1. Introduction
Modern dietary trends, such as the vegetarian or vegan diet, are becoming increasingly popular due to ethical, health, and environmental concerns. One of the main challenges for people following this diet is ensuring an adequate supply of certain nutrients, particularly vitamin B12 (cobalamin (Cbl)). The main sources of vitamin B12 are food products of animal origin, including fish, meat, poultry, eggs, as well as dairy products [
1,
2]. The estimated bioavailability of vitamin B12 from food varies by type of food source and the vitamin dose [
3,
4]. Plant foods do not naturally contain this micronutrient. Therefore, people on a vegan diet are unable to obtain adequate amounts of vitamin B12 through diet alone. Currently, there are some fortified foods (e.g., breakfast cereals and nutritional yeasts) available on the market. These products can be relatively available sources of the vitamin [
3]. However, many vegans feel the negative effects of vitamin deficiency and turn to supplements to complete this important micronutrient [
5].
For people who follow a vegan diet, there are no clear recommendations from government agencies on the amount of vitamin B12 supplementation, but according to the literature, strict vegans are advised to supplement with at least 6.0 μg of vitamin B12 per day [
1,
6]. The European Food Safety Authority (EFSA) recommends an adequate daily intake (AI) of vitamin B12 of 4.0 μg per day for the European population in general. Estimated AIs range from 1.5 μg per day for infants (7–11 months) to 4.0 μg per day for children (15–17 years old) [
7]. For pregnancy and lactation, additional vitamin intakes related to the accumulation of cobalamin in fetal tissues and its transfer into breast milk are considered. Thus, AIs of 4.5 and 5.0 μg per day, respectively, are proposed [
1,
7]. However, some authors point out that daily vitamin B12 losses in apparently healthy adults probably range from 1.4 to 5.1 μg. Based on the relationship between the ingested dose and the amount absorbed, vitamin B12 intakes needed to compensate for these daily losses seem to range from 3.8 to 20.7 μg in apparently healthy adults and elderly people, which is 1.4–8.6 times higher than the amount needed to prevent deficiency [
4]. On the other hand, there is no recommended upper limit for vitamin B12 intake because it is a water-soluble vitamin, meaning that part of it is excreted in the urine. Therefore, to date, no adverse effects associated with excessive supplement intake have been reported in healthy individuals [
8].
Vitamin B12 is a metal complex, constituted by a corrin ring and a central cobalt(III) ion bonded to six ligands, four of which are reduced pyrroles forming the corrin ring [
7]. According to the ligands coordinated around the cobalt atom, four isomers of the vitamin can be listed, including hydroxycobalaminn (OHCbl), cyanocobalamin (CNCbl), methylcobalamin (MeCbl), and adenosylcobalamin (AdCbl or coenzyme B12) [
1,
9]. AdCbl and MeCbl are biochemically active forms of vitamin B12 in the human body. CNCbl is a stable synthetic form, which is added to food, supplements, and drugs [
7]. Other forms of vitamin B12 in supplements are MeCbl, AdCbl, and OHCbl [
3,
10]. Regardless of the form, in cells, they have to be converted either to AdCbl or MeCbl [
7,
11]. Manufacturers advise using MeCbl, as it is a ready-to-use form of the vitamin, whereas CNCbl needs to be activated before being used in metabolism. Hence, it is recommended that vegan individuals should be instructed about vitamin B12 supplementation, the pharmaceutical forms available on the market and their actions, and how to choose the ideal plan to avoid vitamin B12 deficiency [
8].
Additionally, there is a widespread notion that food supplements, which are bought without a prescription, are inherently safe. However, there is a serious risk of ingesting high amounts of various substances together with these products. They may be natural constituents, as is the case with plant toxins such as pyrrolizidine alkaloids [
12,
13]. They may result from contamination of the raw materials, as is the case with many dietary supplements containing elevated levels of toxic metals and metalloids [
12,
14,
15]. These contaminants provide no therapeutic benefit to the consumers, and their levels in drugs and dietary supplements should be strictly controlled.
The determination of vitamin B12 in foods or dietary supplements is of great importance as it allows for the characterization of a diet. A correctly conducted analytical procedure enables the assessment of specific forms of the vitamin and its concentration in the context of food quality, correct labelling, and food regulation [
15]. The approaches for the determination of vitamin B12 include microbiological assays, radioprotein-binding and spectrophotometric methods, optical biosensor-based immunoassays, and LC (liquid chromatography) techniques [
1,
16,
17]. Among these techniques, high-performance liquid chromatography (HPLC) has become one of the most popular, due to its high sensitivity, accuracy, and precision. It can be coupled with UV, fluorescence, or mass spectrometry (MS) detection. Especially, the last-mentioned analytical tool adds a new value of sensitivity and reliability to the method, allowing for precise identification and quantification of vitamin B12 forms [
17]. There is currently a trend towards reducing analysis costs, particularly in small, routine laboratories. Therefore, the low-cost UV-Vis method proposed in this paper seems to be an advantageous approach compared to those previously described in the literature.
Apart from active ingredients, dietary supplements may also contain undesirable contaminants, including metals. Among these metals, Cd, Pb, and Hg are important contaminants due to their negative impact even at very low concentrations [
18,
19,
20]. Especially products containing animal- or mineral-based ingredients may contain metal impurities associated with their local environments [
21,
22]. Here, the use of AAS proposed in the present study seems to be an advantageous approach compared to more expensive methodologies like those using ICP-MS.
Additionally, it is currently difficult to find studies on the determination of metals and vitamin B12 in dietary supplements containing this active ingredient in the available literature. Only reports on the analysis of other food supplements for metal content could be found, which makes it difficult to discuss these data alongside the results obtained in the course of this study [
12,
14,
23,
24,
25,
26]. Therefore, it is necessary to focus on a more detailed analysis of vitamin B12 supplements in terms of the content of metal impurities.
The objective of this work is to carry out a comprehensive analysis of the vitamin B12 supplements available on the market, aimed mainly at people following a vegan and vegetarian diet. To the best of our knowledge, no methodology that uses both LC-MS/MS and UV-Vis has been presented in the literature for verifying the quality of dietary supplements containing vitamin B12. The developed LC-MS/MS method validates the cost-effective UV-Vis method. It shows its suitability for routine sample screening, and at the same time, the LC-MS/MS method may be used to confirm any discrepancies. The research focuses on two key aspects: determining the vitamin B12 content of these products and assessing the presence of potentially harmful contaminants such as Hg, Cd, and Pb in the supplements tested. The analysis of these aspects will allow for a comprehensive assessment of the quality of vitamin B12 supplements for vegans and vegetarians, which is crucial for the health and safety of consumers following this type of diet. This type of study, combining verification of potential risks resulting from insufficient active ingredient content and the presence of heavy metals, has not yet been conducted for supplements containing vitamin B12. Therefore, it allows us to verify whether supplements may contain discrepancies in declared B12 content and unsafe levels of heavy metals.
2. Results and Discussion
Supplements selected for the study contained vitamin B12, which was declared either as MeCbl or CNCbl. Therefore, a method was needed to separately determine these two forms of the vitamin. For this purpose, two techniques were chosen: easily available and inexpensive spectrophotometry (SPF) and more selective but very expensive high-performance liquid chromatography with mass spectrometric detection (LC-MS/MS). Thus, the LC-MS/MS method allowed us to verify whether the SPF method gives reliable results.
2.1. Selection of Analytical Conditions for the Determination of Vitamin B12
2.1.1. Spectrophotometric Analysis
UV-Vis spectrophotometry is a technique that, in addition to determining individual compounds, enables the analysis of multicomponent mixtures in which the compounds being determined absorb radiation at different wavelengths. To select the wavelength of greatest intensity for MeCbl and CNCbl during spectrophotometric determination, the absorbance was measured in a wide wavelength range from 190 to 1100 nm. As a result, in the current work, multicomponent analysis was used to determine MeCbl, which absorbs light at 351 nm, and CNCbl, which absorbs at 361 nm. The measuring speed for this analysis was 50 nm s−1, and the integration time was 0.2 s.
2.1.2. LC-MS/MS Analysis
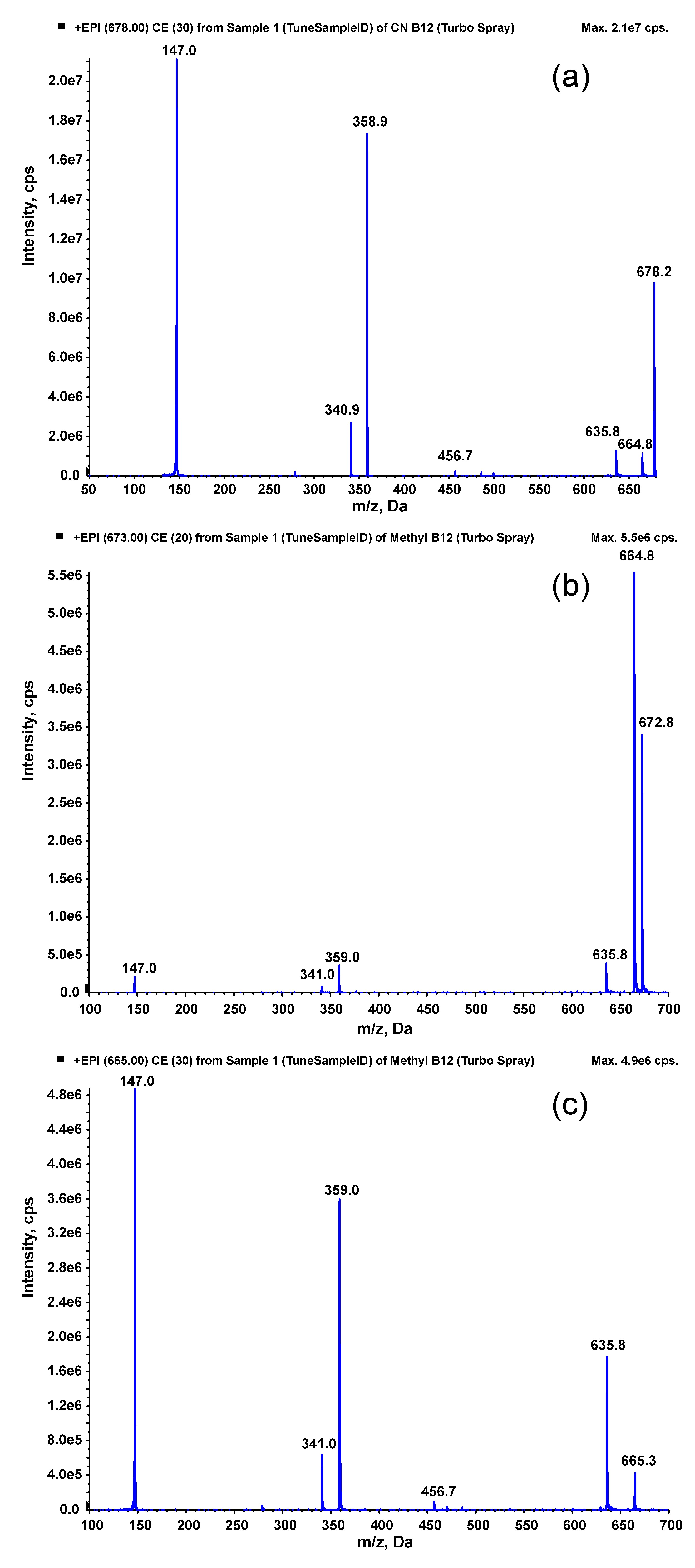
Mass spectrometric conditions were set based on the fragmentation study of MeCbl and CNCbl. For this purpose, standards of MeCbl and CNCbl were injected directly into the mass spectrometer source using a Harvard syringe pump. Mass spectra obtained were in accordance with the literature [
27], showing very intense signals from doubly protonated molecules [M+2H]
2+ (
Figure 1). For CNCbl, the ion at
m/z = 678 was observed, which fragmented to intense ions at
m/z = 147 and
m/z = 359 (
Figure 1a). These two transitions were selected for the determination of CNCbl (as the quantitative and confirmatory transitions). For MeCbl, the [M+2H]
2+ ion at
m/z = 673 fragmented to an intensive ion at
m/z = 665 (
Figure 1b), which fragmented further (
Figure 1c). The transitions to
m/z = 636 and
m/z = 147 were selected for the determination of MeCbl (as the quantitative and confirmatory transitions). After selecting the MS/MS transitions, the chromatographic conditions were set on the HPLC system. For this purpose, an octadecylsilica column was utilized, and the mobile phase containing water/acetonitrile/ammonium formate was selected in a gradient ensuring symmetrical peak shapes and a high MS/MS signal.
2.2. Selection of Sample Preparation Conditions for the Determination of Vitamin B12
In the course of the study, vitamin B12 was determined after ultrasound-assisted extraction. Vitamin B12 is most stable at ca. pH = 4–5 [
1,
3]. Therefore, the extraction was carried out at pH = 4. When synthetic vitamin B12 is added to fortified foods and dietary supplements, it is already in free form and does not require vigorous extraction. Simple aqueous extraction can be carried out. Heating may be used, and clean-up may be required to remove other analytical components [
12]. Therefore, elevated temperature was not used during the tests. Instead, the ultrasound-assisted extraction of MeCbl and CNCbl was performed before their determination in vitamin B12 supplements. Therefore, the ultrasonication time was optimized to ensure maximum extraction efficiency. The study was conducted for vitamin B12 supplements C1 (CNCbl) and M2 (MeCbl). The effect of ultrasonication time was studied within the range of 0–3 min. After extraction, the sample solutions were filtered through 0.2 µm PTFE syringe filters, and both forms of vitamin B12 were determined spectrophotometrically. Maximum absorbance was achieved at a time of 2 min for MeCbl and CNCbl (
Figure 2).
2.3. Analytical Figures of Merit for the Determination of Vitamin B12
The detection limits (LOD) calculated for the spectrophotometric determination of MeCbl and CNCbl were 250 µg L−1 and 100 µg L−1, respectively. The limits of quantification (LOQ) were 833 µg L−1 and 333 µg L−1 for MeCbl and CNCbl, respectively. The relative standard deviations (n = 5), calculated for 1 mg L−1 in a standard solution containing both forms of vitamin B12, were 1.5% and 1.2% for MeCbl and CNCbl, respectively. Calibration was performed by the standard calibration technique. The linear ranges of the calibration functions were 0.5–50 mg L−1 for both forms of vitamin B12. The working ranges of the calibration functions were 0.5–80 mg L−1 for MeCbl and CNCbl. The acceptable correlation coefficients of 0.9997 and 0.9998 were achieved for MeCbl and CNCbl, respectively.
The limits of detection for the LC-MS/MS determination of MeCbl and CNCbl were calculated as 3 times the signal-to-noise ratio, and the limits of quantification as 10 times the signal-to-noise ratio. For MeCbl, LOD was 0.2 µg L−1 and LOQ was 0.8 µg L−1, while for CNCbl, LOD was 0.01 µg L−1 and LOQ was 0.04 µg L−1. Linearity was tested at 9 concentration levels from 0.5 to 1000 µg L−1 for both analytes. Excellent correlation was found for both MeCbl (R2 = 0.9992) and CNCbl (R2 = 0.9997). The high sensitivity of the LC-MS/MS compared to the spectrophotometric determination of vitamin B12 necessitated greater dilution of the sample. However, this had a positive impact, as there was no matrix effect for the samples being tested.
Due to the lack of certified reference materials in the form of dietary supplements, the standard addition was used to ensure the accuracy of the developed procedures. For spectrophotometric determination of MeCbl and CNCbl in capsules, the average recoveries were 99.1 ± 1.2% and 98.8 ± 1.5%, respectively, and in tablets, 98.5 ± 1.5% and 98.7 ± 1.4%, respectively. The average recoveries obtained for LC-MS/MS determination of MeCbl and CNCbl in capsules were 100.6 ± 1.6% and 97.4 ± 4.5%, respectively, and in tablets were 97.7 ± 3.2% and 99.4 ± 5.1%, respectively. The recovery values are within the accuracy range proposed in the Guidelines for Dietary Supplements and Botanicals [
28], which suggests a 90–108% range for supplements containing 0.1% of the active ingredient.
The matrix effect was calculated based on the ratio of the slope of the enriched sample curve to the reference curve. The calculated matrix effect proved to be negligible—below 5%.
2.4. Determination of Vitamin B12 in Supplements
To evaluate the usefulness of the proposed analytical procedures for the determination of MeCbl and CNCbl in vitamin B12 supplements, the contents of these analytes in ten samples were established using the experimental conditions previously optimized. Quantitation of analytes by LC-MS/MS was performed using the external standard technique, for which standards at a concentration of 0.5 µg mL
−1 were used. During the spectrophotometric determination of MeCbl and CNCbl, the standard calibration technique was used. The obtained results of the analysis are given in
Table 1.
One of the aims of the research presented in this paper was to assess the quality of the supplements by determining the content and typical forms of vitamin B12. Supplements containing the declared MeCbl and CNCbl contents were selected for testing. The results indicate that the forms of vitamin B12 declared by the manufacturers were consistent with those determined during testing. However, sample C1 contained significantly higher amounts of MeCbl (7.2 ± 0.5 µg and 6.1 ± 0.1 µg) and very low amounts of CNCbl (0.9 ± 0.1 µg and 0.6 ± 0.1 µg), in addition to the declared CNCbl dose of 10 µg. The declared MeCbl contents ranged from 100 µg to 500 µg per tablet/capsule, depending on the supplement. The content closest to the declared amount was found in sample M3 (97% and 94%), while the lowest amount was found in sample M1 (39% and 39%) and M4 (45% and 41%). All doses determined were lower than the declared amounts, ranging from 39% to 97%. The exception was sample M6, which contained 122% and 108% of the declared vitamin B12 content. In turn, the content of CNCbl declared in the tested samples was from 10 µg to 500 µg per tablet/capsule, depending on the supplement. The content closest to the declared amount was found in samples C2 (106% and 127%) and C3 (111% and 105%). The lowest content was determined in sample C4 (79% and 86%).
There are not many precise requirements on the content of active ingredients in supplements in relation to their labeled declaration. Very precise requirements were defined by the Canadian Food Inspection Agency, stating that each sub-sample should contain ±50% of the labeled active ingredient and ±20% in three composite sub-batches [
29]. Taking into account that only one batch of each supplement was acquired in the present study, the limit ±50% has to be applied. This means that two out of ten samples tested (i.e., M1 and M4) contained too little vitamin B12. Insufficient levels of active ingredients in supplements, as well as their absence or the presence of undeclared ingredients, are a fairly common problem worldwide [
30,
31,
32]. Unfortunately, although the introduction of new supplements must be reported to the supervisory authorities, their control is incomplete. Often, only a few percent of supplements on the market are subject to control [
33,
34].
Of the two forms of vitamin B12 identified during the study, MeCbl is the natural, biochemically active form found in the human body, whereas CNCbl is a stable synthetic form that is often added to supplements [
7]. CNCbl is used frequently in supplements as it is considered more stable and cost-effective than other forms of vitamin B12 [
35,
36]. Once inside the body, CNCbl is converted into either MeCbl or AdCbl, both of which are active forms of the vitamin. Some research suggests that the bioavailability of each form may differ depending on factors such as gastrointestinal pathologies, age, and genetics [
35,
36]. Although some early studies found that the human body absorbed 49.2% of CNCbl (calculated for a 1 μg dose) compared to 44.4% for a 1 μg dose of MeCbl. For a higher dose of 25 µg, these values decrease to 5.6% and 6.1% for CNCbl and MeCbl, respectively [
37]. Additionally, some studies suggest that, for long-term supplementation, the use of natural forms (OHCbl, MeCbl, or AdCbl) should be favored over CNCbl in order to avoid the accumulation of cyanide in the human body. This is particularly important for tobacco smokers, who are exposed to this compound through cigarette smoke [
35,
38,
39,
40]. Consequently, there is a clear trend towards replacing CNCbl supplements, which were previously almost the only option on the market, with their natural forms, especially MeCbl [
35,
38].
It must also be taken into account that the differences in absorption of vitamin B12 are strictly connected with the mechanisms involved. Vitamin B12 liberated from food binds with haptocorrin present in saliva and stomach fluids and moves with it through the gastrointestinal tract. Then, vitamin B12, liberated by proteolytic enzymes, is absorbed through two routes—binding to the intrinsic factor protein and diffusion through the mucosa. Unfortunately, the intrinsic factor protein becomes saturated at 2 µg per meal [
35]. Moreover, there are many conditions influencing vitamin B12 absorption, including autoimmune pernicious anemia and atrophic gastritis, which both lower the production of intrinsic factor, or celiac disease, ulcerative colitis, Crohn’s disease, and tropical sprue, which reduce absorption by endocytosis [
35]. Nevertheless, vitamin B12 can also be absorbed by diffusion without the need for an intrinsic factor. This mechanism, however, works only when high doses of vitamin B12 are administered [
41]. Therefore, supplementing vitamin B12 often requires as much as a 1000 µg daily dose [
35,
41] even though the recommended daily allowance (RDA) is much lower—2.4 µg per day in the United States according to the National Institutes of Health (NIH) [
3,
35]. As a result, constant supplementation is crucial, and supplements of vitamin B12 with content considerably lower than declared, including samples M1 and M4 analyzed in the present study, can lead to health issues.
The European Food Safety Authority (EFSA) Panel (EU) has set an adequate daily intake (AI) of 4.0 μg per day for adults, based on data on different biomarkers of cobalamin status, and taking into account observed intakes in several EU countries, which range between 4.2 and 8.6 μg per day [
7]. For healthy breastfed infants, the AI is the mean intake. For other life stages and gender groups, the AI is believed to cover the needs of all individuals in the group. However, a lack of data or uncertainty in the data prevents being able to specify with confidence the percentage of individuals covered by this intake. RDAs are set to meet the needs of almost all individuals (97–98%) in a group. A comparison of the values determined shows that they significantly exceed the values required by EU and US regulations (
Table 1). These amounts exceed by a large margin the established limits of 2.4 and 4.0 μg per day, and ranged from 6.7 µg per day (sample C1) to 435.0 (sample M2) μg per day assuming that the consumer takes one tablet or capsule per day. It is significant that, in addition to the AI and RDA values, neither the EU nor the US government organizations declare the form of vitamin B12 that dietary supplements should contain.
2.5. Selection of Conditions for the Determination of Cd, Pb, and Hg
An important issue raised in the study is the safety of vitamin B12 supplements, specifically the presence of certain inorganic contaminants. Their determination requires the development of sample preparation methods and the selection of proper analytical conditions for the determination of elements.
2.5.1. Optimization of ET AAS Detection of Cd and Pb
Before Cd and Pb determination, the samples were prepared using microwave-assisted digestion, according to the parameters given in the Materials and Methods Section. Absorbance measurements for Cd were performed at the wavelength of greatest intensity, i.e., 228.8 nm. For Pb, the wavelength of greatest intensity is 217 nm. Unfortunately, at this wavelength, phosphorus oxide can cause spectral interferences. Therefore, the absorbance for Pb was measured at 283.3 nm to obtain accurate results.
The temperature program of the electrothermal atomizer (ET) of the AA spectrometer was optimized for standard solutions containing 5 ng mL
−1 of Cd and 20 ng mL
−1 of Pb. Three drying steps allow for avoiding the spattering of the sample and obtaining a uniform liquid deposit on the graphite surface. The effect of pyrolysis temperature on absorbance was studied within the ranges 600–1200 °C and 800–1400 °C for Cd and Pb, respectively. The absorbance reached a maximum at 1000 °C and 1300 °C for Cd and Pb, respectively, and these values of the pyrolysis temperature were chosen for further experiments. After optimization of pyrolysis conditions, the effect of atomization temperature on analytical signals was studied within the ranges 1200–1600 °C and 1500–2300 °C for Cd and Pb, respectively. Maximum absorbances were achieved at a temperature of 1500 °C and 2200 °C for Cd and Pb, respectively. Therefore, these atomization temperatures were chosen for the determination of Cd and Pb in vitamin B12 supplements. During the experiments, chemical modifiers (magnesium for Cd and phosphate for Pb) were used to ensure stable analytical signals with minimal influence exerted by the matrix. The temperature program of the electrothermal atomizer used for Cd and Pb determination is shown in the
Section 3.
2.5.2. Optimization of CV AAS Detection of Hg
Mercury was determined using cold vapor atomic absorption spectrometry at a wavelength of 253.65 nm, which is commonly used for that purpose. A commercially available automatic mercury analyzer was used for the determination during the tests. The values for the most important parameters for Hg vapor generation were used as recommended by the manufacturer. Before Hg determination, the samples were prepared using microwave-assisted digestion, according to the parameters given in the Materials and Methods Section.
SnCl2 is the most efficient reducing agent for Hg. Therefore, a 2% (w/v) solution of this reagent was used. An acidic medium (2 mol L−1 HCl) was applied to achieve an effective and rapid reaction of vapor generation. The sensitivity was affected by the flow rates of the reducing agent, acid, and sample, since higher flow rates resulted in higher signal intensity. However, too high a flow rate can result in concentrated, unstable vapor; therefore, an ideal sample-to-reagent ratio is required for maximum vapor generation efficiency. The optimum flow rates for SnCl2 and HCl were 1.0 mL min−1. The maximum signal was obtained at a sample flow rate of approximately 1.7 mL min−1.
During Hg determination, the metal ions can be adsorbed onto the surfaces of the tubes and crossflow reactor, resulting in memory effects. To effectively limit this phenomenon, an appropriate flushing medium is required. During the tests, a 0.1% (m/v) solution of NH4OCl was used for this purpose. The flow rate of the carrier gas (Ar) also played an important role in the Hg cold vapor process. The argon flow rate should be high enough to strip the vapor from the crossflow reactor and carry it up to the sample cell, but not so high as to dilute the formed volatile product. The optimum argon flow rate for Hg determination was found to be 4.5 L h−1. Calibration was performed using the standard calibration technique with aqueous standards in an acidic medium. The standard solutions were prepared immediately prior to measurement within the concentration range of 0.1–5.0 μg L−1.
2.6. Selection of Microwave-Assisted Digestion of the Supplements
The metals present in the supplements were quantified after microwave-assisted digestion. Solid supplements (whole tablets and capsule contents) were prepared by digestion in a closed microwave system with focused energy. This type of approach to sample preparation is a widely used technique in spectroscopic analysis [
42,
43]. The samples were prepared using an oxidizing mixture consisting of concentrated HNO
3 and 30% H
2O
2, with the addition of 40% HF. The microwave-assisted acid digestion used allows for rapid and efficient decomposition of the solid matrix with reduced acid consumption, good reproducibility, and lower contamination risk. The mass of the samples was controlled using an analytical balance and was approximately 0.3 g, which ensured stable and safe conditions.
2.7. Analytical Figures of Merit for the Determination of Cd, Hg, and Pb
The limits of detection for inorganic impurities were calculated as the concentration of the analyte yielding a signal equivalent to three times the standard deviation of the blank value (n = 5). The values were 0.05 µg L−1, 0.01 µg L−1, and 1 µg L−1 for Cd, Hg, and Pb, respectively. The limits of quantification were 0.17 µg L−1, 0.03 µg L−1, and 3.3 µg L−1 for Cd, Hg, and Pb, respectively. The relative standard deviations (RSDs), calculated for five replicate measurements of 10 µg mL−1 in a standard solution containing both elements, were 7% and 5% for Cd and Pb, respectively. RSD obtained for Hg, for five replicate measurements of 0.25 µg mL−1 in standard solution, was 4%. For all three analytes, the calibration was performed by the standard calibration technique. The linear ranges of the calibration functions were 0.5–20 µg L−1 for Cd, 10–40 µg L−1 for Pb, and 0.1–5.0 µg L−1 for Hg. The working ranges were 0.5–30 µg L−1 for Cd, 5–60 µg L−1 for Pb, and 0.1–10.0 µg L−1 for Hg. The acceptable correlation coefficients (R2 = 0.9998 for Cd, R2 = 0.9995 for Pb, and R2 = 0.9992 for Hg) were achieved.
Due to the lack of certified reference materials in the form of dietary supplements, the standard addition was used to ensure the accuracy of the developed procedures. The standard addition method was also used to verify the accuracy of the Cd, Pb, and Hg determination procedures. The average recoveries obtained for Cd, Pb, and Hg in capsules were 97.4 ± 6.1%, 96.6 ± 5.1% and 97.2 ± 3.1%, respectively, and in tablets, 96.3 ± 5.3%, 96.6 ± 4.8% and 98.1 ± 2.5%, respectively. The results show that the proposed analytical procedures can be applied to determine inorganic impurities (Cd, Pb, and Hg) in vitamin B12 supplements. For this reason, no further studies on the matrix effect have been carried out.
2.8. Cd, Pb, and Hg Determination in Vitamin B12 Supplements
In the course of the study, Cd and Pb were determined in vitamin B12 supplements by HR-CS ET AAS, and Hg was quantified using CV AAS detection. The results of the analysis are presented in
Table 2.
The developed methods were utilized for the determination of Cd, Pb, and Hg. These heavy metals are sometimes present in pharmaceutical products and supplements and can harm humans. Therefore, their concentrations in these products should be monitored [
23]. Metals can appear in a final product through various routes. For example, they may be residual catalysts added intentionally during synthesis, impurities introduced through interactions with processing equipment or container/closure systems, or they may be present as impurities in active ingredients or excipients. Importantly, products containing ingredients of natural origin may contain heavy metals (such as Cd, Pb, and Hg) due to their accumulation from the soil, water, or air. Thus, both animal- or mineral-based dietary supplements may contain metal impurities associated with their local environments [
21,
22].
To address this issue, the European Medicines Agency (EMA) and the United States Pharmacopeia (USP) have developed guidelines for the risk assessment of elemental impurities [
18,
19,
20]. These agencies have established permitted daily exposure (PDE) levels for these elements. Therefore, identifying the impurity and carrying out a risk assessment according to element classification and route of administration are easier.
The elements included in the ICH (International Conference on Harmonization) EMA guideline have been divided into three classes based on their toxicity (PDE) and likelihood of occurrence in the drug product [
18]. This likelihood was determined based on various factors, including the probability of use in pharmaceutical processes and the probability of co-isolation with other elemental impurities in materials used in pharmaceutical processes. It also took into account the observed natural abundance and environmental distribution of the element. Cd, Pb, and Hg are class 1 elements, which are described as human toxicants with limited or no use in pharmaceutical manufacturing.
Additionally, the elements of toxicological concern are mentioned in Chapter 2232 of the USP [
20]. According to the document, the PDE for both Cd and Pb was defined as 5 µg per day, and for Hg (total and methylmercury) as 15 µg per day and 2 µg per day, respectively.
In Europe, the EU Commission Regulation [
44] sets maximum permissible levels for Cd, Pb, and Hg in various types of food. The values for Cd range from 0.005 mg kg
−1 (in infant formulae) to 3.0 mg kg
−1 (in food supplements consisting of at least 80% dried seaweed). Pb levels vary between 0.010 mg kg
−1 in infant formulae and 3.0 mg kg
−1 in food supplements, and Hg levels range from 1.0 mg kg
−1 in muscle meat from fish to 0.10 mg kg
−1 in food supplements.
Although the amounts of Cd and Pb determined in the supplements are greater than the European limits for infant formulae, one must take into account that these supplements are not intended for infants, and the amount of supplement taken is also restricted to one tablet (or capsule). Therefore, taking into account the amount of Cd and Pb determined and the mass of one tablet (or capsule), none of the supplements analyzed in this study exceeded the limits given in the USP guide (
Table 2).
It is also difficult to draw conclusions about the presence of these elements in the supplements since concentrations of inorganic impurities differ considerably between products (and thus between studies, depending on the products selected) [
12]. For example, some previous studies investigating a large number of food supplements of different origins [
14,
24,
25,
26] reported maximum concentrations of Cd, Pb, and Hg in the ranges 0.940–500 mg kg
−1, 0.036–50 mg kg
−1, and 0.0004–0.550 mg kg
−1, respectively, thus varying by several orders of magnitude.
2.9. Hazard Quotients
Considering all available information, i.e., concentrations, number of tablets (or capsules) consumed, and the reference dose for each element, the hazard connected with the intake of supplements may be calculated and expressed as hazard quotients (HQs). The hazard quotients were calculated to assess the human health risk due to chronic exposure to Cd and Pb [
14]. Values for Hg were not calculated as the content of this element was below the LOD of the analytical method used. The determined quotients show whether the average daily intake is greater than the reference dose. Thus, for HQs greater than one, there is an unacceptable risk due to chronic exposure to Cd or Pb, and adverse effects may arise, while for HQs equal to or lower than one, the risk is acceptable.
Due to both different concentrations and ingestion rates for each kind of tablet or capsule tested, the HQ values were calculated separately for each supplement. The obtained results were summarized in
Table 3 and show no substantial health risk connected with the consumption of the tested products. Also, to summarize the effect of Cd and Pb, the hazard index (HI) was calculated, which is the sum of the HQ values for both metals. The HI values above 1 show unacceptable risk, and for HI ≤ 1, no significant risk is expected [
45]. The results presented in
Table 3 show no risk due to the consumption of the tested food supplements.
The HQ values obtained for Cd and Pb are considerably lower than one, which means that the analyzed supplements are safe for consumption. The calculated values of the hazard index show that the supplements are also safe when taking into account the combined content of determined heavy metals.
4. Conclusions
Analytical methods for determining vitamin B12 and heavy metals in dietary supplements have been developed. The results obtained using an inexpensive spectrophotometric method were found to be consistent with those obtained using the LC-MS/MS technique. This enables inexpensive and rapid verification of the vitamin B12 content of simple dietary supplements that contain vitamin B12 as their only active ingredient.
Most of the supplements tested contained the declared amount of vitamin B12, although one of them contained a different form of the vitamin than that declared on the packaging, i.e., methylcobalamin instead of cyanocobalamin. Unfortunately, in 2 out of 10 samples, significantly lower vitamin B12 content was recorded, approximately 39% and 41%. Such low levels compared to those declared by manufacturers may be a cause for concern for consumers, as they need adequate vitamin B12 supplementation.
The heavy metal content in the samples of the supplements tested was not excessively high. Although cadmium and lead were detected in almost all supplements, their content does not pose a risk to consumers. Both the hazard quotient and hazard index values calculated for all 10supplements were below 1. This indicates that there is no significant risk to consumers associated with the consumption of the supplements tested.
Future research should focus on developing methods to verify the presence of other contaminants in dietary supplements. These methodologies should be designed to be incorporated into the regular work of pharmaceutical and food laboratories.