Encapsulation of Acid Whey in Alginate Microspheres for Application in Skin Microbiome-Friendly Topical Formulations: Optimization Through a Design of Experiments Approach
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
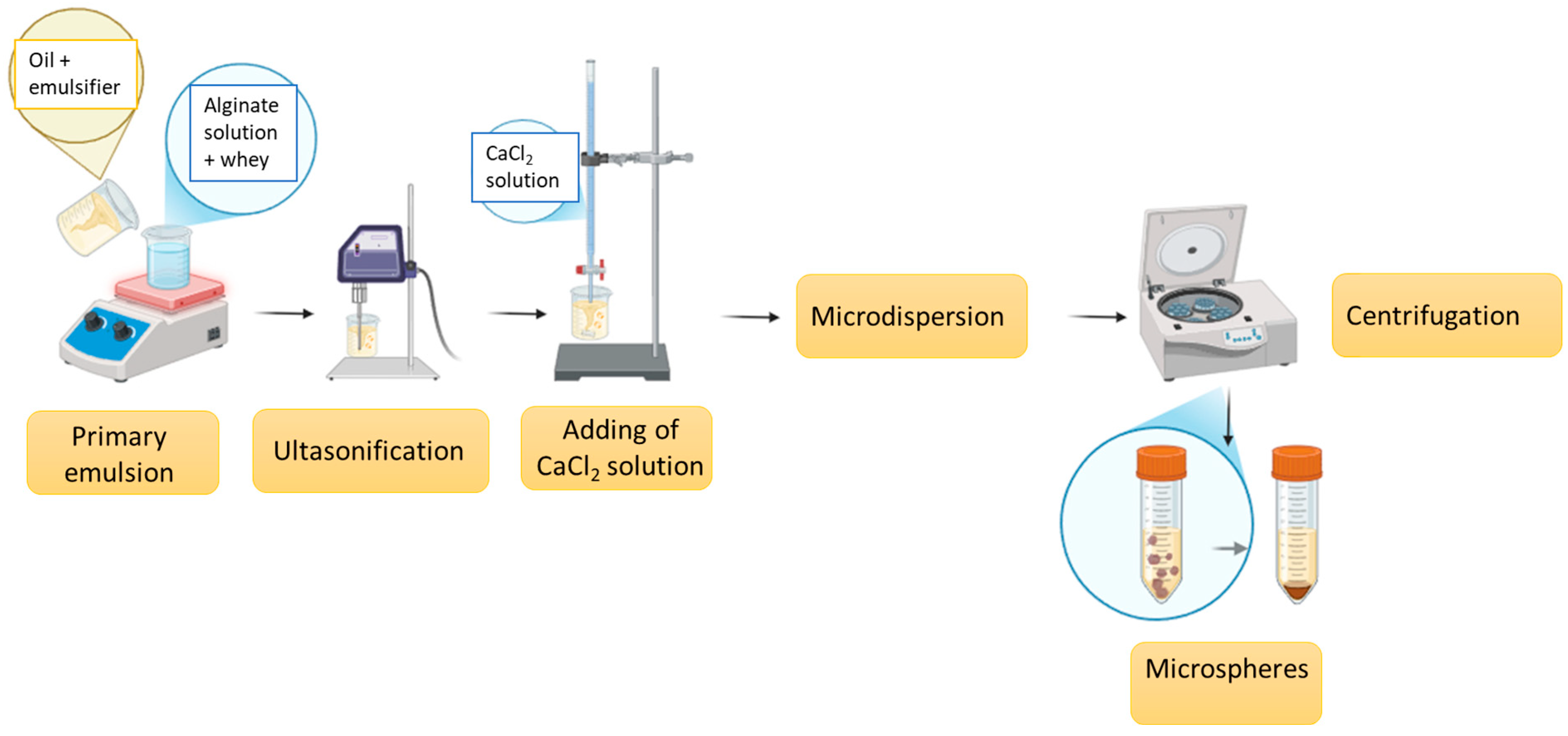
3.2. Encapsulation of Whey
3.3. Optimization Process
3.3.1. Physicochemical Properties
Size and Morphology of the Microspheres
Microdispersions Stability
Viscosity Measurement
3.3.2. Microbiological Properties
Encapsulation Efficiency and Viability over Time
3.4. Preparation of Formulation with Whey-Loaded Microspheres
3.5. Viability of Probiotics in Microencapsulated Whey in Skin Care Product
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernandes, A.; Rodrigues, P.M.; Pintado, M.; Tavaria, F.K. A Systematic Review of Natural Products for Skin Applications: Targeting Inflammation, Wound Healing, and Photo-Aging. Phytomedicine 2023, 115, 154824. [Google Scholar] [CrossRef]
- Haykal, D.; Cartier, H.; Dréno, B. Dermatological Health in the Light of Skin Microbiome Evolution. J. Cosmet. Dermatol. 2024, 23, 3836–3846. [Google Scholar] [CrossRef] [PubMed]
- Knackstedt, R.; Knackstedt, T.; Gatherwright, J. The Role of Topical Probiotics on Wound Healing: A Review of Animal and Human Studies. Int. Wound J. 2020, 17, 1687–1694. [Google Scholar] [CrossRef] [PubMed]
- Puebla-Barragan, S.; Reid, G. Probiotics in Cosmetic and Personal Care Products: Trends and Challenges. Molecules 2021, 26, 1249. [Google Scholar] [CrossRef] [PubMed]
- Hyseni, E.; Glavas Dodov, M. Probiotics in Dermatological and Cosmetic Products—Application and Efficiency. Maced. Pharm. Bull. 2022, 68, 9–26. [Google Scholar] [CrossRef]
- Belbasis, L.; Stefanaki, I.; Stratigos, A.J.; Evangelou, E. Non-Genetic Risk Factors for Cutaneous Melanoma and Keratinocyte Skin Cancers: An Umbrella Review of Meta-Analyses. J. Dermatol. Sci. 2016, 84, 330–339. [Google Scholar] [CrossRef]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The Human Skin Microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Chen, Y.E.; Tsao, H. The Skin Microbiome: Current Perspectives and Future Challenges. J. Am. Acad. Dermatol. 2013, 69, 143–155. [Google Scholar] [CrossRef]
- Górska, A.; Przystupski, D.; Niemczura, M.J.; Kulbacka, J. Probiotic Bacteria: A Promising Tool in Cancer Prevention and Therapy. Curr. Microbiol. 2019, 76, 939–949. [Google Scholar] [CrossRef]
- Skowron, K.; Bauza-Kaszewska, J.; Kraszewska, Z.; Wiktorczyk-Kapischke, N.; Grudlewska-Buda, K.; Kwiecińska-Piróg, J.; Wałecka-Zacharska, E.; Radtke, L.; Gospodarek-Komkowska, E. Human Skin Microbiome: Impact of Intrinsic and Extrinsic Factors on Skin Microbiota. Microorganisms 2021, 9, 543. [Google Scholar] [CrossRef]
- Paetzold, B.; Willis, J.R.; Pereira De Lima, J.; Knödlseder, N.; Brüggemann, H.; Quist, S.R.; Gabaldón, T.; Güell, M. Skin Microbiome Modulation Induced by Probiotic Solutions. Microbiome 2019, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Jeong, S.E.; Lee, S.; Kim, S.; Han, H.; Jeon, C.O. Effects of Cosmetics on the Skin Microbiome of Facial Cheeks with Different Hydration Levels. Microbiologyopen 2018, 7, e00557. [Google Scholar] [CrossRef]
- European Union. Regulation (EC) No 1223/2009 of the European Parliament and of the Council; European Union: Brussels, Belgium, 2009; pp. 1–431. [Google Scholar]
- Sfriso, R.; Egert, M.; Gempeler, M.; Voegeli, R.; Campiche, R. Revealing the Secret Life of Skin-with the Microbiome You Never Walk Alone. Int. J. Cosmet. Sci. 2020, 42, 116–126. [Google Scholar] [CrossRef]
- Jung, Y.-O.; Jeong, H.; Cho, Y.; Lee, E.-O.; Jang, H.-W.; Kim, J.; Nam, K.T.; Lim, K.-M. Lysates of a Probiotic, Lactobacillus Rhamnosus, Can Improve Skin Barrier Function in a Reconstructed Human Epidermis Model. Int. J. Mol. Sci. 2019, 20, 4289. [Google Scholar] [CrossRef] [PubMed]
- Wesołowska-Trojanowska, M.; Targoński, Z. Wykorzystanie Serwatki w Procesach Biotechnogicznych. Eng. Sci. Technol. 2014, 1, 102–119. [Google Scholar] [CrossRef]
- Barba, F.J. An Integrated Approach for the Valorization of Cheese Whey. Foods 2021, 10, 564. [Google Scholar] [CrossRef]
- Kareb, O.; Aïder, M. Whey and Its Derivatives for Probiotics, Prebiotics, Synbiotics, and Functional Foods: A Critical Review. Probiotics Antimicrob. Proteins 2019, 11, 348–369. [Google Scholar] [CrossRef]
- Santa, D.; Srbinovska, S. Whey: Source of Bioactive Peptides, Probiotics, Organic Acids, Aromatic Compounds and Enzymes. In Whey Valorization; Poonia, A., Trajkovska Petkoska, A., Eds.; Springer: Singapore, 2023. [Google Scholar]
- Bjelošević Žiberna, M.; Grilc, B.; Gašperlin, M.; Gosenca Matjaž, M. Exploring the Potential of Cleansing Hydrogel and Shampoo with Whey as a Contemporary Approach to Sustainability. Gels 2025, 11, 374. [Google Scholar] [CrossRef]
- Prokopowicz, M.; Różycki, K.M. Innovation in Cosmetics. Word Sci. News 2017, 72, 448–456. [Google Scholar]
- Smithers, G.W. Whey and Whey Proteins-From “Gutter-to-Gold”. Int. Dairy J. 2008, 18, 695–704. [Google Scholar] [CrossRef]
- Kumar, N.; Heena; Dixit, A.; Mehra, M.; Daniloski, D.; Trajkovska Petkoska, A. Utilization of Whey: Sustainable Trends and Future Developments. In Whey Valorization; Poonia, A., Trajkovska Petkoska, A., Eds.; Springer: Singapore, 2023. [Google Scholar]
- Liu, K.; Kong, X.L.; Li, Q.M.; Zhang, H.L.; Zha, X.Q.; Luo, J.P. Stability and Bioavailability of Vitamin D3 Encapsulated in Composite Gels of Whey Protein Isolate and Lotus Root Amylopectin. Carbohydr. Polym. 2020, 227, 115337. [Google Scholar] [CrossRef]
- Teymoori, F.; Roshanak, S.; Bolourian, S.; Mozafarpour, R.; Shahidi, F. Microencapsulation of Lactobacillus reuteri by Emulsion Technique and Evaluation of Microparticle Properties and Bacterial Viability Under Storage, Processing, and Digestive System Conditions. Food Sci. Nutr. 2024, 12, 10393–10404. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, T. Pharmaceutical and Cosmetic Applications of Protein By-Products. In Protein Byproducts; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128026113. [Google Scholar]
- Tabaszewska, M.; Grega, T.; Sikora, E. Sposób Wytwarzania Preparatu Kosmetycznego Zawierającego Koncentrat Białek Serwatkowych. PL 217398, 31 July 2014. [Google Scholar]
- Dineshbhai, C.K.; Basaiawmoit, B.; Sakure, A.A.; Maurya, R.; Bishnoi, M.; Kondepudi, K.K.; Patil, G.B.; Mankad, M.; Liu, Z.; Hati, S. Exploring the Potential of Lactobacillus and Saccharomyces for Biofunctionalities and the Release of Bioactive Peptides from Whey Protein Fermentate. Food Biosci. 2022, 48, 101758. [Google Scholar] [CrossRef]
- Zhao, C.; Ashaolu, T.J. Bioactivity and Safety of Whey Peptides. LWT 2020, 134, 109935. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Yamauchi, K.; Takase, M. Lactoferrin Research, Technology and Applications. Int. Dairy J. 2006, 16, 1241–1251. [Google Scholar] [CrossRef]
- Caessens, P.; Wouters, W.; De Waard, R.; Walter, A. A Whey Protein Complex for Skin Beauty from the Inside Out. In Nutritional Cosmetics: Beauty from Within; Tabor, A., Blair, M.R., Eds.; William Andrew Publishing: Norwich, NY, USA, 2009; pp. 385–398. ISBN 9780080951881. [Google Scholar]
- Amiri, S.; Rezazadeh-Bari, M.; Alizadeh-Khaledabad, M.; Rezaei-Mokarram, R.; Sowti-Khiabani, M. Fermentation Optimization for Co-Production of Postbiotics by Bifidobacterium lactis BB12 in Cheese Whey. Waste Biomass Valorization 2021, 12, 5869–5884. [Google Scholar] [CrossRef]
- Serum Lab. Whey Benefits for Skin. Available online: https://www.serumlab.it/en/whey-benefits-for-skin/ (accessed on 24 August 2025).
- Marasco, R.; Gazzillo, M.; Campolattano, N.; Sacco, M.; Muscariello, L. Isolation and Identification of Lactic Acid Bacteria from Natural Whey Cultures of Buffalo and Cow Milk. Foods 2022, 11, 233. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, H.; Chen, L.; Cheng, B.; Diao, P.; Dong, L.; Gao, X.; Gu, H.; He, L.; Ji, C.; et al. Consensus of Chinese Experts on Protection of Skin and Mucous Membrane Barrier for Health-Care Workers Fighting against Coronavirus Disease 2019. Dermatol. Ther. 2020, 33, e13310. [Google Scholar] [CrossRef] [PubMed]
- PN-EN ISO 17516:2014-11; Kosmetyki—Mikrobiologia—Limity Mikrobiologiczne. ISO: Geneva, Switzerland, 2014.
- Azeem, M.; Saeed, F.; Afzaal, M.; Ateeq, H.; Ahmad, A.; Liaqat, A.; Busquets, R.; Lorenzo, J.M.; Asif Shah, M. Encapsulation of Probiotics in Solid Lipid Micro Particle for Improved Viability and Stability under Stressed Conditions. Int. J. Food Prop. 2023, 26, 1612–1623. [Google Scholar] [CrossRef]
- Ephrem, E.; Najjar, A.; Charcosset, C.; Greige-Gerges, H. Encapsulation of Natural Active Compounds, Enzymes, and Probiotics for Fruit Juice Fortification, Preservation, and Processing: An Overview. J. Funct. Foods 2018, 48, 65–84. [Google Scholar] [CrossRef]
- Yao, M.; Xie, J.; Du, H.; McClements, D.J.; Xiao, H.; Li, L. Progress in Microencapsulation of Probiotics: A Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 857–874. [Google Scholar] [CrossRef]
- Rokka, S.; Rantamäki, P. Protecting Probiotic Bacteria by Microencapsulation: Challenges for Industrial Applications. Eur. Food Res. Technol. 2010, 231, 1–12. [Google Scholar] [CrossRef]
- Łętocha, A.; Miastkowska, M.; Sikora, E. Preparation and Characteristics of Alginate Microparticles for Food, Pharmaceutical and Cosmetic Applications. Polymers 2022, 14, 3834. [Google Scholar] [CrossRef]
- Trabelsi, I.; Bejar, W.; Ayadi, D.; Chouayekh, H.; Kammoun, R.; Bejar, S.; Ben Salah, R. Encapsulation in Alginate and Alginate Coated-Chitosan Improved the Survival of Newly Probiotic in Oxgall and Gastric Juice. Int. J. Biol. Macromol. 2013, 61, 36–42. [Google Scholar] [CrossRef]
- Han, C.; Xiao, Y.; Liu, E.; Su, Z.; Meng, X.; Liu, B. Preparation of Ca-Alginate-Whey Protein Isolate Microcapsules for Protection and Delivery of L. Bulgaricus and L. Paracasei. Int. J. Biol. Macromol. 2020, 163, 1361–1368. [Google Scholar] [CrossRef]
- Dehkordi, S.S.; Alemzadeh, I.; Vaziri, A.S.; Vossoughi, A. Optimization of Alginate-Whey Protein Isolate Microcapsules for Survivability and Release Behavior of Probiotic Bacteria. Appl. Biochem. Biotechnol. 2020, 190, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.Y.; Chen, M.Y.; Xin, Y.; Qin, X.Y.; Cheng, Z.; Shi, L.E.; Tang, Z.X. Alginate-Based and Protein-Based Materials for Probiotics Encapsulation: A Review. Int. J. Food Sci. Technol. 2013, 48, 1339–1351. [Google Scholar] [CrossRef]
- Wang, X.; Gao, S.; Yun, S.; Zhang, M.; Peng, L.; Li, Y.; Zhou, Y. Microencapsulating Alginate-Based Polymers for Probiotics Delivery Systems and Their Application. Pharmaceuticals 2022, 15, 644. [Google Scholar] [CrossRef]
- Śliwa, K.; Sikora, E.; Ogonowski, J. Application of Waste Whey in Shampoos. Tech. Trans. 2011, 8, 205–211. [Google Scholar]
- Dinkçi, N.; Akdeniz, V.; Akalın, A.S. Probiotic Whey-Based Beverages from Cow, Sheep and Goat Milk: Antioxidant Activity, Culture Viability, Amino Acid Contents. Foods 2023, 12, 610. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.; Zemel, M.B. Functional Properties of Whey, Whey Components, and Essential Amino Acids: Mechanisms Underlying Health Benefits for Active People (Review). J. Nutr. Biochem. 2003, 14, 251–258. [Google Scholar] [CrossRef]
- Kassem, J.M. Future Challenges of Whey Proteins. Int. J. Dairy Sci. 2015, 10, 139–159. [Google Scholar] [CrossRef]
- Khan, I.T.; Nadeem, M.; Imran, M.; Ullah, R.; Ajmal, M.; Jaspal, M.H. Antioxidant Properties of Milk and Dairy Products: A Comprehensive Review of the Current Knowledge. Lipids Health Dis. 2019, 18, 41. [Google Scholar] [CrossRef]
- Lu, Y.; Riyanto, N.; Weavers, L.K. Sonolysis of Synthetic Sediment Particles: Particle Characteristics Affecting Particle Dissolution and Size Reduction. Ultrason. Sonochem. 2002, 9, 181–188. [Google Scholar] [CrossRef]
- Zare, M.; Golmakani, M.T.; Hosseini, S.M. Studying Structural and Rheological Properties of Alginate-Whey Protein Isolate Cold-Set Hybrid Emulgels at Various PH Levels. J. Texture Stud. 2023, 54, 720–735. [Google Scholar] [CrossRef] [PubMed]
- Lauteri, C.; Ferri, G.; Piccinini, A.; Pennisi, L.; Vergara, A. Ultrasound Technology as Inactivation Method for Foodborne Pathogens: A Review. Foods 2023, 12, 1212. [Google Scholar] [CrossRef]
- Akdeniz, V.; Akalın, A.S. Recent Advances in Dual Effect of Power Ultrasound to Microorganisms in Dairy Industry: Activation or Inactivation. Crit. Rev. Food Sci. Nutr. 2022, 62, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Khan, M.K. Applications of Ultrasound in Food Technology: Processing, Preservation and Extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Chandrapala, J.; Zisu, B. Novel Trends in Engineered Milk Products. J. Dairy Res. 2016, 83, 268–280. [Google Scholar] [CrossRef]
- Zanjani, M.A.K.; Tarzi, B.G.; Sharifan, A.; Mohammadi, N. Microencapsulation of Probiotics by Calcium Alginate-Gelatinized Starch with Chitosan Coating and Evaluation of Survival in Simulated Human Gastro-Intestinal Condition. Iran. J. Pharm. Res. IJPR 2014, 13, 843–852. [Google Scholar]
- Annan, N.T.; Borza, A.D.; Hansen, L.T. Encapsulation in Alginate-Coated Gelatin Microspheres Improves Survival of the Probiotic Bifidobacterium adolescentis 15703T during Exposure to Simulated Gastro-Intestinal Conditions. Food Res. Int. 2008, 41, 184–193. [Google Scholar] [CrossRef]
- Łętocha, A.; Michalczyk, A.; Miastkowska, M.; Sikora, E. Design of Alginate Microspheres Formulation as a Probiotics Carrier. Chem. Process Eng. New Front. 2023, 44, e20. [Google Scholar] [CrossRef]
- Łętocha, A.; Michalczyk, A.; Ostrowska, P.; Miastkowska, M.; Sikora, E. Probiotics-Loaded Microspheres for Cosmetic Applications. Appl. Sci. 2024, 14, 1183. [Google Scholar] [CrossRef]
- Burnett, C.L.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Bovine Milk Proteins and Protein Derivatives as Used in Cosmetics. Int. J. Toxicol. 2022, 41, 43S–56S. [Google Scholar] [CrossRef]
- Speer, S.; Amin, S. Sustainable Thermoresponsive Whey Protein- and Chitosan-Based Oil-in-Water for Cosmetic Applications. Int. J. Cosmet. Sci. 2022, 44, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, E.; Mros, S.; McConnell, M.; Cabral, J.D.; Ali, A. Melt-Electrowriting with Novel Milk Protein/PCL Biomaterials for Skin Regeneration. Biomed. Mater. 2019, 14, 055013. [Google Scholar] [CrossRef]
- Kazimierska, K.; Kalinowska-Lis, U. Milk Proteins-Their Biological Activities and Use in Cosmetics and Dermatology. Molecules 2021, 26, 3253. [Google Scholar] [CrossRef]
- Guimarães, R.R.; do Amaral Vendramini, A.L.; dos Santos, A.C.; Leite, S.G.F.; Miguel, M.A.L. Development of Probiotic Beads Similar to Fish Eggs. J. Funct. Foods 2013, 5, 968–973. [Google Scholar] [CrossRef]
- Darjani, P.; Nezhad, H.M.; Kadkhodaee, R.; Milani, E. Influence of Prebiotic and Coating Materials on Morphology and Survival of a Probiotic Strain of Lactobacillus casei Exposed to Simulated Gastrointestinal Conditions. LWT 2016, 73, 162–167. [Google Scholar] [CrossRef]
- Shafizadeh, A.; Golestan, L.; Ahmadi, M.; Darjani, P.; Ghorbani-HasanSaraei, A. Encapsulation of Lactobacillus casei in Alginate Microcapsules: Improvement of the Bacterial Viability under Simulated Gastrointestinal Conditions Using Flaxseed Mucilage. J. Food Meas. Charact. 2020, 14, 1901–1908. [Google Scholar] [CrossRef]
- Łętocha, A.; Michalczyk, A.; Miastkowska, M.; Sikora, E. Formulacja Kosmetyczna Lub Dermokosmetyczna Zawierająca Bakterie Probiotyczne. P.445990, 1 September 2023. [Google Scholar]
- Lasta, E.L.; da Silva Pereira Ronning, E.; Dekker, R.F.H.; da Cunha, M.A.A. Encapsulation and Dispersion of Lactobacillus acidophilus in a Chocolate Coating as a Strategy for Maintaining Cell Viability in Cereal Bars. Sci. Rep. 2021, 11, 20550. [Google Scholar] [CrossRef] [PubMed]
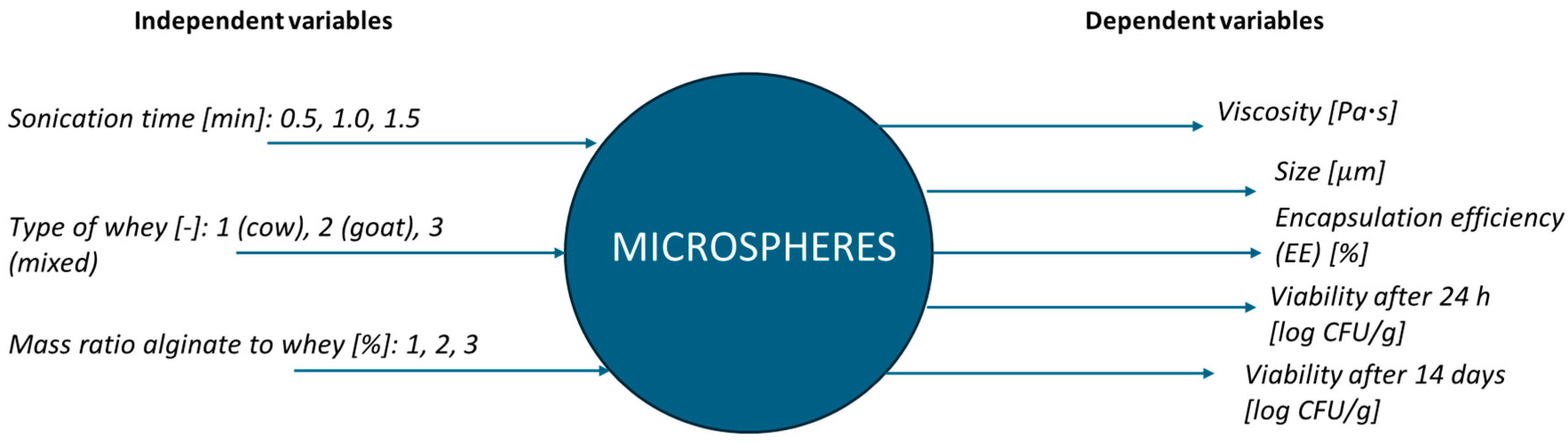
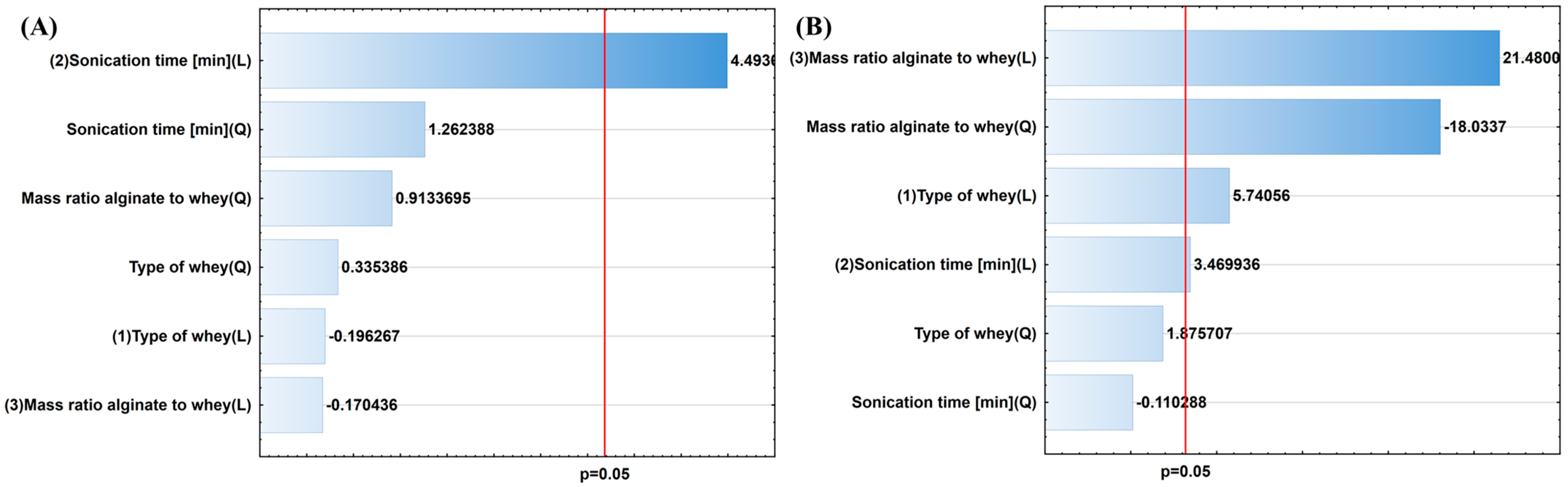
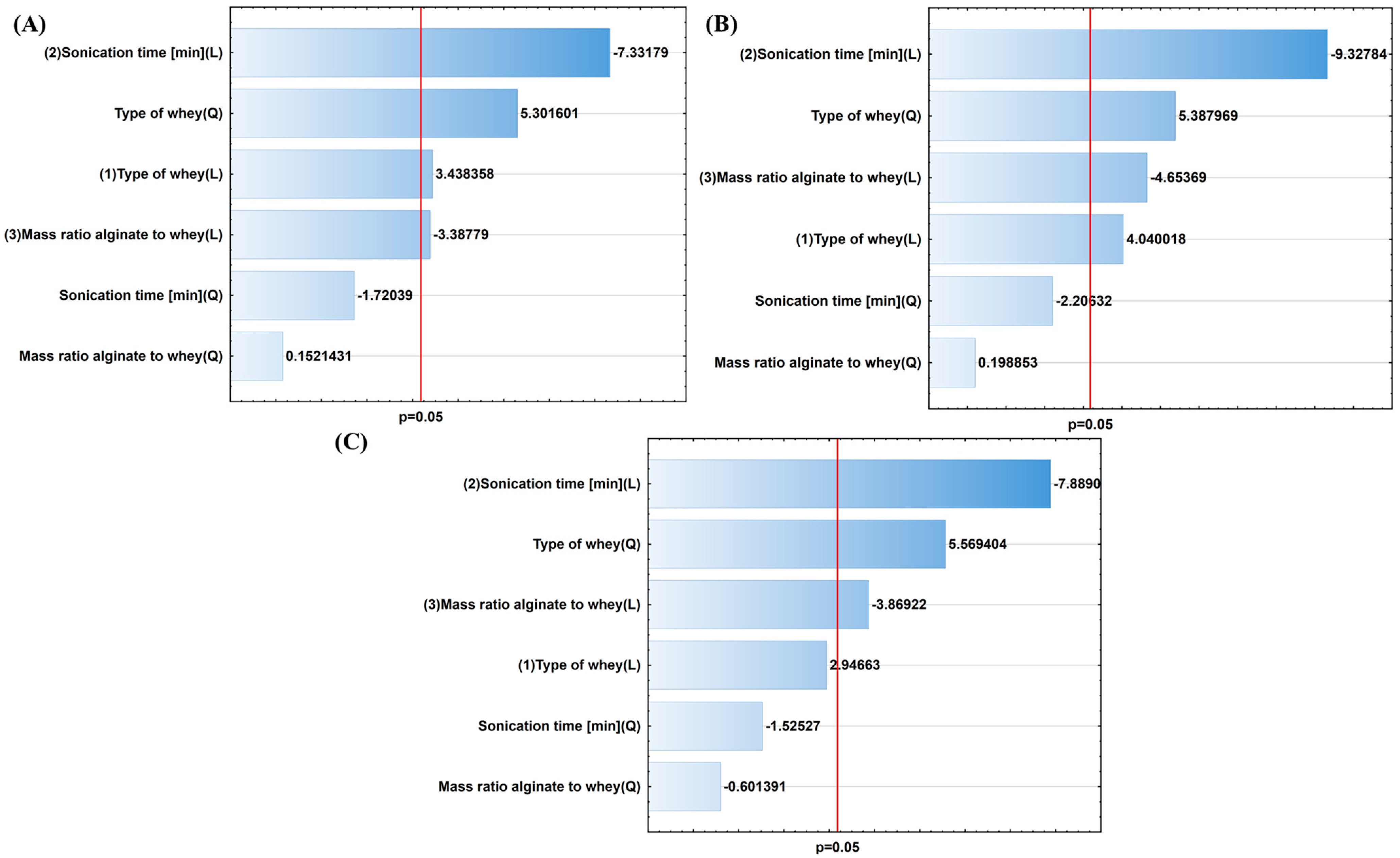
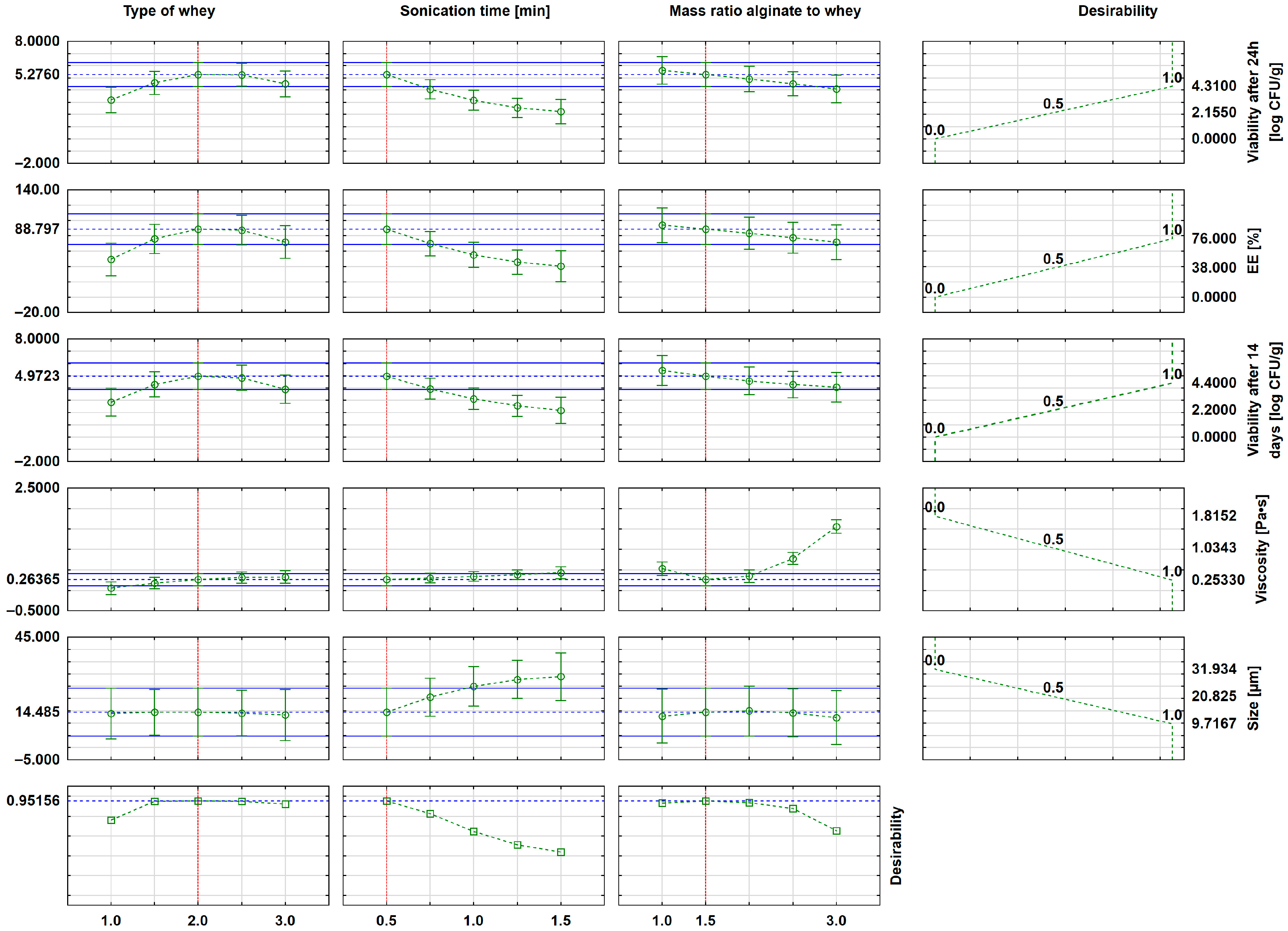
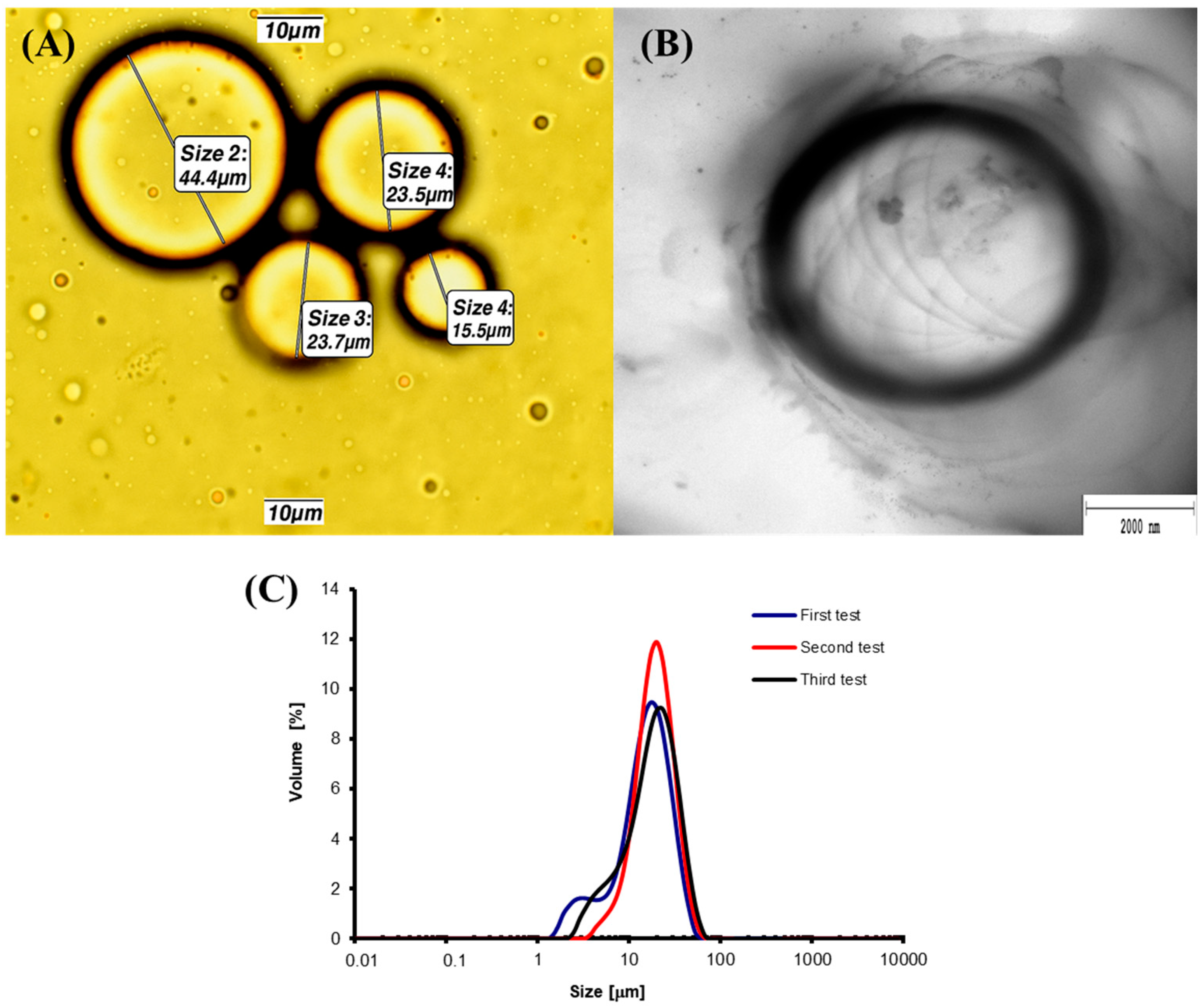

| Independent Variables | Dependent Variables | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample No. | Sonication Time [min] | Type of Whey * | Alginate-to-Whey Mass Ratio [%] | Viscosity [Pa∙s] | Size [µm] | EE ** [%] | Viability After 24 h [log CUF/g] | Viability After 14 Days [log CUF/g] |
| 1 | 1 | 2 | 2 | 0.437 ± 0.18 | 24.4 ± 2.7 | 51 ± 2 | 2.89 ± 0.10 | 2.87 ± 0.12 |
| 2 | 0.5 | 1 | 1 | 0.345 ± 0.20 | 9.7 ± 2.8 | 48 ± 2 | 3.24 ± 0.10 | 3.12 ± 0.10 |
| 3 | 1.5 | 2 | 1 | 0.722 ± 0.12 | 26.7 ± 3.2 | 47 ± 2 | 2.66 ± 0.18 | 2.53 ± 0.12 |
| 4 | 1 | 3 | 1 | 0.626 ± 0.18 | 25.3 ± 4.5 | 48 ± 1 | 2.96 ± 0.16 | 2.86 ± 0.11 |
| 5 | 1.5 | 3 | 3 | 1.815 ± 0.15 | 23.0 ± 2.9 | 0 | 0 | 0 |
| 6 | 1.5 | 1 | 2 | 0.253 ± 0.09 | 31.9 ± 6.2 | 0 | 0 | 0 |
| 7 | 1 | 1 | 3 | 1.453 ± 0.10 | 21.7 ± 3.8 | 0 | 0 | 0 |
| 8 | 1 | 2 | 2 | 0.436 ± 0.12 | 24.0 ± 3.9 | 43 ± 1 | 2.43 ± 0.12 | 2.43 ± 0.15 |
| 9 | 0.5 | 3 | 2 | 0.435 ± 0.12 | 13.2 ± 2.2 | 68 ± 2 | 4.23 ± 0.14 | 3.39 ± 0.15 |
| 10 | 0.5 | 2 | 3 | 1.512 ± 0.08 | 15.4 ± 3.1 | 76 ± 1 | 4.31 ± 0.12 | 4.4 ± 0.14 |
| Coefficients | Size [µm] | Viscosity [Pa∙s] | EE [%] | Viability After 24 h [log CFU/g] | Viability After 14 Days [log CFU/g] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Value | F-Test | p | Value | F-Test | p | Value | F-Test | p | Value | F-Test | p | Value | F-Test | p | |
| a (stała) | −13.9580 | - | 1.47982 | - | 18.313 | - | 2.47750 | - | 1.92250 | - | - | ||||
| b | 3.1767 | 0.03852 | 0.856944 | 0.42865 | 32.9540 | 0.010498 | 124.583 | 11.82231 | 0.041285 | 6.34833 | 16.32174 | 0.027287 | 6.91167 | 8.68263 | 0.060188 |
| c | −0.8730 | 0.11248 | 0.759418 | −0.07279 | 3.5183 | 0.157362 | −28.312 | 28.10697 | 0.013099 | −1.42250 | 29.03021 | 0.012526 | −1.59750 | 31.01827 | 0.011424 |
| d | 40.7255 | 20.18788 | 0.020575 | 0.13198 | 12.0405 | 0.040348 | −121.833 | 53.75519 | 0.005242 | −7.70000 | 87.00858 | 0.002609 | −6.29333 | 62.23728 | 0.004245 |
| e | −13.1441 | 1.59362 | 0.296019 | 0.01712 | 0.0122 | 0.919145 | 36.750 | 2.95973 | 0.183848 | 2.33000 | 4.86785 | 0.114494 | 1.75000 | 2.32644 | 0.224606 |
| f | 9.2362 | 0.02905 | 0.875513 | −2.28478 | 461.3921 | 0.000221 | −7.917 | 11.47715 | 0.042844 | −0.54833 | 21.65684 | 0.018716 | −1.37500 | 14.97084 | 0.030543 |
| g | −2.3775 | 0.83424 | 0.428396 | 0.69982 | 325.2133 | 0.000372 | −0.813 | 0.02315 | 0.888730 | −0.05250 | 0.03954 | 0.855092 | 0.17250 | 0.36167 | 0.589986 |
| R2 | 0.887 | 0.996 | 0.973 | 0.981 | 0.975 | ||||||||||
| Adjusted R2 | 0.661 | 0.989 | 0.918 | 0.944 | 0.925 | ||||||||||
| Sonication Time [min] | Type of Whey * | Alginate-to-Whey Mass Ratio [%] | Viscosity [Pa∙s] | Size [µm] | EE [%] | Viability After 24 h [log CFU/g] | Viability After 14 Days [log CFU/g] |
|---|---|---|---|---|---|---|---|
| 0.5 | 2.0 | 1.5 | 0.437 ± 0.1 | 23.36 ± 2.25 | 72 ± 1 | 4.40 ± 0.32 | 4.31 ± 0.40 |
| Sample No. | Immediately After Preparation | After 7 Days | After 30 Days | |||
|---|---|---|---|---|---|---|
| Cell Viability * [log CFU/g] | Log Reduction | Cell Viability [log CFU/g] | Log Reduction | Cell Viability [log CFU/g] | Log Reduction | |
| Formulation with non-encapsulated whey | 6.38 ± 1.1 | 1.52 | 6.90 ± 0.6 | 1.00 | 0 | 7.9 |
| Formulation with whey-loaded microspheres | 6.35 ± 0.7 | 1.55 | 6.25 ± 0.7 | 1.65 | 4.92 ± 0.9 | 2.98 |
| Emulsion Phase | Component | Concentration [%] |
|---|---|---|
| Water phase | Water | 77 |
| Xanthan gum | ||
| Sodium levulinate; sodium anisate | ||
| Sodium benzoate | ||
| Oil phase | Meadowfoam seed oil | 15 |
| Emulsifiers | Sorbitan stearate; sucrose cocoate | 7 |
| Glyceryl stearate; polyglyceryl-6 palmitate/succinate; cetearyl alcohol | ||
| Active | Whey-loaded microspheres |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikora, E.; Łętocha, A.; Michalczyk, A.; Kozik, A. Encapsulation of Acid Whey in Alginate Microspheres for Application in Skin Microbiome-Friendly Topical Formulations: Optimization Through a Design of Experiments Approach. Molecules 2025, 30, 3907. https://doi.org/10.3390/molecules30193907
Sikora E, Łętocha A, Michalczyk A, Kozik A. Encapsulation of Acid Whey in Alginate Microspheres for Application in Skin Microbiome-Friendly Topical Formulations: Optimization Through a Design of Experiments Approach. Molecules. 2025; 30(19):3907. https://doi.org/10.3390/molecules30193907
Chicago/Turabian StyleSikora, Elżbieta, Anna Łętocha, Alicja Michalczyk, and Agnieszka Kozik. 2025. "Encapsulation of Acid Whey in Alginate Microspheres for Application in Skin Microbiome-Friendly Topical Formulations: Optimization Through a Design of Experiments Approach" Molecules 30, no. 19: 3907. https://doi.org/10.3390/molecules30193907
APA StyleSikora, E., Łętocha, A., Michalczyk, A., & Kozik, A. (2025). Encapsulation of Acid Whey in Alginate Microspheres for Application in Skin Microbiome-Friendly Topical Formulations: Optimization Through a Design of Experiments Approach. Molecules, 30(19), 3907. https://doi.org/10.3390/molecules30193907








