Vitamin D Associated with Exercise Can Be Used as a Promising Tool in Neurodegenerative Disease Protection
Abstract
1. Introduction
2. The Versatile Identity of Vitamin D
3. Neuroinflammation and Neurodegeneration: A Two-Way Route
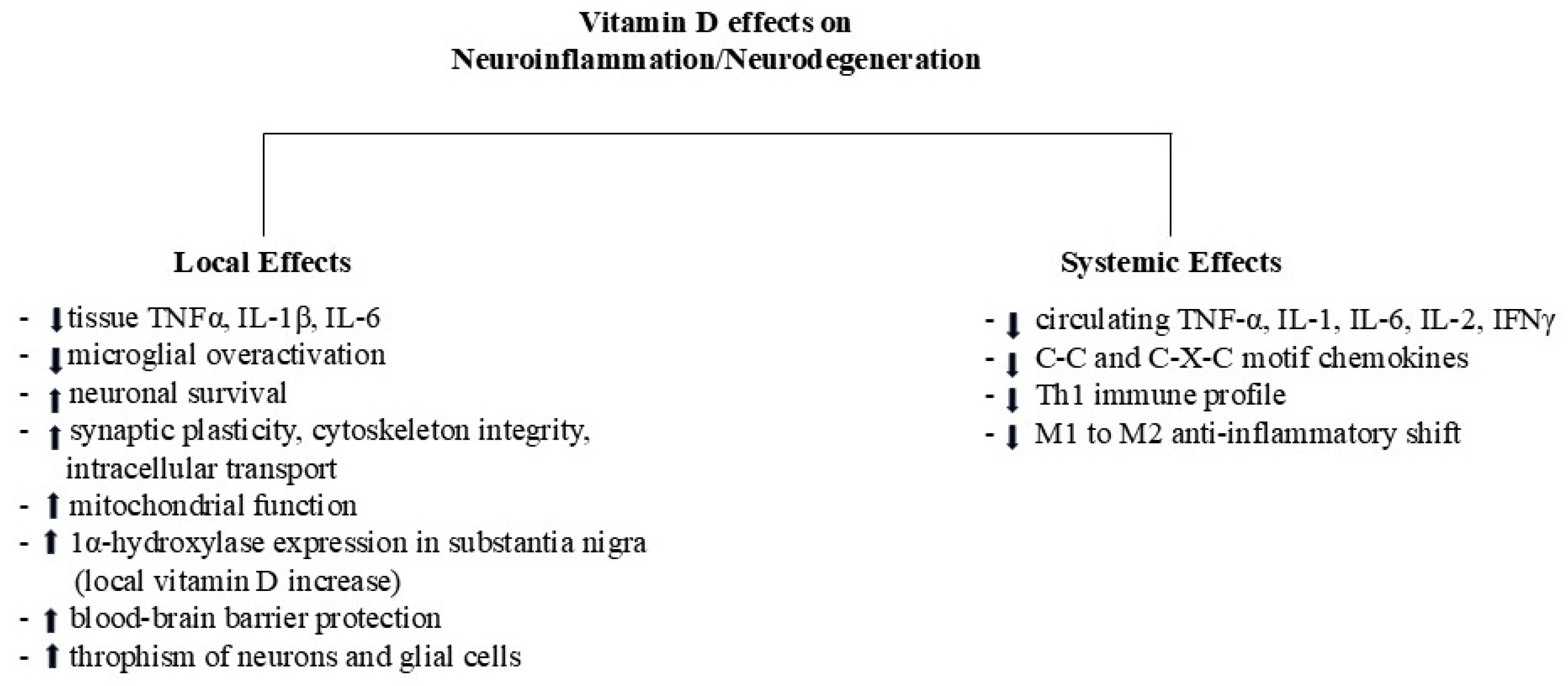
The Systemic-Local Detrimental Loop in CNS
4. Vitamin D Status: Towards Neuroprotection or Neurodegeneration
5. Vitamin D Status and Exercise: A Possible Synergy in Neuroprotection
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Autier, P.; Boniol, M.; Pizot, C.; Mullie, P. Vitamin D status and ill health: A systematic review. Lancet. Diabetes Endocrinol. 2014, 2, 76–89. [Google Scholar] [CrossRef]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Meng, X. Vitamin D and neurodegenerative diseases. Heliyon 2023, 9, e12877. [Google Scholar] [CrossRef]
- Eyles, D.W.; Burne, T.H.; McGrath, J.J. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front. Neuroendocrinol. 2013, 34, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- Giustina, A.; Bouillon, R.; Binkley, N.; Sempos, C.; Adler, R.A.; Bollerslev, J.; Dawson-Hughes, B.; Ebeling, P.R.; Feldman, D.; Heijboer, A.; et al. Controversies in Vitamin D: A Statement From the Third International Conference. JBMR Plus 2020, 4, e10417. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D: Evolutionary, physiological and health perspectives. Curr. Drug Targets 2011, 12, 4–18. [Google Scholar] [CrossRef]
- Bouillon, R.; Okamura, W.H.; Norman, A.W. Structure-function relationships in the vitamin D endocrine system. Endocr. Rev. 1995, 16, 200–257. [Google Scholar] [CrossRef] [PubMed]
- Goltzman, D. Functions of vitamin D in bone. Histochem. Cell Biol. 2018, 149, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Pike, J.W.; Meyer, M.B.; Bishop, K.A. Regulation of target gene expression by the vitamin D receptor—An update on mechanisms. Rev. Endocr. Metab. Disord. 2012, 13, 45–55. [Google Scholar] [CrossRef]
- Buitrago, C.; Boland, R. Caveolae and caveolin-1 are implicated in 1alpha,25(OH)2-vitamin D3-dependent modulation of Src, MAPK cascades and VDR localization in skeletal muscle cells. J. Steroid Biochem. Mol. Biol. 2010, 121, 169–175. [Google Scholar] [CrossRef]
- Khanal, R.C.; Nemere, I. The ERp57/GRp58/1,25 D3-MARRS receptor: Multiple functional roles in diverse cell systems. Curr. Med. Chem. 2007, 14, 1087–1093. [Google Scholar] [CrossRef]
- Penna, G.; Adorini, L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J. Immunol. 2000, 164, 2405–2411. [Google Scholar] [CrossRef]
- Berer, A.; Stöckl, J.; Majdic, O.; Wagner, T.; Kollars, M.; Lechner, K.; Geissler, K.; Oehler, L. 1,25-Dihydroxyvitamin D(3) inhibits dendritic cell differentiation and maturation in vitro. Exp. Hematol. 2000, 28, 575–583. [Google Scholar] [CrossRef]
- Thu, V.T.A.; Hoang, T.X.; Kim, J.Y. 1,25-Dihydroxy Vitamin D3 Facilitates the M2 Polarization and β-Amyloid Uptake by Human Microglia in a TREM2-Dependent Manner. BioMed Res. Int. 2023, 2023, 3483411. [Google Scholar] [CrossRef]
- Ransohoff, R.M.; Brown, M.A. Innate immunity in the central nervous system. J. Clin. Investig. 2012, 122, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Adamu, A.; Li, S.; Gao, F.; Xue, G. The role of neuroinflammation in neurodegenerative diseases: Current understanding and future therapeutic targets. Front. Aging Neurosci. 2024, 16, 1347987. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Kareem, O.; Khushtar, M.; Akbar, M.; Haque, M.R.; Iqubal, A.; Haider, M.F.; Pottoo, F.H.; Abdulla, F.S.; Al-Haidar, M.B.; et al. Neuroinflammation: A Potential Risk for Dementia. Int. J. Mol. Sci. 2022, 23, 616. [Google Scholar] [CrossRef]
- Mayne, K.; White, J.A.; McMurran, C.E.; Rivera, F.J.; de la Fuente, A.G. Aging and Neurodegenerative Disease: Is the Adaptive Immune System a Friend or Foe? Front. Aging Neurosci. 2020, 12, 572090. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Zamani, A.; Dill, L.K.; Sun, M.; Chu, E.; Robinson, M.J.; O’Brien, T.J.; Shultz, S.R.; Semple, B.D. A systemic immune challenge to model hospital-acquired infections independently regulates immune responses after pediatric traumatic brain injury. J. Neuroinflammation 2021, 18, 72. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Ma, Z.; Chen, X.; Shu, S. Microglia activation in central nervous system disorders: A review of recent mechanistic investigations and development efforts. Front. Neurol. 2023, 14, 1103416. [Google Scholar] [CrossRef] [PubMed]
- Ronaldson, P.T.; Davis, T.P. Regulation of blood-brain barrier integrity by microglia in health and disease: A therapeutic opportunity. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2020, 40 (Suppl. 1), S6–S24. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H.; Bohr, V.A. Signaling by cGAS-STING in Neurodegeneration, Neuroinflammation, and Aging. Trends Neurosci. 2021, 44, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Costantini, E.; D’Angelo, C.; Reale, M. The Role of Immunosenescence in Neurodegenerative Diseases. Mediat. Inflamm. 2018, 2018, 6039171. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Hamilton, J.A. GM-CSF in inflammation. J. Exp. Med. 2020, 217, e20190945. [Google Scholar] [CrossRef]
- Codarri, L.; Gyülvészi, G.; Tosevski, V.; Hesske, L.; Fontana, A.; Magnenat, L.; Suter, T.; Becher, B. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat. Immunol. 2011, 12, 560–567. [Google Scholar] [CrossRef]
- Sobue, A.; Komine, O.; Yamanaka, K. Neuroinflammation in Alzheimer’s disease: Microglial signature and their relevance to disease. Inflamm. Regen. 2023, 43, 26. [Google Scholar] [CrossRef]
- Stefanis, L. α-Synuclein in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009399. [Google Scholar] [CrossRef]
- Pajares, M.; IRojo, A.; Manda, G.; Boscá, L.; Cuadrado, A. Inflammation in Parkinson’s Disease: Mechanisms and Therapeutic Implications. Cells 2020, 9, 1687. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, A.G.; Pelucchi, S.; Mertens, J.; Di Luca, M.; Mauceri, D.; Marcello, E. Novel therapeutic approaches to target neurodegeneration. Br. J. Pharmacol. 2023, 180, 1651–1673. [Google Scholar] [CrossRef] [PubMed]
- Landel, V.; Annweiler, C.; Millet, P.; Morello, M.; Féron, F. Vitamin D, Cognition and Alzheimer’s Disease: The Therapeutic Benefit is in the D-Tails. J. Alzheimer’s Dis. JAD 2016, 53, 419–444. [Google Scholar] [CrossRef] [PubMed]
- Laso’ n, W.; Jantas, D.; Leśkiewicz, M.; Regulska, M.; Basta-Kaim, A. The Vitamin D Receptor as a Potential Target for the Treatment of Age-Related Neurodegenerative Diseases Such as Alzheimer’s and Parkinson’s Diseases: A Narrative Review. Cells 2023, 12, 660. [Google Scholar] [CrossRef]
- Lang, F.; Ma, K.; Leibrock, C.B. 1,25(OH)2D3 in Brain Function and Neuropsychiatric Disease. Neuro Signals 2019, 27, 40–49. [Google Scholar] [CrossRef]
- Lasoń, W.; Jantas, D.; Leśkiewicz, M.; Regulska, M.; Basta-Kaim, A. Vitamin D3 and Ischemic Stroke: A Narrative Review. Antioxidants 2022, 11, 2120. [Google Scholar] [CrossRef]
- Smolders, J.; Schuurman, K.G.; van Strien, M.E.; Melief, J.; Hendrickx, D.; Hol, E.M.; van Eden, C.; Luchetti, S.; Huitinga, I. Expression of vitamin D receptor and metabolizing enzymes in multiple sclerosis-affected brain tissue. J. Neuropathol. Exp. Neurol. 2013, 72, 91–105. [Google Scholar] [CrossRef]
- Landel, V.; Stephan, D.; Cui, X.; Eyles, D.; Feron, F. Differential expression of vitamin D-associated enzymes and receptors in brain cell subtypes. J. Steroid Biochem. Mol. Biol. 2018, 177, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, W.E.; O’Brien, L.P. 1,25 (OH)2 vitamin D3 sites of action in the brain. An autoradiographic study. Histochemistry 1987, 87, 393–406. [Google Scholar] [CrossRef]
- Baas, D.; Prüfer, K.; Ittel, M.E.; Kuchler-Bopp, S.; Labourdette, G.; Sarliève, L.L.; Brachet, P. Rat oligodendrocytes express the vitamin D(3) receptor and respond to 1,25-dihydroxyvitamin D(3). Glia 2000, 31, 59–68. [Google Scholar] [CrossRef]
- Zorrilla Veloz, R.I.; McKenzie, T.; Palacios, B.E.; Hu, J. Nuclear hormone receptors in demyelinating diseases. J. Neuroendocrinol. 2022, 34, e13171. [Google Scholar] [CrossRef]
- Holick, M.F.; Mazzei, L.; García Menéndez, S.; Martín Giménez, V.M.; Al Anouti, F.; Manucha, W. Genomic or Non-Genomic? A Question about the Pleiotropic Roles of Vitamin D in Inflammatory-Based Diseases. Nutrients 2023, 15, 767. [Google Scholar] [CrossRef]
- Gómez-Oliva, R.; Geribaldi-Doldán, N.; Domínguez-García, S.; Carrascal, L.; Verástegui, C.; Nunez-Abades, P.; Castro, C. Vitamin D deficiency as a potential risk factor for accelerated aging, impaired hippocampal neurogenesis and cognitive decline: A role for Wnt/β-catenin signaling. Aging 2020, 12, 13824–13844. [Google Scholar] [CrossRef]
- Arrázola, M.S.; Silva-Alvarez, C.; Inestrosa, N.C. How the Wnt signaling pathway protects from neurodegeneration: The mitochondrial scenario. Front. Cell. Neurosci. 2015, 9, 166. [Google Scholar] [CrossRef]
- Uthaiah, C.A.; Beeraka, N.M.; Rajalakshmi, R.; Ramya, C.M.; Madhunapantula, S.V. Role of Neural Stem Cells and Vitamin D Receptor (VDR)-Mediated Cellular Signaling in the Mitigation of Neurological Diseases. Mol. Neurobiol. 2022, 59, 4065–4105. [Google Scholar] [CrossRef] [PubMed]
- Ziebell, F.; Dehler, S.; Martin-Villalba, A.; Marciniak-Czochra, A. Revealing age-related changes of adult hippocampal neurogenesis using mathematical models. Development 2018, 145, dev153544. [Google Scholar] [CrossRef]
- González-Sancho, J.M.; Larriba, M.J.; Muñoz, A. Wnt and Vitamin D at the Crossroads in Solid Cancer. Cancers 2020, 12, 3434. [Google Scholar] [CrossRef]
- Agliardi, C.; Guerini, F.R.; Zanzottera, M.; Bolognesi, E.; Meloni, M.; Riboldazzi, G.; Zangaglia, R.; Sturchio, A.; Casali, C.; Di Lorenzo, C.; et al. The VDR FokI (rs2228570) polymorphism is involved in Parkinson’s disease. J. Neurol. Sci. 2021, 428, 117606. [Google Scholar] [CrossRef] [PubMed]
- Decressac, M.; Volakakis, N.; Björklund, A.; Perlmann, T. NURR1 in Parkinson disease—From pathogenesis to therapeutic potential. Nat. Rev. Neurol. 2013, 9, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Haavik, J.; Toska, K. Tyrosine hydroxylase and Parkinson’s disease. Mol. Neurobiol. 1998, 16, 285–309. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Shirazi, H.A.; Rasouli, J.; Ciric, B.; Rostami, A.; Zhang, G.X. 1,25-Dihydroxyvitamin D3 enhances neural stem cell proliferation and oligodendrocyte differentiation. Exp. Mol. Pathol. 2015, 98, 240–245. [Google Scholar] [CrossRef]
- da Silva Teixeira, S.; Harrison, K.; Uzodike, M.; Rajapakshe, K.; Coarfa, C.; He, Y.; Xu, Y.; Sisley, S. Vitamin D actions in neurons require the PI3K pathway for both enhancing insulin signaling and rapid depolarizing effects. J. Steroid Biochem. Mol. Biol. 2020, 200, 105690. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, W.; Zhang, S.; Gao, N.; Xu, T.; Wang, X.; Zhang, M. Vitamin D Inhibits the Early Aggregation of α-Synuclein and Modulates Exocytosis Revealed by Electrochemical Measurements. Angew. Chem. 2022, 61, e202111853. [Google Scholar] [CrossRef]
- Zheng, Z.; Chen, M.; Feng, S.; Zhao, H.; Qu, T.; Zhao, X.; Ruan, Q.; Li, L.; Guo, J. VDR and deubiquitination control neuronal oxidative stress and microglial inflammation in Parkinson’s disease. Cell Death Discov. 2024, 10, 150. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Selim, H.M.; Alexiou, A.; Papadakis, M.; Negm, W.A.; Batiha, G.E. Does vitamin D protect or treat Parkinson’s disease? A narrative review. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 33–40. [Google Scholar] [CrossRef]
- Sharma, S.; Borski, C.; Hanson, J.; Garcia, M.A.; Link, C.D.; Hoeffer, C.; Chatterjee, A.; Nagpal, P. Identifying an Optimal Neuroinflammation Treatment Using a Nanoligomer Discovery Engine. ACS Chem. Neurosci. 2022, 13, 3247–3256. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, Y.H.; Kim, M.S.; Kwon, B.; Cho, H.R. Efficacy and Safety of Early Anti-inflammatory Drug Therapy for Secondary Injury in Traumatic Brain Injury. World Neurosurg. 2023, 172, e646–e654. [Google Scholar] [CrossRef] [PubMed]
- Boontanrart, M.; Hall, S.D.; Spanier, J.A.; Hayes, C.E.; Olson, J.K. Vitamin D3 alters microglia immune activation by an IL-10 dependent SOCS3 mechanism. J. Neuroimmunol. 2016, 292, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Calvello, R.; Cianciulli, A.; Nicolardi, G.; De Nuccio, F.; Giannotti, L.; Salvatore, R.; Porro, C.; Trotta, T.; Panaro, M.A.; Lofrumento, D.D. Vitamin D Treatment Attenuates Neuroinflammation and Dopaminergic Neurodegeneration in an Animal Model of Parkinson’s Disease, Shifting M1 to M2 Microglia Responses. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2017, 12, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Sayeed, I.; Turan, N.; Stein, D.G.; Wali, B. Vitamin D deficiency increases blood-brain barrier dysfunction after ischemic stroke in male rats. Exp. Neurol. 2019, 312, 63–71. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Xu, S.; Zhao, Y.; Pang, M.; Zhang, X.; Wang, X.; Wang, Y. 1,25-D3 attenuates cerebral ischemia injury by regulating mitochondrial metabolism via the AMPK/AKT/GSK3β pathway. Front. Aging Neurosci. 2022, 14, 1015453. [Google Scholar] [CrossRef] [PubMed]
- Rastegar-Moghaddam, S.H.; Alipour, F.; Hosseini, M.; Ebrahimzadeh-Bideskan, A. Anti-apoptotic and neurogenic properties in the hippocampus as possible mechanisms for learning and memory improving impacts of vitamin D in hypothyroid rats during the growth period. Life Sci. 2023, 312, 121209. [Google Scholar] [CrossRef] [PubMed]
- Calton, E.K.; Keane, K.N.; Soares, M.J. The potential regulatory role of vitamin D in the bioenergetics of inflammation. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 367–373. [Google Scholar] [CrossRef]
- Garcion, E.; Sindji, L.; Montero-Menei, C.; Andre, C.; Brachet, P.; Darcy, F. Expression of inducible nitric oxide synthase during rat brain inflammation: Regulation by 1,25-dihydroxyvitamin D3. Glia 1998, 22, 282–294. [Google Scholar] [CrossRef]
- Garcion, E.; Sindji, L.; Leblondel, G.; Brachet, P.; Darcy, F. 1,25-dihydroxyvitamin D3 regulates the synthesis of gamma-glutamyl transpeptidase and glutathione levels in rat primary astrocytes. J. Neurochem. 1999, 73, 859–866. [Google Scholar] [CrossRef]
- Berridge, M.J. Vitamin D, reactive oxygen species and calcium signalling in ageing and disease. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 2016, 371, 20150434. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.; Almeras, L.; Benech, P.; Patatian, A.; Mackay-Sim, A.; McGrath, J.; Féron, F. Developmental vitamin D deficiency alters the expression of genes encoding mitochondrial, cytoskeletal and synaptic proteins in the adult rat brain. J. Steroid Biochem. Mol. Biol. 2007, 103, 538–545. [Google Scholar] [CrossRef]
- Almeras, L.; Eyles, D.; Benech, P.; Laffite, D.; Villard, C.; Patatian, A.; Boucraut, J.; Mackay-Sim, A.; McGrath, J.; Féron, F. Developmental vitamin D deficiency alters brain protein expression in the adult rat: Implications for neuropsychiatric disorders. Proteomics 2007, 7, 769–780. [Google Scholar] [CrossRef]
- de Abreu, D.A.F.; Eyles, D.; Féron, F. Vitamin D, a neuro-immunomodulator: Implications for neurodegenerative and autoimmune diseases. Psychoneuroendocrinology 2009, 34 (Suppl. 1), S265–S277. [Google Scholar] [CrossRef]
- Kim, H.A.; Perrelli, A.; Ragni, A.; Retta, F.; De Silva, T.M.; Sobey, C.G.; Retta, S.F. Vitamin D Deficiency and the Risk of Cerebrovascular Disease. Antioxidants 2020, 9, 327. [Google Scholar] [CrossRef]
- Farghali, M.; Ruga, S.; Morsanuto, V.; Uberti, F. Can Brain Health Be Supported by Vitamin D-Based Supplements? A Critical Review. Brain Sci. 2020, 10, 660. [Google Scholar] [CrossRef]
- Morello, M.; Landel, V.; Lacassagne, E.; Baranger, K.; Annweiler, C.; Féron, F.; Millet, P. Vitamin D Improves Neurogenesis and Cognition in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 6463–6479. [Google Scholar] [CrossRef]
- Pal, R.; Choudhury, S.; Kumar, H.; Dey, S.; Das, N.; Basu, B.R. Vitamin D deficiency and genetic polymorphisms of vitamin D-associated genes in Parkinson’s disease. Eur. J. Neurosci. 2023, 58, 3362–3377. [Google Scholar] [CrossRef]
- Mirarchi, A.; Albi, E.; Beccari, T.; Arcuri, C. Microglia and Brain Disorders: The Role of Vitamin D and Its Receptor. Int. J. Mol. Sci. 2023, 24, 11892. [Google Scholar] [CrossRef]
- Li, D.; Ma, X.; Zhang, W.; Zhong, P.; Li, M.; Liu, S. Impact of vitamin D3 supplementation on motor functionality and the immune response in Parkinson’s disease patients with vitamin D deficiency. Sci. Rep. 2025, 15, 25154. [Google Scholar] [CrossRef]
- Alcalá-Santiago, Á.; Toscano-Sánchez, R.; Márquez-López, J.C.; González-Jurado, J.A.; Fernández-Pachón, M.S.; García-Villanova, B.; Pedroche, J.; Rodríguez-Martín, N.M. The Synergic Immunomodulatory Effect of Vitamin D and Chickpea Protein Hydrolysate in THP-1 Cells: An In Vitro Approach. Int. J. Mol. Sci. 2024, 25, 12628. [Google Scholar] [CrossRef]
- Zha, X.; Liu, M.; Ruan, S.; Jiang, Y.; Wang, S. Relationship between serum vitamin D levels and pro-inflammatory cytokines in patients with rheumatoid arthritis combined with cardiovascular disease. Clin. Rheumatol. 2025, 44, 3467–3475. [Google Scholar] [CrossRef] [PubMed]
- Colotta, F.; Jansson, B.; Bonelli, F. Modulation of inflammatory and immune responses by vitamin D. J. Autoimmun. 2017, 85, 78–97. [Google Scholar] [CrossRef]
- Naeini, A.E.; Moeinzadeh, F.; Vahdat, S.; Ahmadi, A.; Hedayati, Z.P.; Shahzeidi, S. The Effect of Vitamin D Administration on Intracellular Adhesion Molecule-1 and Vascular Cell Adhesion Molecule-1 Levels in Hemodialysis Patients: A Placebo-controlled, Double-blinded Clinical Trial. J. Res. Pharm. Pract. 2017, 6, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Scolletta, S.; Colletti, M.; Di Luigi, L.; Crescioli, C. Vitamin D receptor agonists target CXCL10: New therapeutic tools for resolution of inflammation. Mediat. Inflamm. 2013, 2013, 876319. [Google Scholar] [CrossRef] [PubMed]
- Sottili, M.; Cosmi, L.; Borgogni, E.; Sarchielli, E.; Maggi, L.; Francalanci, M.; Vannelli, G.B.; Ronconi, E.; Adorini, L.; Annunziato, F.; et al. Immunomodulatory effects of BXL-01-0029, a less hypercalcemic vitamin D analogue, in human cardiomyocytes and T cells. Exp. Cell Res. 2009, 315, 264–273. [Google Scholar] [CrossRef]
- Crescioli, C.; Sottili, M.; Bonini, P.; Cosmi, L.; Chiarugi, P.; Romagnani, P.; Vannelli, G.B.; Colletti, M.; Isidori, A.M.; Serio, M.; et al. Inflammatory response in human skeletal muscle cells: CXCL10 as potential therapeutic target. Eur. J. Cell Biol. 2012, 91, 139–149. [Google Scholar] [CrossRef]
- Morelli, A.; Vignozzi, L.; Filippi, S.; Vannelli, G.B.; Ambrosini, S.; Mancina, R.; Crescioli, C.; Donati, S.; Fibbi, B.; Colli, E.; et al. BXL-628, a vitamin D receptor agonist effective in benign prostatic hyperplasia treatment, prevents RhoA activation and inhibits RhoA/Rho kinase signaling in rat and human bladder. Prostate 2007, 67, 234–247. [Google Scholar] [CrossRef]
- Sagrinati, C.; Sottili, M.; Mazzinghi, B.; Borgogni, E.; Adorini, L.; Serio, M.; Romagnani, P.; Crescioli, C. Comparison between VDR analogs and current immunosuppressive drugs in relation to CXCL10 secretion by human renal tubular cells. Transpl. Int. 2010, 23, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Chel, V.G.; Ooms, M.E.; van der Bent, J.; Veldkamp, F.; Roos, R.A.; Achterberg, W.P.; Lips, P. High prevalence of vitamin D deficiency and insufficiency in patients with manifest Huntington disease: An explorative study. Derm. Endocrinol. 2013, 5, 348–351. [Google Scholar] [CrossRef][Green Version]
- Molnár, M.F.; Török, R.; Szalárdy, L.; Sümegi, E.; Vécsei, L.; Klivényi, P. High-dose 1,25-dihydroxyvitamin D supplementation elongates the lifespan of Huntington’s disease transgenic mice. Acta Neurobiol. Exp. 2016, 76, 176–181. [Google Scholar] [CrossRef][Green Version]
- Rai, S.N.; Singh, P.; Steinbusch, H.W.M.; Vamanu, E.; Ashraf, G.; Singh, M.P. The Role of Vitamins in Neurodegenerative Disease: An Update. Biomedicines 2021, 9, 1284. [Google Scholar] [CrossRef]
- de Miranda, R.C.; Di Lorenzo, N.; Andreoli, A.; Romano, L.; De Santis, G.L.; Gualtieri, P.; De Lorenzo, A. Body composition and bone mineral density in Huntington’s disease. Nutrition 2019, 59, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Goodman, A.O.; Barker, R.A. Body composition in premanifest Huntington’s disease reveals lower bone density compared to controls. PLoS Curr. 2011, 3, RRN1214. [Google Scholar] [CrossRef] [PubMed]
- Duyao, M.; Ambrose, C.; Myers, R.; Novelletto, A.; Persichetti, F.; Frontali, M.; Folstein, S.; Ross, C.; Franz, M.; Abbott, M. Trinucleotide repeat length instability and age of onset in Huntington’s disease. Nat. Genet. 1993, 4, 387–392. [Google Scholar] [CrossRef]
- Zuccato, C.; Ciammola, A.; Rigamonti, D.; Leavitt, B.R.; Goffredo, D.; Conti, L.; MacDonald, M.E.; Friedlander, R.M.; Silani, V.; Hayden, M.R.; et al. Loss of huntingtin-mediated BDNF gene transcription in Huntington’s disease. Science 2001, 293, 493–498. [Google Scholar] [CrossRef]
- Manjari, S.K.V.; Maity, S.; Poornima, R.; Yau, S.Y.; Vaishali, K.; Stellwagen, D.; Komal, P. Restorative Action of Vitamin D3 on Motor Dysfunction Through Enhancement of Neurotrophins and Antioxidant Expression in the Striatum. Neuroscience 2022, 492, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Smolders, J.; Damoiseaux, J.; Menheere, P.; Hupperts, R. Vitamin D as an immune modulator in multiple sclerosis, a review. J. Neuroimmunol. 2008, 194, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Sintzel, M.B.; Rametta, M.; Reder, A.T. Vitamin D and Multiple Sclerosis: A Comprehensive Review. Neurol. Ther. 2018, 7, 59–85. [Google Scholar] [CrossRef]
- Mashayekhi, F.; Salehi, Z. Administration of vitamin D3 induces CNPase and myelin oligodendrocyte glycoprotein expression in the cerebral cortex of the murine model of cuprizone-induced demyelination. Folia Neuropathol. 2016, 54, 259–264. [Google Scholar] [CrossRef]
- Savran, Z.; Baltaci, S.B.; Aladag, T.; Mogulkoc, R.; Baltaci, A.K. Vitamin D and Neurodegenerative Diseases Such as Multiple Sclerosis (MS), Parkinson’s Disease (PD), Alzheimer’s Disease (AD), and Amyotrophic Lateral Sclerosis (ALS): A Review of Current Literature. Curr. Nutr. Rep. 2025, 14, 77. [Google Scholar] [CrossRef]
- Müller, T.; Lohse, L.; Blodau, A.; Frommholz, K. Vitamin D rise enhances blood perfusion in patients with multiple sclerosis. J. Neural Transm. 2019, 126, 1631–1636. [Google Scholar] [CrossRef]
- Perga, S.; Giuliano Albo, A.; Lis, K.; Minari, N.; Falvo, S.; Marnetto, F.; Caldano, M.; Reviglione, R.; Berchialla, P.; Capobianco, M.A.; et al. Vitamin D Binding Protein Isoforms and Apolipoprotein E in Cerebrospinal Fluid as Prognostic Biomarkers of Multiple Sclerosis. PLoS ONE 2015, 10, e0129291. [Google Scholar] [CrossRef]
- Disanto, G.; Ramagopalan, S.V.; Para, A.E.; Handunnetthi, L. The emerging role of vitamin D binding protein in multiple sclerosis. J. Neurol. 2011, 258, 353–358. [Google Scholar] [CrossRef]
- Long, K.V.; Nguyễn, L.T. Roles of vitamin D in amyotrophic lateral sclerosis: Possible genetic and cellular signaling mechanisms. Mol. Brain 2013, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Libonati, L.; Onesti, E.; Gori, M.C.; Ceccanti, M.; Cambieri, C.; Fabbri, A.; Frasca, V.; Inghilleri, M. Vitamin D in amyotrophic lateral sclerosis. Funct. Neurol. 2017, 32, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Cranney, A.; Horsley, T.; O’Donnell, S.; Weiler, H.; Puil, L.; Ooi, D.; Atkinson, S.; Ward, L.; Moher, D.; Hanley, D.; et al. Effectiveness and safety of vitamin D in relation to bone health. Evid. Rep. Technol. Assess. 2007, 158, 1–235. [Google Scholar]
- Bouillon, R.; van Schoor, N.M.; Gielen, E.; Boonen, S.; Mathieu, C.; Vanderschueren, D.; Lips, P. Optimal vitamin D status: A critical analysis on the basis of evidence-based medicine. J. Clin. Endocrinol. Metab. 2013, 98, E1283–E1304. [Google Scholar] [CrossRef]
- Lips, P.; Cashman, K.D.; Lamberg-Allardt, C.; Bischoff-Ferrari, H.A.; Obermayer-Pietsch, B.; Bianchi, M.L.; Stepan, J.; El-Hajj Fuleihan, G.; Bouillon, R. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: A position statement of the European Calcified Tissue Society. Eur. J. Endocrinol. 2019, 180, P23–P54. [Google Scholar] [CrossRef]
- El-Hajj Fuleihan, G.; Bouillon, R.; Clarke, B.; Chakhtoura, M.; Cooper, C.; McClung, M.R.; Singh, R. Serum 25-hydroxyvitamin D levels: Variability, knowledge gaps and the concept of a desirable range. J. Bone Miner. Res. 2015, 30, 1119–1133. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Bilezikian, J.P.; Adler, R.A.; Banfi, G.; Bikle, D.D.; Binkley, N.C.; Bollerslev, J.; Bouillon, R.; Brandi, M.L.; Casanueva, F.F.; et al. Consensus Statement on Vitamin D Status Assessment and Supplementation: Whys, Whens, and Hows. Endocr. Rev. 2024, 45, 625–654. [Google Scholar] [CrossRef]
- Ben Ezzdine, L.; Dhahbi, W.; Dergaa, I.; Ceylan, H.İ.; Guelmami, N.; Ben Saad, H.; Chamari, K.; Stefanica, V.; El Omri, A. Physical activity and neuroplasticity in neurodegenerative disorders: A comprehensive review of exercise interventions, cognitive training, and AI applications. Front. Neurosci. 2025, 19, 1502417. [Google Scholar] [CrossRef]
- Guo, J.; Mo, H.; Zuo, L.; Zhang, X. Association of physical activity and vitamin D deficiency with cognitive impairment in older adults: A population based cross-sectional analysis. Front. Nutr. 2024, 11, 1390903. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization, T. Global recommendations on physical activity for health. In Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010; p. 60. [Google Scholar]
- Bherer, L.; Erickson, K.I.; Liu-Ambrose, T. A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. J. Aging Res. 2013, 2013, 657508. [Google Scholar] [CrossRef]
- Hamer, M.; Chida, Y. Physical activity and risk of neurodegenerative disease: A systematic review of prospective evidence. Psychol. Med. 2009, 39, 3–11. [Google Scholar] [CrossRef]
- Colcombe, S.J.; Erickson, K.I.; Scalf, P.E.; Kim, J.S.; Prakash, R.; McAuley, E.; Elavsky, S.; Marquez, D.X.; Hu, L.; Kramer, A.F. Aerobic exercise training increases brain volume in aging humans. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 1166–1170. [Google Scholar] [CrossRef]
- Hötting, K.; Schickert, N.; Kaiser, J.; Röder, B.; Schmidt-Kassow, M. The Effects of Acute Physical Exercise on Memory, Peripheral BDNF, and Cortisol in Young Adults. Neural Plast. 2016, 2016, 6860573. [Google Scholar] [CrossRef]
- Beltran, G.; Park, K.S.; Arellano, J.; Reyes, S.; Park, J.; Cho, S.K. Effects of exercise intensities on changes in pro-/anti-inflammatory cytokines following 8-weeks of aerobic exercise training. Physiology 2024, 39 (Suppl. 1), 570. [Google Scholar] [CrossRef]
- Seo, D.Y.; Heo, J.W.; Ko, J.R.; Kwak, H.B. Exercise and Neuroinflammation in Health and Disease. Int. Neurourol. J. 2019, 23 (Suppl. 2), S82–S92. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef]
- Nieman, D.C.; Pence, B.D. Exercise immunology: Future directions. J. Sport Health Sci. 2020, 9, 432–445. [Google Scholar] [CrossRef] [PubMed]
- Forte, P.; Branquinho, L.; Ferraz, R. The Relationships between Physical Activity, Exercise, and Sport on the Immune System. Int. J. Environ. Res. Public Health 2022, 19, 6777. [Google Scholar] [CrossRef]
- Lee, J.H.; Jun, H.S. Role of Myokines in Regulating Skeletal Muscle Mass and Function. Front. Physiol. 2019, 10, 42. [Google Scholar] [CrossRef]
- Whitham, M.; Parker, B.L.; Friedrichsen, M.; Hingst, J.R.; Hjorth, M.; Hughes, W.E.; Egan, C.L.; Cron, L.; Watt, K.I.; Kuchel, R.P.; et al. Extracellular Vesicles Provide a Means for Tissue Crosstalk during Exercise. Cell Metab. 2018, 27, 237–251.e4. [Google Scholar] [CrossRef]
- Jin, L.; Han, S.; Lv, X.; Li, X.; Zhang, Z.; Kuang, H.; Chen, Z.; Lv, C.A.; Peng, W.; Yang, Z.; et al. The muscle-enriched myokine Musclin impairs beige fat thermogenesis and systemic energy homeostasis via Tfr1/PKA signaling in male mice. Nat. Commun. 2023, 14, 4257. [Google Scholar] [CrossRef]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle-Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef]
- Bettariga, F.; Taaffe, D.R.; Galvão, D.A.; Lopez, P.; Bishop, C.; Markarian, A.M.; Natalucci, V.; Kim, J.S.; Newton, R.U. Exercise training mode effects on myokine expression in healthy adults: A systematic review with meta-analysis. J. Sport Health Sci. 2024, 13, 764–779. [Google Scholar] [CrossRef]
- Kostka, M.; Morys, J.; Małecki, A.; Nowacka-Chmielewska, M. Muscle-brain crosstalk mediated by exercise-induced myokines—Insights from experimental studies. Front. Physiol. 2024, 15, 1488375. [Google Scholar] [CrossRef]
- Pedersen, B.K. Physical activity and muscle-brain crosstalk. Nat. Rev. Endocrinol. 2019, 15, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Aladdin, N.; Ghareib, S.A. Vitamin D3 Exerts a Neuroprotective Effect in Metabolic Syndrome Rats: Role of BDNF/TRKB/Akt/GS3Kβ Pathway. J. Biochem. Mol. Toxicol. 2024, 38, e70082. [Google Scholar] [CrossRef] [PubMed]
- Skoczek-Rubińska, A.; Cisek-Woźniak, A.; Molska, M.; Heyser, M.; Trocholepsza, M.; Pietrzak, S.; Mruczyk, K. Impact of Vitamin D Status and Supplementation on Brain-Derived Neurotrophic Factor and Mood-Cognitive Outcomes: A Structured Narrative Review. Nutrients 2025, 17, 2655. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Sun, Q.; Chen, S. Oxidative stress: A major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s disease and Alzheimer’s disease. Prog. Neurobiol. 2016, 147, 1–19. [Google Scholar] [CrossRef]
- Guo, S.; Wang, H.; Yin, Y. Microglia Polarization From M1 to M2 in Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 815347. [Google Scholar] [CrossRef]
- Sanesi, L.; Dicarlo, M.; Pignataro, P.; Zerlotin, R.; Pugliese, F.; Columbu, C.; Carnevale, V.; Tunnera, S.; Scillitani, A.; Grano, M.; et al. Vitamin D Increases Irisin Serum Levels and the Expression of Its Precursor in Skeletal Muscle. Int. J. Mol. Sci. 2023, 24, 4129. [Google Scholar] [CrossRef]
- Crescioli, C. Targeting Age-Dependent Functional and Metabolic Decline of Human Skeletal Muscle: The Geroprotective Role of Exercise, Myokine IL-6, and Vitamin D. Int. J. Mol. Sci. 2020, 21, 1010. [Google Scholar] [CrossRef] [PubMed]
- Pojednic, R.M.; Ceglia, L.; Lichtenstein, A.H.; Dawson-Hughes, B.; Fielding, R.A. Vitamin D receptor protein is associated with interleukin-6 in human skeletal muscle. Endocrine 2015, 49, 512–520. [Google Scholar] [CrossRef]
- Mathiasen, I.S.; Foghsgaard, L.; Hansen, C.M.; Jäättelä, M. Sensitization to TNF-induced apoptosis by 1,25-dihydroxy vitamin D3 involves up-regulation of the TNF receptor 1 and cathepsin B. Int. J. Cancer 2001, 93, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Ameri, P.; Giusti, A.; Boschetti, M.; Murialdo, G.; Minuto, F.; Ferone, D. Interactions between vitamin D and IGF-I: From physiology to clinical practice. Clin. Endocrinol. 2013, 79, 457–463. [Google Scholar] [CrossRef]
- da Costa, R.O.; Gadelha-Filho, C.V.J.; de Aquino, P.E.A.; Lima, L.A.R.; de Lucena, J.D.; Ribeiro, W.L.C.; Lima, F.A.V.; Neves, K.R.T.; de Barros Viana, G.S. Vitamin D (VD3) Intensifies the Effects of Exercise and Prevents Alterations of Behavior, Brain Oxidative Stress, and Neuroinflammation, in Hemiparkinsonian Rats. Neurochem. Res. 2023, 48, 142–160. [Google Scholar] [CrossRef]
- Al-Rasheed, N.M.; Al-Rasheed, N.M.; Bassiouni, Y.A.; Hasan, I.H.; Al-Amin, M.A.; Al-Ajmi, H.N.; Mohamad, R.A. Vitamin D attenuates pro-inflammatory TNF-α cytokine expression by inhibiting NF-κB/p65 signaling in hypertrophied rat hearts. J. Physiol. Biochem. 2015, 71, 289–299. [Google Scholar] [CrossRef]
- Radak, Z.; Chung, H.Y.; Goto, S. Systemic adaptation to oxidative challenge induced by regular exercise. Free Radic. Biol. Med. 2008, 44, 153–159. [Google Scholar] [CrossRef]
- Li, J.; Cao, Y.; Xu, J.; Li, J.; Lv, C.; Gao, Q.; Zhang, C.; Jin, C.; Wang, R.; Jiao, R.; et al. Vitamin D Improves Cognitive Impairment and Alleviates Ferroptosis via the Nrf2 Signaling Pathway in Aging Mice. Int. J. Mol. Sci. 2023, 24, 15315. [Google Scholar] [CrossRef]
- Kim, T.; Kim, D.; Kim, Y.; Kim, J.; Kang, S.; Cho, J. Combined Effects of Exercise and Vitamin D on Neuroinflammation, Blood-Brain Barrier Integrity, Oxidative Stress, and Cognitive Function in Nonpathological Mice. Int. Neurourol. J. 2025, 29 (Suppl. 1), S22–S34. [Google Scholar] [CrossRef] [PubMed]
- Riachy, R.; McKinney, K.; Tuvdendorj, D.R. Various Factors May Modulate the Effect of Exercise on Testosterone Levels in Men. J. Funct. Morphol. Kinesiol. 2020, 5, 81. [Google Scholar] [CrossRef]
- Hashimoto, S.; Hosoi, T.; Yakabe, M.; Matsumoto, S.; Hashimoto, M.; Akishita, M.; Ogawa, S. Exercise-induced vitamin D receptor and androgen receptor mediate inhibition of IL-6 and STAT3 in muscle. Biochem. Biophys. Rep. 2023, 37, 101621. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, N.; Nicholson, K.; Isaacs, L.; MacLusky, N.J. Androgen Effects on Neural Plasticity. Androg. Clin. Res. Ther. 2021, 2, 216–230. [Google Scholar] [CrossRef]
- Guo, G.; Kang, L.; Geng, D.; Han, S.; Li, S.; Du, J.; Wang, C.; Cui, H. Testosterone modulates structural synaptic plasticity of primary cultured hippocampal neurons through ERK—CREB signalling pathways. Mol. Cell. Endocrinol. 2020, 503, 110671. [Google Scholar] [CrossRef]
- Foradori, C.D.; Weiser, M.J.; Handa, R.J. Non-genomic actions of androgens. Front. Neuroendocrinol. 2008, 29, 169–181. [Google Scholar] [CrossRef]
- Pike, C.J.; Nguyen, T.V.; Ramsden, M.; Yao, M.; Murphy, M.P.; Rosario, E.R. Androgen cell signaling pathways involved in neuroprotective actions. Horm. Behav. 2008, 53, 693–705. [Google Scholar] [CrossRef]
- Bianchi, V.E.; Rizzi, L.; Bresciani, E.; Omeljaniuk, R.J.; Torsello, A. Androgen Therapy in Neurodegenerative Diseases. J. Endocr. Soc. 2020, 4, bvaa120. [Google Scholar] [CrossRef]
- Monson, N.R.; Klair, N.; Patel, U.; Saxena, A.; Patel, D.; Ayesha, I.E.; Nath, T.S. Association Between Vitamin D Deficiency and Testosterone Levels in Adult Males: A Systematic Review. Cureus 2023, 15, e45856. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jiang, Y.; Yang, A.; Meng, F.; Zhang, J. The Expanding Burden of Neurodegenerative Diseases: An Unmet Medical and Social Need; Advance online publication. Aging Dis. 2024, 16, 2937–2952. [Google Scholar] [CrossRef] [PubMed]
- Bautista, J.; Coral-Riofrio, E.C.; Suárez Urresta, S.; Palacios-Zavala, D.; Echeverría, C.E.; Araujo-Abad, S.; López-Cortés, A. Neurodegeneration rewires the tumor microenvironment via the neuro–immune–cancer axis; Advance online publication. iScience 2025. [Google Scholar] [CrossRef]
- Reichel, H.; Koeffler, H.P.; Norman, A.W. The role of the vitamin D endocrine system in health and disease. N. Engl. J. Med. 1989, 320, 980–991. [Google Scholar] [CrossRef]
- Dallavalasa, S.; Tulimilli, S.V.; Bettada, V.G.; Karnik, M.; Uthaiah, C.A.; Anantharaju, P.G.; Nataraj, S.M.; Ramashetty, R.; Sukocheva, O.A.; Tse, E.; et al. Vitamin D in Cancer Prevention and Treatment: A Review of Epidemiological, Preclinical, and Cellular Studies. Cancers 2024, 16, 3211. [Google Scholar] [CrossRef]
- Marchiani, S.; Bonaccorsi, L.; Ferruzzi, P.; Crescioli, C.; Muratori, M.; Adorini, L.; Forti, G.; Maggi, M.; Baldi, E. The vitamin D analogue BXL-628 inhibits growth factor-stimulated proliferation and invasion of DU145 prostate cancer cells. J. Cancer Res. Clin. Oncol. 2006, 132, 408–416. [Google Scholar] [CrossRef]
- Carlberg, C.; Muñoz, A. An update on vitamin D signaling and cancer. Semin. Cancer Biol. 2022, 79, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Watson, G.; Coyne, Z.; Houlihan, E.; Leonard, G. Exercise oncology: An emerging discipline in the cancer care continuum. Postgrad. Med. 2022, 134, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, D.; Rees-Punia, E.; Newton, R.U.; Sandler, C.X. The expanding role of exercise oncology in cancer care: An editorial highlighting emerging research. JSAMS Plus 2024, 4, 100078. [Google Scholar] [CrossRef]
- Avancini, A.; Borsati, A.; Toniolo, L.; Ciurnelli, C.; Belluomini, L.; Budolfsen, T.; Lillelund, C.; Milella, M.; Quist, M.; Pilotto, S. Physical activity guidelines in oncology: A systematic review of the current recommendations. Crit. Rev. Oncol. Hematol. 2025, 210, 104718. [Google Scholar] [CrossRef]

| Targets/Pathways | PE Effects | Vitamin D Effects | Synergistic Effects |
|---|---|---|---|
| Myokines | ↑ BDNF, ↑ Irisin, ↑ IL-6, ↑ Cathepsin B, ↑ IGF-1 [125,126] ↑ Plasticity, gray matter volume [113], anti-inflammatory effects [114] | ↑ BDNF [127,128], ↑ Irisin [131], ↑ IL-6 [132,133], ↑ Cathepsin B [134], ↑ IGF-1 [135] ↓ molecular derangements [127,128] ↑ neurogeneration and neuroprotection [127,128] | ↑ Microglial shift M1→M2 [129,130] ↓ IL-1β, TNFα, CN Sinflammation [129,130] ↑ neuronal sensitivity via exercise-induced ↑ VDR/Vitamin D [136] |
| VDR | ↑ VDR in the brain (hippocampus, PFC) [136] | ↑ VDR expression in cognitive areas [136] | ↑ neuronal sensitivity to vitamin D and PE-induced effects [136] |
| NF-κB | ↓ proinflammatory signaling [137] | ↓ inflammatory gene expression [137] | ↓ TNFα, CNS inflammation [137] |
| Nrf2 | ↑ antioxidant enzymes [138,139] | ↑ HO-1, GPX4 [139] ↓ ferroptosis [139] | ↓ oxidative and neuroinflammatory stress [138,139] |
| Proinflammatory cytokines | ↓ via immunoregulation [117,118,119] ↑ myokines [120,121,122,123] | ↓ via NF-κB [137], ↑ Nrf2 [138,139] | ↓ TNFα, IL-1β [129,130] |
| Plasticity/Cerebral blood flow | ↑ plasticity and flow [111,112] | ↑ VDR-supported [136] | ↑ multidimensional neuroprotective effects [140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farina, G.; Crescioli, C. Vitamin D Associated with Exercise Can Be Used as a Promising Tool in Neurodegenerative Disease Protection. Molecules 2025, 30, 3823. https://doi.org/10.3390/molecules30183823
Farina G, Crescioli C. Vitamin D Associated with Exercise Can Be Used as a Promising Tool in Neurodegenerative Disease Protection. Molecules. 2025; 30(18):3823. https://doi.org/10.3390/molecules30183823
Chicago/Turabian StyleFarina, Gabriele, and Clara Crescioli. 2025. "Vitamin D Associated with Exercise Can Be Used as a Promising Tool in Neurodegenerative Disease Protection" Molecules 30, no. 18: 3823. https://doi.org/10.3390/molecules30183823
APA StyleFarina, G., & Crescioli, C. (2025). Vitamin D Associated with Exercise Can Be Used as a Promising Tool in Neurodegenerative Disease Protection. Molecules, 30(18), 3823. https://doi.org/10.3390/molecules30183823









