Multi-Target Pharmacological Effects of Asiatic Acid: Advances in Structural Modification and Novel Drug Delivery Systems
Abstract
1. Introduction
2. Pharmacological Effects of Asiatic Acid
2.1. Anti-Inflammatory Effects
2.2. Hepatoprotective Effects
2.3. Anti-Tumor Effects
2.4. Hypoglycemic Effects
2.5. Neuroprotective Effects
2.6. Cardioprotective Effects
2.7. Antibacterial Effects
2.8. Protective Effect on the Skin and Wound Healing Effect
3. Effect of Structural Modification of Asiatic Acid on Pharmacological Effects
3.1. Improvement of Anti-Tumor Effect
3.2. Improvement of Hypoglycemic Effect
3.3. Improvement of Neuroprotective Effect
3.4. Other Effects
4. Toxicity Assessment of Asiatic Acid
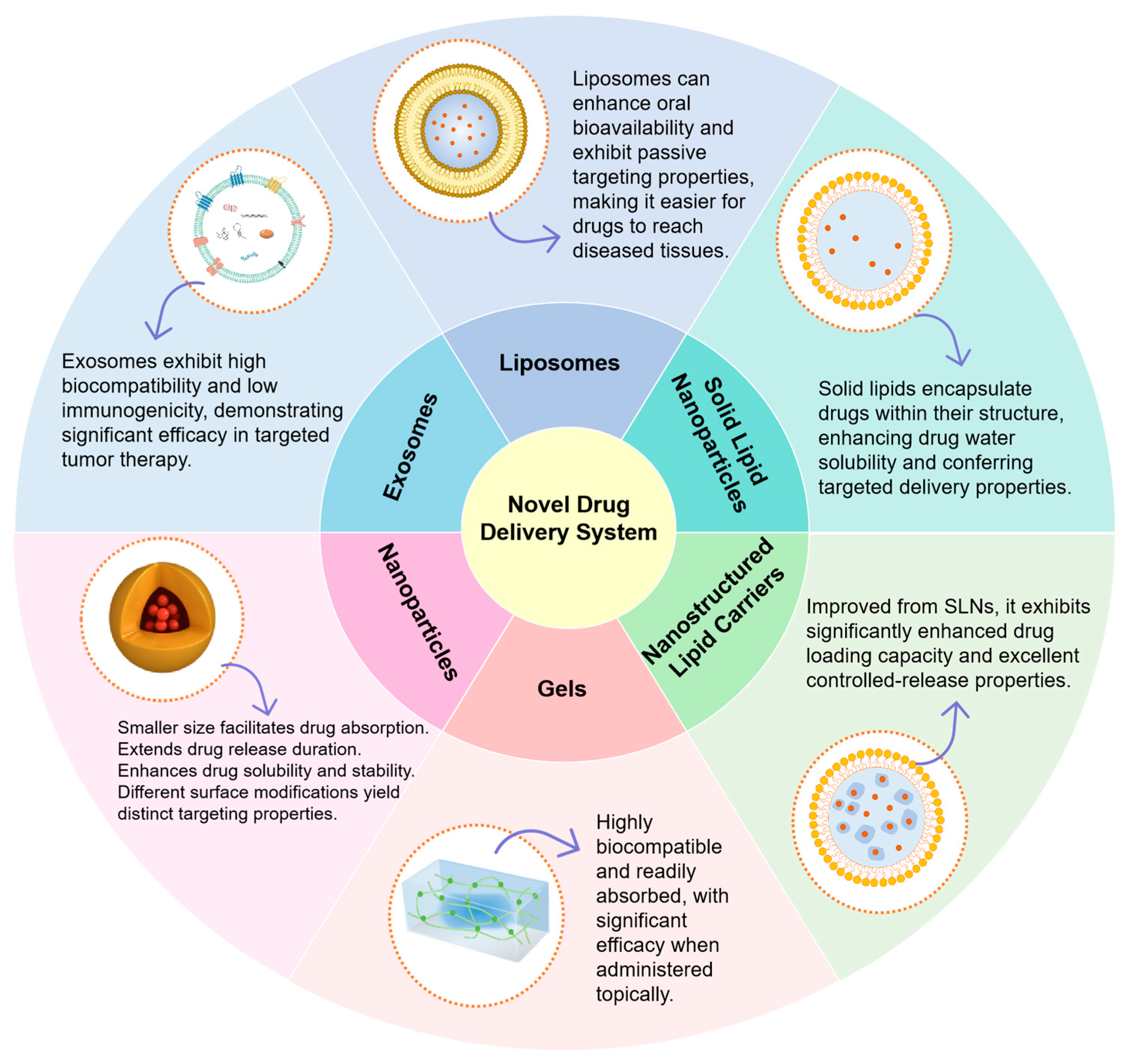
5. Advances in Novel Delivery Systems for Asiatic Acid
5.1. Nanoparticles
5.2. Solid Lipid Nanoparticles (SLN)
5.3. Liposomes
5.4. Nanostructured Lipid Carriers (NLCs)
5.5. Exosomes
5.6. Gel Formulations
6. Clinical Transformation and Challenges
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- James, J.T.; Dubery, I.A. Pentacyclic triterpenoids from the medicinal herb, Centella asiatica (L.) Urban. Molecules 2009, 14, 3922–3941. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, H.; Sun, F.; Sun, S.; Zhu, Z.; Chai, Y. Biopharmaceutical and pharmacokinetic characterization of asiatic acid in Centella asiatica as determined by a sensitive and robust HPLC-MS method. J. Ethnopharmacol. 2015, 163, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Kraft, O.; Hartmann, A.K.; Brandt, S.; Hoenke, S.; Heise, N.V.; Csuk, R.; Mueller, T. Asiatic acid as a leading structure for derivatives combining sub-nanomolar cytotoxicity, high selectivity, and the ability to overcome drug resistance in human preclinical tumor models. Eur. J. Med. Chem. 2023, 250, 115189. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.H.; Chen, M.C.; Liu, F.; Xu, Z.; Tian, X.T.; Xie, Y.; Huang, C.G. Synthesis and Cytotoxic Activity of Novel C-23-Modified Asiatic Acid Derivatives. Molecules 2020, 25, 3709. [Google Scholar] [CrossRef]
- Li, J.F.; Huang, R.Z.; Yao, G.Y.; Ye, M.Y.; Wang, H.S.; Pan, Y.M.; Xiao, J.T. Synthesis and biological evaluation of novel aniline-derived asiatic acid derivatives as potential anticancer agents. Eur. J. Med. Chem. 2014, 86, 175–188. [Google Scholar] [CrossRef]
- Wang, G.; Xiao, Q.; Wu, W.; Wu, Y.; Wei, Y.; Jing, Y.; Gong, Z. Assessment of Toxicity and Absorption of the Novel AA Derivative AA-Pme in SGC7901 Cancer Cells In Vitro and in Zebrafish In Vivo. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 5412–5421. [Google Scholar] [CrossRef]
- Wang, L.; Xu, J.; Zhao, C.; Zhao, L.; Feng, B. Antiproliferative, cell-cycle dysregulation effects of novel asiatic acid derivatives on human non-small cell lung cancer cells. Chem. Pharm. Bull. 2013, 61, 1015–1023. [Google Scholar] [CrossRef]
- Raval, N.; Mistry, T.; Acharya, N.; Acharya, S. Development of glutathione-conjugated asiatic acid-loaded bovine serum albumin nanoparticles for brain-targeted drug delivery. J. Pharm. Pharmacol. 2015, 67, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Dhas, N.; Naha, A.; Rani, U.; Gs, R.; Shetty, A.; Shetty, C.R.; Hebbar, S. Cationic biopolymer decorated Asiatic Acid and Centella asiatica extract incorporated liposomes for treating early-stage Alzheimer’s disease: An In-vitro and In-vivo investigation. F1000Research 2022, 11, 1535. [Google Scholar] [CrossRef]
- Zhao, E.; Tang, X.; Li, X.; Zhao, J.; Wang, S.; Wei, G.; Yang, L.; Zhao, M. Bioactive multifunctional hydrogels accelerate burn wound healing via M2 macrophage-polarization, antioxidant and anti-inflammatory. Mater. Today Bio 2025, 32, 101686. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Zhao, P.; Chen, Y.; Zhou, Y.; Wang, S.; Yin, L. Preparation and evaluation of PEGylated asiatic acid nanostructured lipid carriers on anti-fibrosis effects. Drug Dev. Ind. Pharm. 2020, 46, 57–69. [Google Scholar] [CrossRef]
- Ramalho, M.J.; Bravo, M.; Loureiro, J.A.; Lima, J.; Pereira, M.C. Transferrin-modified nanoparticles for targeted delivery of Asiatic acid to glioblastoma cells. Life Sci. 2022, 296, 120435. [Google Scholar] [CrossRef]
- Libby, P. Inflammatory mechanisms: The molecular basis of inflammation and disease. Nutr. Rev. 2007, 65 Pt 2, S140–S146. [Google Scholar] [CrossRef]
- Zhang, J.; Lv, W.; Zhang, G.; Zeng, M.; Cao, W.; Su, J.; Cao, K.; Liu, J. Nuclear Factor Erythroid 2 Related Factor 2 and Mitochondria Form a Mutually Regulating Circuit in the Prevention and Treatment of Metabolic Syndrome. Antioxid. Redox Signal. 2024, 41, 744–768. [Google Scholar] [CrossRef] [PubMed]
- Cilmiaty, R.; Nurhapsari, A.; Prayitno, A.; Rahma, A.A.; Ilyas, M.F. Asiatic acid reduces lipopolysaccharides-induced pulp inflammation through activation of nuclear factor erythroid 2-related factor 2 in rats. PeerJ 2024, 12, e18004. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Chen, Y.; Liu, S.; Xu, Q.; Zou, W. Study on the mechanism of asiatic acid in alleviatingSalmonella-induced colitis in mice. J. Tradit. Chin. Vet. Med. 2025, 44, 1–6+97+105. [Google Scholar] [CrossRef]
- Cao, S.Y.; Wang, W.; Nan, F.F.; Liu, Y.N.; Wei, S.Y.; Li, F.F.; Chen, L. Asiatic acid inhibits LPS-induced inflammatory response in endometrial epithelial cells. Microb. Pathog. 2018, 116, 195–199. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.Y.; Sun, J.; Cong, Q.J.; Chen, W.X.; Ahsan, H.M.; Gao, J.; Qian, J.J. Asiatic Acid Protects Dopaminergic Neurons from Neuroinflammation by Suppressing Mitochondrial Ros Production. Biomol. Ther. 2019, 27, 442–449. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.N.; Han, X.Y.; Liu, X.; Li, Y. Asiatic acid inhibits rheumatoid arthritis fibroblast-like synoviocyte growth through the Nrf2/HO-1/NF-κB signaling pathway. Chem. Biol. Drug Des. 2024, 103, e14454. [Google Scholar] [CrossRef]
- Yang, Z.; Feng, L.; Huang, J.; Zhang, X.; Lin, W.; Wang, B.; Cui, L.; Lin, S.; Li, G. Asiatic acid protects articular cartilage through promoting chondrogenesis and inhibiting inflammation and hypertrophy in osteoarthritis. Eur. J. Pharmacol. 2021, 907, 174265. [Google Scholar] [CrossRef]
- Jiang, T.; Xu, J.; Lu, Y.; Chen, X.; Li, Y. Network Pharmacology Analysis and Experimental Validation to Explore the Anti-inflammatory Mechanism of Asiatic Acid on Alcoholic Steatohepatitis. Mediat. Inflamm. 2022, 2022, 1708030. [Google Scholar] [CrossRef]
- Moon, G.H.; Lee, Y.; Kim, E.K.; Chung, K.H.; Lee, K.J.; An, J.H. Immunomodulatory and Anti-inflammatory Effects of Asiatic Acid in a DNCB-Induced Atopic Dermatitis Animal Model. Nutrients 2021, 13, 2448. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Zhang, J.; Zhang, K.; Peng, Z.; Xin, R.; Wang, L.; Li, J. Asiatic Acid Attenuates Inflammation Induced by Salmonella via Upregulating LncRNA TVX1 in Microglia. Int. J. Mol. Sci. 2022, 23, 10978. [Google Scholar] [CrossRef]
- Qian, Y.; Xin, Z.; Lv, Y.; Wang, Z.; Zuo, L.; Huang, X.; Li, Y.; Xin, H.B. Asiatic acid suppresses neuroinflammation in BV2 microglia via modulation of the Sirt1/NF-κB signaling pathway. Food Funct. 2018, 9, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; Chiu, C.S.; Chen, H.J.; Hou, W.C.; Sheu, M.J.; Lin, Y.C.; Shie, P.H.; Huang, G.J. Antinociceptive activities and the mechanisms of anti-inflammation of asiatic Acid in mice. Evid.-Based Complement. Altern. Med. 2011, 2011, 895857. [Google Scholar] [CrossRef]
- Guo, W.; Liu, W.; Jin, B.; Geng, J.; Li, J.; Ding, H.; Wu, X.; Xu, Q.; Sun, Y.; Gao, J. Asiatic acid ameliorates dextran sulfate sodium-induced murine experimental colitis via suppressing mitochondria-mediated NLRP3 inflammasome activation. Int. Immunopharmacol. 2015, 24, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Lokman, M.S.; Kassab, R.B.; Salem, F.A.M.; Elshopakey, G.E.; Hussein, A.; Aldarmahi, A.A.; Theyab, A.; Alzahrani, K.J.; Hassan, K.E.; Alsharif, K.F.; et al. Asiatic acid rescues intestinal tissue by suppressing molecular, biochemical, and histopathological changes associated with the development of ulcerative colitis. Biosci. Rep. 2024, 44, BSR20232004. [Google Scholar] [CrossRef]
- Nurhapsari, A.; Cilmiaty, R.; Prayitno, A.; Purwanto, B.; Soetrisno, S. The Role of Asiatic Acid in Preventing Dental Pulp Inflammation: An in-vivo Study. Clin. Cosmet. Investig. Dent. 2023, 15, 109–119. [Google Scholar] [CrossRef]
- Wróbel, A.; Zapała, Ł.; Kluz, T.; Rogowski, A.; Misiek, M.; Juszczak, K.; Sieńko, J.; Gold, D.; Stangel-Wójcikiewicz, K.; Poleszak, E.; et al. The Potential of Asiatic Acid in the Reversion of Cyclophosphamide-Induced Hemorrhagic Cystitis in Rats. Int. J. Mol. Sci. 2021, 22, 5853. [Google Scholar] [CrossRef]
- Ma, X.; McKeen, T.; Zhang, J.; Ding, W.X. Role and Mechanisms of Mitophagy in Liver Diseases. Cells 2020, 9, 837. [Google Scholar] [CrossRef]
- Zhu, W.; Su, H.; Wei, Y.; Huang, Y.; Chen, S.; Shi, Y.; Long, Y.; Qiu, Y.; Wei, J. Asiatic acid ameliorates rifampicin- and isoniazid-induced liver injury in vivo by regulating sphingolipid metabolism and mitogen-activated protein kinase signalling pathways. Basic Clin. Pharmacol. Toxicol. 2023, 133, 402–417. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Qiao, Q.; Vonglorkham, S.; Feng, Z.; Pang, L.; Chen, S.; Wang, D.; Lao, L.; Lin, X.; Wei, J. Asiatic acid ameliorates acute hepatic injury by reducing endoplasmic reticulum stress and triggering hepatocyte autophagy. Biomed. Pharmacother. 2020, 129, 110375. [Google Scholar] [CrossRef]
- Wei, L.; Chen, Q.; Guo, A.; Fan, J.; Wang, R.; Zhang, H. Asiatic acid attenuates CCl4-induced liver fibrosis in rats by regulating the PI3K/AKT/mTOR and Bcl-2/Bax signaling pathways. Int. Immunopharmacol. 2018, 60, 1–8. [Google Scholar] [CrossRef]
- Luo, K.; Geng, Y.; Oosterhuis, D.; de Meijer, V.E.; Olinga, P. Evaluating the antifibrotic potential of naringenin, asiatic acid, and icariin using murine and human precision-cut liver slices. Physiol. Rep. 2024, 12, e16136. [Google Scholar] [CrossRef]
- Li, R.; Wang, C.; Xu, K.; Zhan, Z.; He, S.; Ren, J.; Li, F.; Tao, N.; Li, Z.; Yang, Z.; et al. Asiatic acid inhibits HBV cccDNA transcription by promoting HBx degradation. Virol. J. 2024, 21, 268. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Chen, K.; Huang, J.; Chu, D.; Li, J.; Huang, K.; Ma, C. Asiatic acid inhibits angiogenesis and vascular permeability through the VEGF/VEGFR2 signaling pathway to inhibit the growth and metastasis of breast cancer in mice. Phytother. Res. 2021, 35, 6389–6400. [Google Scholar] [CrossRef]
- Gou, X.J.; Bai, H.H.; Liu, L.W.; Chen, H.Y.; Shi, Q.; Chang, L.S.; Ding, M.M.; Shi, Q.; Zhou, M.X.; Chen, W.L.; et al. Asiatic Acid Interferes with Invasion and Proliferation of Breast Cancer Cells by Inhibiting WAVE3 Activation through PI3K/AKT Signaling Pathway. BioMed Res. Int. 2020, 2020, 1874387. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Cao, Q.X.; Zhai, F.R.; Yang, S.Q.; Zhang, H.X. Asiatic acid exerts anticancer potential in human ovarian cancer cells via suppression of PI3K/Akt/mTOR signalling. Pharm. Biol. 2016, 54, 2377–2382. [Google Scholar] [CrossRef]
- Huang, C.F.; Hung, T.W.; Yang, S.F.; Tsai, Y.L.; Yang, J.T.; Lin, C.L.; Hsieh, Y.H. Asiatic acid from Centella asiatica exert anti-invasive ability in human renal cancer cells by modulation of ERK/p38MAPK-mediated MMP15 expression. Phytomedicine 2022, 100, 154036. [Google Scholar] [CrossRef]
- Singh, J.; Hussain, Y.; Meena, A.; Sinha, R.A.; Luqman, S. Asiatic acid impedes NSCLC progression by inhibiting COX-2 and modulating PI3K signaling. FEBS Lett. 2024, 598, 3036–3052. [Google Scholar] [CrossRef]
- Zhu, Z.; Cui, L.; Yang, J.; Vong, C.T.; Hu, Y.; Xiao, J.; Chan, G.; He, Z.; Zhong, Z. Anticancer effects of asiatic acid against doxorubicin-resistant breast cancer cells via an AMPK-dependent pathway in vitro. Phytomedicine 2021, 92, 153737. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Chuang, Y.C.; Lo, Y.S.; Lin, C.C.; Hsi, Y.T.; Hsieh, M.J.; Chen, M.K. Asiatic Acid, Extracted from Centella asiatica and Induces Apoptosis Pathway through the Phosphorylation p38 Mitogen-Activated Protein Kinase in Cisplatin-Resistant Nasopharyngeal Carcinoma Cells. Biomolecules 2020, 10, 184. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Y.J.; Zhang, L.; Zhou, X.Y.; Jia, X.H. [Reversal Roles and Its Mechanism of Asiatic Acid on Multidrug Resistance in K562/ADR Cells Through the Wnt/β-catenin Pathway]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2024, 32, 1696–1703. [Google Scholar] [CrossRef]
- Meshkovska, Y.; Dzhuraeva, B.; Godugu, C.; Pooladanda, V.; Thatikonda, S. Deciphering the interplay: Circulating cell-free DNA, signaling pathways, and disease progression in idiopathic pulmonary fibrosis. 3 Biotech 2025, 15, 102. [Google Scholar] [CrossRef]
- Lian, G.Y.; Wang, Q.M.; Mak, T.S.; Huang, X.R.; Yu, X.Q.; Lan, H.Y. Inhibition of tumor invasion and metastasis by targeting TGF-β-Smad-MMP2 pathway with Asiatic acid and Naringenin. Mol. Ther. Oncolytics 2021, 20, 277–289. [Google Scholar] [CrossRef]
- Chen, Y.H.; Wu, J.X.; Yang, S.F.; Hsiao, Y.H. Synergistic Combination of Luteolin and Asiatic Acid on Cervical Cancer In Vitro and In Vivo. Cancers 2023, 15, 548. [Google Scholar] [CrossRef]
- Chen, X.C.; Huang, L.F.; Tang, J.X.; Wu, D.; An, N.; Ye, Z.N.; Lan, H.Y.; Liu, H.F.; Yang, C. Asiatic acid alleviates cisplatin-induced renal fibrosis in tumor-bearing mice by improving the TFEB-mediated autophagy-lysosome pathway. Biomed. Pharmacother. 2023, 165, 115122. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar Pathak, A.; Kumar, P.; Kumar Singh, A.; Kumar Sah Gond, M.; Singh Negi, R.; Das, R.; Agrawal, S.; Kumar Mishra, S.; Tiwari, K.N. Effects of Asiatic acid on brain cancer by altering astrocytes and the AKT1-PRKCB signaling pathway: A genomic and network pharmacology perspective. Brain Res. 2025, 1859, 149652. [Google Scholar] [CrossRef]
- Pantia, S.; Kangsamaksin, T.; Janvilisri, T.; Komyod, W. Asiatic Acid Inhibits Nasopharyngeal Carcinoma Cell Viability and Migration via Suppressing STAT3 and Claudin-1. Pharmaceuticals 2023, 16, 902. [Google Scholar] [CrossRef]
- Cheng, Q.; Zhang, S.; Zhong, B.; Chen, Z.; Peng, F. Asiatic acid re-sensitizes multidrug-resistant A549/DDP cells to cisplatin by down regulating long non-coding RNA metastasis associated lung adenocarcinoma transcript 1/β-catenin signaling. Bioengineered 2022, 13, 12972–12984. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.B.; Kim, K.; Bae, S.; Choi, Y.; Cha, H.J.; Kim, S.Y.; Lee, J.H.; Jeon, S.H.; Jung, H.J.; Ahn, K.J.; et al. MicroRNA-1290 promotes asiatic acid-induced apoptosis by decreasing BCL2 protein level in A549 non-small cell lung carcinoma cells. Oncol. Rep. 2014, 32, 1029–1036. [Google Scholar] [CrossRef]
- Wu, T.; Geng, J.; Guo, W.; Gao, J.; Zhu, X. Asiatic acid inhibits lung cancer cell growth in vitro and in vivo by destroying mitochondria. Acta Pharm. Sin. B 2017, 7, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Ren, J.; Zhou, Q.; Yang, Q.; Li, B. Effect of asiatic acid on epithelial-mesenchymal transition of human alveolar epithelium A549 cells induced by TGF-β1. Oncol. Lett. 2019, 17, 4285–4292. [Google Scholar] [CrossRef]
- Li, J.; Chen, K.; Huang, J.; Chu, D.; Tian, M.; Huang, K.; Ma, C. Asiatic Acid Induces Endoplasmic Reticulum Stress and Activates the Grp78/IRE1α/JNK and Calpain Pathways to Inhibit Tongue Cancer Growth. Front. Pharmacol. 2021, 12, 690612. [Google Scholar] [CrossRef]
- Lai, Y.W.; Wang, S.W.; Lin, C.L.; Chen, S.S.; Lin, K.H.; Lee, Y.T.; Chen, W.C.; Hsieh, Y.H. Asiatic acid exhibits antimetastatic activity in human prostate cancer cells by modulating the MZF-1/Elk-1/Snail signaling axis. Eur. J. Pharmacol. 2023, 951, 175770. [Google Scholar] [CrossRef]
- Park, B.C.; Bosire, K.O.; Lee, E.S.; Lee, Y.S.; Kim, J.A. Asiatic acid induces apoptosis in SK-MEL-2 human melanoma cells. Cancer Lett. 2005, 218, 81–90. [Google Scholar] [CrossRef]
- Liu, J.; He, T.; Lu, Q.; Shang, J.; Sun, H.; Zhang, L. Asiatic acid preserves beta cell mass and mitigates hyperglycemia in streptozocin-induced diabetic rats. Diabetes/Metab. Res. Rev. 2010, 26, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Zhang, X.; Chen, J.; Song, S.; Fang, S.; Wang, Z.; Xu, S.; Xu, Y.; Liu, J.; Jiang, C.; et al. Asiatic acid attenuates tubular injury in diabetic kidney disease by regulating mitochondrial dynamics via the Nrf-2 pathway. Phytomedicine 2023, 109, 154552. [Google Scholar] [CrossRef]
- Yamagata, K.; Tsuyama, T.; Sato, Y. Roles of β-Cell Hypoxia in the Progression of Type 2 Diabetes. Int. J. Mol. Sci. 2024, 25, 4186. [Google Scholar] [CrossRef] [PubMed]
- Uddandrao, V.V.S.; Rameshreddy, P.; Brahmanaidu, P.; Ponnusamy, P.; Balakrishnan, S.; Ramavat, R.N.; Swapna, K.; Pothani, S.; Nemani, H.; Meriga, B.; et al. Antiobesity efficacy of asiatic acid: Down-regulation of adipogenic and inflammatory processes in high fat diet induced obese rats. Arch. Physiol. Biochem. 2020, 126, 453–462. [Google Scholar] [CrossRef]
- Wang, X.; Lu, Q.; Yu, D.S.; Chen, Y.P.; Shang, J.; Zhang, L.Y.; Sun, H.B.; Liu, J. Asiatic acid mitigates hyperglycemia and reduces islet fibrosis in Goto-Kakizaki rat, a spontaneous type 2 diabetic animal model. Chin. J. Nat. Med. 2015, 13, 529–534. [Google Scholar] [CrossRef]
- Ding, L.; Liu, T.; Ma, J. Neuroprotective mechanisms of Asiatic acid. Heliyon 2023, 9, e15853. [Google Scholar] [CrossRef] [PubMed]
- Suryavanshi, J.; Prakash, C.; Sharma, D. Asiatic acid attenuates aluminium chloride-induced behavioral changes, neuronal loss and astrocyte activation in rats. Metab. Brain Dis. 2022, 37, 1773–1785. [Google Scholar] [CrossRef]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Rather, M.; Justin Thenmozhi, A.; Manivasagam, T.; Nataraj, J.; Essa, M.M.; Chidambaram, S.B. Asiatic acid nullified aluminium toxicity in in vitro model of Alzheimer’s disease. Front. Biosci. (Elite Ed.) 2018, 10, 287–299. [Google Scholar] [CrossRef]
- Gou, X.; Fu, Y.; Li, J.; Xiang, J.; Yang, M.; Zhang, Y. Impact of nanoplastics on Alzheimer’s disease: Enhanced amyloid-β peptide aggregation and augmented neurotoxicity. J. Hazard. Mater. 2024, 465, 133518. [Google Scholar] [CrossRef]
- Cheng, W.; Chen, W.; Wang, P.; Chu, J. Asiatic acid protects differentiated PC12 cells from Aβ25-35-induced apoptosis and tau hyperphosphorylation via regulating PI3K/Akt/GSK-3β signaling. Life Sci. 2018, 208, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Varada, S.; Chamberlin, S.R.; Bui, L.; Brandes, M.S.; Gladen-Kolarsky, N.; Harris, C.J.; Hack, W.; Neff, C.J.; Brumbach, B.H.; Soumyanath, A.; et al. Oral Asiatic Acid Improves Cognitive Function and Modulates Antioxidant and Mitochondrial Pathways in Female 5xFAD Mice. Nutrients 2025, 17, 729. [Google Scholar] [CrossRef]
- Lu, C.W.; Lin, T.Y.; Pan, T.L.; Wang, P.W.; Chiu, K.M.; Lee, M.Y.; Wang, S.J. Asiatic Acid Prevents Cognitive Deficits by Inhibiting Calpain Activation and Preserving Synaptic and Mitochondrial Function in Rats with Kainic Acid-Induced Seizure. Biomedicines 2021, 9, 284. [Google Scholar] [CrossRef]
- He, J.; Li, X.; Wen, Z. Influence of asiatic acid on ropivacaine induced neurotoxicity in neonatal rats by regulating cAMP/PKA signaling pathway. Chin. J. Birth Health Hered. 2023, 31, 1575–1580. [Google Scholar] [CrossRef]
- Hu, Y.; Gu, J.; Jin, X.; Wu, X.; Li, H.; Bai, L.; Wu, J.; Li, X., Sr. Asiatic acid alleviates subarachnoid hemorrhage-induced brain injury in rats by inhibiting ferroptosis of neurons via targeting acyl-coenzyme a oxidase 1. Neuropharmacology 2025, 262, 110208. [Google Scholar] [CrossRef]
- Hu, X.; Li, B.; Li, L.; Li, B.; Luo, J.; Shen, B. Asiatic Acid Protects against Doxorubicin-Induced Cardiotoxicity in Mice. Oxidative Med. Cell. Longev. 2020, 2020, 5347204. [Google Scholar] [CrossRef]
- Xiang, Q.; Yi, X.; Zhu, X.H.; Wei, X.; Jiang, D.S. Regulated cell death in myocardial ischemia-reperfusion injury. Trends Endocrinol. Metab. 2024, 35, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Si, L.; Xu, J.; Yang, J.; Wang, Q.; Wang, X. Effect and mechanism of asiatic acid on autophagy in myocardial ischemia-reperfusion injury in vivo and in vitro. Exp. Ther. Med. 2020, 20, 54. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtseva, A.V.; Krasnov, G.S.; Dmitriev, A.A.; Alekseev, B.Y.; Kardymon, O.L.; Sadritdinova, A.F.; Fedorova, M.S.; Pokrovsky, A.V.; Melnikova, N.V.; Kaprin, A.D.; et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget 2016, 7, 44879–44905. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Song, M.; Sun, L.; Si, L.; Yu, D.; Li, B.; Lu, P.; Wang, W.; Wang, X. Asiatic Acid Alleviates Myocardial Ischemia-Reperfusion Injury by Inhibiting the ROS-Mediated Mitochondria-Dependent Apoptosis Pathway. Oxidative Med. Cell. Longev. 2022, 2022, 3267450. [Google Scholar] [CrossRef]
- Qiu, F.; Yuan, Y.; Luo, W.; Gong, Y.S.; Zhang, Z.M.; Liu, Z.M.; Gao, L. Asiatic acid alleviates ischemic myocardial injury in mice by modulating mitophagy- and glycophagy-based energy metabolism. Acta Pharmacol. Sin. 2022, 43, 1395–1407. [Google Scholar] [CrossRef]
- Tiwari, A.; Shukla, A.; Kumar Samal, P. Evaluation of Anti-Hyperlipidemic and Anti-Atherogenic Activity of Asiatic Acid and Its Effect on Lipid Peroxidation in Hyperlipidemic Rats. J. Biochem. Mol. Toxicol. 2025, 39, e70255. [Google Scholar] [CrossRef]
- Lv, H.; Qi, Z.; Wang, S.; Feng, H.; Deng, X.; Ci, X. Asiatic Acid Exhibits Anti-inflammatory and Antioxidant Activities against Lipopolysaccharide and d-Galactosamine-Induced Fulminant Hepatic Failure. Front. Immunol. 2017, 8, 785. [Google Scholar] [CrossRef]
- Harnvoravongchai, P.; Chankhamhaengdecha, S.; Ounjai, P.; Singhakaew, S.; Boonthaworn, K.; Janvilisri, T. Antimicrobial Effect of Asiatic Acid Against Clostridium difficile Is Associated With Disruption of Membrane Permeability. Front. Microbiol. 2018, 9, 2125. [Google Scholar] [CrossRef]
- Singh, K.; Sharma, A.; Upadhyay, T.K.; Hayat-Ul-Islam, M.; Khan, M.K.A.; Dwivedi, U.N.; Sharma, R. Structure-based in silico and in vitro Analysis Reveals Asiatic Acid as Novel Potential Inhibitor of Mycobacterium tuberculosis Maltosyl Transferase. Curr. Comput.-Aided Drug Des. 2022, 18, 213–227. [Google Scholar] [CrossRef]
- Kandaswamy, K.; Panda, S.P.; Shaik, M.R.; Hussain, S.A.; Deepak, P.; Thiyagarajulu, N.; Jain, D.; Antonyraj, A.P.M.; Subramanian, R.; Guru, A.; et al. Formulation of Asiatic acid-loaded polymeric chitosan-based hydrogel for effective MRSA infection control and enhanced wound healing in zebrafish models. Int. J. Biol. Macromol. 2024, 293, 137425. [Google Scholar] [CrossRef]
- Sycz, Z.; Tichaczek-Goska, D.; Jezierska-Domaradzka, A.; Wojnicz, D. Are Uropathogenic Bacteria Living in Multispecies Biofilm Susceptible to Active Plant Ingredient-Asiatic Acid? Biomolecules 2021, 11, 1754. [Google Scholar] [CrossRef]
- Maitra, P.; Basak, P.; Okamoto, K.; Miyoshi, S.I.; Dutta, S.; Bhattacharya, S. Asiatic acid inhibits intracellular Shigella flexneri growth by inducing antimicrobial peptide gene expression. J. Appl. Microbiol. 2023, 134, lxac076. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, C.; Zhao, X.; Wang, D.; Liu, Y.; Sun, S. Antifungal activity and potential mechanism of Asiatic acid alone and in combination with fluconazole against Candida albicans. Biomed. Pharmacother. 2021, 139, 111568. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-L.; Lai, Y.-H.; Huang, C.-H.; Lai, J.-Y.; Yao, C.-H. Lumbrokinase-containing gelatin nanofibers with multiple bioactivities for effective skin wound healing. Mater. Today Bio 2025, 32, 101713. [Google Scholar] [CrossRef]
- Park, K.S. Pharmacological Effects of Centella asiatica on Skin Diseases: Evidence and Possible Mechanisms. Evid.-Based Complement. Altern. Med. 2021, 2021, 5462633. [Google Scholar] [CrossRef]
- Hashim, P.; Sidek, H.; Helan, M.H.; Sabery, A.; Palanisamy, U.D.; Ilham, M. Triterpene composition and bioactivities of Centella asiatica. Molecules 2011, 16, 1310–1322. [Google Scholar] [CrossRef] [PubMed]
- Kukula, O.; Kırmızıkan, S.; Tiryaki, E.S.; Çiçekli, M.N.; Günaydın, C. Asiatic acid exerts an anti-psoriatic effect in the imiquimod-induced psoriasis model in mice. Immunopharmacol. Immunotoxicol. 2022, 44, 367–372. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, J.; Zheng, Y.; Lei, J.; Huang, J.; Liu, S.; Liu, F.; Peng, Q.; Zhang, Y.; Wang, J.; et al. Quercetin mediates the therapeutic effect of Centella asiatica on psoriasis by regulating STAT3 phosphorylation to inhibit the IL-23/IL-17A axis. Nan Fang Yi Ke Da Xue Xue Bao = J. South. Med. Univ. 2025, 45, 90–99. [Google Scholar] [CrossRef]
- Soo Lee, Y.; Jin, D.Q.; Beak, S.M.; Lee, E.S.; Kim, J.A. Inhibition of ultraviolet-A-modulated signaling pathways by asiatic acid and ursolic acid in HaCaT human keratinocytes. Eur. J. Pharmacol. 2003, 476, 173–178. [Google Scholar] [CrossRef]
- Maquart, F.X.; Bellon, G.; Gillery, P.; Wegrowski, Y.; Borel, J.P. Stimulation of collagen synthesis in fibroblast cultures by a triterpene extracted from Centella asiatica. Connect. Tissue Res. 1990, 24, 107–120. [Google Scholar] [CrossRef]
- Bian, D.; Zhang, J.; Wu, X.; Dou, Y.; Yang, Y.; Tan, Q.; Xia, Y.; Gong, Z.; Dai, Y. Asiatic acid isolated from Centella asiatica inhibits TGF-β1-induced collagen expression in human keloid fibroblasts via PPAR-γ activation. Int. J. Biol. Sci. 2013, 9, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.S. Structure-activity relationship study of asiatic acid derivatives for new wound healing agent. Arch. Pharmacal Res. 2006, 29, 556–562. [Google Scholar] [CrossRef]
- Masoko, P.; Picard, J.; Howard, R.L.; Mampuru, L.J.; Eloff, J.N. In vivo antifungal effect of Combretum and Terminalia species extracts on cutaneous wound healing in immunosuppressed rats. Pharm. Biol. 2010, 48, 621–632. [Google Scholar] [CrossRef]
- Wang, Z.H. Anti-glycative effects of asiatic acid in human keratinocyte cells. BioMedicine 2014, 4, 19. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, J.; Ye, F.; Dong, X.; Ge, W.; Wang, X.; Zhao, Y.; Dan, G.; Chen, M.; Sai, Y. Asiatic acid improves the damage of HaCaT cells induced by nitrogen mustard through inhibiting endoplasmic reticulum stress. Toxicol. Res. 2025, 14, tfaf019. [Google Scholar] [CrossRef]
- Diniz, L.R.L.; Calado, L.L.; Duarte, A.B.S.; de Sousa, D.P. Centella asiatica and Its Metabolite Asiatic Acid: Wound Healing Effects and Therapeutic Potential. Metabolites 2023, 13, 276. [Google Scholar] [CrossRef]
- Jing, Y.; Wang, G.; Ge, Y.; Xu, M.; Gong, Z. Synthesis, anti-tumor and anti-angiogenic activity evaluations of asiatic Acid amino Acid derivatives. Molecules 2015, 20, 7309–7324. [Google Scholar] [CrossRef] [PubMed]
- Jew, S.S.; Yoo, C.H.; Lim, D.Y.; Kim, H.; Mook-Jung, I.; Jung, M.W.; Choi, H.; Jung, Y.H.; Kim, H.; Park, H.G. Structure-activity relationship study of asiatic acid derivatives against beta amyloid (Aβ)-induced neurotoxicity. Bioorganic Med. Chem. Lett. 2000, 10, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Meng, Y. Synthesis of Asiatic Acid Derivatives and Study on Anti-Tumor Activityin Vitro. J. Shenyang Univ. Chem. Technol. 2022, 36, 326–331+338. [Google Scholar]
- Jing, Y.; Wang, G.; Ge, Y.; Xu, M.; Tang, S.; Gong, Z. AA-PMe, a novel asiatic acid derivative, induces apoptosis and suppresses proliferation, migration, and invasion of gastric cancer cells. OncoTargets Ther. 2016, 9, 1605–1621. [Google Scholar] [CrossRef]
- Wang, G.; Jing, Y.; Cao, L.; Gong, C.; Gong, Z.; Cao, X. A novel synthetic Asiatic acid derivative induces apoptosis and inhibits proliferation and mobility of gastric cancer cells by suppressing STAT3 signaling pathway. OncoTargets Ther. 2017, 10, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Kahnt, M.; Wiemann, J.; Fischer, L.; Sommerwerk, S.; Csuk, R. Transformation of asiatic acid into a mitocanic, bimodal-acting rhodamine B conjugate of nanomolar cytotoxicity. Eur. J. Med. Chem. 2018, 159, 143–148. [Google Scholar] [CrossRef]
- Siewert, B.; Pianowski, E.; Obernauer, A.; Csuk, R. Towards cytotoxic and selective derivatives of maslinic acid. Bioorganic Med. Chem. 2014, 22, 594–615. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Bisht, A.; Avinash, D.; Sahu, K.K.; Patel, P.; Das Gupta, G.; Kurmi, B.D. A comprehensive review on doxorubicin: Mechanisms, toxicity, clinical trials, combination therapies and nanoformulations in breast cancer. Drug Deliv. Transl. Res. 2025, 15, 102–133. [Google Scholar] [CrossRef] [PubMed]
- Siewert, B.; Pianowski, E.; Csuk, R. Esters and amides of maslinic acid trigger apoptosis in human tumor cells and alter their mode of action with respect to the substitution pattern at C-28. Eur. J. Med. Chem. 2013, 70, 259–272. [Google Scholar] [CrossRef]
- Meng, Y.Q.; Wang, Z.Q.; Li, J.M.; Xu, D.P.; Meng, B.B.; Huang, M.Q. Synthesis and anti-tumor activity of asiatic acid derivatives targeting VEGFR. J. Asian Nat. Prod. Res. 2023, 25, 1205–1216. [Google Scholar] [CrossRef]
- Meng, Y.Q.; Wu, Y.J.; Kuai, Z.Y.; Ma, J.J.; Wang, Z.; Meng, B.B.; Wang, Z.Q. Design, synthesis and anti-tumor activity of asiatic acid derivatives as VEGF inhibitors. J. Asian Nat. Prod. Res. 2023, 25, 357–368. [Google Scholar] [CrossRef]
- Filler, R.; Saha, R. Fluorine in medicinal chemistry: A century of progress and a 60-year retrospective of selected highlights. Future Med. Chem. 2009, 1, 777–791. [Google Scholar] [CrossRef]
- Gonçalves, B.M.; Salvador, J.A.; Marín, S.; Cascante, M. Synthesis and anticancer activity of novel fluorinated asiatic acid derivatives. Eur. J. Med. Chem. 2016, 114, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Somsák, L.; Czifrák, K.; Tóth, M.; Bokor, E.; Chrysina, E.D.; Alexacou, K.M.; Hayes, J.M.; Tiraidis, C.; Lazoura, E.; Leonidas, D.D.; et al. New inhibitors of glycogen phosphorylase as potential antidiabetic agents. Curr. Med. Chem. 2008, 15, 2933–2983. [Google Scholar] [CrossRef] [PubMed]
- Oikonomakos, N.G. Glycogen phosphorylase as a molecular target for type 2 diabetes therapy. Curr. Protein Pept. Sci. 2002, 3, 561–586. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, Z.; Quan, M.; Lv, Y.; Li, Y.; Xin, H.B.; Qian, Y. Asiatic acid protests against myocardial ischemia/reperfusion injury via modulation of glycometabolism in rat cardiomyocyte. Drug Des. Dev. Ther. 2018, 12, 3573–3582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, J.; Gong, Y.; Liu, J.; Zhang, L.; Hua, W.; Sun, H. Synthesis and biological evaluation of asiatic acid derivatives as inhibitors of glycogen phosphorylases. Chem. Biodivers. 2009, 6, 864–874. [Google Scholar] [CrossRef]
- Denner, T.C.; Heise, N.V.; Serbian, I.; Angeli, A.; Supuran, C.T.; Csuk, R. An asiatic acid derived trisulfamate acts as a nanomolar inhibitor of human carbonic anhydrase VA. Steroids 2024, 205, 109381. [Google Scholar] [CrossRef]
- Kim, S.R.; Koo, K.A.; Lee, M.K.; Park, H.G.; Jew, S.S.; Cha, K.H.; Kim, Y.C. Asiatic acid derivatives enhance cognitive performance partly by improving acetylcholine synthesis. J. Pharm. Pharmacol. 2004, 56, 1275–1282. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, S.; Guo, F.; Feng, Y.; Li, Y.; Gong, M.; Zhang, Y.; Zhang, C.; Mei, L.; Wang, Y.; et al. Injectable CuS-loaded carboxymethyl and sulfonated chitosan hydrogel with antibacterial and self-healing properties promoting periodontal tissue regeneration. Int. J. Biol. Macromol. 2025, 310, 143205. [Google Scholar] [CrossRef]
- Thamnium, S.; Laomeephol, C.; Pavasant, P.; Osathanon, T.; Tabata, Y.; Wang, C.; Luckanagul, J.A. Osteogenic induction of asiatic acid derivatives in human periodontal ligament stem cells. Sci. Rep. 2023, 13, 14102. [Google Scholar] [CrossRef]
- Sumrejkanchanakij, P.; Fitri, A.R.; Pavasant, P.; Chareonvit, S.; Lin, A.C.K.; Chamni, S. Asiatic acid methyl ester, a new asiaticoside derivative, induces osteogenic differentiation of hPDLCs. Arch. Oral Biol. 2025, 172, 106175. [Google Scholar] [CrossRef]
- Sharma, R.; Banerjee, S.; Sharma, R. Role of Mandukparni (Centella asiatica Linn Urban) in neurological disorders: Evidence from ethnopharmacology and clinical studies to network enrichment analysis. Neurochem. Int. 2024, 180, 105865. [Google Scholar] [CrossRef]
- Yadav, M.K.; Singh, S.K.; Singh, M.; Mishra, S.S.; Singh, A.K.; Tripathi, J.S.; Tripathi, Y.B. In Vivo Toxicity Study of Ethanolic Extracts of Evolvulus alsinoides & Centella asiatica in Swiss Albino Mice. Open Access Maced. J. Med. Sci. 2019, 7, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Oruganti, M.; Roy, B.K.; Singh, K.K.; Prasad, R.; Kumar, S. Safety Assemment of Centella asiatica in albino rats. Pharmacogn. J. 2010, 2, 5–13. [Google Scholar] [CrossRef]
- Yen, G.C.; Chen, H.Y.; Peng, H.H. Evaluation of the cytotoxicity, mutagenicity and antimutagenicity of emerging edible plants. Food Chem. Toxicol. 2001, 39, 1045–1053. [Google Scholar] [CrossRef]
- Ueoka, A.R.; Sufi, B.S.; Magalhães, W.V.; Fernandes, L.; Andreo-Filho, N.; Leite-Silva, V.R.; Lopes, P.S. Flow cytometry as an alternative method to evaluate genotoxicity of natural cosmetic actives. J. Cosmet. Dermatol. 2023, 22, 958–968. [Google Scholar] [CrossRef]
- Junsai, T.; Tangpanithandee, S.; Srimangkornkaew, P.; Suknuntha, K.; Vivithanaporn, P.; Khemawoot, P. Sub-chronic oral toxicity of a water-soluble extract of Centella asiatica (Centell-S) in Wistar rats. Food Chem. Toxicol. 2024, 185, 114509. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Li, Q.; Liang, W.; Zhang, Y.; Jiang, C.; Zhang, Y.; Tan, J.; Zhao, H. Asiatic acid and madecassic acid cause cardiotoxicity via inflammation and production of excessive reactive oxygen species in zebrafish. J. Appl. Toxicol. 2024, 44, 1028–1039. [Google Scholar] [CrossRef]
- Nagoor Meeran, M.F.; Goyal, S.N.; Suchal, K.; Sharma, C.; Patil, C.R.; Ojha, S.K. Pharmacological Properties, Molecular Mechanisms, and Pharmaceutical Development of Asiatic Acid: A Pentacyclic Triterpenoid of Therapeutic Promise. Front. Pharmacol. 2018, 9, 892. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomed. 2016, 11, 673–692. [Google Scholar] [CrossRef]
- Mishra, D.; Hubenak, J.R.; Mathur, A.B. Nanoparticle systems as tools to improve drug delivery and therapeutic efficacy. J. Biomed. Mater. Res. Part A 2013, 101, 3646–3660. [Google Scholar] [CrossRef]
- Dewanjee, S.; Chakraborty, P.; Mukherjee, B.; De Feo, V. Plant-Based Antidiabetic Nanoformulations: The Emerging Paradigm for Effective Therapy. Int. J. Mol. Sci. 2020, 21, 2217. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.; Yan, Y.; Ma, Y.; Tu, L.; Shao, J.; Tang, X.; Chen, L.; Liang, G.; Yin, L. Dual-Targeted Nanoparticle-in-Microparticle System for Ulcerative Colitis Therapy. Adv. Healthc. Mater. 2023, 12, e2301518. [Google Scholar] [CrossRef]
- Dutta, S.; Chakraborty, P.; Basak, S.; Ghosh, S.; Ghosh, N.; Chatterjee, S.; Dewanjee, S.; Sil, P.C. Synthesis, characterization, and evaluation of in vitro cytotoxicity and in vivo antitumor activity of asiatic acid-loaded poly lactic-co-glycolic acid nanoparticles: A strategy of treating breast cancer. Life Sci. 2022, 307, 120876. [Google Scholar] [CrossRef]
- González-Fernández, F.M.; Bianchera, A.; Gasco, P.; Nicoli, S.; Pescina, S. Lipid-Based Nanocarriers for Ophthalmic Administration: Towards Experimental Design Implementation. Pharmaceutics 2021, 13, 447. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Ramalingam, P.; Karthivashan, G.; Ko, Y.T.; Choi, D.K. Recent developments in solid lipid nanoparticle and surface-modified solid lipid nanoparticle delivery systems for oral delivery of phyto-bioactive compounds in various chronic diseases. Int. J. Nanomed. 2018, 13, 1569–1583. [Google Scholar] [CrossRef]
- Chutoprapat, R.; Witarat, J.; Jongpanyangarm, P.; Mang Sung Thluai, L.; Khankaew, P.; Wah Chan, L. Development of solid lipid microparticles (SLMs) containing asiatic acid for topical treatment of acne: Characterization, stability, in vitro and in vivo anti-acne assessment. Int. J. Pharm. 2024, 654, 123980. [Google Scholar] [CrossRef] [PubMed]
- Islamie, R.; Myint, S.L.L.; Rojanaratha, T.; Ritthidej, G.; Wanakhachornkrai, O.; Wattanathamsan, O.; Rodsiri, R. Neuroprotective effect of nose-to-brain delivery of Asiatic acid in solid lipid nanoparticles and its mechanisms against memory dysfunction induced by Amyloid Beta1-42 in mice. BMC Complement. Med. Ther. 2023, 23, 294. [Google Scholar] [CrossRef] [PubMed]
- Garanti, T.; Stasik, A.; Burrow, A.J.; Alhnan, M.A.; Wan, K.W. Anti-glioma activity and the mechanism of cellular uptake of asiatic acid-loaded solid lipid nanoparticles. Int. J. Pharm. 2016, 500, 305–315. [Google Scholar] [CrossRef]
- Patel, P.; Patel, M. Nanostructured Lipid Carriers- A Versatile Carrier for Oral Delivery of Lipophilic Drugs. Recent Pat. Nanotechnol. 2021, 15, 154–164. [Google Scholar] [CrossRef]
- Halder, T.; Patel, B.; Acharya, N. Asiatic Acid Fabricated Nanoconstructs to Mitigate Amyloid Beta1-42 Induced Injury in SH-SY5Y Cells In-Vitro and Ameliorates Cognitive Impairment by Dual Cholinesterase Inhibition and Attenuation of Oxidative Stress In-Vivo. Pharm. Res. 2023, 40, 197–213. [Google Scholar] [CrossRef]
- Zhang, Y.-W.; Tu, L.-L.; Zhang, Y.; Pan, J.-C.; Zheng, G.-L.; Yin, L.-N. Liver-targeted delivery of asiatic acid nanostructured lipid carrier for the treatment of liver fibrosis. Drug Deliv. 2021, 28, 2534–2547. [Google Scholar] [CrossRef]
- Wen, Z.; Zhang, W.; Wu, W. The latest applications of exosome-mediated drug delivery in anticancer therapies. Colloids Surf. B Biointerfaces 2025, 249, 114500. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhu, J.; Chai, J.; Zhao, Y.; Luan, J.; Wang, Y. Application of exosomes as nanocarriers in cancer therapy. J. Mater. Chem. B 2023, 11, 10595–10612. [Google Scholar] [CrossRef]
- Wu, W. Inhibitory Effect of Exosome-Encapsulated Asiatic Acid on Esophageal Cancer and Its Effect on TGF-β/Smad Signaling Pathway. Master’s Thesis, Nanjing Normal University, Nanjing, China, 2019; p. 75. [Google Scholar]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Hong, S.S.; Kim, J.H.; Li, H.; Shim, C.K. Advanced formulation and pharmacological activity of hydrogel of the titrated extract of C. asiatica. Arch. Pharmacal Res. 2005, 28, 502–508. [Google Scholar] [CrossRef]
- Li, M.; Wang, Q.; Chen, N.; Yao, S.; Sun, X.; Quan, P.; Chen, Y. Probing Pharmaceutical Strategies to Promote the Skin Delivery of Asiatic Acid from Hydrogels: Enhancement Effects of Organic Amine Counterions, Chemical Enhancers, and Microneedle Pretreatment. Pharmaceutics 2022, 14, 2532. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Sun, L.; Xu, D.; Miao, S.; Li, N.; Zhao, Y. Melanin-Integrated Structural Color Hybrid Hydrogels for Wound Healing. Adv. Sci. 2023, 10, e2300902. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, S.; Guan, Q.; Li, P.; Fan, Y. Enhancing Chronic Wound Healing through Engineering Mg2+-Coordinated Asiatic Acid/Bacterial Cellulose Hybrid Hydrogels. ACS Appl. Mater. Interfaces 2024, 16, 8238–8249. [Google Scholar] [CrossRef] [PubMed]
- Qadir, A.; Ullah, S.; Gupta, D.K.; Khan, N.; Warsi, M.H.; Kamal, M. Combinatorial drug-loaded quality by design adapted transliposome gel formulation for dermal delivery: In vitro and dermatokinetic study. J. Cosmet. Dermatol. 2023, 22, 2839–2851. [Google Scholar] [CrossRef]
- Opatha, S.A.T.; Titapiwatanakun, V.; Boonpisutiinant, K.; Chutoprapat, R. Preparation, Characterization and Permeation Study of Topical Gel Loaded with Transfersomes Containing Asiatic Acid. Molecules 2022, 27, 4865. [Google Scholar] [CrossRef] [PubMed]
- Mahadev, M.; Ballal, S.; Shetty, A.; Dubey, A.; Shetty, S.S.; Hebbar, S.; El-Zahaby, S.A. Development and evaluation of chitosan-coated virgin coconut oil-asiatic acid-loaded nanoemulgel for enhanced wound management. Int. J. Biol. Macromol. 2025, 299, 140097. [Google Scholar] [CrossRef] [PubMed]
- Batrakova, E.V.; Kim, M.S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J. Control. Release 2015, 219, 396–405. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Y.; Chen, Q.; Li, M.; Xu, L.; Lin, B.; Tan, Y.; Liu, Z. DNA Nanostructures: Advancing Cancer Immunotherapy. Small 2024, 20, e2405231. [Google Scholar] [CrossRef]
- Zheng, M.; Song, W.; Huang, P.; Huang, Y.; Lin, H.; Zhang, M.; He, H.; Wu, J. Drug conjugates crosslinked bioresponsive hydrogel for combination therapy of diabetic wound. J. Control. Release 2024, 376, 701–716. [Google Scholar] [CrossRef]
- Li, J.; Ni, W.; Aisha, M.; Zhang, J.; Sun, M. A rutin nanocrystal gel as an effective dermal delivery system for enhanced anti-photoaging application. Drug Dev. Ind. Pharm. 2021, 47, 429–439. [Google Scholar] [CrossRef]
- Zhao, Z.; Ukidve, A.; Krishnan, V.; Mitragotri, S. Effect of physicochemical and surface properties on in vivo fate of drug nanocarriers. Adv. Drug Deliv. Rev. 2019, 143, 3–21. [Google Scholar] [CrossRef]
- Mittal, P.; Singla, M.; Smriti; Kapoor, R.; Kumar, D.; Gupta, S.; Gupta, G.; Bhattacharya, T. Paclitaxel loaded Capmul MCM and tristearin based nanostructured lipid carriers (NLCs) for glioblastoma treatment: Screening of formulation components by quality by design (QbD) approach. Discov. Nano 2024, 19, 175. [Google Scholar] [CrossRef]
- Mauser, A.; Waibel, I.; Banerjee, K.; Mujeeb, A.A.; Gan, J.; Lee, S.; Brown, W.; Lang, N.; Gregory, J.; Raymond, J.; et al. Controlled Delivery of Paclitaxel via Stable Synthetic Protein Nanoparticles. Adv. Ther. 2024, 7, 2400208. [Google Scholar] [CrossRef] [PubMed]
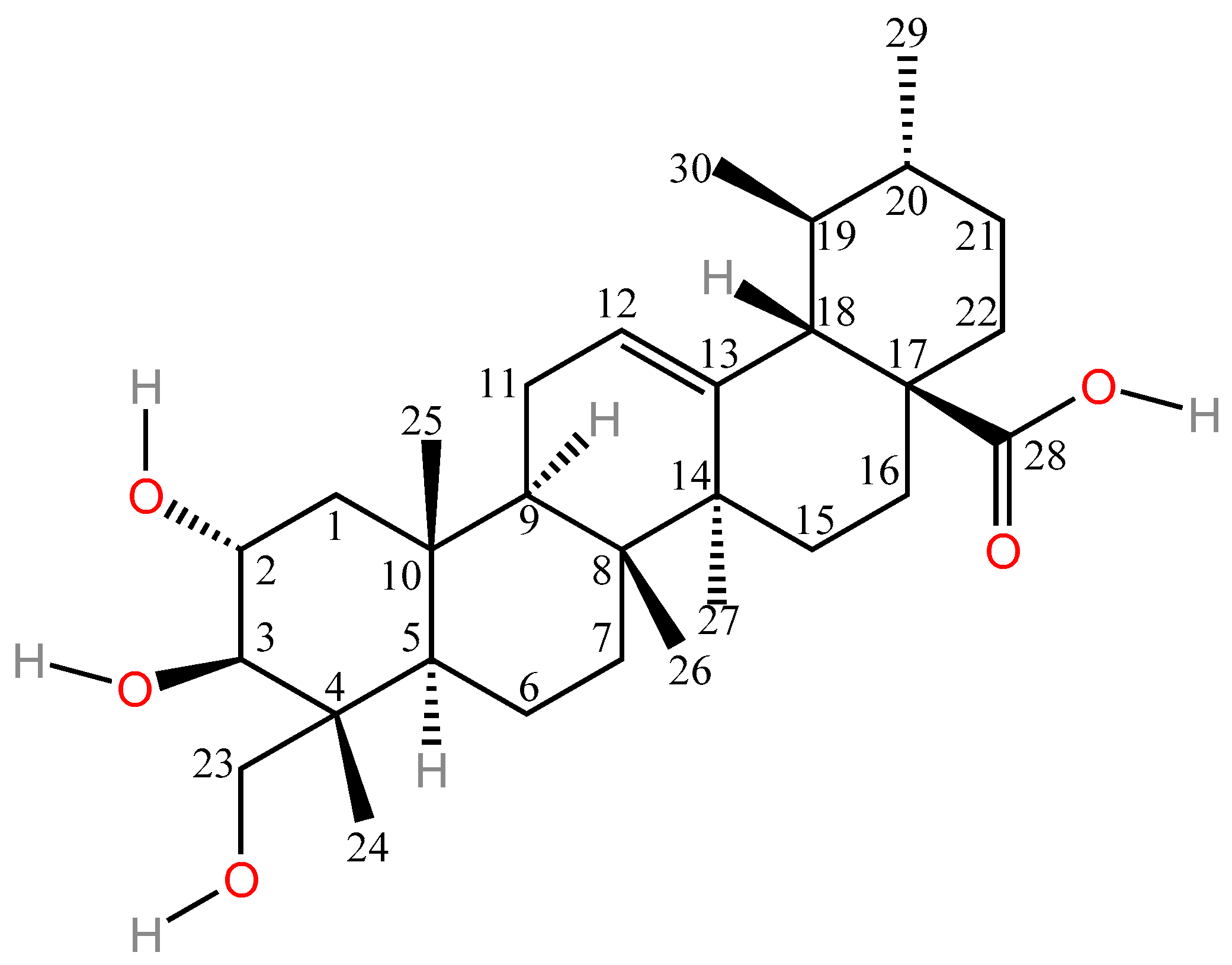

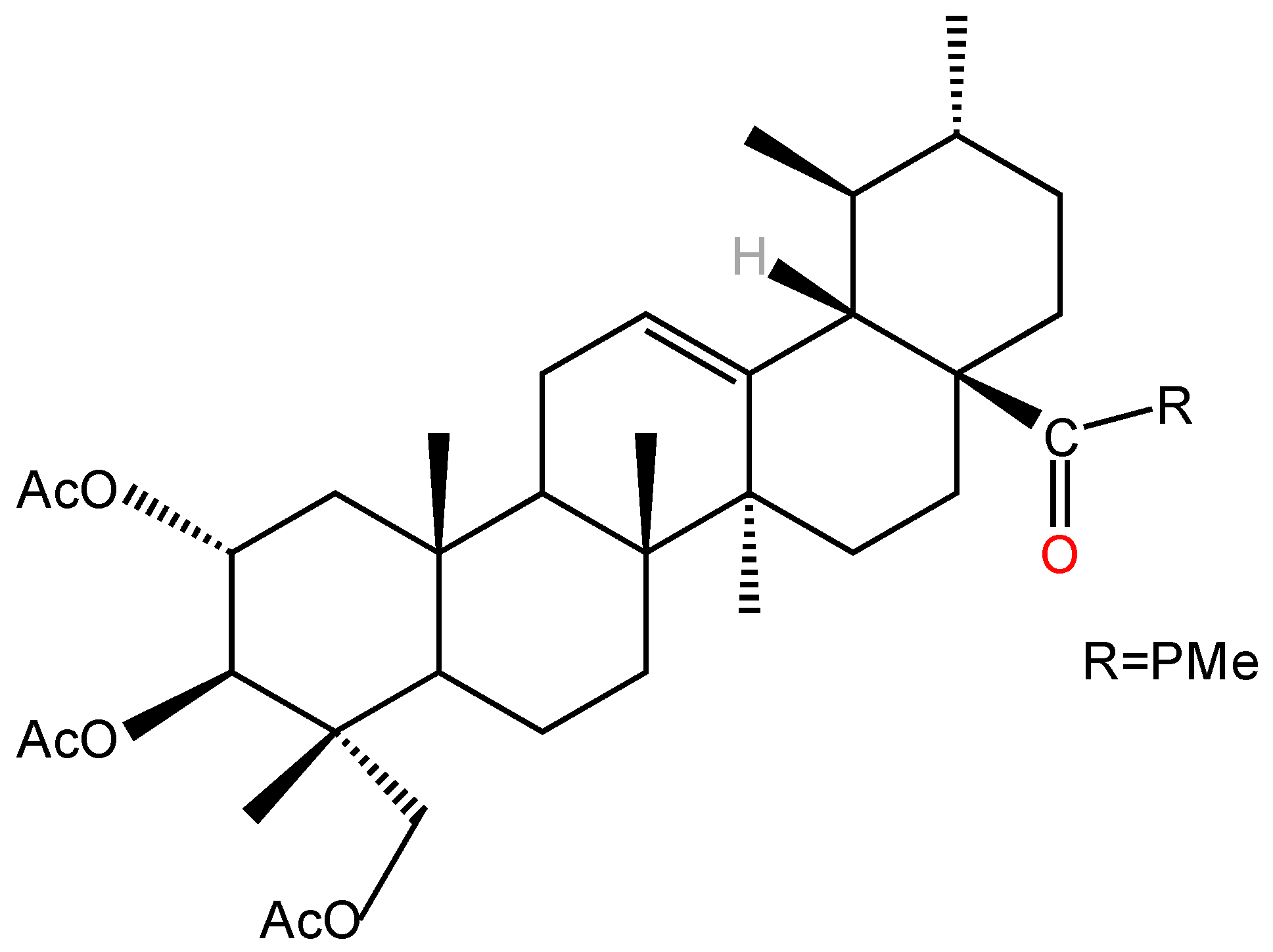
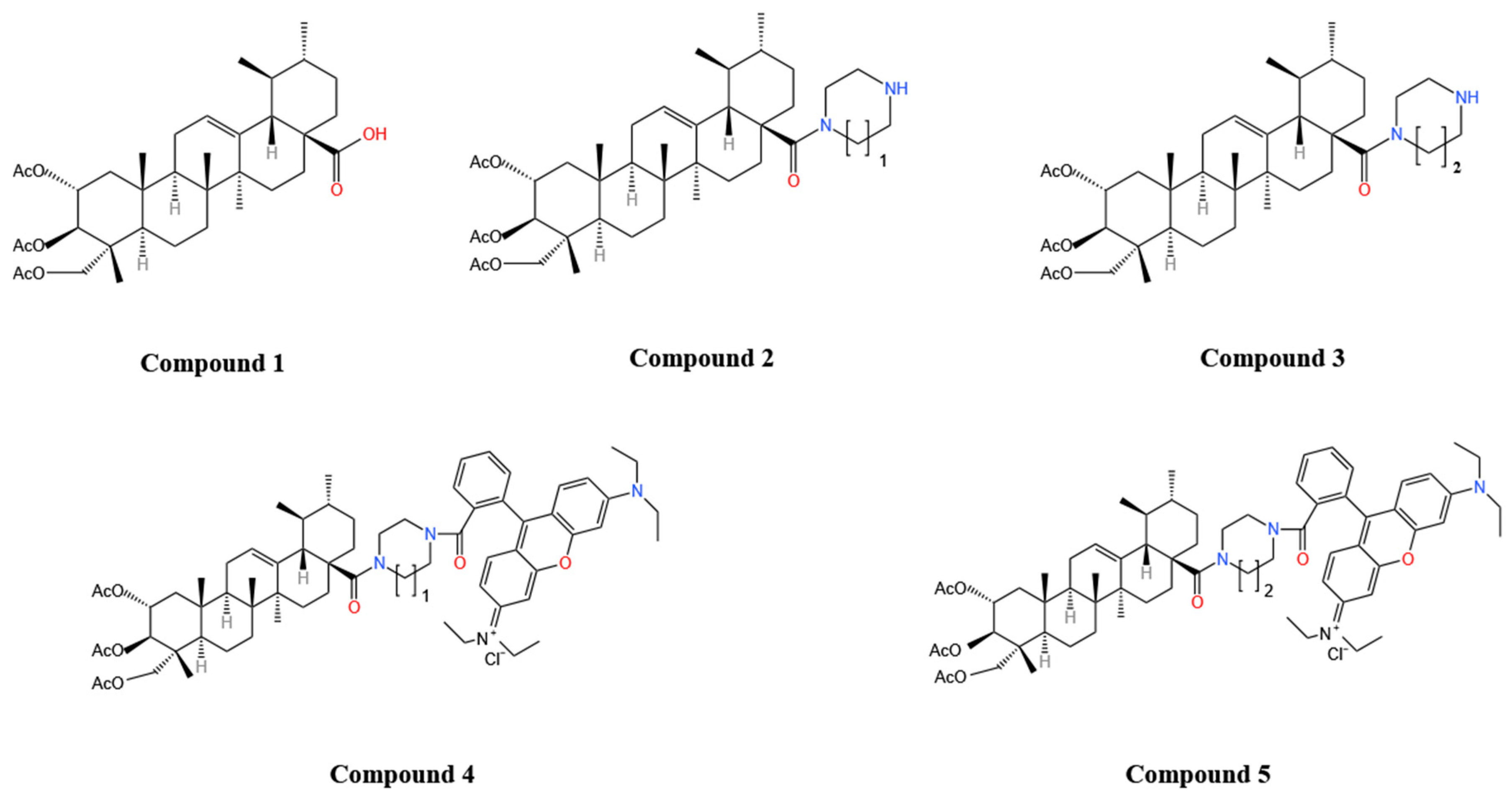
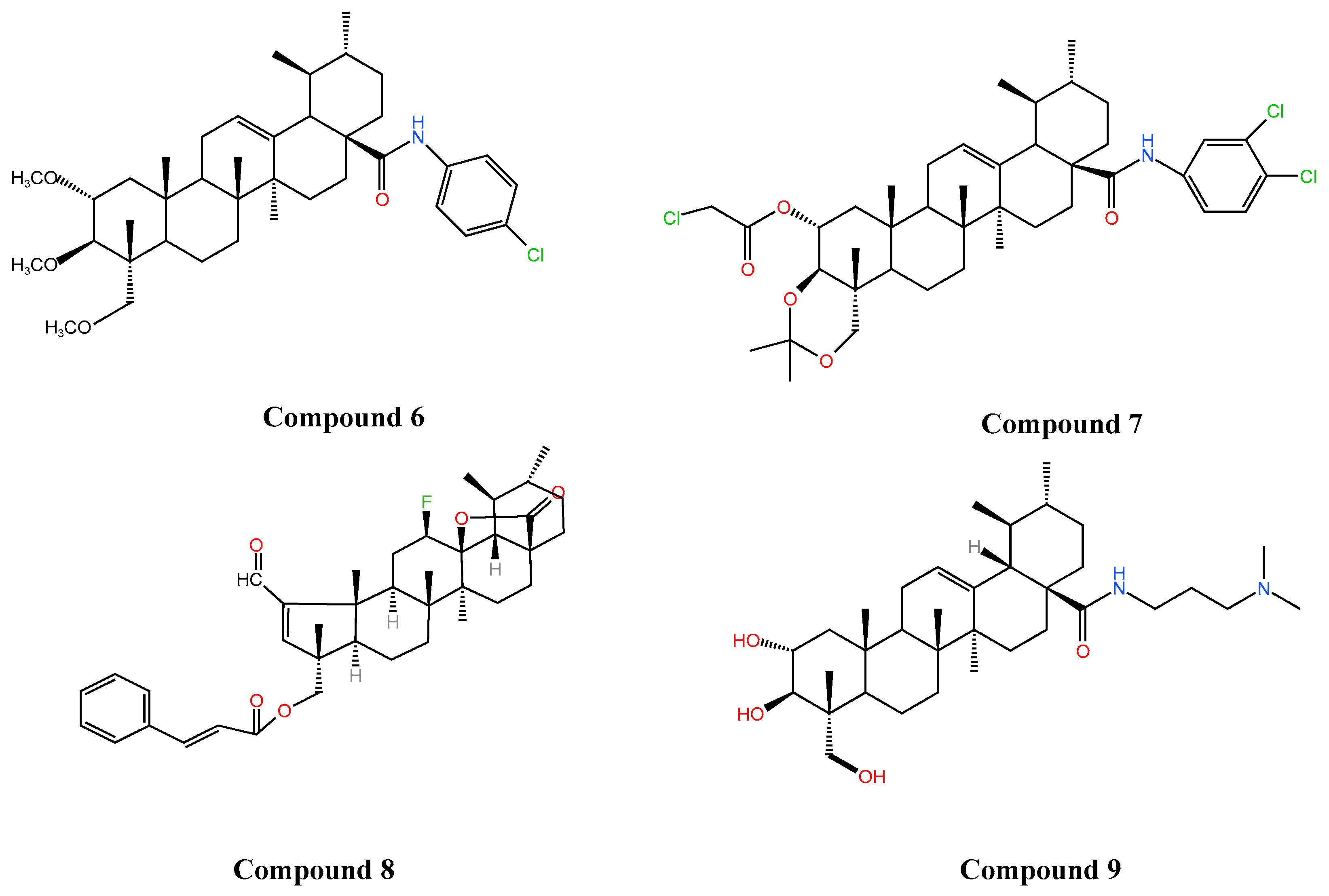
| Types of Inflammation | Animal/Cell | Dosages | Pathway | Results | Ref. |
|---|---|---|---|---|---|
| Endometritis | mouse endometrial epithelial cells | 20, 40, 80 µM | TLR4/NF-κB, PPARγ | TNFα ↓, IL1β ↓, PGE2 ↓, NO ↓ | [17] |
| Neuroinflammation | SH-SY5Y cells | 10 nM | NLRP3 | IL-1β ↓, TNFα ↓, Caspase-1 ↓, mtROS ↓ | [18] |
| Rheumatoid arthritis | RA-FLS cells | 80, 100 µM | Nrf2/HO-1, NF-κB | NF-κB ↓, Bax ↑, caspase-3 ↑, Bcl-2 ↓, Proliferation of RA-FLS ↓ | [19] |
| Osteoarthritis | Chondrocytes cells | 5, 10, 25 µM | PPARγ, NF-κB | iNOS ↓, Cox2 ↓, Mmp13 ↓ | [20] |
| Alcoholic fatty hepatitis | Raw264.7 cells C57BL/6J mice | 5, 25, 50 mg·kg−1 | NF-κB | NF-κB-Pp65 ↓, TNF-α ↓, IL-1β ↓, IL-6 ↓ | [21] |
| Atopic dermatitis | HaCaT cells BALB/c mice | 5, 10, 20 μg·mL−1 30, 75 mg·kg−1 | NF-κB, MAPK | COX-2 ↓, CXCL9 ↓, IL-6 ↓, TNF-α ↓, IL-8 ↓, NF-κB ↓, MAPK ↓, p-p38 ↓, p-JNK ↓, p-ERK1/2 ↓ | [22] |
| Inflammations | BV-2 cells | 12.5 µM | NF-κB, NLRP3 | IL-1β ↓, IL-6 ↓, IL-18 ↓, lncRNA ↑, TVX1 ↑, p-p65 ↓ Caspase 1 ↓ | [23] |
| BV-2 cells | 1, 10, 100 µM | Sirt1/NF-κB | Sirt1 ↑, NF-κB-p65 ↓, TNF-α ↓, IL-1β ↓, IL-6 ↓, NO ↓, iNOS ↓ | [24] | |
| ICR mice | 1, 5, 10 mg·kg−1 | NF-κB | CAT ↑, SOD ↑, GPx ↑ MDA ↓, iNOS ↓, COX-2 ↓, NF-κB ↓ | [25] | |
| Ulcerative colitis | THP-1 Cells female C57BL/6 mice | 15, 30, 60 µM 3, 10, 30 mg·kg−1 | — | TNF-α ↓, IL-1β ↓, IL-6 ↓, IFN-γ ↓, NLRP3 ↓ | [26] |
| Salmonella-induced colitis | Balb/c mice | 10 mg·kg−1 | — | claudin-2 ↑, claudin-7 ↑ IL-1β ↓, IL-6 ↓, TNF-α mRNA ↓ | [16] |
| Ulcerative colitis | Wistar rats | 20, 40 mg·kg−1 | NF-κB | TNF-α ↓, IL-1β ↓, PGE2 ↓, MCP-1 ↓, NF-κB p65 ↓ | [27] |
| Acute pulpitis | Wistar rats | 0.5%, 1%, 2.0% (v/w) | Nrf2/ARE, NF-κB/MAPK | NF-κB ↓, MAPK ↓, TNF-α ↓, MDA ↓, CGRP ↑, SOD ↑, β-endorphin ↑ | [28] |
| Cystitis | Wistar rats | 30 mg·kg−1·day−1 | NF-κB, NLRP3 | IL-1β ↑, IL-6 ↑, NGF ↑, TNF-α ↑, ORM1 ↑, HPX ↑, MDA ↓ | [29] |
| Types of Cancer | Animal/Cell | Dosages | Pathway | Results | Ref. |
|---|---|---|---|---|---|
| Breast cancer | HUVE Cells BALB/c mice | 40 µM 50 mg·kg−1 | VEGF/VEGFR2 | VEGF ↓, VEGFR2 ↓, ERK1/2 ↓, p-Src ↓, p-FAK ↓ | [36] |
| MCF-7, MDA-MB-231 Cells | — | PI3K/AKT | WAVE3 ↓, P53 ↓, p-PI3K ↓, p-AKT ↓ | [37] | |
| MCF-7 Cells | 40, 80, 160 µM | AMPK | AMPKα ↓, ROS ↑, ATP ↓, P-gp ↑ | [41] | |
| Nasopharyngeal carcinoma | NPC-bm, NPC-039 Cells | 50, 75 µM | p38/MAPK | Bax ↑, p-38 ↑, JNK ↑, Caspase-3 ↑ | [42] |
| TW-01, SUNE5-8F Cells | — | — | STAT3 ↓, Claudin-1 ↓, caspase-3 phosphorylation ↑ | [49] | |
| Non-small cell lung cancer | A549, DDP Cells | — | MALAT1/miR-1297/p300/β-catenin ↓, | MALAT1 ↓, p300 ↓, β-catenin ↓, MDR1 ↓, cleaved caspase-3 ↑, miR-1297 ↑, | [50] |
| A549, NSCLC Cells | 50, 100 µM | — | miR-1290 ↑BCL2 ↓ | [51] | |
| A549, H460, HNSCLC Cells | 30, 60 µM | PI3K/Akt/mTOR, MAPK/ERK | MAPK/ERK ↓, Akt ↓, VEGF ↓, COX-2 ↓, PI3K ↓, mTOR ↓, HIF-1 ↓ | [40] | |
| Lung cancer | A549 cells, H1299 cells C57BL/6J mice | 20, 40, 80 µM 50, 100 mg·kg−1 | — | Mitochondrial functions ↓, PARP ↑, caspase-9 ↑, caspase-3 ↑ | [52] |
| A549 Cells | 40 µmol·L−1 | TGF-β1/Snail, Wnt/β-catenin | E-cadherin ↑, Snail ↓, N-cadherin ↓, vimentin ↓, β-catenin ↓, p-GSK-3β ↓, | [53] | |
| Ovarian cancer | SKOV3, OVCAR-3 cells | 10, 40 μg·mL−1 | PI3K/Akt/mTOR | PI3K ↓, Akt ↓, mTOR ↓ | [38] |
| Renal cell carcinoma | 786-O, A-498, Caki-1, ACHN Cells | 40 µM | ERK/p38MAPK | p-ERK1/2 ↓, p-p38MAPK ↓, MMP-15 ↓ | [39] |
| Tongue cancer | BALB/cANNCjr nu/nu mice | 40 µM 15 mg·kg·d−1 | Grp78/IRE1α/JNK | Grp78 ↑, P-JNK ↑, P-IRE1α ↑, caspase-3 ↑, Bcl-2 ↓ | [54] |
| Prostate cancer | 22Rv1, PC3, DU145 Cells | 20, 30 µM | MZF-1/Elk-1/Snail | MZF-1 ↓, Elk-1 ↓, Snail ↓, MEK3/6-p38/MAPK ↓ | [55] |
| Skin cancer | SK-MEL-2 Cells | 20 µM | — | ROS ↑, bax ↑, caspase-3 ↑ | [56] |
| Modification Site | Modification Type | Physical Property Changes | Pharmacological Effect Changes | Ref. |
|---|---|---|---|---|
| C-2, C-3, C-23; C-28 | Acetylation; Esterification | Stability ↑ (No decomposition at 37 °C and −20 °C) | Anti-tumor activity ↑ (Decreased IC50 for multiple tumor cells); Inhibition of angiogenesis ↑; Toxicity to normal cells ↓; Ability to induce cancer cell apoptosis ↑ | [99,102,103] |
| C-2, C-3, C-23; C-28 | Acetylation; Rhodamine B conjugation | Liposolubility ↑ | Anti-tumor activity ↑ | [104] |
| C-23 | Esterification | Liposolubility ↑ | Anti-tumor activity ↑ (The smaller the amide group at the C-23 position, the stronger the antitumor activity of the derivative.) | [3] |
| C-11; C-28 | Aniline substituent; Amidation | Water solubility ↑; Membrane permeability ↑ | Anti-tumor activity ↑ (After C-28 amidation, the IC50 of the compound against HepG2 liver cancer cells decreased from 34.9 µM to 5.97 µM.) | [5] |
| C-28 | Amidation | — | Anti-tumor activity ↑ (Increased toxicity to HepG2 and SGC7901 cells); Inhibition of VEGF secretion and VEGFR phosphorylation | [109,110] |
| C-2, C-3, C-23; C-12; C-28 | Acetylation; Fluorination; Amidation | — | Anti-proliferative activity ↑ | [112] |
| C-28 | Esterification; Amidation | Liposolubility ↑; Water solubility ↑ | Activity of the derivatives against Rabbit Muscle GPa ↑ (When a lipophilic derivative is introduced at the C-28 position); Activity of the derivatives against Rabbit Muscle GPa ↓ (When amino acid derivatives are introduced at the C-28 site) | [116] |
| C-2, C-3, C-23; C-28 | Sulfonylation; Amidation | Water solubility ↑ | Inhibitory activity against hCA VA ↑ | [117] |
| C-2; C-28 | Oxidation; Anhydride formation | — | Cognitive enhancement ability ↑ | [118] |
| C-2, C-3, C-23; C-28 | Acetylation; Amidation | Water solubility ↑ | Induce osteogenic differentiation of hPDLSCs cells ↑ (Osteogenic activity when dimethylaminopropylamine was introduced at C-28 site ↑; Osteogenic activity when introducing long-chain alkyls ↓) | [120] |
| C-28 | Esterification | Water solubility ↑ | Cytotoxicity ↓; Induction of osteogenic potential ↑ (Osteogenesis can be induced at low concentrations (1–10 µM)) | [121] |
| Types of Formulations | Formulation Name | Carrier Material/Structure | Preparation Technology | Release Model | Particle Size | Drug Loading/Content | Encapsulation Efficiency | Route of Administration | Effect | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Nanoparticle | AA/CDM-BT-ALG | CDM-BT-ALG | Solvent evaporation technique | Anomalous Diffusion | 37.8 ± 7.1 nm | 13.0 ± 1.0% | 99.3 ± 7.5% | Oral administration | Increase intracellular drug concentration; prolong drug release time in the body | [133] |
| AA-loaded BSA NPs | Bovine Serum Albumin, BSA | Modified desolvation technique | Biphasic Release | 228.66 ± 2.51 nm | 16.33 ± 1.52% | 60.00 ± 1.00% | Injection administration | Extended drug release time in the body; 10-fold increase in drug bioavailability in the brain | [8] | |
| AA-PLGA NPs | PLGA (polylactic acid-hydroxyacetic acid copolymer) | Multiple emulsion solvent evaporation technique | Biphasic Release | 359.6 nm | 6.08 ± 0.29% | 65.63 ± 1.88% | Injection administration | Selective cytotoxicity, reducing the volume and mass of breast tumors in mice; prolonging the release time of drugs in the body. | [134] | |
| Tf-AA-PLGA NPs | PLGA; Transferrin (Tf) | Single Emulsion-Solvent Evaporation method | Biphasic Release | 149 ± 2 nm | 3.3 ± 0.1% | 66 ± 3% | Injection administration | Tf modification significantly enhances the uptake of nanoparticles in U87 cells, improving antitumor activity and reducing toxicity to healthy cells. | [12] | |
| Solid lipid nanoparticle | AA-SLN | Glyceryl monostearate | Hot Melt emulsification | Anomalous Diffusion | 189.27 ± 4.22 nm | 2.26 mg·mL−1 | — | Intranasal administration | Combined with intranasal administration, this avoids the first-pass effect and increases the concentration of the drug in the brain. | [138] |
| AA-MS-SLNs; AA-DS-SLNs: AA-TS-SLNs | Glyceryl monostearate; Glyceryl distearate; Glyceryl tristearate | Solvent evaporation and hot homogenisation technique. | Anomalous Diffusion | 141.7 ± 1.7 nm; 141.3 ± 2.5 nm; 126.9 ± 0.5 nm | 5.0 ± 0.25%; 2.1 ± 0.30%; 3.1 ± 0.12% | 98. ± 0.05%; 98.1 ± 0.60%; 99.9 ± 0.04% | Injection administration | Extending drug action time; SLNs enhance AA’s targeted toxicity to glioblastoma cells while reducing damage to normal cells. | [139] | |
| Liposomes | CLAA | Soybean lecithin; Chitosan; Cholesterol | Solvent evaporation technique | Higuchi model | 209.8 nm | 68 ± 0.04% | 71.2 ± 0.1% | Oral administration | Extended drug retention time in the intestine, AUC increased by 2.9 times, T1/2 extended to 3.49 h; improved drug permeability in the intestine. | [9] |
| Nanostructured lipid carriers | AA-NLC | Glyceryl Monostearate; Oleic Acid; Soybean Lecithin | Hot-melt emulsification technique | Anomalous Diffusion | 44.1 ± 12.4 nm | 20% | 73.41 ± 2.53% | Injection administration | Enhance the penetration and absorption of AA through the blood–brain barrier; Extending the retention time of the drug in the body, the C max and AUC0–t in the brain were increased by 2.28 and 2.99 times, respectively, compared to the AA suspension, and T1/2 was extended to 15.57 h. | [141] |
| P-AA-NLC | Glyceryl Monostearate; Oleic Acid; PEG2000-SA | Solvent Diffusion method | Anomalous Diffusion | 160.50 ± 4.16 nm | 19.03 ± 0.18% | 93.3 ± 0.9% | Oral administration | Protect drugs from dissolution by stomach acid, improve drug bioavailability, and give drugs a certain degree of liver targeting. | [11] | |
| UP-AA-NLC | UA-PEG-SA (Ursodeoxycholic acid-polyethylene glycol-stearic acid); Glyceryl Monostearate; Oleic Acid | Solvent Diffusion method | Ritger–Peppas model | 159.7 ± 4.9 nm | 10.53 ± 0.10% | 77.44 ± 0.69% | Oral administration | Increases drug concentration in the liver (6.2 times higher than free AA) and prolongs drug retention time in the body. | [142] | |
| Exosomes | AA-loaded EXOs-K; AA-loaded EXOs-T | EXOs-K;EXOs-T | Differential Ultracentrifugation | Biphasic Release | 122.7 ± 2.8 nm; 111.2 ± 3.4 nm | 7.9 ± 1.2%; 7.5 ± 0.8% | — | Oral administration | Slow and small release in blood or normal cell environment, continuous release in tumor sites | [145] |
| Gel formulations | AA hydrogel | Hydrogel containing 3.5% hyaluronic acid | Physical mixing method | Zero-level release pattern | — | 2.0% | — | Transdermal administration | Improve the penetration of drugs into the deep layers of the skin | [148] |
| Hybrid structural color hydrogel patch | FMA; AG; MNPs | Thermal Melting Infusion | Light/NIR-triggered release | — | 1 mg·mL−1 | — | Topical administration | Combining photothermal effects to control drug release; promoting wound healing and remodeling. | [149] | |
| AA-gel | Chitosan; Gelatin | Solvent casting method | Higuchi model | — | 20 µg·mL−1 | — | Topical administration | Accelerate wound healing, control drug release, and enhance antibacterial capacity. | [82] | |
| AA-Mg self-assembled hydrogels | Mg2+; Bacterial Cellulose | Self-assembly technology | Anomalous Diffusion | — | 7.7 mg·mL−1 | — | Topical administration | Maintain the sustained release of AA; accelerate wound healing rate; synergize with Mg2+ to exert anti-inflammatory and antibacterial effects. | [150] | |
| AA-TL | Lipoid S100; cholesterol; triethanolamine; Carbopol 934 | Thin-Film Hydration -Sonication | Higuchi model | — | — | 87.66 ± 2.12% (In the liposomes in the gel) | Topical administration | Improve the penetration efficiency of drugs into the skin; form a drug deposit layer in the skin to prolong the duration of action. | [151] | |
| TW80AATG | Soybean Lecithin; Tween 80; Span 80; sodium deoxycholate | High-Pressure homogenization method; | Higuchi model | — | 2.80 ± 0.05 mg·g−1 | — | Topical administration | Improve drug penetration and flux into the skin; | [152] | |
| CS-ASA-NEG | Oil Phase; Aspirin, ASA; Chitosan; Carbopol 934 | High-pressure homogenization method; Nano-emulsion gelation | Higuchi model | 131.80 ± 0.33 nm (In the liposomes in the gel) | 0.3% | 131.80 ± 0.33 nm (In the liposomes in the gel) | Topical administration | Improve drug penetration into the skin; increase drug retention time at the site of inflammation; improve biocompatibility. | [153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Wang, T.; Gao, C.; Cui, Y.; Li, L. Multi-Target Pharmacological Effects of Asiatic Acid: Advances in Structural Modification and Novel Drug Delivery Systems. Molecules 2025, 30, 3688. https://doi.org/10.3390/molecules30183688
Dong X, Wang T, Gao C, Cui Y, Li L. Multi-Target Pharmacological Effects of Asiatic Acid: Advances in Structural Modification and Novel Drug Delivery Systems. Molecules. 2025; 30(18):3688. https://doi.org/10.3390/molecules30183688
Chicago/Turabian StyleDong, Xiaofan, Tianyi Wang, Chenjia Gao, Yulong Cui, and Lingjun Li. 2025. "Multi-Target Pharmacological Effects of Asiatic Acid: Advances in Structural Modification and Novel Drug Delivery Systems" Molecules 30, no. 18: 3688. https://doi.org/10.3390/molecules30183688
APA StyleDong, X., Wang, T., Gao, C., Cui, Y., & Li, L. (2025). Multi-Target Pharmacological Effects of Asiatic Acid: Advances in Structural Modification and Novel Drug Delivery Systems. Molecules, 30(18), 3688. https://doi.org/10.3390/molecules30183688








