Next-Generation Wastewater-Based Epidemiology: Green Automation for Detecting 69 Multiclass Pharmaceutical and Personal Care Products in Wastewater Using 96-Well Plate Solid-Phase Extraction by LC-MS/MS
Abstract
1. Introduction
2. Results and Discussion
2.1. Outlines of Method Optimization and Validation
2.2. Outcome of Method Optimization
2.3. Method Validation
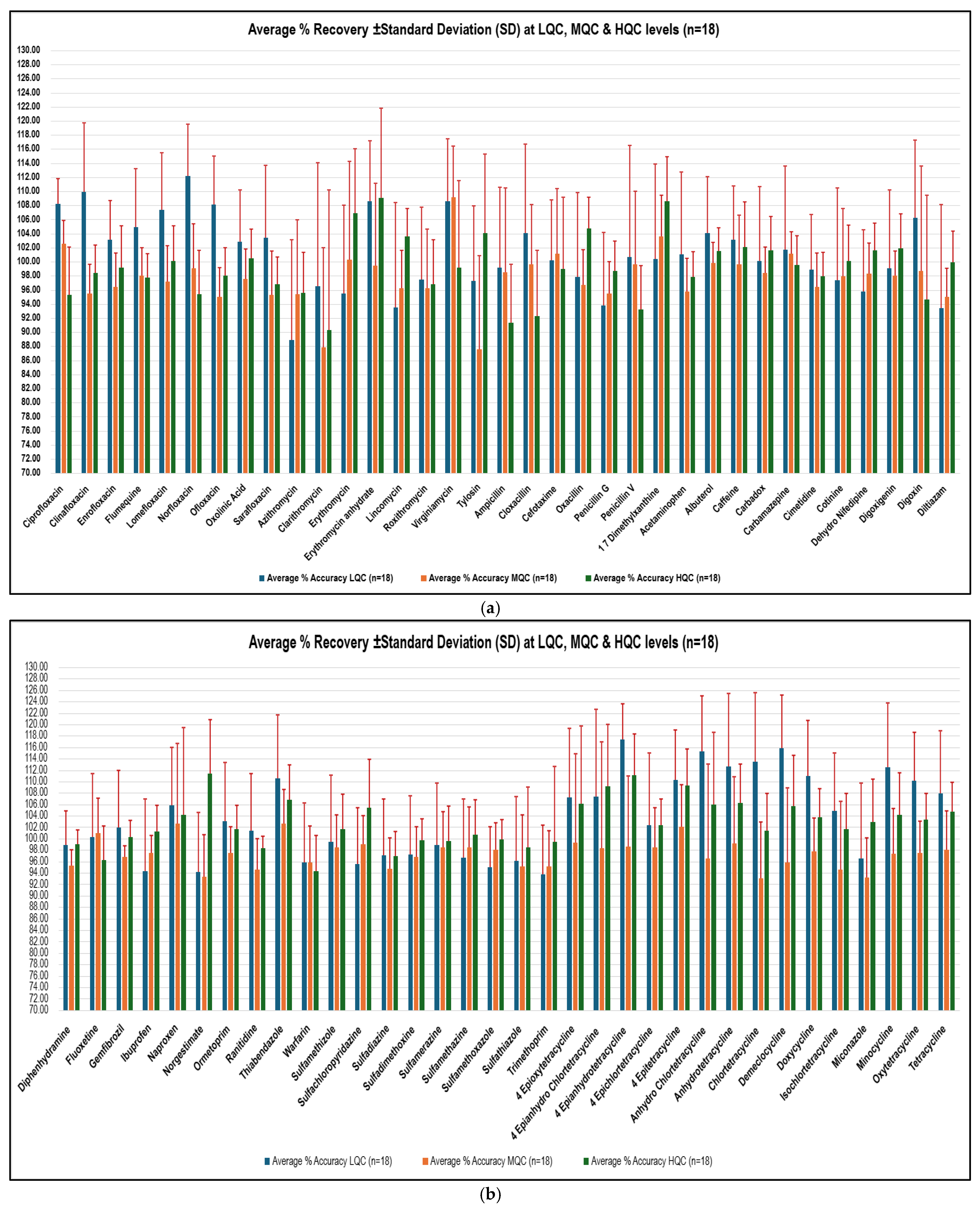
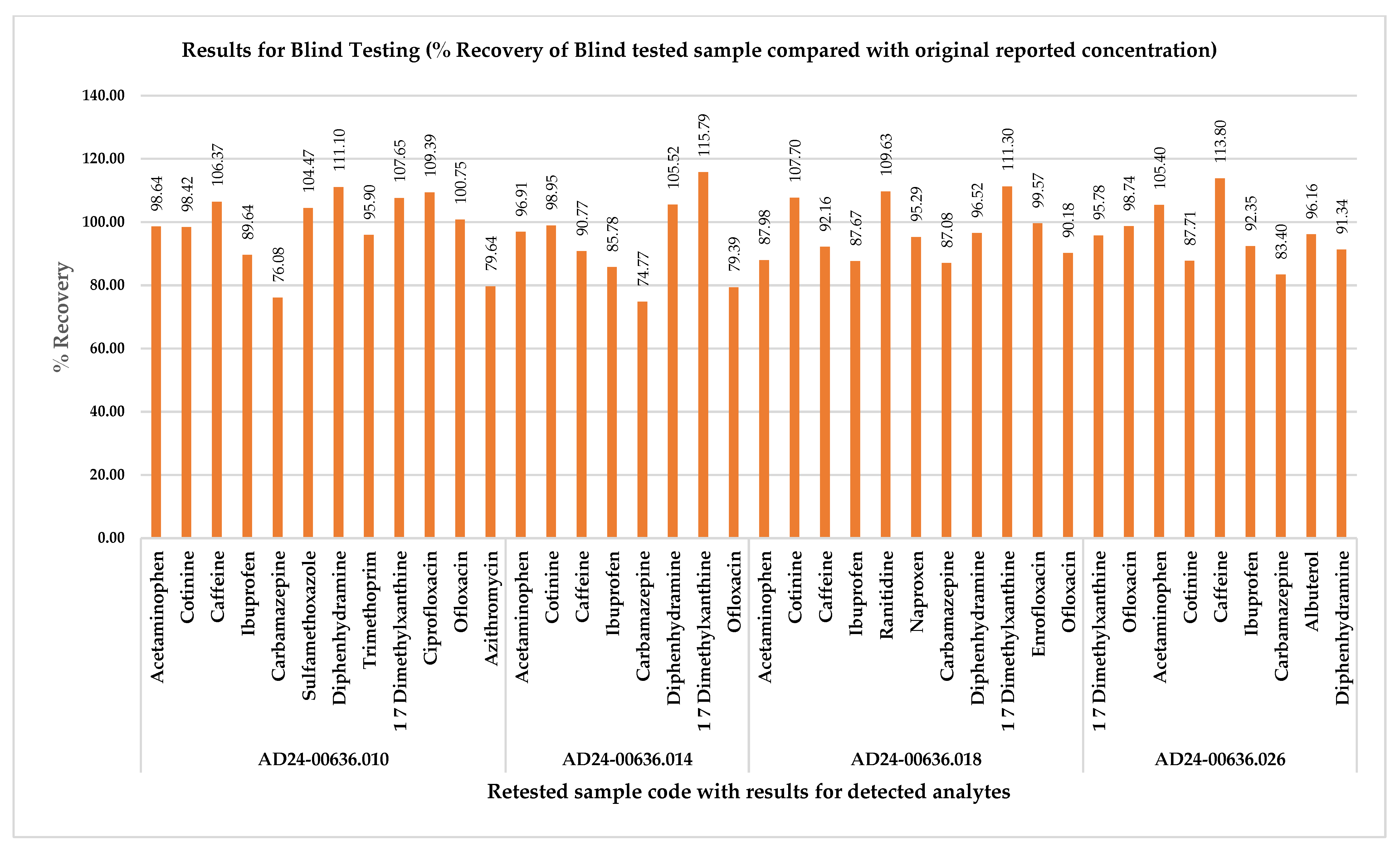
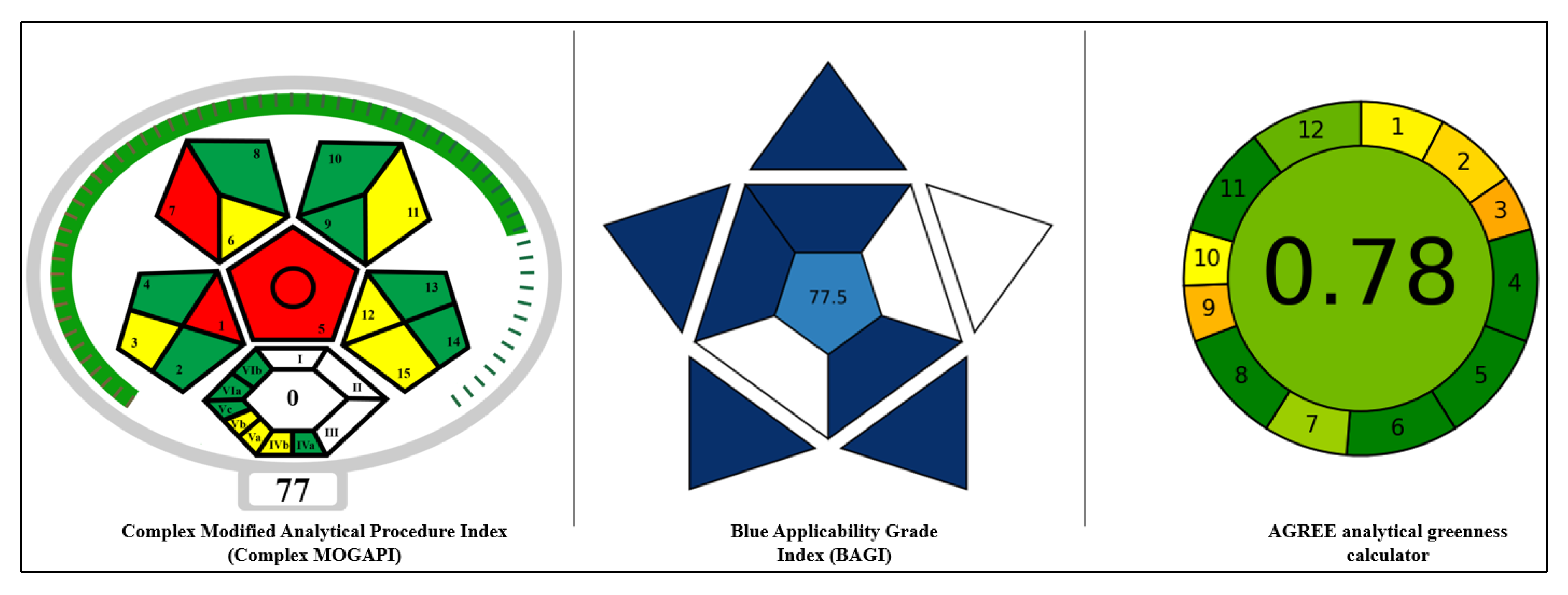
2.4. Method Greenness and Its Practicality
3. Materials and Methods
3.1. Materials and Reagents
3.2. Instrumentation
3.3. Mass Spectrometric and Liquid Chromatographic Method Optimization
3.4. Wastewater Matrix Blank Preparation Protocol
3.5. Evaluation of SPE Cartridges for Extraction Efficiency
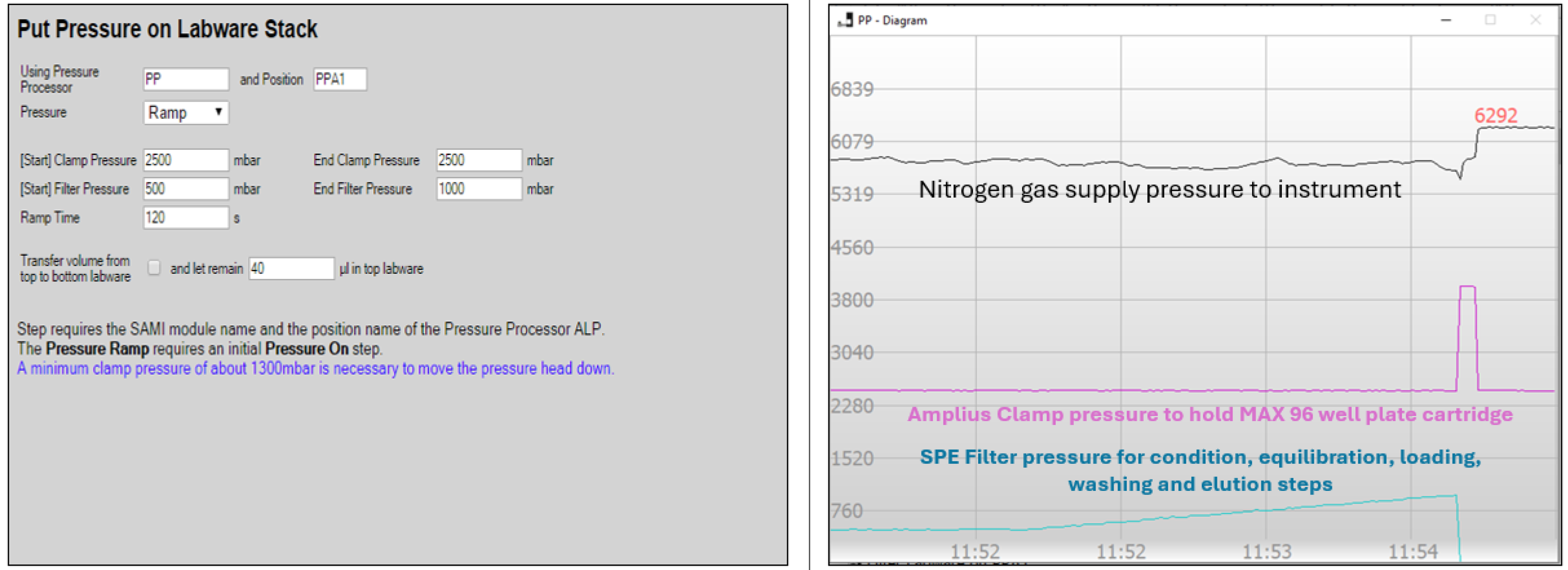
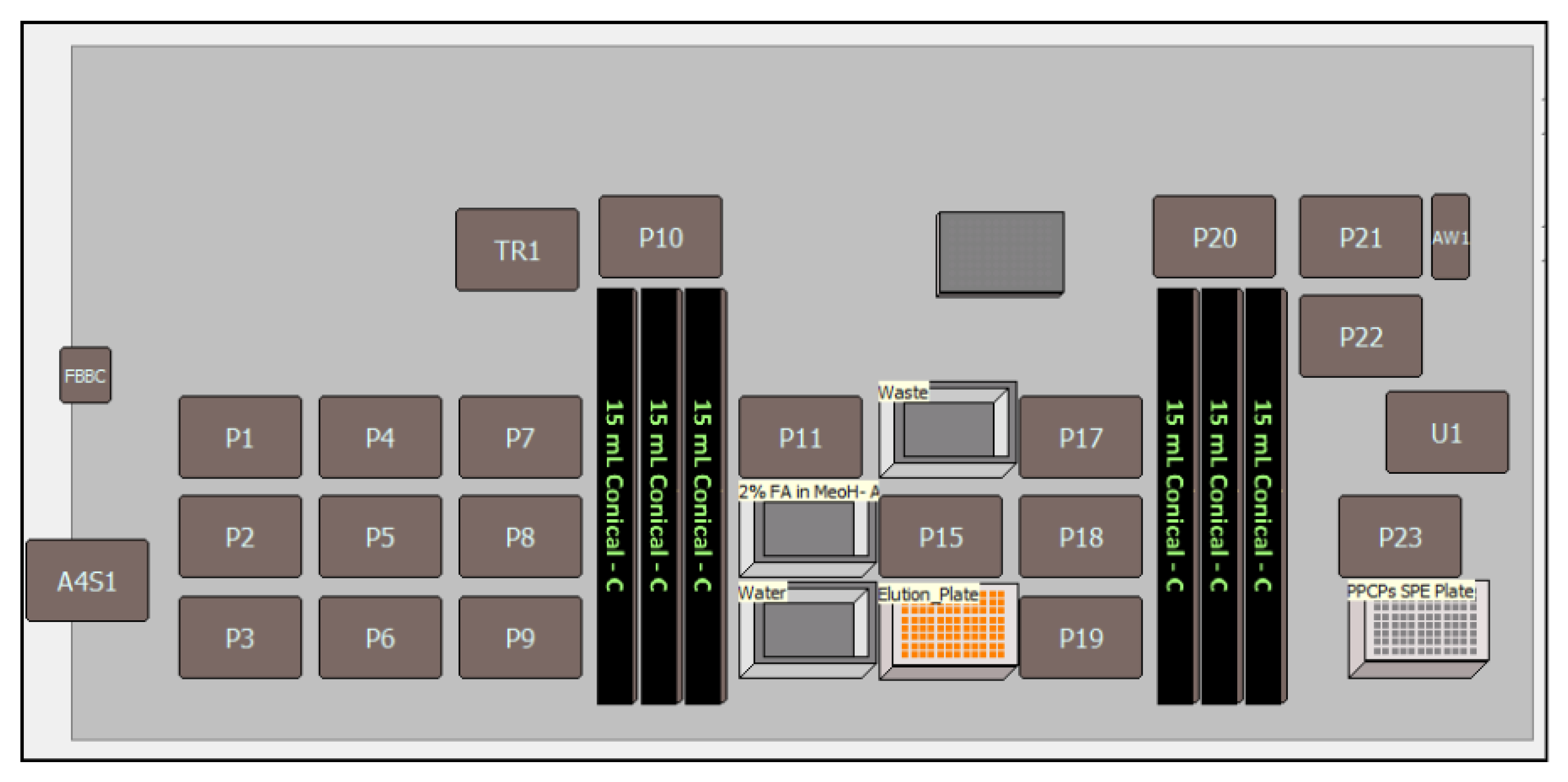
3.6. Sample Extraction Automation
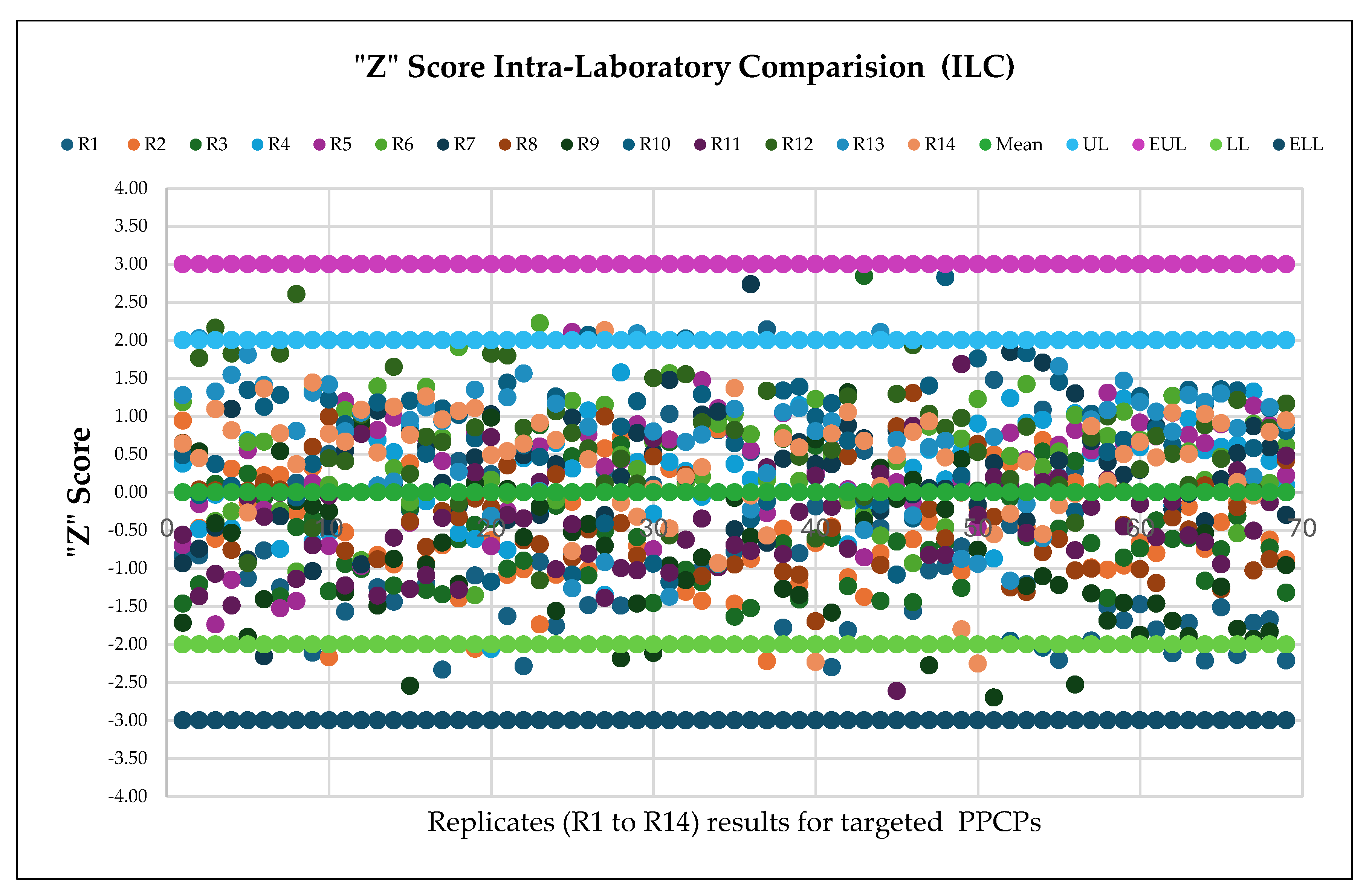
3.7. Estimation of Measurement Uncertainty (MU)
3.8. Data Analysis, Calculation, and Representations
3.9. Evaluation of the Method Greenness and Its Applicability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nandi, A.; Pecetta, S.; Bloom, D.E. Global Antibiotic Use during the COVID-19 Pandemic: Analysis of Pharmaceutical Sales Data from 71 Countries, 2020–2022. eClinicalMedicine 2023, 57, 101848. [Google Scholar] [CrossRef]
- Quireyns, M.; Boogaerts, T.; Wichelen, N.V.; Pussig, B.; Loof, H.D.; Covaci, A.; Nuijs, A.V. Temporal Monitoring of Pharmaceutical Consumption Using a Wastewater-Based Epidemiologic Approach. Ann. Toxicol. Anal. 2022, 34, S72. [Google Scholar] [CrossRef]
- Ortúzar, M.; Esterhuizen, M.; Olicón-Hernández, D.R.; González-López, J.; Aranda, E. Pharmaceutical Pollution in Aquatic Environments: A Concise Review of Environmental Impacts and Bioremediation Systems. Front. Front. Front. Microbiol. 2022, 13, 869332. [Google Scholar] [CrossRef]
- Gewurtz, S.B.; Auyeung, A.S.; Teslic, S.; Smyth, S.A. Pharmaceuticals and Personal Care Products in Canadian Municipal Wastewater and Biosolids: Occurrence, Fate, and Time Trends 2010–2013 to 2022. Environ. Sci. Pollut. Res. 2025, 32, 5022–5039. [Google Scholar] [CrossRef] [PubMed]
- Ohoro, C.R.; Adeniji, A.O.; Okoh, A.I.; Okoh, O.O. Distribution and Chemical Analysis of Pharmaceuticals and Personal Care Products (PPCPs) in the Environmental Systems: A Review. Int. J. Environ. Res. Public Health 2019, 16, 3026. [Google Scholar] [CrossRef] [PubMed]
- BIO Intelligence Service. Study on the Environmental Risks of Medicinal Products; Final Report prepared for Executive Agency for Health and Consumers; BIO Intelligence Service: Paris, France, 2013; Available online: https://ec.europa.eu/health/sites/health/files/files/environment/study_environment.pdf (accessed on 19 December 2024).
- Omuferen, L.O.; Maseko, B.; Olowoyo, J.O. Occurrence of Antibiotics in Wastewater from Hospital and Convectional Wastewater Treatment Plants and Their Impact on the Effluent Receiving Rivers: Current Knowledge between 2010 and 2019. Environ. Monit. Assess. 2022, 194, 306. [Google Scholar] [CrossRef]
- Yang, X.; Flowers, R.C.; Weinberg, H.S.; Singer, P.C. Occurrence and Removal of Pharmaceuticals and Personal Care Products (PPCPs) in an Advanced Wastewater Reclamation Plant. Water Res. 2011, 45, 5218–5228. [Google Scholar] [CrossRef]
- Molnarova, L.; Halesova, T.; Tomesova, D.; Vaclavikova, M.; Bosakova, Z. Monitoring Pharmaceuticals and Personal Care Products in Healthcare Effluent Wastewater Samples and the Effectiveness of Drug Removal in Wastewater Treatment Plants Using the UHPLC-MS/MS Method. Mol. Annu. 2024, 29, 1480. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.-H. Occurrence and Fate of Emerging Contaminants in Municipal Wastewater Treatment Plants from Different Geographical Regions-a Review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef]
- Nasri, E.; Cristina, A.; Martí, C.B.; Mansour, H.B.; Diaz-Cruz, M.S. Pharmaceuticals and Personal Care Products in Tunisian Hospital Wastewater: Occurrence and Environmental Risk. Environ. Sci. Pollut. Res. Int. 2023, 31, 2716–2731. [Google Scholar] [CrossRef]
- Murray, C.J.L. Global Burden of Bacterial Antimicrobial Resistance 1990–2021: A Systematic Analysis with Forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- Daughton, C.G. Pharmaceuticals and Personal Care Products in the Environment: Overarching Issues and Overview. In Pharmaceuticals and Care Products in the Environment; ACS Symposium Series; ACS Publications: Washington, DC, USA, 2001; pp. 2–38. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Proctor, K.; Jagadeesan, K.; Lopardo, L.; O’Daly, K.J.; Standerwick, R.; Barden, R. Estimation of Community-Wide Multi-Chemical Exposure via Water-Based Chemical Mining: Key Research Gaps Drawn from a Comprehensive Multi-Biomarker Multi-City Dataset. Environ. Int. 2021, 147, 106331. [Google Scholar] [CrossRef]
- Boogaerts, T.; Ahmed, F.; Choi, P.M.; Tscharke, B.; O’Brien, J.; De Loof, H.; Gao, J.; Thai, P.; Thomas, K.; Mueller, J.F.; et al. Current and Future Perspectives for Wastewater-Based Epidemiology as a Monitoring Tool for Pharmaceutical Use. Sci. Total Environ. 2021, 789, 148047. [Google Scholar] [CrossRef]
- Duan, L.; Zhang, Y.; Wang, B.; Yu, G.; Gao, J.; Cagnetta, G.; Huang, C.; Zhai, N. Wastewater Surveillance for 168 Pharmaceuticals and Metabolites in a WWTP: Occurrence, Temporal Variations and Feasibility of Metabolic Biomarkers for Intake Estimation. Water Res. 2022, 216, 118321. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Bello, D.; Pagsuyoin, S. Long-Term Wastewater-Based Surveillance and Impacts of the COVID-19 Pandemic on Drug Use Trends in a U.S. Northeast Rural Town. Sci. Total Environ. 2023, 877, 162806. [Google Scholar] [CrossRef]
- Ting, T.-T.; Chen, P.-C.; Chang, Y.-C.; Chiang, P.-J.; Li, H.-C.; Chen, S.-H.; Chen, P.-C.; Chu, H.-T.; Chuang, P.-Y.; Liu, Y.-H.; et al. Wastewater-Based Epidemiology to Monitor 68 NPS/Conventional Drug Use in Taipei Metropolitan Area in Taiwan during and after COVID-19 Pandemic. J. Hazard. Mater. 2024, 476, 135020. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency—Safe Drinking Water Act. Contaminant Candidate List 4-CCL 4. Available online: https://www.epa.gov/ccl/ccl-4-chemical-contaminants (accessed on 22 February 2021).
- United States Environmental Protection Agency—Safe Drinking Water Act Contaminant Candidate List 3—CCL 3|US EPA. Available online: https://www.epa.gov/ccl/contaminant-candidate-list-3-ccl-3#ccl3-list (accessed on 4 January 2016).
- EU Commission Implementing Decision (EU) 2022/1307 of 22 July 2022. Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council (Notified under Document C(2022) 5098). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32022D1307 (accessed on 19 December 2024).
- Belay, M.H.; Precht, U.; Mortensen, P.; Marengo, E.; Robotti, E. A Fully Automated Online SPE-LC-MS/MS Method for the Determination of 10 Pharmaceuticals in Wastewater Samples. Toxics 2022, 10, 103. [Google Scholar] [CrossRef]
- Wilschnack, M.; Homer, B.; Cartmell, E.; Yates, K.; Petrie, B. Targeted Multi-Analyte UHPLC-MS/MS Methodology for Emerging Contaminants in Septic Tank Wastewater, Sludge and Receiving Surface Water. Anal. Methods 2024, 16, 709–720. [Google Scholar] [CrossRef]
- Batt, A.L.; Kostich, M.S.; Lazorchak, J.M. Analysis of Ecologically Relevant Pharmaceuticals in Wastewater and Surface Water Using Selective Solid-Phase Extraction and UPLC−MS/MS. Anal. Chem. 2008, 80, 5021–5030. [Google Scholar] [CrossRef]
- Yao, W.; Ge, J.; Hu, Q.; Ma, J.; Yuan, D.; Fu, X.; Qi, Y.; Volmer, D.A. An Advanced LC–MS/MS Protocol for Simultaneous Detection of Pharmaceuticals and Personal Care Products in the Environment. Rapid Commun. Mass Spectrom. 2022, 37, e9397. [Google Scholar] [CrossRef]
- Semreen, M.; Shanableh, A.; Semerjian, L.; Alniss, H.; Mousa, M.; Bai, X.; Acharya, K. Simultaneous Determination of Pharmaceuticals by Solid-Phase Extraction and Liquid Chromatography-Tandem Mass Spectrometry: A Case Study from Sharjah Sewage Treatment Plant. Molecules 2019, 24, 633. [Google Scholar] [CrossRef]
- Fokunang, E.T.; Nguidjoe, E.M.; Kathleenngu, K.; Fokunang, E.T.; Fokunang, C.N. Detection and Analysis of Pharmaceutical Products from the Waste Water System at the University Teaching Hospital of Yaoundé, Cameroon. MOJ Toxicol. 2018, 4, 1. [Google Scholar] [CrossRef]
- EPA Method 1694: Pharmaceuticals and Personal Care Products in Water, Soil, Sediment, and Biosolids by HPLC/MS/MS. EPA, 2007. Available online: https://www.epa.gov/sites/default/files/2015-10/documents/method_1694_2007.pdf (accessed on 19 December 2024).
- ISO/IEC 17025:2017; General Requirements for the Competence of Testing and Calibration Laboratories. International Organization for Standardization: Geneva, Switzerland, 2017.
- Oertel, R.; Baldauf, J.; Rossmann, J. Development and Validation of a Hydrophilic Interaction Liquid Chromatography-Tandem Mass Spectrometry Method for the Quantification of the Antidiabetic Drug Metformin and Six Others Pharmaceuticals in Wastewater. J. Chromatogr. A J. Chromatogr. 2018, 1556, 73–80. [Google Scholar] [CrossRef]
- de Jongh, C.M.; Kooij, P.J.; de Voogt, P.; ter Laak, T.L. Screening and Human Health Risk Assessment of Pharmaceuticals and Their Transformation Products in Dutch Surface Waters and Drinking Water. Sci. Total Environ. 2012, 427–428, 70–77. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef] [PubMed]
- Mansour, F.R.; Omer, K.M.; Płotka-Wasylka, J. A Total Scoring System and Software for Complex Modified GAPI (ComplexMoGAPI) Application in the Assessment of Method Greenness. Green Anal. Chem. 2024, 10, 100126. [Google Scholar] [CrossRef]
- Manousi, N.; Wojnowski, W.; Płotka-Wasylka, J.; Samanidou, V. Blue Applicability Grade Index (BAGI) and Software: A New Tool for the Evaluation of Method Practicality. Green Chem. Chem. 2023, 25, 7598–7604. [Google Scholar] [CrossRef]
- Safont, D.F.; Marín, E.G.; Ibáñez, M.; Pitarch, E.; Hernández, F. Analytical Key Issues and Challenges in the LC-MS/MS Determination of Antibiotics in Wastewater. Anal. Chim. Acta 2023, 1239, 340739. [Google Scholar] [CrossRef]
- Bozlee, M. Novel Sample Preparation and GC–MS/MS Analysis of Triclosan and Methyl Triclosan in Biosolids. Chromatogr. Online 2018, 35, 28–33. Available online: https://www.chromatographyonline.com/view/novel-sample-preparation-and-gc-msms-analysis-triclosan-and-methyl-triclosan-biosolids-0 (accessed on 19 December 2024).
- Tohidi, F.; Cai, Z. GC/MS Analysis of Triclosan and Its Degradation By-Products in Wastewater and Sludge Samples from Different Treatments. Environ. Sci. Pollut. Res. 2015, 22, 11387–11400. [Google Scholar] [CrossRef]
- Baker, D.R.; Kasprzyk-Hordern, B. Multi-Residue Analysis of Drugs of Abuse in Wastewater and Surface Water by Solid-Phase Extraction and Liquid Chromatography–Positive Electrospray Ionisation Tandem Mass Spectrometry. J. Chromatogr. A 2011, 1218, 1620–1631. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.A.; Shrivastav, P.S.; Sharma, V.; Yadav, M. Challenges in Simultaneous Extraction and Chromatographic Separation of Metformin and Three SGLT-2 Inhibitors in Human Plasma Using LC–MS/MS. J. Pharm. Biomed. Anal. 2019, 175, 112790. [Google Scholar] [CrossRef]
- Shah, P.A.; Shrivastav, P.S. Ion-Pair Solid Phase Extraction for the Simultaneous Separation and Quantitation of Metformin and Canagliflozin in Human Plasma by LC-MS/MS. Microchem. J. 2018, 143, 181–189. [Google Scholar] [CrossRef]
- Rozman, D.; Hrkal, Z.; Váňa, M.; Vymazal, J.; Boukalová, Z. Occurrence of Pharmaceuticals in Wastewater and Their Interaction with Shallow Aquifers: A Case Study of Horní Beřkovice, Czech Republic. Water 2017, 9, 218. [Google Scholar] [CrossRef]
- Patrolecco, L.; Capri, S.; Ademollo, N. Occurrence of Selected Pharmaceuticals in the Principal Sewage Treatment Plants in Rome (Italy) and in the Receiving Surface Waters. Environ. Sci. Pollut. Res. 2014, 22, 5864–5876. [Google Scholar] [CrossRef] [PubMed]
- Shraim, A.; Diab, A.; Alsuhaimi, A.; Niazy, E.; Metwally, M.; Amad, M.; Sioud, S.; Dawoud, A. Analysis of Some Pharmaceuticals in Municipal Wastewater of Almadinah Almunawarah. Arab. J. Chem. 2017, 10, S719–S729. [Google Scholar] [CrossRef]
- Asimakopoulos, A.G.; Kannan, P.; Higgins, S.; Kannan, K. Determination of 89 Drugs and Other Micropollutants in Unfiltered Wastewater and Freshwater by LC-MS/MS: An Alternative Sample Preparation Approach. Anal. Bioanal. Chem. 2017, 409, 6205–6225. [Google Scholar] [CrossRef]
- SANTE 11312/2021 v2 2024, Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed, EURL for Residues of Pesticides. 2024. Available online: http://food.ec.europa.eu/system/files/2023-11/pesticides_mrl_guidelines_wrkdoc_2021-11312.pdf (accessed on 19 December 2024).
- ISO 13528:2022; Statistical Methods for Use in Proficiency Testing by Interlaboratory Comparison. International Organization for Standardization: Geneva, Switzerland, 2022. Available online: https://www.iso.org/standard/78879.html (accessed on 19 December 2024).
- Gómez-Canela, C.; Edo, S.; Rodríguez, N.; Gotor, G.; Lacorte, S. Comprehensive Characterization of 76 Pharmaceuticals and Metabolites in Wastewater by LC-MS/MS. Chemosensors 2021, 9, 273. [Google Scholar] [CrossRef]
- Paíga, P.; Correia, M.; Fernandes, M.J.; Silva, A.; Carvalho, M.; Vieira, J.; Jorge, S.; Silva, J.G.; Freire, C.; Delerue-Matos, C. Assessment of 83 Pharmaceuticals in WWTP Influent and Effluent Samples by UHPLC-MS/MS: Hourly Variation. Sci. Total Environ. 2019, 648, 582–600. [Google Scholar] [CrossRef]
- Shinde, D.; Murugan, V.; Dhagat, U.; Gupta, P.; Tadala, R.; Karubothula, B.; Golebiewska, I.; Salem, S.B.; Elamin, W.; Brudecki, G. Introduction of a Small Volume Ethyl Acetate Based Liquid-Liquid Extraction Procedure for Analysis of Polycyclic Aromatic Hydrocarbons in Wastewater by Atmospheric Pressure Gas Chromatography-Mass Spectrometry and Evaluation of Method Greenness. J. Chromatogr. A 2025, 1740, 465563. [Google Scholar] [CrossRef]
- Evaluation of Measurement Data—Guide to the Expression of Uncertainty in Measuremen; Joint Committee for Guides in Metrology (JCGM): Sèvres, France, 2008. [CrossRef]
- EURACHEM/CITAC Guide CG 4, 2012. Quantifying Uncertainty in Analytical Measurement Third Edition. URL. Available online: https://www.eurachem.org/index.php/publications/guides/quam (accessed on 19 December 2024).
| Sr. No. | Name of the PPCPs (Ionization) | Class of PPCPs | Details of MRM Parameters, Retention Time (RT), and Ion Ratios (PPCPs and IS) | Evaluation of SPE Efficiency of Targeted PPCPs (Average Area at MQC, n = 4) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parent Ion |
Product Ion (Q1, Q2) | CV (V) | CE (Q1, Q2) (eV) | Within Batch Stability (n = 26) | Details of the Internal Standard (IS) Used for Quantification | |||||||||||
| Ion Ratio (Q2/Q1 ± % RSD) | RT ± Stdev | Name of IS Used for Quantification | MRM Transition of IS | RT ± Stdev (IS) | CV (V) | CE (eV) | MAX | HLB | MCX | |||||||
| 1 | Ciprofloxacin | Fluoroquinolones | 332.1 | 288.1, 314.1 | 35 | 22, 18 | 0.9802 ± 3.39 | 3.11 ± 0.007 | Flumequine-13C3 | 265.1 > 247.03 | 5.84 ± 0.006 | 30 | 15 | 1,056,301 a | 645,745 b | 143,687 c |
| 2 | Clinafloxacin | 366.0 | 304.94, 347.79 | 15 | 20, 18 | 0.4452 ± 9.69 | 3.56 ± 0.012 | Flumequine-13C3 | 265.1 > 247.03 | 5.84 ± 0.006 | 30 | 15 | 414,501 a | 300,808 b | 88,168 c | |
| 3 | Enrofloxacin | 360.3 | 316.16, 245.11 | 12 | 19, 25 | 0.5257 ± 5.18 | 3.26 ± 0.013 | Flumequine-13C3 | 265.1 > 247.03 | 5.84 ± 0.006 | 30 | 15 | 1,255,182 a | 930,841 b | 295,111 c | |
| 4 | Flumequine | 262.1 | 201.96, 125.95 | 15 | 31, 45 | 0.3903 ± 3.05 | 5.84 ± 0.007 | Flumequine-13C3 | 265.1 > 247.03 | 5.84 ± 0.006 | 30 | 15 | 2,210,158 a | 1,648,106 b | 665,288 c | |
| 5 | Lomefloxacin | 352.1 | 265.02, 236.93 | 44 | 22, 34 | 0.3844 ± 4.40 | 3.22 ± 0.013 | Flumequine-13C3 | 265.1 > 247.03 | 5.84 ± 0.006 | 30 | 15 | 1,114,359 a | 979,694 b | 280,779 c | |
| 6 | Norfloxacin | 320.1 | 233.05, 204.83 | 15 | 24, 30 | 0.3726 ± 9.92 | 3.05 ± 0.005 | Flumequine-13C3 | 265.1 > 247.03 | 5.84 ± 0.006 | 30 | 15 | 250,767 a | 132,066 b | 15,024 c | |
| 7 | Ofloxacin | 362.1 | 261.02, 218.62 | 44 | 34, 26 | 0.0654 ± 8.84 | 3.04 ± 0.003 | Flumequine-13C3 | 265.1 > 247.03 | 5.84 ± 0.006 | 30 | 15 | 1,400,463 a | 1,106,513 b | 310,326 c | |
| 8 | Oxolinic Acid | 262.1 | 243.92, 159.96 | 25 | 20, 36 | 0.1559 ± 4.13 | 4.75 ± 0.005 | Flumequine-13C3 | 265.1 > 247.03 | 5.84 ± 0.006 | 30 | 15 | 3,521,015 a | 2,622,939 b | 308,513 c | |
| 9 | Sarafloxacin | 386.1 | 342.18, 299.09 | 35 | 18, 25 | 0.8187 ± 3.72 | 3.47 ± 0.014 | Flumequine-13C3 | 265.1 > 247.03 | 5.84 ± 0.006 | 30 | 15 | 239,738 a | 146,017 b | 66,503 c | |
| 10 | Azithromycin | Macrolides | 749.5 | 158.04, 82.98 | 45 | 40, 38 | 0.8659 ± 4.99 | 3.88 ± 0.004 | Azithromycin-13C D3 | 753.7 > 115.92 | 3.88 ± 0.006 | 35 | 36 | 1,346,648 c | 2,792,538 b | 3,235,748 a |
| 11 | Clarithromycin | 748.3 | 590.14, 158.09 | 20 | 16, 16 | 1.1556 ± 6.67 | 5.83 ± 0.005 | Roxithromycin-D7 | 844.7 > 157.99 | 5.91 ± 0.002 | 35 | 27 | 4,986,458 b | 6,314,206 a | 4,720,949 c | |
| 12 | Erythromycin (Negative) | 734.4 | 157.98, 576.37 | 40.0 | 32, 18 | 0.4046 ± 7.55 | 5.18 ± 0.005 | Erythromycin 13C D3 | 738.4 > 162.4 | 5.18 ± 0.005 | 15 | 30 | 697,088 b | 1,088,683 a | 450,116 c | |
| 13 | Erythromycin Anhydrate | 716.4 | 158.0, 116.0 | 33.0 | 45, 30 | 0.5518 ± 2.85 | 5.58 ± 0.005 | Erythromycin 13C D3 | 738.4 > 162.4 | 5.18 ± 0.005 | 15 | 30 | 7,158,761 c | 10,217,700 a | 8,098,076 b | |
| 14 | Lincomycin | 407.1 | 125.99, 359.12 | 38.0 | 24, 18 | 0.0858 ± 4.97 | 2.89 ± 0.004 | NA | NA | NA | NA | NA | 3,781,706 a | 1,610,207 b | 3,905,783 a | |
| 15 | Roxithromycin | 837.5 | 679.43, 158.04 | 44.0 | 20, 34 | 0.5391 ± 4.10 | 5.94 ± 0.006 | Roxithromycin-D7 | 844.7 > 157.99 | 5.91 ± 0.002 | 35 | 27 | 4,139,132 c | 7,468,155 a | 6,084,984 b | |
| 16 | Virginiamycin | 526.1 | 508.13, 355.05 | 30.0 | 12, 17 | 0.4833 ± 5.35 | 6.61 ± 0.003 | Roxithromycin-D7 | 844.7 > 157.99 | 5.91 ± 0.002 | 35 | 27 | 2,320,324 b | 6,819,360 a | 7,299,741 a | |
| 17 | Tylosin | 916.5 | 174.1, 101.1 | 45.0 | 40, 45 | 0.2002 ± 6.57 | 5.41 ± 0.005 | NA | NA | NA | NA | NA | 2,639,936 b | 5,072,795 a | 2,023,093 c | |
| 18 | Ampicillin | Penicillins | 350.0 | 105.95, 159.94 | 28.0 | 16, 10 | 0.3503 ± 4.08 | 3.07 ± 0.006 | NA | NA | NA | NA | NA | 74,200 c | 281,386 a | 235,753 b |
| 19 | Cloxacillin | 436.2 | 160.0, 277.0 | 50.0 | 22, 40 | NA | 2.21 ± 0.006 | NA | NA | NA | NA | NA | 722,367 c | 1,060,749 b | 1,327,762 a | |
| 20 | Cefotaxime | 456.0 | 395.89, 323.92 | 25.0 | 10, 14 | 0.6454 ± 3.45 | 3.77 ± 0.008 | NA | NA | NA | NA | NA | 586,648 c | 2,698,762 b | 4,045,124 a | |
| 21 | Oxacillin | 402.3 | 143.97, 185.98 | 69.0 | 20, 15 | 0.3323 ± 5.57 | 6.49 ± 0.005 | NA | NA | NA | NA | NA | 2,009,376 a | 1,988,415 a | 2,339,547 b | |
| 22 | Penicillin G | 335.0 | 217.13, 220.14 | 61.0 | 14, 13 | 0.2187 ± 3.59 | 5.65 ± 0.005 | NA | NA | NA | NA | NA | 3,299,258 b | 3,444,161 ab | 3,667,462 a | |
| 23 | Penicillin V | 351.1 | 106.00, 160.01 | 30.0 | 20, 14 | 0.3252 ± 6.85 | 3.06 ± 0.006 | NA | NA | NA | NA | NA | 8752 c | 33,885 a | 28,001 b | |
| 24 | 1 7 Dimethylxanthine | Other PPCPs | 181.1 | 124.00, 68.92 | 30.0 | 18, 18 | 0.1207 ± 13.19 | 2.84 ± 0.006 | NA | NA | NA | NA | NA | 23,050,434 b | 27,725,049 a | 23,068,096 b |
| 25 | Acetaminophen | 152.1 | 110.14, 64.84 | 35.0 | 14, 25 | 0.1474 ± 4.13 | 2.70 ± 0.005 | Acetaminophen-13C2 | 155.0 > 111 | 2.70 ± 0.005 | 15 | 16 | 53,102,347 c | 62,178,936 a | 56,092,384 b | |
| 26 | Albuterol | 240.2 | 147.99, 165.94 | 24.0 | 16, 12 | 0.2856 ± 3.33 | 2.39 ± 0.003 | NA | NA | NA | NA | NA | 2,656,223 a | 324,846 c | 2,443,745 b | |
| 27 | Caffeine | 195.0 | 137.97,123.2 | 15.0 | 20, 20 | 0.0164 ± 6.32 | 3.24 ± 0.009 | Caffeine-13C3 | 198 > 140 | 3.24 ± 0.008 | 20 | 15 | 4,528,989 b | 5,095,626 a | 4,440,410 b | |
| 28 | Carbadox | 263.0 | 230.8, 129.88 | 30.0 | 13, 20 | 0.8011 ± 5.64 | 3.53 ± 0.009 | NA | NA | NA | NA | NA | 889,938 b | 701,716 c | 1,156,898 a | |
| 29 | Carbamazepine | 237.0 | 178.9, 165.04 | 38.0 | 34, 36 | 0.7634 ± 3.25 | 5.91 ± 0.006 | NA | NA | NA | NA | NA | 1,356,458 b | 1,310,734 b | 1,427,801 a | |
| 30 | Cimetidine | 253.1 | 158.97, 94.96 | 26 | 12, 24 | 1.0375 ± 3.03 | 2.41 ± 0.005 | Cimetidine-D3 | 256.1 > 162.04 | 2.41 ± 0.005 | 25 | 14 | 1,531,346 a | 380,811 b | 1,479,030 a | |
| 31 | Cotinine | 176.7 | 79.86, 97.86 | 35 | 20, 20 | 0.2922 ± 11.53 | 2.30 ± 0.010 | Cotinine-D3 | 180 > 101 | 2.30 ± 0.008 | 15 | 16 | 15,740,823 b | 1,712,875 c | 18,535,503 a | |
| 32 | Dehydro Nifedipine | 344.9 | 283.98, 267.84 | 94 | 28, 28 | 0.3650 ± 4.32 | 6.47 ± 0.005 | NA | NA | NA | NA | NA | 1,033,433 a | 929,161 b | 1,088,647 a | |
| 33 | Digoxigenin | 391.5 | 355.3, 373.3 | 30 | 15, 10 | 0.6746 ± 2.82 | 4.55 ± 0.005 | NA | NA | NA | NA | NA | 653,080 c | 769,604 b | 911,624 a | |
| 34 | Digoxin | 803.3 | 282.9 | 49 | 45 | NA | 6.33 ± 0.003 | NA | NA | NA | NA | NA | 33,110 a | 25,353 c | 27,905 b | |
| 35 | Diltiazam | 415.1 | 177.95, 149.92 | 36 | 24, 28 | 0.1044 ± 13.18 | 4.94 ± 0.006 | Diltiazam-D3 | 418.2 > 177.9 | 4.93 ± 0.006 | 25 | 22 | 2,965,188 c | 5,532,001 b | 5,924,272 a | |
| 36 | Diphenhydramine | 256.2 | 166.97, 151.88 | 15 | 12, 32 | 0.2653 ± 2.88 | 4.63 ± 0.006 | Diphenhydramine-D3 | 259.2 > 166.97 | 4.63 ± 0.006 | 20 | 20 | 2,445,338 b | 2,827,255 a | 2,904,373 a | |
| 37 | Fluoxetine | 310.2 | 251.99, 163.05 | 30 | 17, 17 | 0.9506 ± 3.39 | 6.83 ± 0.004 | Fluoxetine-D5 | 315.2 > 43.88 | 5.57 ± 0.006 | 25 | 8 | 1,720,777 a | 1,303,781 b | 1,828,129 a | |
| 38 | Gemfibrozil (Negative) | 249.0 | 121.0, 127.0 | 25 | 13, 10 | 0.0732 ± 1.50 | 8.22 ± 0.005 | NA | NA | NA | NA | NA | 902,455 a | 536,656 c | 730,348 b | |
| 39 | Ibuprofen (Negative) | 205.0 | 161.02 | 20 | 8.0 | NA | 7.57 ± 0.007 | NA | NA | NA | NA | NA | 195,532 a | 173,539 b | 196,722 a | |
| 40 | Naproxen | 231.1 | 184.99, 169.97 | 18 | 12, 24 | 0.3163 ± 13.33 | 6.69 ± 0.005 | NA | NA | NA | NA | NA | 1,468,498 a | 799,771 b | 1,508,501 a | |
| 41 | Norgestimate | 370.2 | 123.97, 90.99 | 40 | 32, 48 | 0.6474 ± 4.69 | 8.27 ± 0.007 | NA | NA | NA | NA | NA | 1,030,506 a | 750,127 b | 1,213,558 a | |
| 42 | Ormetoprim | 275.0 | 123.02, 259.08 | 30 | 25, 25 | 0.7967 ± 3.52 | 3.13 ± 0.006 | NA | NA | NA | NA | NA | 2,624,786 ab | 2807173 a | 2561420 b | |
| 43 | Ranitidine | 315.1 | 175.99, 124 | 20 | 17, 17 | 0.2723 ± 4.92 | 2.45 ± 0.005 | Ranitidine-D6 | 321.2 > 176.05 | 2.44 ± 0.005 | 30 | 16 | 2,757,507 b | 857,502 c | 3,015,978 a | |
| 44 | Thiabendazole | 202.0 | 130.96, 174.95 | 31 | 40, 40 | 0.2368 ± 6.10 | 3.42 ± 0.011 | NA | NA | NA | NA | NA | 1,197,504 a | 1,190,961 a | 1,311,527 a | |
| 45 | Warfarin (Negative) | 307.1 | 161.0, 250.0 | 35 | 20, 25 | 0.5237 ± 1.11 | 6.84 ± 0.004 | Warfarin-D5 | 312.1 > 160.82 | 6.82 ± 0.00 | 15 | 20 | 6431222 a | 5217781 b | 6727270 a | |
| 46 | Sulfamethizole | Sulfonamides | 271.1 | 156.0, 92.0 | 30 | 15, 25 | 0.6451 ± 3.36 | 3.61 ± 0.010 | Sulfamethizole-13C6 | 276.90 >162.02 | 3.61 ± 0.001 | 25 | 14 | 2,010,358 c | 2,261,471 b | 2,490,050 a |
| 47 | Sulfachloropyridazine | 285.1 | 155.93, 91.94 | 30 | 13, 29 | 0.6132 ± 4.50 | 4.01 ± 0.007 | NA | NA | NA | NA | NA | 1,943,737 a | 2,071,357 a | 1,986,850 a | |
| 48 | Sulfadiazine | 251.0 | 156.0, 92.0 | 30 | 15, 27 | 0.8029 ± 4.48 | 2.93 ± 0.006 | Sulfadiazine-13C6 | 257.0 > 162.04 | 2.93 ± 0.005 | 20 | 15 | 1,551,625 b | 2,002,372 a | 1,627,019 b | |
| 49 | Sulfadimethoxine | 311.1 | 156.0, 92.0 | 36 | 20, 32 | 0.4828 ± 3.19 | 4.87 ± 0.004 | Sulfadimethoxine-D6 | 317 > 162.15 | 4.84 ± 0.005 | 25 | 22 | 3,313,191 a | 3,058,455 a | 3,043,851 a | |
| 50 | Sulfamerazine | 265.1 | 156.0, 92.0 | 35 | 15, 25 | 0.9283 ± 11.57 | 3.33 ± 0.008 | Sulfamearazine_13C6 | 271. 1> 171.9 | 3.33 ± 0.008 | 25 | 16 | 1,334,396 b | 1,581,985 a | 1,409,520 b | |
| 51 | Sulfamethazine | 279.1 | 186.0, 124.1 | 40 | 15, 25 | 0.5960 ± 8.50 | 3.70 ± 0.007 | Sulfamethazine-13C6 | 285 > 186.03 | 3.69 ± 0.007 | 25 | 16 | 2,326,698 b | 2,511,412 a | 2,372,135 ab | |
| 52 | Sulfamethoxazole | 254.1 | 156.0, 92.0 | 30 | 15, 25 | 0.9546 ± 3.97 | 4.14 ± 0.007 | Sulfamethoxazole-13C6 | 260.1 > 161.96 | 4.14 ± 0.007 | 35 | 16 | 1,447,175 b | 1,601,116 a | 1,536,744 ab | |
| 53 | Sulfathiazole | 256.0 | 156.0, 92.0 | 31 | 15, 25 | 0.6875 ± 5.78 | 3.08 ± 0.003 | Sulfamethoxazole-13C6 | 260.1 > 161.96 | 4.14 ± 0.007 | 35 | 16 | 2,110,959 b | 2,716,660 a | 2,338,768 b | |
| 54 | Trimethoprim | 291.1 | 229.99, 123 | 45 | 22, 24 | 0.8770 ± 3.38 | 2.96 ± 0.006 | Trimethoprim 13C3 | 294 > 233 | 2.97 ± 0.006 | 45 | 25 | 1,501,819 a | 1,594,025 a | 1,525,977 a | |
| 55 | 4 Epioxytetracycline | Tetracyclines | 460.9 | 425.9, 443.15 | 28 | 18, 13 | 0.2978 ± 3.92 | 3.20 ± 0.009 | NA | NA | NA | NA | NA | 8,026,750 b | 8,922,119 a | 53,390 c |
| 56 | 4 Epianhydrochlortetracycline | 461.0 | 443.91, 97.88 | 25 | 20, 35 | 0.2380 ± 10.52 | 5.22 ± 0.006 | NA | NA | NA | NA | NA | 1,962,967 a | 2,263,661 a | 375,931 b | |
| 57 | 4 Epianhydrotetracycline | 427.2 | 410.2, 154.0 | 36 | 18, 34 | 0.0367 ± 5.39 | 4.45 ± 0.006 | NA | NA | NA | NA | NA | 17,573,393 a | 19,431,498 a | 1,535,024 b | |
| 58 | 4 Epichlortetracycline | 479.3 | 461.85, 97.88 | 25 | 18, 41 | 0.0543 ± 4.89 | 3.35 ± 0.012 | NA | NA | NA | NA | NA | 11,270,071 a | 9,947,026 b | 2,860,411 c | |
| 59 | 4 Epitetracycline | 445.5 | 410.0, 154.0 | 25 | 20, 25 | 0.0741 ± 5.99 | 3.12 ± 0.004 | NA | NA | NA | NA | NA | 12,960,601 b | 17,010,339 a | 107,167 c | |
| 60 | Anhydro Chlortetracycline | 461.0 | 443.92, 153.9 | 12 | 25, 25 | 0.7813 ± 11.68 | 6.68 ± 0.005 | NA | NA | NA | NA | NA | 1,552,990 a | 399,176 b | 374,544 b | |
| 61 | Anhydrotetracycline | 427.2 | 410.2, 154.0 | 36 | 16, 34 | 0.2444 ± 2.21 | 5.52 ± 0.005 | NA | NA | NA | NA | NA | 31,317,995 a | 11,519,591 b | 3,591,401 c | |
| 62 | Chlortetracycline | 479.3 | 461.85, 97.88 | 25 | 18, 41 | 1.2606 ± 7.42 | 4.25 ± 0.004 | NA | NA | NA | NA | NA | 1,659,364 b | 3,486,243 a | 17,343 c | |
| 63 | Demeclocycline | 465.1 | 430.01, 153.88 | 2 | 20, 28 | 0.9855 ± 4.01 | 3.82 ± 0.007 | NA | NA | NA | NA | NA | 3,952,343 b | 6,117,631 a | 35,931 c | |
| 64 | Doxycycline | 445.1 | 154.0, 428.1 | 20 | 28, 15 | 0.1852 ± 4.38 | 3.51 ± 0.008 | NA | NA | NA | NA | NA | 11,572,856 b | 16,328,640 a | 101,422 c | |
| 65 | Isochlortetracycline | 479.3 | 461.85, 97.88 | 25 | 18, 41 | 0.1985 ± 7.75 | 3.69 ± 0.009 | NA | NA | NA | NA | NA | 19,874,394 a | 15,761,242 b | 7,370,522 c | |
| 66 | Miconazole | 417.0 | 159.0, 161.0 | 20 | 19, 19 | 0.9912 ± 3.13 | 6.69 ± 0.001 | NA | NA | NA | NA | NA | 1,911,547 c | 3,001,115 b | 4,197,732 a | |
| 67 | Minocycline | 458.0 | 441.0, 352.0 | 20 | 15, 30 | 0.2533 ± 6.56 | 3.62 ± 0.010 | NA | NA | NA | NA | NA | 3,065,017 b | 4,035,484 a | 67,515 c | |
| 68 | Oxytetracycline | 461.0 | 426.03, 336.99 | 30 | 15, 28 | 0.2216 ± 2.93 | 3.44 ± 0.012 | NA | NA | NA | NA | NA | 11,368,429 b | 12,319,767 a | 141,176 c | |
| 69 | Tetracycline | 445.5 | 410.0, 154.0 | 25 | 20, 25 | 0.8208 ± 2.86 | 3.51 ± 0.010 | NA | NA | NA | NA | NA | 14,294,950 b | 20,725,639 a | 127,031 c | |
| Automated extraction of PPCPs in wastewater using Biomek i7 Workstation | Steps | Sample Preparation Task | ||
| Sample Preparation | Unknown wastewater samples and spiked (QC) wastewater samples with ISTD (0.100 mL) and extraction buffer (0.400 mL of 5% ammonia in ASTM type I water for PPCPs) were loaded in the 15 mL conical tube racks (samples 10.0 mL) and precleared by centrifugation at 4500 rpm in a model (Eppendorf). | |||
| Conditioning | Condition the MAX 96-well plate cartridge with 1.0 mL 100% MeOH. | |||
| Equilibration | Equilibrate the cartridge with 1.0 mL of 100% ASTM type I water. | |||
| Sample Loading | Load 1.5 mL of the sample and quality controls three times (4.5 mL) on respective MAX 96-well plate cartridge. | |||
| Washing | Wash 96 wells with 1.0 mL of 5% ammonia in ASTM type I water. Dry the cartridge at 1000 mbar for up to 10 min. | |||
| Elution | Elute 96-well plate with 0.5 mL of 2% formic acid in methanol–acetonitrile (50:50, v/v). | |||
| Dilution, Well Plates Sealing | Dilute the final elute sample with 0.750 mL of water and seal the cartridge plate with a4S 4titude automated role heat sealer and vortex mixture at lower rpm. | |||
| LC-MS/MS | Load sealed 96-well plate cartridge LC-MS/MS autosampler and inject 4 μL of sample volume. | |||
| Equipment Details | Ion Source | Z Spray XEVO Ion Source | ||
| Pump | Acquity UPLC-I Class Plus | |||
| Autosampler | FTN Sample Manager | |||
| Column Oven | Acquity UPLC Column Heater | |||
| LC Column | ACQUITY Premier BEH C18 Column, 1.7 µm, 2.1 mm × 100 mm | |||
| LC Parameters | Mobile Phase A | 0.1% acetic acid in water | ||
| Mobile Phase B | 0.1% acetic acid in methanol–acetonitrile [50/50, v/v] | |||
| Sample Purge | Methanol–acetonitrile–water–IPA [1:1:1:1, v/v/v/v] with 0.5% acetic acid | |||
| Sample Wash | Methanol–acetonitrile–water–IPA [1:1:1:1, v/v/v/v] with 0.5% acetic acid | |||
| Seal Wash | Methanol–water [10/90, v/v] | |||
| Flow Rate | 0.350 mL/minute | |||
| Column Oven | 55 ± 5 °C | |||
| Sample Manager | 10 ± 3 °C | |||
| Injection | 4.0 µL volume | |||
| LC Gradient | Flow | Time (min) | Pump A % | Pump B % |
| 0.35 | Initial | 100.00 | 0.00 | |
| 0.35 | 0.60 | 100.00 | 0.00 | |
| 0.35 | 2.00 | 80.00 | 20.00 | |
| 0.35 | 5.00 | 50.00 | 50.00 | |
| 0.35 | 5.50 | 50.00 | 50.00 | |
| 0.35 | 5.60 | 30.00 | 70.00 | |
| 0.35 | 8.00 | 10.00 | 90.00 | |
| 0.35 | 9.00 | 10.00 | 90.00 | |
| 0.35 | 9.10 | 2.00 | 98.00 | |
| 0.35 | 10.00 | 2.00 | 98.00 | |
| 0.35 | 10.10 | 100.00 | 0.00 | |
| 0.35 | 12.00 | 100.00 | 0.00 | |
| MS Parameters | Mode and polarity | ESI +/− | ||
| Scan type | (Segmented MRM) | |||
| Source temperature (°C) | 150.00 | |||
| Disolvation gas temp. (°C) | 600.00 | |||
| Disolvation gas flow (L/h) | 1100.00 | |||
| Cone gas flow (L/h) | 150.00 | |||
| Capillary voltage (kV) | 1.20 (Positive)/2.80 (Negative) | |||
| Nebulizer gas flow (bar) | 7.00 | |||
| Sr. No. | Name of the PPCPs | Class (PPCPs) | Results for Method Validation | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Specificity (%) | Matrix Effect (%) | Linearity Range ( µg/L) |
Coefficient of Determination (r2) and % Deviation from Back-Calculated Concentration of Linear Calibration Curve (Average of Results from Day 1, 2, and 3 Validation Trials) | E-LOD ( µg/L) | E-LOQ ( µg/L) | T-LOQ ( µg/L) | MU (@ Mean Calculated Concentration at LOQ ± MU) µg/L | |||||||||||
| r2 | L1 | L2 | L3 | L4 | L5 | L6 | L7 | L8 | ||||||||||
| 1 | Ciprofloxacin | Fluoroquinolones | 0.85 | 21.62 | 0.50–20.0 | 0.9870 | 5.73 | −16.03 | 5.13 | 8.68 | 8.46 | −0.71 | −4.76 | −6.56 | 0.022 | 0.067 | 0.500 | 0.530 ± 0.025 |
| 2 | Clinafloxacin | 0.99 | 8.61 | 0.10–4.0 | 0.9937 | −0.67 | −0.33 | 1.00 | 5.67 | 1.20 | −4.84 | −1.41 | −0.88 | 0.011 | 0.032 | 0.100 | 0.114 ± 0.009 | |
| 3 | Enrofloxacin | 0.34 | 15.36 | 0.05–2.0 | 0.9984 | 0.67 | −2.33 | −1.50 | 1.80 | 4.80 | 0.24 | 2.67 | −7.07 | 0.006 | 0.018 | 0.050 | 0.053 ± 0.005 | |
| 4 | Flumequine | 0.34 | −1.86 | 0.05–2.0 | 0.9973 | −2.67 | 5.33 | −4.33 | 2.73 | 4.50 | −1.92 | −0.53 | −3.75 | 0.009 | 0.027 | 0.050 | 0.050 ± 0.007 | |
| 5 | Lomefloxacin | 1.49 | 2.82 | 0.05–2.0 | 0.9969 | 1.33 | −3.00 | −2.83 | 4.80 | −0.97 | −0.80 | 0.51 | 0.27 | 0.009 | 0.028 | 0.050 | 0.054 ± 0.008 | |
| 6 | Norfloxacin | 6.32 | 18.17 | 0.50–20.0 | 0.9835 | 7.07 | −18.65 | 8.42 | 8.43 | 6.62 | −1.50 | −2.34 | −6.57 | 0.044 | 0.135 | 0.500 | 0.554 ± 0.038 | |
| 7 | Ofloxacin | 2.22 | 10.44 | 0.05–2.0 | 0.9923 | 4.00 | −9.67 | 0.17 | 6.40 | 3.37 | −2.80 | 0.38 | −2.10 | 0.008 | 0.024 | 0.050 | 0.054 ± 0.005 | |
| 8 | Oxolinic Acid | 0.79 | 0.68 | 0.05–2.0 | 0.9978 | −4.00 | 12.33 | −7.67 | −0.13 | 1.20 | −0.24 | 0.53 | −1.85 | 0.004 | 0.012 | 0.050 | 0.051 ± 0.003 | |
| 9 | Sarafloxacin | 1.48 | 5.84 | 0.10–4.0 | 0.9930 | 0.00 | 1.83 | −5.67 | 7.97 | 3.68 | −2.25 | −1.74 | −3.53 | 0.016 | 0.050 | 0.100 | 0.104 ± 0.013 | |
| 10 | Azithromycin | Macrolides | 0.22 | 22.12 | 1.0–40.0 | 0.9838 | −5.67 | 8.75 | 4.87 | 3.53 | 4.77 | −3.60 | −6.97 | −5.68 | 0.280 | 0.848 | 1.000 | 0.997 ± 0.212 |
| 11 | Clarithromycin | 0.11 | 4.83 | 1.0–40.0 | 0.9933 | 3.90 | −9.05 | −2.83 | −13.57 | −6.50 | 13.63 | 1.60 | 10.12 | 0.240 | 0.727 | 1.000 | 1.092 ± 0.184 | |
| 12 | Erythromycin | 0.00 | 38.29 | 1.0–40.0 | 0.9806 | 5.43 | −5.28 | −10.92 | −4.67 | −1.00 | −0.82 | 7.00 | 10.26 | 0.171 | 0.517 | 1.000 | 1.019 ± 0.135 | |
| 13 | Erythromycin Anhydrate | 0.02 | −0.02 | 1.0–40.0 | 0.9742 | −1.07 | 4.03 | −3.83 | −1.32 | 1.43 | 0.11 | 3.97 | −3.35 | 0.241 | 0.731 | 1.000 | 1.131 ± 0.183 | |
| 14 | Lincomycin | 0.12 | 1.09 | 0.05–2.0 | 0.9923 | −3.33 | 3.50 | −9.00 | −0.73 | 2.37 | −4.83 | 0.44 | 3.28 | 0.006 | 0.019 | 0.050 | 0.051 ± 0.005 | |
| 15 | Roxithromycin | 0.00 | 2.20 | 1.0–40.0 | 0.9943 | −2.20 | −3.10 | −7.58 | −3.67 | 7.03 | −2.54 | −3.68 | 4.18 | 0.118 | 0.359 | 1.000 | 1.042 ± 0.095 | |
| 16 | Virginiamycin | 1.19 | 1.78 | 1.0–40.0 | 0.9858 | 1.97 | 1.75 | −2.91 | −8.71 | 4.94 | 15.16 | 0.46 | 0.20 | 0.306 | 0.926 | 1.000 | 1.056 ± 0.242 | |
| 17 | Tylosin | 0.00 | 3.78 | 1.0–40.0 | 0.9812 | −0.97 | 7.32 | −9.08 | −15.58 | 1.54 | −1.61 | 0.41 | 6.31 | 0.264 | 0.800 | 1.000 | 1.041 ± 0.199 | |
| 18 | Ampicillin | Penicillin | 0.04 | −3.33 | 0.50–20.0 | 0.9935 | −4.33 | 7.13 | 2.33 | 6.76 | −3.69 | −1.43 | −1.12 | −5.58 | 0.107 | 0.325 | 0.500 | 0.530 ± 0.083 |
| 19 | Cloxacillin | 1.92 | 1.59 | 2.5–100.0 | 0.9724 | −7.72 | 14.79 | −0.55 | 10.42 | −0.65 | −6.84 | −4.62 | −4.82 | 0.703 | 2.131 | 2.500 | 2.616 ± 0.532 | |
| 20 | Cefotaxime | 0.41 | −0.39 | 1.0–40.0 | 0.9957 | −1.77 | 4.77 | −3.20 | 4.12 | −6.170 | 0.47 | 5.71 | −3.90 | 0.122 | 0.370 | 1.000 | 0.984 ± 0.097 | |
| 21 | Oxacillin | 0.32 | −6.39 | 0.50–20.0 | 0.9951 | −0.07 | 6.67 | −12.87 | −4.24 | 4.563 | −2.44 | 4.30 | 4.08 | 0.080 | 0.243 | 0.500 | 0.496 ± 0.061 | |
| 22 | Penicillin G | 0.07 | −2.34 | 0.50–20.0 | 0.9942 | −4.67 | −0.38 | −5.27 | −3.47 | 3.926 | −3.17 | 1.50 | 1.66 | 0.058 | 0.175 | 0.500 | 0.484 ± 0.045 | |
| 23 | Penicillin V | 1.35 | −3.26 | 2.5–100.0 | 0.9891 | −2.85 | 5.01 | −0.84 | 9.65 | −3.093 | −3.56 | −0.44 | −3.88 | 0.647 | 1.959 | 2.500 | 2.874 ± 0.49 | |
| 24 | 1 7 Dimethylxanthine | Others | 14.79 | −2.14 | 0.05–2.0 | 0.9980 | −1.33 | 7.33 | −7.50 | −0.87 | 1.400 | −0.35 | 2.24 | −0.50 | 0.008 | 0.023 | 0.050 | 0.051 ± 0.005 |
| 25 | Acetaminophen | 9.47 | −1.53 | 0.05–2.0 | 0.9960 | −2.67 | 9.00 | −6.33 | −1.47 | 4.533 | −1.09 | −2.47 | 0.70 | 0.009 | 0.027 | 0.050 | 0.050 ± 0.008 | |
| 26 | Albuterol | 0.08 | 0.40 | 0.05–2.0 | 0.9985 | −4.00 | 9.33 | −5.00 | 0.93 | 2.667 | −0.99 | −1.93 | −1.57 | 0.005 | 0.015 | 0.050 | 0.054 ± 0.005 | |
| 27 | Caffeine | 10.52 | −1.40 | 0.5–20.0 | 0.9959 | 2.47 | −5.15 | −4.75 | 2.42 | 2.757 | 0.19 | 1.62 | −1.31 | 0.066 | 0.200 | 0.500 | 0.505 ± 0.052 | |
| 28 | Carbadox | 1.96 | −2.50 | 0.10–4.0 | 0.9894 | −8.67 | 10.50 | −4.67 | 3.60 | −3.500 | −1.63 | −0.91 | −3.50 | 0.025 | 0.077 | 0.100 | 0.103 ± 0.02 | |
| 29 | Carbamazepine | 0.35 | −3.49 | 0.10–4.0 | 0.9977 | −6.67 | 2.50 | −2.17 | 7.23 | −0.050 | −1.43 | −3.43 | −7.36 | 0.016 | 0.048 | 0.100 | 0.103 ± 0.013 | |
| 30 | Cimetidine | 3.46 | −0.91 | 0.05–2.0 | 0.9953 | −4.00 | 3.00 | −8.50 | −1.20 | 2.400 | −0.16 | −3.44 | 2.28 | 0.006 | 0.018 | 0.050 | 0.050 ± 0.005 | |
| 31 | Cotinine | 5.47 | 30.53 | 0.10–4.0 | 0.9827 | −0.67 | 6.33 | −10.33 | −1.30 | 8.117 | −0.80 | −4.02 | 2.73 | 0.021 | 0.064 | 0.100 | 0.104 ± 0.015 | |
| 32 | Dehydro Nifedipine | 0.24 | −2.36 | 0.10–4.0 | 0.9974 | −2.00 | 8.33 | −9.25 | 1.60 | −0.967 | 0.59 | 1.37 | 0.32 | 0.013 | 0.041 | 0.100 | 0.101 ± 0.01 | |
| 33 | Digoxigenin | 0.97 | −6.19 | 0.10–4.0 | 0.9988 | −5.33 | 1.25 | −3.58 | 3.27 | −1.967 | −1.56 | −2.46 | −0.61 | 0.017 | 0.052 | 0.100 | 0.100 ± 0.013 | |
| 34 | Digoxin | 1.59 | 2.76 | 2.5–100.0 | 0.9831 | −7.17 | 14.12 | 13.10 | 8.18 | −0.879 | −0.71 | −11.58 | −15.52 | 0.444 | 1.345 | 2.500 | 2.565 ± 0.339 | |
| 35 | Diltiazam | 1.90 | −0.85 | 0.10–4.0 | 0.9946 | −3.00 | 10.67 | −7.42 | −5.13 | 2.000 | 2.45 | 1.91 | −1.31 | 0.029 | 0.087 | 0.100 | 0.102 ± 0.022 | |
| 36 | Diphenhydramine | 0.39 | 0.93 | 0.05–2.0 | 0.9945 | −2.00 | −3.00 | −9.50 | −4.60 | 3.367 | 0.83 | 0.22 | 2.60 | 0.005 | 0.014 | 0.050 | 0.048 ± 0.003 | |
| 37 | Fluoxetine | 0.25 | −4.08 | 0.10–4.0 | 0.9935 | −2.00 | 6.00 | −5.42 | 5.27 | 4.033 | 1.67 | −3.14 | −6.23 | 0.013 | 0.039 | 0.100 | 0.093 ± 0.008 | |
| 38 | Gemfibrozil | 0.08 | −0.02 | 0.50–20.0 | 0.9994 | −3.13 | 0.85 | −2.78 | −1.85 | 1.755 | −0.92 | 1.57 | 1.02 | 0.049 | 0.148 | 0.500 | 0.508 ± 0.04 | |
| 39 | Ibuprofen | 2.93 | 0.18 | 1.0–40.0 | 0.9977 | −2.90 | −2.47 | −5.86 | −1.15 | 0.640 | 0.22 | −0.39 | 0.55 | 0.123 | 0.373 | 1.000 | 1.071 ± 0.098 | |
| 40 | Naproxen | 0.93 | −12.72 | 1.0–40.0 | 0.9881 | 9.60 | 10.58 | −8.63 | −1.77 | −4.845 | 2.42 | −8.57 | 8.23 | 0.189 | 0.573 | 1.000 | 1.062 ± 0.145 | |
| 41 | Norgestimate | 0.71 | −0.31 | 0.50–20.0 | 0.9631 | 2.73 | 7.37 | −21.70 | −16.23 | −5.107 | −4.57 | 13.69 | 16.09 | 0.093 | 0.283 | 0.500 | 0.473 ± 0.071 | |
| 42 | Ormetoprim | 0.12 | −4.90 | 0.05–2.0 | 0.9940 | −3.33 | 1.50 | −8.83 | 3.00 | 4.967 | −3.23 | −1.13 | −2.15 | 0.006 | 0.018 | 0.050 | 0.055 ± 0.005 | |
| 43 | Ranitidine | 0.25 | 22.29 | 0.10–4.0 | 0.9959 | −1.33 | 5.33 | −5.08 | 0.47 | 0.367 | 0.20 | −1.32 | 1.55 | 0.016 | 0.047 | 0.100 | 0.108 ± 0.013 | |
| 44 | Thiabendazole | 0.91 | −2.65 | 0.10–4.0 | 0.9938 | 3.00 | −8.25 | −2.25 | 3.07 | −0.100 | 0.53 | 1.14 | 0.26 | 0.033 | 0.099 | 0.100 | 0.111 ± 0.024 | |
| 45 | Warfarin | 0.02 | −0.24 | 0.50–20.0 | 0.9948 | −6.73 | −0.48 | 0.69 | 1.76 | 2.873 | −2.05 | −5.17 | −5.27 | 0.018 | 0.054 | 0.500 | 0.524 ± 0.021 | |
| 46 | Sulfamethizole | Sulfonamides | 2.89 | −3.55 | 0.05–2.0 | 0.9907 | 13.33 | 4.33 | −9.33 | −0.87 | 2.800 | 0.56 | 0.93 | 0.12 | 0.006 | 0.020 | 0.050 | 0.05 ± 0.005 |
| 47 | Sulfachloropyridazine | 0.46 | −3.44 | 0.05–2.0 | 0.9980 | −2.00 | 8.67 | −7.00 | −0.47 | −0.167 | −1.55 | 0.64 | 2.53 | 0.006 | 0.018 | 0.050 | 0.048 ± 0.005 | |
| 48 | Sulfadiazine | 0.42 | −1.88 | 0.05–2.0 | 0.9971 | −4.00 | 10.67 | −5.50 | 0.13 | 0.567 | −1.36 | −2.96 | 2.23 | 0.009 | 0.027 | 0.050 | 0.050 ± 0.008 | |
| 49 | Sulfadimethoxine | 0.33 | −1.42 | 0.05–2.0 | 0.9981 | −3.33 | 0.00 | −7.67 | −3.20 | 3.833 | 0.83 | −1.93 | 0.90 | 0.008 | 0.023 | 0.050 | 0.051 ± 0.005 | |
| 50 | Sulfamerazine | 0.66 | −2.22 | 0.05–2.0 | 0.9889 | −0.67 | 6.33 | −8.83 | 1.60 | 3.533 | 7.44 | −0.02 | −8.52 | 0.012 | 0.038 | 0.050 | 0.051 ± 0.01 | |
| 51 | Sulfamethazine | 1.41 | −4.22 | 0.05–2.0 | 0.9951 | −2.00 | 7.67 | −7.83 | −5.67 | −3.133 | −0.11 | −1.22 | 11.52 | 0.008 | 0.025 | 0.050 | 0.049 ± 0.007 | |
| 52 | Sulfamethoxazole | 0.87 | −1.77 | 0.05–2.0 | 0.9961 | −2.00 | 7.33 | −7.00 | −2.20 | −0.300 | 3.79 | 0.44 | −0.17 | 0.006 | 0.018 | 0.050 | 0.050 ± 0.005 | |
| 53 | Sulfathiazole | 0.64 | −2.06 | 0.05–2.0 | 0.9974 | −4.67 | 14.67 | −12.17 | −0.47 | −0.200 | −0.67 | 6.04 | −2.92 | 0.011 | 0.033 | 0.050 | 0.049 ± 0.008 | |
| 54 | Trimethoprim | 0.78 | −7.22 | 0.05–2.0 | 0.9981 | −2.67 | 9.00 | −8.67 | −1.53 | 2.267 | 1.60 | −2.13 | 1.62 | 0.003 | 0.010 | 0.050 | 0.049 ± 0.003 | |
| 55 | 4 Epioxytetracycline | Tetracyclines | 1.35 | 8.47 | 1.0–40.0 | 0.9882 | 1.10 | 1.07 | −5.81 | −2.24 | −6.415 | 1.25 | 5.93 | 5.13 | 0.140 | 0.424 | 1.000 | 1.196 ± 0.134 |
| 56 | 4 Epianhydro Chlortetracycline | 8.11 | −4.23 | 1.0–40.0 | 0.9763 | 8.93 | −12.33 | −18.36 | −5.13 | −7.580 | 5.36 | 15.54 | 11.15 | 0.075 | 0.226 | 1.000 | 1.256 ± 0.070 | |
| 57 | 4 Epianhydrotetracycline | 3.26 | −0.08 | 1.0–40.0 | 0.9750 | 0.53 | 6.65 | −12.73 | −10.02 | −3.462 | −1.93 | 8.04 | 12.94 | 0.123 | 0.374 | 1.000 | 1.221 ± 0.099 | |
| 58 | 4 Epichlortetracycline | 0.36 | 8.66 | 1.0–40.0 | 0.9925 | −2.73 | 11.37 | −11.65 | −1.93 | 0.610 | −0.38 | 2.81 | 1.91 | 0.174 | 0.528 | 1.000 | 1.096 ± 0.152 | |
| 59 | 4 Epitetracycline | 1.72 | −0.33 | 1.0–40.0 | 0.9952 | −0.13 | 2.13 | −3.38 | −1.71 | −3.357 | 1.85 | 5.31 | −0.73 | 0.198 | 0.599 | 1.000 | 1.181 ± 0.152 | |
| 60 | Anhydro Chlortetracycline | 13.74 | −3.05 | 1.0–40.0 | 0.9662 | 6.50 | −8.90 | −11.27 | −8.49 | −5.318 | 8.15 | 15.54 | 5.70 | 0.230 | 0.697 | 1.000 | 1.220 ± 0.179 | |
| 61 | Anhydrotetracycline | 3.88 | −4.85 | 1.0–40.0 | 0.9805 | −0.30 | 5.68 | −9.51 | −2.03 | −5.295 | −3.61 | 7.18 | 7.86 | 0.085 | 0.257 | 1.000 | 1.233 ± 0.077 | |
| 62 | Chlortetracycline | 3.90 | −7.00 | 1.0–40.0 | 0.9779 | 2.93 | −0.45 | −10.58 | −1.94 | −7.475 | 0.67 | 6.86 | 10.00 | 0.218 | 0.662 | 1.000 | 1.208 ± 0.172 | |
| 63 | Demeclocycline | 5.15 | −6.97 | 1.0–40.0 | 0.9654 | 4.97 | −5.18 | −10.10 | −0.38 | −8.512 | 3.63 | 9.30 | 6.27 | 0.191 | 0.577 | 1.000 | 1.212 ± 0.148 | |
| 64 | Doxycycline | 1.99 | −1.23 | 1.0–40.0 | 0.9825 | −1.17 | 5.47 | −6.17 | 0.20 | −3.197 | −3.51 | 5.49 | 2.89 | 0.187 | 0.566 | 1.000 | 1.168 ± 0.147 | |
| 65 | Isochlortetracycline | 0.74 | −1.69 | 1.0–40.0 | 0.9727 | 1.67 | 2.72 | −13.06 | 1.70 | −6.35 | 1.92 | 4.65 | 6.77 | 0.200 | 0.607 | 1.000 | 1.120 ± 0.154 | |
| 66 | Miconazole | 0.60 | −1.94 | 0.50–20.0 | 0.9527 | 3.67 | 11.30 | −18.48 | −8.31 | −1.61 | −7.02 | 9.30 | 12.00 | 0.073 | 0.222 | 0.500 | 0.540 ± 0.057 | |
| 67 | Minocycline | 5.09 | 53.68 | 1.0–40.0 | 0.9674 | 5.77 | −4.87 | −13.81 | −3.67 | 2.56 | −3.35 | 9.00 | 8.38 | 0.157 | 0.474 | 1.000 | 1.249 ± 0.122 | |
| 68 | Oxytetracycline | 0.98 | −0.71 | 1.0–40.0 | 0.9882 | −0.50 | 5.35 | −9.00 | 0.71 | −5.02 | 0.23 | 4.73 | 3.50 | 0.089 | 0.270 | 1.000 | 1.172 ± 0.078 | |
| 69 | Tetracycline | 1.99 | −1.53 | 1.0–40.0 | 0.9820 | −0.17 | 4.28 | −8.19 | 0.75 | −3.33 | −0.51 | 2.23 | 4.95 | 0.211 | 0.640 | 1.000 | 1.179 ± 0.164 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karubothula, B.; Kota, V.V.; Shinde, D.; Tadala, R.; Cheerala, V.; Salem, S.B.; Elamin, W.F.; Brudecki, G. Next-Generation Wastewater-Based Epidemiology: Green Automation for Detecting 69 Multiclass Pharmaceutical and Personal Care Products in Wastewater Using 96-Well Plate Solid-Phase Extraction by LC-MS/MS. Molecules 2025, 30, 3694. https://doi.org/10.3390/molecules30183694
Karubothula B, Kota VV, Shinde D, Tadala R, Cheerala V, Salem SB, Elamin WF, Brudecki G. Next-Generation Wastewater-Based Epidemiology: Green Automation for Detecting 69 Multiclass Pharmaceutical and Personal Care Products in Wastewater Using 96-Well Plate Solid-Phase Extraction by LC-MS/MS. Molecules. 2025; 30(18):3694. https://doi.org/10.3390/molecules30183694
Chicago/Turabian StyleKarubothula, Bhaskar, Veera Venkataramana Kota, Dnyaneshwar Shinde, Raghu Tadala, Vishnu Cheerala, Samara Bin Salem, Wael Faroug Elamin, and Grzegorz Brudecki. 2025. "Next-Generation Wastewater-Based Epidemiology: Green Automation for Detecting 69 Multiclass Pharmaceutical and Personal Care Products in Wastewater Using 96-Well Plate Solid-Phase Extraction by LC-MS/MS" Molecules 30, no. 18: 3694. https://doi.org/10.3390/molecules30183694
APA StyleKarubothula, B., Kota, V. V., Shinde, D., Tadala, R., Cheerala, V., Salem, S. B., Elamin, W. F., & Brudecki, G. (2025). Next-Generation Wastewater-Based Epidemiology: Green Automation for Detecting 69 Multiclass Pharmaceutical and Personal Care Products in Wastewater Using 96-Well Plate Solid-Phase Extraction by LC-MS/MS. Molecules, 30(18), 3694. https://doi.org/10.3390/molecules30183694







