 146, 875–882. [Google Scholar] [CrossRef]
- Yang, G.; Liu, K.; Wang, Y.; Pan, X.; Ye, J.; Li, Y.; Du, F.; Feng, T.; Yuan, X. Phosphorylation of NIR-II Emitting Au Nanoclusters for Targeted Bone Imaging and Improved Rheumatoid Arthritis Therapy. Aggregate 2024, 5, e435. [Google Scholar] [CrossRef]
- Yan, Y.; Lv, J.; Wang, M.; Xu, J.; Xia, Y.; Wei, R.; Hua, L.; Xie, J.; Chen, Y. Multifunctional Silver Nanoclusters with Hyaluronic Acid for Dual-Targeted Tumor Imaging and ROS-Mediated Therapy. Colloids Surf. B Biointerfaces 2025, 255, 114913. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, H.; Li, H.; Zhang, T.; Zhang, J.; Guo, W.; Fu, K.; Du, G. Magnetic and Near-Infrared-II Fluorescence Au–Gd Nanoclusters for Imaging-Guided Sensitization of Tumor Radiotherapy. Nanoscale Adv. 2022, 4, 1815–1826. [Google Scholar] [CrossRef]
- El-Mageed, H.R.A.; Mustafa, F.M.; Abdel-Latif, M.K. The Ability of Gold Nanoclusters as a New Nanocarrier for D-Penicillamine Anticancer Drug: A Computational Chemistry Study. Struct. Chem. 2020, 31, 781–793. [Google Scholar] [CrossRef]
- Mohseni, N.; Moodi, M.; Kefayat, A.; Shokati, F.; Molaabasi, F. Challenges and Opportunities of Using Fluorescent Metal Nanocluster-Based Colorimetric Assays in Medicine. ACS Omega 2024, 9, 3143–3163. [Google Scholar] [CrossRef]
- Zhang, G.; Fang, B.; Peng, J.; Deng, S.; Hu, L.; Lai, W. Luminescent Gold Nanoclusters from Synthesis to Sensing: A Comprehensive Review. Chem. Eng. J. 2025, 503, 158294. [Google Scholar] [CrossRef]
- Zhao, R.-X.; Liu, A.-Y.; Wen, Q.-L.; Wu, B.-C.; Wang, J.; Hu, Y.-L.; Pu, Z.-F.; Ling, J.; Cao, Q. Glutathione Stabilized Green-Emission Gold Nanoclusters for Selective Detection of Cobalt Ion. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 254, 119628. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Majhi, S.; Sharma, K.; Ali, M.; Sharma, S.; Choudhary, D.; Tripathi, C.S.P.; Guin, D. BSA Stabilized Copper Nanoclusters as a Highly Sensitive and Selective Probe for Fluorescence Sensing of Fe3+ Ions. Chem. Phys. Lett. 2022, 787, 139226. [Google Scholar] [CrossRef]
- Zhang, C.; Shao, C.; Wang, J.; Li, Z.; Liang, M.; Wang, Y.; Liu, D.; Lu, S. Multifunctional Fluorescent Copper Nanoclusters for Ag+ Sensing, Anticounterfeiting, and Blue/White Light-Emitting Diodes. ACS Appl. Nano Mater. 2022, 5, 7449–7459. [Google Scholar] [CrossRef]
- Desai, M.L.; Basu, H.; Saha, S.; Singhal, R.K.; Kailasa, S.K. One Pot Synthesis of Fluorescent Gold Nanoclusters from Curcuma Longa Extract for Independent Detection of Cd2+, Zn2+ and Cu2+ Ions with High Sensitivity. J. Mol. Liq. 2020, 304, 112697. [Google Scholar] [CrossRef]
- Saleh, S.M.; Almotiri, M.K.; Ali, R. Green Synthesis of Highly Luminescent Gold Nanoclusters and Their Application in Sensing Cu(II) and Hg(II). J. Photochem. Photobiol. A Chem. 2022, 426, 113719. [Google Scholar] [CrossRef]
- Hada, A.-M.; Zetes, M.; Focsan, M.; Nagy-Simon, T.; Craciun, A.-M. Novel Paper-Based Sensing Platform Using Photoluminescent Gold Nanoclusters for Easy, Sensitive and Selective Naked-Eye Detection of Cu2+. J. Mol. Struct. 2021, 1244, 130990. [Google Scholar] [CrossRef]
- Yang, Y.; Xing, X.; Zou, T.; Wang, Z.; Zhao, R.; Hong, P.; Peng, S.; Zhang, X.; Wang, Y. A Novel and Sensitive Ratiometric Fluorescence Assay for Carbendazim Based on N-Doped Carbon Quantum Dots and Gold Nanocluster Nanohybrid. J. Hazard. Mater. 2020, 386, 121958. [Google Scholar] [CrossRef]
- Yang, Y.; Ghalandari, B.; Lin, L.; Sang, X.; Su, W.; Divsalar, A.; Ding, X. A Turn-on Fluorescence Sensor Based on Cu2+ Modulated DNA-Templated Silver Nanoclusters for Glyphosate Detection and Mechanism Investigation. Food Chem. 2022, 367, 130617. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Chen, H.; Fan, T.; Guo, Z.; Liu, F. Fluorescence Turn-off Strategy for Sensitive Detection of DNA Methyltransferase Activity Based on DNA-Templated Gold Nanoclusters. Heliyon 2023, 9, e17724. [Google Scholar] [CrossRef]
- Qin, W.; Wang, J.; Tang, Z.; Tian, H.; Wu, Z. Tris(2-Carboxyethyl)Phosphine-Mediated Immobilization of Thiolated DNA on Gold Nanoclusters and Its Application in Multiplex microRNA Detection. Sens. Actuators B Chem. 2024, 401, 135028. [Google Scholar] [CrossRef]
- Qi, L.; Liu, Q.; Chen, G.; Chen, J. Fluorescence “Turn-on” of Sanguinarine-Templated Copper Nanoclusters for Sensing Ascorbic Acid in Orange Drinks and Tablets. Food Chem. 2025, 491, 145160. [Google Scholar] [CrossRef]
- Bin Jardan, Y.A.; Elmasry, M.R.; Ali, A.-M.B.H.; El-Wekil, M.M. Dual Modification of Nickel Nanoclusters for Selective Detection of Glutathione through a Competitive Displacement Mechanism. RSC Adv. 2025, 15, 18826–18835. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, Z.; Meng, G.; Yang, C. Smartphone-Assisted Copper Nanoclusters Fluorescent Sensing Platform for Visual Determination of Congo Red. Colloids Surf. B Biointerfaces 2025, 251, 114610. [Google Scholar] [CrossRef]
- Evstigneeva, S.S.; Chumakov, D.S.; Tumskiy, R.S.; Khlebtsov, B.N.; Khlebtsov, N.G. Detection and Imaging of Bacterial Biofilms with Glutathione-Stabilized Gold Nanoclusters. Talanta 2023, 264, 124773. [Google Scholar] [CrossRef]
- Song, Y.; Wang, L.; Zhao, J.; Li, H.; Yang, X.; Fu, S.; Qin, X.; Chen, Q.; Jiang, Y.; Man, C. A Novel Colorimetric Sensor Using Aptamers to Enhance Peroxidase-like Property of Gold Nanoclusters for Detection of Escherichia Coli O157:H7 in Milk. Int. Dairy J. 2022, 128, 105318. [Google Scholar] [CrossRef]
- Pang, L.; Li, S.; Liu, B.; Su, Q.; Qu, B.; Zhang, W.; Yang, X.; Jiang, Y. Colorimetric Biosensor Based on Aptamer Recognition-Induced Multi-DNA Release and Peroxidase-Mimicking Three-Way Junction DNA-Ag/PtNCs for the Detection of Salmonella Typhimurium. Talanta 2024, 274, 125930. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.-F.; Zhao, K.-R.; Liu, Z.-J.; Wang, L.; Ye, S.-Y.; Liang, G.-X. Cas12a-Based Electrochemiluminescence Biosensor for Target Amplification-Free DNA Detection. Biosens. Bioelectron. 2021, 176, 112954. [Google Scholar] [CrossRef]
- Tao, Y.; Yi, K.; Wang, H.; Li, K.; Li, M. Metal Nanoclusters Combined with CRISPR-Cas12a for Hepatitis B Virus DNA Detection. Sens. Actuators B Chem. 2022, 361, 131711. [Google Scholar] [CrossRef]
- Wu, N.-N.; Chen, L.-G.; Xiao, M.-Z.; Yuan, R.-Y.; Wang, H.-B. Determination of Trypsin Using Protamine Mediated Fluorescent Enhancement of DNA Templated Au Nanoclusters. Microchim. Acta 2023, 190, 158. [Google Scholar] [CrossRef]
- Niu, X.; Suo, Z.; Li, J.; Wei, M.; Jin, H.; He, B. Self-Assembled Programmable DNA Nanoflower for in Situ Synthesis of Gold Nanoclusters and Integration with Mn-MOF to Sensitively Detect AFB1. Chem. Eng. J. 2024, 479, 147806. [Google Scholar] [CrossRef]
- Guan, Z.; Li, J.; Hu, F.; Wang, Q. Structural Engineering toward Gold Nanocluster Catalysis. Angew. Chem. Int. Ed. 2022, 61, e202209725. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Guo, C.; Qin, L.; Wang, L.; Qiao, L.; Chi, K.; Tang, Z. Atomically Precise Cu Nanoclusters: Recent Advances, Challenges, and Perspectives in Synthesis and Catalytic Applications. Nano-Micro Lett. 2025, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.-Z.; Wang, Y.-J.; Li, S.; Wang, R.; Wu, J.; Zang, S.-Q. Integrating Homogeneous and Heterogeneous Catalysis in a Copper Nanocluster with Lewis Acid–Base Sites for Chemical Conversion of CO2 and Propargylamine. CCS Chem. 2024, 6, 2131–2141. [Google Scholar] [CrossRef]
- Du, Y.; Li, C.; Dai, Y.; Yin, H.; Zhu, M. Recent Progress in Atomically Precise Metal Nanoclusters for Photocatalytic Application. Nanoscale Horiz 2024, 9, 1262–1278. [Google Scholar] [CrossRef]
- Li, S.; Du, X.; Liu, Z.; Li, Y.; Shao, Y.; Jin, R. Size Effects of Atomically Precise Gold Nanoclusters in Catalysis. Precis. Chem. 2023, 1, 14–28. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, H.; Hu, Y.; Yang, S.; Xue, X.; He, L.; Liu, X.; Ma, J.; Jin, Z. Photodriven Catalytic Hydrogenation of CO2 to CH4 with Nearly 100% Selectivity over Ag25 Clusters. Nano Lett. 2021, 21, 8693–8700. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yu, Y.; Zhang, X.; Weinert, M.; Song, X.; Ai, J.; Han, L.; Fei, H. N-Heterocyclic Carbene-Stabilized Ultrasmall Gold Nanoclusters in a Metal-Organic Framework for Photocatalytic CO2 Reduction. Angew. Chem. Int. Ed. 2021, 60, 17388–17393. [Google Scholar] [CrossRef]
- Tian, J.; Zhong, K.; Zhu, X.; Yang, J.; Mo, Z.; Liu, J.; Dai, J.; She, Y.; Song, Y.; Li, H.; et al. Highly Exposed Active Sites of Au Nanoclusters for Photocatalytic CO2 Reduction. Chem. Eng. J. 2023, 451, 138392. [Google Scholar] [CrossRef]
- Dong, J.; Xu, Y.; Zhang, X.; Zhang, H.; Yao, L.; Wang, R.; Zang, S. Copper-Sulfur-Nitrogen Cluster Providing a Local Proton for Efficient Carbon Dioxide Photoreduction. Angew. Chem. Int. Ed. 2023, 62, e202313648. [Google Scholar] [CrossRef]
- Dai, S.; Kajiwara, T.; Ikeda, M.; Romero-Muñiz, I.; Patriarche, G.; Platero-Prats, A.E.; Vimont, A.; Daturi, M.; Tissot, A.; Xu, Q.; et al. Ultrasmall Copper Nanoclusters in Zirconium Metal-Organic Frameworks for the Photoreduction of CO2. Angew. Chem. Int. Ed. 2022, 61, e202211848. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Wang, Q.; Quick, M.; Kovalenko, S.A.; Chen, Q.; Koch, N.; Pinna, N. Insights into Charge Transfer at an Atomically Precise Nanocluster/Semiconductor Interface. Angew. Chem. Int. Ed. 2020, 59, 7748–7754. [Google Scholar] [CrossRef]
- Fu, X.-Y.; Wei, Z.-Q.; Xu, S.; Lin, X.; Hou, S.; Xiao, F.-X. Maneuvering Intrinsic Instability of Metal Nanoclusters for Boosted Solar-Powered Hydrogen Production. J. Phys. Chem. Lett. 2020, 11, 9138–9143. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.-C.; Huang, M.-H.; Li, Y.-B.; Li, T.; Hou, S.; Wei, Z.-Q.; Xiao, F.-X. Probing the Advantageous Photosensitization Effect of Metal Nanoclusters over Plasmonic Metal Nanocrystals in Photoelectrochemical Water Splitting. J. Phys. Chem. C 2020, 124, 4989–4998. [Google Scholar] [CrossRef]
- Kawawaki, T.; Kataoka, Y.; Hirata, M.; Akinaga, Y.; Takahata, R.; Wakamatsu, K.; Fujiki, Y.; Kataoka, M.; Kikkawa, S.; Alotabi, A.S.; et al. Creation of High-Performance Heterogeneous Photocatalysts by Controlling Ligand Desorption and Particle Size of Gold Nanocluster. Angew. Chem. Int. Ed. 2021, 60, 21340–21350. [Google Scholar] [CrossRef]
- Huang, X.; Li, X.; Luan, Q.; Zhang, K.; Wu, Z.; Li, B.; Xi, Z.; Dong, W.; Wang, G. Highly Dispersed Pt Clusters Encapsulated in MIL-125-NH2 via in Situ Auto-Reduction Method for Photocatalytic H2 Production under Visible Light. Nano Res. 2021, 14, 4250–4257. [Google Scholar] [CrossRef]
- Lu, X.; Tong, A.; Luo, D.; Jiang, F.; Wei, J.; Huang, Y.; Jiang, Z.; Lu, Z.; Ni, Y. Confining Single Pt Atoms from Pt Clusters on Multi-Armed CdS for Enhanced Photocatalytic Hydrogen Evolution. J. Mater. Chem. A 2022, 10, 4594–4600. [Google Scholar] [CrossRef]
- Li, Y.; Yang, L.; He, H.; Sun, L.; Wang, H.; Fang, X.; Zhao, Y.; Zheng, D.; Qi, Y.; Li, Z.; et al. In Situ Photodeposition of Platinum Clusters on a Covalent Organic Framework for Photocatalytic Hydrogen Production. Nat. Commun. 2022, 13, 1355. [Google Scholar] [CrossRef] [PubMed]
- Bootharaju, M.S.; Lee, C.W.; Deng, G.; Kim, H.; Lee, K.; Lee, S.; Chang, H.; Lee, S.; Sung, Y.; Yoo, J.S.; et al. Atom-Precise Heteroatom Core-Tailoring of Nanoclusters for Enhanced Solar Hydrogen Generation. Adv. Mater. 2023, 35, 2207765. [Google Scholar] [CrossRef]
- Sun, Y.; Pei, W.; Xie, M.; Xu, S.; Zhou, S.; Zhao, J.; Xiao, K.; Zhu, Y. Excitonic Au4 Ru2 (PPh3 )2 (SC2 H4 Ph)8 Cluster for Light-Driven Dinitrogen Fixation. Chem. Sci. 2020, 11, 2440–2447. [Google Scholar] [CrossRef]
- Cheng, X.; Sui, X.; Xu, J.; Liu, X.; Chen, M.; Zhu, Y. On the Photocatalysis Evolution of Heteroatom-Doped Ag4 M2 Nanoclusters. RSC Adv. 2021, 11, 32526–32532. [Google Scholar] [CrossRef]
- Xue, Q.; Wang, Z.; Han, S.; Liu, Y.; Dou, X.; Li, Y.; Zhu, H.; Yuan, X. Ligand Engineering of Au Nanoclusters with Multifunctional Metalloporphyrins for Photocatalytic H2O2 Production. J. Mater. Chem. A 2022, 10, 8371–8377. [Google Scholar] [CrossRef]

| Sample | Capping Ligand | Synthesis Method | Time | λexc/λem(nm) | Ref. |
|---|---|---|---|---|---|
| His-AuNCs | histidine | Microwave assisted | 30 min | 380/471 | [19] |
| His-AuNCs | histidine | Microwave assisted | 30 min | 400/475–520 | [20] |
| GSH-AuNCs | glutathione | Microwave assisted | 20 min | 405/610–800 | [21] |
| His-AgNCs | histidine | Microwave assisted | 8 min | 356/440 | [22] |
| Pep-CuNCs | pepsin | Microwave assisted | 36 min | 349/409 | [23] |
| Ag25 | (1,5-bis-(diphenylphosphino)pentane) | Photochemical assisted | 24 h | 588/n.r. | [24] |
| Ag12Cu7 | (4-t BuPhC≡C)14(Dpppe)3 | Photochemical assisted | 24 h | 345/665 | [25] |
| Ni10(4-MePhS)20 Ni11(PhS)22 Pd9(PhS)18 Pd10(PhS)20 | phenyl disulfide radicals | Photochemical assisted | 8 h | 467/n.r. | [26] |
| Cu(acac)2 | monoethanolamine | Photochemical assisted | 180 min | 390/490 | [27] |
| NAC-CuNCs | N-acetyl-L-cysteine | Sonochemical assisted | 15 min | 340/630 | [29] |
| Ni/Ti3C2Tx | Ti3C2Tx MXene | Sonochemical assisted | 3–7 h | n.r. | [30] |
| PPh4[Au13(TBBT)4 (Dppe)4]Br2 | phosphine (Dppe) ligands | Catalytic assisted | 10 h | 600/n.r. | [31] |
| Sample | Capping Ligand | λex/λem (nm) | QY (%) | Imaging Model | Ref. |
|---|---|---|---|---|---|
| FA-BSA-AuNCs | bovine serum albumin | 530/670 | 6 | targerted in vitro imaging of NIH:OVCAR-3 cells | [40] |
| MUC1-AuNCs | thiolated MUC1 aptamer | 420/655 | 4.2 | targerted in vitro imaging of 4T1 cells | [41] |
| LAuNC | modified lycosin-I peptide | 347/782 | 9.1 | targerted in vitro imaging of 4T1 cells | [42] |
| GS-AuNPs | gluthatione | 350.730–800 | - | ex vivo imaging of kidney, brain, and small intestine tissue | [43] |
| BSA-AuNCs | bovine serum albumin | 810TPE/655 | 6 | ex vivo imaging of tissue-mimicking phantom | [44] |
| BSA-AuNCs | bovine serum albumin | 820TPE/670 | - | ex vivo imaging of tissue-mimicking phantom | [45] |
| GSH-AuNCs | gluthatione | 405/610–800 | 9.9 | ex vivo imaging of tissue-mimicking phantom | [21] |
| CD-Au NCs | thiolated cyclodextrin | 808/1050 | 0.11 | in vivo imaging of BALB/c nude mice bearing MCF-7 breast tumors | [46] |
| AS1411-AuNPs | Phosphorothioate-modified AS1411 DNA aptamer | 808–980/1030 | - | in vivo imaging of BALB/c mice bearing 4T1 breast cancer xenografts | [47] |
| TPPTS-AuNPs | triphenylphosphine-3,3′,3″-trisulfonic acid | 808/1026 | - | in vivo imaging of BALB/c mice with early-stage kidney injury | [48] |
| Au7Cd1-MHA/MPA | 6-mercaptohexanoic acid (MHA) and 3-mercaptopropionic acid (MPA) | 808/1000–1050 | - | in vivo imaging of C57BL/6 male mice’ vessels | [49] |
| Au25(SG)18 | gluthatione | 730–800/850–950 and 1150–1400 | - | in vivo imaging of healthy mice and mice with radiation-induced intestinal injury | [50] |
| Sample | λex/λem (nm) | Biological Model | Therapeutic Mechanism | Performance | Ref. |
|---|---|---|---|---|---|
| ICG4−GS-Au25 | 808/820 | female BALB/c mice bearing subcutaneous TUBO murine breast tumors | photothermal therapy triggered by LASER | complete tumor ablation | [51] |
| Min 23@AuNCs | 808/1050 | BALB/c mice with subcutaneous 4T1 breast tumors | Photodynamic therapy triggered by smartphone LED | ~90% tumor growth inhibition; effective with low-cost light activation | [52] |
| AuNC–PTEN | - | BALB/c nude mice with HepG2 tumor | targeted therapeutic gene delivery | Strong tumor targeting (peak after 6h); significant tumor growth inhibition; | [53] |
| Au44MBA26-P NCs | 808/1080–1280 | mice with cattle-derived type II collagen immunization-induced rheumatoid arthritis | Anti-inflammatory and immunomodulatory | superior rheumatoid arthritis outcomes compared to methotrexate and non-phosphorylated Au44 | [54] |
| Ag@PEG2000-HA NCs | -/600–800 | BALB/c mice with 4T1 tumors | reactive oxygen species-mediated mitochondrial apoptosis | Early tumor signal; robust tumor inhibition; survival extended to 47–73 days vs. 26–50 days (control) | [55] |
| Au–Gd NCs | 808/ >1000 | BALB/c mice with subcutaneous TUBO breast tumors | photothermal therapy triggered by LASER | 3× longer survival compared to controls; significant tumor volume reduction | [56] |
| AunNCs-DPA | - | - computational | Drug delivery feasibility | solvation lowers binding energies; predicted facile release from Au surfaces | [57] |
| Sample | Analyte | Detection Strategy | Linear Range | Limit of Detection | Real Samples Performance | Ref. |
|---|---|---|---|---|---|---|
| GSH-Au NCs | Cobalt ion (Co2⁺) | Fluorescence quenching | 2.0–50.0 µM | 0.124 µM | 102.8–108.3% | [60] |
| BSA-CuNCs | Ferric ion (Fe3⁺) | Fluorescence quenching | 0.2–2.4 µM | 10 nM | 93.8–104.0% | [61] |
| MMI-CuNCs | Silver ion (Ag+) | Fluorescence quenching | 0.025–50 µM | 6.7 nM | 97.0–104.0 | [62] |
| 11-MUA-AuNCs | Cadmium ion (Cd2+); Zinc ion (Zn2+); Copper ion (Cu2+) | Fluorescence enhancement for Cd2+ and Zn2+; Fluorescence quenching for Cu2+ | Cd2+: 0.01–2.5 µM Zn2+: 0.025–5.0 µM Cu2+: 0.05–10 µM | Cd2+: 0.012 µM Zn2+: 0.016 µM Cu2+: 0.026 µM | Cd2+: 87.74–100.24% Zn2+: 91.51–103.18% Cu2+: 98.71–101.16% | [63] |
| CASE-AuNCs | Copper ion (Cu2+) Mercury ion (Hg2+) | Fluorescence quenching | Cu2+: 0–7 µM Hg2+: 0–14 µM | Cu2+: 14.78 nM Hg2+: 35.21 nM | Cu2+: 96.4–99.4% Hg2+: 96.3–98.9% | [64] |
| BSA-AuNCs | Copper ion (Cu2+) | Fluorescence quenching | - | 5 µM | - | [65] |
| His-AuNCs | Ferrous ion (Fe2+) Ferric ion (Fe3+) | Fluorescence quenching | 9−97 µM | 3.2 µM | 102.0–105.4% | [19] |
| AuNCs | Carbendazim | Fluorescence resonance energy transfer turn-on | 1−100 µM; 150−1000 µM | 0.83 µM; 37.25 µM | 92.0–97.3% | [66] |
| DNA-AgNCs/Cu2+ | glyphosate | Fluorescence turn-on | 15–100 µg/L | 5 µg/L | 80.0–115.8% | [67] |
| DNA-AuNC | DNA methyltransferase | Fluorescence turn-off | 0.5–40 U mL−1 | 0.178 U mL−1 | 92.5–110.5% | [68] |
| Fe3O4NPs@SiO2@AuNCs | microRNA-21 and microRNA-141 | 21: Fluorescence quenching 141: Fluorescence enhancement | 21: 0.1 pM–10 nM 141: 0.1 pM–1 nM | 21: 0.02 pM 141: 0.017 pM | 21: 98.9–103% 141: 93.5–99.2% | [69] |
| SAN-CuNCs | Ascorbic acid | Fluorescence turn-on | 25–400 µM | 6.9 µM | 94.8–105.3% | [70] |
| PEI/DTH@NiNCs | glutathione | Fluorescence enhancement | 0–250 µM | 0.007 µM | 95.2–104.5% | [71] |
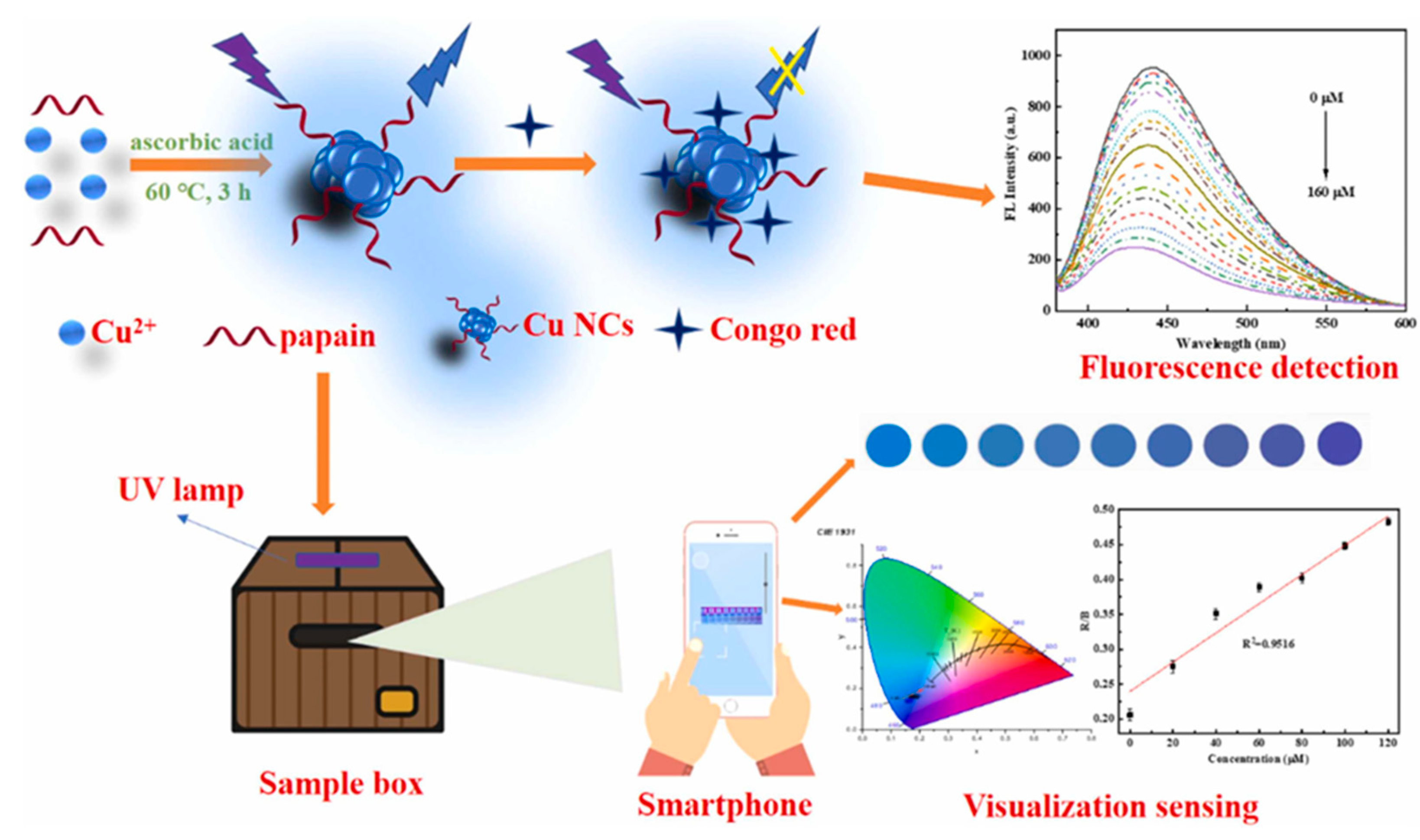
| Cu NCs@PP | Congo red | change in fluorescence color | 0.5–160 µM | 0.085 µM | 97.2–110.8% | [72] |
| GSH-AuNCs | Staphylococcus aureus and Escherichia coli biofilms | Fluorescence enhancement | 2.6 × 105–6.7 × 107 CFU/mL | 1.7 × 105 CFU/mL | - | [73] |
| aptamers@papain@AuNCs | Escherichia coli O157:H7 | Fluorescence enhancement | 101–106 CFU/mL | Pure culture: 39 CFU/mL | high sensitivity in ultra-high temperature (UHT), pasteurized, and raw milk (LODs ~500 CFU/mL) | [74] |
| 3WJ/DNA-Ag/PtNCs | Salmonella typhimurium | Solution color change | 2.6 × 102–2.6 × 106 CFU/mL | 2.6 × 102 CFU/mL | 96.5–107.7% | [75] |
| Met-AuNCs | human papillomavirus | Cas12a-based electrochemiluminescence | 1 pM–10 nM | 0.48 pM | 95.4–101.3% | [76] |
| CuNCs | hepatitis B virus DNA | Fluorescence quenching | 0.5–100 pM | 0.54 pM | 99.1–102.1% | [77] |
| ssDNA-AuNCs | trypsin | Fluorescence turn-off | 5 ng/mL–60 ng/mL | 1.5 ng/mL | 98.7%–103.5% | [78] |
| DNF@AuNCs | Aflatoxin B1 | Fluorescence quenching | 0.01–200 ng/mL | 7 pg/mL | 95.3–108.6% | [79] |
| Sample | Co-Catalyst | Catalytic Reaction | Resulting Product | Performance | Ref. |
|---|---|---|---|---|---|
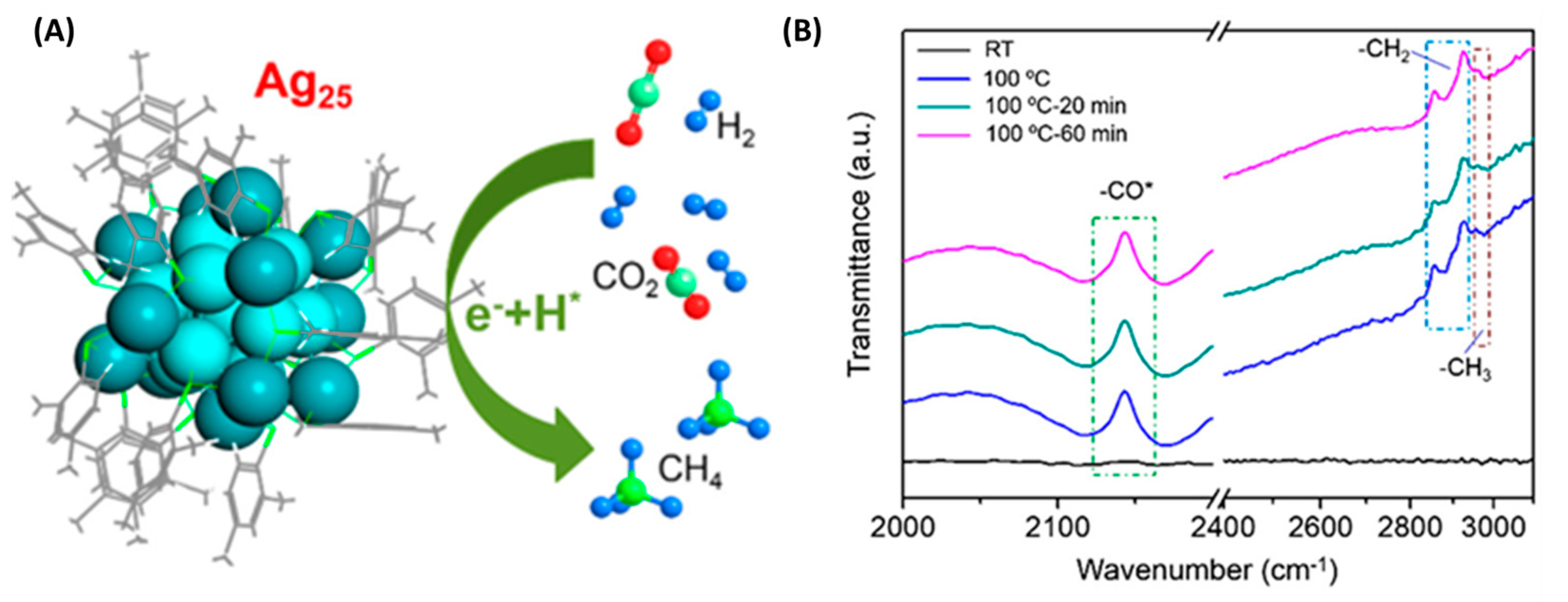
| Ag25(SPhMe2)18 NCs | - | CO2 reduction | CH4 | 28.95 µmol h−1 mg−1 CH4; 100% selectivity; 5.19% after 10h illumination | [87] |
| AuNCs | MOF | CO2 reduction | CO Trace of CH4 and H2 | 57.6 mmol g−1 h−1 CO over 5 h; maintains > 90% activity after 3 catalytic cycles; | [88] |
| Au25 NCs | BiOBr nanosheets | CO2 reduction | CO | Superior to previous BiOBr-based catalysts: 43.57 µmol CO g−1 h−1 (2.7× higher than unmodified BiOBr) | [89] |
| Cu6–NH NCs | - | CO2 reduction | CO | 148.8 µmol g−1 h−1 CO superior to non-protonated ligand (Cu6N)-25.8 µmol g−1 h−1 CO; 5-cycle reuse with no significant loss of activity | [90] |
| Cu NCs | Zr-MOFs | CO2 reduction | HCOOH and CO | Cu NCs@MOF-801: 94 µmol h−1 g−1 HCOOH (66% selectivity) and 32 µmol h−1 g−1 CO; Cu NCs@UiO-66-NH2:128 µmol h−1 g−1 HCOOH (86% selectivity) | [91] |
| Cu6-NH2 NCs | - | CO2 fixation | oxazolidinones | 1.54 g product at 97% yield | [82] |
| Ag44(SR)30 | TiO2 NPs | Water splitting | H2 | 7.4 mmol h−1 g−1 (10 times higher than pure TiO2 and 5× higher than TiO2/Ag NPs); maintained 83% activity after 5 cycles | [90] |
| Aux@GSH NCs | PDDA layer with a CdTe shell over CdS nanowires | Water splitting | H2 | 4.42 mmol g−1 h−1 (14 times higher than CdS alone); increasing activity over multiple cycles | [91] |
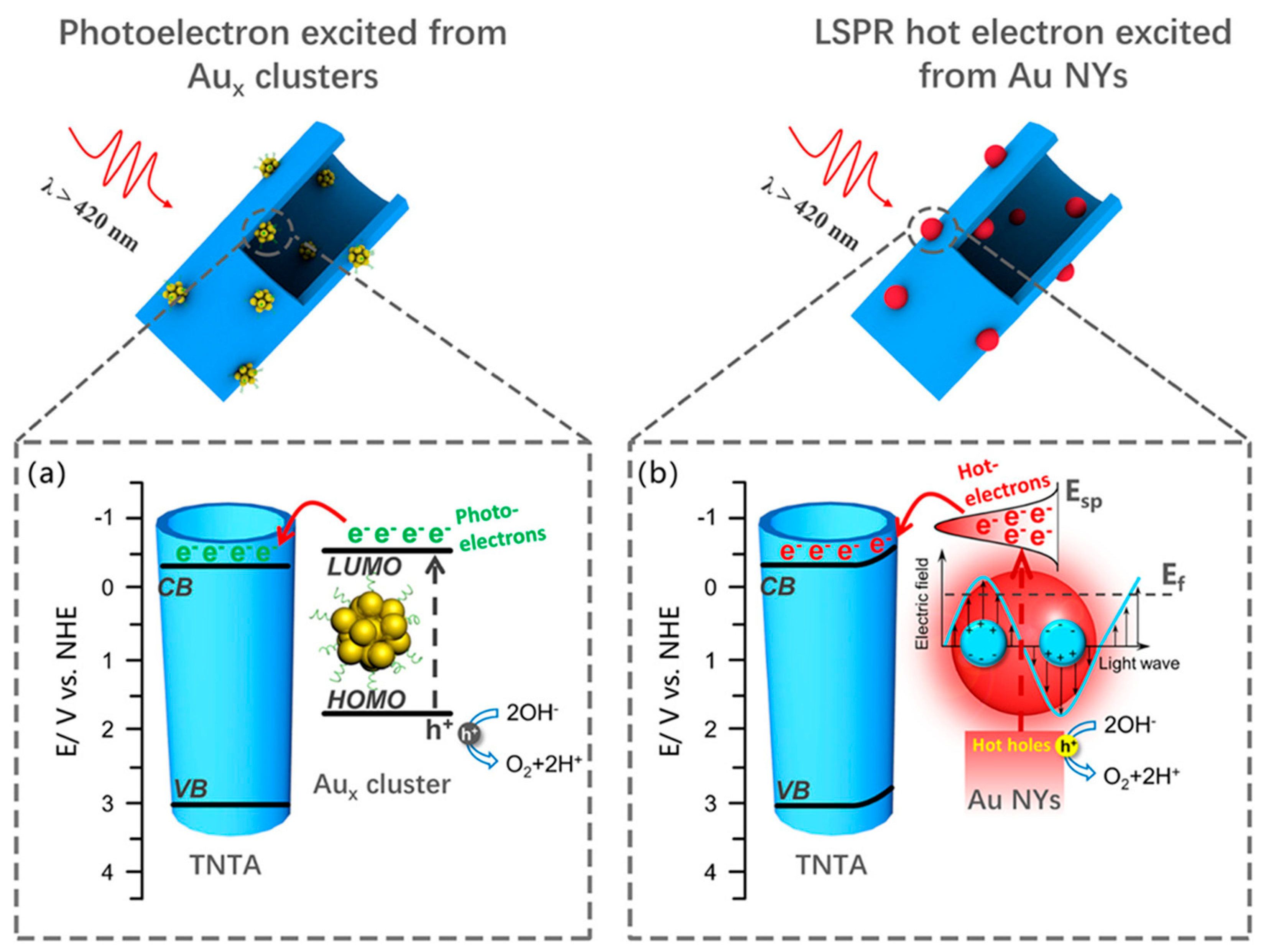
| Aux@GSH NCs | TiO2 nanotube | Water splitting | - | outperformed their plasmonic counterparts in terms of photocurrent generation, charge carrier density, and applied bias photon-to-current efficiency | [92] |
| Au25(PET,p-MBA)18 | BaLa4Ti4O15 or Cr(OH)3/BaLa4Ti4O15 semiconductors | Water splitting | H2 | highly active heterogeneous catalysts; long-term stability | [93] |
| PtNCs | MIL-125-NH-CH2OH | Water splitting | H2 | 4496.4 µmol·g−1·h−1 (31 times higher than MIL-125-NH2 alone) | [94] |
| Pt5(GS)10 NCs | CdS nanorods | Water splitting | H2 | 13.0 mmol g−1 h−1 (6 times higher than CdS nanorods); 25.08% efficiency; | [95] |
| Pt NCs | π-conjugated 2D covalent organic framework (PY-DHBD-COF) | Water splitting | H2 | 71,160 µmol·g−1·h−1; 8.4% efficiency; stable for 60 h | [96] |
| Au12Ag32(SePh)30 | TiO2 support | Water splitting | H2 | 6810 µmol·g−1·h−1; 0.96% efficiency; ~90% after 16 h operation | [97] |
| Au4Ru2NCs | TiO2 nanocrystals | N2 fixation | NH3 | 44.5 µmol·g−1·h−1 | [98] |
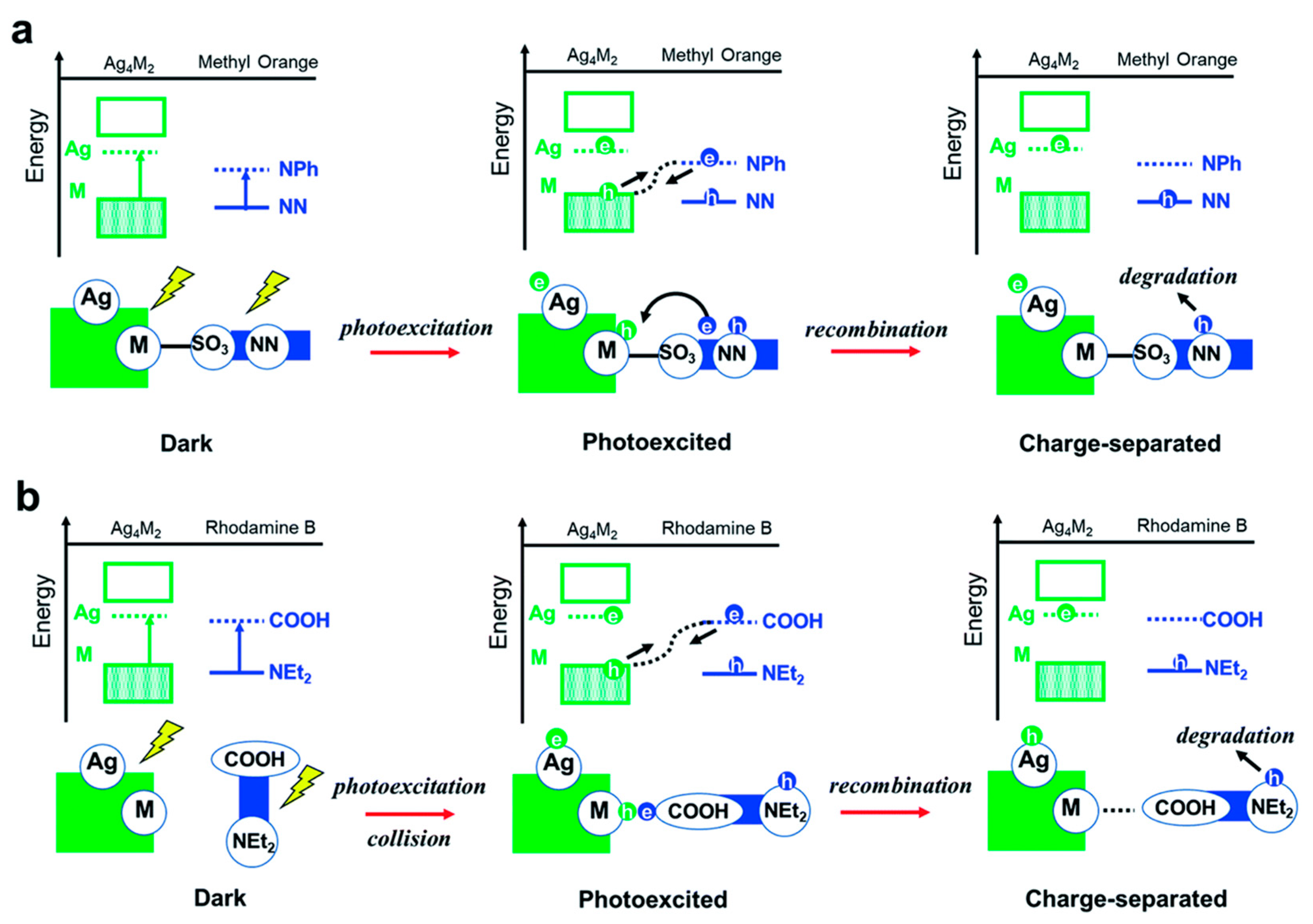
| Ag4M2(SPhMe2)8 NCs (M is Ni or Pd or Pt) | TiO2 support | Methyl orange (MO) and Rhodamine B degradation (RhB) | - | Ag4Pd2/TiO2 Complete degradation of MO in 18 minutes; Ag4Ni2/TiO2: Fastest degradation of RhB | [99] |
| Au-Co-TCPP | - | O2 reduction | H2O2 | 235.93 mM in 60 min (2 times higher than bare AuNCs | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hada, A.-M.; Lamy de la Chapelle, M.; Focsan, M.; Astilean, S. Recent Advances in Metal Nanoclusters: From Novel Synthesis to Emerging Applications. Molecules 2025, 30, 3848. https://doi.org/10.3390/molecules30193848
Hada A-M, Lamy de la Chapelle M, Focsan M, Astilean S. Recent Advances in Metal Nanoclusters: From Novel Synthesis to Emerging Applications. Molecules. 2025; 30(19):3848. https://doi.org/10.3390/molecules30193848
Chicago/Turabian StyleHada, Alexandru-Milentie, Marc Lamy de la Chapelle, Monica Focsan, and Simion Astilean. 2025. "Recent Advances in Metal Nanoclusters: From Novel Synthesis to Emerging Applications" Molecules 30, no. 19: 3848. https://doi.org/10.3390/molecules30193848
APA StyleHada, A.-M., Lamy de la Chapelle, M., Focsan, M., & Astilean, S. (2025). Recent Advances in Metal Nanoclusters: From Novel Synthesis to Emerging Applications. Molecules, 30(19), 3848. https://doi.org/10.3390/molecules30193848









