Abstract
Ru/TiO2 catalysts are well known for their high activity in the hydrogenation of CO2 to CH4 (the Sabatier reaction). This activity is commonly attributed to strong metal–support interactions (SMSIs), associated with reducible oxide layers partly covering the Ru-metal particles. Moreover, isothermal rates of formation of CH4 can be significantly enhanced by the exposure of Ru/TiO2 to light of UV/visible wavelengths, even at relatively low intensities. In this study, we confirm the significant enhancement in the rate of formation of methane in the conversion of CO2, e.g., at 200 °C from ~1.2 mol gRu−1·h−1 to ~1.8 mol gRu−1·h−1 by UV/Vis illumination of a hydrogen-treated Ru/TiOx catalyst. The activation energy does not change upon illumination—the rate enhancement coincides with a temperature increase of approximately 10 °C in steady state (flow) conditions. In-situ DRIFT experiments, performed in batch mode, demonstrate that the Ru–CO absorption frequency is shifted and the intensity reduced by combined UV/Vis illumination in the temperature range of 200–350 °C, which is more significant than can be explained by temperature enhancement alone. Moreover, exposing the catalyst to either UV (predominantly exciting TiO2) or visible illumination (exclusively exciting Ru) at small intensities leads to very similar effects on Ru–CO IR intensities, formed in situ by exposure to CO2. This further confirms that the temperature increase is likely not the only explanation for the enhancement in the reaction rates. Rather, as corroborated by photophysical studies reported in the literature, we propose that illumination induces changes in the electron density of Ru partly covered by a thin layer of TiOx, lowering the CO coverage, and thus enhancing the methane formation rate upon illumination.
1. Introduction
Combustion and the utilization of fossil fuels have introduced significant quantities of CO2 into the atmosphere [1,2,3,4], leading to increasing global temperatures and causing the acidification of oceans [5,6]. To prevent future emissions of CO2, alternative resources and routes to chemicals and fuels need to be developed. Since methane has a higher compatibility with existing energy infrastructure than H2, a possible strategy is to use electrochemically produced H2, and (air-captured) CO2 to sustainably produce CH4, following the Sabatier reaction [2,7]:
Methanization of CO2 is an exothermic process, and a low temperature favors the thermodynamic equilibrium to CH4. However, high temperatures (typically > 250 °C) are required to yield considerable methane production rates [7].
An innovative means for the sustainable conversion of CO2 to methane is the employment of photocatalysis [4,8,9,10,11,12]. Semiconductors can be excited by photons, thus creating electron/hole-pairs that could be spatially separated over the distinct components of the material [13]. Provided that they do not recombine, these electrons and holes can subsequently be used for reduction and oxidation reactions, respectively. Titanium dioxide is most often investigated in photocatalysis [14,15], even though UV excitation is required for activation. Doping can be used to introduce new electronic levels, allowing for visible light absorption [16,17,18], but dopants typically induce recombination centers, lowering the photonic efficiency.
A promising emerging technology is the combination of light and temperature in so-called photothermal catalysis, driven by light at mildly elevated temperatures [1,2,4,19,20,21,22], requiring combinations of semiconducting supports and light-sensitive nanoparticles. While plasmonic metal nanoparticles (Ag, Au) are particularly effective [22,23,24,25], interestingly catalytically active particles of Ru have also been reported to be sensitive to photon-activation [26,27,28,29,30,31,32]. For Ru/TiO2, UV illumination excites TiO2, while, simultaneously, visible photons can be utilized to induce two physical phenomena in the plasmonic nanoparticles: (i) the formation of excited states followed by charge transfer to the conduction band of (n-type) semiconductors, such as TiO2 [23], or TiOx overlayers; and (ii) localized heating by the decay of localized surface plasmon resonances (LSPRs) [24,25].
Interesting recent studies employing these principles include the investigation of several Group VIII elements on a light-inactive Al2O3 support for photothermal CO2 reduction to CH4 and CO by Meng et al. [26]. From the investigated metals, Rh and, in particular, Ru nanostructures were demonstrated to provide excellent activities for CO2 conversion, with selectivity values above 99% towards methane [26]. In the same year, O’Brien et al. demonstrated photothermal CO2 methanization over Ru-loaded silicon nanowires [27]. In both the studies of Meng et al. and O’Brien et al., the absorption window of the catalyst is remarkably broad, allowing for light harvesting in the visible region and even in the (near-) infrared. Multiple other reports have been published, demonstrating the suitability of Ru nanoparticles (sometimes proposed to be in an oxidized form) for photothermal methanization of CO2 [28,29,30,31,32,33,34]. Photo-methanization by Ru on TiO2 was already reported in 1987 (with a more than 60-fold increase in activity when the temperature was raised from room temperature to 90 °C) [35], but the number of studies aimed at understanding of the photo-physics [36], and the mechanistic implications of illumination, is limited [3,5].
In this manuscript, we show that Ru on anatase TiO2, prepared by NaBH4-mediated deposition, is very effective in the photothermal hydrogenation of CO2 by performing transient analysis (light-on, light-off cycles). Furthermore, we provide convincing evidence for changes in the electron density of Ru nanoparticles deposited on anatase TiO2 upon illumination, through a thorough analysis and assessment of the temperature- and illumination-dependent position of in-situ-generated CO on Ru/TiO2 in DRIFT spectra. We discuss the charge transfer effects upon illumination in relation to analyses of the photophysical properties of Ru/TiO2 catalysts.
2. Results
2.1. Characterization of the Photocatalyst
The actual metal loading of the as-prepared catalysts was determined by XRF, and confirmed, on average, to be the targeted loading of Ru of 1 wt-% on TiO2, STO, and SiO2, respectively. However, the particle size distribution is different for each support, as evident from the TEM images shown in Figure 1.
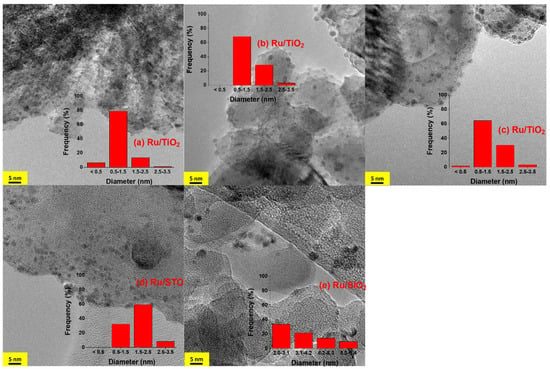
Figure 1.
TEM images of Ru/TiO2 after: (a) H2 pretreatment; (b) reaction at 250 °C with on-off light cycles (20 min each) for a duration of 24 h; (c) stepwise increase in temperature from 250 to 450 °C and cool down to 250 °C; (d) Ru/STO; and (e) Ru/SiO2 after H2 pretreatment.
Analysis of the particles shows uniform Ru nanoparticles are present on TiO2, of which ~80% consists of a size of around 1 nm diameter (Figure 1a), with some larger particles in the 2 nm range (~15%), and finally some smaller particles below 1 nm (~5%) after preparation. Treating the sample at 250 °C in H2/CO2 under illumination did not significantly change the particle size distribution. Nonetheless, ~70% of the Ru particles consist of a diameter of around 1 nm, although some particles in the 3 nm range are now visible (contributing to less than 5%). Prolonged exposure to 450 °C under illumination in H2/CO2 did not lead to significant changes either, with ~65% of the Ru particles consisting of a diameter of around 1 nm. Ru/TiO2 catalysts have been previously synthesized and analyzed in a multitude of studies aimed at catalytic hydrogenation of CO, CO2, or mixtures thereof. Several recent studies aimed at achieving a better understanding of the complex interactions between Ru nanoparticles (NPs) and oxide supports (typically TiO2), typically assigned to strong metal–support interactions (SMSIs) associated with structural modifications such as layers of TiOx on Ru particles [29,30]. In Figure S3, we show images of the Ru/TiOx sample after H2 treatment, which are similar to the particles reported by Zhang et al. [37] and Abdel-Mageed et al. [38]. The overlayer is indicated by the blue arrows inserted in the picture (Figure S3). Finally, Abdel-Mageed and coworkers have been able to determine the size distribution of Ru nanoparticles on P25 TiO2 at a high resolution [38], which unfortunately was not feasible using the acquired images in this study.
After H2 treatment at 450 °C, inhomogeneous and bigger Ru particles are observed on STO and SiO2, respectively. On STO, only approximately 35% of the particles are present in the size range of 1 nm, while 50% of the particles contain diameters of ~2 nm (Figure 1d). On SiO2, the Ru particle size distribution is even less homogeneous, with sizes extending from 2 to 6 nm (Figure 1e). In both cases, evidence for overlayers of oxides is absent, which is likely associated with limitations in reducibility (for SrTiO3), or the absence of reducibility of the oxides (for SiO2).
X-ray photoelectron spectroscopy studies were performed to study the surface oxidation state of the Ru/TiO2, Ru/SrTiO3 (STO), and Ru/SiO2 catalysts after preparation, including H2 treatment at 450 °C; the spectra are shown in Figure 2a. For all three catalysts, the intensity profile can be deconvoluted by three peaks. The peak at ~284.8 eV (green trace) is attributed to adventitious carbon species on the surface. The peaks at 283.8 eV and 279.6 eV are assigned to metallic Ru (Ru 3d3/2, yellow trace, and Ru 3d5/2, blue trace, respectively), demonstrating that the preparation procedure—using NaBH4 and 50 °C for 2 h—results in the complete reduction of the Ru precursor salt. The spectra are comparable to spectra reported by Abdel-Mageed et al. [38] and Cisneros et al. [39], but seem to contain significantly less carbon contaminant, suggesting that the washing and reductive treatment is very affective in the removal of carbon-containing residues. Figure 2b shows the UV-Vis absorbance spectra of the catalysts. The pure TiO2 (anatase phase) nanoparticles only exhibit strong absorption below 400 nm, corresponding to a bandgap of 3.0 eV (slightly lower than the expected 3.2 eV bandgap) [15], while the Ru-containing catalysts additionally show a broad absorption band from 400 to 1000 nm, with a shallow optimum at around 500 nm, which is particularly visible for the Ru/TiO2 sample. Similar behavior can be observed for SrTiO3 (bandgap of 3.1 eV in this study, in line with the expected 3.2 eV) [15]. The nature of the strong visible light absorption of Ru is still under debate. In some studies, the intensive broad absorption from 400 to beyond 800 nm is assigned to RuO2 absorptions [29,30]. Other research groups suggest that light attenuation by small, well-dispersed Ru nanoparticles is responsible for the strong light absorption [27,28]. Given that the XPS spectra conclusively show the presence of Ru in the metallic state, and the observed size of the Ru0 particles in Figure 1 is in the order of 1 nm, we favor the assignment of strong light absorption at visible wavelengths to well-dispersed Ru0 nanoparticles. The width of the absorption band centered around 490 nm likely results from structural inhomogeneity, as the Ru nanoparticles show a distribution in sizes (see Figure 1).
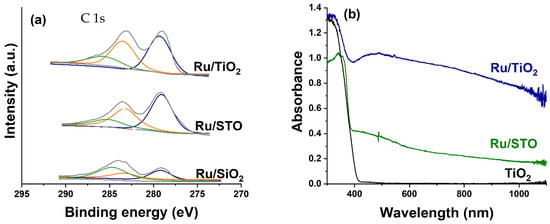
Figure 2.
(a) XPS analysis of as-prepared Ru particles supported on TiO2, STO, and SiO2. The C 1s, Ru 3d 3/2, and Ru 3d 5/2 deconvolution corresponds to the green, yellow, and blue traces, respectively. (b) UV-vis absorbance spectra of the Ru/TiO2, Ru/STO, and TiO2 catalysts after H2 pretreatment at 450 °C.
2.2. Photothermal Activity—Transient MS Analysis
We will now discuss the performance of the three supported and reductively treated Ru catalysts in a relatively low temperature range, where CH4 formation is not significantly thermodynamically limited.
Figure 3 and Figure 4 show the CH4 formation rates for a stepwise increase between 120 °C and 220 °C (see top of the curve). The bare TiO2 support is inactive for the production of methane in the investigated temperature range, both in the dark and under illumination. Ru nanoparticles are clearly necessary to induce the formation of CH4 by conversion of CO2 and H2. The activity of Ru/TiO2 is significantly larger than that of Ru/STO and Ru/SiO2, which is in agreement with the literature investigating catalytic performance of (TiO2)-supported Ru catalysts, typically assigned to strong or electronic metal–support interactions. For example, at 220 °C, the Ru-normalized CH4 formation rate of Ru/TiO2 amounts to 1.8 mol gRu−1 h−1 under UV-vis irradiation, which is 4.5 times higher than that obtained for Ru/SrTiO3 (STO, 0.4 mol gRu−1 h−1). In agreement with the obtained (low) rate for Ru/STO, Mateo et al. report for STO-supported—oxidized—RuO2 nanoparticles (which likely are reduced in situ in the process conditions) a steady state production of 50 mmol gRu−1 h−1 at 150 °C under UV-vis irradiation (1 sun, 300 W Xe lamp) [29]. As shown in Table 1, Wang et. al. report an activity for Ru/TiO2 in the formation of CH4 of 172 mmol gRu−1 h−1 at 150 °C under 1 sun irradiation (AM 1.5). They also report stability of the system for at least 1000 min at 200 °C in the photothermal reduction of CO2 [5]. Finally, Novoa-Cid et al. demonstrate that small Ru nanoparticles (1.5 nm) supported on Titanate nanotubes produce CH4 at a rate of 110.7 mmol gRu−1 h−1 at 210 °C in a pressured batch reactor under UV-vis-NIR illumination (1 sun) [30]. The strongest light enhancement is observed around 200 °C in Figure 3, where the rate rapidly increases from ~1.2 mol gRu−1 h−1 to ~1.8 mol gRu−1 h−1 upon illumination.
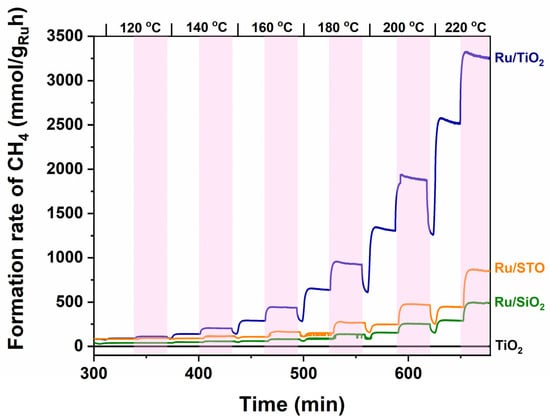
Figure 3.
The Ru-normalized CH4 formation rates (left axis) from 120 °C to 220 °C (temperature indicated above graph) as determined for Ru/TiO2, Ru/STO, and Ru/SiO2 catalysts, respectively. Exposure to UV-vis irradiation is indicated by the pink rectangular areas—see the legend, as indicated in the Figure, for the respective catalysts. The TiO2 support does not show any activity—even when illuminated. Ilumination was provided according to the spectrum shown in Figure S1, with an intensity of 360 mW/cm2.
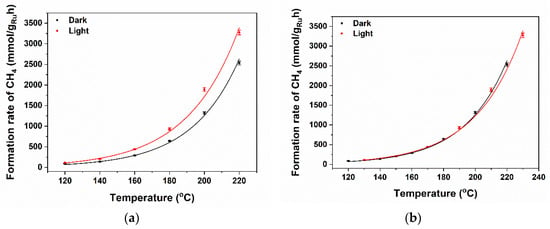
Figure 4.
(a) The Ru-normalized CH4 formation rates (left axis) plotted as a function of temperature with light off (black) and light on (red curve). (b) The curves can be overlapped if the light-on curve is shifted to higher temperature values by ~10 °C. By comparing the Arrhenius plots (See Figure S2), the activation energies can be estimated to be quite similar in dark conditions and upon illumination—suggesting that light enhances the number of available sites. Based on the DRIFT analysis to follow, this is proposed to be due to changes in CO coverage.

Table 1.
Comparison of performance data in this study and as reported in the literature for light activated Ru catalysts.
In the following section, we will address the performance of Ru/TiO2 at higher temperatures, in the temperature range of 200 °C to 450 °C, where thermodynamic limitations in the formation of CH4 begin to play a role. Figure 5 shows the transients in CO2 (blue), CH4 (red), CO (orange), and H2 (black) of the ion currents recorded by the Mass Spectrometer, as a function of temperature and a transient in light-on, light off cycles. Upon light-on, up to 300 °C, a positive response of the CH4 signal to light is observed. The response of the formation of CH4 to light is most dominant at 250 °C, as further illustrated in Figure S5—comparing the response of the formation of CH4 to light at 200, 250, and 300 °C, respectively. It should be noted that, at 300 °C, conversion of CO2 and H2 to CH4 becomes significantly affected by thermodynamic equilibrium [30].
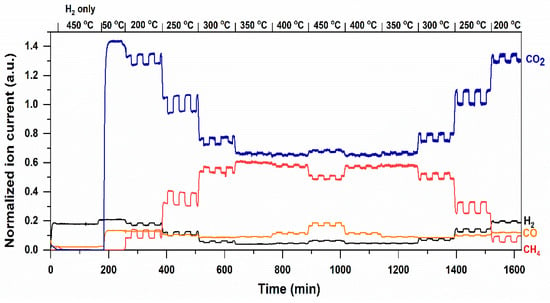
Figure 5.
Mass spectrometry results (normalized ion current—y-axis) of photothermal hydrogenation of CO2 on Ru/TiO2 in the dark and under UV-vis irradiation, by stepwise increasing temperature from 50 to 450 °C, and decreasing from 450 to 200 °C, respectively. Temperature steps are indicated above the figure.
Thus, at higher temperatures than 250 °C, the light effect on CH4 production diminishes, interestingly to become negative at 450 °C. This is obviously because the CO2 conversion to CH4 is now significantly limited by thermodynamic equilibrium [38]. At this point, exposure to light shows a promotion in the formation of CO—an intermediate in the formation of CH4—with a concomitant decrease in the production of CH4, again in agreement with a (local) temperature increase. At each temperature, the light-promoted formation of CH4 or CO can be well reproduced. Lowering the temperature shows the high stability of the catalyst, since the light-induced transients and the temperature-determined quantities in CH4 are comparable to the amounts obtained in the increasing temperature curve. In other words, a significant hysteresis in performance of the Ru/TiO2 catalyst at all temperatures and reaction conditions is absent under the process conditions investigated here. In Figure S6, enhancement in the production of CH4 is compared to the Ru/TiO2 catalyst exposed to UV/Vis or Vis light (using a cut-off filter for wavelengths < 420 nm), respectively (See Figure S1 for light emission spectrum). UV wavelengths apparently do contribute significantly to the promotion of the conversion in the applied flow reactor. This implies that light absorption by the Ru particles is predominantly responsible for the promotion in the rate of formation of CH4.
The high photothermal stability of Ru/TiO2 was further investigated at 250 °C under chopped UV-vis irradiation for a total period of 24 h (Figure S7). The CO2 conversion amounts to 30% at 250 °C in the dark, which increases to 40% under illumination. A corresponding (higher) increase in H2 conversion can be observed. Figure S7 also demonstrates the excellent stability of (H2-treated) Ru/TiO2 in time at 250 °C for at least 24 h. Notably, in the work of Garcia and coworkers, the performance of Ru(O2)/STO decreases significantly under UV-vis illumination in a period of 3 h [29]. Hence, the H2-pretreated Ru/TiO2 catalyst in our study not only shows a high mass-based activity, but also a high stability in performance, probably related to the strong metal–support interaction achieved by the H2 treatment. We assign the increase in conversion of CO2 (and H2) predominantly to an increase in the catalyst temperature of 10 °C upon illumination. To corroborate this increase in temperature, we also provide some back-on-the-envelope calculations on page 9 of the SI, confirming that such an increase is entirely possible considering the light intensity (360 mW/cm−2) exposed to the sample. We hypothesize that the increase in CO2 conversion and CH4 productivity is due to a reduction in Ru–CO surface coverage. To analyze the effect of illumination on the Ru–CO coverage, we will now discuss the results of in-situ DRIFT measurements, as well as extensively examining the assignment of the observed IR absorption bands.
2.3. DRIFT Analysis
The results of the DRIFT (static gas—no flow) measurements are shown in Figure 6 (for Ru/TiO2), using the same light source as used for the flow experiments illustrated in Figure 3. It should be noted that the illumination measurements need to be considered in situ (and not operando), since the spectra were recorded in the absence of flow (the gas composition equilibrates to a certain composition—please consider that H2 is the limiting reactant (a ratio of CO2:H2 of 1:3 was applied—see reaction (1)). After H2 pretreatment, CO2 gas was introduced into the cell at 50 °C, and infrared bands at 1628, 1566, and 1363 cm−1 become apparent—of which the intensity stabilizes after 30 min. These bands are very similar to the bands obtained in the experiment with the bare TiO2 support (see Figure S8) and have been assigned to carbonate and carboxylate species of different conformation (monodentate, bidentate) [40,41], adsorbed on titania. The small band at ~1420 cm−1 can be assigned to carbonate. In addition, for Ru/TiO2 (Figure 6), bands of low intensity appear at 2072 and 2018 cm−1, which can be attributed to surface-adsorbed CO on partly-oxidized Ru and metallic Ru, respectively, likely coordinating via the carbon atom [41]. The observation of CO adsorption suggests that the Ru particles are incompletely covered by TiOx, and significant interaction of Ru0 with the gas phase is possible. This adsorption also seems to indicate that dissociation of CO2 into CO takes place at 50 °C, likely accompanied by partial oxidation of the Ru particles, explaining the presence of the 2072 cm−1 band [40]. After introduction of H2/CO2, and stabilizing for 30 min, the peak at 2072 cm−1 almost completely disappears (in agreement with oxide reduction) and the intensity of the band at 2018 cm−1 significantly broadens and increases. Obviously, hydrogen promotes the conversion of CO2 to (surface-adsorbed) CO, without significantly changing the intensity of the carboxylate peaks—only the peak at ~1420 cm−1 seems to decrease by the introduction of hydrogen. The effect of UV-Vis irradiation on the reaction is quite limited at 50 °C, showing few spectral changes.
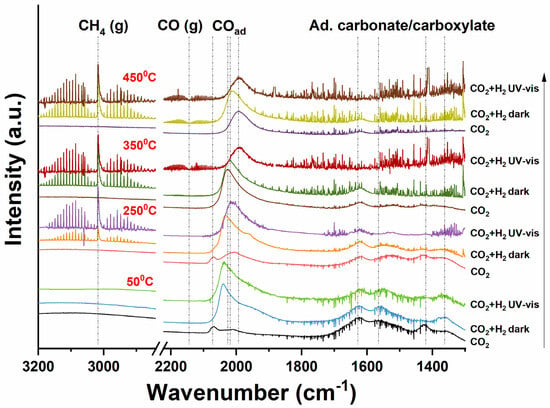
Figure 6.
In-situ DRIFT spectra of photothermal CO2 reduction on Ru/TiO2 in the dark and under UV-vis irradiation. At each temperature (as indicated in the figure), three conditions are compared: exposure to CO2; exposure to H2/CO2; and exposure to H2/CO2 and UV-vis light.
At 250 °C, similar peaks to those observed at 50 °C can be observed after CO2 treatment, with a slightly higher intensity of the Ru0-CO band at 2018 cm−1. Introducing H2 into the CO2 gas flow again causes the 2018 cm−1 band to significantly broaden and increase in intensity. The rotational spectral signature of a band at 3017 cm−1 is attributed to CH4 gas [42]. UV-vis irradiation greatly enhances the intensity of the CH4 signature. Clearly, UV-vis irradiation promotes the conversion of CO2 (and the intermediate CO) into CH4 by reaction with H2, which agrees well with the activity tests. Most importantly, the Ru–CO band seems to decrease in intensity upon illumination, and significantly shifts to lower wavenumbers as a result of decreasing coverage with CO.
At 350 °C, a much higher quantity of CO on Ru appears at 2026 cm−1 (Ru0-CO) already in a CO2 environment in the absence of H2, in comparison to the low intensity CO signature at 50 and 250 °C. This suggests that 350 °C is sufficient for significant CO2 dissociation. Similar to 250 °C, introducing H2 into CO2 results in CH4 gas formation, but does not dramatically change the peak profile of adsorbed Ru0-CO. Upon irradiation with UV-Vis light, the rotational CH4 signature now decreases slightly in intensity and gas-phase CO can be observed (in the range of ~2100 to 2200 cm−1) [43]. Again, a significant decrease in the Ru–CO intensity can be observed upon illumination, accompanied by a shift to a lower wavenumber. At 450 °C, the observations are similar to those at 350 °C, with illumination lowering and shifting the CO band intensity. The amount of gas-phase product is not significantly affected, in agreement with the transient MS analysis.
In addition to illumination with UV/Vis radiation, we also exposed the catalyst in the IR Cell either to exclusively UV (370 nm) irradiation or to visible irradiation (550 nm)—see Figures S9 and S10. The development of the spectra of the catalyst when exposed to CO2 in the dark is very similar to that observed in Figure 6, with the CO band on both oxidized and reduced Ru clusters obvious at 50 °C, as well as the formation of (bi)carbonate. At an elevated temperature (250, 350, and 450 °C, respectively), the broad band of CO adsorbed on Ru0 clusters develops. The introduction of H2 at these temperatures again results in the formation of methane, accompanied by a decrease in intensity of the band of adsorbed CO. Surprisingly, in the presence of H2, illumination with 370 (Figure S9) or 550 nm (Figure S10) does not now significantly affect the position and intensity of the IR band assigned to CO adsorbed on Ru0 nanoclusters, nor the intensity of the methane signature (band at 3017 cm−1). Interestingly, differences in the absence or presence of illumination can be observed in the absence of H2, where the effect of UV radiation and visible light excitation (550 nm) is most pronounced at 450 °C (Figures S9 and S10).
3. Discussion
3.1. Metal–Support Interactions
As stated in the introduction, the high activity of Ru/TiO2 in methanation of CO2 is explained by the so-called SMSI effect, which has been extensively discussed in the literature [38,39,40]. Close inspection of the comparison between different supports shown in Figure 3 reveals that titania, known for this effect, results in dramatically higher performance than the other supports—and TEM characterization confirms that the preparation procedure, followed by treatment in H2 atmosphere, leads to the formation of an amorphous overlayer, only for the Ru/TiO2 catalyst—which is predominantly responsible for the higher activity (see SI Figure S3). The temperature of 450 °C in H2 atmosphere used here is high enough to induce titania migration onto the ruthenium particles, as also shown by Xu et al. [44]. We acknowledge that the particle size determination in TEM, combined with the assumption of spherical (or hemi-spherical) particles, yields a three-times higher dispersion on titania compared to silica, while the dispersion on STO is 1.7 times higher than on silica, but we assume this has only minor consequences for the observed rate [38]. The performance variation between Ru/STO and Ru/SiO2 could stem from support oxygen vacancies in STO, which would require further XPS valence state analysis and oxygen defect analysis of the STO (SrTiO3) used [29]. Ru/SiO2 is known to show little (photo)catalytic activity, due to the lack of SMSI effects [28,29,30,31,32,33,34,35,36,37,38,39,40], and the absence of charge transfer phenomena between Ru particles and the SiO2 support upon illumination of Ru [36].
In our IR studies, we observed a significant quantity of CO adsorbed on ruthenium metal, showing that TiOx likely does not fully cover the surface. We therefore envision that Ru0 clusters are in strong interaction with (reduced) TiOx sites, (with some of) the reaction steps likely occurring at sites located near the overlayer or present at the perimeter between TiOx and Ru.
3.2. Surface Coverage—Assessed by Literature Evaluation
For the description of the reaction rate and reaction selectivity, we need to consider the surface-mediated reactions given below, where * denotes a vacant site on the Ru surface and (H) indicates the reactions in the presence of H2:
CO2 (g) + * ↔ CO2 ad
CO2 (+H) + * ↔ COad + O(H)
COad (+x H) + * ↔ C(H)ad + O(H)ad
C(H)ad + 3 Had → CH4 (g) + 4 *
O(H)ad + Had ↔ H2O (g) + 2 *
COad ↔ CO (g) + *
Reactions (2) and (3) describe the adsorption and dissociation of CO2 on the catalyst surface. CO2 adsorption studies on silica-supported ruthenium by Zağli and Falconer [45] show that direct CO2 dissociation already occurs at room temperature. In line with this, we do indeed find some adsorbed CO after exposure to pure CO2 at 50 °C for Ru/TiO2. The amount of CO at 50 °C increases substantially when hydrogen is added, and this may point to a hydrogen-assisted route in which CO2 dissociation proceeds. We note that we do not observe formate species in IR, which would appear around 1590 cm−1 and which are particularly prominently seen at 400 °C on ~1.8 nm Ni particles on silica [46]. Irrespective of the contribution from hydrogen-assisted routes, we conclude that CO2 dissociation is a fast reaction and does not limit the overall reaction rate. This is in line with isotope exchange experiments reported by Mansour and Iglesia [47], who show that the CO2 dissociation step is equilibrated on Ru/SiO2 at 300 °C and is thus not rate-limiting for the reaction. Our IR measurements at 350 °C under pure CO2 show the dynamic equilibrium in action: the spectrum shows a clear band due to adsorbed CO, which we attribute to CO2 dissociation (likely concurrently oxidizing oxygen vacancies) at the Ru_TiOx interface.
Likewise, the dissociation of CO, step (4), is not a very difficult reaction. Surface science studies show that step sites [48], as well as other types of undercoordinated sites [49], are active for direct CO dissociation, a reaction that occurs below 127 °C on Ru. More recent work on mass-selected ruthenium nanoparticles [50] as well as an STM study on stepped ruthenium [51] identified undercoordinated sites as the locus where direct CO dissociation can occur. These studies also show that the reverse reaction, carbon + oxygen to form CO, occurs around 277 °C, which implies that Reaction (4) becomes reversible above this temperature. In the previously mentioned study by Zağli and Falconer [45], silica-supported ruthenium was exposed to either CO2 or CO at room temperature and subsequently heated in the presence of hydrogen (temperature-programmed hydrogenation, TPH). The onset of methane was found around 127 °C, irrespective of the precursor used, which shows that CO dissociation must have occurred below this temperature already. These authors also deposited carbon and oxygen on the surface by adsorbing either CO or CO2 at room temperature and heating to high temperature in an inert atmosphere to induce dissociation. In the TPH performed afterwards, the formation of methane was already found at room temperature, a finding that corroborates the earlier report of methane formation at room temperature by Low and Bell [52] on alumina-supported ruthenium. This shows, firstly, that Reaction (5), hydrogenation of surface carbon, is a very easy reaction that can occur at room temperature already. It also means that the onset temperature of 127 °C for methane formation during the TPH of the CO-covered sample is either determined by the onset temperature of CO dissociation or instead caused by CO poisoning of dissociative hydrogen adsorption.
The same study [52] also informs us about Reaction (6), the removal of oxygen from the catalyst surface. The TPH experiments consistently show that the water formation peak is shifted to 10–15 degrees relative to the methane formation peak, and it also extends to a much higher temperature than the methane peak. Water formation thus requires a higher temperature to proceed than any of the other reactions, which suggests that oxygen removal has the lowest rate constant of all the reactions listed above. This is consistent with the positive order in hydrogen pressure for CO2 hydrogenation on ruthenium, as reported by Mansour and Iglesia [47]. In this view, the promoting role of titania is to provide an alternative, slightly easier (5 kJ mol−1) route to remove oxygen from the surface.
The adsorbed hydrogen atoms required in Reactions (5) and (6) stem from dissociative adsorption of the H2 reactant. The maximum hydrogen coverage is determined by the number of available sites left open for hydrogen to adsorb, multiplied by the fractional occupancy of those sites as determined by the hydrogen adsorption–desorption equilibrium. Considering the hydrogen adsorption energy on ruthenium of around 100 kJ mol−1 [53], and the relatively low H2 pressure of 300 mbar used here, the hydrogen coverage at the highest temperatures studied, 450 °C, is expected to be significantly lower than at lower temperatures. We therefore attribute the observed drop in conversion above 400 °C and concomitant selectivity change to CO, the less-hydrogenated product, to a decreased hydrogen coverage.
Dissociative hydrogen adsorption (as well as a number of other reactions from the list) requires vacant surface sites that are rather scarce at low temperature since the adsorption of carbon monoxide, the primary product of CO2 dissociation, is rather strong. The desorption temperature of around 227 °C on Ru(0001) for CO coverages below 0.33 ML translates to an adsorption energy of 150–160 kJ mol−1. CO desorption from ruthenium nanoparticles as well as from more open ruthenium surfaces [49] occurs at a significantly lower temperature. CO desorption from size-selected ruthenium nanoparticles [54] as small as 2.5 nm was found around 127 °C, a temperature that translates to an adsorption energy of 125 kJ mol−1 using a Redhead equation with ν = 1 × 1015 s−1. We will hereafter use this value as characteristic of the CO desorption barrier from ruthenium nanoparticles.
3.3. Explaining Temperature-Dependent (Surface) Chemistry
The IR spectrum at 50 °C in CO2/H2 shows a significant amount of adsorbed CO, which is a clear indication that CO2 dissociation is possible at this temperature. However, the adsorption energy of 125 kJ mol−1 translates to a CO residence time of (1/kdes) of 1.5 × 105 s at 50 °C, which means that CO is essentially irreversibly adsorbed and poisons the surface for adsorption of reactants—so that no steady state reaction is possible. Figure 3 shows that the onset of the methanation reaction is around 140 °C, which is essentially the onset temperature where CO starts to desorb from ruthenium nanoparticles.
The CO residence time at this temperature has dropped to 6 s, which is fast enough so that CO adsorption, Reaction (7), has now become reversible. The CO coverage is still very high and blocks the majority of the surface sites, but as there is now a dynamic adsorption–desorption equilibrium, (short-lived) vacancies now randomly appear on the surface so that adsorption and dissociation of hydrogen and CO2 is possible, and the steady state reaction can occur.
The large gain in activity seen upon increasing the temperature can be understood as a lowering of the CO equilibrium coverage. This is directly visible in the IR spectra, showing that the intensity of the CO absorption band decreases as the temperature increases. The frequency also shifts to lower wavenumbers with increasing temperature, which can be attributed to a coverage effect. Adsorption studies on silica-supported ruthenium nanoparticles show that the frequency at which adsorbed CO appears depends on the CO coverage, where a higher frequency means a higher CO coverage [55]. Using the combination of intensity and peak position as a probe for the CO surface concentration, we find that the decrease in the CO equilibrium concentration from 250 °C to 350 °C is much larger than a decrease from 350–450 °C. This matches the trend in activity seen in Figure 4, where the step change from 250 °C to 350 °C causes a significant gain in activity while the step from 350 °C to 450 °C actually causes a loss of (methane formation) activity. This shows that CO poisoning is dominant at a lower temperature while it ceases to play a role above 350 °C (under the low-pressure conditions used here). As mentioned before, the surface hydrogen concentration instead appears to become the limiting factor at high temperatures. The conclusion that CO poisoning is rate-limiting for CO2 hydrogenation below 350 °C is consistent with the previously mentioned kinetic studies performed at 300 °C on silica-supported ruthenium [47], from which it was also concluded that the rate at this temperature is inhibited by the CO reaction product.
3.4. Effect of Photoexcitation on Performance
The experiments show that irradiation has the most prominent positive effect on the Ru particles deposited on TiO2. We first of all note that the Arrhenius plot of CH4 formation in the dark and under illumination, as derived from the low temperature range reported in Figure 3, yields a similar apparent activation energy, while the pre-exponential factor (Figure S4 in Spporting Information) is different, indicating that irradiation does not change the reaction mechanism [56]. The absolute gain in CO2 conversion is by far the largest at 250 °C, in the temperature window where CO poisoning is the dominant rate-limiting factor. The IR measurements show that irradiation in this temperature window causes a substantial lowering of the CO coverage, and this rationalizes the strong light-induced promotion at this temperature.
The light-induced changes are in fact quite similar to a temperature increase, which raises the question of whether the light selectively deposits heat in the catalyst particles so that their local temperature is about 10 °C higher than the average temperature of the catalyst bed. We can test this hypothesis by using the position and area of the CO absorption band in the IR spectrum as an in-situ probe of the CO equilibrium coverage. The peak position and peak area at 250 °C under illumination approximately corresponds to the position and area found at 450 °C without illumination. In case of a purely thermal effect of light, to explain these spectral data, it would mean that the average temperature of the particles should be 450 °C and one would thus expect to obtain the CH4/CO selectivity characteristic of 450 °C under illumination at 250 °C. This is not the case, since at 450 °C significant CO production is observed (see the rotational signature of gas-phase CO), whereas this is completely absent at 250 °C under illumination (see the spectra and product distribution).
Non-thermal weakening of the Ru—CO bond is also achieved at relatively high temperatures in conditions without H2, when exclusively UV or green light illumination is applied, while the effect is similar at both wavelengths: see Figures S9 and S10. The physical phenomena at play in non-thermal weaking of the Ru–CO could be as follows.
3.4.1. Further Consideration of the Effect of Illumination on CH4 Productivity and Ru–CO Surface Coverage
Further considerations for excluding the possibility of the thermal heating effects to account for our spectral data, implying a temperature rise of ~200 degrees, include a back-of-the-envelope calculation of the temperature increase based on complete absorption of all the light energy by the catalyst bed (see Supplementary Information). We only calculate a maximum rate of temperature increase of ~2.9 K s−1, which agrees with the activity data obtained in our flow reactor but does not agree with the 200 degree rise required to explain the differences in CO-infrared absorption intensities and peak positions. Finally, a recent study helpfully illustrates the heating effects of illumination of a catalyst bed [57]. Analyzing the reported temperature increase as a function of intensity of illumination, the increase at the intensity of light used in our study can indeed be expected to be limited to ~10 °C, implying that additional non-thermal weakening of the Ru–CO bond is at play in our infrared experiments.
3.4.2. Non-Thermal Effects
The question remains as to why light leads to such lower CO coverage at intermediate temperatures, if this cannot be entirely explained by heat effects. One explanation can be that the light-induced electric field associated with the Ru particles may polarize adsorbed surface species [58] and reduce CO poisoning [59]. Electron transfer phenomena have also been reported for Ru clusters on semiconductor entities, which are indeed temperature-dependent, in line with the temperature dependence of the lowering of the CO surface coverage upon illumination [60]. In line with this hypothesis, the following situations can be proposed. Although we cannot exclude absorbate–metal electronic transitions as described in the literature, for example for Pt–CO and Ru–CO [61,62], Figure 7a,b illustrates the photoinduced hole (or electron) transfer from a Ru nanocluster towards the TiOx, depending on the position of the HOMO and LUMO energy levels of the Ru nanoclusters relative to the valence band and conduction band of the TiOx [36]. The photoexcited electron in the Ru could promote CO desorption (Figure 7b) [63]. A derived third scenario, which is possible due to the hydrogen treatment preceding the photothermal catalysis partly reducing the TiOx, is shown in Figure 7c. Photoexcitation of the Ru nanocluster is followed by a hole transfer towards the reduced TiOx, while the excited electron promotes CO desorption. Finally, Figure 7d illustrates a fourth possibility, with excitation of the electronic transition between hybridized absorbate–metal bonding and antibonding states destabilizing the metal–CO bond [62]. Considering the major difference in activity we observe between Ru/SiO2 and Ru/TiO2 and the weak activity of the first (Figure 3), we believe the latter is unlikely to play a significant role here.
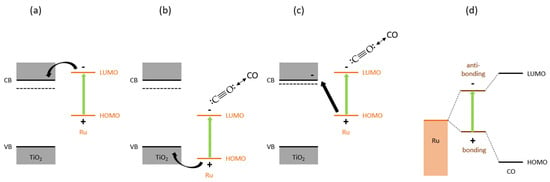
Figure 7.
Charge transfer processes upon light activation of the Ru nanoparticles on TiOx. (a) and (b) illustrate photoinduced electron or hole transfer from the Ru towards the TiOx, depending on the position of the HOMO and LUMO energy levels of the Ru nanoclusters relative to the valence band (VB) and conduction band (CB) of the TiOx. (c) shows photoexcitation of the Ru nanoclusters, followed by the electron promoting CO desorption and hole transfer towards partly reduced TiOx induced by the hydrogen treatment preceding the photothermal catalysis. (d) presents photoexcitation of a hybrid bonding state formed between Ru and CO towards the antibonding state, destabilizing the Ru–CO bond. The latter is unlikely to play a significant role here, as in that case Ru/SiO2 should have shown a higher performance.
Sa and coworkers recently reported phonon-assisted hot carrier generation for Au/ZrO2 and Au/TiO2 systems, in contrast to the regular thermally deactivated electron transfer observed in non-plasmonic systems [64]. It is important to note here that the Ru nanostructures in this study are very small; consequently, their behavior is likely at the border of plasmonic nanoparticles, and rather agrees with non-plasmonic nanoclusters showing molecular-type properties [65]. Ultrafast (luminescence) studies at in-situ conditions are currently being performed to unravel the temperature-dependency of the light-induced charge separation and recombination dynamics of Ru/TiO2 catalysts, and if these processes occur between the Ru nanoparticles and TiO2 and/or between Ru nanoparticles and surface-adsorbed species.
3.4.3. Simplified View of Photon-Induced Changes in CO Coverage
A simplified view of the effect of illumination on CO coverage is shown in Figure 8. The left figure shows that UV irradiation results in electron transfer via reduced TiOx at the perimeter, to the Ru nanoparticles, causing enhanced electron density, thereby reducing the surface coverage of CO according to rate constant k2—formed by thermal conversion of CO2 (according to rate constant k1). In the presence of hydrogen, the formation of CO is very fast (k1 is large by coproduction of H2O); hence an effect on the CO coverage was not observed upon excitation with UV light alone. The right scheme shows that visible irradiation results in hole transfer from Ru to the (reduced) TiOx at the perimeter of the particles, again causing enhanced electron density, and reducing the surface coverage of Ru–CO according to a rate constant k2. We would like to state that positive charge transfer from Ru to TiO2 is not in agreement with localized surface plasmon resonance (LSPR), which can still create “hot electrons” in the electronic band of the plasmonic metal, which are then injected into the conduction band of the semiconducting TiO2. In fact, the transfer of holes in Ru to TiO2 is counter-intuitive because of the depletion zone, characteristic of a Schottky barrier at a metal–semiconductor interface, which lowers the concentration of electrons in the TiO2 conduction band near the interface. Nevertheless, the observed shift in CO band upon illumination to lower frequencies is in agreement with a larger electron density on the Ru particles. When both UV and Vis illumination are applied, apparently the increase in charge density of the Ru particles is sufficient to even observe an effect on the surface coverage of CO in the presence of H2.
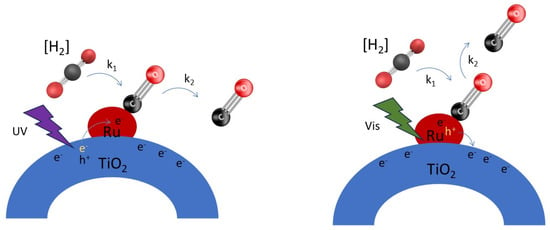
Figure 8.
Effect of charge transfer processes on Ru–CO coverage—the left shows the situation upon UV light activation of the Ru nanoparticles on TiOx, and the right shows the situation upon green light activation of the Ru nanoparticles on TiOx.
While CO poisoning dominates the reaction rate to methane in the range of 200–300 °C, as we have substantiated by citing the relevant literature, several hypotheses can be proposed to explain the limited extent to which the methane formation rate can be enhanced. As we have stated previously (see steps (4) and (5)), other surface adsorbates also play a key role in the mechanism, and non-thermal effects might also cause changes in the Ru–H coverage, for example, which not only affects the CH4 formation rate (step 4), but also the oxygen removal rate to form water (step 5). We are currently assessing the options to investigate the effect of illumination on the likely decrease in Ru–H coverage.
We would like to note, finally, that the effect of metal loading on catalyst performance merits further investigation. Increasing the loading could lead to various effects from a (thermal) catalytic perspective: a higher Ru loading leads to more active sites, enhancing performance, but at the same time it could reduce the stability of the catalyst by increasing the probability of sintering. From the perspective of light absorption by the TiOx support, a higher Ru loading could shield the TiO2 surface from light, leading to lower UV absorption, and thus possibly a lower effect of illumination on enhancement of the methane formation rate. Studying the effect of loading on performance and light enhancement is part of our ongoing research activities, with a focus on irradiation at relatively low intensities. This is contrary to reports in the literature where light of more than one order of magnitude higher intensity has been utilized, and thermal effects are likely dominant in enhancing rates upon illumination [66,67,68].
4. Experimental
4.1. Catalyst Preparation
Ruthenium (III) chloride hydrate (0.103 g, Sigma-Aldrich, Burlington, MA, USA, 99.98% trace metals basis) and polyvinylpyrrolidone (0.523 g PVP, average molecular weight 55,000, Sigma-Aldrich) were dissolved in a mixture of methanol (200 mL, Sigma-Aldrich) and MilliQ water (160 mL) in a glass beaker while continuously stirring with a stir-bar. Then, titanium dioxide (1.0 g, Sigma-Aldrich, anatase, 99.7%, <25 nm particle size) was added to the above solution, which was stirred continuously at room temperature for 1 h. Subsequently, sodium borohydride (0.185 g NaBH4, Sigma-Aldrich, Purum p.a., ≥96%) was added instantly into the above suspension, which turned dark black immediately. After continuous stirring for 2 h at room temperature, the suspension was heated on a heating plate to 50 °C and stirred for another 2 h. Then, the suspension was filtered with the aid of filter paper. After liquid removal was completed, the filter cake was washed thoroughly with MilliQ water, collected in a crucible, and dried overnight by inserting the crucible in a heating stove kept at 90 °C. The deposition of Ru on SrTiO3 (Aldrich) and SiO2 (Degussa, Germany) was performed the same way, by the introduction of 1.0 g of each of these supports in the solution containing PVP, methanol, Ruthenium (III) chloride hydrate, and water, followed by reduction with NaBH4, filtration, and drying at 90 °C overnight. All samples were consecutively treated at elevated temperatures in a commercial flow-bed microreactor (Linkam, Redhill, UK, CCR1000) at 450 °C (ramp 10 °C.min−1) for 2 h in a flow of 5 Ml min−1 consisting of 30 vol% H2 in He. Before opening and collecting the samples in vials, the flow composition was changed to 5 mL He min−1 and the samples were cooled to room temperature.
4.2. Characterization
A selection of samples after reductive thermal treatment was analyzed by transmission electron microscopy (TEM, Philips CM300ST-FEG, Amsterdam, The Netherlands) to determine the size, distribution, and morphology. Ex situ X-ray photoelectron spectroscopy (XPS, Quantera SXM, Physical Electronics, Chanhassen, MN, USA) using a monochromatic Al Kα x-ray source (1486.6 eV) was performed to determine the oxidation state of the Ru. The measurements were performed at 120 eV pass energy and 0.4 eV step size, and the working pressure in the chamber was typically lower than 7 × 10−9 Torr. The results were analyzed using the Multipak software (version 9.8) and the binding energy of all the spectra was calibrated using the adventitious carbon peak at 284.8 eV. UV-vis spectra were recorded using an Avantes probe and spectrometer (AvaSpec-2048, Apeldoorn, The Netherlands) to evaluate the absorbance spectra of the various catalysts. The metal loading was quantified by X-Ray Fluorescence spectroscopy (XRF, Philips PW 1480, Amsterdam, The Netherlands).
4.2.1. Diffuse Reflectance Fourier Transform Infrared (DRIFT) Spectroscopy
DRIFT analysis was performed using a VERTEX 70 spectrometer (Bruker, Singapore) in combination with a diffuse reflectance spectroscopy cell (Harrick, Pleasantville, NY, USA, Praying Mantis, Bengaluru, India), equipped with a three-window dome and a temperature controller (Harrick, Anadis Instruments, Almere, The Netherlands). The measurements were performed at a spectral resolution of 4 cm−1. Before each measurement, the powdered samples of ~10 mg were pretreated in a flow of 50 mL min−1 containing 30 vol-% H2 in He, at 450 °C for 2 h, identical to the procedure followed prior to the activity measurements and sample characterization. This was carried out to ensure that any potentially present RuOx was reduced to Ru and that any carbon was removed from the sample. During the cooling down of the sample, background spectra were recorded at 450 °C, 350 °C, 250 °C, and 50 °C. After cooling down to 50 °C, the reactor was purged with 50 mL min−1 He for 30 min to remove any remaining H2 gas. Then, a flow of 35 mL min−1 of 14.3% CO2 in He was introduced into the reactor for a duration of 30 min, to complete the interaction of the catalyst surface with CO2, which was confirmed by the recording of several spectra. Then, the first DRIFT spectrum of a series at each temperature was recorded. Subsequently, hydrogen was introduced (adding 15 mL min−1, total flow now 50 mL min−1 containing 10% CO2 and 30% H2), followed by 30 min of stabilization—and the second DRIFT spectrum was recorded. The gas flow was then closed, and light was subsequently introduced. A 120 W Hg lamp (HP-120, Dr. Grobel UV-Elektronik GmbH, Ettlingen, Germany, with the UV/Vis spectrum provided in Figure S1 of the Supporting Information) was used to illuminate the samples via a fiber optic cable at a distance of ~6 cm from the quartz window of the three-window dome, resulting in exposure of the window of the dome to approximately 360 mW/cm2. A third DRIFT spectrum was recorded—after 30 min of illumination. Then, the gas flow was opened again at 35 mL min−1 containing 14.3% CO2 in He, and the temperature increased to 250 °C, followed by stabilization, recording of the first DRIFT spectrum at this temperature, introduction of 15 mL min−1 of H2, recording of the second spectrum, closure of the flow, illumination for 30 min, and recording of the third spectrum. This cycle was repeated with the same catalyst at 350 °C and 450 °C.
4.2.2. (DRIFT) Spectroscopy During LED Illumination at 530 nm and 365 nm
The pretreatment of a fresh sample and introduction of gases of at each wavelength was identical to the UV/Vis experiments. Narrow band exposure to 365 nm (UV) or (maximum at) 530 nm (green) was independently investigated. The intensity vs. distance curves are shown in Figure S2—resulting in exposure of the window of the dome to ~7 mW/cm2 when positioning the UV-LED at ~1 cm away from the window, and ~8.5 mW/cm2 to green light, again positioned at ~1 cm away from the window.
In addition to a temperature-dependent series recording spectra during illumination in the presence of CO2 and H2, a complete temperature series was also recorded during illumination and exposure of the catalyst to CO2; thus without the addition and involvement of H2. After the introduction of CO2, the flow was discontinued, the first spectrum recorded in the dark, and LED illumination initiated for 30 min, after which a second spectrum was recorded during illumination. Then, the flow of gas (35 mL min−1, 14.6% CO2 in He) was reinitiated and the sample heated to the next desired temperature.
4.3. Photothermal Reactivity by Transient Analysis
Photothermal catalytic reduction of CO2 by H2 was investigated in a commercial flow-bed microreactor (Linkam, CCR1000, Redhill, UK) and CH4, CO, and H2O were detected by mass spectrometry (MS, PFEIFFER, OmniStar GSD320, Aßlar, Germany). The temperature was measured via an S-type platinum/rhodium thermocouple—positioned adjacent to the small ceramic cup—holding the sample. The thickness of the sample bed amounted to a few mm. First, 50 mg of sample was pretreated in situ in a 1.5 mL min−1 H2/3.5 mL min−1 He gas flow at 450 °C with a heating ramp of 10 °C min−1. Then, the catalyst was kept at this temperature for 2 h and subsequently cooled in the gas mixture to 50 °C. Again, this was done to reduce any potentially present RuOx to Ru and to remove possible carbon contaminations from the sample. Subsequently, at this temperature, a reactant gas flow containing 0.5 mL min−1 CO2/1.5 mL min−1 H2/3 mL min−1 He was switched on. Purging with this gas flow was continued for 60 min. before heating up the samples. A 120 W Hg lamp (HP-120, Dr. Grobel UV-Elektronik GmbH, Ettlingen, Germany, with the spectrum provided in Figure S1 of the Supporting Information) was used to illuminate the samples via a fiber optic cable at a distance of 6 cm, resulting in exposure of a quartz window of the microreactor from the top to an intensity of 360 mW/cm2.
To compare the intrinsic reactivities of different catalysts, kinetic measurements were first carried out between 120 and 220 °C, thus outside the range of equilibrium constraints. At each reaction temperature, the samples were kept for 30 min. in the dark, and then exposed to light-on, light off cycles for 30 min each.
The photothermal performance was studied further by increasing the temperature from 220 to 450 °C and then stepwise decreasing the temperature to 250 °C, to assess the presence of hysteresis and/or deactivation of the catalyst. At each temperature, the performance in the dark was analyzed for 20 min, followed by performance under irradiation for 20 min. This procedure was repeated three times. The photothermal stability of the Ru/TiO2 catalyst was also tested at 250 °C for 24 h by continuously alternating between dark and (UV-vis) irradiation conditions.
5. Conclusions
The experimental data provided in this study clearly support the following conclusions:
- The Ru/TiO2 catalyst used for methanation of CO2 shows a photo-response, which is larger than that of the catalysts reported in the literature. This is likely due to the strong metal (Ru) support (TiO2) interactions induced by the high temperature treatment in H2, preceding photothermal catalysis.
- Using a fixed illumination energy of 360 mW/cm2, the strongest light-induced enhancement in conversion of CO2 was obtained in the range of 200–250 °C. At 200 °C, the rate rapidly increases from ~1.2 mol gRu−1 h−1 to ~1.8 mol gRu−1 h−1 upon illumination. Light does not induce a change in mechanism given the similar activation energy, while the increase in rate would agree with a global temperature rise of the catalyst of ~10 °C.
- A change in selectivity from CH4 towards CO was observed at 450 °C—enhanced by illumination.
- DRIFT spectroscopic analysis in static gas conditions shows a diminishing intensity and shift in the Ru–CO absorption frequency upon illumination in isothermal conditions—indicative of light-induced desorption of CO equivalent to a temperature rise of several 100 s of degrees.
- Using the CO IR spectrum as an in-situ probe, localized heating (by Ru visible light absorption) cannot explain the observed decreasing CO coverage. Rather, interfacial charge transfer processes should play a role, in agreement with recent spectroscopic observations [36].
- To explain the limited increase in CH4 formation rate by non-thermal lowering of the CO coverage, we hypothesize that the surface concentrations of other adsorbates, relevant in the reaction mechanism, such as Ru–H, are also affected by illumination.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30122577/s1, Figure S1. Emission spectrum of the Hg lamp used in this work for irradiation. Figure S2. Intensity vs distance curves for the two LED light sources used in this study. Figure S3. Enlargement of the TEM image of the Ru/TiO2 particles, obtained after reduction in H2 flow. Figure S4. (a). Arrhenius plots of CH4 formation on Ru/TiO2 in dark and under UV-vis irradiation. From the slope of the curve (equal to 1000* Ea/R) and the intersection with the y-axis (equal to ln(A)), we calculate in the dark Ea = 64.1 ± 0.3 kJ mol−1 and A = (15.9 ± 1.1)*109 s−1, while under UV-vis illumination we find Ea = 59.6 ± 1.2 kJ mol−1 and A = (6.9 ± 2.1)*109 s−1 (1st order kinetics is assumed). Although minor differences are found, we believe that the differences are too small to justify that it originates from a change in reaction mechanism. (b). Comparison of the difference in ln r as a function of 1/T. Considering the figures of the main text (shifting by 10 °C resulting in large overlap) we anticipate the difference is relatively constant as a function of temperature. Figure S5. Zoom in of the CH4 response of the Mass Spectrometer in photothermal conversion of CO2 and H2 in dark, and under UV-vis irradiation as a function of temperature, the largest response is obtained at 250 °C. Figure S6. Comparing the transient in CH4 response of the mass spectrometer induced by visible light (pink), or UV/Vis (purple) illumination in the temperature range from 50 °C to 300 °C. Figure S7. Stability assessment of the Ru/TiO2 using repetition of light-on, light-off cycles at 250 °C under chopped UV/Vis irradiation for 24 h. Figure S8. In situ DRIFT spectra of photothermal hydrogenation of CO2 on TiO2 in the dark and under UV-vis irradiation. Figure S9. (a): In situ DRIFT spectra of Ru-CO formed by exposure of Hydrogen- reduced Ru/TiO2 to CO2 only (for 30 min). Spectra in the absence (‘dark’-blue lines) and under UV irradiation (‘UV-light–red lines) are compared at various temperatures. Please note that in particular at the highest temperature of 450 °C, a small difference in the intensity of the CO absorption band can be observed; (b): In situ DRIFT spectra of Ru-CO formed by exposure of Hydrogen-reduced Ru/TiO2 to CO2 and Hydrogen (for 30 min in batch mode). UV LED illumination has no effect on the adsorbed CO intensity—nor on the amount of methane that is formed (see the rotational bands of gas phase methane at ~3000 cm−1. Illumination was performed with a UV LED maximizing intensity at 370 nm—to the amount of ~7 mW/cm2. Figure S10. (a): In situ DRIFT spectra of Ru-CO formed by exposure of hydrogen-reduced Ru/TiO2 to CO2 only (for 30 min). Spectra in the absence (‘dark’-blue lines) and under Visible irradiation (Visible-light–red lines) are compared at various temperatures. Please note that in particular at the highest temperature of 450 °C, a small decrease in the intensity of the CO absorption band can be observed upon illumination; (b): In situ DRIFT spectra of Ru-CO formed by exposure of hydrogen-reduced Ru/TiO2 to CO2 and hydrogen (for 30 min in batch mode). Now visible light illumination has no effect on the adsorbed CO intensity—nor on the amount of methane that is formed (see the rotational bands of gas phase methane at ~3000 cm−1. Illumination was performed with a Visible light LED maximizing intensity at 530 nm (green light)—to the amount of ~8.5 mW/cm2.
Author Contributions
Methodology, Y.B. and A.H.; Writing—original draft, K.W., N.T.C., K.-J.C.J.W. and G.M. All authors have read and agreed to the published version of the manuscript.
Funding
We would like to extend our gratitude to the Dutch Science Foundation (NWO) for its funding for the framework of the solar to products project Solar to Products Program 733.000.001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to thank Niemantsverdriet and Fredriksson of SynGasChem BV for their financial support and fruitful discussions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ulmer, U.; Dingle, T.; Duchesne, P.N.; Morris, R.H.; Tavasoli, A.; Wood, T.; Ozin, G.A. Fundamentals and applications of photocatalytic CO2 methanation. Nat. Commun. 2019, 10, 3169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, Y.-H.; Qi, M.-Y.; Yamada, Y.M.A.; Anpo, M.; Tang, Z.-R.; Xu, Y.-J. Photothermal catalytic CO2 reduction over nanomaterials. Chem Catal. 2021, 1, 272–297. [Google Scholar] [CrossRef]
- Yang, X.; Tan, F.; Wang, D.; Feng, Q.; Qiu, D.; Dang, D.; Wang, X. Entrapping Ru nanoparticles into TiO2 nanotube: Insight into the confinement synergy on boosting pho-thermal CO2 methanation activity. Ceram. Int. 2021, 47, 27316–27323. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Mul, G.; Baltrusaitis, J.; Larrazábal, G.O.; Pérez-Ramírez, J. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energy Environ. Sci. 2013, 6, 3112–3135. [Google Scholar] [CrossRef]
- Wang, C.; Fang, S.; Xie, S.; Zheng, Y.; Hu, Y.H. Thermo-photo catalytic CO2 hydrogenation over Ru/TiO2. J. Mater. Chem. A 2020, 8, 7390–7394. [Google Scholar] [CrossRef]
- Yang, Z.; Qi, Y.; Wang, F.; Han, Z.; Jiang, Y.; Han, H.; Liu, J.; Zhang, X.; Ong, W.J. State-of-the-art advancements in photo-assisted CO2 hydrogenation: Recent progress in catalyst development and reaction mechanisms. J. Mater. Chem. A 2020, 8, 24868–24894. [Google Scholar] [CrossRef]
- Wang, Y.; Winter, L.R.; Chen, J.G.; Yan, B. CO2 hydrogenation over heterogeneous catalysts at atmospheric pressure: From electronic properties to product selectivity. Green Chem. 2021, 23, 249–267. [Google Scholar] [CrossRef]
- Shehzad, N.; Tahir, M.; Johari, K.; Murugesan, T.; Hussain, M. A critical review on TiO2 based photocatalytic CO2 reduction system: Strategies to improve efficiency. J. CO2 Util. 2018, 26, 98–122. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Schmidt-Mende, L.; Stolarczyk, J.K. Photocatalytic Reduction of CO2 on TiO2 and Other Semiconductors. Angew. Chem. Int. Ed. 2013, 52, 7372–7408. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jaroniec, M.; Chen, X. Cocatalysts for Selective Photoreduction of CO2 into Solar Fuels. Chem. Rev. 2019, 119, 3962–4179. [Google Scholar] [CrossRef]
- Wenderich, K.; Mul, G. Methods, Mechanism, and Applications of Photodeposition in Photocatalysis: A Review. Chem. Rev. 2016, 116, 14587–14619. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Yin, W.; Wang, L.; Li, Z.; Xiong, Y. Surface and interface design in cocatalysts for photocatalytic water splitting and CO2 reduction. RSC Adv. 2016, 6, 57446–57463. [Google Scholar] [CrossRef]
- Xu, P.; McCool, N.S.; Mallouk, T.E. Water splitting dye-sensitized solar cells. Nano Today 2017, 14, 42–58. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Medhi, R.; Marquez, M.D.; Lee, T.R. Visible-Light-Active Doped Metal Oxide Nanoparticles: Review of their Synthesis, Properties, and Applications. ACS Appl. Nano Mater. 2020, 3, 6156–6185. [Google Scholar] [CrossRef]
- Nah, Y.-C.; Paramasivam, I.; Schmuki, P. Doped TiO2 and TiO2 Nanotubes: Synthesis and Applications. ChemPhysChem 2010, 11, 2698–2713. [Google Scholar] [CrossRef]
- Ghoussoub, M.; Xia, M.; Duchesne, P.N.; Segal, D.; Ozin, G. Principles of photothermal gas-phase heterogeneous CO2 catalysis. Energy Environ. Sci. 2019, 12, 1122–1142. [Google Scholar] [CrossRef]
- Keller, N.; Ivanez, J.; Highfield, J.; Ruppert, A.M. Photo-/thermal synergies in heterogeneous catalysis: Towards low-temperature (solar-driven) processing for sustainable energy and chemicals. Appl. Catal. B Environ. 2021, 296, 120320. [Google Scholar] [CrossRef]
- Mateo, D.; Cerrillo, J.L.; Durini, S.; Gascon, J. Fundamentals and applications of photo-thermal catalysis. Chem. Soc. Rev. 2021, 50, 2173–2210. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Gao, M.; Peh, C.K.N.; Ho, G.W. Solar-driven photothermal nanostructured materials designs and prerequisites for evaporation and catalysis applications. Mater. Horiz. 2018, 5, 323–343. [Google Scholar] [CrossRef]
- Sa, J.; Tagliabue, G.; Friedli, P.; Szlachetko, J.; Rittmann-Frank, M.H.; Santomauro, F.G.; Milne, C.J.; Sigg, H. Direct observation of charge separation on Au localized surface plasmons. Energy Environ. Sci. 2013, 6, 3584–3588. [Google Scholar] [CrossRef]
- Yu, Y.; Williams, J.D.; Willets, K.A. Quantifying photothermal heating at plasmonic nanoparticles by scanning electrochemical microscopy. Faraday Discuss. 2018, 210, 29–39. [Google Scholar] [CrossRef]
- Kamarudheen, R.; Aalbers, G.J.W.; Hamans, R.F.; Kamp, L.P.J.; Baldi, A. Distinguishing Among All Possible Activation Mechanisms of a Plasmon-Driven Chemical Reaction. ACS Energy Lett. 2020, 5, 2605–2613. [Google Scholar] [CrossRef]
- Meng, X.; Wang, T.; Liu, L.; Ouyang, S.; Li, P.; Hu, H.; Kako, T.; Iwai, H.; Tanaka, A.; Ye, J. Photothermal Conversion of CO2 into CH4 with H2 over Group VIII Nanocatalysts: An Alternative Approach for Solar Fuel Production. Angew. Chem. Int. Ed. 2014, 53, 11478–11482. [Google Scholar] [CrossRef]
- O’Brien, P.G.; Sandhel, A.; Wood, T.E.; Jelle, A.A.; Hoch, L.B.; Perovic, D.D.; Mims, C.A.; Ozin, G.A. Photomethanation of Gaseous CO2 over Ru/Silicon Nanowire Catalysts with Visible and Near-Infrared Photons. Adv. Sci. 2014, 1, 1400001. [Google Scholar] [CrossRef]
- Kong, N.; Han, B.; Li, Z.; Fang, Y.; Feng, K.; Wu, Z.; Wang, S.; Xu, A.-B.; Yu, Y.; Li, C.; et al. Ruthenium Nanoparticles Supported on Mg(OH)2 Microflowers as Catalysts for Photothermal Carbon Dioxide Hydrogenation. ACS Appl. Nano Mater. 2020, 3, 3028–3033. [Google Scholar] [CrossRef]
- Mateo, D.; Albero, J.; García, H. Titanium-Perovskite-Supported RuO2 Nanoparticles for Photocatalytic CO2 Methanation. Joule 2019, 3, 1949–1962. [Google Scholar] [CrossRef]
- Novoa-Cid, M.; Baldovi, H.G. Study of the Photothermal Catalytic Mechanism of CO2 Reduction to CH4 by Ruthenium Nanoparticles Supported on Titanate Nanotubes. Nanomaterials 2020, 10, 2212. [Google Scholar] [CrossRef]
- Sastre, F.; Versluis, C.; Meulendijks, N.; Rodríguez-Fernández, J.; Sweelssen, J.; Elen, K.; Van Bael, M.K.; den Hartog, T.; Verheijen, M.A.; Buskens, P. Sunlight-Fueled, Low-Temperature Ru-Catalyzed Conversion of CO2 and H2 to CH4 with a High Photon-to-Methane Efficiency. ACS Omega 2019, 4, 7369–7377. [Google Scholar] [CrossRef] [PubMed]
- Jelle, A.A.; Ghuman, K.K.; O’Brien, P.G.; Hmadeh, M.; Sandhel, A.; Perovic, D.D.; Singh, C.V.; Mims, C.A.; Ozin, G.A. Highly Efficient Ambient Temperature CO2 Photomethanation Catalyzed by Nanostructured RuO2 on Silicon Photonic Crystal Support. Adv. Energy Mater. 2018, 8, 1702277. [Google Scholar]
- O’Brien, P.G.; Ghuman, K.K.; Jelle, A.A.; Sandhel, A.; Wood, T.E.; Loh, J.Y.Y.; Jia, J.; Perovic, D.; Singh, C.V.; Kherani, N.P.; et al. Enhanced photothermal reduction of gaseous CO2 over silicon photonic crystal supported ruthenium at ambient temperature. Energy Environ. Sci. 2018, 11, 3443–3451. [Google Scholar] [CrossRef]
- Ren, J.; Ouyang, S.; Xu, H.; Meng, X.; Wang, T.; Wang, D.; Ye, J. Targeting Activation of CO2 and H2 over Ru-Loaded Ultrathin Layered Double Hydroxides to Achieve Efficient Photothermal CO2 Methanation in Flow-Type System. Adv. Energy Mater. 2017, 7, 1601657. [Google Scholar] [CrossRef]
- Thampi, K.R.; Kiwi, J.; Grätzel, M. Methanation and photo-methanation of carbon dioxide at room temperature and atmospheric pressure. Nature 1987, 327, 506–508. [Google Scholar] [CrossRef]
- Wenderich, K.; Zhu, K.J.; Bu, Y.B.; Tichelaar, F.D.; Mul, G.; Huijser, A. Photophysical Characterization of Ru Nanoclusters on Nanostructured TiO2 by Time-Resolved Photoluminescence Spectroscopy. J. Phys. Chem. C 2023, 127, 14353–14362. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, W.; Qi, H.; Su, X.; Su, Y.; Liu, X.; Li, L.; Yang, X.; Huang, Y.; Zhang, T. Strong Metal–Support Interaction of Ru on TiO2 Derived from the Co-Reduction Mechanism of RuxTi1–xO2 Interphase. ACS Catal. 2022, 12, 1697–1705. [Google Scholar] [CrossRef]
- Abdel-Mageed, A.M.; Wiese, K.; Parlinska-Wojtan, M.; Rabeah, J.; Brückner, A.; Behm, R.J. Encapsulation of Ru nanoparticles: Modifying the reactivity toward CO and CO2 methanation on highly active Ru/TiO2 catalysts. Appl. Catal. B Environ. 2020, 270, 118846. [Google Scholar] [CrossRef]
- Cisneros, S.; Abdel-Mageed, A.; Mosrati, J.; Bartling, S.; Rockstroh, N.; Atia, H.; Abed, H.; Rabeah, J.; Brückner, A. Oxygen vacancies in Ru/TiO2—Drivers of low-temperature CO2 methanation assessed by multimodal operando spectroscopy. iScience 2022, 25, 103886. [Google Scholar] [CrossRef]
- Muroyama, H.; Tsuda, Y.; Asakoshi, T.; Masitah, H.; Okanishi, T.; Matsui, T.; Eguchi, K. Carbon dioxide methanation over Ni catalysts supported on various metal oxides. J. Catal. 2016, 343, 178–184. [Google Scholar] [CrossRef]
- Ortelli, E.E.; Weigel, J.M.; Wokaun, A. Methanol synthesis pathway over Cu/ZrO2 catalysts: A time-resolved DRIFT C-13-labelling experiment. Catal. Lett. 1998, 54, 41–48. [Google Scholar] [CrossRef]
- Falbo, L.; Visconti, C.G.; Lietti, L.; Szanyi, J. The effect of CO on CO2 methanation over Ru/Al2O3 catalysts: A combined steady-state reactivity and transient DRIFT spectroscopy study. Appl. Catal. B-Environ. 2019, 256, 117791. [Google Scholar] [CrossRef]
- Scire, S.; Crisafulli, C.; Maggiore, R.; Minico, S.; Galvagno, S. Influence of the support on CO2 methanation over Ru catalysts: An FT-IR study. Catal. Lett. 1998, 51, 41–45. [Google Scholar] [CrossRef]
- Xu, J.H.; Su, X.; Duan, H.M.; Hou, B.L.; Lin, Q.Q.; Liu, X.Y.; Pan, X.L.; Pei, G.X.; Geng, H.R.; Huang, Y.Q.; et al. Influence of pretreatment temperature on catalytic performance of rutile TiO2-supported ruthenium catalyst in CO2 methanation. J. Catal. 2016, 333, 227–237. [Google Scholar] [CrossRef]
- Zagli, E.; Falconer, J.L. Carbon Dioxide Adsorption and Methanation on Ruthenium. J. Catal. 1981, 69, 1–8. [Google Scholar]
- Vogt, C.; Groeneveld, E.; Kamsma, G.; Nachtegaal, M.; Lu, L.; Kiely, C.J.; Berben, P.H.; Meirer, F.; Weckhuysen, B.M. Unravelling structure sensitivity in CO2 hydrogenation over nickel. Nat. Catal. 2018, 1, 163. [Google Scholar] [CrossRef]
- Mansour, H.; Iglesia, E. Mechanistic Connections between CO2 and CO Hydrogenation on Dispersed Ruthenium Nanoparticles. J. Am. Chem. Soc. 2021, 143, 11582–11594. [Google Scholar] [CrossRef]
- Vendelbo, S.B.; Johansson, M.; Mowbray, D.J.; Andersson, M.P.; Abild-Pedersen, F.; Nielsen, J.H.; Norskov, J.K.; Chorkendorff, I. Self Blocking of CO Dissociation on a Stepped Ruthenium Surface. Top. Catal. 2010, 53, 357–364. [Google Scholar] [CrossRef]
- Fan, C.Y.; Bonzel, H.P.; Jacobi, K. CO adsorption on the multiple-site Ru(11(2)over-bar1) surface: The role of bonding competition. J. Chem. Phys. 2003, 118, 9773–9782. [Google Scholar] [CrossRef]
- Strebel, C.; Murphy, S.; Nielsen, R.M.; Nielsen, J.H.; Chorkendorff, I. Probing the active sites for CO dissociation on ruthenium nanoparticles. Phys. Chem. Chem. Phys. 2012, 14, 8005–8012. [Google Scholar] [CrossRef]
- Tison, Y.; Nielsen, K.; Mowbray, D.J.; Bech, L.; Holse, C.; Calle-Vallejo, F.; Andersen, K.; Mortensen, J.J.; Jacobsen, K.W.; Nielsen, J.H. Scanning Tunneling Microscopy Evidence for the Dissociation of Carbon Monoxide on Ruthenium Steps. J. Phys. Chem. C 2012, 116, 14350–14359. [Google Scholar] [CrossRef]
- Low, G.G.; Bell, A.T. Studies of CO desoprtion and reaction with H2 on Alumina Supported Ru. J. Catal. 1979, 57, 397–405. [Google Scholar] [CrossRef]
- Lin, H.Y.; Chen, Y.W. The kinetics of H-2 adsorption on supported ruthenium catalysts. Thermochim. Acta 2004, 419, 283–290. [Google Scholar] [CrossRef]
- Alves, O.B.; Hoster, H.E.; Behm, R.J. Electrochemistry at Ru(0001) in a flowing CO-saturated electrolyte-reactive and inert adlayer phases. Phys. Chem. Chem. Phys. 2011, 13, 6010–6021. [Google Scholar] [CrossRef]
- Yokomizo, G.H.; Louis, C.; Bell, A.T. An Infrared Study of CO Adsorption on Reduced and Oxidized Ru/SiO2. J. Catal. 1989, 120, 1–14. [Google Scholar] [CrossRef]
- Devasia, D.; Das, A.; Mohan, V.; Jain, P.K. Control of Chemical Reaction Pathways by Light–Matter Coupling. Annu. Rev. Phys. Chem. 2021, 72, 423–443. [Google Scholar] [CrossRef]
- Mascaretti, L.; Schirato, A.; Montini, T.; Alabastri, A.; Naldoni, A.; Fornasiero, P. Challenges in temperature measurements in gas-phase photothermal catalysis. Joule 2022, 6, 1727–1732. [Google Scholar] [CrossRef]
- Nelson, D.A.; Schultz, Z.D. The impact of optically rectified fields on plasmonic electrocatalysis. Faraday Discuss. 2019, 214, 465–477. [Google Scholar] [CrossRef]
- Jia, C.Y.; Zhong, W.H.; Deng, M.S.; Jiang, J. CO oxidation on Ru-Pt bimetallic nanoclusters supported on TiO2(101): The effect of charge polarization. J. Chem. Phys. 2018, 148, 124701. [Google Scholar] [CrossRef]
- Huang, S.P.; Inerbaev, T.M.; Kilin, D.S. Excited State Dynamics of Ru-10 Cluster Interfacing Anatase TiO2(101) Surface and Liquid Water. J. Phys. Chem. Lett. 2014, 5, 2823–2829. [Google Scholar] [CrossRef]
- Frischkorn, C.; Wolf, M. Femtochemistry at metal surfaces: Nonadiabatic reaction dynamics. Chem. Rev. 2006, 106, 4207–4233. [Google Scholar] [CrossRef] [PubMed]
- Kale, M.J.; Avanesian, T.; Xin, H.L.; Yan, J.; Christopher, P. Controlling Catalytic Selectivity on Metal Nanoparticles by Direct Photoexcitation of Adsorbate-Metal Bonds. Nano Lett. 2014, 14, 5405–5412. [Google Scholar] [CrossRef] [PubMed]
- Bonn, M.; Funk, S.; Hess, C.; Denzler, D.N.; Stampfl, C.; Scheffler, M.; Wolf, M.; Ertl, G. Phonon- versus electron-mediated desorption and oxidation of CO on Ru(0001). Science 1999, 285, 1042–1045. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Meng, J.; Zheng, K.B.; de Andrade, A.M.; Kullgren, J.; Broqvist, P.; Nordlander, P.; Sa, J. Phonon-Assisted Hot Carrier Generation in Plasmonic Semiconductor Systems. Nano Lett. 2021, 21, 1083–1089. [Google Scholar] [CrossRef]
- Zhou, M.; Jin, R.C. Optical Properties and Excited-State Dynamics of Atomically Precise Gold Nanoclusters. Annu. Rev. Phys. Chem. 2021, 72, 121–142. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.J.; Shi, R.; Waterhouse, G.I.N.; Wen, X.-D.; Zhang, T. Fe-Based Catalysts for the Direct Photohydrogenation of CO2 to Value-Added Hydrocarbons. Adv. Energy Mater. 2021, 11, 2002783. [Google Scholar] [CrossRef]
- Li, Z.; Shi, R.; Zhao, J.; Zhang, T. Ni-based catalysts derived from layered-double-hydroxide nanosheets for efficient photothermal CO2 reduction under flow-type system. Nano Res. 2021, 14, 4828. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Zhao, Z.-W.; Liu, H.; Wang, H.-Q. Core–shell nanostructure for supra-photothermal CO2 catalysis. Rare Met. 2022, 41, 1403–1405. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).