Removal of Oxyanions and Trace Metals from River Water Samples Using Magnetic Biopolymer/Halloysite Nanocomposites
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterisation of the Hydrogel Materials
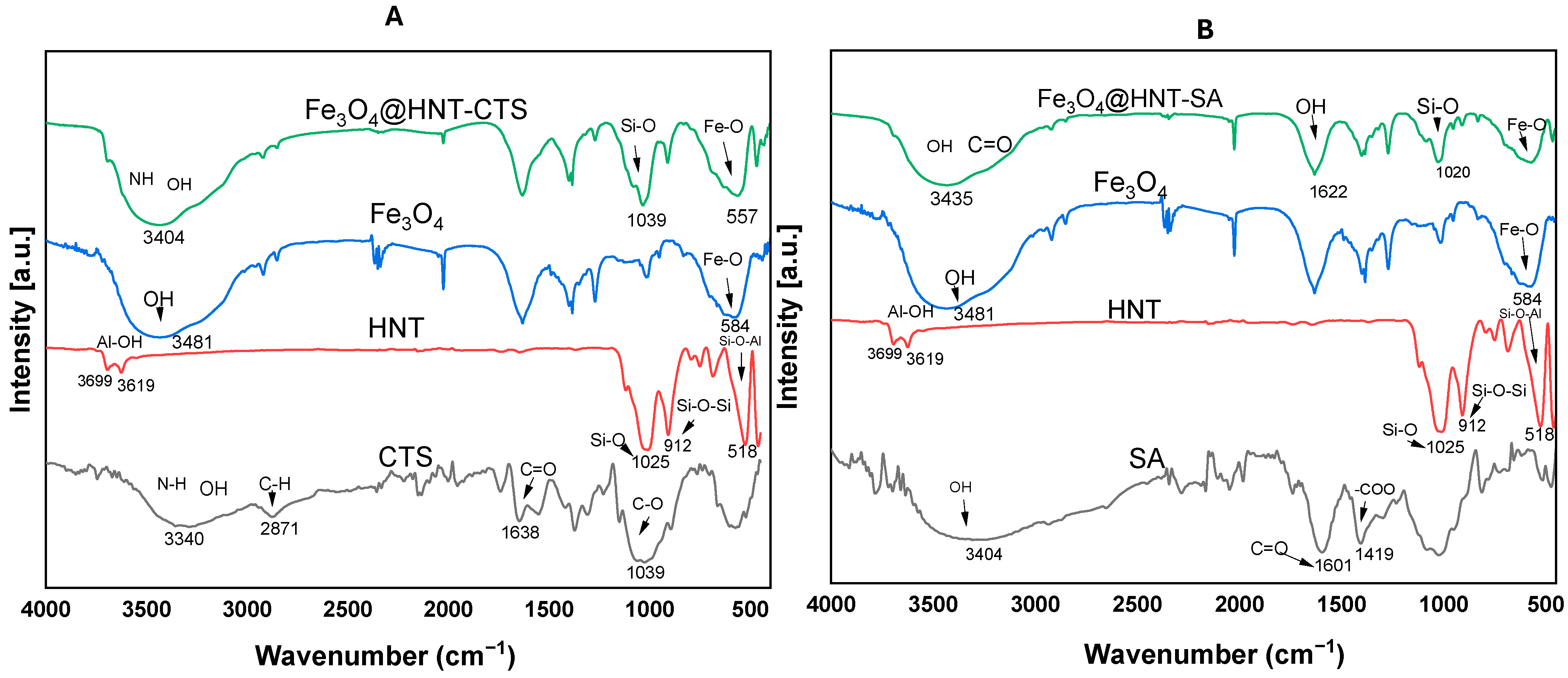
2.1.1. Fourier-Transform Infrared Spectroscopy (FTIR)
2.1.2. Transmission Electron Microscopy (TEM)
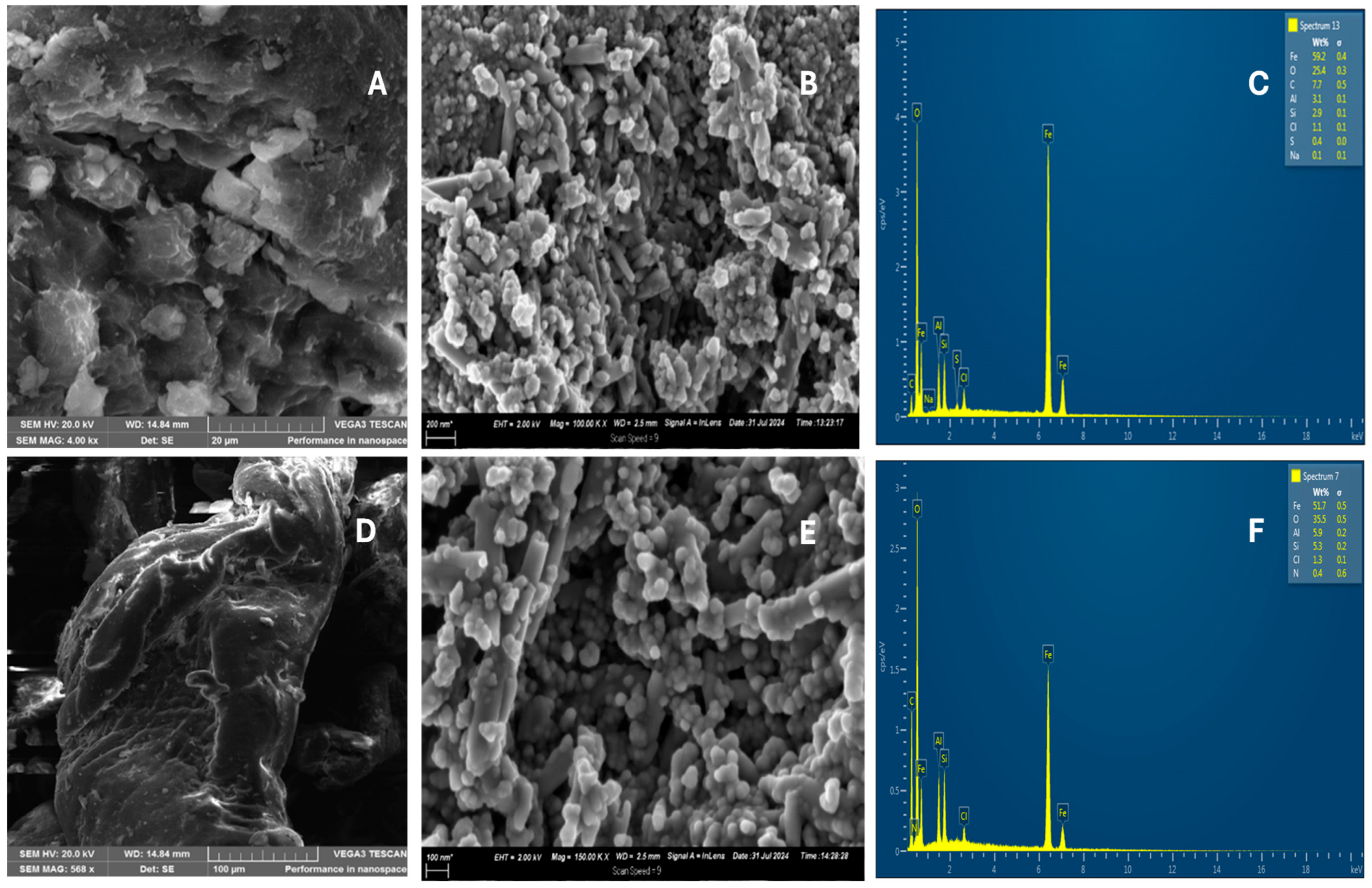
2.1.3. Scanning Electron Microscopy (SEM)
2.1.4. Brunauer–Emmett–Teller (BET)
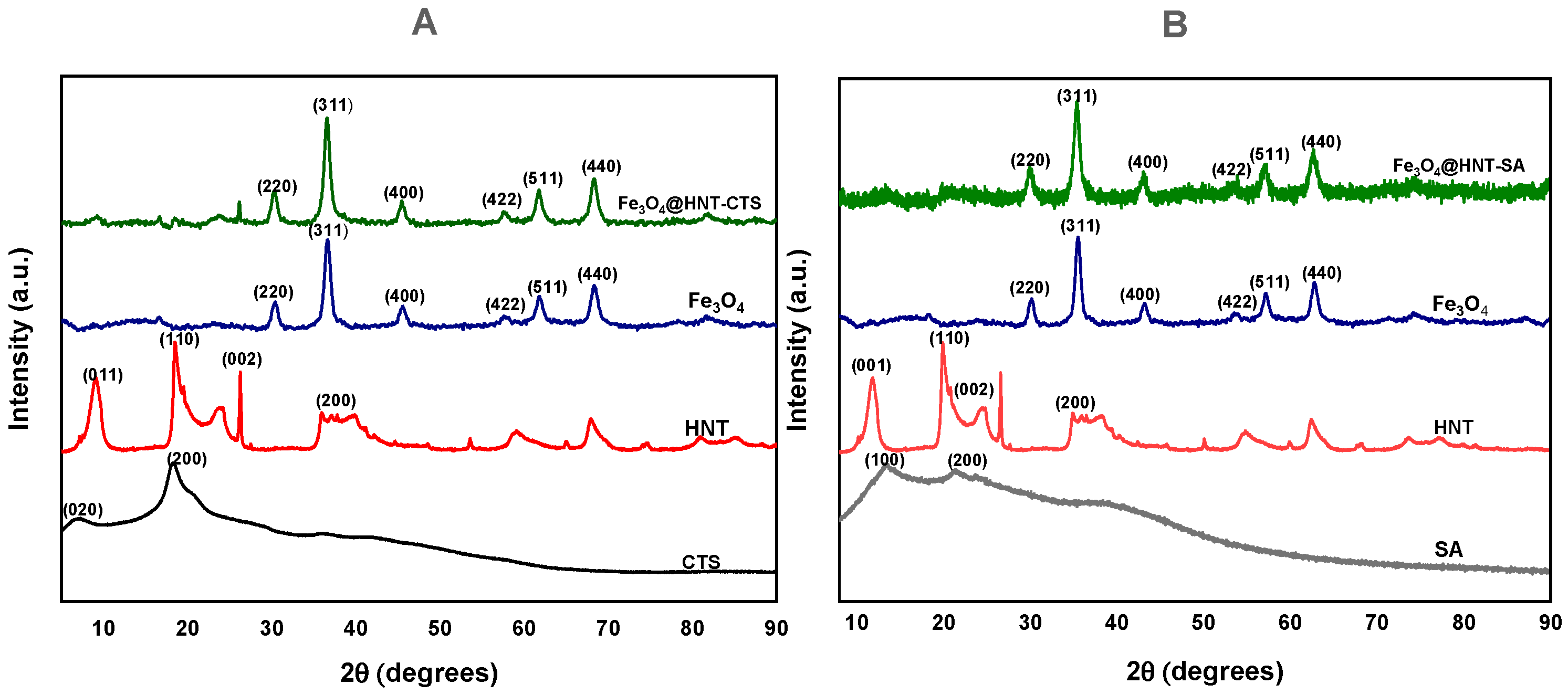
2.1.5. X-Ray Diffraction Analysis (XRD)
2.1.6. Zeta Potential
2.2. Adsorbent Selection
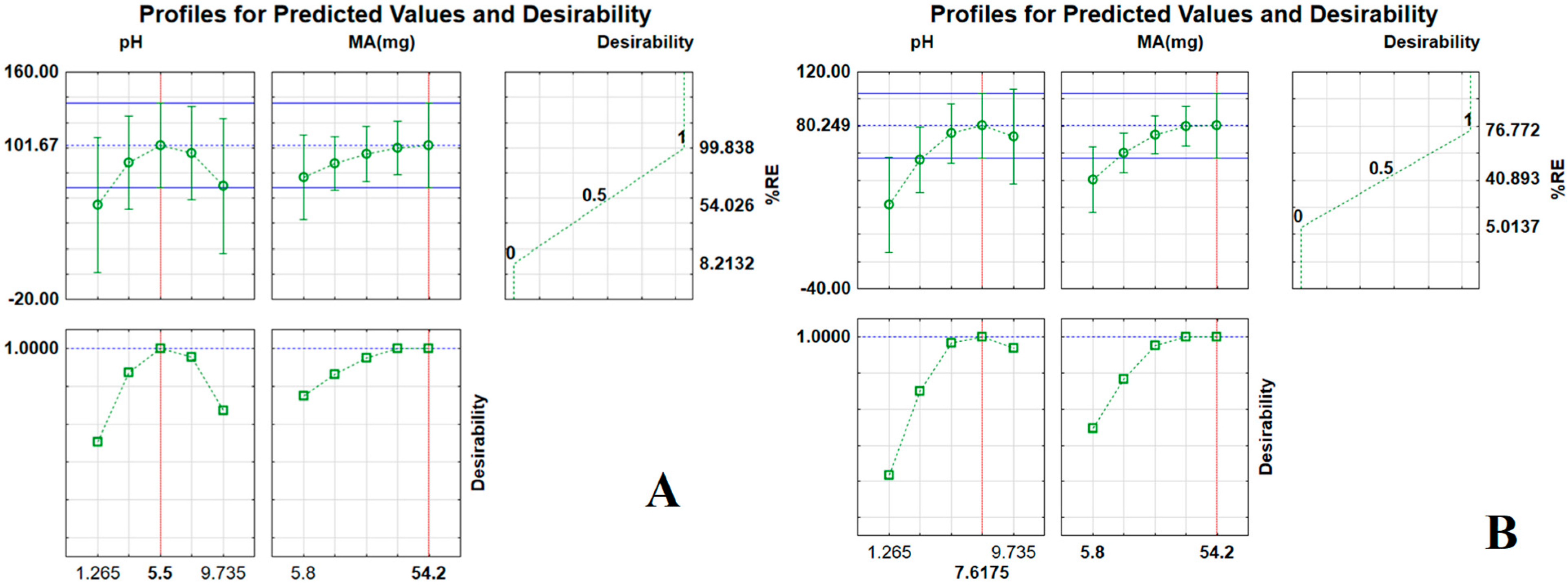
2.3. Optimisation of Batch Adsorption Experiments Using Central Composite Design
2.4. Adsorption Studies
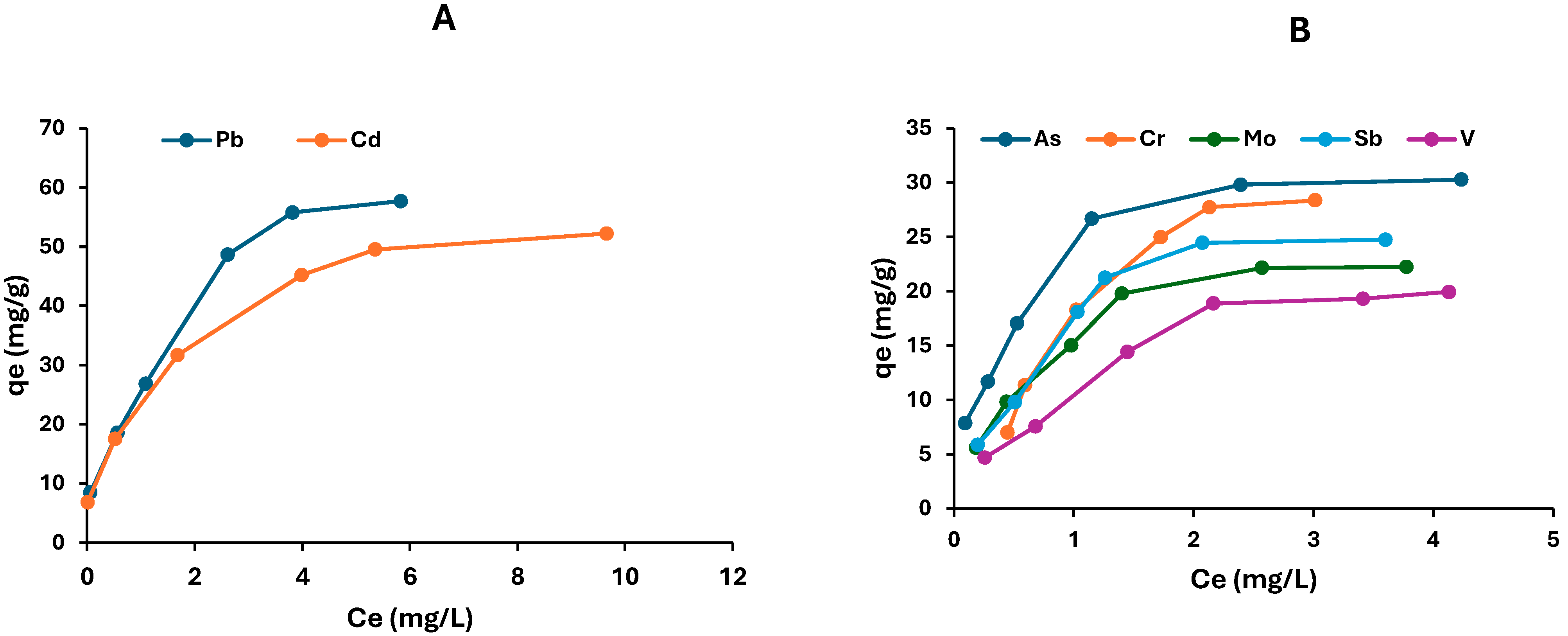
2.4.1. Adsorption Isotherms Studies
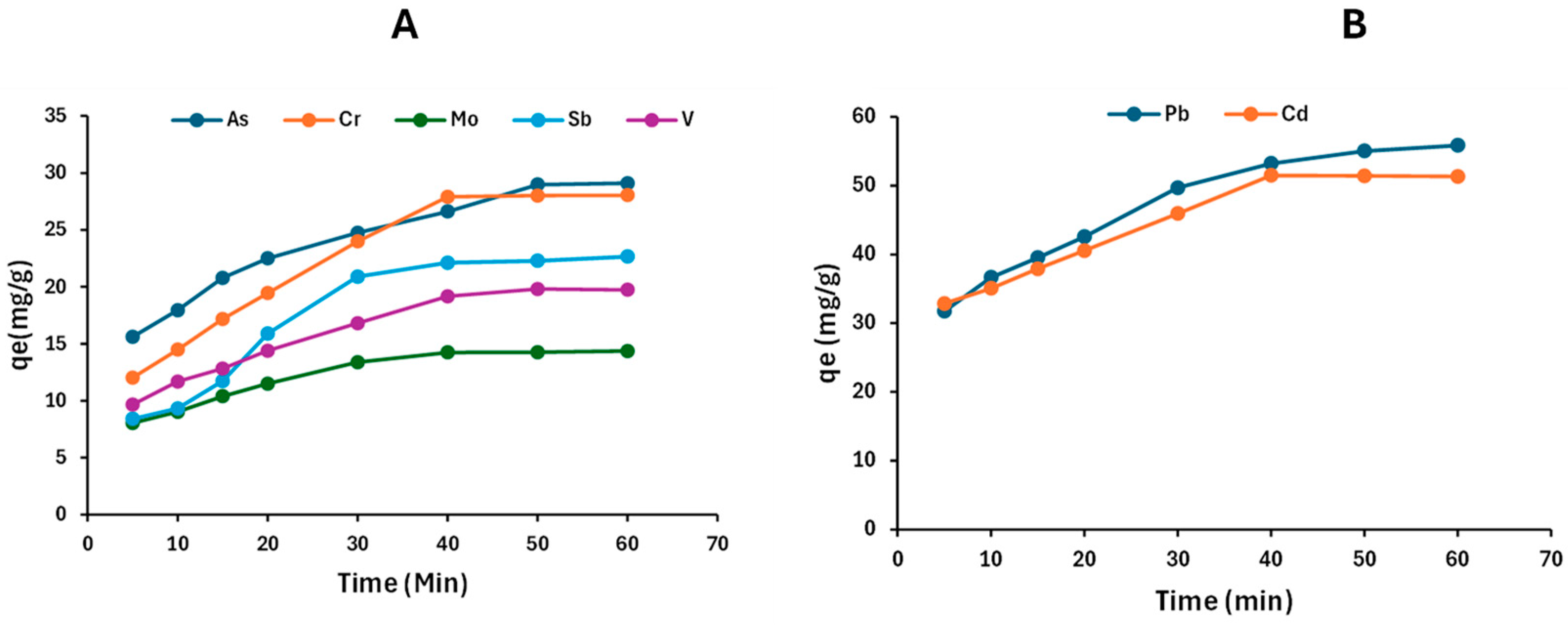
2.4.2. Adsorption Kinetics Studies
2.4.3. Thermodynamics
2.5. Interference Studies
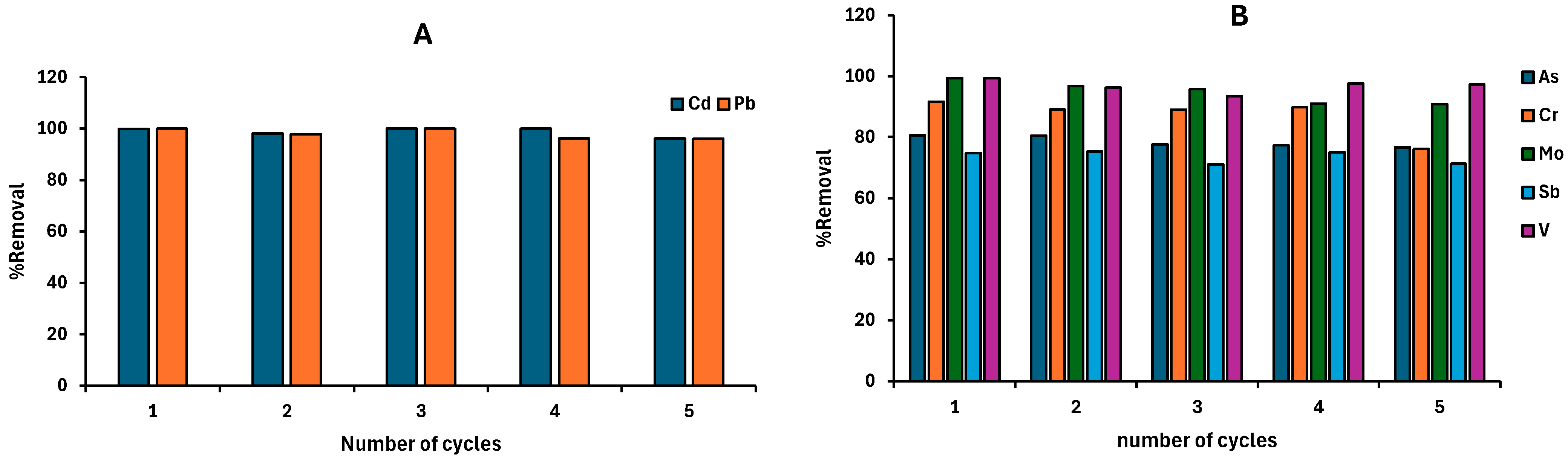
2.6. Reusability Studies
2.7. Application in Real Water Samples
2.8. Comparison Studies
3. Experimental
3.1. Materials and Chemicals
3.2. Instrumentation
3.3. Synthesis of Magnetic Halloysite Nanotube (HNT@Fe3O4)
3.4. Synthesis of HNT@Fe3O4-CTS
3.5. Preparation of Fe3O4@HNT-SA
3.6. Batch Adsorption Experiments
3.7. Adsorption Kinetics and Isotherm Studies
3.8. Reusability
3.9. Application to Real Water Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CCD | Central composite design |
| CTS | Chitosan |
| Fe3O4@HNT-CTS | Magnetic halloysite nanoclay chitosan |
| HNT | Halloysite nanotubes |
| ICP-OES | Inductively coupled plasma optical emission spectroscopy |
| Fe3O4@HNT-SA | Magnetic halloysite nanoclay alginate |
| NIP | Non-imprinted polymer |
| SA | Alginate |
References
- Padhiary, M.; Kumar, R. Assessing the Environmental Impacts of Agriculture, Industrial Operations, and Mining on Agro-Ecosystems. In Smart Internet of Things for Environment and Healthcare; Springer Nature: Cham, Switzerland, 2024; pp. 107–126. [Google Scholar]
- Wang, C.; Jiang, A.; Liu, X.; Yuen Koh, K.; Yang, Y.; Chen, J.P.; Li, K. Amorphous Metal-Organic Framework UiO-66-NO2 for Removal of Oxyanion Pollutants: Towards Improved Performance and Effective Reusability. Sep. Purif. Technol. 2022, 295, 121014. [Google Scholar] [CrossRef]
- Çelebi, H.; Gök, G.; Gök, O. Adsorption Capability of Brewed Tea Waste in Waters Containing Toxic Lead(II), Cadmium (II), Nickel (II), and Zinc(II) Heavy Metal Ions. Sci. Rep. 2020, 10, 17570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Duan, X. Chemical Precipitation of Heavy Metals from Wastewater by Using the Synthetical Magnesium Hydroxy Carbonate. Water Sci. Technol. 2020, 81, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Liu, C.; Feng, L.; Yang, S.; Shan, Y.; Xiao, F. Quantitatively Ion-Exchange between Mg(II) and Pb(II)/Cd(II) during the Highly Efficient Adsorption by MgO-Loaded Lotus Stem Biochar. J. Taiwan Inst. Chem. Eng. 2023, 144, 104736. [Google Scholar] [CrossRef]
- Harharah, R.H.; Abdalla, G.M.T.; Elkhaleefa, A.; Shigidi, I.; Harharah, H.N. A Study of Copper (II) Ions Removal by Reverse Osmosis under Various Operating Conditions. Separations 2022, 9, 155. [Google Scholar] [CrossRef]
- Elboughdiri, N. The Use of Natural Zeolite to Remove Heavy Metals Cu (II), Pb (II) and Cd (II), from Industrial Wastewater. Cogent Eng. 2020, 7, 1782623. [Google Scholar] [CrossRef]
- Ayouch, I.; Kassem, I.; Kassab, Z.; Barrak, I.; Barhoun, A.; Jacquemin, J.; Draoui, K.; Achaby, M. El Crosslinked Carboxymethyl Cellulose-Hydroxyethyl Cellulose Hydrogel Films for Adsorption of Cadmium and Methylene Blue from Aqueous Solutions. Surf. Interfaces 2021, 24, 101124. [Google Scholar] [CrossRef]
- Hnamte, M.; Pulikkal, A.K. Clay-Polymer Nanocomposites for Water and Wastewater Treatment: A Comprehensive Review. Chemosphere 2022, 307, 135869. [Google Scholar] [CrossRef]
- Gul, S.; Ahmad, Z.; Asma, M.; Ahmad, M.; Rehan, K.; Munir, M.; Bazmi, A.A.; Ali, H.M.; Mazroua, Y.; Salem, M.A.; et al. Effective Adsorption of Cadmium and Lead Using SO3H-Functionalized Zr-MOFs in Aqueous Medium. Chemosphere 2022, 307, 135633. [Google Scholar] [CrossRef]
- Maqbool, A.; Shahid, A.; Jahan, Z.; Bilal Khan Niazi, M.; Ali Inam, M.; Tawfeek, A.M.; M Kamel, E.; Saeed Akhtar, M. Development of ZnO-GO-NiO Membrane for Removal of Lead and Cadmium Heavy Metal Ions from Wastewater. Chemosphere 2023, 338, 139622. [Google Scholar] [CrossRef]
- Kończyk, J.; Kluziak, K.; Kołodyńska, D. Adsorption of Vanadium (V) Ions from the Aqueous Solutions on Different Biomass-Derived Biochars. J. Environ. Manag. 2022, 313, 114958. [Google Scholar] [CrossRef]
- Moges, A.; Nkambule, T.T.I.; Fito, J. The Application of GO-Fe3O4 Nanocomposite for Chromium Adsorption from Tannery Industry Wastewater. J. Environ. Manag. 2022, 305, 114369. [Google Scholar] [CrossRef]
- Mutar, R.F.; Saleh, M.A. Optimization of Arsenic Ions Adsorption and Removal from Hospitals Wastewater by Nano-Bentonite Using Central Composite Design. Mater. Today Proc. 2022, 60, 1248–1256. [Google Scholar] [CrossRef]
- Nie, J.; Yao, Z.; Shao, P.; Jing, Y.; Bai, L.; Xing, D.; Yi, G.; Li, D.; Liu, Y.; Yang, L.; et al. Revisiting the Adsorption of Antimony on Manganese Dioxide: The Overlooked Dissolution of Manganese. Chem. Eng. J. 2022, 429, 132468. [Google Scholar] [CrossRef]
- del Mar Orta, M.; Martín, J.; Santos, J.L.; Aparicio, I.; Medina-Carrasco, S.; Alonso, E. Biopolymer-Clay Nanocomposites as Novel and Ecofriendly Adsorbents for Environmental Remediation. Appl. Clay Sci. 2020, 198, 105838. [Google Scholar] [CrossRef]
- Alver, E.; Metin, A.Ü.; Brouers, F. Methylene Blue Adsorption on Magnetic Alginate/Rice Husk Bio-Composite. Int. J. Biol. Macromol. 2020, 154, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Sun, S.; Xu, K.; Zhao, W.; Hao, R.; Zhang, J.; Li, D. Iron-Loaded Magnetic Alginate-Chitosan Double-Gel Interpenetrated Porous Beads for Phosphate Removal from Water: Preparation, Adsorption Behavior and PH Stability. React. Funct. Polym. 2022, 177, 105328. [Google Scholar] [CrossRef]
- ALSamman, M.T.; Sánchez, J. Recent Advances on Hydrogels Based on Chitosan and Alginate for the Adsorption of Dyes and Metal Ions from Water. Arab. J. Chem. 2021, 14, 103455. [Google Scholar] [CrossRef]
- Majiya, H.; Clegg, F.; Sammon, C. Bentonite-Chitosan Composites or Beads for Lead (Pb) Adsorption: Design, Preparation, and Characterisation. Appl. Clay Sci. 2023, 246, 107180. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, B.; Jie, P.; Zhu, J.; Cheng, F. Preparation of Chitosan/Lignosulfonate for Effectively Removing Pb(II) in Water. Polymer 2021, 228, 123878. [Google Scholar] [CrossRef]
- Rahaman, M.H.; Islam, M.A.; Islam, M.M.; Rahman, M.A.; Alam, S.M.N. Biodegradable Composite Adsorbent of Modified Cellulose and Chitosan to Remove Heavy Metal Ions from Aqueous Solution. Curr. Res. Green Sustain. Chem. 2021, 4, 100119. [Google Scholar] [CrossRef]
- Zia, Q.; Tabassum, M.; Meng, J.; Xin, Z.; Gong, H.; Li, J. Polydopamine-Assisted Grafting of Chitosan on Porous Poly (L-Lactic Acid) Electrospun Membranes for Adsorption of Heavy Metal Ions. Int. J. Biol. Macromol. 2021, 167, 1479–1490. [Google Scholar] [CrossRef]
- Alsuhaibani, A.M.; Alayyafi, A.A.A.; Albedair, L.A.; El-Desouky, M.G.; El-Bindary, A.A. Efficient Fabrication of a Composite Sponge for Cr(VI) Removal via Citric Acid Cross-Linking of Metal-Organic Framework and Chitosan: Adsorption Isotherm, Kinetic Studies, and Optimization Using Box-Behnken Design. Mater. Today Sustain. 2024, 26, 100732. [Google Scholar] [CrossRef]
- Ferreira, D.C.M.; dos Santos, T.C.; dos Reis Coimbra, J.S.; de Oliveira, E.B. Chitosan/Carboxymethylcellulose Polyelectrolyte Complexes (PECs) Are an Effective Material for Dye and Heavy Metal Adsorption from Water. Carbohydr. Polym. 2023, 315, 120977. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, R.; Shetty, K.; Jeevananda, T.; Ananda Murthy, H.C.; Sillanpaa, M.; Nhat, T. Novel Polyaniline–Halloysite Nanoclay Hybrid Composites: Synthesis, Physico-Chemical, Thermal and Electrical Properties. Inorg. Chem. Commun. 2023, 148, 110328. [Google Scholar] [CrossRef]
- Queiroz, M.F.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the Use of Chitosan Contribute to Oxalate Kidney Stone Formation? Mar. Drugs 2015, 13, 141. [Google Scholar] [CrossRef]
- Drabczyk, A.; Kudłacik-Kramarczyk, S.; Głab, M.; Kedzierska, M.; Jaromin, A.; Mierzwiński, D.; Tyliszczak, B. Physicochemical Investigations of Chitosan-Based Hydrogels Containing Aloe Vera Designed for Biomedical Use. Materials 2020, 13, 3073. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Q.; Xu, S.; Zheng, Q.; Cao, X. Preparation and Properties of 3D Printed Alginate-Chitosan Polyion Complex Hydrogels for Tissue Engineering. Polymers 2018, 10, 664. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Abdel-Fadeel, M.A.; Aljohani, N.S.; Al-Mhyawi, S.R.; Halawani, R.F.; Aljuhani, E.H.; Salam, M.A. A Simple Method for Removal of Toxic Dyes Such as Brilliant Green and Acid Red from the Aquatic Environment Using Halloysite Nanoclay. J. Saudi Chem. Soc. 2022, 26, 101475. [Google Scholar] [CrossRef]
- Faaliyan, K.; Abdoos, H.; Borhani, E.; Afghahi, S.S.S. Magnetite-Silica Nanoparticles with Core-Shell Structure: Single-Step Synthesis, Characterization and Magnetic Behavior. J. Sol-Gel Sci. Technol. 2018, 88, 609–617. [Google Scholar] [CrossRef]
- Podgorbunskikh, E.; Kuskov, T.; Rychkov, D.; Lomovskii, O.; Bychkov, A. Mechanical Amorphization of Chitosan with Different Molecular Weights. Polymers 2022, 14, 4438. [Google Scholar] [CrossRef]
- Shameem, A.; Devendran, P.; Siva, V.; Venkatesh, K.S.; Manikandan, A.; Bahadur, S.A.; Nallamuthu, N. Dielectric Investigation of NaLiS Nanoparticles Loaded on Alginate Polymer Matrix Synthesized by Single Pot Microwave Irradiation. J. Inorg. Organomet. Polym. Mater. 2018, 28, 671–678. [Google Scholar] [CrossRef]
- Ramanery, F.P.; Mansur, A.A.P.; Mansur, H.S. One-Step Colloidal Synthesis of Biocompatible Water-Soluble ZnS Quantum Dot/Chitosan Nanoconjugates. Nanoscale Res. Lett. 2013, 8, 512. [Google Scholar] [CrossRef]
- Nargis, A.; Habib, A.; Rashid, H.O.; Harun, H.B.; Islam Sarker, M.S.; Jin, R.; Liu, G.; Liu, W.; Al-Razee, A.N.M.; Chen, K.; et al. Status of Multielement in Water of the River Buriganga, Bangladesh: Aquatic Chemistry of Metal Ions in Polluted River Water. Emerg. Contam. 2021, 7, 99–115. [Google Scholar] [CrossRef]
- Cabrerizo, A.; Bulteel, D.; Waligora, J.; Landrot, G.; Fonda, E.; Olard, F. Chemical, Mineralogical, and Environmental Characterization of Tunnel Boring Muds for Their Valorization in Road Construction: A Focus on Molybdenum Characterization. Environ. Sci. Pollut. Res. 2020, 27, 44314–44324. [Google Scholar] [CrossRef]
- Lim, M.; Han, G.C.; Ahn, J.W.; You, K.S.; Kim, H.S. Leachability of Arsenic and Heavy Metals from Mine Tailings of Abandoned Metal Mines. Int. J. Environ. Res. Public Health 2009, 6, 2865. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Li, J.; Luo, L.; Zhong, Z.; Xie, X. Advances in Sorptive Removal of Hexavalent Chromium (Cr(VI)) in Aqueous Solutions Using Polymeric Materials. Polymers 2023, 15, 388. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, P.; Abraham, M. Adsorption of Cadmium (Cd) and Lead (Pb) Using Powdered Activated Carbon Derived from Cocos Nucifera Waste: A Kinetics and Equilibrium Study for Long-Term Sustainability. Sustain. Energy Technol. Assess. 2022, 53, 102709. [Google Scholar] [CrossRef]
- Ramutshatsha-Makhwedzha, D.; Mbaya, R.; Mavhungu, M.L. Application of Activated Carbon Banana Peel Coated with Al2O3-Chitosan for the Adsorptive Removal of Lead and Cadmium from Wastewater. Materials 2022, 15, 860. [Google Scholar] [CrossRef]
- Kochar, C.; Taneja, L.; Yadav, P.K.; Tripathy, S.S. RSM-CCD Approach for Optimization Study on Effective Remediation of Lead and Cadmium from Water Using Surface-Modified Water Caltrop Peel Biochar. Biomass Convers. Biorefinery 2024, 14, 17985–18003. [Google Scholar] [CrossRef]
- Karrat, A.; Palacios-Santander, J.M.; Amine, A.; Cubillana-Aguilera, L. A Novel Magnetic Molecularly Imprinted Polymer for Selective Extraction and Determination of Quercetin in Plant Samples. Anal. Chim. Acta 2022, 1203, 339709. [Google Scholar] [CrossRef]
- Fu, C.; Zhu, X.; Dong, X.; Zhao, P.; Wang, Z. Study of Adsorption Property and Mechanism of Lead(II) and Cadmium(II) onto Sulfhydryl Modified Attapulgite. Arab. J. Chem. 2021, 14, 102960. [Google Scholar] [CrossRef]
- Yasin, S.R.; Jasim, S.S.; Al-Baldawi, I.A. Removal of Lead, Cadmium, and Copper from Wastewater Using Cinnamon Bark Waste to Introduce It as a Value-Added Product: Removal, Kinetics and Thermodynamics Study. Desalination Water Treat. 2025, 323, 101345. [Google Scholar] [CrossRef]
- Saleem, M.; Rasheed, A.; Haider, F.; Raja, S.S. Evaluation of Cadmium Remediation Studies by Cobalt-Doped Magnetic Biochar Composite (CoFe2O4@MBC) Derived from Corn Stalk. Int. J. Environ. Sci. Technol. 2025, 3, 1–14. [Google Scholar] [CrossRef]
- Semysim, F.A.; Ridha, R.K.; Azooz, E.A.; Snigur, D. Switchable Hydrophilicity Solvent-Assisted Solidified Floating Organic Drop Microextraction for Separation and Determination of Arsenic in Water and Fish Samples. Talanta 2024, 272, 125782. [Google Scholar] [CrossRef]
- Ghorbani, Y.A.; Ghoreishi, S.M.; Ghani, M. Derived N-Doped Carbon through Core-Shell Structured Metal-Organic Frameworks as a Novel Sorbent for Dispersive Solid Phase Extraction of Cr(III) and Pb(II) from Water Samples Followed by Quantitation through Flame Atomic Absorption Spectrometry. Microchem. J. 2020, 155, 104786. [Google Scholar] [CrossRef]
- Zeng, W.; Hu, Z.; Luo, J.; Hou, X.; Jiang, X. Highly Sensitive Determination of Trace Antimony in Water Samples by Cobalt Ion Enhanced Photochemical Vapor Generation Coupled with Atomic Fluorescence Spectrometry or ICP-MS. Anal. Chim. Acta 2022, 1191, 339361. [Google Scholar] [CrossRef] [PubMed]
- Tuzen, M.; Altunay, N.; Hazer, B.; Mogaddam, M.R.A. Synthesis of Polystyrene-Polyricinoleic Acid Copolymer Containing Silver Nano Particles for Dispersive Solid Phase Microextraction of Molybdenum in Water and Food Samples. Food Chem. 2022, 369, 130973. [Google Scholar] [CrossRef] [PubMed]
- Vasseghian, Y.; Sadeghi Rad, S.; Vilas-Boas, J.A.; Khataee, A. A Global Systematic Review, Meta-Analysis, and Risk Assessment of the Concentration of Vanadium in Drinking Water Resources. Chemosphere 2021, 267, 128904. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, C.; Padilla-Ortega, E.; Robledo-Cabrera, A.; López-Valdivieso, A. Cr(VI) Adsorption on Activated Carbon: Mechanisms, Modeling and Limitations in Water Treatment. J. Environ. Chem. Eng. 2020, 8, 104031. [Google Scholar] [CrossRef]
- Bagdat, S.; Tokay, F.; Demirci, S.; Yilmaz, S.; Sahiner, N. Removal of Cd(II), Co(II), Cr(III), Ni(II), Pb(II) and Zn(II) Ions from Wastewater Using Polyethyleneimine (PEI) Cryogels. J. Environ. Manag. 2023, 329, 117002. [Google Scholar] [CrossRef]
- El-Feky, H.H.; Behiry, M.S.; Amin, A.S.; Nassar, M.Y. Facile Fabrication of Nano-Sized SiO2 by an Improved Sol–Gel Route: As an Adsorbent for Enhanced Removal of Cd(II) and Pb(II) Ions. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1129–1141. [Google Scholar] [CrossRef]
- Ali, M.M.S.; Imam, D.M.; El-Nadi, Y.A. Vanadium(V) Removal and Recovery by Adsorption onto Modified Activated Carbon Derived from Natural Hydroxyapatite. J. Iran. Chem. Soc. 2021, 18, 2771–2784. [Google Scholar] [CrossRef]
- Ma, M.Y.; Ke, X.; Liu, Y.C.; Ha, Z.H.; Wang, T.; Li, J.; Zhang, F.; Zhang, T.C. A Novel Electrolytic-Manganese-Residues-and-Serpentine-Based Composite (S-EMR) for Enhanced Cd(II) and Pb(II) Adsorption in Aquatic Environment. Rare Metals 2023, 42, 346–358. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Wang, S.; Fu, L.; Zhang, L. The One-Step Synthesis of a Novel Metal–Organic Frameworks for Efficient and Selective Removal of Cr(VI) and Pb(II) from Wastewater: Kinetics, Thermodynamics and Adsorption Mechanisms. J. Colloid. Interface Sci. 2023, 640, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Feng, J.; Yan, W.; Zhang, L.; Liu, Y.; Mu, R. Dual-Functional Sites for Synergistic Adsorption of Cr(VI) and Sb(V) by Polyaniline-TiO2 Hydrate: Adsorption Behaviors, Sites and Mechanisms. Front. Environ. Sci. Eng. 2022, 16, 105. [Google Scholar] [CrossRef]
- Xu, R.; Li, Q.; Nan, X.; Jiang, G.; Wang, L.; Xiong, J.; Yang, Y.; Xu, B.; Jiang, T. Simultaneous Removal of Antimony(III/V) and Arsenic(III/V) from Aqueous Solution by Bacteria–Mediated Kaolin@Fe–Mn Binary (Hydr)Oxides Composites. Appl. Clay Sci. 2022, 217, 106392. [Google Scholar] [CrossRef]
- Wang, Z.; Tian, T.; Xu, K.; Jia, Y.; Zhang, C.; Li, J.; Wang, Z. Removal of Antimony(III) by Magnetic MIL-101(Cr)-NH2 Loaded with SiO2: Optimization Based on Response Surface Methodology and Adsorption Properties. Chem. Pap. 2022, 76, 2733–2745. [Google Scholar] [CrossRef]
- Li, L.; Wang, F.; Lv, Y.; Liu, J.; Zhang, D.; Shao, Z. Halloysite Nanotubes and Fe3O4 Nanoparticles Enhanced Adsorption Removal of Heavy Metal Using Electrospun Membranes. Appl. Clay Sci. 2018, 161, 225–234. [Google Scholar] [CrossRef]
- Türkeş, E.; Sağ Açıkel, Y. Synthesis and Characterization of Magnetic Halloysite–Chitosan Nanocomposites: Use in the Removal of Methylene Blue in Wastewaters. Int. J. Environ. Sci. Technol. 2020, 17, 1281–1294. [Google Scholar] [CrossRef]

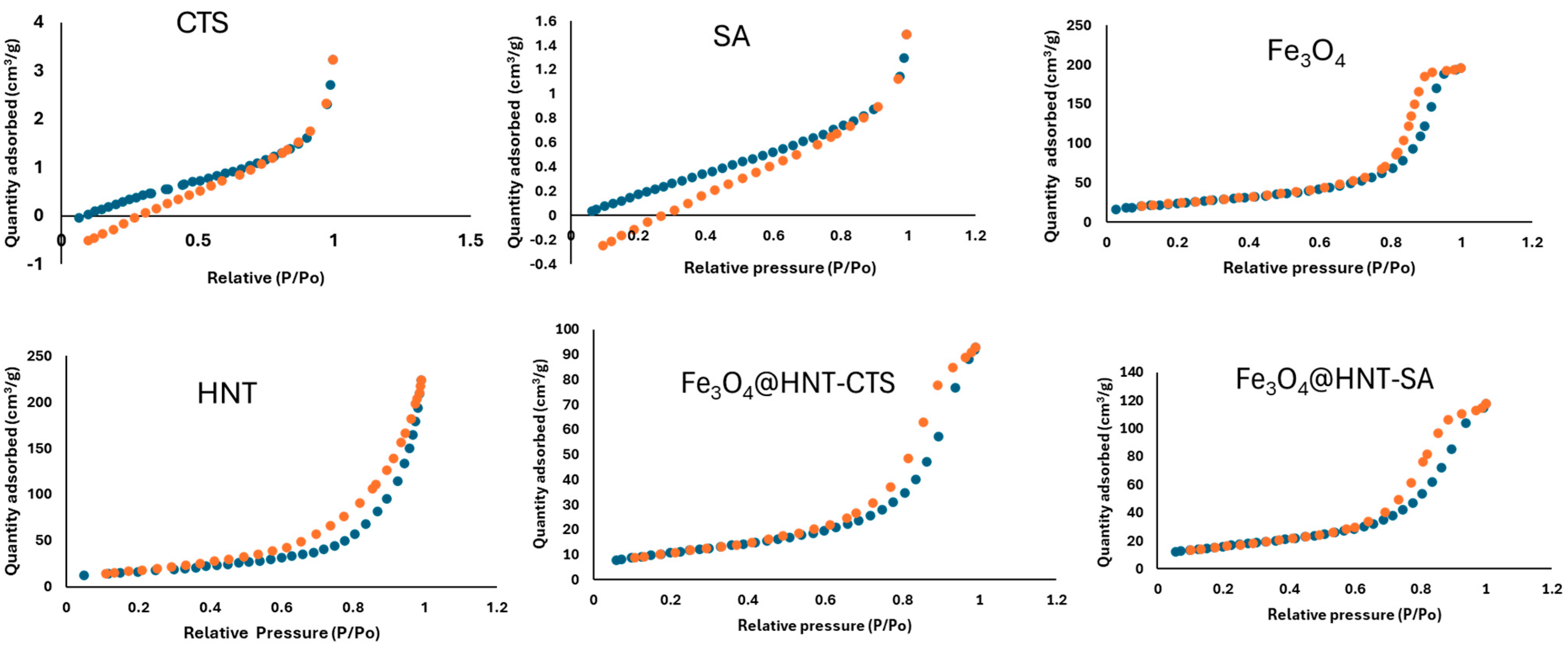




| Adsorbent | Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| CTS | 1.60 | 0.00499 | 8.57 |
| SA | 3.82 | 0.00231 | 7.46 |
| HNT | 60.4 | 0.345 | 13.1 |
| Fe3O4 | 86.0 | 0.302 | 12.5 |
| Fe3O4@HNT-CTS | 58.4 | 0.179 | 9.85 |
| Fe3O4@HNT-SA | 40.7 | 0.144 | 11.2 |
| Models | Parameters | As | V | Cr | Mo | Sb | Cd | Pb |
|---|---|---|---|---|---|---|---|---|
| Fe3O4@HNT-CTS Hydrogel | Fe3O4@HNT-SA Hydrogel | |||||||
| qe (expt) (mg/g) | 30.3 | 19.9 | 28.4 | 22.2 | 24.7 | 52.2 | 57.7 | |
| Langmuir | qmax (mg/g) | 33.60 | 27.2 | 69.9 | 27.0 | 31.7 | 56.2 | 69.4 |
| KL(L/mg) | 2.48 | 0.74 | 0.246 | 1.46 | 1.19 | 1.22 | 59.9 | |
| RL | 0.0464 | 0.166 | 0.375 | 0.0922 | 0.109 | 0.0472 | 0.0735 | |
| R2 | 0.9951 | 0.9693 | 0.6667 | 0.9920 | 0.9720 | 0.9861 | 0.9589 | |
| Freundlich | KF (mg/g) | 11.6 | 11.1 | 21.9 | 10.6 | 13.50 | 16.4 | 26.0 |
| N | 0.331 | 0.429 | 0.385 | 0.378 | 0.367 | 0.304 | 0.301 | |
| R2 | 0.9459 | 0.9574 | 0.9441 | 0.942 | 0.9254 | 0.9774 | 0.9788 | |
| Models | Parameters | Cd | Pb | As | V | Cr | Mo | Sb |
|---|---|---|---|---|---|---|---|---|
| qe, expt (mg/g) | 52.2 | 57.7 | 30.3 | 19.9 | 28.4 | 22.2 | 24.7 | |
| Pseudo-first-order | k1 (1/min) | 0.0706 | 0.0570 | 0.0302 | 0.0834 | 0.0843 | 0.0115 | 0.0429 |
| qe,calc. | 34.14 | 36.34 | 20.2 | 21.9 | 33.3 | 14.0 | 20.3 | |
| R2 | 0.8799 | 0.9935 | 0.9709 | 0.9310 | 0.9254 | 0.8873 | 0.9259 | |
| Pseudo-second-order | k2 (g/mg∙min) | 0.00298 | 0.0220 | 0.0000575 | 0.00426 | 0.00219 | 0.00881 | 0.00243 |
| qe (mg/g) | 57.4 | 62.5 | 32.68 | 23.26 | 34.84 | 16.2 | 26.2 | |
| R2 | 0.9937 | 0.9953 | 0.9946 | 0.9908 | 0.9857 | 0.9959 | 0.9598 |
| Analytes | ΔG° (kj, mol−1) | ΔH° (kj, mol−1) | ΔS° (j, mol−1k−1) | ||
|---|---|---|---|---|---|
| 298 K | 308 K | 313 K | |||
| Fe3O4@HNT-Cd | −4.30 | −1.95 | −0.768 | −74.6 | −236 |
| Fe3O4@HNT-SA-Pb | −5.32 | −6.36 | −6.88 | 25.7 | 104 |
| Fe3O4@HNT-CTS-As | −4.46 | −4.85 | −5.05 | 7.29 | 39.4 |
| Fe3O4@HNT-CTS-Cr | −1.78 | −2.18 | −2.39 | 133 | 451 |
| Fe3O4@HNT-CTS-Mo | −2.85 | −4.51 | −5.44 | 48.6 | 173 |
| Fe3O4@HNT-CTS-Sb | −4.62 | −2.52 | −1.48 | −67.1 | −210 |
| Fe3O4@HNT-CTS-V | −3.43 | −5.04 | −5.58 | 44.6 | 161 |
| Adsorbent | Analyte | Maximum Uptake (mg/g) | References |
|---|---|---|---|
| Nano-sized silicon dioxide | Cd and Pb | 42.2 and 34.2 | [54] |
| Polyethyleneimine (PEI) cryogels | Cd, Co, Cr, Ni, Pb, and Zn | 19.88–24.39 | [53] |
| manganese-residues-and-serpentine-based composite | Cd and Pb | 98.05 and 565.81 | [56] |
| Polyaniline-TiO2 hydrate: | Cr and Sb | 394 and 48.5 | [58] |
| Bacteria–mediated kaolin@Fe–Mn binary hydroxides | Sb(III), Sb(V), As(III) and As(V) | 177.19, 56.26, 62.92 and 42.18 | [59] |
| metal–organic frameworks | Cr and Pb | 188.12 and 349.09 | [57] |
| Sb | 30.26, 86.35 | [60] | |
| modified activated carbon | V | 19.45 | [55,58] |
| Fe3O4@HNT-CTS | As, Cr, Mo, Sb, and V | 30.3, 19.9, 28.4, 22.2, 24.7 | This study |
| Fe3O4@HNT-SA | Cd and Pb | 52.2 and 57.7 | This study |
| Factors | −α | −1 | 0 | + | +α |
|---|---|---|---|---|---|
| Mass adsorbent (MA, mg) | 5.78 | 10 | 30 | 50 | 54.2 |
| Sample pH | 1.26 | 2.0 | 5.5 | 9.0 | 9.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mabaso, N.B.; Nomngongo, P.N.; Nyaba, L. Removal of Oxyanions and Trace Metals from River Water Samples Using Magnetic Biopolymer/Halloysite Nanocomposites. Molecules 2025, 30, 3777. https://doi.org/10.3390/molecules30183777
Mabaso NB, Nomngongo PN, Nyaba L. Removal of Oxyanions and Trace Metals from River Water Samples Using Magnetic Biopolymer/Halloysite Nanocomposites. Molecules. 2025; 30(18):3777. https://doi.org/10.3390/molecules30183777
Chicago/Turabian StyleMabaso, Nyeleti Bridget, Philiswa Nosizo Nomngongo, and Luthando Nyaba. 2025. "Removal of Oxyanions and Trace Metals from River Water Samples Using Magnetic Biopolymer/Halloysite Nanocomposites" Molecules 30, no. 18: 3777. https://doi.org/10.3390/molecules30183777
APA StyleMabaso, N. B., Nomngongo, P. N., & Nyaba, L. (2025). Removal of Oxyanions and Trace Metals from River Water Samples Using Magnetic Biopolymer/Halloysite Nanocomposites. Molecules, 30(18), 3777. https://doi.org/10.3390/molecules30183777








