Studies on the Use of Loan Extraction to Produce Natural Shower Gels (Cosmetic) Based on Grape Pomace Extracts—The Effect of the Type of Surfactant Borrowed
Abstract
1. Introduction
2. Result and Discussion
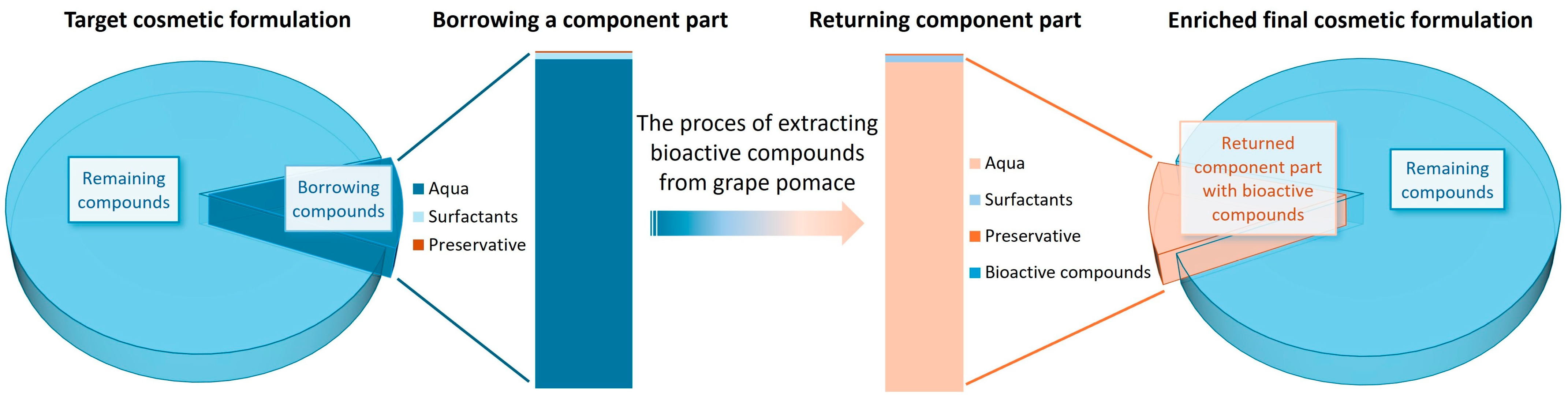
2.1. Development of Loan Extraction Process to Obtain Cosmetically Valuable Compounds from Waste Plant Material, Using Borrowed Components from the Final Product
2.1.1. Extraction Process
2.1.2. Microbiological Stability
2.1.3. Determination of Selected Compounds by UPLC-MS/MS
2.1.4. Total Phenolic Content (TPC) and Antioxidant Capacity (DPPH, ABTS)
2.2. Characterization of Cosmetics Products
2.2.1. Sensory Properties
2.2.2. Stability
2.2.3. Viscosity
2.2.4. Foaming Properties
2.2.5. Irritating Potential
3. Materials and Methods
3.1. Materials
3.2. Plant Material
3.3. Determination of Bioactive Compounds by UPLC-ESI–MS/MS
Quantitative Analysis of Selected Compounds in Grape Pomace Extracts by UPLC-ESI-MS/MS
3.4. Determination of Antioxidant Properties
3.4.1. Total Phenolics Content (TPC)
3.4.2. Antioxidant Activity (DPPH Test)
3.4.3. Antioxidant Activity (Abts Test)
3.5. Preparation of Micellar Extracts from Grape
3.6. Characterization of Cosmetic Product (Shower Gel)
3.6.1. Viscosity
3.6.2. Foaming Properties
3.6.3. Determination of Irritant Potential—Zein Value
3.6.4. Microbiological Stability
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SCS | Sodium Coco-Sulfate |
| DSCG | Disodium Cocoyl Glutamate |
| DG | Decyl Glucoside |
| CB | Cocamidopropyl Betaine |
| GPE | Grape pomace extract |
| GPE_DG | Grape pomace extract obtained using Decyl Glucoside solution |
| GPE_CB | Grape pomace extract obtained using Cocamidopropyl Betaine solution |
| GPE_SCS | Grape pomace extract obtained using Sodium Coco-Sulfate solution |
| GPE_DSCG | Grape pomace extract obtained using Disodium Cocoyl Glutamate solution |
| SG_E_0p | Shower gel without extract |
| SG_E_10p | Shower gel with addition of 10% of extract |
References
- Rico, F.; Mazabel, A.; Egurrola, G.; Pulido, J.; Barrios, N.; Marquez, R.; García, J. Meta-Analysis and Analytical Methods in Cosmetics Formulation: A Review. Cosmetics 2024, 11, 1. [Google Scholar] [CrossRef]
- Pérez-Rivero, C.; López-Gómez, J.P. Unlocking the Potential of Fermentation in Cosmetics: A Review. Fermentation 2023, 9, 463. [Google Scholar] [CrossRef]
- Rischard, F.; Gore, E.; Flourat, A.; Savary, G. The challenges faced by multifunctional ingredients: A critical review from sourcing to cosmetic applications. Adv. Colloid Interface Sci. 2025, 340, 103463. [Google Scholar] [CrossRef]
- Bujak, T.; Niziol-Lukaszewska, Z.; Wasilewski, T. Effect of molecular weight of polymers on the properties of delicate facial foams. Tenside Surfactants Deterg. 2018, 55, 96–102. [Google Scholar] [CrossRef]
- Nizioł-Łukaszewska, Z.; Bujak, T.; Wasilewski, T.; Szmuc, E. Inulin as an effectiveness and safe ingredient in cosmetics. Polish J. Chem. Technol. 2019, 21, 44–49. [Google Scholar] [CrossRef]
- Bujak, T.; Nizioł-Łukaszewska, Z.; Wasilewski, T. Sodium lauryl sulfate vs. Sodium coco sulfate. Study of the safety of use anionic surfactants with respect to their interaction with the skin. Tenside Surfactants Deterg. 2019, 56, 126–133. [Google Scholar] [CrossRef]
- Mahesh, S.K.; Fathima, J.; Veena, V.G. Cosmetic Potential of Natural Products: Industrial Applications. In Natural Bio-Active Compounds: Volume 2: Chemistry, Pharmacology and Health Care Practices; Swamy, M.K., Akhtar, M.S., Eds.; Springer: Singapore, 2019; pp. 215–250. [Google Scholar]
- Morea, D.; Fortunati, S.; Martiniello, L. Circular economy and corporate social responsibility: Towards an integrated strategic approach in the multinational cosmetics industry. J. Clean. Prod. 2021, 315, 128232. [Google Scholar] [CrossRef]
- Sharma, M.; Trivedi, P.; Deka, J. A paradigm shift in consumer behaviour towards green cosmetics: An empirical study. Int. J. Green Econ. 2021, 15, 1–19. [Google Scholar] [CrossRef]
- Luengo, G.S.; Fameau, A.-L.; Léonforte, F.; Greaves, A.J. Surface science of cosmetic substrates, cleansing actives and formulations. Adv. Colloid Interface Sci. 2021, 290, 102383. [Google Scholar] [CrossRef]
- Hernández-Rivas, M.; Guzmán, E.; Fernández-Peña, L.; Akanno, A.; Greaves, A.; Léonforte, F.; Ortega, F.G.; Rubio, R.; Luengo, G.S. Deposition of Synthetic and Bio-Based Polycations onto Negatively Charged Solid Surfaces: Effect of the Polymer Cationicity, Ionic Strength, and the Addition of an Anionic Surfactant. Colloids Interfaces 2020, 4, 33. [Google Scholar] [CrossRef]
- Guzmán, E.; Lucia, A. Essential Oils and Their Individual Components in Cosmetic Products. Cosmetics 2021, 8, 114. [Google Scholar] [CrossRef]
- Laneri, S.; Di Lorenzo, R.M.; Bernardi, A.; Sacchi, A.; Dini, I. Aloe barbadensis: A plant of nutricosmetic interest. Nat. Prod. Commun. 2020, 15. [Google Scholar] [CrossRef]
- González-Minero, F.J.; Bravo-Díaz, L. The Use of Plants in Skin-Care Products, Cosmetics and Fragrances: Past and Present. Cosmetics 2018, 5, 50. [Google Scholar] [CrossRef]
- Zorić, M.; Banožić, M.; Aladić, K.; Vladimir-Knežević, S.; Jokić, S. Supercritical CO2 extracts in cosmetic industry: Current status and future perspectives. Sustain. Chem. Pharm. 2022, 27, 100688. [Google Scholar] [CrossRef]
- Baroi, A.M.; Popitiu, M.; Fierascu, I.; Sărdărescu, I.-D.; Fierascu, R.C. Grapevine Wastes: A Rich Source of Antioxidants and Other Biologically Active Compounds. Antioxidants 2022, 11, 393. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.G.; Peixoto, J.; Oliveria, J.M.; Oliveria, J.A.; Costa, C.; Nogueira, R.; Rodrigues, A. Brewery and winery wastewater treatment: Some focal points of design and operation. In Utilization of By-Products and Treatment of Waste in the Food Industry; Oreopoulou, V., Russ, W., Eds.; Springer Science + Business Media LLC.: New York, NY, USA, 2007; pp. 109–131. [Google Scholar]
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Polyphenolic content and in vitro antioxidant characteristics of wine industry and other agri-food solid waste extracts. J. Food Compos. Anal. 2007, 20, 125–132. [Google Scholar] [CrossRef]
- Xia, E.; Deng, G.; Guo, Y.; Li, H. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Murthy, K.N.C.; Singh, R.P.; Jayaprakasha, G.K. Antioxidant activities of grape Vitis vinifera pomace extracts. J. Agric. Food Chem. 2002, 50, 5909–5914. [Google Scholar] [CrossRef]
- Ilyas, T.; Chowdhary, P.; Chaurasia, D.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Sustainable green processing of grape pomace for the production of value-added products: An overview. Environ. Technol. Innov. 2021, 23, 101592. [Google Scholar] [CrossRef]
- Kalli, E.; Lappa, I.; Bouchagier, P.; Tarantilis, P.A.; Skotti, E. Novel application and industrial exploitation of winery by-products. Bioresour. Bioprocess. 2018, 5, 46. [Google Scholar] [CrossRef]
- Tikhonova, A.; Ageeva, N.; Globa, E. Grape pomace as a promising source of biologically valuable components. BIO Web Conf. 2021, 34, 06002. [Google Scholar] [CrossRef]
- Hoss, I.; Rajha, H.N.; El Khoury, R.; Youssef, S.; Manca, M.L.; Manconi, M.; Louka, N.; Maroun, R.G. Valorization of Wine-Making By-Products’ Extracts in Cosmetics. Cosmetics 2021, 8, 109. [Google Scholar] [CrossRef]
- Cádiz-Gurrea, M.; Silva, A.; Delerue-Matos, C.; Rodrigues, F. Cosmetics—Food waste recovery. In Food Waste Recovery; Ademic Press: Cambridge, MA, USA, 2021; pp. 503–528. ISBN 978-0-12-820563-1. [Google Scholar]
- Matos, M.S.; Romero-Díez, R.; Álvarez, A.; Bronze, M.R.; Rodríguez-Rojo, S.; Mato, R.B.; Cocero, M.J.; Matias, A.A. Polyphenol-Rich Extracts Obtained from Winemaking Waste Streams as Natural Ingredients with Cosmeceutical Potential. Antioxidants 2019, 8, 355. [Google Scholar] [CrossRef]
- Negro, C.; Aprile, A.; Luvisi, A.; De Bellis, L.; Miceli, A. Antioxidant Activity and Polyphenols Characterization of Four Monovarietal Grape Pomaces from Salento (Apulia, Italy). Antioxidants 2021, 10, 1406. [Google Scholar] [CrossRef]
- Jin, Q.; O’Hair, J.; Stewart, A.C.; O’Keefe, S.F.; Neilson, A.P.; Kim, Y.-T.; McGuire, M.; Lee, A.; Wilder, G.; Huang, H. Compositional Characterization of Different Industrial White and Red Grape Pomaces in Virginia and the Potential Valorization of the Major Components. Foods 2019, 8, 667. [Google Scholar] [CrossRef]
- Fontana, A.; Antoniolli, A.; Fernández, M.A.D.; Bottini, R. Phenolics profiling of pomace extracts from different grape varieties cultivated in Argentina. RSC Adv. 2017, 7, 29446–29457. [Google Scholar] [CrossRef]
- Yammine, S.; Delsart, C.; Vitrac, X.; Peuchot, M.M.; Ghidossi, R. Characterisation of polyphenols and antioxidant potential of red and white pomace by-product extracts using subcritical water extraction. OENO One 2020, 54, 263–278. [Google Scholar] [CrossRef]
- Gerardi, G.; Cavia-Saiz, M.; Rivero-Pérez, M.D.; González-SanJosé, M.L.; Muñiz, P. The dose–response effect on polyphenol bioavailability after intake of white and red wine pomace products by Wistar rats. Food Funct. 2020, 11, 1661–1671. [Google Scholar] [CrossRef]
- de la Cerda-Carrasco, A.; López-Solís, R.; Nuñez-Kalasic, H.; Peña-Neira, Á.; Obreque-Slier, E. Phenolic composition and antioxidant capacity of pomaces from four grape varieties (Vitis vinifera L.). J. Sci. Food. Agric. 2015, 95, 1521–1527. [Google Scholar] [CrossRef]
- Fitri, A.; Obitsu, T.; Sugino, T. Effect of ensiling persimmon peel and grape pomace as tannin-rich byproduct feeds on their chemical composition and in vitro rumen fermentation. Anim. Sci. J. 2021, 92, e13524. [Google Scholar] [CrossRef] [PubMed]
- Torre, E.; Iviglia, G.; Cassinelli, C.; Morra, M.; Russo, N. Polyphenols from grape pomace induce osteogenic differentiation in mesenchymal stem cells. Int. J. Mol. Med. 2020, 45, 1721–1734. [Google Scholar] [CrossRef] [PubMed]
- Denev, P.; Ciz, M.; Ambrozova, G.; Lojek, A.; Yanakieva, I.; Kratchanova, M. Solid-phase extraction of berries’ anthocyanins and evaluation of their antioxidative properties. Food Chem. 2010, 123, 1055–1061. [Google Scholar] [CrossRef]
- Ruiz-García, Y.; Silva, C.L.; Gómez-Plaza, E.; Câmara, J.S. A Powerful Analytical Strategy Based on QuEChERS-Dispersive Solid-Phase Extraction Combined with Ultrahigh Pressure Liquid Chromatography for Evaluating the Effect of Elicitors on Biosynthesis of trans-Resveratrol in Grapes. Food Anal. Methods 2016, 9, 670–679. [Google Scholar] [CrossRef]
- Sokač Cvetnić, T.; Krog, K.; Benković, M.; Jurina, T.; Valinger, D.; Gajdoš Kljusurić, J.; Radojčić Redovniković, I.; Jurinjak Tušek, A. Solid–Liquid Extraction of Bioactive Molecules from White Grape Skin: Optimization and Near-Infrared Spectroscopy. Separations 2023, 10, 452. [Google Scholar] [CrossRef]
- da Rocha, B.C.; Noreña, Z.C.P. Microwave-Assisted Extraction and Ultrasound-Assisted Extraction of Bioactive Compounds from Grape Pomace. Int. J. Food Eng. 2020, 16, 20190191. [Google Scholar] [CrossRef]
- Vinatour, M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 2001, 8, 303–313. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I.; Azhari, N.H. Vernonia cinerea leaves as the source of phenolic compounds, antioxidants, and anti-diabetic activity using microwave-assisted extraction technique. Ind. Crops Prod. 2018, 122, 533–544. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Wang, H.; Huo, S. Evaluation of extraction technologies and optimization of microwave and ultrasonic assisted consecutive extraction of phenolic antioxidants from winery byproducts. J. Food Process Eng. 2019, 42, e13064. [Google Scholar] [CrossRef]
- Pereira, D.T.V.; Tarone, A.G.; Cazarin, C.B.B.; Barbero, G.F.; Martínez, J. Pressurized liquid extraction of bioactive compounds from grape marc. J. Food Eng. 2019, 240, 105–113. [Google Scholar] [CrossRef]
- Casazza, A.A.; Aliakbarian, B.; Sannita, E.; Perego, P. High-pressure high-temperature extraction of phenolic compounds from grape skins. Int. J. Food Sci. Technol. 2012, 47, 399–405. [Google Scholar] [CrossRef]
- Machado, T.d.O.X.; Portugal, I.; Kodel, H.d.A.C.; Fathi, A.; Fathi, F.; Oliveira, M.B.P.P.; Dariva, C.; Souto, E.B. Pressurized liquid extraction as an innovative high-yield greener technique for phenolic compounds recovery from grape pomace. Sustain. Chem. Pharm. 2024, 40, 101635. [Google Scholar] [CrossRef]
- Toepfl, S.; Mathys, A.; Heinz, V.; Knorr, D. Review: Potential of high hydrostatic pressure and pulsed electric fields for en-ergy efficiency and environmentally friendly food processing. Food Rev. Int. 2006, 22, 405–423. [Google Scholar] [CrossRef]
- Ju, Z.Y.; Howard, L.R. Effects of Solvent and Temperature on Pressurized Liquid Extraction of Anthocyanins and Total Phe-nolics from Dried Red Grape Skin. J. Agric. Food Chem. 2003, 51, 5207–5213. [Google Scholar] [CrossRef]
- Liazid, A.; Barbero, G.F.; Azaroual, L.; Palma, M.; Barroso, C.G. Stability of Anthocyanins from Red Grape Skins under Pressurized Liquid Extraction and Ultrasound-Assisted Extraction Conditions. Molecules 2014, 19, 21034–21043. [Google Scholar] [CrossRef]
- Tomaz, I.; Maslov, L.; Stupić, D.; Preiner, D.; Ašperger, D.; Kontić, J.K. Recovery of flavonoids from grape skins by enzyme-assisted extraction. Sep. Sci. Technol. 2015, 51, 255–268. [Google Scholar] [CrossRef]
- Streimikyte, P.; Viskelis, P.; Viskelis, J. Enzymes-Assisted Extraction of Plants for Sustainable and Functional Applications. Int. J. Mol. Sci. 2022, 23, 2359. [Google Scholar] [CrossRef] [PubMed]
- Stanek-Wandzel, N.; Krzyszowska, A.; Zarębska, M.; Gębura, K.; Wasilewski, T.; Hordyjewicz-Baran, Z.; Tomaka, M. Evaluation of Cellulase, Pectinase, and Hemicellulase Effectiveness in Extraction of Phenolic Compounds from Grape Pomace. Int. J. Mol. Sci. 2024, 25, 13538. [Google Scholar] [CrossRef]
- Gomez-García, R.; Martínez-Avila, G.C.G.; Aguilar, C.N. Enzyme-assisted extraction of antioxidative phenolics from grape (Vitis vinifera L.) residues. Biotech 2012, 3, 297–300. [Google Scholar] [CrossRef]
- de Camargo, A.C.; Regitano-d’Arce, M.A.; Biasoto, A.C.; Shahidi, F. Enzyme assisted extraction of phenolics from winemaking by-products: Antioxidant potential and inhibition of alpha-glucosidase and lipase activities. Food Chem. 2016, 212, 395–402. [Google Scholar] [CrossRef]
- Gaur, R.; Sharma, A.; Khare, S.K.; Gupta, M.N. A novel process for extraction of edible oils: Enzyme assisted three phase partitioning (EATPP). Bioresour. Technol. 2007, 98, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Duba, K.; Fiori, L. Supercritical CO2 extraction of grape seeds oil: Scale-up and economic analysis. Int. J. Food Sci. Technol. 2019, 54, 1306–1312. [Google Scholar] [CrossRef]
- Meireles, A.; Angela, M. Supercritical Extraction from Solid: Process Design Data (2001–2003). Curr. Opin. Solid State Mater. Sci. 2003, 7, 321–330. [Google Scholar] [CrossRef]
- Baron, G.; Ferrario, G.; Marinello, C.; Carini, M.; Morazzoni, P.; Aldini, G. Effect of Extraction Solvent and Temperature on Polyphenol Profiles, Antioxidant and Anti-Inflammatory Effects of Red Grape Skin By-Product. Molecules 2021, 26, 5454. [Google Scholar] [CrossRef] [PubMed]
- Pintać, D.; Majkić, T.; Torović, L.; Orčić, D.; Beara, I.; Simin, N.; Mimica–Dukić, N.; Lesjak, M. Solvent selection for efficient extraction of bioactive compounds from grape pomace. Ind. Crops Prod. 2018, 111, 379–390. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Oluwaseun, R.A.; Nour, H.A.; Chinonso, I.U. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef]
- Eyiz, V.; Tontul, I.; Turker, S. Optimization of green extraction of phytochemicals from red grape pomace by homogenizer assisted extraction. J. Food Meas. Charact. 2020, 14, 39–47. [Google Scholar] [CrossRef]
- Vichapong, J.; Santaladchaiyakit, Y.; Burakham, R.; Supalax, S. Cloud-point extraction and reversed-phase high performance liquid chromatography for analysis of phenolic compounds and their antioxidant activity in Thai local wines. J. Food Sci. Technol. 2014, 51, 664–672. [Google Scholar] [CrossRef]
- Arya, S.S.; Kaimal, A.M.; Chib, M.; Sonawane, S.K.; Show, P.L. Novel, energy efficient and green cloud point extraction: Technology and applications in food processing. J. Food Sci. Technol. 2019, 56, 524–534. [Google Scholar] [CrossRef]
- Alibade, A.; Batra, G.; Bozinou, E.; Salakidou, C.; Lalas, S. Optimization of the extraction of antioxidants from winery wastes using cloud point extraction and a surfactant of natural origin (lecithin). Chem. Pap. 2020, 74, 4517–4524. [Google Scholar] [CrossRef]
- Wasilewski, T.; Hordyjewicz-Baran, Z.; Zarębska, M.; Stanek, N.; Zajszły-Turko, E.; Tomaka, M.; Bujak, T.; Nizioł-Łukaszewska, Z. Sustainable Green Processing of Grape Pomace Using Micellar Extraction for the Production of Value-Added Hygiene Cosmetics. Molecules 2022, 27, 2444. [Google Scholar] [CrossRef]
- Hordyjewicz-Baran, Z.; Wasilewski, T.; Zarębska, M.; Stanek-Wandzel, N.; Zajszły-Turko, E.; Tomaka, M.; Zagórska-Dziok, M. Micellar and Solvent Loan Chemical Extraction as a Tool for the Development of Natural Skin Care Cosmetics Containing Substances Isolated from Grapevine Buds. Appl. Sci. 2024, 14, 1420. [Google Scholar] [CrossRef]
- Samant, B.S.; Kaliappan, R. The use of surfactants in the extraction of active ingredients from natural resources: A comprehensive revie. RSC Adv. 2025, 15, 23569–23587. [Google Scholar] [CrossRef]
- Mourtzinos, I.; Christaki, S.; Tsetsilas, A.; Kyriakoudi, A. Surfactant-Assisted Extraction of Bioactive Compounds from Turmeric (Curcuma longa L.). Proceedings 2024, 105, 9. [Google Scholar] [CrossRef]
- Bujak, T.; Wasilewski, T.; Nizioł-Łukaszewska, Z. Role of macromolecules in the safety of use of body wash cosmetics. Colloids Surf. B Biointerfaces 2015, 135, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Cornwell, P.A. A review of shampoo surfactant technology: Consumer benefits, raw materials and recent developments. Int. J. Cosmet. Sci. 2018, 40, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Huanbutta, K.; Sripirom, P.; Phetthong, P.; Thalerngkiatsiri, P.; Kabthong, N.; Sangnim, T.; Sriamornsak, P. Dissolvable shower gel tablets with enhanced skin benefits. Int. J. Cosmet Sci. 2023, 45, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Seok, J.K.; Kim, M.; Choi, S.; Hong, J.; Yoon, Y.A.; Chung, H.; Bae, O.-N.; Kwack, S.J.; Kim, K.-B.; et al. Safety assessment of cocamidopropyl betaine, a cosmetic ingredient. Toxicol. Res. 2024, 40, 361–375. [Google Scholar] [CrossRef]
- Hosseinzadeh, R.; Khorsandi, K.; Hemmaty, S. Study of the Effect of Surfactants on Extraction and Determination of Polyphenolic Compounds and Antioxidant Capacity of Fruits Extracts. PLoS ONE 2013, 8, e57353. [Google Scholar] [CrossRef]
- Skrypnik, L.; Novikova, A. Response Surface Modeling and Optimization of Polyphenols Extraction from Apple Pomace Based on Nonionic Emulsifiers. Agronomy 2020, 10, 92. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.; Cruz, A.P.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, C.M.; Dias, M.I.; Alves, M.J.; Calhelha, R.C.; Barros, L.; Pinho, S.P.; Ferreira, I.C. Grape pomace as a source of phenolic compounds and diverse bioactive properties. Food Chem. 2018, 253, 132–138. [Google Scholar] [CrossRef]
- Hogervorst, J.C.; Miljić, U.; Puškaš, V. Extraction of bioactive compounds from grape processing by-products. In Handbook of Grape Processing By-Products; Galanakis, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 105–135. [Google Scholar]
- Su, E.; Herman, S. Beyond Sulfate-Free Personal Cleansing Technology. Cosmetics 2025, 12, 14. [Google Scholar] [CrossRef]
- Hill, K.; LeHen-Ferrenbach, C. Sugar-Based Surfactants for Consumer Products and Technical Applications. In Sugar-Based Surfactants: Fundamentals and Applications; Carnero Ruiz, C., Ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 1–21. [Google Scholar]
- Kjellin, M.; Johansson, I. Surfactants from Renewable Resources; Kjellin, M., Johansson, I., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; pp. 63–84. [Google Scholar]
- ISO 16128-1:2016; Guidelines on Technical Definitions and Criteria for Natural and Organic Cosmetic Ingredients and Products Part 1: Definitions for Ingredients. ISO: Geneva, Switzerland, 2016.
- ISO 16128-2:2017; Cosmetics—Guidelines on Technical Definitions and Criteria for Natural and Organic Cosmetic Ingredients Part 2: Criteria for Ingredients and Products. ISO: Geneva, Switzerland, 2017.
- Leanpolchareanchai, J.; Teeranachaideekul, V. Topical Microemulsions: Skin Irritation Potential and Anti-Inflammatory Effects of Herbal Substances. Pharmaceuticals 2023, 16, 999. [Google Scholar] [CrossRef]
- McKim, J.M., Jr.; Keller, D.J., 3rd; Gorski, J.R. An in vitro method for detecting chemical sensitization using human reconstructed skin models and its applicability to cosmetic, pharmaceutical, and medical device safety testing. Cutan. Ocul. Toxicol. 2012, 31, 292–305. [Google Scholar] [CrossRef]
- de Lima Sá, L.; Rodrigues, R.V.; Vani, M.A.; Gaspar, L.R. Strategies for the evaluation of the eye irritation potential of different types of surfactants and silicones used in cosmetic products. Toxicol. Vitr. 2022, 81, 105351. [Google Scholar] [CrossRef] [PubMed]
- Rivero, M.N.; Lenze, M.; Izaguirre, M.; Damonte, S.H.P.; Aguilar, A.; Wikinski, S.; Gutiérrez, M.L. Comparison between HET-CAM protocols and a product use clinical study for eye irritation evaluation of personal care products including cosmetics according to their surfactant composition. Food Chem. Toxicol. 2021, 153, 112229. [Google Scholar] [CrossRef] [PubMed]
- Kuo-Yann, L. Liquid Detergents; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Shinoda, K.; Yamaguchi, T.; Hori, R. The Surface Tension and the Critical Micelle Concentration in Aqueous Solution of β-d-Alkyl Glucosides and their Mixtures. Bull. Chem. Soc. Jpn. 1961, 34, 237–241. [Google Scholar] [CrossRef]
- Staszak, K.; Wieczorek, D.; Michocka, K. Effect of Sodium Chloride on the Surface and Wetting Properties of Aqueous Solutions of Cocamidopropyl Betaine. J. Surfactants Deterg. 2015, 18, 321–328. [Google Scholar] [CrossRef]
- Motin, A.; Mia, H.; Islam, N. Thermodynamic properties of Sodium Dodecyl Sulfate aqueous solutions with Methanol, Ethanol, n-Propanol and iso-Propanol at different temperatures. J. Saudi Chem. Soc. 2015, 19, 172–180. [Google Scholar] [CrossRef]
- Arakawa, T.; Niikura, T.; Kita, Y.; Akuta, T. Sodium Dodecyl Sulfate Analogs as a Potential Molecular Biology Reagent. Curr. Issues Mol. Biol. 2024, 46, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.G.; Shah, D. The effect of micellar lifetime on the rate of solubilization and detergency in sodium dodecyl sulfate solutions. J. Am. Oil Chem. 1993, 70, 673–678. [Google Scholar] [CrossRef]
- Hadgiivanova, R.; Diamant, H.; Andelman, D. Kinetics of surfactant micellization: A free energy approach. J. Phys. Chem. B 2011, 115, 7268–7280. [Google Scholar] [CrossRef]
- Rangel-Yagui, C.O.; Pessoa, A.; Tavares, L.C. Micellar solubilization of drugs. J. Pharm. Pharm. Sci. 2005, 8, 147–163. [Google Scholar]
- Jackson, C.T.; Paye, M.; Maibach, H. Mechanism of Skin Irritation by Surfactants and Anti-Irritants for Surfactants Base Products. In Handbook of Cosmetic Science and Technology, 4th ed.; Barel, A., Paye, M., Maibach, H., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 353–365. [Google Scholar]
- Farn, R.J. Chemistry and Technology of Surfactants; Blackwell Publishing: Hoboken, NY, USA, 2006; pp. 46–90. [Google Scholar]
- Rosen, M.J. Surfactants and Interfacial Phenomena, 3rd ed.; John Wiley & Sons Inc.: New York, NY, USA, 2006. [Google Scholar]
- Dominguez, J.G.; Balaguer, F.; Parra, J.L.; Pelejero, C.M. The inhibitory effect of some amphoteric surfactants on the irritation potential of alkylsulphates. Int. J. Cosmet. Sci. 1981, 3, 57–68. [Google Scholar] [CrossRef]
- Moore, P.N.; Puvvada, S.; Blankschtein, D. Challenging the surfactant monomer skin penetration model: Penetration of sodium dodecyl sulfate micelles into the epidermis. J. Cosmet. Sci. 2003, 54, 29–46. [Google Scholar]
- Dasilva, S.C.; Sahu, R.P.; Konger, R.L.; Perkins, S.M.; Kaplan, M.H.; Travers, J.B. Increased skin barrier disruption by sodium lauryl sulfate in mice expressing a constitutively active STAT6 in T cells. Arch. Dermatol. Res. 2012, 304, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Faucher, J.A.; Goddard, E.D. Interaction of keratinous substrates with sodium lauryl sulfate. I. Sorption. J. Soc. Cosmet. Chem. 1978, 29, 323–337. [Google Scholar]
- Hall-Manning, T.J.; Holland, G.H.; Rennie, G.; Revell, P.; Hines, J.; Barratt, M.D.; Basketter, D.A. Skin irritation potential of mixed surfactant systems. Food Chem. Toxicol. 1998, 36, 233–238. [Google Scholar] [CrossRef]
- Nielsen, G.D.; Nielsen, J.B.; Andersen, K.E.; Grandjean, P. Effects of industrial detergents on the barier function of human skin. Int. J. Occup. Environ. Health 2000, 6, 138–142. [Google Scholar] [CrossRef]
- Cohen, L.; Martin, M.; Soto, F.; Trujillo, F.; Sanchez, E. The Effect of Counterions of Linear Alkylbenzene Sulfonate on Skin Compatibility. J. Surfactants Deterg. 2016, 19, 219–222. [Google Scholar] [CrossRef]
- Ciurleo, A.; Cinelli, S.; Guidi, M.; Bonincontro, A.; Onori, G.; Mesa, C.L. Some Properties of Lysozyme− Lithium Perfluorononanoate Complexes. Biomacromolecules 2007, 8, 399. [Google Scholar] [CrossRef]
- Ghosh, S. Interaction of trypsin with sodium dodecyl sulfate in aqueous medium: A conformational view. Colloids Surf. B 2008, 66, 178. [Google Scholar] [CrossRef]
- Salomon, G.; Giordano-Labadie, F. Surfactant irritations and allergies. Eur. J. Dermatol. 2022, 32, 677–681. [Google Scholar] [CrossRef]
- Chiavaroli, A.; Balaha, M.; Acquaviva, A.; Ferrante, C.; Cataldi, A.; Menghini, L.; Rapino, M.; Orlando, G.; Brunetti, L.; Leone, S.; et al. Phenolic Characterization and Neuroprotective Properties of Grape Pomace Extracts. Molecules 2021, 26, 6216. [Google Scholar] [CrossRef] [PubMed]
- Brazinha, C.; Cadima, M.; Crespo, J.G. Optimization of Extraction of Bioactive Compounds from Different Types of Grape Pomace Produced at Wineries and Distilleries. J. Food Sci. 2014, 79, E1142–E1149. [Google Scholar] [CrossRef] [PubMed]
- Mita, S.R.; Husni, P.; Putriana, N.A.; Maharani, R.; Hendrawan, R.P.; Dewi, D.A. A Recent Update on the Potential Use of Catechins in Cosmeceuticals. Cosmetics 2024, 11, 23. [Google Scholar] [CrossRef]
- Atanacković Krstonošić, M.; Sazdanić, D.; Ćirin, D.; Maravić, N.; Mikulić, M.; Cvejić, J.; Krstonošić, V. Aqueous solutions of non-ionic surfactant mixtures as mediums for green extraction of polyphenols from red grape pomace. Sustain. Chem. Pharm. 2023, 33, 101069. [Google Scholar] [CrossRef]
- Parus, A. Antioxidant and Pharmacological Properties of Phenolic Acids. Postępy Fitoter. 2012. Available online: https://www.czytelniamedyczna.pl/4338,przeciwutleniajace-i-farmakologiczne-wlasciwosci-kwasow-fenolowych.html (accessed on 1 December 2023).
- Noguchi, A.; Djerassi, D. Chapter 15-Amino Acids and Peptides: Building Blocks for Skin Proteins. Nutr. Cosmet. 2009, 15, 287. [Google Scholar]
- Sharma, S.; Kori, S.; Parmar, A. Surfactant mediated extraction of total phenolic contents (TPC) and antioxidants from fruits juices. Food Chem. 2015, 185, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Sazdanić, D.; Atanacković, K.M.; Ćirin, D.; Cvejić, J.; Alamri, A.; Galanakis, C.M.; Krstonošić, V. Non-ionic surfactants-mediated green extraction of polyphenols from red grape pomace. J. Appl. Res. Med. Aromat. Plants 2023, 32, 100439. [Google Scholar] [CrossRef]
- Śliwa, K.; Śliwa, P. The Accumulated Effect of the Number of Ethylene Oxide Units and/or Carbon Chain Length in Surfactants Structure on the Nano-Micellar Extraction of Flavonoids. J. Funct. Biomater. 2020, 11, 57. [Google Scholar] [CrossRef]
- Bujak, T.; Nizioł-Łukaszewska, Z.; Zan, A. Amphiphilic cationic polymers as effective substances improving the safety of use of body wash gels. Int. J. Biol. Macromol. 2020, 15, 973–979. [Google Scholar] [CrossRef]
- Lips, A.; Ananthapadmanabhan, K.P.; Vethamuthu, M.; Hua, X.Y.; Yang, L.; Vincent, C.; Deo, N.; Somasundaran, P. Role of Surfactant Micelle Charge in Protein Denaturation and Surfactant-Induced Skin Irritation. In Surfactants in Personal Care Products and Decorative Cosmetics, 3rd ed.; Rhein, L., Schlossman, M., Lenick, A., Somasundaran, P., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2007; pp. 177–189. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
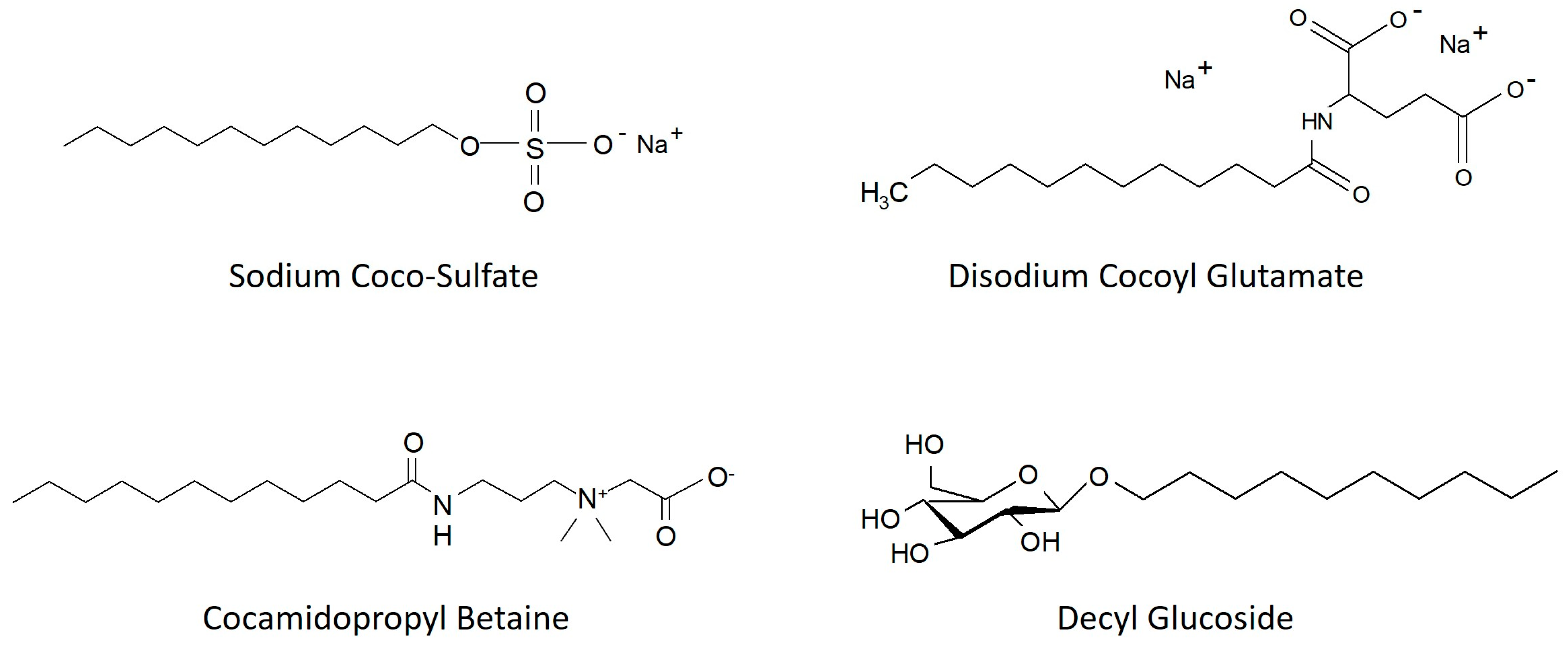

| Ingredient (INCI Name) | SG [%] | |
|---|---|---|
| 1 | Sodium Coco-Sulfate | 4.5 |
| 2 | Aqua | to 100 |
| 3 | Decyl Glucoside | 4.5 |
| 4 | Benzyl Alcohol, Benzoic Acid, Dehydroacetic Acid, Tocopherol | 0.5 |
| 5 | Disodium Cocoyl Glutamate | 1 |
| 6 | Citric Acid | to pH 5.5 |
| 7 | Parfum | 0.3 |
| 8 | Cocamidopropyl Betaine | 2 |
| 9 | Sodium Chloride | 3 |
| Ingredient (INCI Name) | GPE_DG | GPE_CB | GPE_SCS | GPE_DSCG | |
|---|---|---|---|---|---|
| % (w/w) | |||||
| 1 | Decyl Glucoside | 2 | |||
| Cocamidopropyl Betaine | 2 | ||||
| Sodium Coco-Sulfate | 2 | ||||
| Disodium Cocoyl Glutamate | 2 | ||||
| 2 | Benzyl Alcohol, Benzoic Acid, Dehydroacetic Acid, Tocopherol | 0.5 | 0.5 | 0.5 | 0.5 |
| 3 | Aqua | 97.5 | 97.5 | 97.5 | 97.5 |
| Ingredient (INCI Name) | CMC mmol/L | MW g/mol | CMC %, (w/w) | NOI % | Zein Number mgN/100 mL |
|---|---|---|---|---|---|
| Decyl Glucoside | 2.2 [88] | 320 | 0.07 | 100 | 7.7 |
| Cocamidopropyl Betaine | 0.28 [89] | 342 | 0.01 | 87 | 33.3 |
| Sodium Coco-Sulfate | 8.5 [90] | 330 | 0.28 | 100 | 632.1 |
| Disodium Cocoyl Glutamate | 10.6 [91] | 191 | 0.20 | 100 | 359.1 |
| Compound | Quantification/Confirmation Transition | Family | GPE_DG [mg/L] | GPE_CB [mg/L] | GPE_SCS [mg/L] | GPE_DSCG [mg/L] | |
|---|---|---|---|---|---|---|---|
| 1 | (+)-Catechin | 290.9 > 139.0 290.9 > 123.0 | flavonols | 142 ± 3.88 | 267 ± 6.56 | 82.2 ± 7.16 | 218 ± 4.18 |
| 2 | (−)-Epicatechin | 290.9 > 139.0 290.9 > 123.0 | flavonols | 283 ± 1.28 | 651 ± 8.52 | 164 ± 2.13 | 525 ± 16.1 |
| 3 | (−)-Gallocatechin | 306.9 > 288.8 306.9 > 163.0 | flavonols | 1.46 ± 0.06 | 1.29 ± 0.12 | 1.19 ± 0.05 | 1.36 ± 0.15 |
| 4 | Rutin | 608.9 > 299.9 608.9 > 270.9 | flavonols | 0.619 ± 0.03 | 0.529 ± 0.002 | 0.549 ± 0.03 | 0.588 ± 0.04 |
| 5 | Quercetin | 300.8 > 151.0 300.8 > 179.0 | flavonols | 4.78 ± 0.001 | 4.78 ± 0.002 | 4.80 ± 0.004 | 4.80 ± 0.002 |
| 6 | Syringic acid | 196.9 > 120.9 196.9 > 181.9 | phenolic acid | 0.103 ± 0.001 | 0.116 ± 0.001 | 0.113 ± 0.001 | 0.107 ± 0.001 |
| 7 | Trans-Resweratrol | 226.9 > 185.0 226.9 > 143.0 | stilbenes | 0.197 ± 0.003 | 0.199 ± 0.001 | 0.197 ± 0.002 | 0.199 ± 0.001 |
| 8 | Gallic acid | 168.9 > 124.8 168.9 > 78.9 | phenolic acid | n.d. * | 0.265 ± 0.004 | n.d. * | 0.191 ± 0.05 |
| Sum of phenolic compounds | 433 | 925 | 253 | 750 | |||
| 9 | L-phenyloalanine | 163.9 > 147.0 163.9 > 103.0 | amino acid | 1.95 ± 0.27 | 1.89 ± 0.01 | 2.33 ± 0.03 | 1.96 ± 0.03 |
| 10 | L-Aspartic acid | 131.8 > 88.0 131.8 > 114.9 | amino acid | 4.48 ± 0.58 | 4.53 ± 0.14 | 4.77 ± 0.42 | 4.64 ± 0.01 |
| 11 | L-Valine | 118.1 > 72.0 118.1 > 55.0 | amino acid | 9.80 ± 0.39 | 8.03 ± 0.11 | 10.8 ± 0.06 | 10.2 ± 0.33 |
| 12 | L-Lisyne | 147.1 > 84.0 147.1 > 130.0 | amino acid | 27.2 ± 0.95 | 23.7 ± 0.56 | 28.4 ± 0.06 | 26.0 ± 0.51 |
| 13 | L-Tryptophan | 205.0 > 188.0 205.0 > 145.9 | amino acid | 13.4 ± 0.51 | 15.1 ± 0.12 | 11.8 ± 0.43 | 13.7 ± 0.43 |
| 14 | L-Leucine | 132.1 > 86.0 132.1 > 44.0 | amino acid | 11.4 ± 0.38 | 8.41 ± 0.05 | 12.0 ± 0.24 | 11.3 ± 0.001 |
| 15 | L-Threonine | 120.1 > 74.0 120.1 > 56.0 | amino acid | 4.54 ± 0.15 | 4.06 ± 0.001 | 4.50 ± 0.006 | 4.24 ± 0.07 |
| 16 | L-Methionine | 150.1 > 103.9 150.1 > 132.9 | amino acid | 0.268 ± 0.05 | 0.350 ± 0.004 | 0.243 ± 0.008 | 0.370 ± 0.005 |
| 17 | L-Histidine | 156.1 > 110.0 156.1 > 82.9 | amino acid | 3.50 ± 0.15 | 1.78 ± 0.07 | 0.25 ± 0.04 | 0.51 ± 0.08 |
| Sum of amino acids | 76.6 | 67.9 | 75.1 | 72.9 | |||
| 19 | D-(+)-glucose | 178.9 > 58.9 178.9 > 88.9 | sugars | 967 ± 28.3 | 787 ± 13 | 982 ± 16 | 830 ± 12.1 |
| 21 | D-(-)-fructose | 179.9 > 59.0 179.9 > 90.0 | sugars | 202 ± 14 | 170 ± 7.01 | 186 ± 12 | 236 ± 13 |
| 23 | D-(+)-mannose | 178.9 > 58.9 178.9 > 88.9 | sugars | 250 ± 26 | 196 ± 6.50 | 217 ± 4.50 | 193 ± 11 |
| 24 | D-sorbitol | 180.9 > 58.9 180.9 > 70.8 | sugars | 102 ± 0.41 | 112 ± 5.34 | 107 ± 0.41 | 80 ± 5.34 |
| Sum of sugars | 1521 | 1266 | 1492 | 1339 | |||
| Grape Pomace Extract | TPC [mg GAE/L] | DPPH [mg TE/L] | ABTS [mg TE/L] |
|---|---|---|---|
| GPE_DG | 856.2 ± 32.2 | 1480.8 ± 31.3 | 1983.8 ± 10.5 |
| GPE_CB | 587. 9± 16.0 | 694.5 ± 34.3 | 1676.0 ± 11.8 |
| GPE_SCS | 510.1 ± 12.7 | 579.8 ± 28.1 | 1245.0 ± 15.2 |
| GPE_DSCG | 877.3 ± 25.8 | 1791.1 ± 29.3 | 2233.7 ± 9.1 |
| Ingredient (INCI Name) | [% m/m] | ||
|---|---|---|---|
| SG_E_0p | SG_E_10p | ||
| 1 | Sodium Coco-Sulfate | 4.5 | 4.5 |
| 2 | Aqua | do 100 | do 100 |
| 3 | Extract | 0 | 10 |
| Decyl Glucoside | 0 | 0.2 | |
| Benzyl Alcohol, Benzoic Acid,
Dehydroacetic Acid, Tocopherol | 0 | 0.05 | |
| Aqua | 0 | 9.75 | |
| 4 | Decyl Glucoside | 4.5 | 4.3 |
| 5 | Disodium Cocoyl Glutamate | 1 | 1 |
| 6 | Citric Acid | to pH 5.5 | to pH 5.5 |
| 7 | Benzyl Alcohol, Benzoic Acid, Dehydroacetic Acid, Tocopherol | 0.5 | 0.45 |
| 8 | Cocamidopropyl Betaine | 2 | 2 |
| 9 | Sodium Chloride | 3 | 3 |
| Cosmetics Product | Stability | Viscosity mPa·s | Foaming Ability cm3 | Foam Stability % | Irritating Potential mgN/100 mL |
|---|---|---|---|---|---|
| SG_E_0p | stable | 5184 ± 50 | 530 ± 25 | 98 | 90.7 ± 0.5 |
| SG_E_10p | stable | 4224 ± 30 | 496 ± 20 | 97 | 74.2 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasilewski, T.; Hordyjewicz-Baran, Z.; Malorna, K.; Dresler, E.; Sabura, E.; Zegarski, M.; Stanek-Wandzel, N. Studies on the Use of Loan Extraction to Produce Natural Shower Gels (Cosmetic) Based on Grape Pomace Extracts—The Effect of the Type of Surfactant Borrowed. Molecules 2025, 30, 3709. https://doi.org/10.3390/molecules30183709
Wasilewski T, Hordyjewicz-Baran Z, Malorna K, Dresler E, Sabura E, Zegarski M, Stanek-Wandzel N. Studies on the Use of Loan Extraction to Produce Natural Shower Gels (Cosmetic) Based on Grape Pomace Extracts—The Effect of the Type of Surfactant Borrowed. Molecules. 2025; 30(18):3709. https://doi.org/10.3390/molecules30183709
Chicago/Turabian StyleWasilewski, Tomasz, Zofia Hordyjewicz-Baran, Katarzyna Malorna, Ewa Dresler, Ewa Sabura, Maciej Zegarski, and Natalia Stanek-Wandzel. 2025. "Studies on the Use of Loan Extraction to Produce Natural Shower Gels (Cosmetic) Based on Grape Pomace Extracts—The Effect of the Type of Surfactant Borrowed" Molecules 30, no. 18: 3709. https://doi.org/10.3390/molecules30183709
APA StyleWasilewski, T., Hordyjewicz-Baran, Z., Malorna, K., Dresler, E., Sabura, E., Zegarski, M., & Stanek-Wandzel, N. (2025). Studies on the Use of Loan Extraction to Produce Natural Shower Gels (Cosmetic) Based on Grape Pomace Extracts—The Effect of the Type of Surfactant Borrowed. Molecules, 30(18), 3709. https://doi.org/10.3390/molecules30183709








