Malted Soybeans as a Substrate for Plant-Based Beverages—Analysis of Nutritional Properties, Antioxidant Activity, and Volatiles
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Soy Malting Procedure
3.2.2. Soy Beverage Production
3.2.3. Chemical Composition Testing Methods
Essential Nutrient Content
Total Polyphenol Content and Antioxidant Activity
Chromatographic Analysis of Volatiles
3.2.4. Organoleptic Evaluation
3.2.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PM | Pilsener-type malt |
| CM | Caramel-type malt |
| SAba | Soybean seeds of the Abaca variety |
| SAbe | Soybean seeds of the Abelina variety |
| SAu | Soybean seeds of the Aurelina variety |
| PMAba | Pilsener-type malt of the Abaca variety |
| PMAbe | Pilsener-type malt of the Abelina variety |
| PMAu | Pilsener-type malt of the Aurelina variety |
| CMAba | Caramel-type malt of the Abaca variety |
| CMAbe | Caramel-type malt of the Abelina variety |
| CMAu | Caramel-type malt of the Aurelina variety |
References
- Sharma, N.; Yeasmen, N.; Dube, L.; Orsat, V. A review on current scenario and key challenges of plant-based functional beverages. Food Biosci. 2024, 60, 104320. [Google Scholar] [CrossRef]
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-based milk alternatives an emerging segment of functional beverages: A review. J. Food Sci. Technol. 2016, 53, 3408–3423. [Google Scholar] [CrossRef]
- Riaz, M.N. Soy Base Extract: Soymilk and Dairy Alternatives. In Soy Applications in Food; CRC Press: Boca Raton, FL, USA, 2005; pp. 111–134. [Google Scholar]
- Briggs, D.E. Malts and Malting, 1st ed.; Blackie Academic & Professional: London, UK, 1998. [Google Scholar]
- Tricase, C.; Amicarelli, V.; Lamonaca, E.; Rana, R.L. Economic analysis of the barley market and related uses. Grasses Food Feed 2018, 10, 25–46. [Google Scholar]
- Schwarz, P.; Li, Y. Malting and brewing uses of barley. In Barley: Production, Improvement, and Uses, 1st ed.; Ullrich, S.E., Ed.; Blackwell Publishing Ltd.: Chichester, UK, 2011; pp. 478–521. [Google Scholar]
- Mäkinen, O.E.; Arendt, E.K. Oat malt as a baking ingredient–A comparative study of the impact of oat, barley and wheat malts on bread and dough properties. J. Cereal Sci. 2012, 56, 747–753. [Google Scholar] [CrossRef]
- Mäkinen, O.E.; Arendt, E.K. Nonbrewing applications of malted cereals, pseudocereals, and legumes: A review. J. Am. Soc. Brew. Chem. 2015, 73, 223–227. [Google Scholar] [CrossRef]
- Błażewicz, J.; Kawa-Rygielska, J.; Gasior, J. Słody specjalne z nasion roślin strączkowych. Przemysł Ferment. I Owocowo Warzywny 2019, 11. [Google Scholar] [CrossRef]
- Gasiński, A.; Kawa-Rygielska, J. Malting—A method for modifying volatile composition of black, brown and green lentil seeds. PLoS ONE 2023, 18, e0290616. [Google Scholar]
- Czubaszek, A.; Gertchen, M.; Gasiński, A.; Miedzianka, J.; Kawa-Rygielska, J. Nutritional Quality of Rye Bread with the Addition of Selected Malts from Beans. Molecules 2025, 30, 1006. [Google Scholar] [CrossRef]
- Gasiński, A.; Noguera-Artiaga, L.; Kawa-Rygielska, J. Influence of Malted Chickpea on the Composition of Volatiles in Hummus. Molecules 2025, 30, 1231. [Google Scholar] [CrossRef] [PubMed]
- Trugo, L.C.; Muzquiz, M.; Pedrosa, M.M.; Ayet, G.; Burbano, C.; Cuadrado, C.; Cavieres, E. Influence of malting on selected components of soya bean, black bean, chickpea and barley. Food Chem. 1999, 65, 85–90. [Google Scholar] [CrossRef]
- Ma, M.; Zhang, H.; Xie, Y.; Yang, M.; Tang, J.; Wang, P.; Yang, R.; Gu, Z. Response of nutritional and functional composition, anti-nutritional factors and antioxidant activity in germinated soybean under UV-B radiation. LWT 2020, 118, 108709. [Google Scholar]
- Gasiński, A.; Mikulski, D.; Kłosowski, G.; Kawa-Rygielska, J. Influence of malting procedure on the isoflavonoid content of soybeans. Sci. Rep. 2024, 14, 7184. [Google Scholar] [CrossRef] [PubMed]
- Yahya, H.; Linforth, R.S.; Cook, D.J. Flavour generation during commercial barley and malt roasting operations: A time course study. Food Chem. 2014, 145, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Gąsior, J.; Kawa-Rygielska, J.; Kucharska, A.Z. Carbohydrates Profile, Polyphenols Content and Antioxidative Properties of Beer Worts Produced with Different Dark Malts Varieties or Roasted Barley Grains. Molecules 2020, 25, 3882. [Google Scholar] [CrossRef]
- Obatolu, V.A. Nutrient and sensory qualities of extruded malted or unmalted millet/soybean mixture. Food Chem. 2002, 76, 129–133. [Google Scholar] [CrossRef]
- Mezgebo, K.; Belachew, T.; Satheesh, N. Optimization of red teff flour, malted soybean flour, and papaya fruit powder blending ratios for better nutritional quality and sensory acceptability of porridge. Food Sci. Nutr. 2018, 6, 891–903. [Google Scholar] [CrossRef]
- Ayo, J.A.; Ayo, V.A.; Popoola, C.; Omosebi, M.; Joseph, L. Production and evaluation of malted soybean-acha composite flour bread and biscuit. Afr. J. Food Sci. Technol. 2014, 5, 21–28. [Google Scholar]
- Uvere, P.O.; Amazikwu, U.C. Processing and evaluation of instant kunun zaki from millet-cowpea malt and millet-soybean malt. Afr. J. Food Sci. 2011, 5, 761–768. [Google Scholar]
- Gasiński, A.; Kawa-Rygielska, J. Mashing quality and nutritional content of lentil and bean malts. LWT 2022, 169, 113927. [Google Scholar] [CrossRef]
- de Paiva Gonçalves, J.; Gasparini, K.; de Toledo Picoli, E.A.; Costa, M.D.B.L.; Araujo, W.L.; Zsögön, A.; Ribeiro, D.M. Metabolic control of seed germination in legumes. J. Plant Physiol. 2024, 295, 154206. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, M.; Wang, H.; Devahastin, S. Effect of carbon dots in combination with aqueous chitosan solution on shelf life and stability of soy milk. Int. J. Food Microbiol. 2020, 326, 108650. [Google Scholar] [CrossRef]
- Ikya, J.K.; Gernah, I.; Ojobo, E.; Oni, K. Effect of cooking temperature on some quality characteristics of soy milk. Adv. J. Food Sci. Technol. 2013, 5, 543–546. [Google Scholar] [CrossRef]
- Scholz-Ahrens, K.E.; Ahrens, F.; Barth, C.A. Nutritional and health attributes of milk and milk imitations. Eur. J. Nutr. 2020, 59, 19–34. [Google Scholar] [PubMed]
- Bricarello, L.P.; Kasinski, N.; Bertolami, M.C.; Faludi, A.; Pinto, L.A.; Relvas, W.G.M.; Izar, M.C.O.; Ihara, S.S.M.; Tufik, S.; Fonseca, F.A.H. Comparison between the effects of soy milk and non-fat cow milk on lipid profile and lipid peroxidation in patients with primary hypercholesterolemia. Nutrition 2004, 20, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Walther, B.; Guggisberg, D.; Badertscher, R.; Egger, L.; Portmann, R.; Dubois, S.; Haldimann, M.; Kopf-Bolanz, K.; Rhyn, P.; Zoller, O.; et al. Comparison of nutritional composition between plant-based drinks and cow’s milk. Front. Nutr. 2022, 9, 2645. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Rani, A.; Solanki, S.; Hussain, S.M. Influence of growing environment on the biochemical composition and physical characteristics of soybean seed. J. Food Compos. Anal. 2006, 19, 188–195. [Google Scholar] [CrossRef]
- Bøhn, T.; Cuhra, M.; Traavik, T.; Sanden, M.; Fagan, J.; Primicerio, R. Compositional differences in soybeans on the market: Glyphosate accumulates in Roundup Ready GM soybeans. Food Chem. 2014, 153, 207–215. [Google Scholar] [CrossRef]
- Han, H.; Choi, J.K.; Park, J.; Im, H.C.; Han, J.H.; Huh, M.H.; Lee, Y.B. Recent innovations in processing technologies for improvement of nutritional quality of soymilk. CyTA-J. Food 2021, 19, 287–303. [Google Scholar]
- Naresh, S.; Ong, M.K.; Thiagarajah, K.; Muttiah, N.B.S.J.; Kunasundari, B.; Lye, H.S. Engineered soybean-based beverages and their impact on human health. In Non-Alcoholic Beverages; Woodhead Publishing: Montgomery, IL, USA, 2019; pp. 329–361. [Google Scholar]
- Hajirostamloo, B.; Mahastie, P. Comparison of soymilk and cow milk nutritional parameter. Res. J. Biol. Sci. 2008, 3, 1324–1326. [Google Scholar]
- Chen, K.I.; Erh, M.H.; Su, N.W.; Liu, W.H.; Chou, C.C.; Cheng, K.C. Soyfoods and soybean products: From traditional use to modern applications. Appl. Microbiol. Biotechnol. 2012, 96, 9–22. [Google Scholar] [CrossRef]
- García, M.; Marina, M.; Laborda, F.; Torre, M. Chemical characterization of commercial soybean products. Food Chem. 1998, 62, 325–331. [Google Scholar] [CrossRef]
- Kudełka, W.; Kowalska, M.; Popis, M. Quality of soybean products in terms of essential amino acids composition. Molecules 2021, 26, 5071. [Google Scholar] [CrossRef]
- Krishnan, H.B.; Jez, J.M. The promise and limits for enhancing sulfur-containing amino acid content of soybean seed. Plant Sci. 2018, 272, 14–21. [Google Scholar] [CrossRef]
- De, B.; Shrivastav, A.; Das, T.; Goswami, T.K. Physicochemical and nutritional assessment of soy milk and soymilk products and comparative evaluation of their effects on blood gluco-lipid profile. Appl. Food Res. 2022, 2, 100146. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.B. Legumes as a source of natural antioxidants. Eur. J. Lipid Sci. Technol. 2008, 110, 865–878. [Google Scholar] [CrossRef]
- López-Amorós, M.L.; Hernández, T.; Estrella, I. Effect of germination on legume phenolic compounds and their antioxidant activity. J. Food Compos. Anal. 2006, 19, 277–283. [Google Scholar] [CrossRef]
- Huang, X.; Cai, W.; Xu, B. Kinetic changes of nutrients and antioxidant capacities of germinated soybean (Glycine max L.) and mung bean (Vigna radiata L.) with germination time. Food Chem. 2014, 143, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Huang, H. Characterisation and comparison of phenols, flavonoids and isoflavones of soymilk and their correlations with antioxidant activity. Int. J. Food Sci. Technol. 2014, 49, 2290–2298. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, S.K. Trypsin inhibitor activity, phenolic content and antioxidant capacity of soymilk as affected by grinding temperatures, heating methods and soybean varieties. LWT 2022, 153, 112424. [Google Scholar] [CrossRef]
- Niki, E. Assessment of antioxidant capacity in vitro and in vivo. Free Radic. Biol. Med. 2010, 49, 503–515. [Google Scholar] [CrossRef]
- Nilsson, J.; Pillai, D.; Önning, G.; Persson, C.; Nilsson, Å.; Åkesson, B. Comparison of the 2,2′-azinobis-3-ethylbenzotiazo-line-6-sulfonic acid (ABTS) and ferric reducing anti-oxidant power (FRAP) methods to assess the total antioxidant capacity in extracts of fruit and vegetables. Mol. Nutr. Food Res. 2005, 49, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Fröhlich, K.; Böhm, V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem. 2011, 129, 139–148. [Google Scholar] [CrossRef]
- Xu, B.; Chang, S.K. Characterization of phenolic substances and antioxidant properties of food soybeans grown in the North Dakota−Minnesota region. J. Agric. Food Chem. 2008, 56, 9102–9113. [Google Scholar] [CrossRef]
- Carvalho, D.O.; Correia, E.; Lopes, L.; Guido, L.F. Further insights into the role of melanoidins on the antioxidant potential of barley malt. Food Chem. 2014, 160, 127–133. [Google Scholar] [CrossRef]
- Xia, N.; Yan, W.; Wang, X.; Shao, Y.; Yang, M.; Wang, Z.; Zhan, Y.; Teng, W.; Han, Y.; Shi, Y. Genetic dissection of hexanol content in soybean seed through genome-wide association analysis. J. Integr. Agric. 2019, 18, 1222–1229. [Google Scholar] [CrossRef]
- Boué, S.M.; Shih, B.Y.; Carter-Wientjes, C.H.; Cleveland, T.E. Identification of volatile compounds in soybean at various developmental stages using solid phase microextraction. J. Agric. Food Chem. 2003, 51, 4873–4876. [Google Scholar] [CrossRef]
- Achouri, A.; Boye, J.I.; Zamani, Y. Identification of volatile compounds in soymilk using solid-phase microextraction-gas chromatography. Food Chem. 2006, 99, 759–766. [Google Scholar] [CrossRef]
- Nacchio, B.L.; Avila Hael, N.; Medina, R.B.; Garro, M.S. Aroma compounds and consumer acceptability of soybean paste fermented by lactobacilli. J. Food Sci. Technol. 2022, 59, 1948–1957. [Google Scholar] [CrossRef]
- Guo, L.; Huang, L.; Cheng, X.; Gao, Y.; Zhang, X.; Yuan, X.; Xue, C.; Chen, X. Volatile Flavor Profile and Sensory Properties of Vegetable Soybean. Molecules 2022, 27, 939. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, J.; He, L.; Xing, R.; Yu, N.; Chen, Y. New insight into the evolution of volatile profiles in four vegetable oils with different saturations during thermal processing by integrated volatolomics and lipidomics analysis. Food Chem. 2023, 403, 134342. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Takemoto, H.; Koeduka, T.; Ohnishi, T. 1-Octen-3-ol is formed from its glycoside during processing of soybean [Glycine max (L.) Merr.] seeds. J. Agric. Food Chem. 2018, 66, 7409–7416. [Google Scholar] [CrossRef]
- Xiao, L.; Li, C.; Chai, D.; Chen, Y.; Wang, Z.; Xu, X.; Wang, Y.; Geng, Y.; Dong, L. Volatile compound profiling from soybean oil in the heating process. Food Sci. Nutr. 2020, 8, 1139–1149. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Hua, Y.; Chen, Y.; Kong, X.; Zhang, C. Effects of water absorption of soybean seed on the quality of soymilk and the release of flavor compounds. RSC Adv. 2019, 9, 2906–2918. [Google Scholar] [CrossRef]
- Kern, S.; Granier, T.; Dkhil, H.; Haupt, T.; Ellis, G.; Natsch, A. Stability of limonene and monitoring of a hydroperoxide in fragranced products. Flavour Fragr. J. 2014, 29, 277–286. [Google Scholar] [CrossRef]
- ISO 20483:2013; Cereals and Pulses—Determination of the Nitrogen Content and Calculation of the Crude Protein Content—Kjeldahl Method. International Organization for Standardization: Geneva, Switzerland, 2013.
- Association of Official Analytical Chemists. AOAC Official Methods of Analysis, 18th ed.; AOAC: Arlington, TX, USA, 2012. [Google Scholar]
- AACC. AACC Approved Methods of the AACC, 10th ed.; American Association of Cereal Chemists: St. Paul, MN, USA, 2000. [Google Scholar]
- Miedzianka, J.; Walkowiak, K.; Zielińska-Dawidziak, M.; Zambrowicz, A.; Wolny, S.; Kita, A. The functional and physicochemical properties of rice protein concentrate subjected to acetylation. Molecules 2023, 28, 770. [Google Scholar] [CrossRef]
- Gao, X.; Ohlander, M.; Jeppsson, N.; Björk, L.; Trajkovski, V. Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophae rhamnoides L.) during maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.C.; Chen, H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
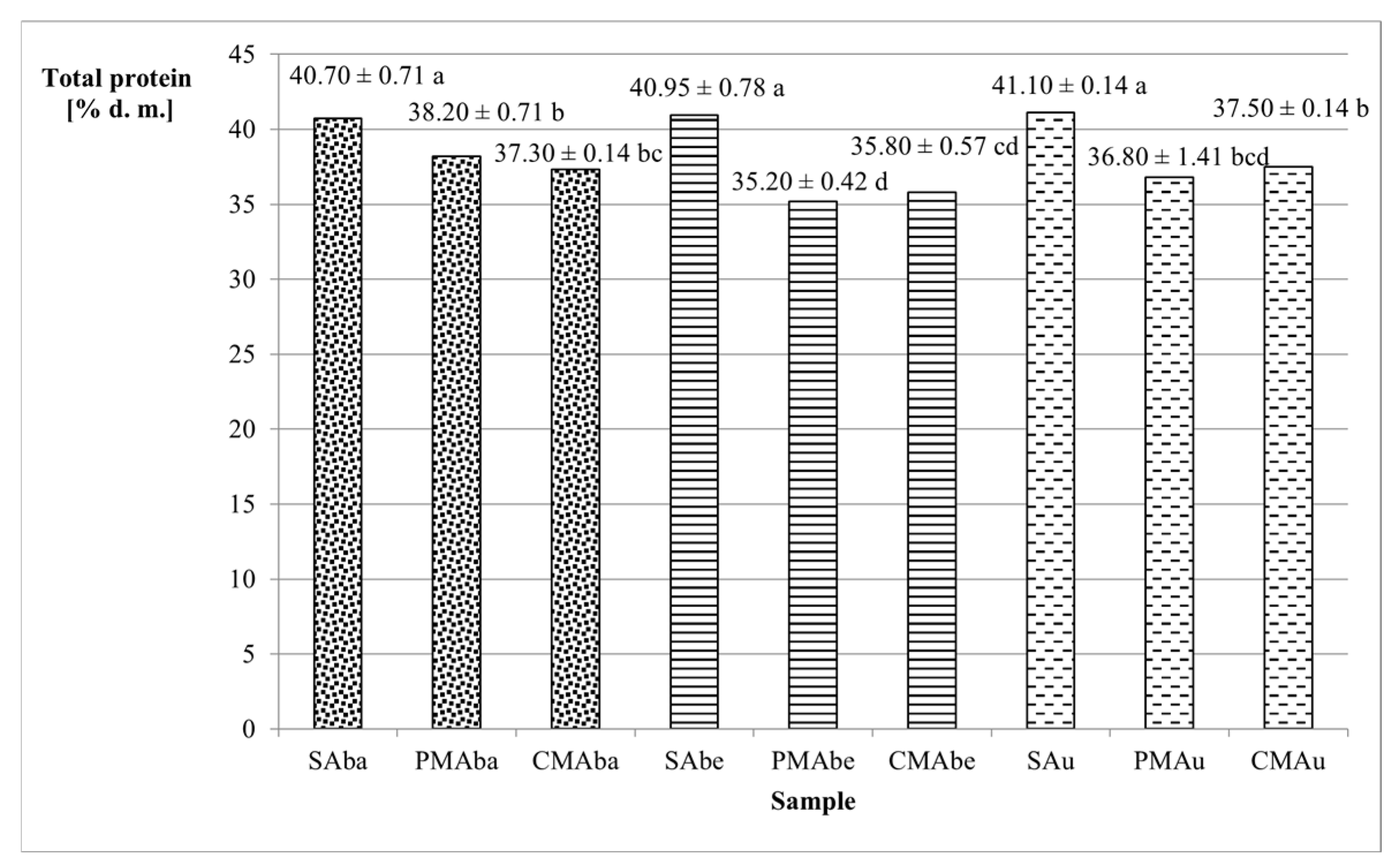
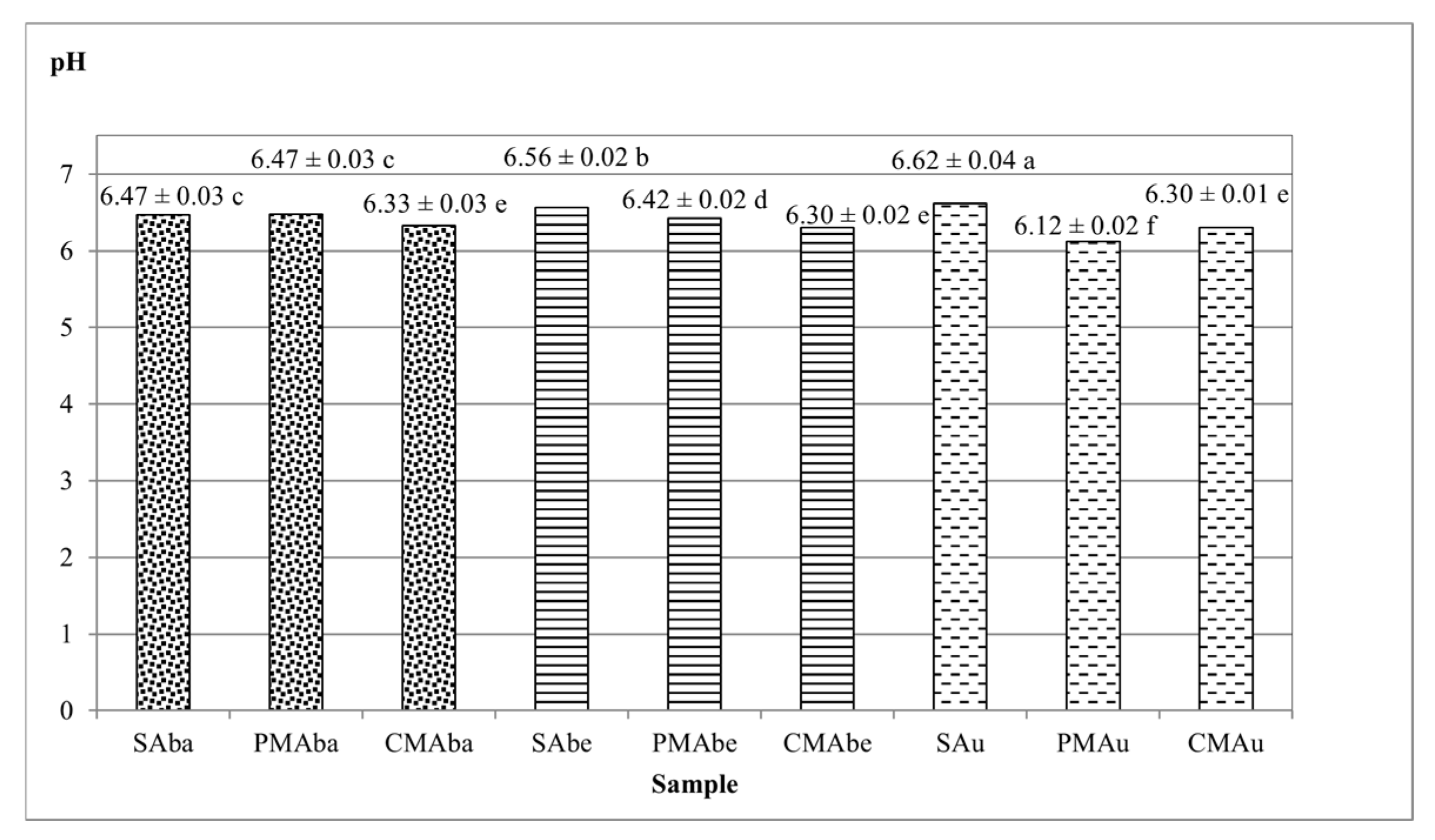
| Sample | Total Protein | Total Dietary Fiber | Lipid | Ash |
|---|---|---|---|---|
| [g/100 g] | [g/100 g] | [g/100 g] | [g/100 g] | |
| SAba | 2. 48 ± 0.06 b,1 | 1.45 ± 0.10 ab | 2.31 ± 0.18 c | 0.30 ± 0.00 b |
| PMAba | 2.53 ± 0.02 ab | 1.12 ± 0.01 b | 2.30 ± 0.13 c | 0.24 ± 0.00 d |
| CMAba | 2.55 ± 0.01 a | 1.17 ± 0.08 b | 2.72 ± 0.09 b | 0.22 ± 0.01 e |
| SAbe | 2.34 ± 0.01 d | 1.65 ± 0.35 a | 2.39 ± 0.10 c | 0.31 ± 0.00 b |
| PMAbe | 2. 40 ± 0.01 c | 1.27 ± 0.00 b | 3.12 ± 0.22 a | 0.23 ± 0.00 d |
| CMAbe | 2.41 ± 0.02 c | 1.19 ± 0.06 b | 2.92 ± 0.07 ab | 0.24 ± 0.00 d |
| SAu | 2.17 ± 0.01 f | 1.62 ± 0.13 a | 2.20 ± 0.05 c | 0.33 ± 0.00 a |
| PMAu | 2.24 ± 0.01 e | 1.12 ± 0.09 b | 3.04 ± 0.09 a | 0.26 ± 0.00 c |
| CMAu | 2.37 ± 0.00 cd | 1.70 ± 0.11 a | 1.88 ± 0.13 d | 0.27 ± 0.00 c |
| Sample | SAba [mg/g] | PMAba [mg/g] | CMAba [mg/g] | SAbe [mg/g] | PMAbe [mg/g] | CMAbe [mg/g] | SAu [mg/g] | PMAu [mg/g] | CMAu [mg/g] | |
|---|---|---|---|---|---|---|---|---|---|---|
| Amino Acid | ||||||||||
| Asp | 44.46 ± 0.36 g,1 | 59.43 ± 0.26 ab | 60.86 ± 1.39 a | 46.79 ± 0.18 f | 57.54 ± 0.21 bc | 53.36 ± 2.18 de | 42.98 ± 0.04 g | 55.43 ± 0.75 cd | 52.80 ± 1.35 e | |
| Thr * | 14.72 ± 0.02 c | 16.20 ± 0.13 b | 16.98 ± 0.27 a | 15.94 ± 0.03 b | 16.08 ± 0.13 b | 14.97 ± 0.66 c | 14.53 ± 0.13 c | 15.22 ± 0.26 c | 15.10 ± 0.38 c | |
| Ser | 18.36 ± 0.07 d | 21.53 ± 0.13 b | 22.46 ± 0.37 a | 20.17 ± 0.10 c | 21.34 ± 0.02 b | 19.51 ± 0.95 c | 18.36 ± 0.18 d | 19.97 ± 0.27 c | 19.80 ± 0.44 c | |
| Glu | 85.06 ± 1.87 cd | 91.48 ± 2.17 ab | 95.06 ± 2.86 a | 81.41 ± 0.43 d | 89.26 ± 1.50 bc | 83.08 ± 2.88 d | 84.97 ± 0.09 cd | 83.46 ± 0.27 d | 94.57 ± 2.42 a | |
| Pro | 14.10 ± 0.62 b | 13.73 ± 0.53 b | 13.99 ± 1.38 b | 13.79 ± 0.67 b | 14.06 ± 0.29 b | 15.21 ± 0.45 b | 14.43 ± 1.00 b | 14.62 ± 0.90 b | 20.85 ± 0.13 a | |
| Gly | 16.23 ± 0.19 c | 17.92 ± 0.15 ab | 18.59 ± 0.38 a | 17.48 ± 0.04 b | 17.51 ± 0.09 b | 16.35 ± 0.59 c | 15.86 ± 0.04 c | 16.54 ± 0.21 c | 16.25 ± 0.52 c | |
| Ala | 15.58 ± 0.17 d | 18.30 ± 0.10 b | 19.04 ± 0.32 a | 16.97 ± 0.04 c | 17.82 ± 0.11 b | 16.63 ± 0.58 c | 15.51 ± 0.01 d | 16.97 ± 0.21 c | 16.86 ± 0.47 c | |
| Cys * | 3.17 ± 0.09 f | 3.46 ± 0.03 bcd | 3.58 ± 0.05 ab | 3.71 ± 0.10 a | 3.54 ± 0.13 abc | 3.31 ± 0.10 cdef | 3.19 ± 0.04 ef | 3.42 ± 0.07 bcde | 3.25 ± 0.19 def | |
| Val * | 17.62 ± 0.51 de | 20.53 ± 0.13 ab | 21.04 ± 0.45 a | 18.88 ± 0.10 c | 20.07 ± 0.42 b | 18.94 ± 0.62 c | 17.30 ± 0.01 e | 18.73 ± 0.29 c | 18.23 ± 0.49 cd | |
| Met * | 3.78 ± 0.05 a | 2.98 ± 0.01 c | 3.21 ± 0.09 b | 2.98 ± 0.06 c | 3.07 ± 0.05 bc | 2.77 ± 0.12 d | 2.17 ± 0.00 e | 3.09 ± 0.07 bc | 3.25 ± 0.14 b | |
| Ile * | 16.37 ± 0.10 d | 18.84 ± 0.06 b | 19.64 ± 0.54 a | 17.20 ± 0.09 c | 18.58 ± 0.08 b | 17.37 ± 0.57 c | 15.94 ± 0.06 d | 17.51 ±0.30 c | 17.37 ± 0.45 c | |
| Leu * | 29.38 ± 0.43 c | 34.46 ± 0.22 a | 35.71 ± 0.80 a | 27.84 ± 0.73 d | 34.24 ± 0.30 a | 31.82 ± 1.05 b | 28.82 ± 0.05 cd | 32.07 ± 0.36 b | 31.69 ± 0.89 b | |
| Tyr * | 11.73 ± 0.17 e | 13.30 ± 0.13 ab | 13.88 ± 0.44 a | 12.85 ± 0.03 bc | 13.43 ± 0.08 ab | 12.15 ± 0.51 de | 11.61 ± 0.05 e | 12.64 ± 0.14 cd | 12.61 ± 0.33 cd | |
| Phe * | 20.52 ± 0.27 d | 24.58 ± 0.13 ab | 25.25 ± 0.54 a | 21.70 ± 0.07 c | 23.86 ± 0.08 b | 22.11 ± 0.72 c | 19.94 ± 0.12 d | 22.51 ± 0.19 c | 21.92 ± 0.75 c | |
| His | 9.55 ± 0.15 c | 10.96 ± 0.07 a | 10.86 ± 0.35 a | 10.35 ± 0.02 b | 10.90 ± 0.10 a | 9.41 ± 0.39 c | 9.45 ± 0.02 c | 10.15 ± 0.17 b | 9.12 ± 0.28 c | |
| Lys * | 23.71 ± 0.26 b | 25.65 ± 0.03 a | 25.01 ± 0.54 a | 22.48 ± 0.10 c | 25.68 ± 0.19 a | 22.37 ± 0.93 c | 23.53 ± 0.04 b | 23.71 ± 0.18 b | 22.12 ± 0.63 c | |
| Arg | 26.72 ± 0.38 bc | 28.99 ± 0.16 a | 29.44 ± 1.27 a | 27.92 ± 0.23 ab | 28.85 ± 0.17 a | 25.31 ± 0.78 cd | 25.30 ± 0.08 cd | 25.33 ± 0.58 cd | 24.79 ± 0.87 d | |
| Total amino acids | 371.04 | 422.33 | 434.60 | 378.46 | 415.81 | 384.67 | 363.89 | 391.39 | 400.61 | |
| Sample | Total Phenolic Compounds Content [mg GAE/100 mL] | FRAP [μmol Trolox/mL] | DPPH● [μmol Trolox/mL] | ABTS●+ [μmol Trolox/mL] |
|---|---|---|---|---|
| SAba | 6.73 ± 0.16 d,1 | 0.17 ± 0.00 d | 0.01 ± 0.01 f | 0.13 ± 0.03 d |
| PMAba | 7.47 ± 0.36 c | 0.18 ± 0.01 cd | 0.04 ± 0.00 d | 0.14 ± 0.01 d |
| CMAba | 8.35 ± 0.22 b | 0.24 ± 0.01 b | 0.08 ± 0.00 b | 0.36 ± 0.01 b |
| SAbe | 6.96 ± 0.15 d | 0.19 ± 0.01 c | 0.04 ± 0.00 e | 0.14 ± 0.01 d |
| PMAbe | 7.44 ± 0.17 c | 0.19 ± 0.01 c | 0.05 ± 0.00 b | 0.32 ± 0.01 c |
| CMAbe | 8.45 ± 0.18 b | 0.23 ± 0.00 b | 0.08 ± 0.00 c | 0.35 ± 0.01 b |
| SAu | 5.55 ± 0.13 e | 0.15 ± 0.00 e | 0.03 ± 0.00 e | 0.09 ± 0.01 e |
| PMAu | 8.19 ± 0.10 b | 0.19 ± 0.00 c | 0.06 ± 0.00 c | 0.32 ± 0.00 c |
| CMAu | 10.33 ± 0.27 a | 0.30 ± 0.01 a | 0.09 ± 0.00 a | 0.45 ± 0.01 a |
| RT | Sample | SAba | PMAba | CMAba | SAbe | PMAbe | CMAbe | SAu | PMAu | CMAu |
|---|---|---|---|---|---|---|---|---|---|---|
| Volatile Compounds/Chemical Family | ppb | ppb | ppb | ppb | ppb | ppb | ppb | ppb | ppb | |
| 4.549 | Hexanol | 0.00 ± 0.00 c,1 | 80.09 ± 11.68 b | 7.05 ± 0.71 c | 0.00 ± 0.00 c | 16.71 ± 2.18 c | 7.83 ± 1.34 c | 0.00 ± 0.00 c | 978.08 ± 48.93 a | 19.84 ± 1.99 c |
| 6.607 | 1-Octen-3-ol | 8.49 ± 1.45 e | 209.35 ± 48.00 b | 55.13 ± 2.76 d | 10.31 ± 1.14 e | 47.23 ± 3.78 d | 73.81 ± 3.69 cd | 8.04 ± 1.45 e | 1017.64 ± 30.54 a | 92.90 ± 3.72 c |
| 6.954 | 3-Octanol | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 2.58 ± 0.62 b | 0.00 ± 0.00 b | 3.68 ± 0.52 b | 3.36 ± 0.81 b | 0.00 ± 0.00 b | 82.09 ± 9.86 a | 4.87 ± 1.03 b |
| 7.540 | 1-Hexanol. 2-ethyl- | 5.79 ± 1.39 bc | 13.09 ± 0.27 b | 7.69 ± 1.54 bc | 6.08 ± 0.91 bc | 6.26 ± 0.82 bc | 7.14 ± 1.29 bc | 4.50 ± 0.59 c | 82.08 ± 11.51 a | 8.70 ± 1.74 bc |
| Total alcohols | 14.28 | 302.52 | 72.45 | 16.39 | 73.88 | 92.14 | 12.54 | 2159.89 | 126.31 | |
| % of all volatiles | 10.04 | 50.98 | 43.09 | 11.87 | 45.49 | 46.82 | 34.60 | 46.35 | 41.38 | |
| 5.136 | Heptanal | 0.00 ± 0.00 d | 34.05 ± 4.43 b | 3.58 ± 0.72 d | 0.00 ± 0.00 d | 6.12 ± 1.10 cd | 5.17 ± 0.68 d | 0.00 ± 0.00 d | 139.82 ± 12.59 a | 13.64 ± 1.78 c |
| 6.170 | 2-Heptenal | 0.00 ± 0.00 c | 12.62 ± 0.99 b | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 2.21 ± 0.51 c | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 32.36 ± 4.21 a | 0.00 ± 0.00 c |
| 6.260 | Benzaldehyde | 0.00 ± 0.00 e | 0.00 ± 0.00 e | 9.29 ± 1.30 c | 0.00 ± 0.00 e | 2.68 ± 0.73 de | 10.71 ± 1.50 bc | 3.63 ± 0.04 d | 26.26 ± 4.74 a | 13.10 ± 1.97 b |
| 7.057 | Octanal | 17.31 ± 1.73 c | 35.38 ± 1.26 b | 5.58 ± 1.07 de | 14.02 ± 1.41 cd | 4.82 ± 0.25 de | 7.38 ± 1.55 de | 2.86 ± 0.29 e | 116.33 ± 15.14 a | 12.72 ± 2.93 cde |
| 9.031 | Nonanal | 22.87 ± 1.60 c | 53.43 ± 2.03 a | 8.72 ± 2.27 e | 22.76 ± 0.91 c | 8.22 ± 0.91 e | 6.83 ± 0.96 e | 6.36 ± 0.64 e | 36.12 ± 4.70 b | 13.24 ± 1.59 d |
| 10.937 | Decanal | 62.62 ± 3.14 b | 27.01 ± 0.99 c | 2.04 ± 0.35 d | 63.61 ± 1.28 b | 3.39 ± 0.17 d | 2.57 ± 0.46 d | 6.99 ± 1.54 d | 202.71 ± 16.23 a | 5.79 ± 0.76 d |
| 12.749 | Undecanal | 4.86 ± 0.98 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 4.05 ± 0.69 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 348.57 ± 38.38 a | 0.00 ± 0.00 b |
| 14.454 | Dodecanal | 9.24 ± 2.04 b | 4.64 ± 0.24 c | 1.79 ± 0.47 d | 5.31 ± 0.64 c | 1.66 ± 0.45 d | 1.43 ± 0.39 d | 1.68 ± 0.22 d | 11.73 ± 1.64 a | 2.13 ± 0.45 d |
| Total aldehydes | 116.90 | 167.14 | 30.99 | 109.75 | 29.10 | 34.09 | 21.51 | 913.90 | 60.61 | |
| % of all volatiles | 82.20 | 28.17 | 18.43 | 79.54 | 17.92 | 17.32 | 59.36 | 19.61 | 19.86 | |
| 4.904 | 2-Heptanone | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 13.52 ±2.30 b | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 14.26 ±1.14 b | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 24.62 ±1.97 a |
| 6.720 | 3-Octanone | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 11.44 ± 1.49 c | 0.00 ± 0.00 c | 36.28 ± 2.18 b | 12.00 ± 1.33 c | 0.00 ± 0.00 c | 665.04 ± 33.27 a | 12.63 ± 1.27 c |
| 7.740 | 3-Octen-2-one | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 1.83 ± 0.55 b | 0.00 ± 0.00 b | 42.20 ± 8.45 a | 4.57 ± 0.69 b |
| Total ketones | 0.00 | 0.00 | 24.96 | 0.00 | 36.28 | 28.09 | 0.00 | 707.24 | 41.81 | |
| % of all volatiles | 0.00 | 0.00 | 14.84 | 0.00 | 22.34 | 14.27 | 0.00 | 15.18 | 13.70 | |
| 6.810 | Furan. 2-pentyl- | 0.00 ± 0.00 e | 123.74 ± 14.63 b | 38.02 ± 2.28 d | 7.53 ± 0.99 e | 20.33 ± 2.04 de | 42.49 ± 2.98 d | 2.19 ± 0.15 e | 750.45 ± 37.54 a | 76.51 ± 2.30 c |
| Total furans | 0.00 | 123.74 | 38.02 | 7.53 | 20.33 | 42.49 | 2.19 | 750.45 | 76.51 | |
| % of all volatiles | 0.00 | 20.85 | 22.61 | 5.46 | 12.52 | 21.59 | 6.04 | 16.11 | 25.07 | |
| 7.581 | D-Limonene | 11.04 ± 1.44 b | 0.00 ± 0.00 d | 1.73 ± 0.31 cd | 4.32 ± 1.13 c | 2.82 ± 0.57 cd | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 29.55 ± 5.03 a | 0.00 ± 0.00 d |
| Total terpenes | 11.04 | 0.00 | 1.73 | 4.32 | 2.82 | 0.00 | 0.00 | 29.55 | 0.00 | |
| % of all volatiles | 7.76 | 0.00 | 1.03 | 3.13 | 1.73 | 0.00 | 0.00 | 0.65 | 0.00 | |
| Total volatiles | 142.22 | 593.40 | 168.15 | 137.99 | 162.41 | 196.80 | 36.24 | 4561.03 | 305.24 | |
| Sample | Flavor | Aroma | Color | Texture | Mouthfeel | Average Score |
|---|---|---|---|---|---|---|
| SAba | 3.10 a,1 | 3.50 a | 4.50 a | 3.80 a | 3.10 abc | 3.60 a |
| PMAba | 3.13 a | 3.80 a | 3.87 ab | 3.53 a | 3.07 abc | 3.48 a |
| CMAba | 3.29 a | 3.86 a | 3.50 bc | 3.29 a | 3.00 bc | 3.39 a |
| SAbe | 2.80 a | 3.50 a | 3.00 c | 3.10 a | 2.80 bc | 3.04 a |
| PMAbe | 3.13 a | 3.53 a | 3.80 abc | 3.27 a | 2.80 bc | 3.31 a |
| CMAbe | 3.07 a | 3.43 a | 3.57 bc | 3.43 a | 3.14 abc | 3.33 a |
| SAu | 3.70 a | 3.40 a | 4.20 ab | 3.90 a | 3.30 ab | 3.70 a |
| PMAu | 2.53 a | 3.07 a | 3.87 ab | 3.20 a | 2.53 c | 3.04 a |
| CMAu | 3.64 a | 3.71 a | 3.43 bc | 3.86 a | 3.71 a | 3.67 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Opiela, E.; Czubaszek, A.; Gasiński, A.; Miedzianka, J.; Kawa-Rygielska, J. Malted Soybeans as a Substrate for Plant-Based Beverages—Analysis of Nutritional Properties, Antioxidant Activity, and Volatiles. Molecules 2025, 30, 3845. https://doi.org/10.3390/molecules30193845
Opiela E, Czubaszek A, Gasiński A, Miedzianka J, Kawa-Rygielska J. Malted Soybeans as a Substrate for Plant-Based Beverages—Analysis of Nutritional Properties, Antioxidant Activity, and Volatiles. Molecules. 2025; 30(19):3845. https://doi.org/10.3390/molecules30193845
Chicago/Turabian StyleOpiela, Ewelina, Anna Czubaszek, Alan Gasiński, Joanna Miedzianka, and Joanna Kawa-Rygielska. 2025. "Malted Soybeans as a Substrate for Plant-Based Beverages—Analysis of Nutritional Properties, Antioxidant Activity, and Volatiles" Molecules 30, no. 19: 3845. https://doi.org/10.3390/molecules30193845
APA StyleOpiela, E., Czubaszek, A., Gasiński, A., Miedzianka, J., & Kawa-Rygielska, J. (2025). Malted Soybeans as a Substrate for Plant-Based Beverages—Analysis of Nutritional Properties, Antioxidant Activity, and Volatiles. Molecules, 30(19), 3845. https://doi.org/10.3390/molecules30193845





