Cytocompatible FRET Assembly of CdTe@GSH Quantum Dots and Au@BSA Nanoclusters: A Novel Ratiometric Strategy for Dopamine Detection
Abstract
1. Introduction
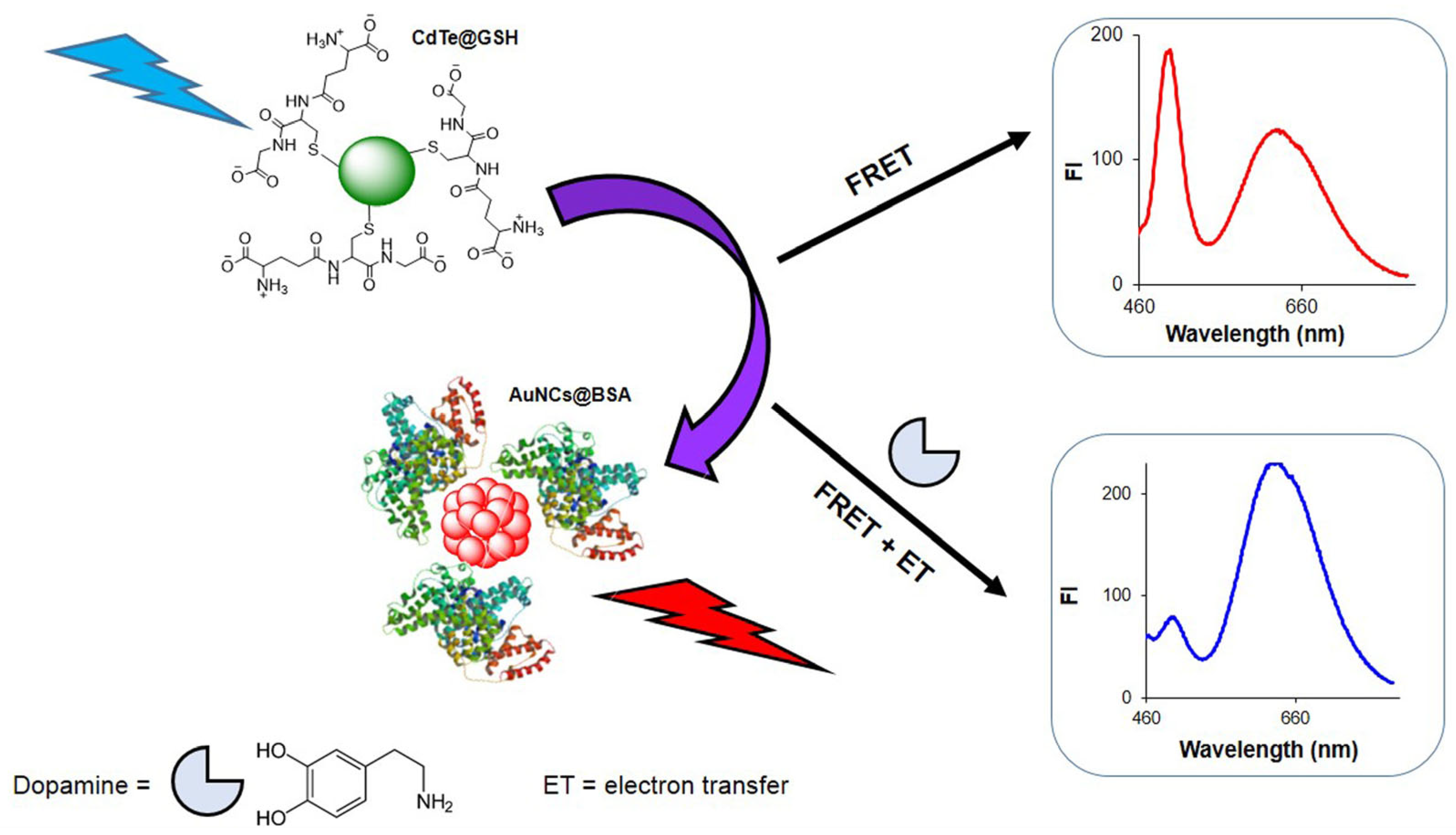
2. Results and Discussion
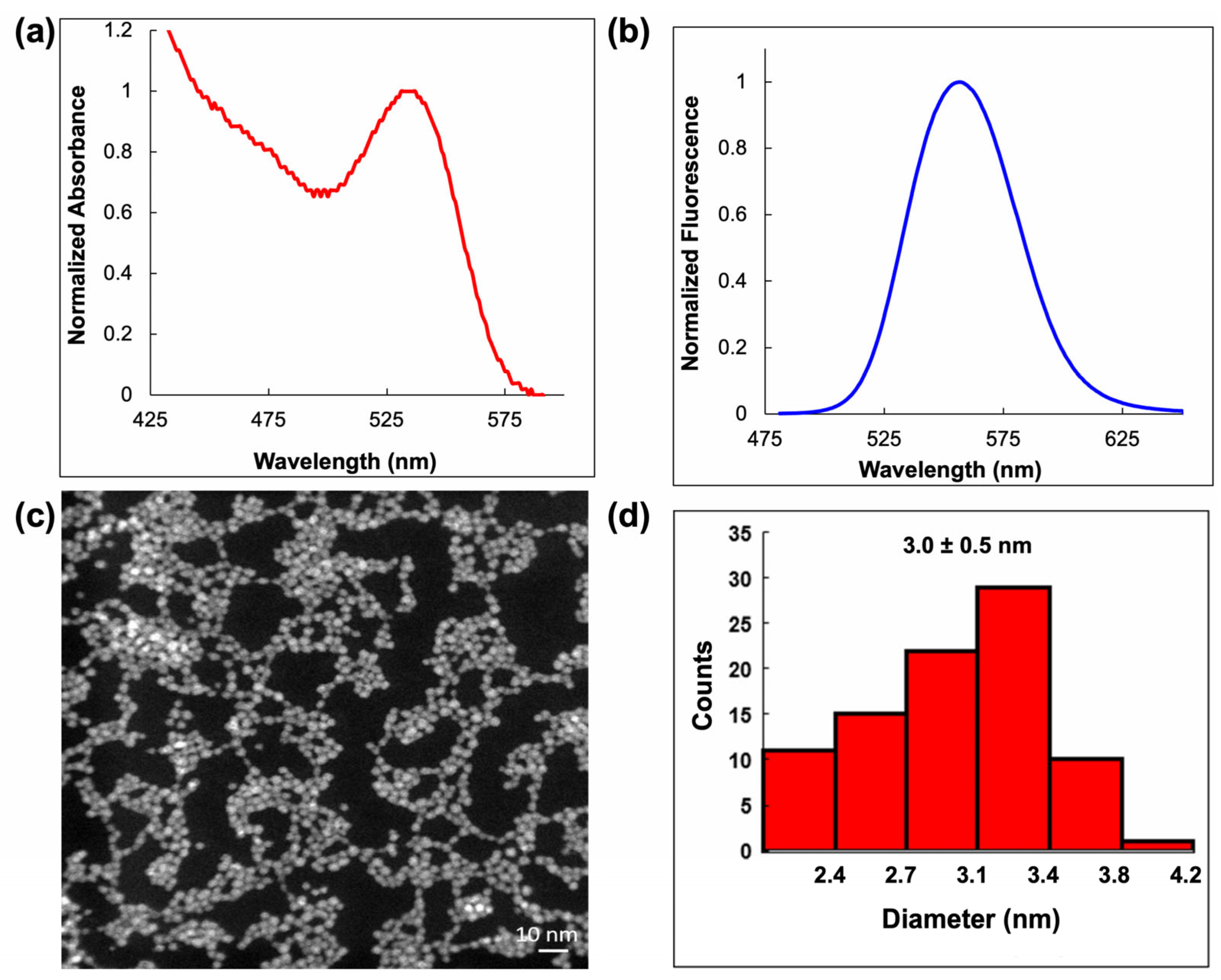
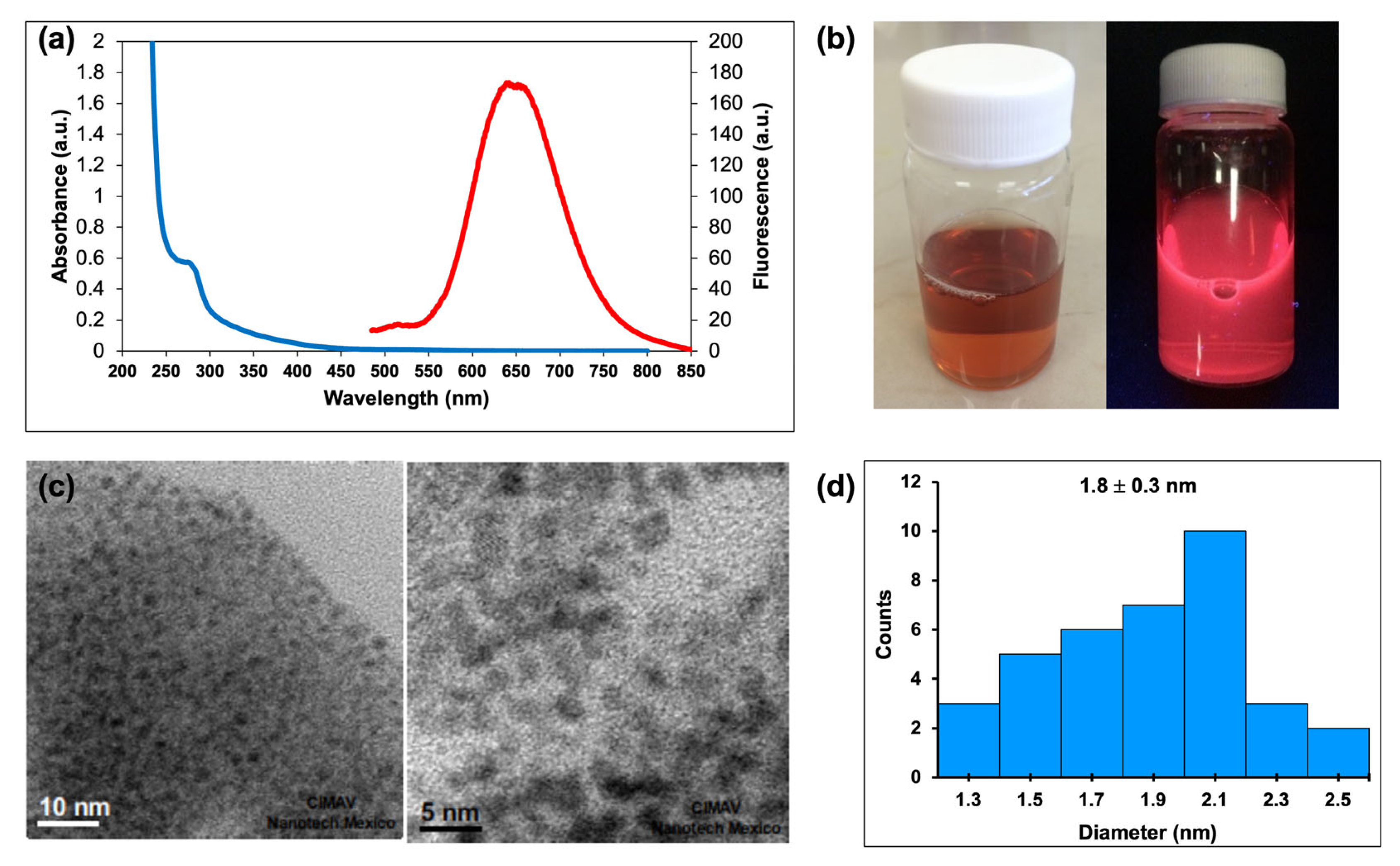
2.1. Synthesis and Characterization of CdTe/GSH QDs and AuNCs/BSA
2.1.1. Synthesis and Spectral Characteristics of CdTe/GSH QDs
2.1.2. Synthesis and Spectral Characteristics of AuNCs/BSA
2.2. Optical Stability of CdTe/GSH and AuNCs/BSA
2.3. Study of FRET Process Between CdTe/GSH QDs and AuNCs/BSA Assemblies
Selection of Optimal Conditions of the FRET Process
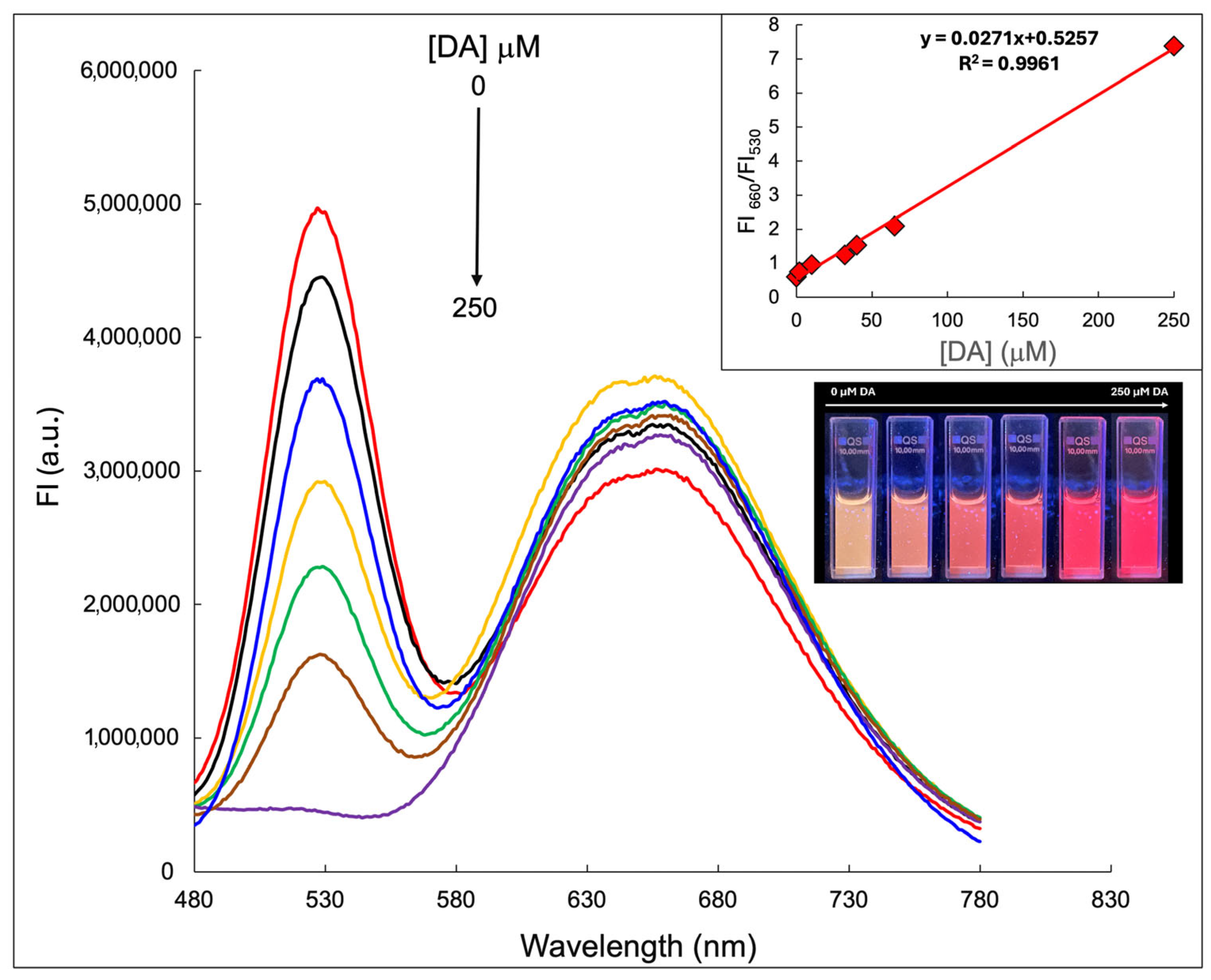
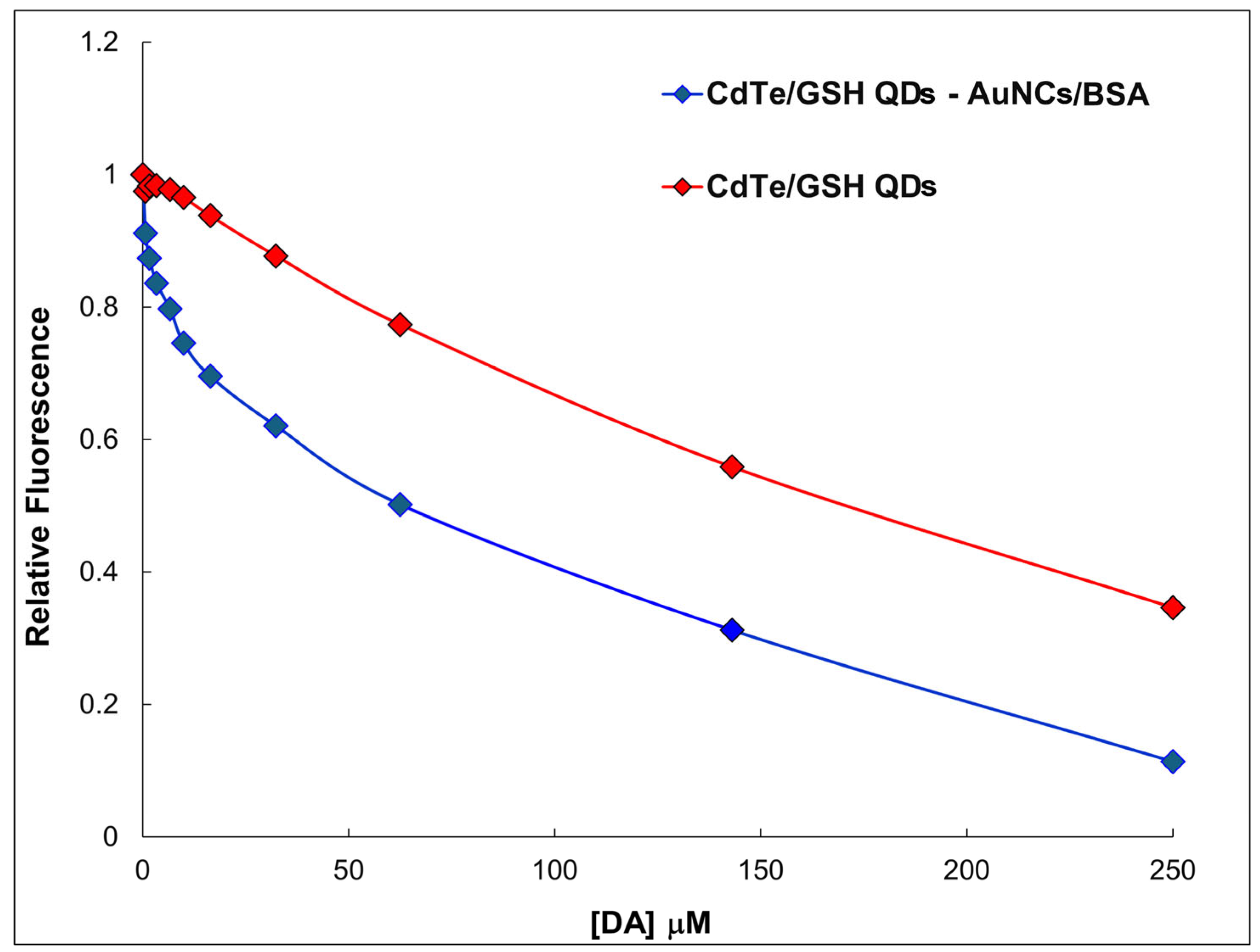
2.4. The Detection of DA by Using CdTe/GSH-AuNCs/BSA Assembly
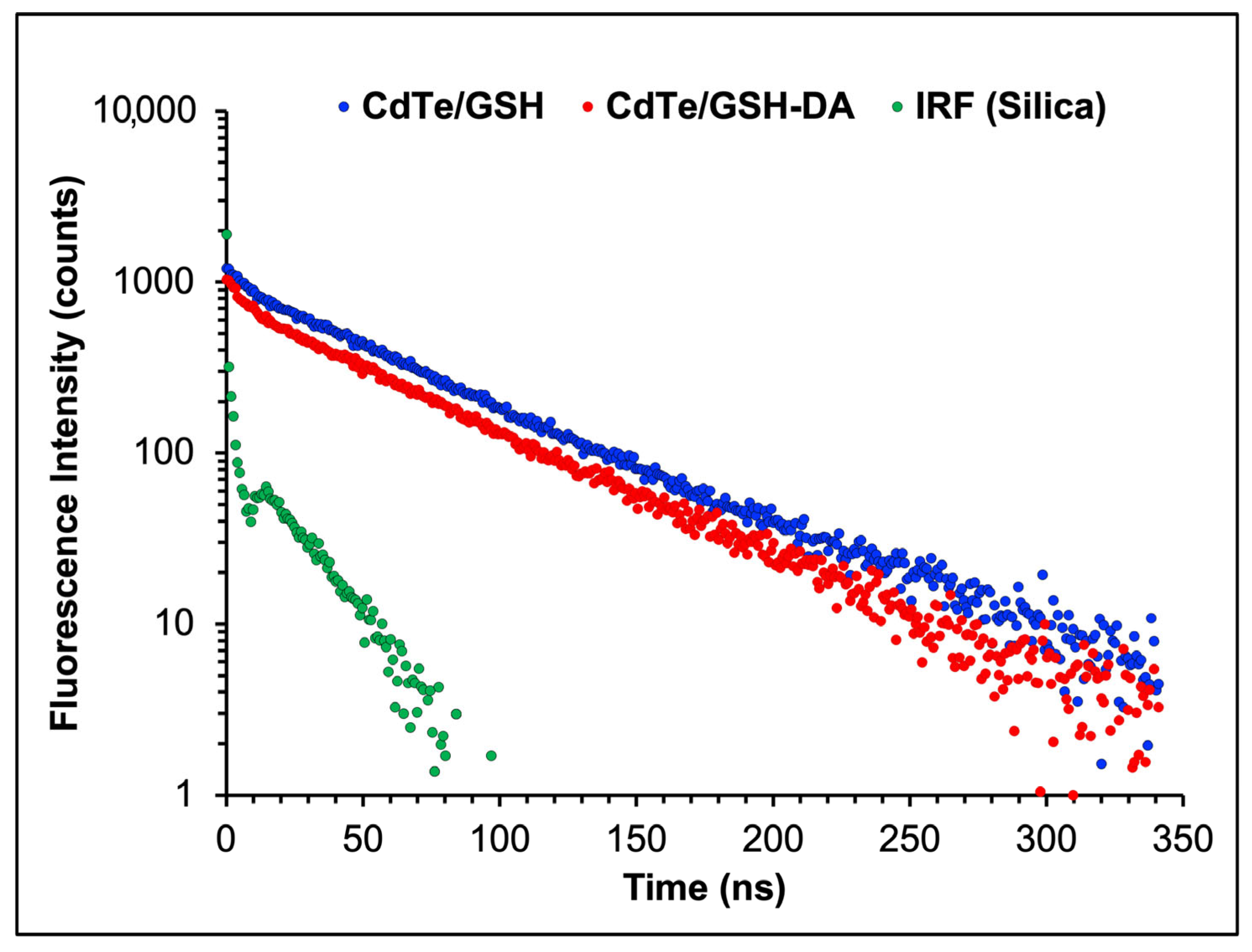
2.5. Response Mechanism of CdTe/GSH-AuNCs/BSA Assembly to DA
2.6. Selectivity of CdTe/GSH-AuNCs/BSA Assembly for the DA Detection
2.7. Comparison with Other DA Sensors
2.8. The Ratiometric Determination of DA in Real Sample
2.9. Screening Tests of Cytocompatibility
3. Materials and Methods
3.1. Reagents and Apparatus
3.2. Preparation of BSA-Coated Au Nanocluster
3.3. Preparation of GSH-Coated CdTe QDs
3.4. FRET Assays Between CdTe/GSH QDs and AuNCs/BSA
Selection of Optimal Condition to FRET Process
3.5. Stability Study
3.6. Lifetime Measurements
3.7. Calibration Curve
3.8. Interference Study
3.9. Detection of DA in Real Samples
3.10. Cell Culture Conditions
3.11. Screening Test for Cytocompatibility
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Govindaraju, S.; Reddy, A.S.; Kim, J.; Yun, K. sensitive detection of epinephrine in human serum via fluorescence enhancement of gold nanoclusters. Appl. Surf. Sci. 2019, 498, 143837. [Google Scholar] [CrossRef]
- Laruelle, M. Schizophrenia: From dopaminergic to glutamatergic interventions. Curr. Opin. Pharmacol. 2014, 14, 97–102. [Google Scholar] [CrossRef]
- Calabresi, P.; Castrioto, A.; Di Filippo, M.; Picconi, B. New experimental and clinical links between the hippocampus and the dopaminergic system in parkinson’s disease. Lancet Neurol. 2013, 12, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Barbato, G.; Fichele, M.; Senatore, I.; Casiello, M.; Muscettola, G. Increased dopaminergic activity in restricting-type anorexia nervosa. Psychiatry Res. 2006, 142, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Frank, G.K.; Bailer, U.F.; Henry, S.E.; Drevets, W.; Meltzer, C.C.; Price, J.C.; Mathis, C.A.; Wagner, A.; Hoge, J.; Ziolko, S.; et al. Increased dopamine D2/D3 receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [11c] Raclopride. Biol. Psychiatry 2005, 58, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Oppolzer, D.; Moreno, I.; da Fonseca, B.; Passarinha, L.; Barroso, M.; Costa, S.; Queiroz, J.A.; Gallardo, E. Analytical approach to determine biogenic amines in urine using microextraction in packed syringe and liquid chromatography coupled to electrochemical detection. Biomed. Chromatogr. BMC 2013, 27, 608–614. [Google Scholar] [CrossRef]
- Kanamori, T.; Isokawa, M.; Funatsu, T.; Tsunoda, M. Development of analytical method for catechol compounds in mouse urine using hydrophilic interaction liquid chromatography with fluorescence detection. J. Chromatogr. B 2015, 985, 142–148. [Google Scholar] [CrossRef]
- Huang, H.-M.; Lin, C.-H. Methanol plug assisted sweeping-micellar electrokinetic chromatography for the determination of dopamine in urine by violet light emitting diode-induced fluorescence detection. J. Chromatogr. B 2005, 816, 113–119. [Google Scholar] [CrossRef]
- Safavi, A.; Maleki, N.; Moradlou, O.; Tajabadi, F. Simultaneous determination of dopamine, ascorbic acid, and uric acid using carbon ionic liquid electrode. Anal. Biochem. 2006, 359, 224–229. [Google Scholar] [CrossRef]
- Goyal, R.N.; Gupta, V.K.; Bachheti, N.; Sharma, R.A. Electrochemical sensor for the determination of dopamine in presence of high concentration of ascorbic acid using a fullerene-C60 coated gold electrode. Electroanalysis 2008, 20, 757–764. [Google Scholar] [CrossRef]
- Yu, D.; Zeng, Y.; Qi, Y.; Zhou, T.; Shi, G. A novel electrochemical sensor for determination of dopamine based on aunps@sio2 core-shell imprinted composite. Biosens. Bioelectron. 2012, 38, 270–277. [Google Scholar] [CrossRef]
- Goyal, R.N.; Gupta, V.K.; Oyama, M.; Bachheti, N. Gold nanoparticles modified indium tin oxide electrode for the simultaneous determination of dopamine and serotonin: Application in pharmaceutical formulations and biological fluids. Talanta 2007, 72, 976–983. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, S.; Huang, J.; Ye, F. Quantum dot-enhanced chemiluminescence detection for simultaneous determination of dopamine and epinephrine by capillary electrophoresis. Talanta 2011, 85, 2650–2654. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Hui, Q.S.; Xu, L.X.; Jiang, J.G.; Sun, Y. Fluorimetric determination of dopamine in pharmaceutical products and urine using ethylene diamine as the fluorigenic reagent. Anal. Chim. Acta 2003, 497, 93–99. [Google Scholar] [CrossRef]
- Abbasi, S.; Valinezhad, R.; Khani, H. A novel kinetic spectrophotometric method for the determination of ultra trace amount of cyanide. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 2010, 77, 112–116. [Google Scholar] [CrossRef]
- Moghadam, M.R.; Dadfarnia, S.; Shabani, A.M.H.; Shahbazikhah, P. Chemometric-assisted kinetic–spectrophotometric method for simultaneous determination of ascorbic acid, uric acid, and dopamine. Anal. Biochem. 2011, 410, 289–295. [Google Scholar] [CrossRef]
- Zetterström, T.; Sharp, T.; Marsden, C.A.; Ungerstedt, U. In vivo measurement of dopamine and its metabolites by intracerebral dialysis: Changes after d-amphetamine. J. Neurochem. 1983, 41, 1769–1773. [Google Scholar] [CrossRef]
- Chen, J.-L.; Yan, X.-P.; Meng, K.; Wang, S.-F. Graphene oxide based photoinduced charge transfer label-free near-infrared fluorescent biosensor for dopamine. Anal. Chem. 2011, 83, 8787–8793. [Google Scholar] [CrossRef]
- Zhou, X.; Ma, P.; Wang, A.; Yu, C.; Qian, T.; Wu, S.; Shen, J. Dopamine fluorescent sensors based on polypyrrole/graphene quantum dots core/shell hybrids. Biosens. Bioelectron. 2015, 64, 404–410. [Google Scholar] [CrossRef]
- Weng, S.; Liang, D.; Qiu, H.; Liu, Z.; Lin, Z.; Zheng, Z.; Liu, A.; Chen, W.; Lin, X. A Unique turn-off fluorescent strategy for sensing dopamine based on formed polydopamine (pDA) using graphene quantum dots (GQDS) as fluorescent probe. Sens. Actuators B. Chem. 2015, 221, 7–14. [Google Scholar] [CrossRef]
- Teng, Y.; Jia, X.; Li, J.; Wang, E. Ratiometric fluorescence detection of tyrosinase activity and dopamine using thiolate-protected gold nanoclusters. Anal. Chem. 2015, 87, 4897–4902. [Google Scholar] [CrossRef]
- Wu, H.-P.; Cheng, T.-L.; Tseng, W.-L. phosphate-modified TiO2 nanoparticles for selective detection of dopamine, levodopa, adrenaline, and catechol based on fluorescence quenching. Langmuir 2007, 23, 7880–7885. [Google Scholar] [CrossRef]
- Mu, Q.; Xu, H.; Li, Y.; Ma, S.; Zhong, X. Adenosine capped qds based fluorescent sensor for detection of dopamine with high selectivity and sensitivity. Analyst 2013, 139, 93–98. [Google Scholar] [CrossRef]
- Kim, J.Y.; Voznyy, O.; Zhitomirsky, D.; Sargent, E.H. 25th Anniversary article: Colloidal quantum dot materials and devices: A quarter-century of advances. Adv. Mater. 2013, 25, 4986–5010. [Google Scholar] [CrossRef]
- Algar, W.R.; Susumu, K.; Delehanty, J.B.; Medintz, I.L. Semiconductor quantum dots in bioanalysis: Crossing the valley of death. Anal. Chem. 2011, 83, 8826–8837. [Google Scholar] [CrossRef] [PubMed]
- Algar, W.R.; Tavares, A.J.; Krull, U.J. Beyond labels: A review of the application of quantum dots as integrated components of assays, bioprobes, and biosensors utilizing optical transduction. Anal. Chim. Acta 2010, 673, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Jin, R. Atomically precise metal nanoclusters: Stable sizes and optical properties. Nanoscale 2015, 7, 1549–1565. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, W. Sub-nanometre sized metal clusters: From synthetic challenges to the unique property discoveries. Chem. Soc. Rev. 2012, 41, 3594–3623. [Google Scholar] [CrossRef]
- Zheng, J.; Nicovich, P.R.; Dickson, R.M. Highly fluorescent noble-metal quantum dots. Annu. Rev. Phys. Chem. 2007, 58, 409–431. [Google Scholar] [CrossRef] [PubMed]
- Goswami, N.; Yao, Q.; Luo, Z.; Li, J.; Chen, T.; Xie, J. Luminescent metal nanoclusters with aggregation-induced emission. J. Phys. Chem. Lett. 2016, 7, 962–975. [Google Scholar] [CrossRef]
- Yu, W.W.; Qu, L.; Guo, W.; Peng, X. Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals. Chem. Mater. 2003, 15, 2854–2860. [Google Scholar] [CrossRef]
- Xie, J.; Zheng, Y.; Ying, J.Y. Protein-directed synthesis of highly fluorescent gold nanoclusters. J. Am. Chem. Soc. 2009, 131, 888–889. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Zhou, X.; Fang, T.; Liu, P.; Liu, P.; Min, X.; Li, X. Interaction of different thiol-capped CdTe quantum dots with bovine serum albumin. J. Lumin. 2012, 132, 1695–1700. [Google Scholar] [CrossRef]
- Ramírez-Herrera, D.E.; Tirado-Guízar, A.; Paraguay-Delgado, F.; Pina-Luis, G. Ratiometric arginine assay based on FRET between CdTe quantum dots and Cresyl violet. Microchim. Acta 2017, 184, 1997–2005. [Google Scholar] [CrossRef]
- Zhang, Q.; Ni, Y.; Kokot, S. Binding interaction of dopamine with bovine serum albumin: A biochemical study. J. Liq. Chromatogr. Relat. Technol. 2012, 45, 85–92. [Google Scholar] [CrossRef]
- Rajamanikandan, R.; Ilanchelian, M. Highly selective and sensitive biosensing of dopamine based on glutathione coated silver nanoclusters enhanced fluorescence. New J. Chem. 2017, 41, 15244–15250. [Google Scholar] [CrossRef]
- Xiangzhao, A.; Qiang, M.; Xingguang, S. Nanosensor for dopamine and glutathione based on the quenching and recovery of the fluorescence of silica-coated quantum dots. Microchim. Acta 2013, 180, 269–277. [Google Scholar] [CrossRef]
- Medintz, I.L.; Stewart, M.H.; Trammell, S.A.; Susumu, K.; Delehanty, J.B.; Mei, B.C.; Melinger, J.S.; Blanco-Canosa, J.B.; Dawson, P.E.; Mattoussi, H. Quantum-dot/dopamine bioconjugates function as redox coupled assemblies for in vitro and intracellular ph sensing. Nat. Mater. 2010, 9, 676–684. [Google Scholar] [CrossRef]
- Ji, X.; Palui, G.; Avellini, T.; Na, H.B.; Yi, C.; Knappenberger, K.L., Jr.; Mattoussi, H. On the pH-dependent quenching of quantum dot photoluminescence by redox active dopamine. J. Am. Chem. Soc. 2012, 134, 6006–6017. [Google Scholar] [CrossRef]
- Tao, Y.; Lin, Y.; Ren, J.; Qu, X. A dual fluorometric and colorimetric sensor for dopamine based on bsa-stabilized Au nanoclusters. Biosens. Bioelectron. 2013, 42, 41–46. [Google Scholar] [CrossRef]
- Ma, N.; Yang, J.; Stewart, K.M.; Kelley, S.O. DNA-Passivated CdS nanocrystals: luminescence, bioimaging, and toxicity profiles. Langmuir 2007, 23, 12783–12787. [Google Scholar] [CrossRef]
- Baruah, U.; Gogoi, N.; Konwar, A.; Jyoti Deka, M.; Chowdhury, D.; Majumdar, G. Carbon dot based sensing of dopamine and ascorbic acid. J. Nanoparticles 2014, 2014, 178518. [Google Scholar] [CrossRef]
- Shamsipur, M.; Shanehasz, M.; Khajeh, K.; Mollania, N.; Kazemi, S.H. A Novel quantum dot–laccase hybrid nanobiosensor for low level determination of dopamine. Analyst 2012, 137, 5553–5559. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tian, F.; Wen, S.; Xu, H.; Zhang, L.; Zeng, J. Selective determination of dopamine in pharmaceuticals and human urine using carbon quantum dots as a fluorescent probe. Processes 2021, 9, 170. [Google Scholar] [CrossRef]
- Chatterjee, M.; Nath, P.; Kadian, S.; Kumar, A.; Kumar, V.; Roy, P.; Manik, G.; Satapathi, S. Highly sensitive and selective detection of dopamine with boron and sulfur co-doped graphene quantum dots. Sci. Rep. 2022, 12, 13016. [Google Scholar] [CrossRef]
- Naik, V.; Zantye, P.; Gunjal, D.; Gore, A.; Anbhule, P.; Kowshik, M.; Bhosale, S.V.; Kolekar, G. Nitrogen-doped carbon dots via hydrothermal synthesis: Naked eye fluorescent sensor for dopamine and used for multicolor cell imaging. ACS Appl. Bio. Mater. 2019, 2, 2096–2105. [Google Scholar] [CrossRef]
- Kumar, A.; Kumari, A.; Mukherjee, P.; Saikia, T.; Pal, K.; Sahu, S.K. A Design of fluorescence-based sensor for the detection of dopamine via fret as well as live cell imaging. Microchem. J. 2020, 159, 105590. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, H.; Yang, Y.; Zhu, F.; Pu, Y.; You, X.; Liao, X. Efficient fluorescence resonance energy transfer-based ratiometric fluorescent probe for detection of dopamine using a dual-emission carbon dot-gold nanocluster nanohybrid. J. Photochem. Photobiol. Chem. 2021, 411, 113195. [Google Scholar] [CrossRef]
- Vikraman, A.E.; Jose, A.R.; Jacob, M.; Kumar, K.G. Thioglycolic acid capped cds quantum dots as a fluorescent probe for the nanomolar determination of dopamine. Anal. Methods 2015, 7, 6791–6798. [Google Scholar] [CrossRef]
- Gekle, M.; Drumm, K.; Mildenberger, S.; Freudinger, R.; Gaßner, B.; Silbernagl, S. Inhibition of Na+−H+ exchange impairs receptor-mediated albumin endocytosis in renal proximal tubule-derived epithelial cells from opossum. J. Physiol. 1999, 520, 709–721. [Google Scholar] [CrossRef]
- Lee, E.M.; Pollock, C.A.; Drumm, K.; Barden, J.A.; Poronnik, P. Effects of pathophysiological concentrations of albumin on NHE3 activity and cell proliferation in primary cultures of human proximal tubule cells. Am. J. Physiol. Renal. Physiol. 2003, 285, F748–F757. [Google Scholar] [CrossRef][Green Version]
- Traverso, N.; Ricciarelli, R.; Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Role of glutathione in cancer progression and chemoresistance. Oxid. Med. Cell. Longev. 2013, 2013, 972913. [Google Scholar] [CrossRef]
- De Souza, A.O. Overview of nanomaterials and cellular interactions. Biointerface Res. Appl. Chem. 2022, 13, 367. [Google Scholar] [CrossRef]
- Thomas, N.G.; Varghese, N.; Kalarikkal, N.; Thomas, S.; Sreedharan, M.; George, S.; John, S.; Varghese, M.G.; George, V.T. Toxicity evaluation and biocompatibility of nanostructured biomaterials. In Cytotoxicity—Understanding Cellular Damage and Response; Sukumaran, A., Mansour, M.A., Eds.; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Raja, I.S.; Kim, C.; Kang, M.S.; Joung, Y.K.; Lee, J.H.; Han, D.-W. Studies on cytocompatibility of human dermal fibroblasts on carbon nanofiber nanoparticle-containing bioprinted constructs. Discov. Nano 2024, 19, 149. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Herrera, D.E.; Reyes-Cruzaley, A.P.; Domínguez, G.; Paraguay-Delgado, F.; Tirado-Guízar, A.; Pina-Luis, G. CdTe quantum dots modified with cysteamine: A new efficient nanosensor for the determination of folic acid. Sensors 2019, 19, 4548. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization (ISO). Tests for in Vitro Cytotoxicity. In Biological Evaluation of Medical Devices, 3rd ed.; ISO: Geneva, Switzerland, 2009; Available online: https://www.iso.org/standard/36406.html (accessed on 30 August 2025).
- Topete, A.; Melgar, D.; Alatorre-Meda, M.; Iglesias, P.; Argibay, B.; Vidawati, S.; Barbosa, S.; Costoya, J.A.; Taboada, P.; Mosquera, V. Nir-light active hybrid nanoparticles for combined imaging and bimodal therapy of cancerous cells. J. Mater. Chem. B 2014, 2, 6967–6977. [Google Scholar] [CrossRef]
- Barbosa, S.; Topete, A.; Alatorre-Meda, M.; Villar-Alvarez, E.M.; Pardo, A.; Alvarez-Lorenzo, C.; Concheiro, A.; Taboada, P.; Mosquera, V. Targeted combinatorial therapy using gold nanostars as theranostic platforms. J. Phys. Chem. C 2014, 118, 26313–26323. [Google Scholar] [CrossRef]
- Topete, A.; Alatorre-Meda, M.; Villar-Alvarez, E.M.; Carregal-Romero, S.; Barbosa, S.; Parak, W.J.; Taboada, P.; Mosquera, V. Polymeric-gold nanohybrids for combined imaging and cancer therapy. Adv. Healthc. Mater. 2014, 3, 1309–1325. [Google Scholar] [CrossRef]
- Rodríguez-Velázquez, E.; Silva, M.; Taboada, P.; Mano, J.F.; Suárez-Quintanilla, D.; Alatorre-Meda, M. Enhanced cell affinity of chitosan membranes mediated by superficial cross-linking: A straightforward method attainable by standard laboratory procedures. Biomacromolecules 2014, 15, 291–301. [Google Scholar] [CrossRef]
- Sotolongo-García, R.; Rodríguez-Velázquez, E.; Alatorre-Meda, M.; Oropeza-Guzmán, M.T.; Tirado-Guízar, A.; Pina-Luis, G. Optimizing the efficiency of a cytocompatible carbon-dots-based FRET platform and its application as a riboflavin sensor in beverages. Nanomaterials 2021, 11, 1981. [Google Scholar] [CrossRef]
- Zumbardo-Bacelis, G.A.; Peponi, L.; Vargas-Coronado, R.F.; Rodríguez-Velázquez, E.; Alatorre-Meda, M.; Chevallier, P.; Copes, F.; Mantovani, D.; Abraham, G.A.; Cauich-Rodríguez, J.V. A comparison of three-layer and single-layer small vascular grafts manufactured via the roto-evaporation method. Polymers 2024, 16, 1314. [Google Scholar] [CrossRef]
- Karakaş, D.; Ari, F.; Ulukaya, E. The MTT viability assay yields strikingly false-positive viabilities although the cells are killed by some plant extracts. Turk. J. Biol. Turk. Biyol. Derg. 2017, 41, 919–925. [Google Scholar] [CrossRef]
- Huang, K.T.; Chen, Y.H.; Walker, A.M. Inaccuracies in MTS assays: Major distorting effects of medium, serum albumin, and fatty acids. BioTechniques 2004, 37, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Eastman, A. Improving anticancer drug development begins with cell culture: Misinformation perpetrated by the misuse of cytotoxicity assays. Oncotarget 2017, 8, 8854–8866. [Google Scholar] [CrossRef] [PubMed]
- Funk, D.; Schrenk, H.-H.; Frei, E. Serum albumin leads to false-positive results in the XTT and the MTT assay. BioTechniques 2007, 43, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Granada, A.E.; Jiménez, A.; Stewart-Ornstein, J.; Blüthgen, N.; Reber, S.; Jambhekar, A.; Lahav, G. The effects of proliferation status and cell cycle phase on the responses of single cells to chemotherapy. Mol. Biol. Cell 2020, 31, 845–857. [Google Scholar] [CrossRef]



| Donor (D) | Acceptor (A) | J (cm3Lmol−1) | E | R0 (nm) | r (nm) | K (M−1) |
|---|---|---|---|---|---|---|
| CdTe/GSH | AuNCs/BSA | 5.9 × 10−13 | 0.78 | 5.9 | 4.8 | 8.4 × 103 |
| Sample | τ1 (ns) | A1 (%) | τ2 (ns) | A2 (%) | χ2 | ⟨τ⟩ (ns) |
|---|---|---|---|---|---|---|
| CdTe/GSH | 11.39 ± 0.63 | 17.8 | 50.56 ± 0.81 | 82.2 | 0.967 | 38.4 |
| QDs/GSH-DA | 9.52 ± 0.53 | 18.7 | 46.02 ± 0.74 | 81.3 | 0.967 | 33.9 |
| Sensor | Lineal Range | Detection Limit | Reference |
|---|---|---|---|
| NCs Au/BSA | 1–10 µM | 10 nM | [40] |
| CdSe/ZnS-DNA | 2 × 10−5 –1 × 10−4 M | 29.3 nM | [41] |
| Carbon Dots (CDs) | 0.1 × 10−3–0.5 × 10−3 M | 33 µM | [42] |
| CdTe/TGA | 0.1–0.3 × 10−3 M | 6 × 10−3 M | [43] |
| CDs | 0.3–100 µM | 93 nM | [44] |
| B,S co-doped graphene QDs | 0–340 μM | 3.6 μM | [45] |
| Silica coated QDs | 0.0005–0.1 mM | 2.41 × 10−4 mM | [37] |
| N-doped CDs | 2–20 μg/mL | 1.97 μg/mL | [46] |
| Carbon dots FRET | 1–50 μM | 20 nM | [47] |
| CDs-AuNCs FRET | 5–180 nM | 2.9 nM | [48] |
| CdS/thioglycolic acid | 3.94 × 10−7–4.67 × 10−8 M | 2.55 × 10−9 M | [49] |
| CdTe/GSH-NCsAu/BSA FRET | 0–250 µM | 6.9 nM | (this work) |
| Sample | Found (µM) | Added (µM) | Found (µM) | Recovery (%) |
|---|---|---|---|---|
| Urine | 1.8 | 3.3 | 5.3 | 103.5 |
| 9.9 | 12.2 | 104.2 | ||
| 32.3 | 34.9 | 102.3 | ||
| 62.5 | 63.7 | 99.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavón-Hernández, A.I.; Ramírez-Herrera, D.; Rodríguez-Velázquez, E.; Alatorre-Meda, M.; Ramos-Heredia, M.; Tirado-Guízar, A.; Pina-Luis, G. Cytocompatible FRET Assembly of CdTe@GSH Quantum Dots and Au@BSA Nanoclusters: A Novel Ratiometric Strategy for Dopamine Detection. Molecules 2025, 30, 4169. https://doi.org/10.3390/molecules30214169
Pavón-Hernández AI, Ramírez-Herrera D, Rodríguez-Velázquez E, Alatorre-Meda M, Ramos-Heredia M, Tirado-Guízar A, Pina-Luis G. Cytocompatible FRET Assembly of CdTe@GSH Quantum Dots and Au@BSA Nanoclusters: A Novel Ratiometric Strategy for Dopamine Detection. Molecules. 2025; 30(21):4169. https://doi.org/10.3390/molecules30214169
Chicago/Turabian StylePavón-Hernández, Arturo Iván, Doris Ramírez-Herrera, Eustolia Rodríguez-Velázquez, Manuel Alatorre-Meda, Miguel Ramos-Heredia, Antonio Tirado-Guízar, and Georgina Pina-Luis. 2025. "Cytocompatible FRET Assembly of CdTe@GSH Quantum Dots and Au@BSA Nanoclusters: A Novel Ratiometric Strategy for Dopamine Detection" Molecules 30, no. 21: 4169. https://doi.org/10.3390/molecules30214169
APA StylePavón-Hernández, A. I., Ramírez-Herrera, D., Rodríguez-Velázquez, E., Alatorre-Meda, M., Ramos-Heredia, M., Tirado-Guízar, A., & Pina-Luis, G. (2025). Cytocompatible FRET Assembly of CdTe@GSH Quantum Dots and Au@BSA Nanoclusters: A Novel Ratiometric Strategy for Dopamine Detection. Molecules, 30(21), 4169. https://doi.org/10.3390/molecules30214169







