A Novel Schiff Base Probe Based on Fluorescein for Fluorometric and Colorimetric Dual-Mode Rapid Detection of Cu2+
Abstract
1. Introduction
2. Results
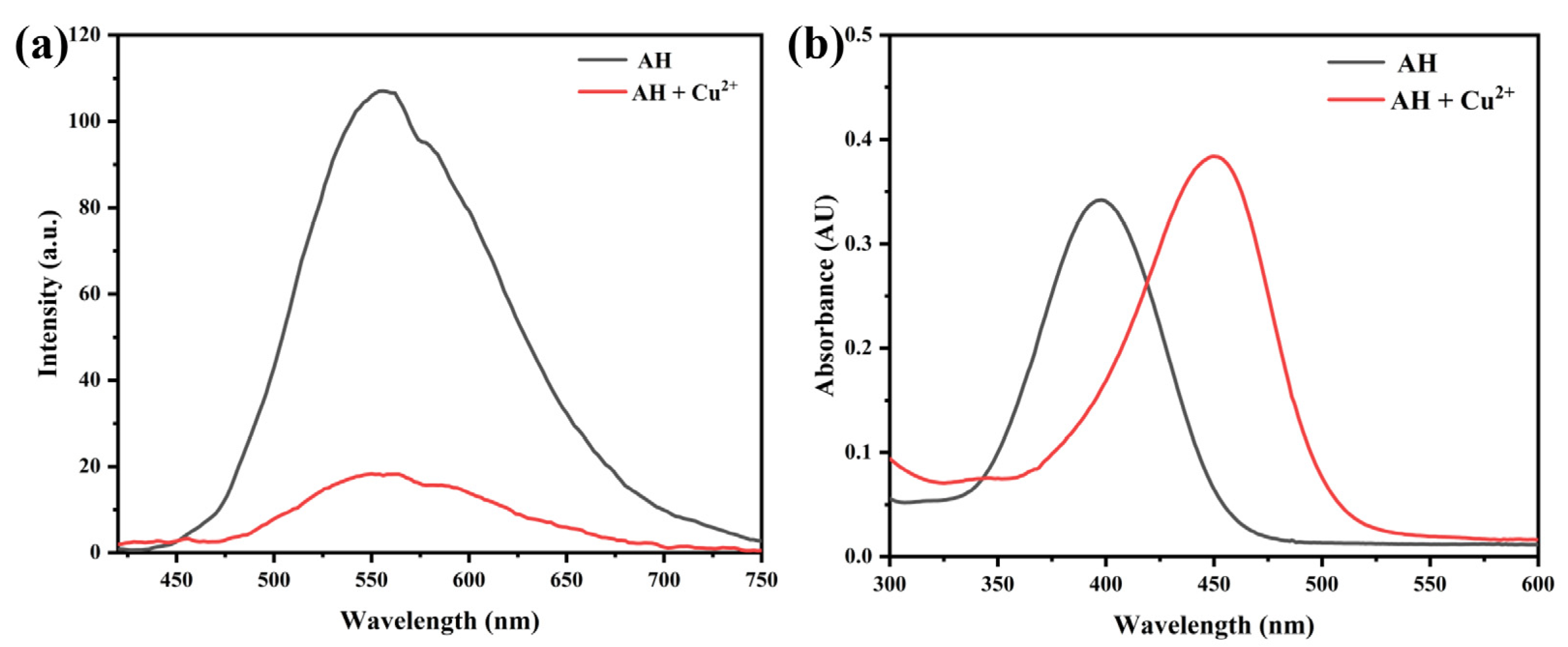
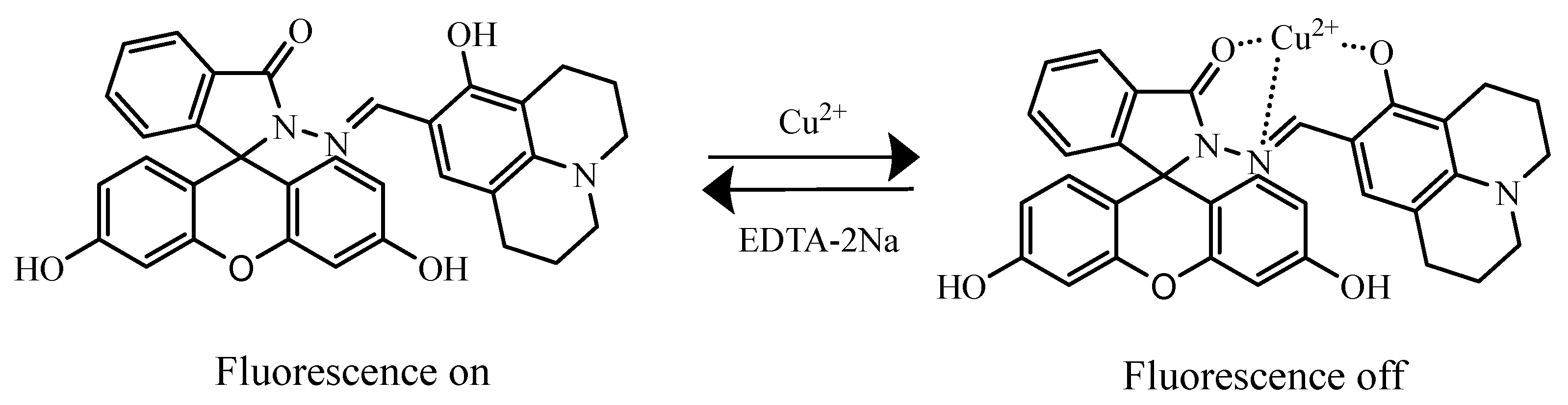
2.1. Feasibility Study of Probe AH
2.2. Optimization of Detection Conditions
2.2.1. Effect of Solvent Type on the Luminescence Properties of Probe AH
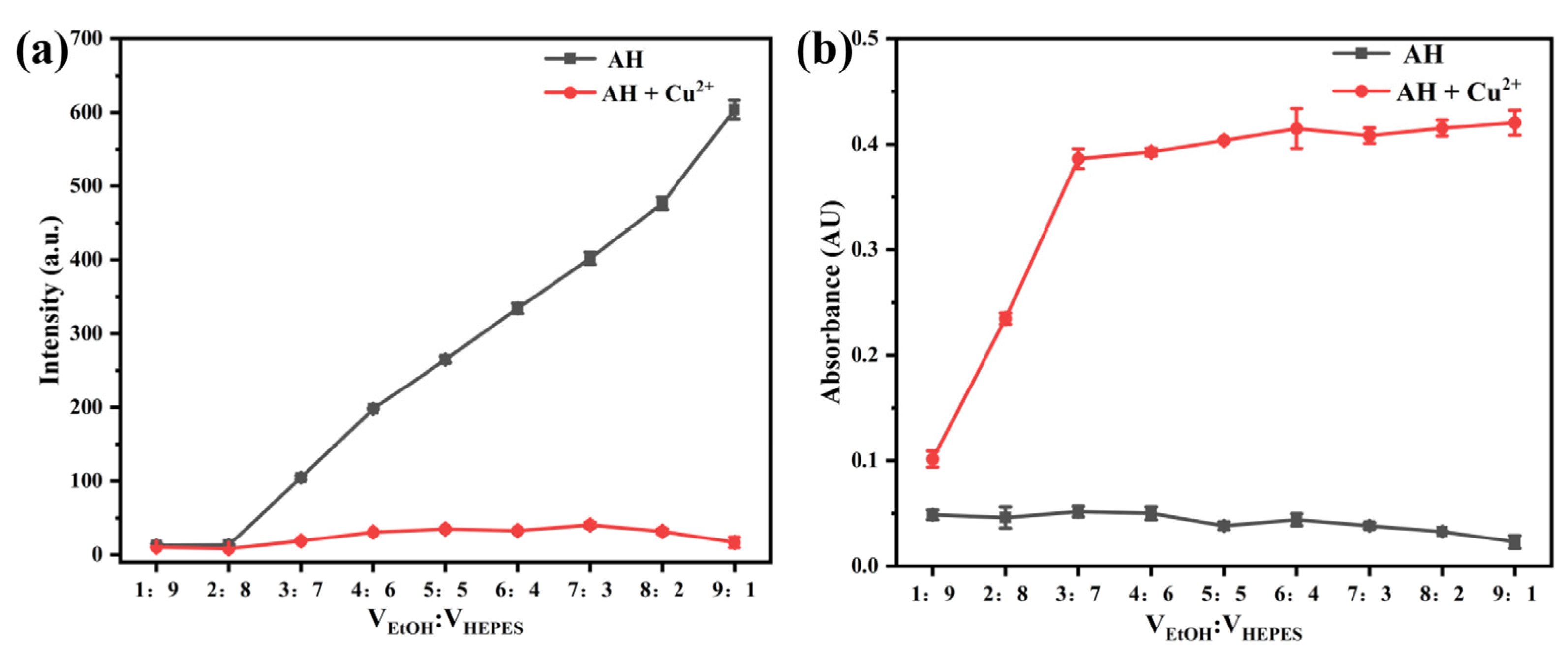
2.2.2. Effect of Solvent Ratio on the Luminescence Properties of Probe AH
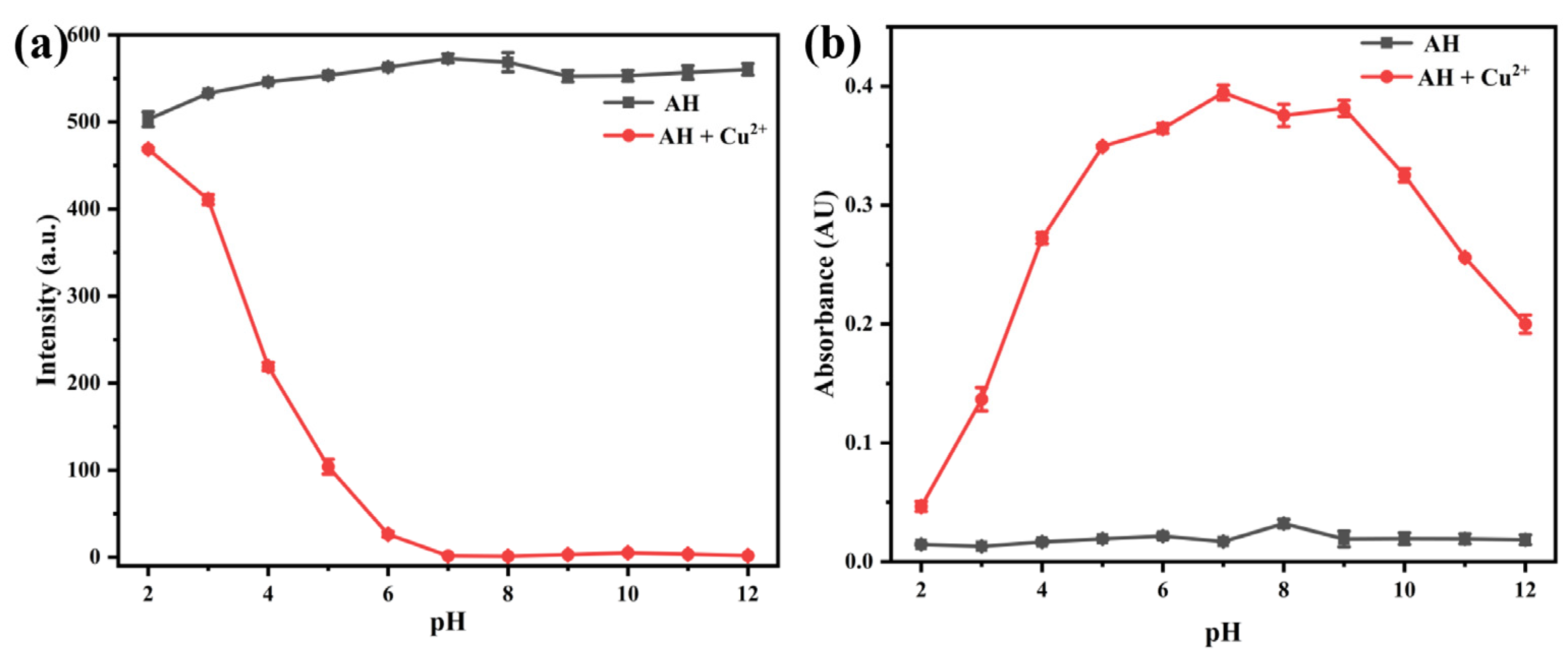
2.2.3. The Effect of pH on the Luminescence Performance of Probe AH
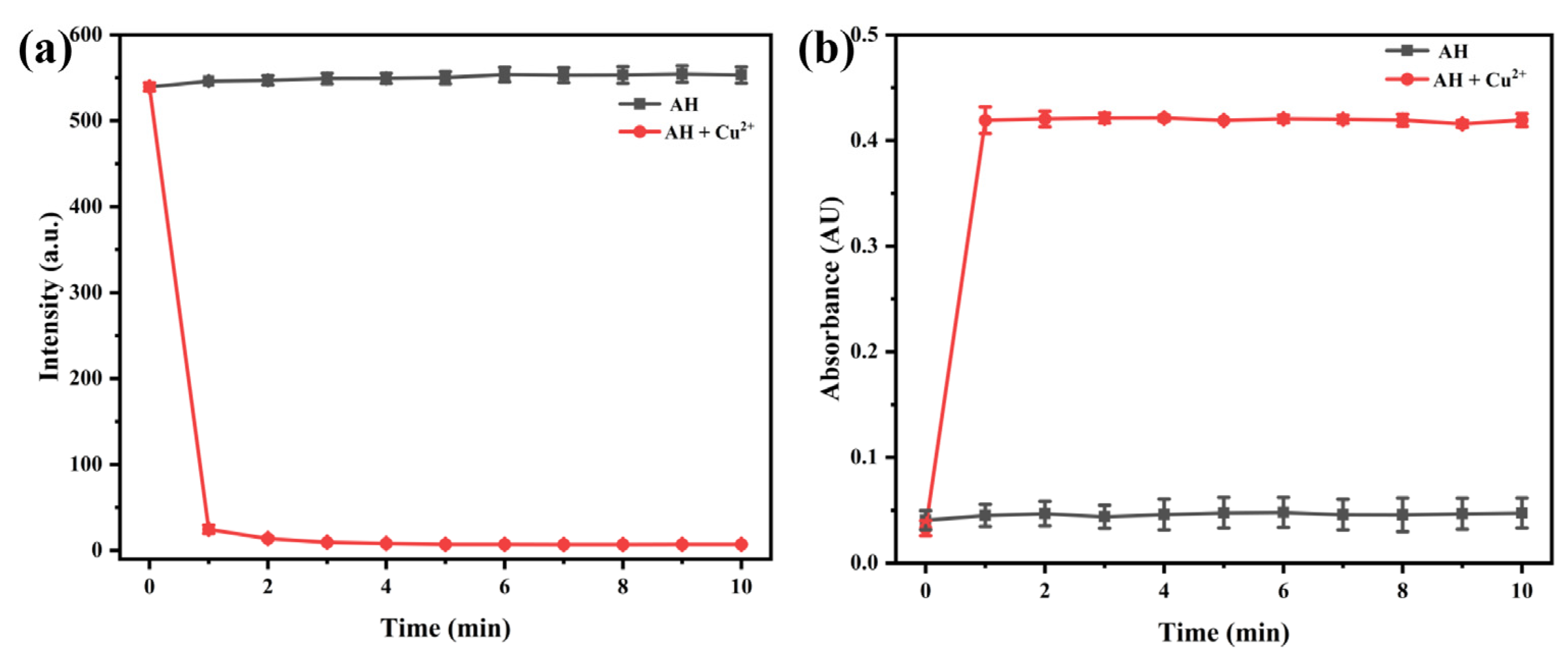
2.2.4. Response Time of Probe AH to Cu2+
2.3. Concentration Titration Experiments of Probe AH with Cu2+
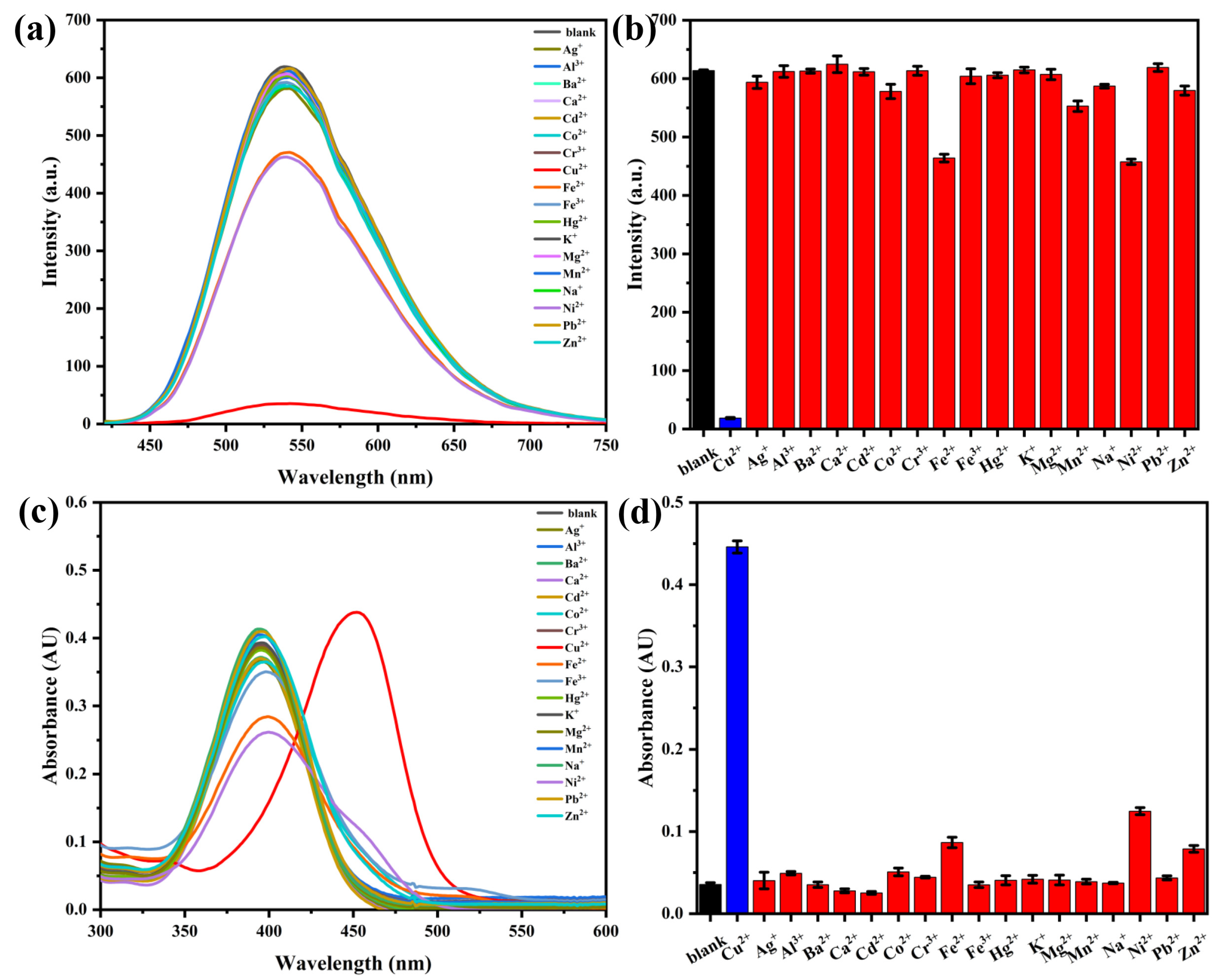
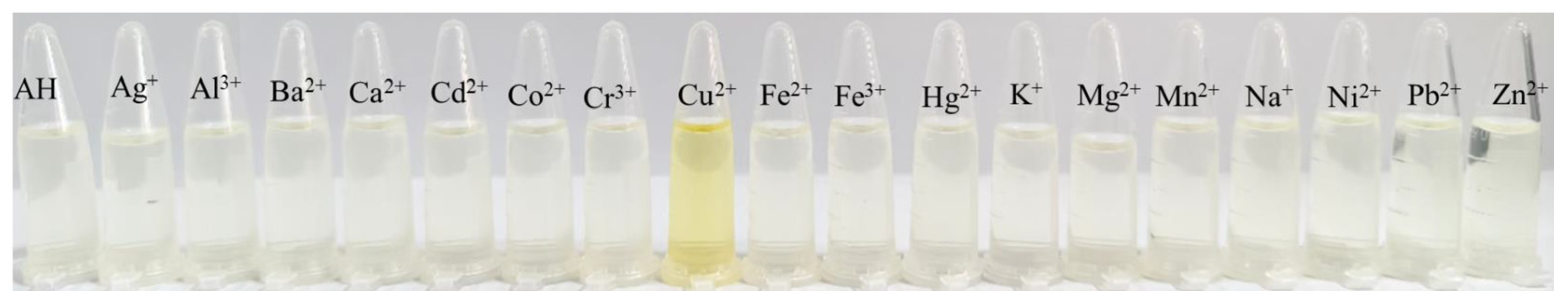
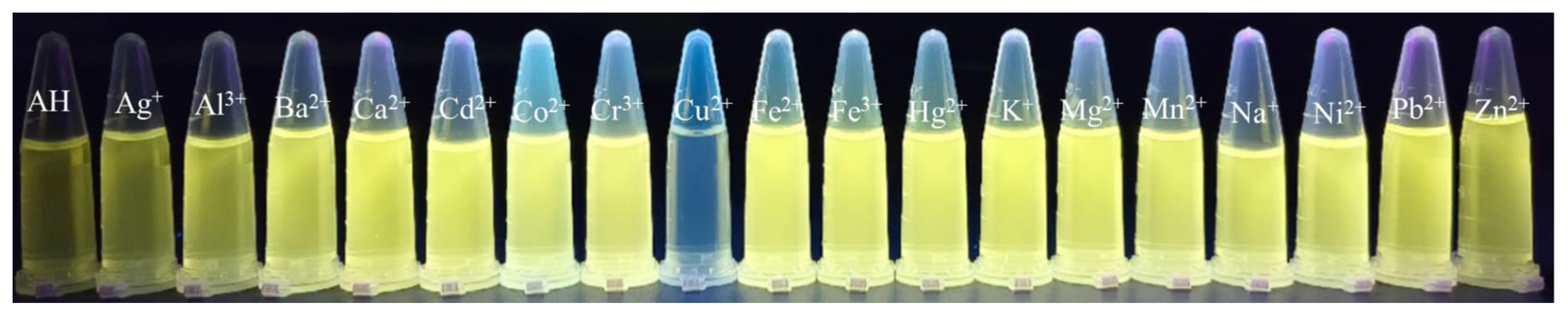
2.4. Selectivity Study of Probe AH for Cu2+ Detection
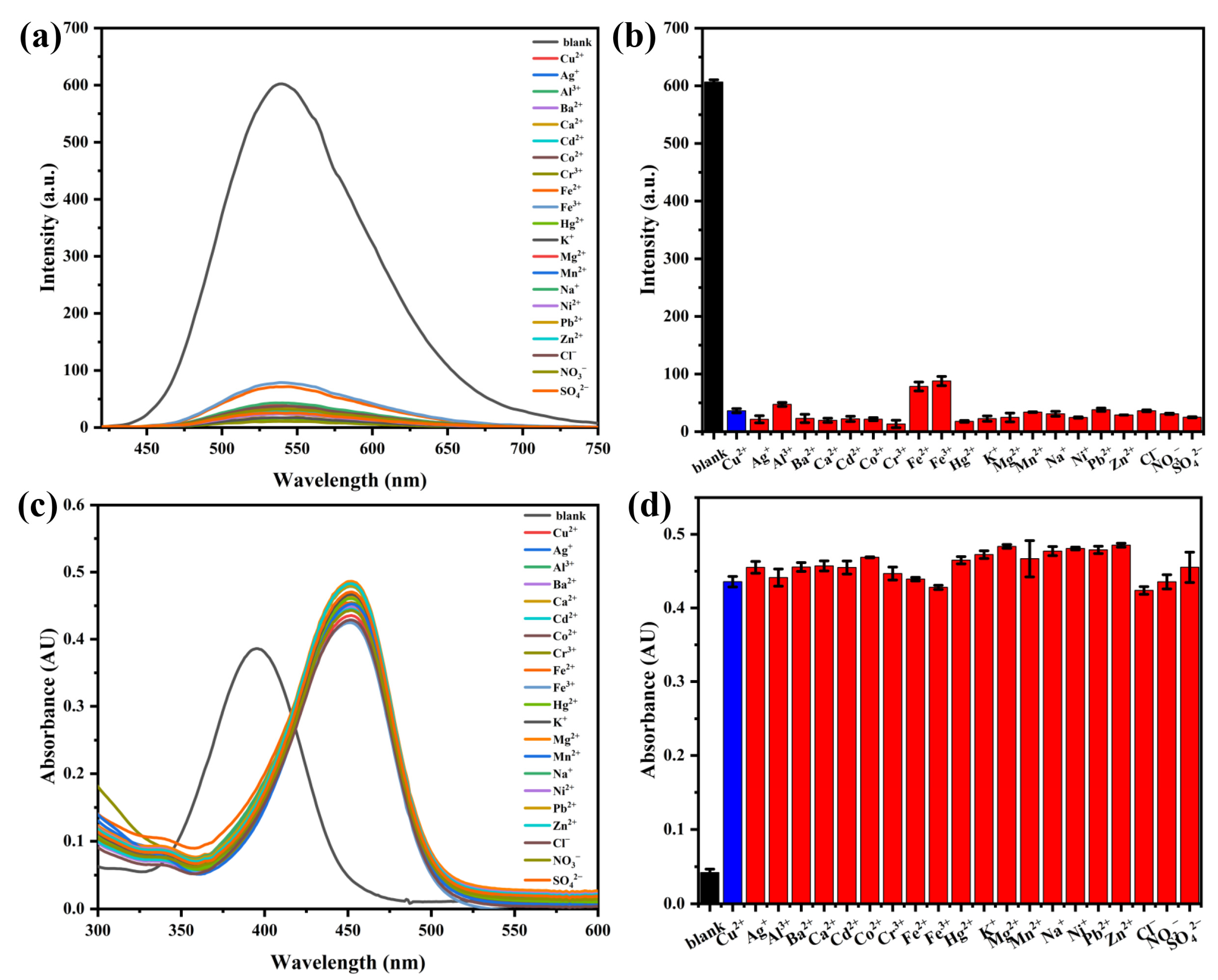
2.5. Interference Resistance Study of Probe AH for Cu2+ Detection
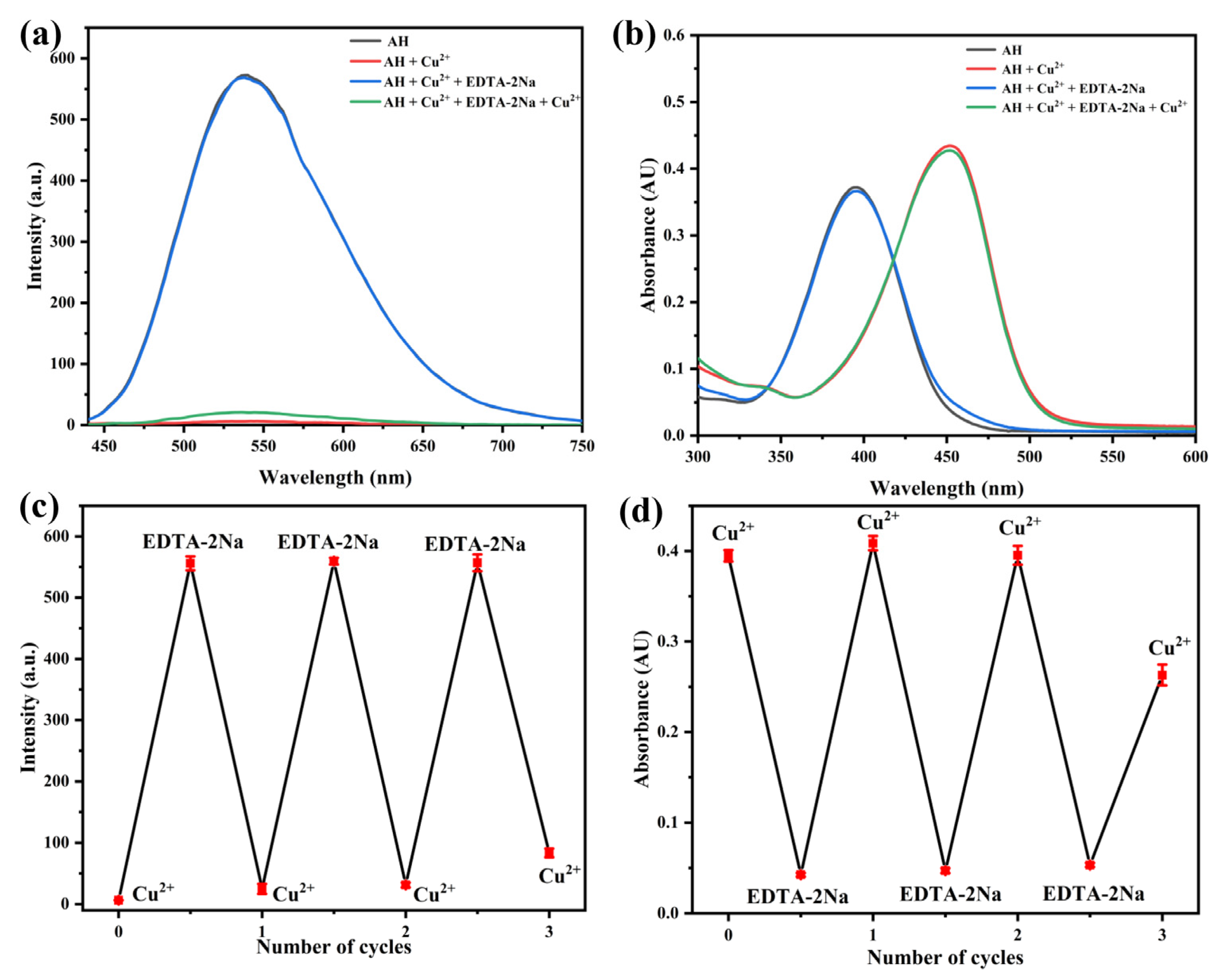
2.6. Reversibility Study of Probe AH
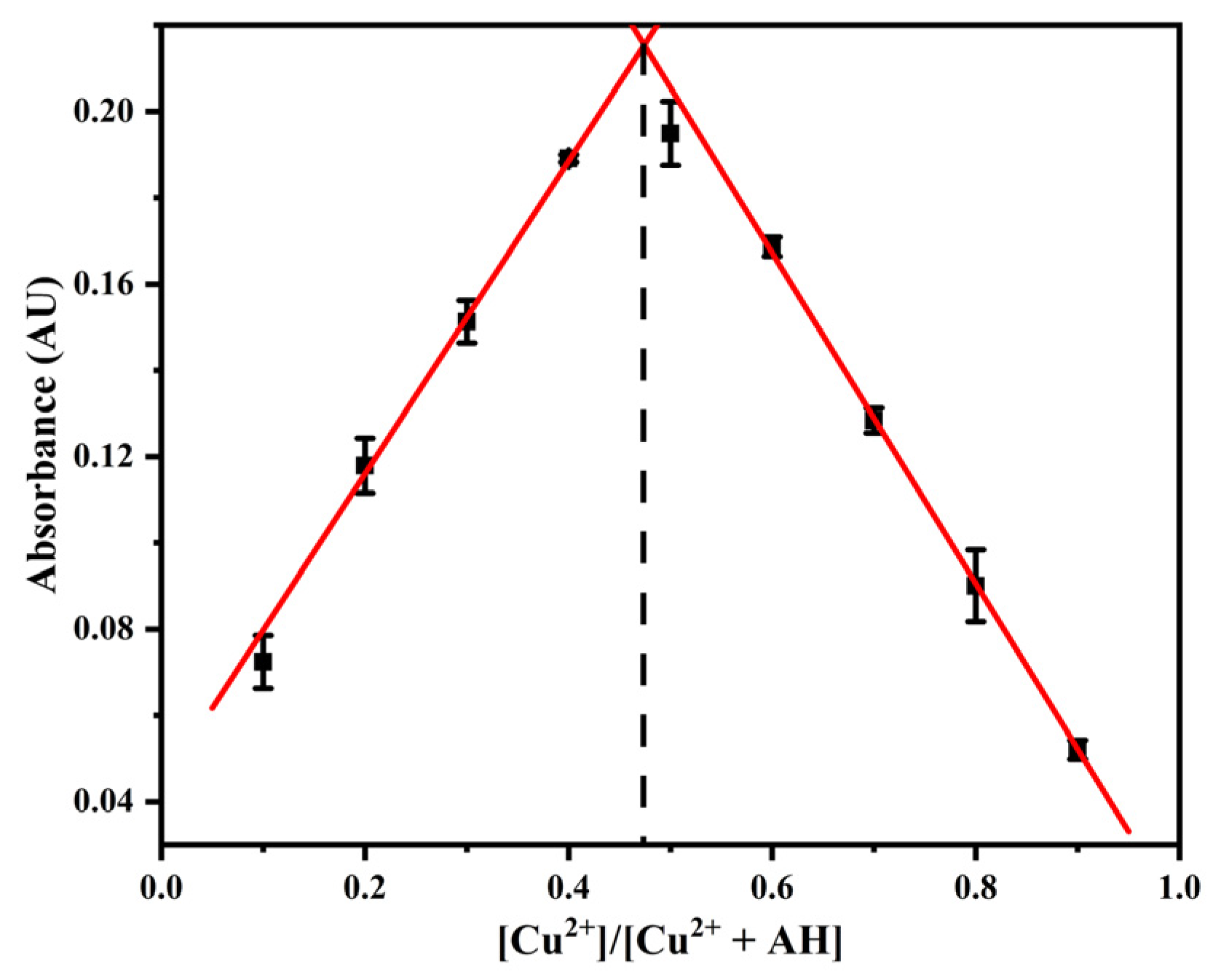
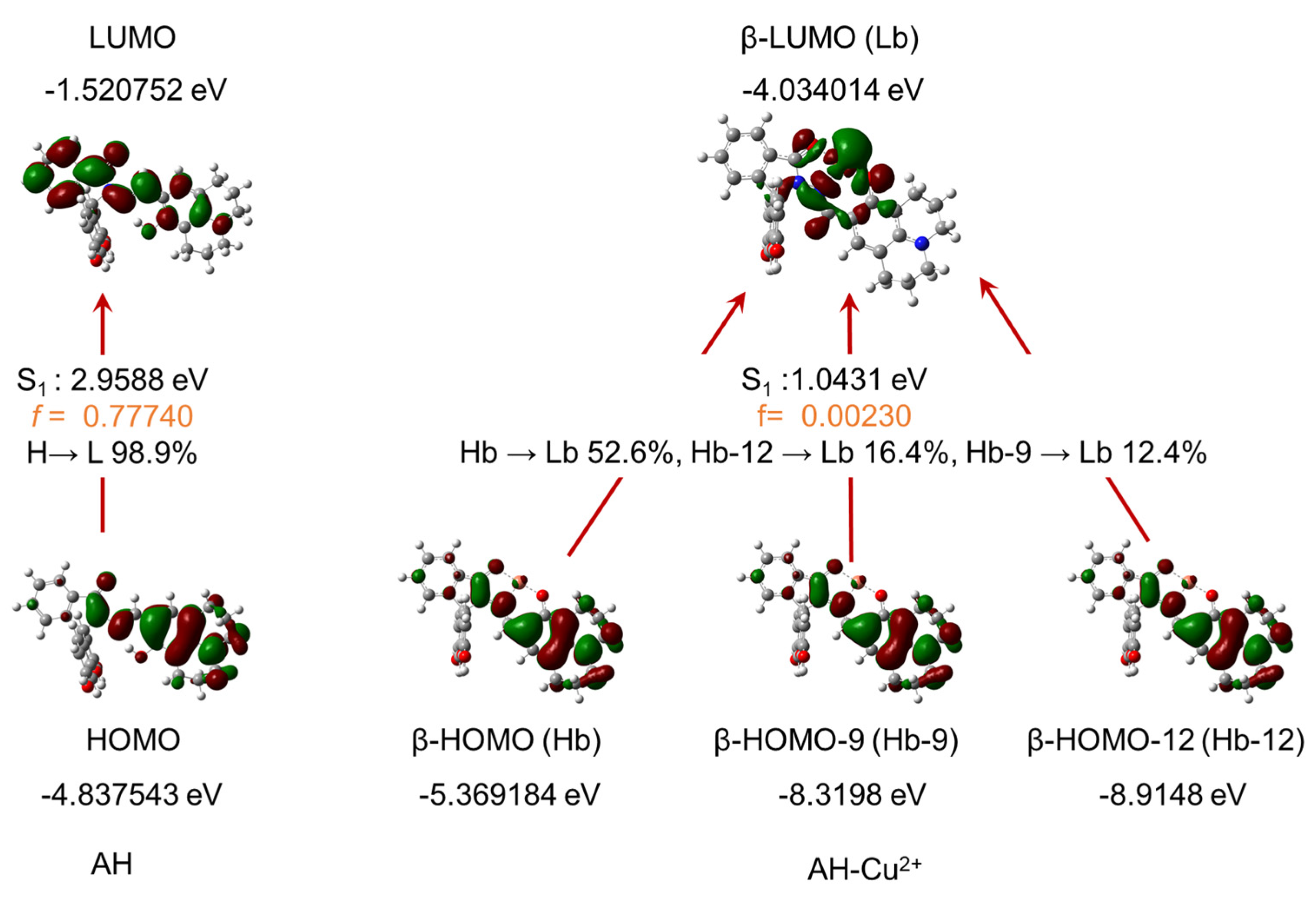
2.7. Study on the Recognition Mechanism of Cu2+ by Probe AH
2.8. Visual Detection of Cu2+ by Probe AH
2.9. Analysis of Real Samples and Cellular Bioimaging
2.10. Comparison with Other Probes
3. Materials and Methods
3.1. Reagents and Instruments
3.2. Synthesis of Compound 1
3.3. Synthesis of Probe AH
3.4. UV Visible and Fluorescence Spectroscopy Study
3.5. Calculation of Detection Limit
3.6. Analysis of Water Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- You, J.; Lee, S.; Tark, H.J.; Nang, M.J.; Oh, J.H.; Choi, I. Optical Detection of Copper Ions via Structural Dissociation of Plasmonic Sugar Nanoprobes. Anal. Chem. 2022, 94, 5521–5529. [Google Scholar] [CrossRef]
- Xie, S.; Xia, S.; Xu, Z.; Li, W.; Wang, E.; Wang, S. An imidazole-based fluorescent probe for cupric ion. Synth. Met. 2021, 277, 116784. [Google Scholar] [CrossRef]
- Du, B.; Li, Q.; Huang, K.; Liang, L. A quinoline-fluoran hybrid fluorescent probe for selectively and sensitively sensing copper ions and fluorescence imaging application. J. Mol. Struct. 2023, 1271, 134015. [Google Scholar] [CrossRef]
- Mohanasundaram, D.; Bhaskar, R.; Sankarganesh, M.; Nehru, K.; Kumar, G.G.V.; Rajesh, J. A simple pyridine based fluorescent chemosensor for selective detection of copper ion. Spectrochim. Acta Part A 2022, 265, 120395. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cui, J.; Fang, X.; Zhang, W.; Wang, J.; Chen, S.; Qian, J. Fluorescent detection of copper ions with acylhydrazine-based probes: Effects of substitute and its position. Dye. Pigment. 2022, 197, 109954. [Google Scholar] [CrossRef]
- Mohanasundaram, D.; Bhaskar, R.; Lenin, N.; Nehru, K.; Rajagopal, G.; Kumar, G.G.V.; Rajesh, J. A simple triphenylamine based turn-off fluorescent sensor for copper (II) ion detection in semi-aqueous solutions. J. Photochem. Photobiol. A 2022, 427, 113850. [Google Scholar] [CrossRef]
- Zhou, W.; Hu, Z.; Wei, J.; Lu, H.; Dai, H.; Zhao, J.; Zhang, W.; Guo, R. A ratiometric fluorescent probe based on PCN-224 for rapid and ultrasensitive detection of copper ions. Compos. Commun. 2022, 33, 101221. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, S.; Jin, Y.; Pang, X.-f.; Zhao, Q.; Zhang, T.; Zhang, J. A near-infrared “turn-on” fluorescent probe for selective detection of copper(II) ions in aqueous media and its application in cell imaging. Spectrochim. Acta Part A 2025, 336, 126036. [Google Scholar] [CrossRef]
- Chen, J.; Liu, S.; Deng, D.; Guo, G.; Min, Y. A highly-sensitive fluorescent probe for the detection of copper ions and its applications in water quality monitoring, neural cell imaging and plant imaging. Spectrochim. Acta Part A 2025, 329, 125613. [Google Scholar] [CrossRef]
- Chen, J.; Luo, R.; Li, S.; Shao, J.; Wang, T.; Xie, S.; Xu, L.; You, Q.; Feng, S.; Feng, G. A novel NIR fluorescent probe for copper(ii) imaging in Parkinson’s disease mouse brain. Chem. Sci. 2024, 15, 13082–13089. [Google Scholar] [CrossRef]
- Zhou, Z.; Tang, H.; Chen, S.; Huang, Y.; Zhu, X.; Li, H.; Zhang, Y.; Yao, S. A turn-on red-emitting fluorescent probe for determination of copper(II) ions in food samples and living zebrafish. Food Chem. 2021, 343, 128513. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.; Yuan, K.; Lv, L.; Liu, K.; Li, Z. Fluorescent Mechanism of a Highly Selective Probe for Copper(II) Detection: A Theoretical Study. ACS Omega 2023, 8, 17171–17180. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zong, K.; Yan, D.; Du, Y.; Wang, Z.; Gao, W.; Li, M.; Lin, J.; Zhang, W.; Chen, H.; et al. Accurate determination of trace silver in geological reference materials by inductively coupled plasma-tandem mass spectrometry (ICP-MS/MS). J. Anal. At. Spectrom. 2023, 38, 1984–1994. [Google Scholar] [CrossRef]
- Wippermann, D.; Zonderman, A.; Zimmermann, T.; Pröfrock, D. Determination of technology-critical elements in seafood reference materials by inductively coupled plasma-tandem mass spectrometry. Anal. Bioanal. Chem. 2024, 416, 2797–2807. [Google Scholar] [CrossRef]
- Isaguirre, A.C.; Moyano, M.F.; Gil, R.A.; Moglia, M.M. A Novel and Simple Method for Elements Determination in Aerobiological Samples by Inductively Coupled Plasma Mass Spectrometry (ICP-MS) Analysis. Water Air Soil Pollut. 2020, 231, 70. [Google Scholar] [CrossRef]
- Costa, L.M.; Borges, F.A.; da Silva Cavalcanti, M.H.; do Lago, A.C.; Tarley, C.R.T.; de Fátima Lima Martins, G.; Figueiredo, E.C. Direct magnetic sorbent sampling flame atomic absorption spectrometry (DMSS-FAAS) for highly sensitive determination of trace metals. Anal. Chim. Acta 2023, 1251, 340709. [Google Scholar] [CrossRef]
- Wu, D.; Hu, Y.; Cheng, H.; Ye, X. Detection Techniques for Lead Ions in Water: A Review. Molecules 2023, 28, 3601. [Google Scholar] [CrossRef]
- Ahmed, N.; Liu, J.; Xu, X.; Hussain, A.; Mustafai, A.; Yar, M.; Ayub, K.; Alothman, A.A.; Mohammad, S.; Ye, Y.; et al. An activatable NIR turn-on fluorescent probe for copper (II) ion and live cell imaging. Sci. Rep. 2024, 14, 19068. [Google Scholar] [CrossRef]
- Gümrah, Ö.; Güçoğlu, M.; Şatıroğlu, N. Smartphone-assisted chemosensor based on thiocarbohydrazide Schiff base for selective detection of Hg2+ and Cu2+ ions. J. Mol. Struct. 2024, 1316, 138859. [Google Scholar] [CrossRef]
- Genc, H.N.; Guctekin Yasar, O.; Karuk Elmas, S.N.; Arslan, F.N.; Yilmaz, I.; Sirit, A. Naked-eye colorimetric and switch-on fluorescence chemosensor based on a rhodamine derivative for Hg2+: Smartphone device, test-kit and food sample applications. J. Photochem. Photobiol. A 2023, 438, 114558. [Google Scholar] [CrossRef]
- Gomes, L.J.; Outis, M.; Gomes, C.S.B.; Tomé, A.C.; Moro, A.J. Development of Fluorescent Chemosensors for Calcium and Lead Detection. Molecules 2024, 29, 527. [Google Scholar] [CrossRef] [PubMed]
- Diana, R.; Caruso, U.; Gentile, F.S.; Di Costanzo, L.; Panunzi, B. A Novel L-Shaped Fluorescent Probe for AIE Sensing of Zinc (II) Ion by a DR/NIR Response. Molecules 2021, 26, 7347. [Google Scholar] [CrossRef] [PubMed]
- Phromsiri, N.; Abiodun, S.L.; Manipuntee, C.; Leeladee, P.; Greytak, A.B.; Insin, N. Fluorescent responses of CdSe and Si QDs toward Copper (II) ion and the mixed-QDs probe for Cu2+ ion sensing. J. Mol. Struct. 2023, 1271, 134050. [Google Scholar] [CrossRef]
- Pang, J.; Shu, L.; Li, M.; Hu, X. Theoretical insights into a colorimetric azo-based probe to detect copper ions. RSC Adv. 2020, 10, 23196–23202. [Google Scholar] [CrossRef]
- Sahu, S.; Sikdar, Y.; Bag, R.; Drew, M.G.B.; Cerón-Carrasco, J.P.; Goswami, S. A Quinoxaline−Naphthaldehyde Conjugate for Colorimetric Determination of Copper Ion. Molecules 2022, 27, 2908. [Google Scholar] [CrossRef]
- Moafi, L.; Eshghi, P.; Alidoosti, M. A highly selective colorimetric chemosensor based on Salicylaldehyde Schiff base for Cu2+ and F− ions detection with a turn-off response for copper (II) ion: Experimental and theoretical studies. J. Mol. Struct. 2025, 1325, 141025. [Google Scholar] [CrossRef]
- Gogoi, K.D.; Gohain, B.R.; Talukdar, D.; Singh, R. Benzil based simple and selective Schiff base probe for rapid colorimetric detection of Cu2+ ions. J. Mol. Struct. 2025, 1331, 141635. [Google Scholar] [CrossRef]
- Li, C.; Niu, Q.; Wang, J.; Wei, T.; Li, T.; Chen, J.; Qin, X.; Yang, Q. Bithiophene-based fluorescent sensor for highly sensitive and ultrarapid detection of Hg2+ in water, seafood, urine and live cells. Spectrochim. Acta Part A 2020, 233, 118208. [Google Scholar] [CrossRef]
- Lv, H.; Yuan, G.; Zhang, G.; Ren, Z.; He, H.; Sun, Q.; Zhang, X.; Wang, S. A novel benzopyran-based colorimetric and near-infrared fluorescent sensor for Hg2+ and its imaging in living cell and zebrafish. Dye. Pigment. 2020, 172, 107658. [Google Scholar] [CrossRef]
- Kathuria, I.; Kaur, P.; Kumar, S. A light-responsive, highly sensitive probe to perceive mercuric ions in both water and biological samples using fluorescence, colorimetric & electrochemical techniques. J. Photochem. Photobiol. A 2023, 436, 114413. [Google Scholar] [CrossRef]
- Zavalishin, M.N.; Gamov, G.A.; Kiselev, A.N.; Nikitin, G.A. A fluorescein conjugate as colorimetric and red-emissive fluorescence chemosensor for selective recognition Cu2+ ions. Opt. Mater. 2024, 153, 115580. [Google Scholar] [CrossRef]
- Zavalishin, M.N.; Pogonin, A.E.; Gamov, G.A. Hg2+-induced hydrolysis of fluorescein hydrazone: A new fluorescence probe for selective recognition Hg2+ in an aqueous solution. J. Mol. Struct. 2025, 1334, 141930. [Google Scholar] [CrossRef]
- Roy, S.; Mondal, T.; Dey, D.; Mane, M.V.; Panja, S.S. A New Thiophene-Appended Fluorescein-Hydrazone-Based Chromo-Fluorogenic Sensor for the Screening of Hg2+ Ions in Real Water Samples. ChemistrySelect 2021, 6, 10464–10479. [Google Scholar] [CrossRef]
- Tang, L.; Li, P.; Han, Y.; Yang, G.; Xin, H.; Zhao, S.; Guan, R.; Liu, Z.; Cao, D. A fluorescein-based fluorescent probe for real-time monitoring hypochlorite. J. Photochem. Photobiol. A 2023, 438, 114511. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, H.; Yang, B.; Leng, X. Development of a Fluorescein-Based Probe with an “Off–On” Mechanism for Selective Detection of Copper (II) Ions and Its Application in Imaging of Living Cells. Biosensors 2023, 13, 301. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Du, Z.; Zhang, W.; Zhang, R.; Song, B.; Yuan, J. Development of a fluorescein modified ruthenium(II) complex probe for lysosome-targeted ratiometric luminescence detection and imaging of peroxynitrite in living cells. Anal. Chim. Acta 2022, 1205, 339784. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B. 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Weigend, F.; and Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Scalmani, G.; Frisch, M.J. Continuous surface charge polarizable continuum models of solvation. I. General formalism. J. Chem. Phys. 2010, 132, 114110. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, PA, USA, 2016. [Google Scholar]
- Legault, C.Y. CYL View, version 20; Universite de Sherbrooke: Sherbrooke, QC, Canada, 2020; Available online: https://www.cylview.org (accessed on 16 June 2025).
- Luchini, G.; Alegre-Requena, J.V.; Funes-Ardoiz, I.; Paton, R.S. GoodVibes: Automated thermochemistry for heterogeneous computational chemistry data. F1000Research 2020, 9, 291. [Google Scholar] [CrossRef]
- Shen, Y.; Zheng, W.; Yao, Y.; Wang, D.; Lv, G.; Li, C. Phenoxazine-based Near-infrared Fluorescent Probes for the Specific Detection of Copper (II) Ions in Living Cells. Chemistry—An Asian Journal 2020, 15, 2864–2867. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Deng, L.; Xue, L.; Gao, Y.; Cheng, Y.; Wang, H. A coumarin based “turn-on” type fluorescent probe for the detection of Cu2+ with mechanochromic property. J. Mol. Struct 2025, 1334, 141938. [Google Scholar] [CrossRef]
- Shruthi, B.; Revanasiddappa, H.D.; Jayalakshmi, B.; Syed, A.; Elgorban, A.M.; Eswaramoorthy, R.; Amachawadi, R.G.; Shivamallu, C.; Kollur, S.P. Hydrobenzoic acid-based ‘Turn-Off’ fluorescent sensor for selective detection of Cu2+ ions: Chemical preparation, characterization and photophysical studies. Inorg. Chem. Commun. 2023, 150, 110467. [Google Scholar] [CrossRef]
- Hao, C.; Zhang, F.; Jiang, T.; Ma, Y.; Ji, W.; Shi, Z. Perylene tetra-(alkoxycarbonyl) based ‘turn-on’ fluorescent probe for selective recognition of Cu(II) and its fluorescence imaging in living cells. Tetrahedron 2022, 117–118, 132840. [Google Scholar] [CrossRef]
- Ge, C.; Pei, F.; Wang, X.; Zhang, P.; Li, H.; Sai, Z.; Yang, Y.; Chang, K.; Ni, T.; Yang, Z. Coumarin-based fluorescent probes for the detection of copper (II) and imaging in mice of Wilson’s disease. Bioorganic Chem. 2025, 154, 108051. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, H.; Fang, Y.; Liu, C.; Wang, K.; Zhang, X.; Li, X.; Ma, L.; Yu, M.; Sheng, W.; et al. 3D-printed colorimetric copper ion detection kit and portable fluorescent sensing device using smartphone based on ratiometric fluorescent probes. Anal. Chim. Acta 2024, 1286, 341980. [Google Scholar] [CrossRef]
- Yao, W.; Zhu, D.; Ye, Y.; Wang, B.; Xie, W.; Ren, A. A novel colorimetric and ratiometric fluorescent probe for detection of Cu2+ with large stokes shift in complete aqueous solution. J. Mol. Struct. 2023, 1278, 134970. [Google Scholar] [CrossRef]




| Sample Type | Spiked (μM) | Detected (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Tap Water | 0 | Not detected | - | - |
| 4 | 4.28 | 107.00 | 3.09 | |
| 8 | 7.89 | 98.63 | 4.00 | |
| 12 | 12.34 | 102.83 | 3.61 | |
| River Water | 0 | Not detected | - | - |
| 4 | 3.72 | 93.00 | 2.84 | |
| 8 | 8.06 | 100.75 | 1.15 | |
| 12 | 12.38 | 103.17 | 2.84 | |
| Lake water | 0 | Not detected | - | - |
| 4 | 3.75 | 93.75 | 0.16 | |
| 8 | 8.36 | 104.50 | 3.87 | |
| 12 | 12.32 | 102.67 | 3.49 |
| Sample Type | Spiked (μM) | Detected (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Tap Water | 0 | Not detected | - | - |
| 4 | 4.13 | 103.25 | 0.57 | |
| 8 | 8.24 | 103.00 | 4.98 | |
| 12 | 12.16 | 101.33 | 0.29 | |
| River Water | 0 | Not detected | - | - |
| 4 | 3.97 | 99.25 | 1.31 | |
| 8 | 8.39 | 104.88 | 2.34 | |
| 12 | 11.96 | 99.67 | 0.31 | |
| Lake water | 0 | Not detected | - | - |
| 4 | 4.13 | 103.35 | 0.33 | |
| 8 | 8.47 | 105.88 | 1.45 | |
| 12 | 12.06 | 100.50 | 0.32 |
| Probe | Method | Solvent medium | λem/λmax | Response time | LOD | Reference |
|---|---|---|---|---|---|---|
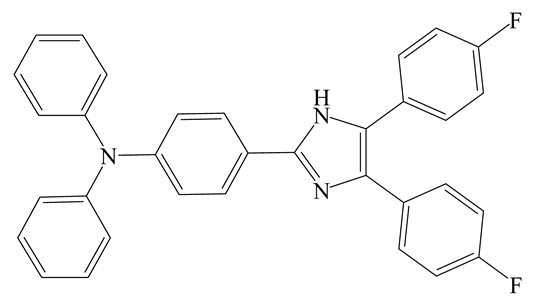 | FL | MeCN | 408 nm | 4 min | 3.93 µM | [2] |
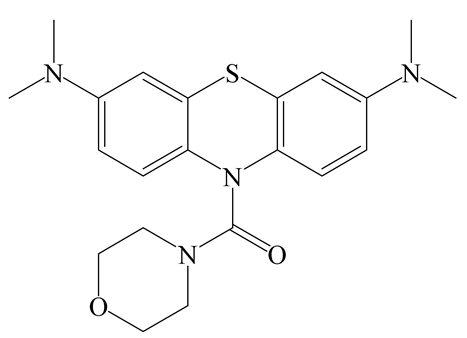 | FL | MeCN/Tris-HCl (4:6) | 689 nm | 5 min | 0.33 µM | [18] |
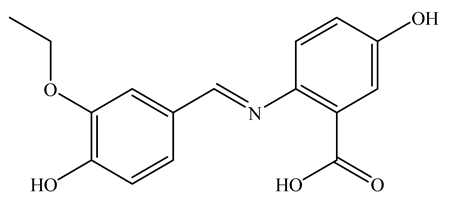 | UV | EtOH/H2O (8:2) | 275 nm (λmax) | Not mentioned | 0.39 µM | [48] |
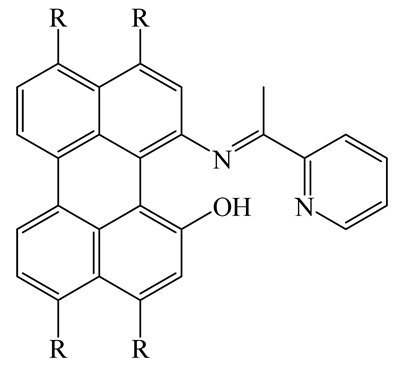 | FL | DMSO | 490 nm | 3 min | 0.89 µM | [49] |
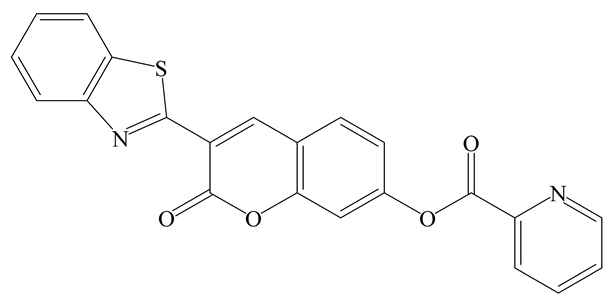 | FL | DMSO/PBS (3:7) | 493 nm | 30 min | 0.57 µM | [50] |
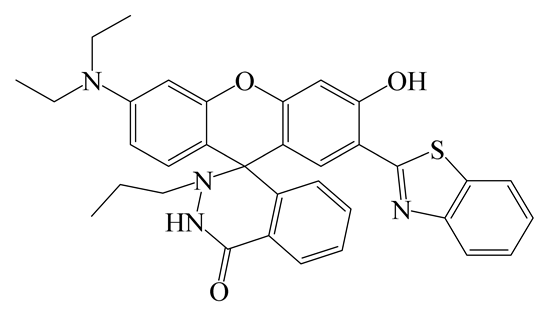 | FL | MeCN/HEPES (3:7) | 580 nm | 40 min | 0.25 µM | [51] |
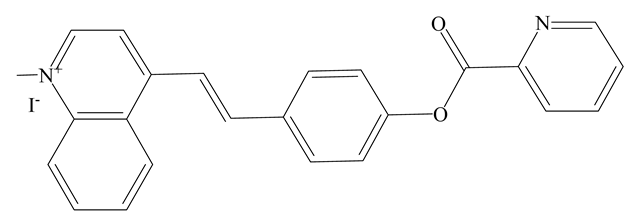 | FL | HEPES | F560 nm/F495 nm | 2 min | 0.62 µM | [52] |
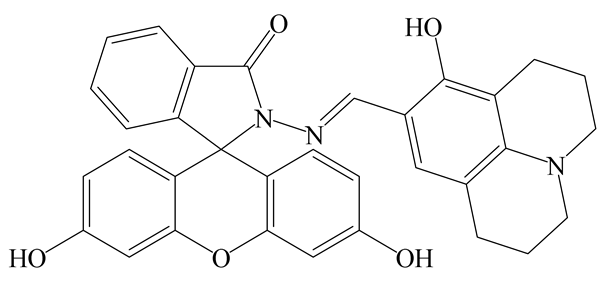 | FL UV | EtOH/HEPES (9:1) | 540 nm 452 nm (λmax) | 1 min 1 min | 0.22 µM 0.38 µM | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Lei, C.; Wang, Q.; He, Y.; Tian, S. A Novel Schiff Base Probe Based on Fluorescein for Fluorometric and Colorimetric Dual-Mode Rapid Detection of Cu2+. Molecules 2025, 30, 3824. https://doi.org/10.3390/molecules30183824
Yang Z, Lei C, Wang Q, He Y, Tian S. A Novel Schiff Base Probe Based on Fluorescein for Fluorometric and Colorimetric Dual-Mode Rapid Detection of Cu2+. Molecules. 2025; 30(18):3824. https://doi.org/10.3390/molecules30183824
Chicago/Turabian StyleYang, Zhi, Chaojie Lei, Qian Wang, Yonghui He, and Senlin Tian. 2025. "A Novel Schiff Base Probe Based on Fluorescein for Fluorometric and Colorimetric Dual-Mode Rapid Detection of Cu2+" Molecules 30, no. 18: 3824. https://doi.org/10.3390/molecules30183824
APA StyleYang, Z., Lei, C., Wang, Q., He, Y., & Tian, S. (2025). A Novel Schiff Base Probe Based on Fluorescein for Fluorometric and Colorimetric Dual-Mode Rapid Detection of Cu2+. Molecules, 30(18), 3824. https://doi.org/10.3390/molecules30183824






