Chemical Composition and Bioactivity of Essential Oils from Magnolia pugana, an Endemic Mexican Magnoliaceae Species
Abstract
1. Introduction
2. Results and Discussion
2.1. Essential Oil Extraction
2.2. Chemical Analysis
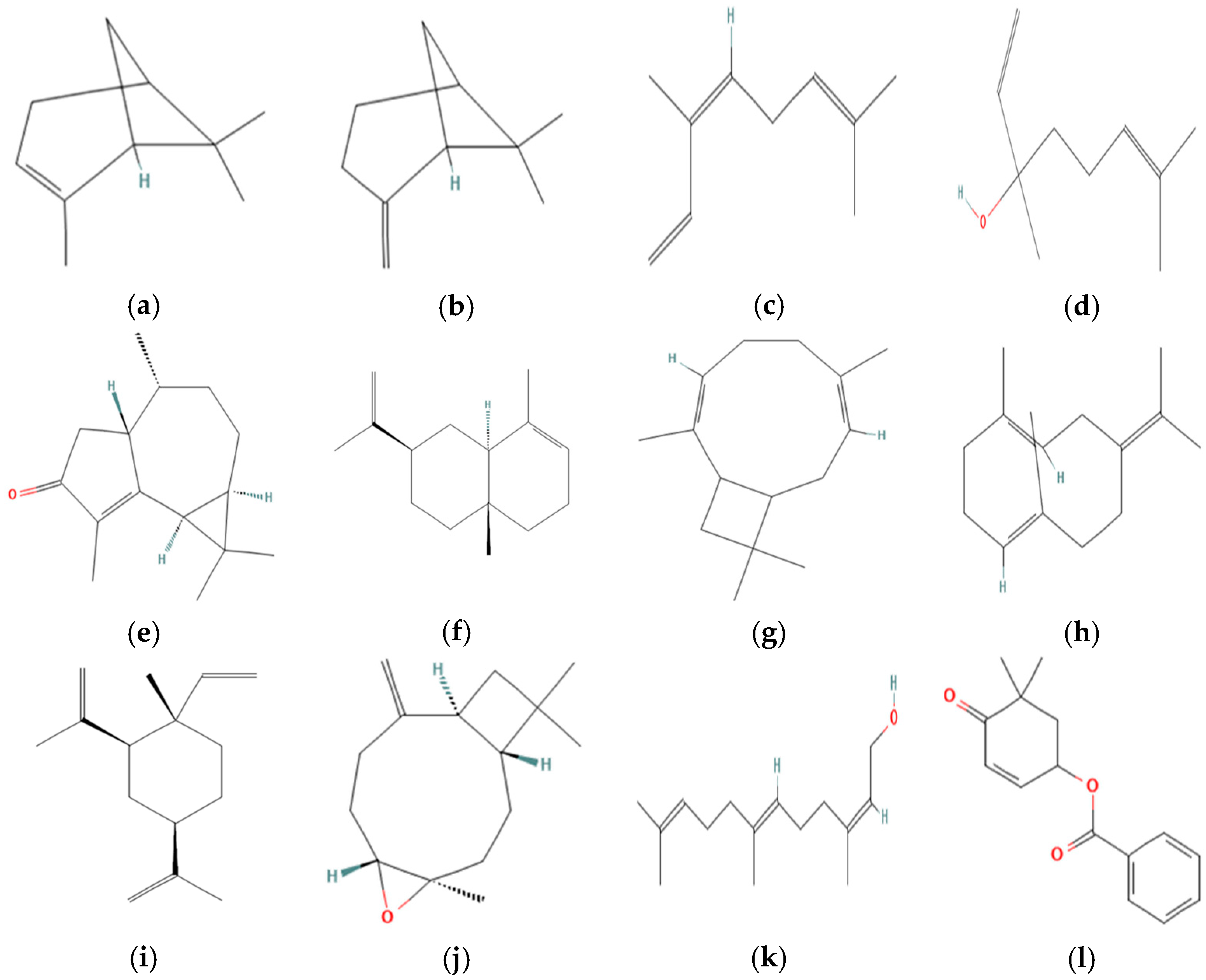
2.3. Antioxidant Activity
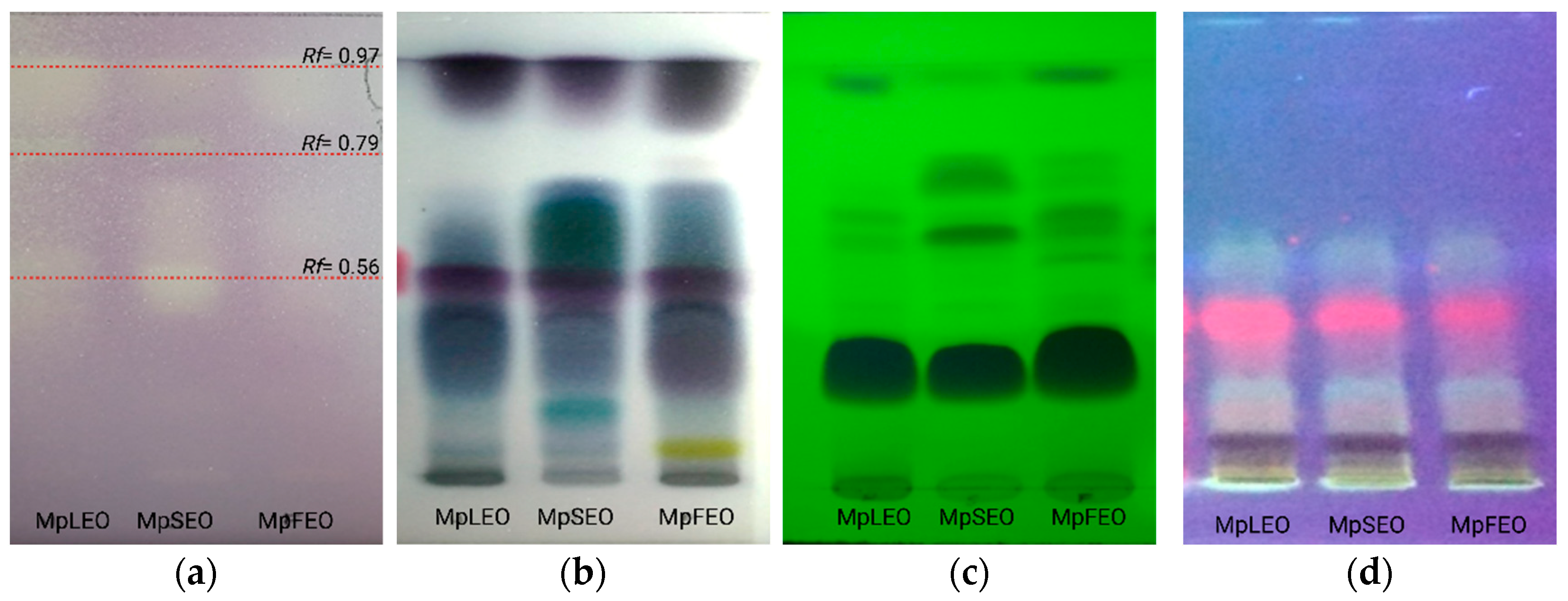
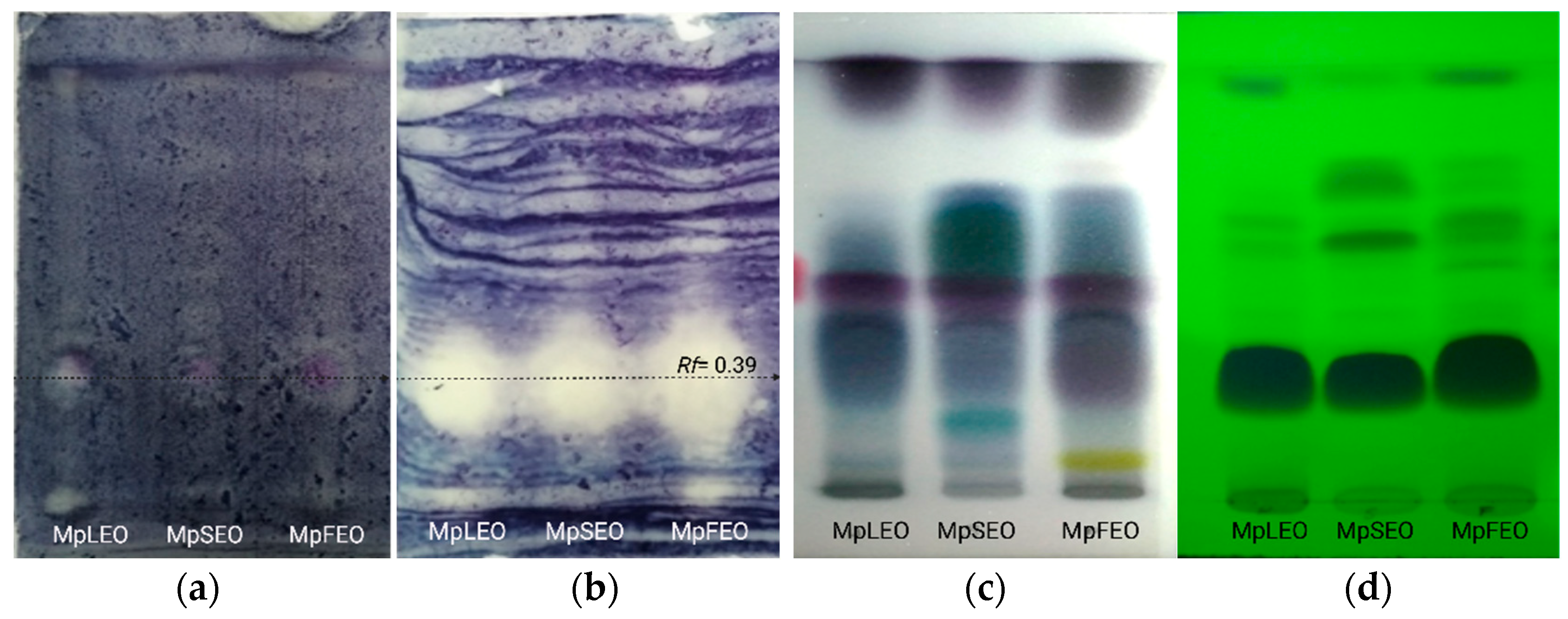
Antioxidant Bioautography
2.4. Antibacterial Activity
Antibacterial Bioautography
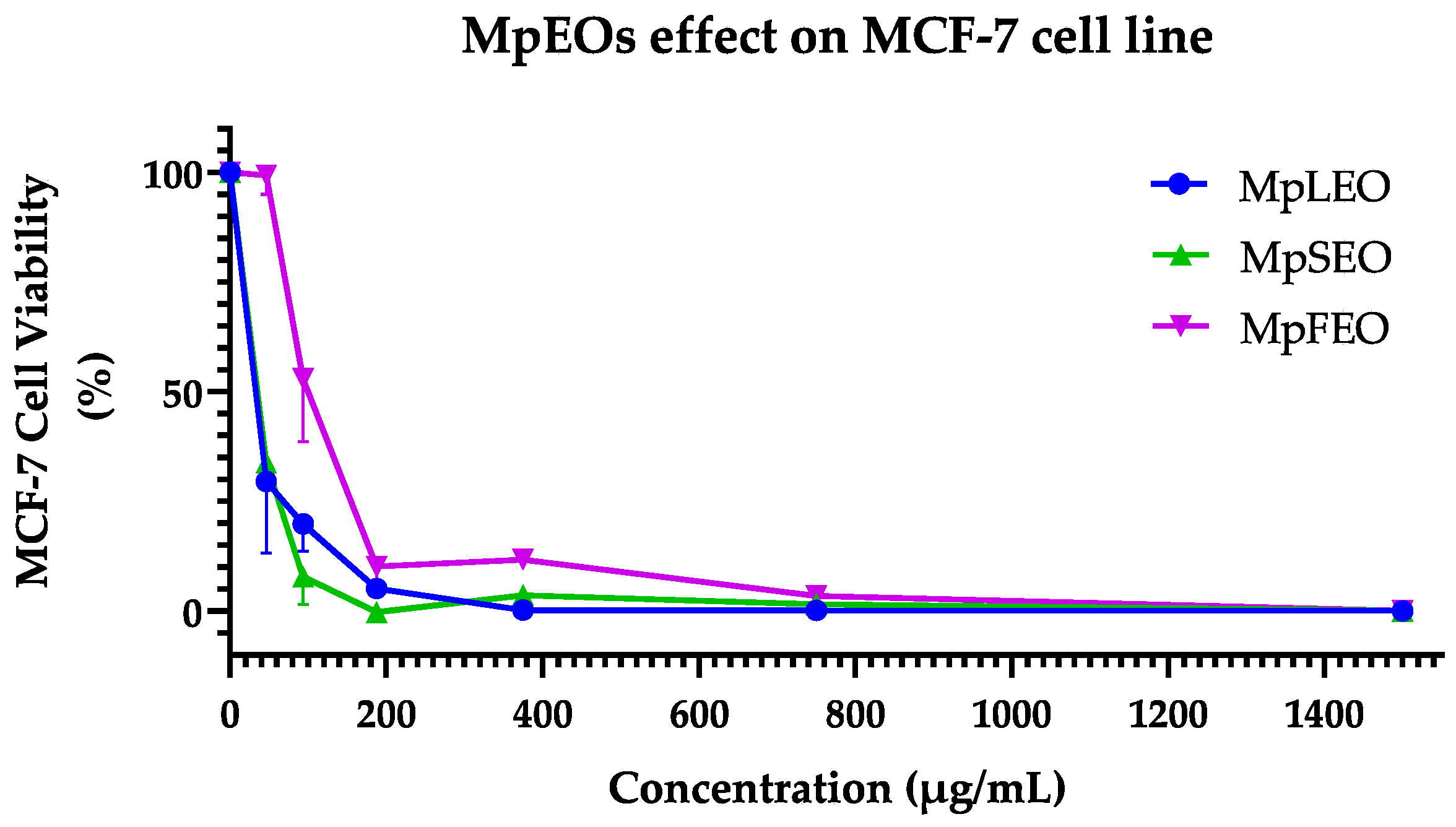
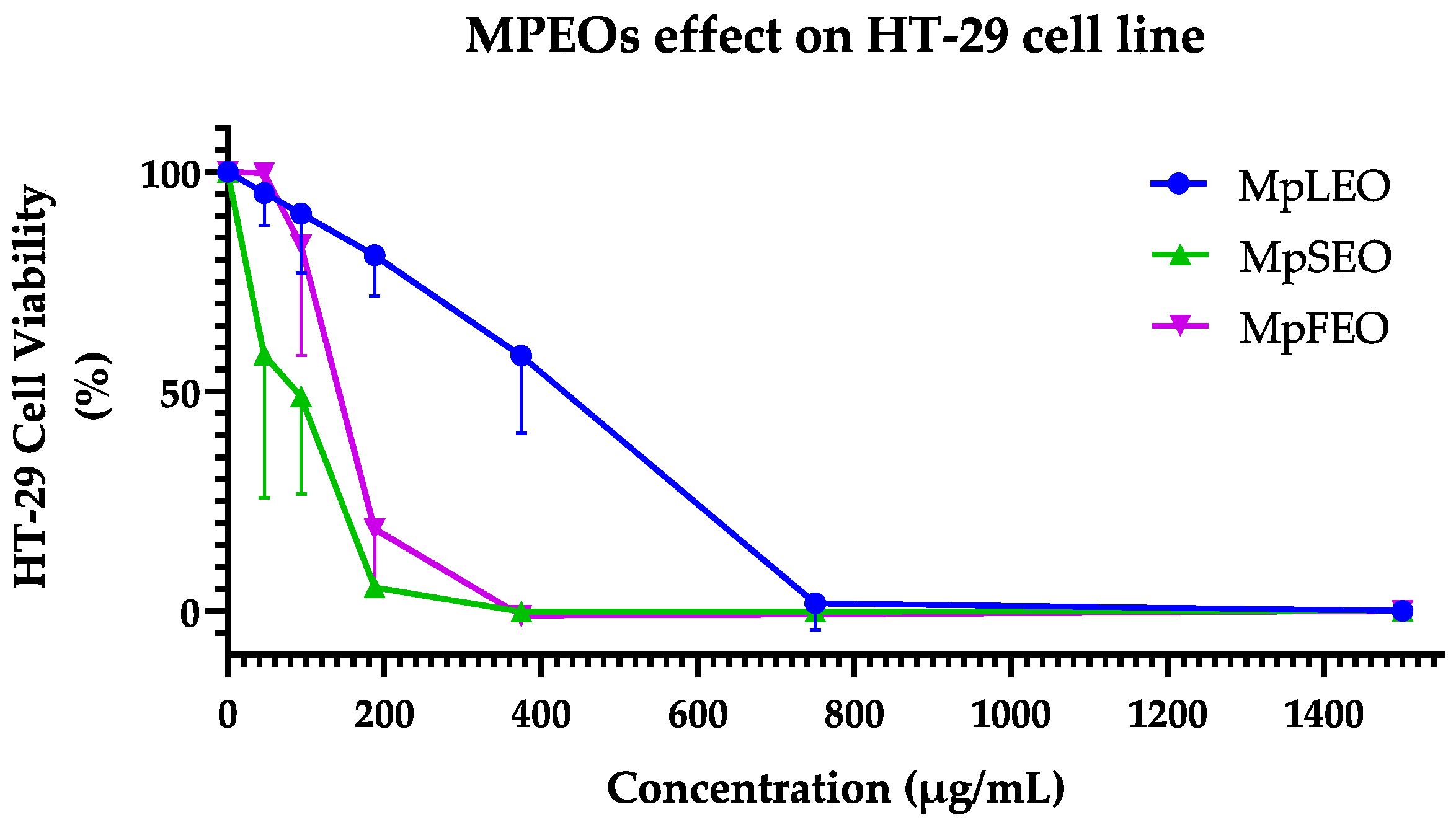
2.5. Cytotoxic Activity
3. Materials and Methods
3.1. Plant Material
3.2. Extraction of Essential Oils
3.3. Chemical Composition of Essential Oils
3.4. Antioxidant Activity
Autobiographic Antioxidant Activity
3.5. Antibacterial Activity
Autographic Antibacterial Activity
3.6. Cytotoxic Activity
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EOs | Essential oils |
| MpEOs | Magnolia pugana essential oils |
| MpLEO | Magnolia pugana leaf essential oil |
| MpFEO | Magnolia pugana flower essential oil |
| MpSEO | Magnolia pugana seed essential oil |
| TLC | Thin Layer chromatography |
| GC-FID | Gas chromatography-flame ionization detector |
| GC-MS | Gas chromatography coupled to mass spectrometry |
| MIC | Minimal inhibitory concentration |
| IC50 | Inhibitory concentration 50% |
| TLC-DB | Thin Layer chromatography-Direct Bioautography |
References
- Vázquez-García, J.A.; Domínguez-Yescas, R.; Pedraza-Ruíz, R.; Muñiz-Castro, M. Magnolia Rzedowskiana (Magnoliaceae), a New Species of Section Macrophylla from the Central Sierra Madre Oriental, Mexico. Acta Botánica Mex. 2015, 112, 17. [Google Scholar]
- Yao, W.; Fan, Y.; Wang, Z.; Liu, D.; Ding, Z.; Ou, J. Diversity and Geographic Distribution Patterns of Wild Magnoliaceae Species in China. Sustainability 2024, 16, 9448. [Google Scholar] [CrossRef]
- Antonio Vázquez-García, J.; Angel Muñiz-Castro, M.; Dahua-Machoa, A.; Antonio Osorio-Muñoz, E.; Hernández-Vera, G.; Salomé Ortega-Peña, A.; de Lourdes Romo-Campos, R.; Jacobo-Pereira, C.; Álvarez de Román, N.; Shalisko, V. How to Save Endangered Magnolias? From Population Biology to Conservation Action: The Case of Allopatric Radiation in Western Mexico. In Endangered Plants; IntechOpen: London, UK, 2021. [Google Scholar]
- Sarker, S.; Maruyama, Y. Magnolia the Genus Magnolia; Taylor & Francis: London, UK, 2002; ISBN 0-203-21665-2. [Google Scholar]
- Vázquez-García, J.A.; Neill, D.; Asanza, M.; Pérez, Á.J.; Arroyo, F.; Dahua, A.; Merino-Santi, R.E. Magnolias de Ecuador: En Riesgo de Extinción; Universidad Estatal Amazónica: Puyo, Ecuador, 2015. [Google Scholar]
- Vázquez-García, J.A.; Carvajal, S.; Hernandez-Lopez, L. Magnolia pugana (Magnoliaceae): A New Combination in the Complex M. pacifica. Novon 2002, 12, 137–141. [Google Scholar]
- Carranza, A.S. Diversidad y Diferenciación Genética de Magnolia pugana y Magnolia pacifica, Especies Endémicas Del Occidente de México. Bachelor’s Thesis, Universidad de Guadalajara, Guadalajara, Mexico, 2014. [Google Scholar]
- Jacobo-Pereira, C.; Muñiz-Castro, M.Á.; Muñoz-Urias, A.; Huerta-Martínez, F.M.; Vázquez-García, J.A.; Flores, J. Effect of Temperature and Drought Stress on Germination of Magnolia pugana, an Endangered Species from Western Mexico. Bot. Sci. 2023, 101, 1115–1127. [Google Scholar] [CrossRef]
- Rivers, M.; Beech, E.; Lydia, M.; Oldfield, S. The Red List of Magnoliaceae—Revised and Extended; Botanic Gardens Conservation International: Richmond, UK, 2016; ISBN 1-905164-64-5. [Google Scholar]
- Dahua, A.N. Temporalidad de Fenofases y Micropropagación In Vitro de Tres Especies Relictuales de Magnolia Del Occidente de México: Implicaciones Para Su Conservación in situ y ex situ. Master’s Thesis, Universidad de Guadalajara, Guadalajara, Mexico, 2018. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 2017; ISBN 0931710855. [Google Scholar]
- Morsy, N.F.S. Chemical Structure, Quality Indices and Bioactivity of Essential Oil Constituents. In Active Ingredients from Aromatic and Medicinal Plants; InTech: London, UK, 2017. [Google Scholar]
- Calvo-Irabien, L. Native Mexican Aromatic Flora and Essential Oils: Current Research Status, Gaps in Knowledge and Agro-Industrial Potential. Ind. Crops Prod. 2018, 111, 807–822. [Google Scholar] [CrossRef]
- Zheng, Y.-F.; Ren, F.; Liu, X.-M.; Lai, F.; Ma, L. Comparative Analysis of Essential Oil Composition from Flower and Leaf of Magnolia Kwangsiensis Figlar & Noot. Nat. Prod. Res. 2016, 30, 1552–1556. [Google Scholar] [CrossRef]
- Pergentino de Sousa, D. Bioactive Essential Oils and Cancer; de Sousa, D.P., Ed.; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-19143-0. [Google Scholar]
- Gilardoni, G.; Malagon, O.; Morocho, V.; Negri, R.; Tosi, S.; Guglielminetti, M.; Vidari, G.; Finzi, P.V. Phytochemical Researches and Antimicrobial Activity of Clinopodium nubigenum Kunth (Kuntze) Raw Extracts. Braz. J. Pharmacogn. 2011, 21, 850–855. [Google Scholar] [CrossRef]
- Aljaafari, M.; Sultan Alhosani, M.; Abushelaibi, A.; Lai, K.-S.; Erin Lim, S.-H. Essential Oils: Partnering with Antibiotics. In Essential Oils—Oils of Nature; IntechOpen: London, UK, 2019. [Google Scholar]
- Noriega, P.; Mosquera, T.; Paredes, E.; Parra, M.; Zappia, M.; Herrera, M.; Villegas, A.; Osorio, E. Antimicrobial and Antioxidant Bioautography Activity of Bark Essential Oil from Ocotea quixos (Lam.) Kosterm. JPC—J. Planar Chromatogr.—Mod. TLC 2018, 31, 163–168. [Google Scholar] [CrossRef]
- Hu, M.; Bai, M.; Ye, W.; Wang, Y.; Wu, H. Variations in Volatile Oil Yield and Composition of “Xin-Yi” (Magnolia Biondii Pamp. Flower Buds) at Different Growth Stages. J. Oleo Sci. 2018, 67, 779–787. [Google Scholar] [CrossRef]
- Aldulaimi, O.; Wu Li, W. Antibacterial Effects of the Essential Oil from Flower Buds of Magnolia Biondii Pamp. Planta Med. 2016, 8, 2. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology. NIST/EPA/NIH Mass Spectral Library (NIST 14); (1A 2.2); NIST: Gaithersburg, MD, USA, 2014. [Google Scholar]
- Al Muzaini, O.; Al Dulaimi, O.; Al Qahtani, T.; Li, W. Antimicrobial Compounds from Magnolia liliflora Desr. Hamdan Med. J. 2015, 82, S1–S381. [Google Scholar] [CrossRef]
- Scharf, D.; Simionato, E.; Mello-Silva, R.; Carvalho, J.; Salvador, M.; Stefanello, M. Cytotoxicity and Chemical Composition of the Essential Oils of Magnolia ovata. Lat. Am. J. Pharm. 2016, 35, 4. [Google Scholar]
- Calancea, M.; Carret, S.; Deprés, J.-P. Short Access to the Aromadendrane Family: Highly Efficient Stereocontrolled Total Synthesis of (±)-Cyclocolorenone and (±)-α-Gurjunene. Eur. J. Org. Chem. 2009, 2009, 3134–3137. [Google Scholar] [CrossRef]
- Davé, P.C.; Vogler, B.; Setzer, W.N. Composition of the Floral Essential Oil of Magnolia grandiflora L. (Magnoliaceae): Intraspecific and Floral Maturity Variations. J. Essent. Oil Bear. Plants 2012, 15, 694–702. [Google Scholar] [CrossRef]
- Durán, R.C.; Flores, K.V.; Ramírez, J.J. Magnolia vazquezii (Magnoliaceae), Una Especie Nueva Del Estado De Guerrero, México. Novon A J. Bot. Nomencl. 2008, 18, 21–24. [Google Scholar] [CrossRef]
- NCBI Pubchem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 8 September 2025).
- Henderson, P.; Seaby, R. Community Analysis Package; Version 4.0; Pisces Conservation Ltd.: Lymington, UK, 1999. [Google Scholar]
- Kapetanos, C.; Karioti, A.; Bojović, S.; Marin, P.; Veljić, M.; Skaltsa, H. Chemical and Principal-Component Analyses of the Essential Oils of Apioideae Taxa (Apiaceae) from Central Balkan. Chem. Biodivers. 2008, 5, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Bartosz, G. Evaluation of The Antioxidant Capacity of Food Products: Methods, Applications and Limitations. Processes 2022, 10, 2031. [Google Scholar] [CrossRef]
- Nie, J.-Y.; Li, R.; Jiang, Z.-T.; Wang, Y.; Tan, J.; Tang, S.-H.; Zhang, Y. Screening and Evaluation of Radical Scavenging Active Compounds in the Essential Oil from Magnolia biondii Pamp by Electronic Nose Coupled with Chemical Methodology. Ind. Crops Prod. 2020, 144, 112060. [Google Scholar] [CrossRef]
- Malik, S. Essential Oil Research: Trends in Biosynthesis, Analytics, Industrial Applications and Biotechnological Production; Malik, S., Ed.; Springer International Publishing: San Luis, Brazil, 2019; ISBN 978-3-030-16545-1. [Google Scholar]
- Luo, M.; Sun, J.; Zhang, B.; Jiang, L. Chemical Composition and Antioxidant Activity of Essential Oil from Magnolia grandiflora L. Seed. Wuhan Univ. J. Nat. Sci. 2012, 17, 249–254. [Google Scholar] [CrossRef]
- Chang, D.; Sharma, L.; Dela Cruz, C.S.; Zhang, D. Clinical Epidemiology, Risk Factors, and Control Strategies of Klebsiella Pneumoniae Infection. Front. Microbiol. 2021, 12, 750662. [Google Scholar] [CrossRef]
- Gill, A.; Tamber, S.; Yang, X. Standard Methods for the Bacteriological Analysis of Meat. In Encyclopedia of Meat Sciences; Elsevier: Amsterdam, The Netherlands, 2024; pp. 137–153. [Google Scholar]
- Dubay, M.M.; Johnston, N.; Wronkiewicz, M.; Lee, J.; Lindensmith, C.A.; Nadeau, J.L. Quantification of Motility in Bacillus subtilis at Temperatures up to 84 °C Using a Submersible Volumetric Microscope and Automated Tracking. Front. Microbiol. 2022, 13, 836808. [Google Scholar] [CrossRef]
- El-Demerdash, A.S.; Alfaraj, R.; Farid, F.A.; Yassin, M.H.; Saleh, A.M.; Dawwam, G.E. Essential Oils as Capsule Disruptors: Enhancing Antibiotic Efficacy against Multidrug-Resistant Klebsiella pneumoniae. Front. Microbiol. 2024, 15, 14. [Google Scholar] [CrossRef]
- Illenseher, M.; Hentschker, C.; Salazar, M.G.; Busch, L.M.; Zierke, L.; Reder, A.; Michalik, S.; Völker, U.; Hammerschmidt, S.; Surmann, K. Global Quantitative Proteome Analysis of a Multi-Resistant Klebsiella Pneumoniae Strain. Front. Microbiol. 2025, 16, 1528869. [Google Scholar] [CrossRef] [PubMed]
- Grabley, S.; Thiericke, R. (Eds.) Drug Discovery from Nature; Springer: Berlin/Heidelberg, Germany, 2000; ISBN 978-3-540-66947-0. [Google Scholar]
- Schühly, W.; Khan, I.; Fischer, N.H. The Ethnomedicinal Uses of Magnoliaceae from the Southeastern United States as Leads in Drug Discovery. Pharm. Biol. 2001, 39, 63–69. [Google Scholar] [CrossRef]
- Yan, X.; Lin, J.; Liu, Z.; David, S.D.; Liang, D.; Nie, S.; Ge, M.; Xue, Z.; Li, W.; Qiao, J. The Recent Progress of Tricyclic Aromadendrene-Type Sesquiterpenoids: Biological Activities and Biosynthesis. Biomolecules 2024, 14, 1133. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.O.; Bouwmeester, H.J.; Bülow, N.; König, W.A. Isolation, Characterization, and Mechanistic Studies of (−)-α-Gurjunene Synthase From Solidago canadensis. Arch. Biochem. Biophys. 1999, 364, 167–177. [Google Scholar] [CrossRef]
- Malićanin, M.; Karabegović, I.; Đorđević, N.; Mančić, S.; Stojanović, S.S.; Brković, D.; Danilović, B. Influence of the Extraction Method on the Biological Potential of Solidago virgaurea L. Essent Oil Hydrolates. Plants 2024, 13, 2187. [Google Scholar] [CrossRef]
- la Torre Fabiola, V.-D.; Ralf, K.; Gabriel, B.; Victor Ermilo, A.-A.; Martha, M.-G.; Mirbella, C.-F.; Rocio, B.-A. Anti-Inflammatory and Immunomodulatory Effects of Critonia aromatisans Leaves: Downregulation of pro-Inflammatory Cytokines. J. Ethnopharmacol. 2016, 190, 174–182. [Google Scholar] [CrossRef]
- Jacyno, J.M.; Montemurro, N.; Bates, A.D.; Cutler, H.G. Phytotoxic and Antimicrobial Properties of Cyclocolorenone from Magnolia grandiflora L. J. Agric. Food Chem. 1991, 39, 1166–1168. [Google Scholar] [CrossRef]
- Vasconcelos, N.G.; Croda, J.; Silva, K.E.; Motta, M.L.L.; Maciel, W.G.; Limiere, L.C.; Simionatto, S. Origanum vulgare L. Essential Oil Inhibits the Growth of Carbapenem-Resistant Gram-Negative Bacteria. Rev. Soc. Bras. Med. Trop. 2019, 52, e20180502. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; D’Acierno, A.; Coppola, R.; Jesus Ayala-Zavala, F.; Gomez da Cruz, A.; De Feo, V. Essential Oils and Microbial Communication. In Essential Oils—Oils of Nature [Working Title]; IntechOpen: London, UK, 2019. [Google Scholar]
- Flatt, V.; Campos, C.; Kraemer, M.; Bailey, B.; Satyal, P.; Setzer, W. Compositional Variation and Bioactivity of the Leaf Essential Oil of Montanoa guatemalensis from Monteverde, Costa Rica: A Preliminary Investigation. Medicines 2015, 2, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.R.F.; Schuh, R.S.; Jacques, A.L.B.; Augustin, O.A.; Bordignon, S.A.L.; Dias, D.O.; Kelmann, R.G.; Koester, L.S.; Gehring, M.P.; Morrone, F.B.; et al. Citotoxic Activity Evaluation of Essential Oils and Nanoemulsions of Drimys angustifolia and D. Brasiliensis on Human Glioblastoma (U-138 MG) and Human Bladder Carcinoma (T24) Cell Lines in Vitro. Rev. Bras. Farmacogn. 2013, 23, 259–267. [Google Scholar] [CrossRef]
- Kuete, V.; Karaosmanoğlu, O.; Sivas, H. Anticancer Activities of African Medicinal Spices and Vegetables. In Medicinal Spices and Vegetables from Africa; Elsevier: Amsterdam, The Netherlands, 2017; pp. 271–297. [Google Scholar]
- Ranganathan, S.; Halagowder, D.; Sivasithambaram, N.D. Quercetin Suppresses Twist to Induce Apoptosis in MCF-7 Breast Cancer Cells. PLoS ONE 2015, 10, e0141370. [Google Scholar] [CrossRef]
- Verhoeckx, K.; Cotter, P.; López-Expósito, I.; Kleiveland, C.; Lea, T.; Mackie, A.; Requena, T.; Swiatecka, D.; Wichers, H. (Eds.) The Impact of Food Bioactives on Health; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-15791-7. [Google Scholar]
- Buriani, A.; Fortinguerra, S.; Sorrenti, V.; Dall’Acqua, S.; Innocenti, G.; Montopoli, M.; Gabbia, D.; Carrara, M. Human Adenocarcinoma Cell Line Sensitivity to Essential Oil Phytocomplexes from Pistacia Species: A Multivariate Approach. Molecules 2017, 22, 1336. [Google Scholar] [CrossRef]
- Russo, R.; Corasaniti, M.T.; Bagetta, G.; Morrone, L.A. Exploitation of Cytotoxicity of Some Essential Oils for Translation in Cancer Therapy. Evid.-Based Complement. Altern. Med. 2015, 2015, 397821. [Google Scholar] [CrossRef]
- Raut, J.S.; Karuppayil, S.M. A Status Review on the Medicinal Properties of Essential Oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Ramos, E.H.S.; Moraes, M.M.; Nerys, L.L.d.A.; Nascimento, S.C.; Militão, G.C.G.; de Figueiredo, R.C.B.Q.; da Câmara, C.A.G.; Silva, T.G. Chemical Composition, Leishmanicidal and Cytotoxic Activities of the Essential Oils from Mangifera indica L. Var. Rosa and Espada. Biomed Res. Int. 2014, 2014, 734946. [Google Scholar] [CrossRef]
- Scalvenzi, L.; Grandini, A.; Spagnoletti, A.; Tacchini, M.; Neill, D.; Ballesteros, J.; Sacchetti, G.; Guerrini, A. Myrcia splendens (Sw.) DC. (Syn. M. Fallax (Rich.) DC.) (Myrtaceae) Essential Oil from Amazonian Ecuador: A Chemical Characterization and Bioactivity Profile. Molecules 2017, 22, 1163. [Google Scholar] [CrossRef]
- Noriega, P.; Calderón, L.; Ojeda, A.; Paredes, E. Chemical Composition, Antimicrobial and Antioxidant Bioautography Activity of Essential Oil from Leaves of Amazon Plant Clinopodium brownei (Sw.). Molecules 2023, 28, 1741. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas—Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Jiang, L.; Tang, X.; Peng, L.; Li, X.; Zhao, G.; Zhong, L. Chemical Composition, Antimicrobial and Antioxidant Activities of the Flower Volatile Oils of Fagopyrum esculentum, Fagopyrum tataricum and Fagopyrum cymosum. Molecules 2018, 23, 182. [Google Scholar] [CrossRef] [PubMed]
- Torres, P.; Pires, J.; dos Santos, D.; Chow, F. Ensayo de Potencial Antioxidante de Extractos de Algas Através de Secuestro de ABTS° En Microplaca; Instituto de Biociências, Universidade de São Paulo: São Paulo, Brasil, 2017; 4p. [Google Scholar]
- Semeniuc, C.A.; Pop, C.R.; Rotar, A.M. Antibacterial Activity and Interactions of Plant Essential Oil Combinations against Gram-Positive and Gram-Negative Bacteria. J. Food Drug Anal. 2017, 25, 403–408. [Google Scholar] [CrossRef]
- Clinical Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 9th ed.; CLSI: Berwyn, PA, USA, 2012. [Google Scholar]
- Jesionek, W.; Móricz, Á.M.; Ott, P.G.; Kocsis, B.; Horváth, G.; Choma, I.M. TLC-Direct Bioautography and LC/MS as Complementary Methods in Identification of Antibacterial Agents in Plant Tinctures from the Asteraceae Family. J. AOAC Int. 2015, 98, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.; Mirokhin, Y. NIST MS Search (2.0); National Institute of Standards and Technology: Gaithersburg, MD, USA, 2002. [Google Scholar]
- NIST. Libro del Web de Química del NIST. 2018. Available online: https://webbook.nist.gov/ (accessed on 8 September 2025).

| Plant Part | Weight (g) | EO (mL) | Yield | Density | RI | Odor | Color |
|---|---|---|---|---|---|---|---|
| Leaves 1 | 150 | ≅0.49 | 0.33 ± 0.3% | 0.923 | 1.505 | Pungent, woody, bitter | Pale yellow |
| Flowers 1 | ≅0.42 | 0.28 ± 0.4% | 0.962 | 1.510 | Sweet, citric | Light yellow | |
| Seeds 2 | ≅6.00 | 4.00 ± 0.2% | 0.956 | 1.509 | Sweet, woody | Light yellow |
| No. | Compound | Apolar DB-5 Column | DB-Wax Column | Total (%) | S.D. | ||
|---|---|---|---|---|---|---|---|
| AI Teo | AI Exp | AI Teo | AI Exp | ||||
| 1 | α-pinene | 932 | 928 | 1025 | 1022 | 3.73 | ±1.50 |
| 2 | Camphene | 946 | 943 | 1068 | 1060 | 2.87 | ±0.83 |
| 3 | β-pinene | 974 | 972 | 1110 | 1107 | 20.45 | ±4.00 |
| 4 | Myrcene | 988 | 985 | 1160 | 1157 | 0.41 | ±0.06 |
| 5 | δ-3-carene | 1008 | 1005 | 1147 | 1140 | 2.20 | ±0.32 |
| 6 | Limonene | 1024 | 1020 | 1198 | 1195 | 0.85 | ±0.03 |
| 7 | (Z)-β-ocimene | 1032 | 1027 | 1235 | 1233 | 3.37 | ±0.35 |
| 8 | Linalool | 1095 | 1094 | 1543 | 1540 | 5.75 | ±1.64 |
| 9 | 2-ethyl-1,1-dimethyl-3 methylene-cyclohexene | - | 1109 | - | 1561 | 0.57 | ±0.09 |
| 10 | Bornyl acetate | 1284 | 1274 | 1580 | 1575 | 0.94 | ± 0.38 |
| 11 | β-elemene | 1389 | 1386 | 1591 | 1588 | 1.97 | ±0.81 |
| 12 | E-caryophyllene | 1417 | 1404 | 1598 | 1592 | 0.87 | ±0.31 |
| 13 | Germacrene D | 1480 | 1472 | 1710 | 1703 | 0.87 | ±0.13 |
| 14 | δ-cadinene | 1522 | 1498 | 1756 | 1748 | 0.43 | ±0.18 |
| 15 | (E)-γ-bisabolene | 1577 | 1569 | 1745 | 1750 | 1.11 | ±0.31 |
| 16 | Caryophyllene oxide | 1582 | 1573 | 1987 | 1981 | 2.94 | ±1.06 |
| 17 | Viridiflorol | 1592 | 1595 | 2090 | 2082 | 1.13 | ±0.37 |
| 18 | τ-muurolol | 1640 | 1639 | 2186 | 2179 | 0.97 | ±0.27 |
| 19 | α-cadinol | 1652 | 1650 | 2227 | 2219 | 2.70 | ±0.67 |
| 20 | Cyclocolorenone | 1759 | 1748 | - | 2321 | 45.34 | ±2.48 |
| Class | Total (%) | 99.47 | |||||
| Hydrocarbon monoterpenes | 33.88 | ||||||
| Oxygenated monoterpenes | 5.75 | ||||||
| 39.63 | |||||||
| Hydrocarbon sesquiterpenes | 4.14 | ||||||
| Oxygenated sesquiterpenes | 54.20 | ||||||
| 58.34 | |||||||
| Others | 1.51 | ||||||
| Not identified | 0.53 | ||||||
| No. | Compound | Apolar DB-5 Column | DB-Wax Column | Total (%) | S.D. | ||
|---|---|---|---|---|---|---|---|
| AI Teo | AI Exp | AI Teo | AI Exp | ||||
| 1 | α-pinene | 932 | 949 | 1025 | 1022 | 1.07 | ±0.15 |
| 2 | β-pinene | 974 | 1003 | 1110 | 1107 | 7.58 | ±0.90 |
| 3 | Isobutyl isovalerate | 1014 | 1013 | 1294 | 1220 | 0.13 | ±0.05 |
| 4 | β-elemene | 1389 | 1395 | 1591 | 1510 | 1.97 | ±0.07 |
| 5 | α-gurjunene | 1409 | 1407 | 1529 | 1532 | 0.86 | ±0.04 |
| 6 | E-caryophyllene | 1417 | 1420 | 1598 | 1581 | 1.27 | ±0.04 |
| 7 | α-bermagotene | 1437 | 1442 | 1575 | - | 0.28 | ±0.03 |
| 8 | α-humulene | 1452 | 1459 | 1667 | 1654 | 0.26 | ±0.01 |
| 9 | Germacrene D | 1480 | 1484 | 1709 | 1703 | 0.48 | ±0.02 |
| 10 | α-selinene | 1495 | 1499 | 1725 | - | 6.30 | ±0.03 |
| 11 | δ-cadinene | 1522 | 1525 | 1756 | 1748 | 1.08 | ±0.02 |
| 12 | (E)-γ-bisabolene | 1500 | 1528 | 1745 | 1750 | 1.36 | ±0.09 |
| 13 | cis,trans-nerolidol | 1533 | 1530 | 2007 | - | 0.16 | ±0.06 |
| 14 | (I3)-caryophyleine | 1538 | 1539 | - | - | 10.84 | ±0.15 |
| 15 | E-nerolidol | 1561 | 1573 | 2036 | - | 0.58 | ±0.03 |
| 16 | Longipinocarvone | 1561 | 1575 | - | - | 2.87 | ±0.32 |
| 17 | Germacrene B | 1566 | 1582 | 1824 | - | 5.52 | ±0.09 |
| 18 | Germacrene D- 4-ol | 1574 | 1584 | 2057 | - | 1.17 | ±0.02 |
| 19 | Caryophyllene oxide | 1582 | 1587 | 1986 | 1981 | 1.80 | ±0.01 |
| 20 | Ledol | 1590 | 1610 | 2039 | 2027 | 0.73 | ±0.01 |
| 21 | β-cedren-9-one | 1630 | 1617 | - | - | 3.30 | ±0.03 |
| 22 | Allo-aromadendrene epoxide | 1639 | 1632 | - | - | 0.15 | ±0.00 |
| 23 | (5β,7β,10β)-3,11-eudesmadien-2-one | 1640 | 1639 | - | - | 0.16 | ±0.01 |
| 24 | τ-muurolol | 1640 | 1654 | 2186 | 2179 | 0.55 | ±0.10 |
| 25 | Ent-spathulenol | 1577 | 1659 | 2133 | 2125 | 0.27 | ±0.17 |
| 26 | α-cadinol | 1652 | 1666 | 2227 | 2219 | 1.07 | ±0.01 |
| 27 | Helifolenol A | - | 1684 | - | - | 2.22 | ±0.01 |
| 28 | 8-cedren-13-ol | 1688 | 1698 | - | - | 0.44 | ±0.00 |
| 29 | Eudesm-7(11)-en-4-ol | 1700 | 1705 | 2282 | 2285 | 1.12 | ±0.01 |
| 30 | 14-hydroxy-α-humulene | 1690 | 1714 | - | - | 0.22 | ±0.01 |
| 31 | Isobicyclogermacrenal | 1733 | 1740 | - | - | 0.16 | ±0.00 |
| 32 | Eremophilone | 1734 | 1744 | - | - | 1.78 | ±0.05 |
| 33 | Cyclocolorenone | 1759 | 1767 | - | 2321 | 39.03 | ±1.15 |
| 34 | Dehidrosaussurea lactone | 1838 | 1833 | - | 2401 | 0.40 | ±0.04 |
| Class | Total (%) | 97.20 | |||||
| Hydrocarbon monoterpenes | 10.62 | ||||||
| Oxygenated monoterpenes | 0.86 | ||||||
| 11.48 | |||||||
| Hydrocarbon sesquiterpenes | 32.17 | ||||||
| Oxygenated sesquiterpenes | 53.42 | ||||||
| 85.59 | |||||||
| Others | 0.13 | ||||||
| Not identified | 2.80 | ||||||
| No. | Compound | Apolar DB-5 Column | DB-Wax Column | Total (%) | S.D. | ||
|---|---|---|---|---|---|---|---|
| AI Teo | AI Exp | AI Teo | AI Exp | ||||
| 1 | α-pinene | 932 | 951 | 1025 | 1022 | 0.34 | ±0.10 |
| 2 | β-pinene | 974 | 1008 | 1110 | 1107 | 2.21 | ±0.58 |
| 3 | (Z)-β-ocimene | 1032 | 1064 | 1234 | 1230 | 0.68 | ±0.09 |
| 4 | (E)-β-ocimene | 1044 | 1075 | 1250 | 1233 | 0.77 | ±0.36 |
| 5 | Cis-linalool oxide | 1170 | 1185 | 1446 | 1428 | 0.19 | ±0.05 |
| 6 | β-elemene | 1389 | 1392 | 1591 | 1510 | 3.85 | ±0.60 |
| 7 | α-gurjunene | 1409 | 1404 | 1529 | 1532 | 0.61 | ±0.05 |
| 8 | E-caryophyllene | 1417 | 1418 | 1598 | 1592 | 2.62 | ±0.19 |
| 9 | α-humulene | 1452 | 1456 | - | 1654 | 0.50 | ±0.06 |
| 10 | E-germacrene D | 1480 | 1482 | 1708 | 1703 | 2.07 | ±0.96 |
| 11 | β-selinene | 1489 | 1488 | 1716 | 1709 | 0.41 | ±0.35 |
| 12 | α-selinene | 1498 | 1495 | 1725 | 1712 | 1.21 | ±0.12 |
| 13 | Benzyl tiglate | 1497 | 1500 | - | - | 0.29 | ±0.19 |
| 14 | β-bisabolene | 1505 | 1512 | 1727 | 1721 | 0.77 | ±0.30 |
| 15 | δ-cadinene | 1511 | 1521 | 1755 | 1748 | 0.91 | ±0.10 |
| 16 | (E)-γ-bisabolene | 1528 | 1531 | 1744 | 1750 | 0.83 | ±0.02 |
| 17 | 10-isopropenyl-3,7- cyclodecadien-1-one | 1533 | 1534 | - | - | 1.39 | ±0.20 |
| 18 | Elemol | 1548 | 1559 | 2036 | 2013 | 0.27 | ±0.19 |
| 19 | E-nerolidol | 1561 | 1570 | 2078 | 2022 | 0.31 | ±0.02 |
| 20 | Caryophyllene oxide | 1582 | 1583 | 1986 | 1981 | 2.65 | ±0.00 |
| 21 | Ledol | 1602 | 1606 | 2039 | 2027 | 0.56 | ±0.08 |
| 22 | γ-gurjunenepoxide-(2) | 1626 | 1622 | - | - | 0.37 | ±0.28 |
| 23 | Allo-aromadendrene epoxide | 1639 | 1631 | - | - | 0.26 | ±0.10 |
| 24 | Cis-guaia-3,9-dien-11-ol | 1648 | 1644 | - | - | 0.10 | ±0.05 |
| 25 | τ-muurolol | 1640 | 1650 | 2186 | 2179 | 0.95 | ±0.10 |
| 26 | Ent-spathulenol | 1577 | 1655 | 2133 | 2125 | 0.69 | ±0.32 |
| 27 | α-cadinol | 1652 | 1662 | 2227 | 2219 | 1.53 | ±0.35 |
| 28 | 14-hydroxycaryophyllene | 1668 | 1678 | - | - | 0.89 | ±0.67 |
| 29 | Helifolenol A | 1674 | 1684 | - | - | 1.35 | ±0.17 |
| 30 | 8-cedren-13-ol | 1688 | 1694 | - | - | 0.25 | ±0.17 |
| 31 | Eudesm-7(11)-en-4-ol | 1700 | 1700 | 2300 | 2285 | 0.47 | ±0.13 |
| 32 | 14-hydroxy-α-humulene | 1713 | 1710 | - | - | 0.35 | ±0.02 |
| 33 | 2Z,6E-farnesol | 1722 | 1727 | 2324 | 2302 | 8.47 | ±0.69 |
| 34 | Isobicyclogermacrenal | 1733 | 1735 | - | - | 0.42 | ±0.20 |
| 35 | Cyclocolorenone | 1759 | 1764 | - | 2321 | 41.95 | ±1.72 |
| 36 | Benzyl benzoate | 1759 | 1774 | 2612 | 2584 | 1.12 | ±0.04 |
| 37 | Epi-cyclocolorenone | 1774 | 1781 | - | - | 0.12 | ±0.00 |
| 38 | 2α-acetoxyamorfa-4,7(11)-diene | 1805 | 1818 | - | - | 0.38 | ±0.09 |
| 39 | Benzoic acid (5,5-dimethyl-4-oxo- 2-cyclohexenyl)-ester | 1874 | 1862 | - | - | 8.41 | ±0.21 |
| Class | Total (%) | 91.51 | |||||
| Hydrocarbon monoterpenes | 3.99 | ||||||
| Oxygenated monoterpenes | 0.19 | ||||||
| 4.18 | |||||||
| Hydrocarbon sesquiterpenes | 13.78 | ||||||
| Oxygenated sesquiterpenes | 70.37 | ||||||
| 84.15 | |||||||
| Others | 3.19 | ||||||
| Not identified | 8.49 | ||||||
| DPPH 1 | ABTS 1 | |||
|---|---|---|---|---|
| IC50 | S.D. | IC50 | S.D. | |
| MpLEO | 37.40 c | ±4.60 | 14.80 c | ±4.20 |
| MpSEO | 21.50 b | ±4.50 | 17.40 c | ±1.20 |
| MpFEO | 27.90 b | ±2.80 | 9.04 b | ±0.56 |
| BHT | 1.66 a | ±0.15 | 0.24 a | ±0.02 |
| Fraction | f1 | f2 | f3 |
|---|---|---|---|
| Rf | 0.97 | 0.79 | 0.56 |
| MpLEO | β-elemene E-caryophyllene Germacrene D (E)-γ-bisabolene | Caryophyllene oxide τ-muurolol | - |
| MpSEO | β-elemene α-gurjunene E-caryophyllene Germacrene D (E)-γ-Bisabolene Ledol 8-cedren-13-ol | Longipinocarvone, Caryophyllene oxide τ-Muurolol Ent-spathulenol | α-selinene (I3)-Caryophyleine Germacrene B β-Cedren-9-one Eudesm-7(11)-en-4-ol 14-hydroxy-α-humulene |
| MpFEO | β-elemene α-gurjunene E-caryophyllene E-germacrene D β-bisabolene (E)-γ-bisabolene Ledol 8-cedren-13-ol 2α-acetoxyamorpha-4,7(11)-diene | Caryophyllene oxide τ-muurolol Ent-spathulenol | Eudesm-7(11)-en-4-ol 14-hydroxy-α-humulene |
| MIC (mg/mL) | |||||
|---|---|---|---|---|---|
| Bacteria | Strain | MpLEO | MpSEO | MpFEO | Ampicillin 1 |
| E. coli | ATCC® 25922 | 22.73 b | 22.73 b | 22.73 b | 5.01 × 10−3 a |
| E. coli | ATCC® 8739 | 22.73 b | 22.73 b | 22.73 b | 5.01 × 10−3 a |
| K. pneumoniae | ATCC® 10031 | 5.68 c | 2.84 b | 5.68 c | 8.01 × 10−2 a |
| S. typhimurium | ATCC® 14028 | 11.36 b | 11.36 b | 11.36 b | 2.51 × 10−3 a |
| S. aureus | ATCC® 6538p | 1.42 c | 0.71 b | 0.71 b | 6.30 × 10−4 a |
| S. epidermidis | ATCC® 14990 | 0.71 c | 0.35 b | 0.71 c | 1.25 × 10−3 a |
| B. subtilis | ATCC® 6633 | 11.36 b | 11.36 b | 11.36 b | 5.01 × 10−3 a |
| Cell line | MCF-7 (ATCC® HTB-22) | HT-29 (ATCC® HTB-38) | ||
|---|---|---|---|---|
| Tested Agent | IC50 | S.D. | IC50 | S.D. |
| MpLEO | 27.25 a | ±3.80 | 377.6 c | ±87.80 |
| MpSEO | 36.26 a | ±5.02 | 54.01 a | ±1.72 |
| MpFEO | 98.87 c | ±15.00 | 134.8 b | ±4.00 |
| Cyclophosphamide 1 | 51.56 b | ±8.31 | 59.98 a | ±2.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osorio, E.; Vázquez-García, J.A.; Noriega, P.; Reynoso-Orozco, R.; Huizar, R.; Noa, M.; Cabrera-Diaz, E.; Barrientos-Ramírez, L.; Cerda, H.; Ruíz-López, M.A. Chemical Composition and Bioactivity of Essential Oils from Magnolia pugana, an Endemic Mexican Magnoliaceae Species. Molecules 2025, 30, 3778. https://doi.org/10.3390/molecules30183778
Osorio E, Vázquez-García JA, Noriega P, Reynoso-Orozco R, Huizar R, Noa M, Cabrera-Diaz E, Barrientos-Ramírez L, Cerda H, Ruíz-López MA. Chemical Composition and Bioactivity of Essential Oils from Magnolia pugana, an Endemic Mexican Magnoliaceae Species. Molecules. 2025; 30(18):3778. https://doi.org/10.3390/molecules30183778
Chicago/Turabian StyleOsorio, Edison, José A. Vázquez-García, Paco Noriega, Ramón Reynoso-Orozco, Rosario Huizar, Mario Noa, Elisa Cabrera-Diaz, Lucía Barrientos-Ramírez, Hugo Cerda, and Mario A. Ruíz-López. 2025. "Chemical Composition and Bioactivity of Essential Oils from Magnolia pugana, an Endemic Mexican Magnoliaceae Species" Molecules 30, no. 18: 3778. https://doi.org/10.3390/molecules30183778
APA StyleOsorio, E., Vázquez-García, J. A., Noriega, P., Reynoso-Orozco, R., Huizar, R., Noa, M., Cabrera-Diaz, E., Barrientos-Ramírez, L., Cerda, H., & Ruíz-López, M. A. (2025). Chemical Composition and Bioactivity of Essential Oils from Magnolia pugana, an Endemic Mexican Magnoliaceae Species. Molecules, 30(18), 3778. https://doi.org/10.3390/molecules30183778









