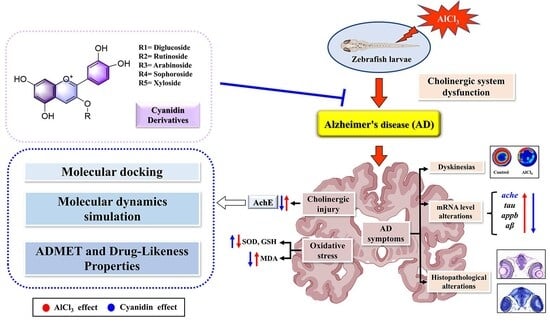
The Neuroprotective Effects of Cyanidin Derivatives on AlCl3-Induced Zebrafish Model of Alzheimer’s Disease
Abstract
1. Introduction
2. Results
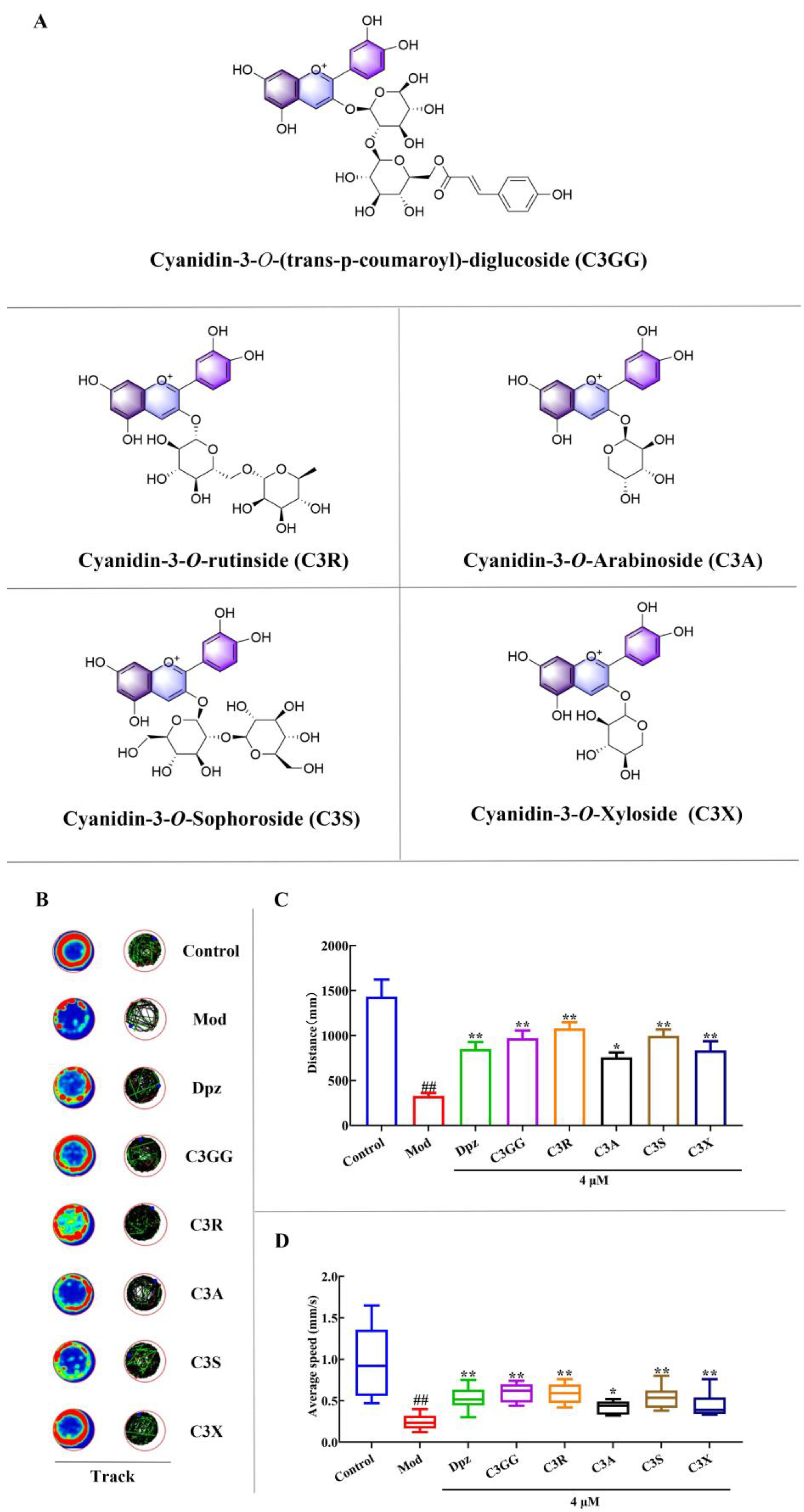
2.1. The Effects of Cyanidin Derivatives on AlCl3-Induced Locomotor Deficits of Zebrafish Larvae
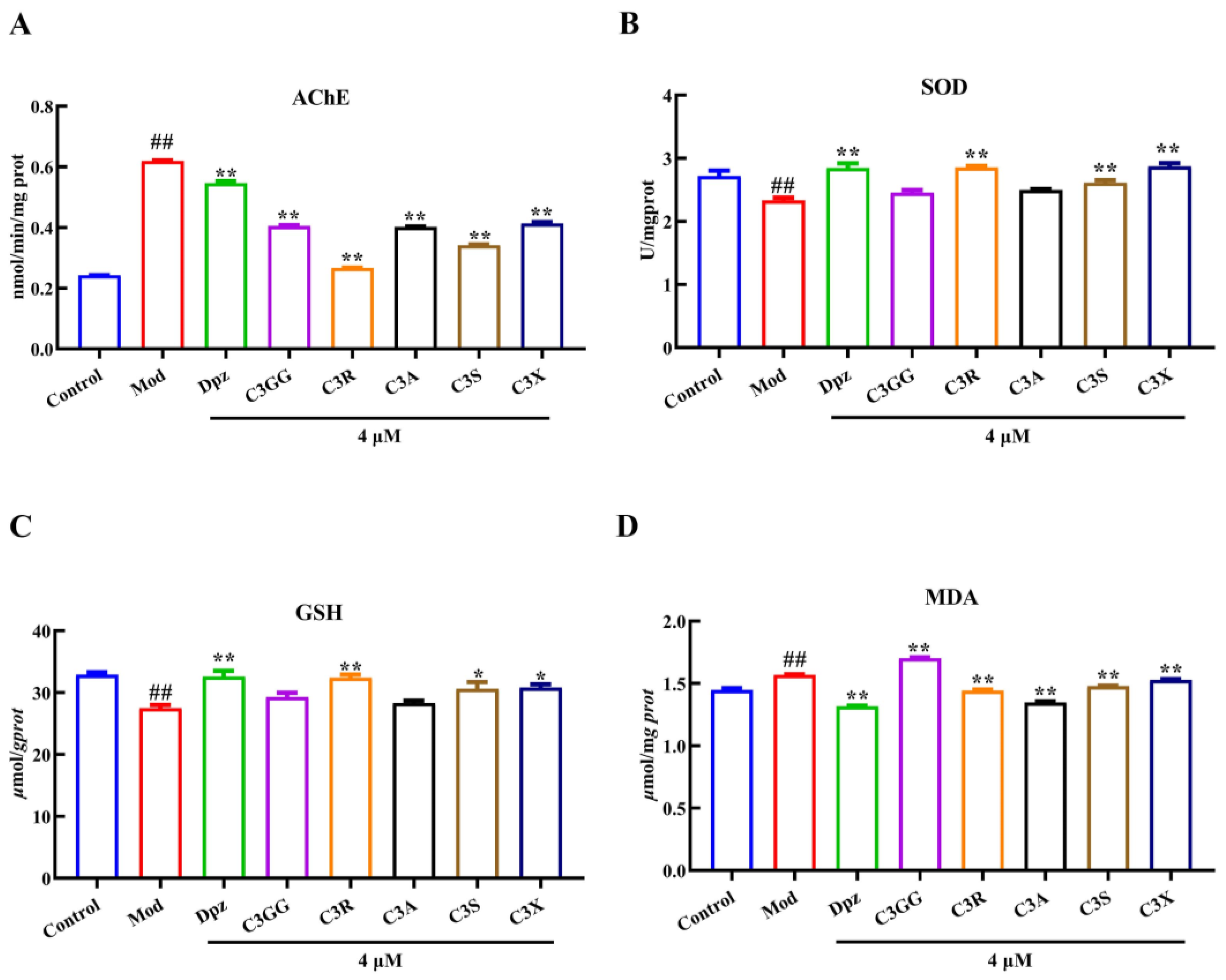
2.2. The Effects of Cyanidin Derivatives on AlCl3-Induced the Dysfunction of Cholinergic System and Oxidative Stress
2.3. The Effects of Cyanidin Derivatives on the Expression of AD-Related Genes
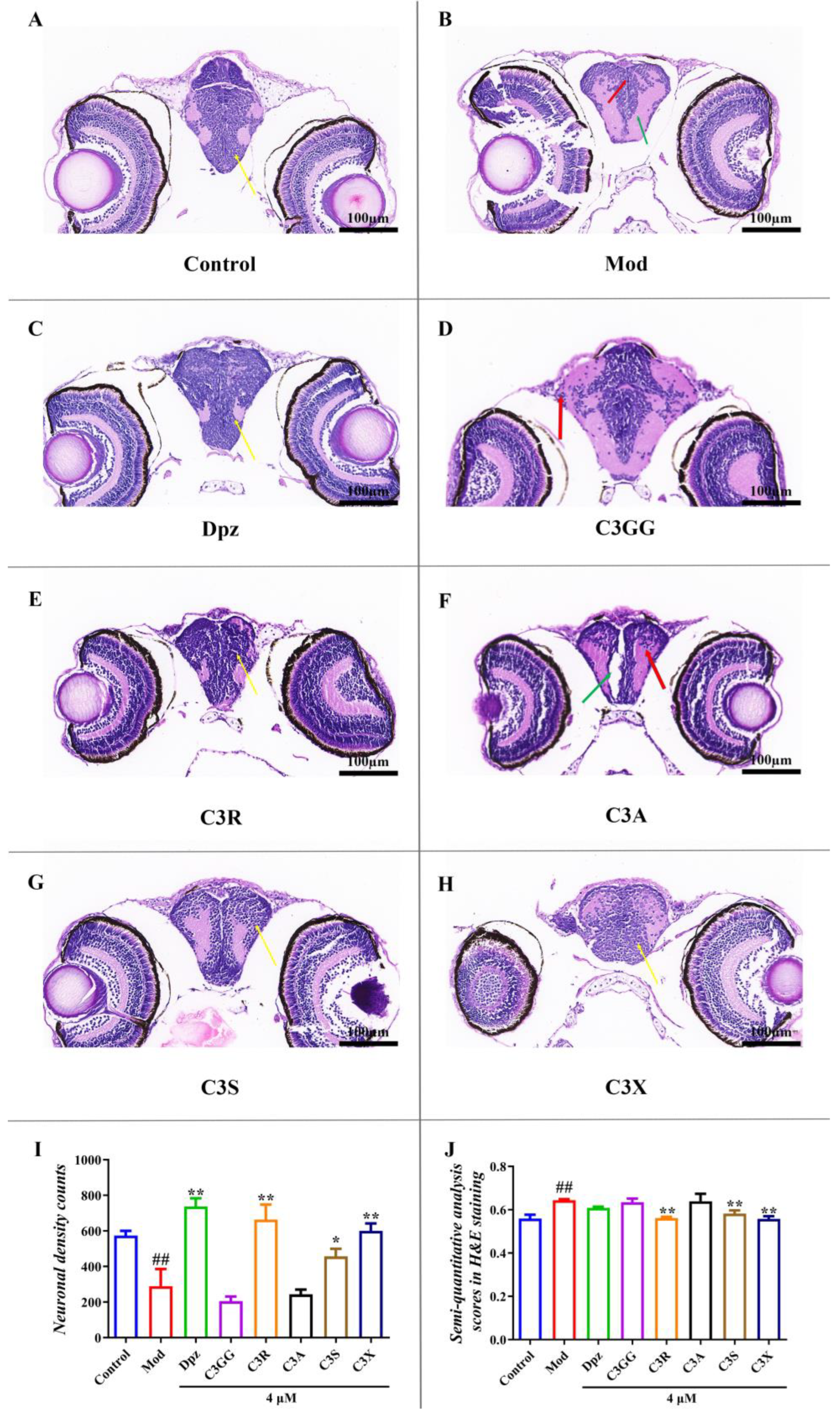
2.4. The Protect Effects of Cyanidin Derivatives on AlCl3-Induced Histopathological Lesions in the Larval Brain
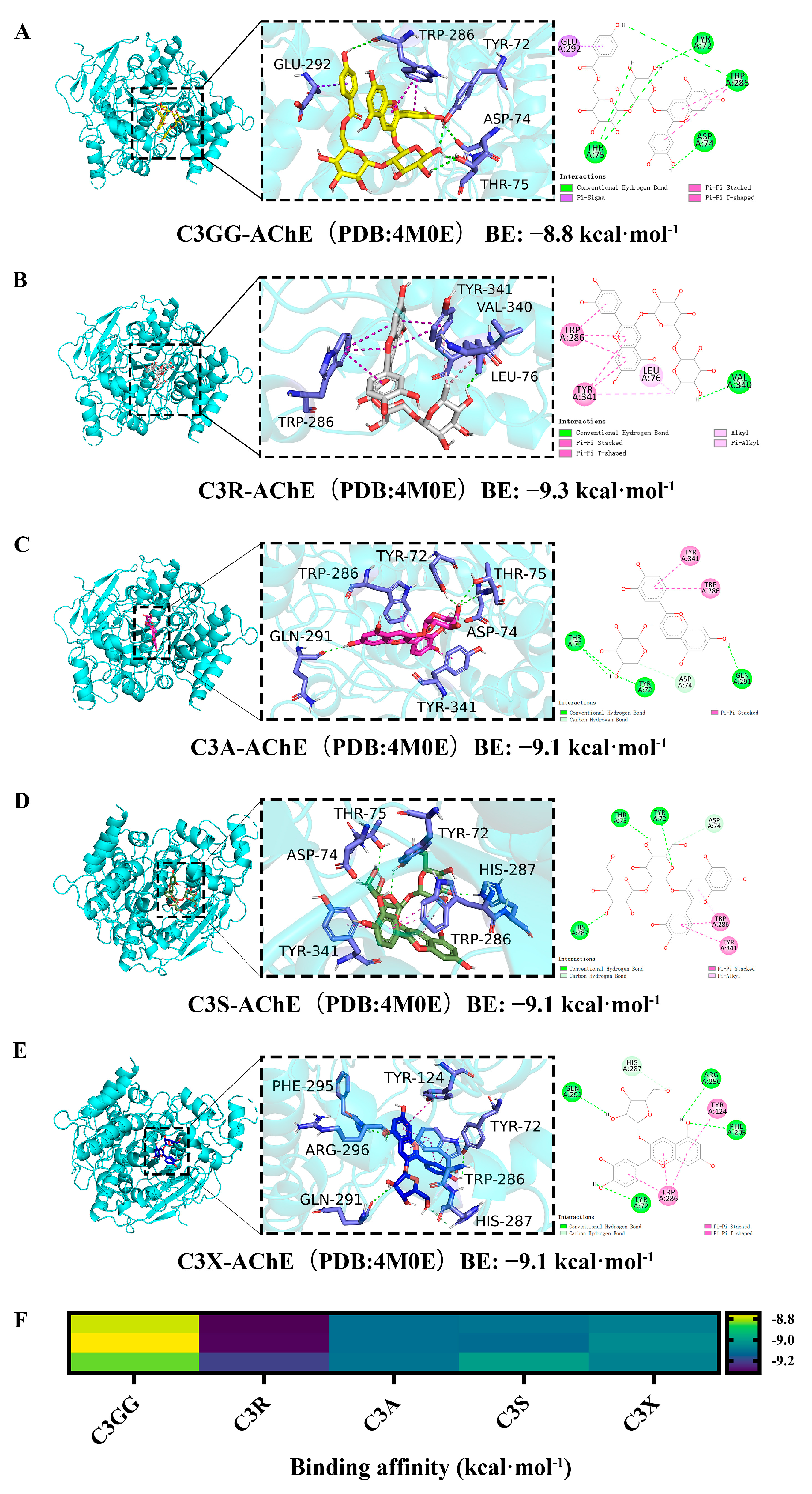
2.5. Molecular Docking Results
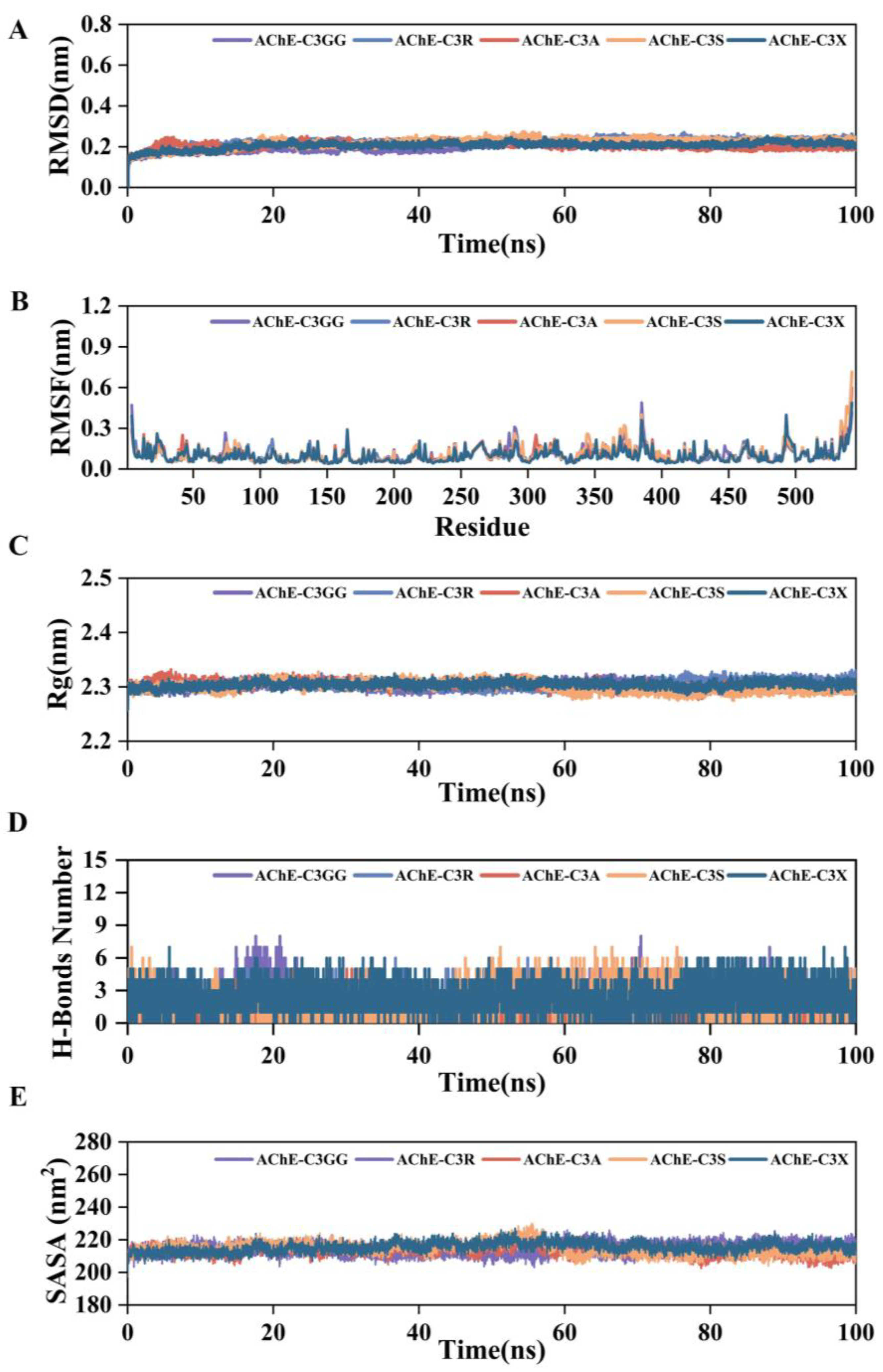
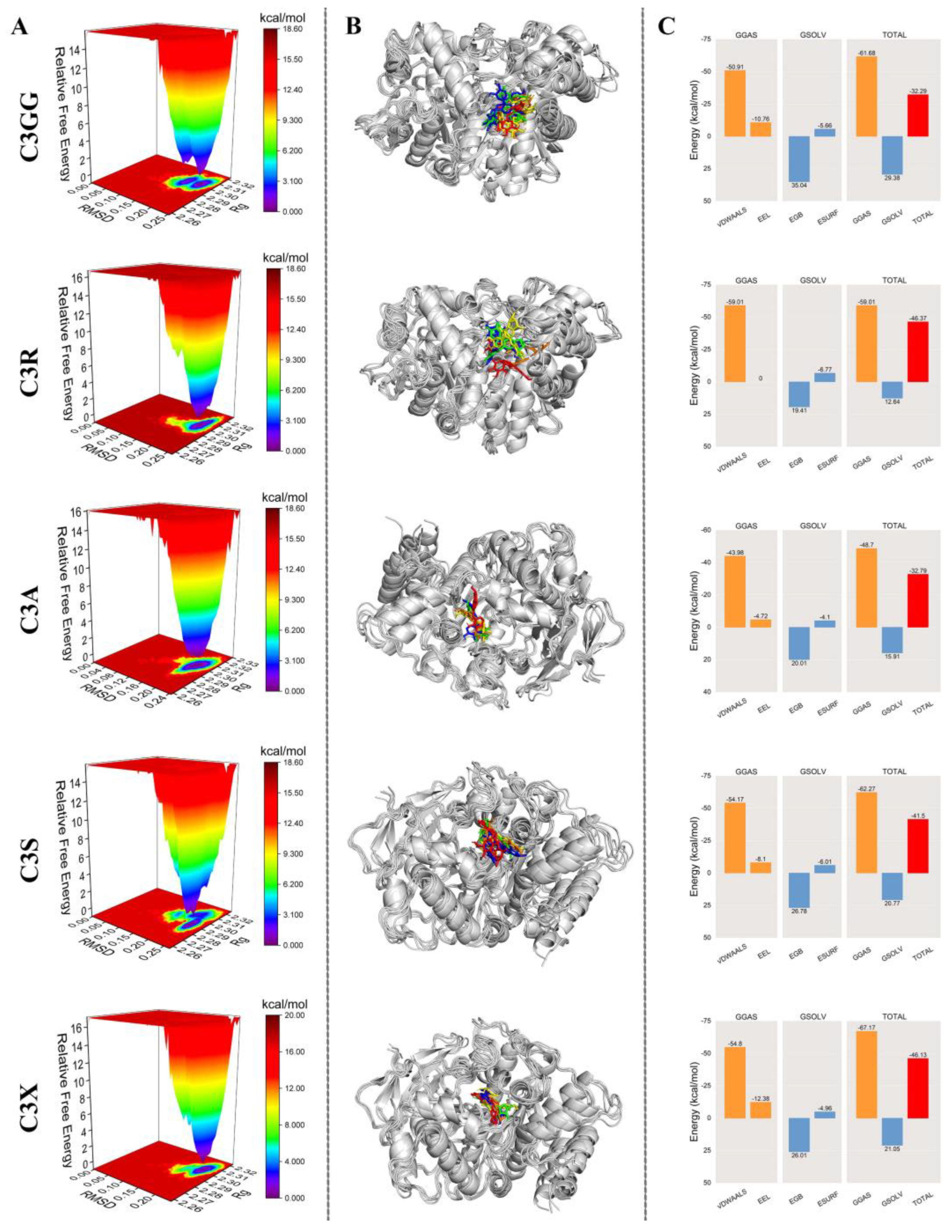
2.6. MD Simulation Results
2.7. Prediction of ADMET and Drug-Likeness Properties
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals
4.3. Establishment of Zebrafish AD Model and Grouping
4.4. Behavioral Analysis
4.5. Determination of Biochemical Indicator
4.6. Detection of Gene Expression
4.7. Hematoxylin-Eosin Staining (H&E) Staining and Nissl Staining
4.8. Molecular Docking
4.9. Molecular Dynamics Simulation
4.10. In Silico Prediction of ADMET and Drug-Likeness Properties of Cyanidin Derivatives
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AChE | Acetylcholinesterase |
| AD | Alzheimer’s disease |
| ADMET | Absorption, Distribution, Metabolism, Elimination, Toxicity |
| AlCl3 | Aluminum chloride |
| APP | amyloid precursor protein |
| BBB | Blood-brain barrier |
| BCA | Bicinchoninic acid |
| C3A | Cyanidin-3-O-arabinoside |
| C3GG | Cyanidin-3-O-(trans-p-coumaroyl)-diglucoside |
| C3R | Cyanidin-3O-rutinoside |
| C3S | Cyanidin-3-O-sophoroside |
| C3X | Cyanidin-3-O-xyloside |
| DILI | Drug-Induced Liver Injury |
| dpf | Days post-fertilization |
| Dpz | donepezi |
| GSH | Glutathione |
| H-Bonds | hydrogen bonds |
| H-HT | Human hepatotoxicity |
| MDA | Malondialdehyde |
| MDCK | Madin-Darby Canine Kidney cell |
| PBS | Phosphate buffered saline |
| PDB | Protein data bank |
| qRT-PCR | Real-time fluorescence quantitative PCR |
| RMSD | Root mean square deviation |
| RMSF | Root mean square fluctuation |
| Rg | Radius of gyration |
| SASA | solvent accessible surface area |
| SAR | structure-activity relationship |
| SOD | Superoxide dismutase |
References
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Kokkali, M.; Karali, K.; Thanou, E.; Papadopoulou, M.A.; Zota, I.; Tsimpolis, A.; Efstathopoulos, P.; Calogeropoulou, T.; Li, K.W.; Sidiropoulou, K.; et al. Multimodal beneficial effects of BNN27, a nerve growth factor synthetic mimetic, in the 5xFAD mouse model of Alzheimer’s disease. Mol. Psychiatry 2024, 30, 2265–2283. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cao, Z.; Nandi, A.; Counts, N.; Jiao, L.; Prettner, K.; Kuhn, M.; Seligman, B.; Tortorice, D.; Vigo, D. The global macroeconomic burden of Alzheimer’s disease and other dementias: Estimates and projections for 152 countries or territories. Lancet Glob. Health 2024, 12, e1534–e1543. [Google Scholar] [CrossRef]
- Khan, H.T.A.; Addo, K.M.; Findlay, H. Public Health Challenges and Responses to the Growing Ageing Populations. Public Health Chall. 2024, 3, e213. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- Coyle, J.T.; Price, D.L.; DeLong, M.R. Alzheimer’s disease: A disorder of cortical cholinergic innervation. Science 1983, 219, 1184–1190. [Google Scholar] [CrossRef]
- Akıncıoğlu, H.; Gülçin, İ. Potent Acetylcholinesterase Inhibitors: Potential Drugs for Alzheimer’s Disease. Mini Rev. Med. Chem. 2020, 20, 703–715. [Google Scholar] [CrossRef]
- Vecchio, I.; Sorrentino, L.; Paoletti, A.; Marra, R.; Arbitrio, M. The State of The Art on Acetylcholinesterase Inhibitors in the Treatment of Alzheimer’s Disease. J. Cent. Nerv. Syst. Dis. 2021, 13, 11795735211029113. [Google Scholar] [CrossRef]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Khachaturian, A.S.; Vergallo, A.; Farlow, M.R.; Snyder, P.J.; Giacobini, E.; Khachaturian, Z.S. Revisiting the Cholinergic Hypothesis in Alzheimer’s Disease: Emerging Evidence from Translational and Clinical Research. J. Prev. Alzheimer’s Dis. 2019, 6, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Nasr, N.N.; El-Hagrassi, A.M.; Ahmed, Y.R.; Hamed, M.A. GC/MS and LC-ESI-MS Analysis of Conocarpus erectus Leaves Extract via Regulating Amyloid-β-Peptide, Tau Protein, Neurotransmitters, Inflammation and Oxidative Stress against AlCl(3)-Induced Alzheimer’s Disease in Rats. Chem. Biodivers. 2025, 22, e202401960. [Google Scholar] [CrossRef]
- Senger, M.R.; Seibt, K.J.; Ghisleni, G.C.; Dias, R.D.; Bogo, M.R.; Bonan, C.D. Aluminum exposure alters behavioral parameters and increases acetylcholinesterase activity in zebrafish (Danio rerio) brain. Cell Biol. Toxicol. 2011, 27, 199–205. [Google Scholar] [CrossRef]
- Dey, M.; Singh, R.K. Chronic oral exposure of aluminum chloride in rat modulates molecular and functional neurotoxic markers relevant to Alzheimer’s disease. Toxicol. Mech. Methods 2022, 32, 616–627. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, F.; Xiu, C.; Zhang, J.; Li, Y. Hypericum perforatum extract attenuates behavioral, biochemical, and neurochemical abnormalities in Aluminum chloride-induced Alzheimer’s disease rats. Biomed. Pharmacother. 2017, 91, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Saba, K.; Rajnala, N.; Veeraiah, P.; Tiwari, V.; Rana, R.K.; Lakhotia, S.C.; Patel, A.B. Energetics of Excitatory and Inhibitory Neurotransmission in Aluminum Chloride Model of Alzheimer’s Disease: Reversal of Behavioral and Metabolic Deficits by Rasa Sindoor. Front. Mol. Neurosci. 2017, 10, 323. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, B.V.; Sudhakar, M.; Prakash, K.S. Protective effect of selenium against aluminum chloride-induced Alzheimer’s disease: Behavioral and biochemical alterations in rats. Biol. Trace Elem. Res. 2015, 165, 67–74. [Google Scholar] [CrossRef]
- Walton, J.R. Aluminum involvement in the progression of Alzheimer’s disease. J. Alzheimer’s Dis. 2013, 35, 7–43. [Google Scholar] [CrossRef] [PubMed]
- Vaz, R.; Hofmeister, W.; Lindstrand, A. Zebrafish Models of Neurodevelopmental Disorders: Limitations and Benefits of Current Tools and Techniques. Int. J. Mol. Sci. 2019, 20, 1296. [Google Scholar] [CrossRef]
- Bashirzade, A.A.; Zabegalov, K.N.; Volgin, A.D.; Belova, A.S.; Demin, K.A.; de Abreu, M.S.; Babchenko, V.Y.; Bashirzade, K.A.; Yenkoyan, K.B.; Tikhonova, M.A.; et al. Modeling neurodegenerative disorders in zebrafish. Neurosci. Biobehav. Rev. 2022, 138, 104679. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.B.; He, K.J.; Wang, F.; Liu, C.F. Advances of Zebrafish in Neurodegenerative Disease: From Models to Drug Discovery. Front. Pharmacol. 2021, 12, 713963. [Google Scholar] [CrossRef]
- Newman, M.; Ebrahimie, E.; Lardelli, M. Using the zebrafish model for Alzheimer’s disease research. Front. Genet. 2014, 5, 189. [Google Scholar] [CrossRef]
- Chia, K.; Klingseisen, A.; Sieger, D.; Priller, J. Zebrafish as a model organism for neurodegenerative disease. Front. Mol. Neurosci. 2022, 15, 940484. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Zhang, Y. Animal models of Alzheimer’s disease: Applications, evaluation, and perspectives. Zool. Res. 2022, 43, 1026–1040. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, H.; Yin, X.; Liu, Y.; Zhang, T.; Wu, H.; Kang, G.; Yu, Y.; Bai, M.; Bao, L.; et al. The therapeutic effect of Zhenbao pills on behavioral changes in zebrafish caused by aluminum chloride. Biomed. Pharmacother. 2023, 160, 114399. [Google Scholar] [CrossRef]
- Nadiga, A.P.R.; Suman; Krishna, K.L. A novel Zebrafish model of Alzheimer’s disease by Aluminium chloride; involving nitro-oxidative stress, neuroinflammation and cholinergic pathway. Eur. J. Pharmacol. 2024, 965, 176332. [Google Scholar] [CrossRef]
- Wang, B.; Tang, X.; Mao, B.; Zhang, Q.; Tian, F.; Zhao, J.; Chen, W.; Cui, S. Effects of in vitro fecal fermentation on the metabolism and antioxidant properties of cyanidin-3-O-glucoside. Food Chem. 2024, 431, 137132. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Fu, Y.; Yang, L.; Chen, J.; Lei, H.; Liu, Q. Cyanidin-3-O-Glucoside and Cyanidin Protect Against Intestinal Barrier Damage and 2,4,6-Trinitrobenzenesulfonic Acid-Induced Colitis. J. Med. Food 2020, 23, 90–99. [Google Scholar] [CrossRef]
- Fan, Z.; Wen, H.; Zhang, X.; Li, J.; Zang, J. Cyanidin-3-O-β-Galactoside Alleviated Cognitive Impairment in Mice by Regulating Brain Energy Metabolism During Aging. J. Agric. Food Chem. 2022, 70, 1111–1121. [Google Scholar] [CrossRef]
- Choi, M.J.; Kim, B.K.; Park, K.Y.; Yokozawa, T.; Song, Y.O.; Cho, E.J. Anti-aging effects of cyanidin under a stress-induced premature senescence cellular system. Biol. Pharm. Bull. 2010, 33, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Chen, W.; Huang, X.F.; Yan, F.J.; Deng, S.G.; Zheng, X.D.; Shan, P.F. Anti-diabetic effect of anthocyanin cyanidin-3-O-glucoside: Data from insulin resistant hepatocyte and diabetic mouse. Nutr. Diabetes 2024, 14, 7. [Google Scholar] [CrossRef]
- Ye, X.; Chen, W.; Yan, F.J.; Zheng, X.D.; Tu, P.C.; Shan, P.F. Exploring the Effects of Cyanidin-3-O-Glucoside on Type 2 Diabetes Mellitus: Insights into Gut Microbiome Modulation and Potential Antidiabetic Benefits. J. Agric. Food Chem. 2023, 71, 20047–20061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yu, Z.; Zhao, W.; Liu, J. Assessment of the anti-tumor activity of cyanidin-3-O-arabinoside from apple against APN, JAK, and EZH2 target proteins. Food Biosci. 2022, 48, 101788. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, L.; Li, Z.; Liu, Q. Cyanidin-3-O-glucoside, cyanidin, and oxidation products of cyanidin protect neuronal function through alleviating inflammation and oxidative damage. J. Food Sci. 2022, 87, 2159–2172. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Arumuggam, N.; Amararathna, M.; De Silva, A. The potential health benefits of haskap (Lonicera caerulea L.): Role of cyanidin-3-O-glucoside. J. Funct. Foods 2018, 44, 24–39. [Google Scholar] [CrossRef]
- Gutierres, J.M.; Carvalho, F.B.; Schetinger, M.R.; Marisco, P.; Agostinho, P.; Rodrigues, M.; Rubin, M.A.; Schmatz, R.; da Silva, C.R.; Cognato, G.D.P.; et al. Anthocyanins restore behavioral and biochemical changes caused by streptozotocin-induced sporadic dementia of Alzheimer’s type. Life Sci. 2014, 96, 7–17. [Google Scholar] [CrossRef]
- Suresh, S.; Begum, R.F.; Singh, S.A. Anthocyanin as a therapeutic in Alzheimer’s disease: A systematic review of preclinical evidences. Ageing Res. Rev. 2022, 76, 101595. [Google Scholar] [CrossRef] [PubMed]
- Bharathy, P.; Thanikachalam, P.V. Pharmacological relevance of anthocyanin derivative: A review. Pharmacol. Res.-Mod. Chin. Med. 2025, 14, 100565. [Google Scholar] [CrossRef]
- Nalivaeva, N.N.; Turner, A.J. AChE and the amyloid precursor protein (APP)—Cross-talk in Alzheimer’s disease. Chem. Biol. Interact. 2016, 259 Pt B, 301–306. [Google Scholar] [CrossRef]
- Majdi, A.; Sadigh-Eteghad, S.; Rahigh Aghsan, S.; Farajdokht, F.; Vatandoust, S.M.; Namvaran, A.; Mahmoudi, J. Amyloid-β, tau, and the cholinergic system in Alzheimer’s disease: Seeking direction in a tangle of clues. Rev. Neurosci. 2020, 31, 391–413. [Google Scholar] [CrossRef]
- García-Ayllón, M.S.; Small, D.H.; Avila, J.; Sáez-Valero, J. Revisiting the Role of Acetylcholinesterase in Alzheimer’s Disease: Cross-Talk with P-tau and β-Amyloid. Front. Mol. Neurosci. 2011, 4, 22. [Google Scholar] [CrossRef]
- Colović, M.B.; Krstić, D.Z.; Lazarević-Pašti, T.D.; Bondžić, A.M.; Vasić, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Ivanenkov, Y.A.; Zagribelnyy, B.A.; Aladinskiy, V.A. Are We Opening the Door to a New Era of Medicinal Chemistry or Being Collapsed to a Chemical Singularity? J. Med. Chem. 2019, 62, 10026–10043. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.D.; Blagg, J.; Price, D.A.; Bailey, S.; Decrescenzo, G.A.; Devraj, R.V.; Ellsworth, E.; Fobian, Y.M.; Gibbs, M.E.; Gilles, R.W.; et al. Physiochemical drug properties associated with in vivo toxicological outcomes. Bioorg. Med. Chem. Lett. 2008, 18, 4872–4875. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.; Milatovic, D.; Andrade, V.; Batoreu, M.C.; Aschner, M.; Marreilha dos Santos, A.P. The inhibitory effect of manganese on acetylcholinesterase activity enhances oxidative stress and neuroinflammation in the rat brain. Toxicology 2012, 292, 90–98. [Google Scholar] [CrossRef]
- Marucci, G.; Buccioni, M.; Ben, D.D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology 2021, 190, 108352. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, S.; Ba, S.; Dang, J.; Ren, Q.; Zhu, Y.; Liu, K.; Jin, M. Eucommia ulmoides Olive Male Flower Extracts Ameliorate Alzheimer’s Disease-Like Pathology in Zebrafish via Regulating Autophagy, Acetylcholinesterase, and the Dopamine Transporter. Front. Mol. Neurosci. 2022, 15, 901953. [Google Scholar] [CrossRef]
- Khan, H.; Marya; Amin, S.; Kamal, M.A.; Patel, S. Flavonoids as acetylcholinesterase inhibitors: Current therapeutic standing and future prospects. Biomed. Pharmacother. 2018, 101, 860–870. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, W.; Chen, X.; Xiao, J. Inhibition of flavonoids on acetylcholine esterase: Binding and structure-activity relationship. Food Funct. 2014, 5, 2582–2589. [Google Scholar] [CrossRef]
- Guedri, M.M.; Krir, N.; Terol, C.C.; Romdhane, M.; Boulila, A.; Guetat, A. Phytochemical Analysis, Acetylcholinesterase Inhibition, Antidiabetic and Antioxidant Activities of Atriplex halimus L. (Amaranthaceae Juss.). Chem. Biodivers. 2024, 21, e202301941. [Google Scholar] [CrossRef]
- Mohamed, H.E.; Abo, E.D.M.; Mesbah, N.M.; Saleh, S.M.; Ali, A.A.; Sakr, A.T. Raspberry ketone preserved cholinergic activity and antioxidant defense in obesity induced Alzheimer disease in rats. Biomed. Pharmacother. 2018, 107, 1166–1174. [Google Scholar] [CrossRef]
- Suresh, S.; Vellapandian, C. Cyanidin Ameliorates Bisphenol A-Induced Alzheimer’s Disease Pathology by Restoring Wnt/β-Catenin Signaling Cascade: An In Vitro Study. Mol. Neurobiol. 2024, 61, 2064–2080. [Google Scholar] [CrossRef]
- Haake, A.; Nguyen, K.; Friedman, L.; Chakkamparambil, B.; Grossberg, G.T. An update on the utility and safety of cholinesterase inhibitors for the treatment of Alzheimer’s disease. Expert. Opin. Drug Saf. 2020, 19, 147–157. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, M.; Li, Y.; Xue, H.Y.; Si, X.; Shu, C.; Zhou, Y.; Yang, B.R.; Makarov, S.S.; Chudetsky, A.I.; et al. Multispectral analysis and molecular docking simulation of the inhibition mechanism of dietary anthocyanins on acetylcholinesterase. Food Biosci. 2025, 71, 107136. [Google Scholar] [CrossRef]
- Arruda, H.S.; Neri-Numa, I.A.; Kido, L.A.; Marostica Junior, M.R.; Pastore, G.M. Recent advances and possibilities for the use of plant phenolic compounds to manage ageing-related diseases. J. Funct. Foods 2020, 75, 104203. [Google Scholar] [CrossRef]
- Martearena, M.R.; Daz, M.; Ellenrieder, G. Synthesis of rutinosides and rutinose by reverse hydrolysis catalyzed by fungal α-L-rhamnosidases. Biocatal. Biotransform. 2008, 26, 177–185. [Google Scholar] [CrossRef]
- Kasbe, P.; Jangra, A.; Lahkar, M. Mangiferin ameliorates aluminium chloride-induced cognitive dysfunction via alleviation of hippocampal oxido-nitrosative stress, proinflammatory cytokines and acetylcholinesterase level. J. Trace Elem. Med. Biol. 2015, 31, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.Y.; Wang, T.; Ma, H.X.; Chen, S.; Chang, Z.Y.; Li, F. Synergistic effect of osthole and notopterol combination against Alzheimer’s disease and osteoporosis by applying zebrafish AD/OP comorbidity model. Eur. J. Pharmacol. 2024, 979, 176829. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.; Iliffe, S. Alzheimer’s disease. BMJ 2009, 338, b158. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Zhang, J.; Wang, Y.; Cui, K.; Cao, Y.; Wang, L.; Wu, Y. Linarin improves the dyskinesia recovery in Alzheimer’s disease zebrafish by inhibiting the acetylcholinesterase activity. Life Sci. 2019, 222, 112–116. [Google Scholar] [CrossRef]
- Abdel-Aal, R.A.; Hussein, O.A.; Elsaady, R.G.; Abdelzaher, L.A. Naproxen as a potential candidate for promoting rivastigmine anti-Alzheimer activity against aluminum chloride-prompted Alzheimer’s-like disease in rats; neurogenesis and apoptosis modulation as a possible underlying mechanism. Eur. J. Pharmacol. 2022, 915, 174695. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Narang, R.K.; Singh, S. AlCl(3) induced learning and memory deficit in zebrafish. Neurotoxicology 2022, 92, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Zheng, J.; Li, W.; Suo, Y. Isolation, Stability, and Antioxidant Activity of Anthocyanins from Lycium ruthenicum Murray and Nitraria tangutorum Bobr of Qinghai-Tibetan Plateau. Sep. Sci. Technol. 2014, 49, 2897–2906. [Google Scholar] [CrossRef]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Gao, Y.; Tie, F.; Wang, R.; Hu, N.; Dong, Q.; Fu, C.; Wang, H. The Neuroprotective Effects of Cyanidin Derivatives on AlCl3-Induced Zebrafish Model of Alzheimer’s Disease. Molecules 2025, 30, 3686. https://doi.org/10.3390/molecules30183686
Wu Y, Gao Y, Tie F, Wang R, Hu N, Dong Q, Fu C, Wang H. The Neuroprotective Effects of Cyanidin Derivatives on AlCl3-Induced Zebrafish Model of Alzheimer’s Disease. Molecules. 2025; 30(18):3686. https://doi.org/10.3390/molecules30183686
Chicago/Turabian StyleWu, Yun, Yidan Gao, Fangfang Tie, Ruinan Wang, Na Hu, Qi Dong, Chunxiang Fu, and Honglun Wang. 2025. "The Neuroprotective Effects of Cyanidin Derivatives on AlCl3-Induced Zebrafish Model of Alzheimer’s Disease" Molecules 30, no. 18: 3686. https://doi.org/10.3390/molecules30183686
APA StyleWu, Y., Gao, Y., Tie, F., Wang, R., Hu, N., Dong, Q., Fu, C., & Wang, H. (2025). The Neuroprotective Effects of Cyanidin Derivatives on AlCl3-Induced Zebrafish Model of Alzheimer’s Disease. Molecules, 30(18), 3686. https://doi.org/10.3390/molecules30183686