Bioactive Compounds and Traditional Uses of Tripleurospermum disciforme (C.A.Mey.) Sch.Bip.: A Comprehensive Study on Its Therapeutic Potential
Abstract
1. Introduction
2. Results and Discussion
2.1. Ethnobotanical Surveys
Quantitative Statistical Analysis of Ethnobotanical Results
2.2. Tripleurospermum Disciforme
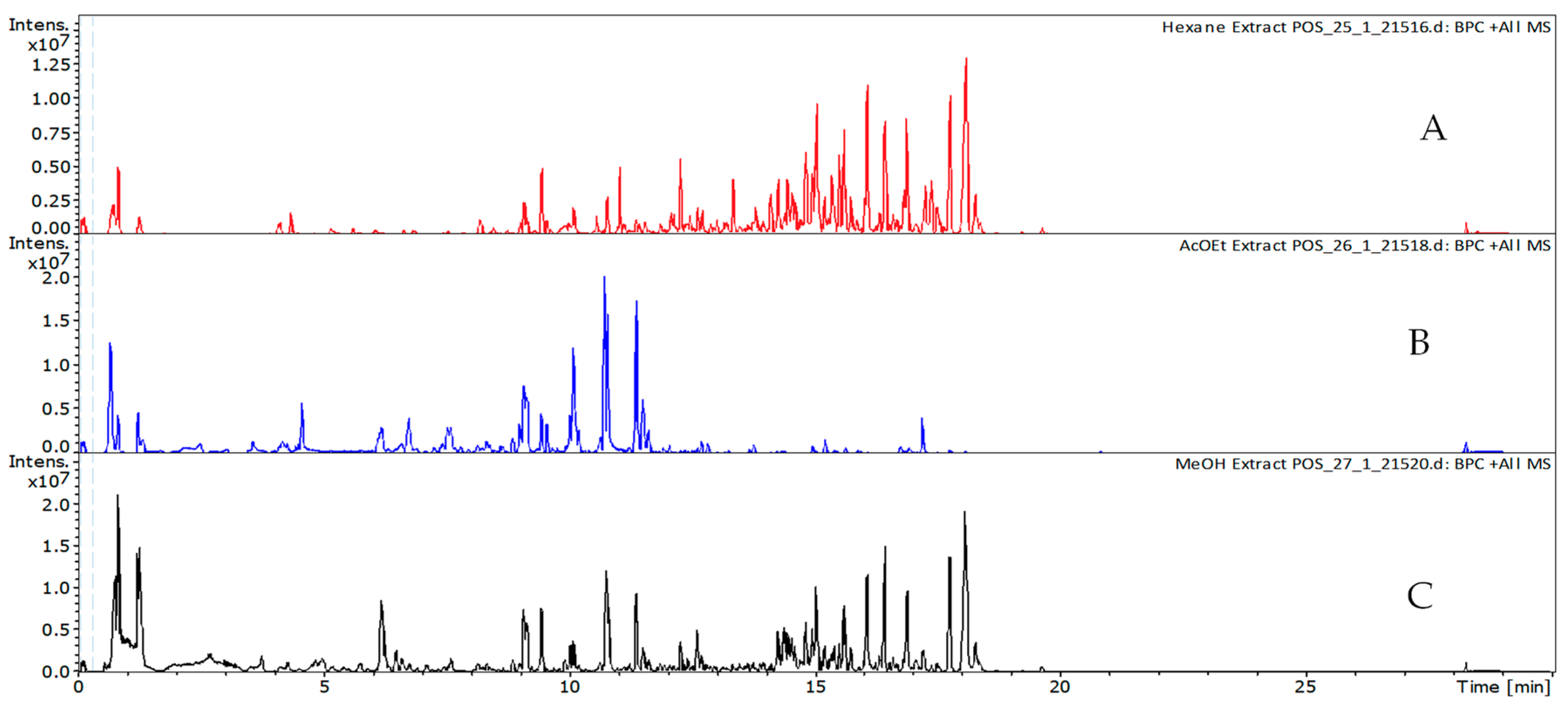
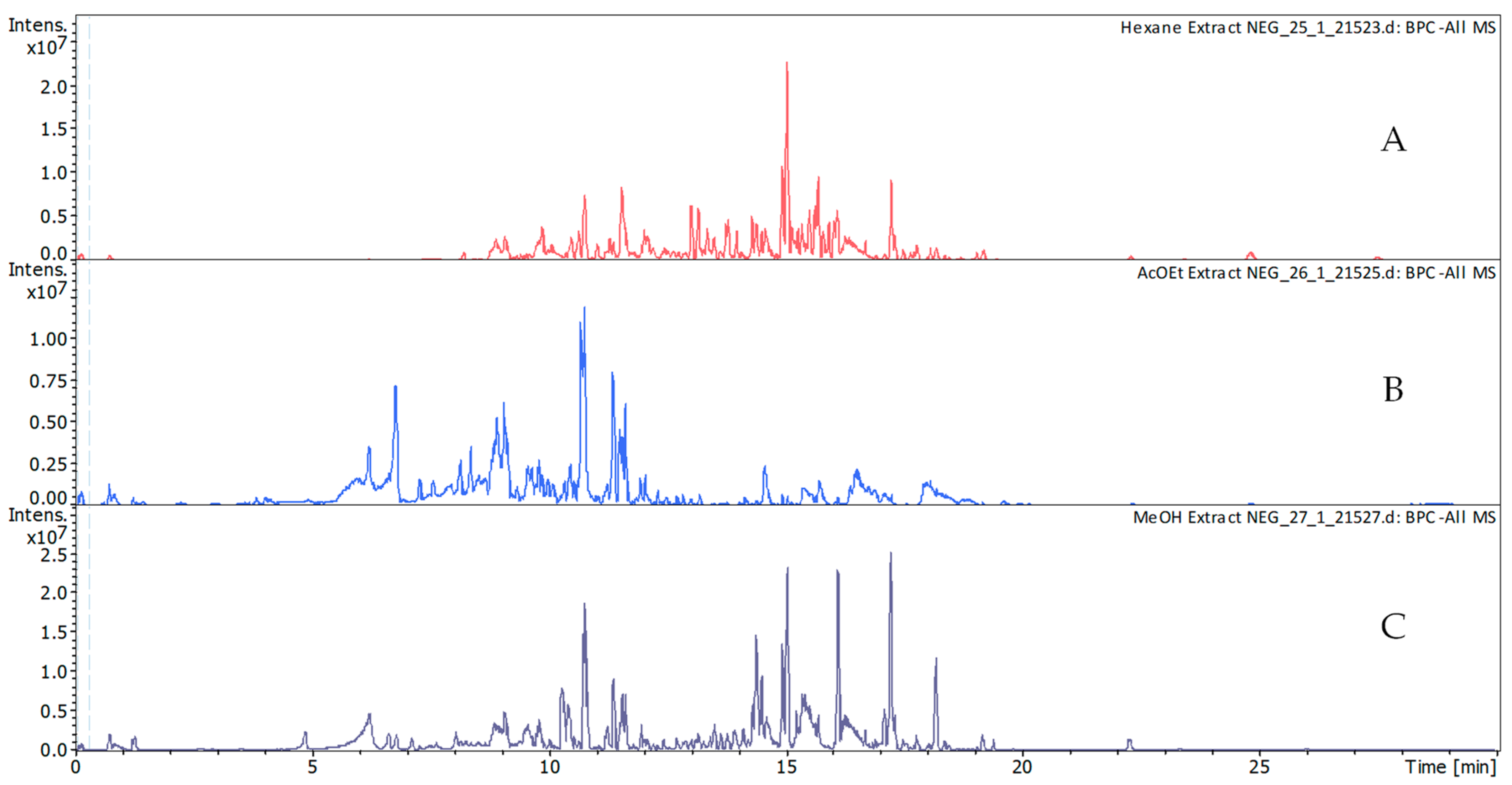
2.2.1. Phytochemical Characterization
2.2.2. Antioxidant Activity
2.3. Antimicrobial Activity
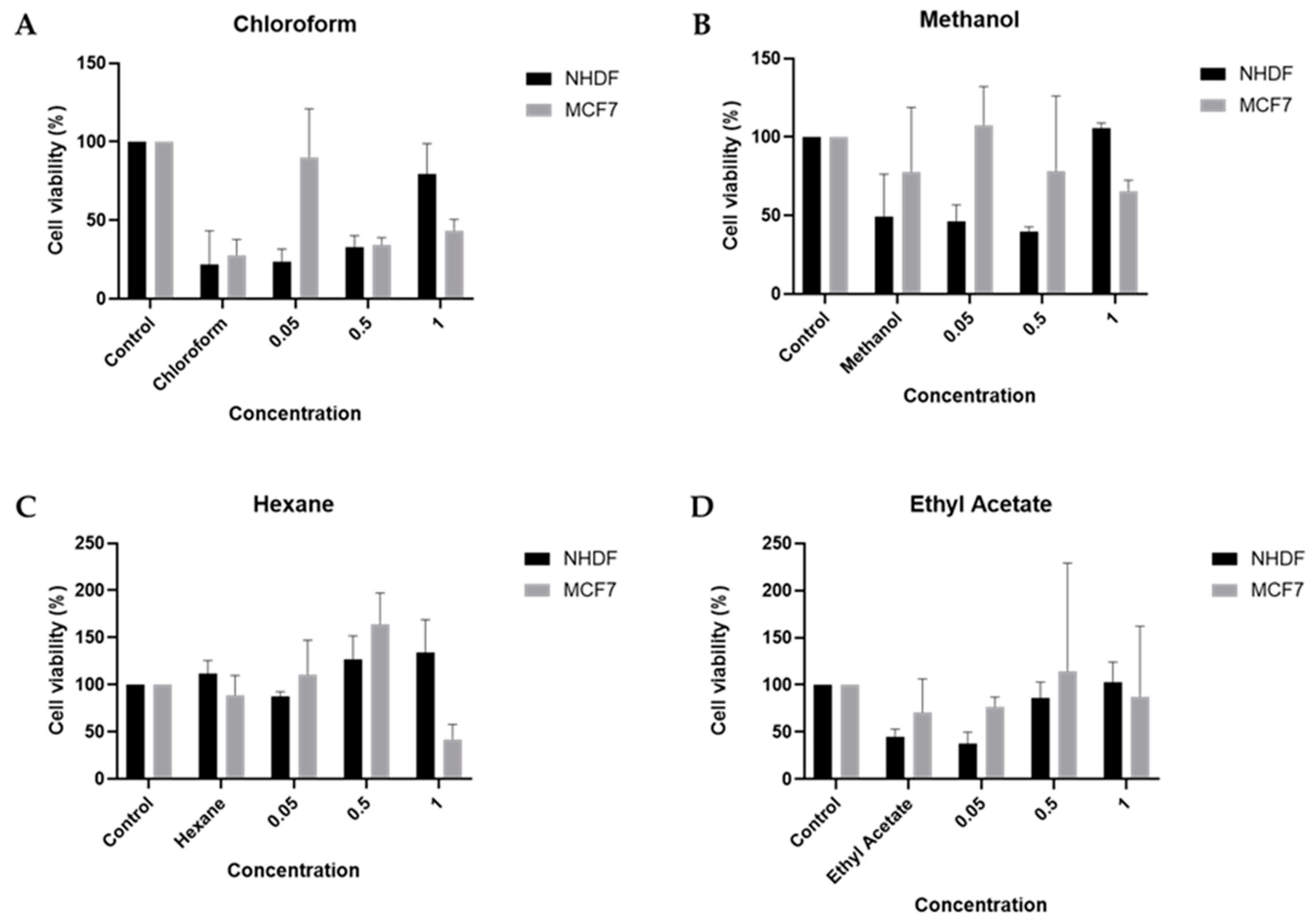
2.4. Cellular Viability
3. Materials and Methods
3.1. Ethnobotanical Surveys
3.1.1. Guilan, Qazvin, and Alborz Provinces Geo-Ethnographical Profile
3.1.2. Field Interview
3.1.3. Quantitative Analysis of the Ethnobotanical Results
3.2. Collection and Identification of Plant Materials
3.3. Extraction (Preparation of Extracts)
3.4. Phytochemical Characterization and Phenolic Profile
3.5. Biological Activities Evaluation
3.5.1. Antioxidant Activity
3.5.2. Antimicrobial Activity
Microorganisms and Media
Determination of Disc Diffusion Assay
Determination of Minimum Inhibitory Concentration (MIC)
3.6. In Vitro Studies Using NHDF and MCF-7 Cells
3.6.1. Cell Culture
3.6.2. Preparation of the Solutions of the Compounds Under Study
3.6.3. Cytotoxicity Assay and Protein Quantification
3.6.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Omidbeigi, R. Production Plants and Products of Medicinal Plants; Designers Publishing: Singapore, 2015; Volume 2, 442p. [Google Scholar]
- Sadat-Hosseini, M.; Farajpour, M.; Boroomand, N.; Solaimani-Sardou, F. Ethnopharmacological studies of indigenous medicinal plants in the south of Kerman, Iran. J. Ethnopharmacol. 2017, 199, 194–204. [Google Scholar] [CrossRef]
- Bremer, K.; Anderberg, A.A. Asteraceae: Cladistics and Classification; Timber Press: Portland, OR, USA, 1994. [Google Scholar]
- Ghahreman, A.; Maassoumi, A.; Pakravan, M. Notes on the genus Astragalus L.(sect. Xiphidium Bge.) in Iran. Iran. J. Bot. 1996, 7, 45–50. [Google Scholar]
- Hooper, D. Useful Plants and Drugs of Iran and Iraq; University of Illinois: Champaign, IL, USA, 1937. [Google Scholar]
- Amin, G. Popular Medicinal Plants of Iran; Ministry of Health: Tehran, Iran, 1991; pp. 40–47. [Google Scholar]
- Oberprieler, C.; Himmelreich, S.; Vogt, R. A new subtribal classification of the tribe Anthemideae (Compositae). Willdenowia 2007, 37, 89–114. [Google Scholar] [CrossRef]
- Inceer, H.; Bal, M.; Ceter, T.; Pinar, N.M. Fruit structure of 12 Turkish endemic Tripleurospermum Sch. Bip.(Asteraceae) taxa and its taxonomic implications. Plant Syst. Evol. 2012, 298, 845–855. [Google Scholar] [CrossRef]
- Inceer, H.; Garnatje, T.; Hayırlıoğlu-Ayaz, S.; Pascual-Díaz, J.P.; Vallès, J.; Garcia, S. A genome size and phylogenetic survey of Mediterranean Tripleurospermum and Matricaria (Anthemideae, Asteraceae). PLoS ONE 2018, 13, e0203762. [Google Scholar] [CrossRef] [PubMed]
- Plants of the World Online; Royal Botanic Gardens, Kew: Richmond, UK, 2021.
- Pobedimova, E. Galium L. Flora USSR 2000, 23, 345–459. [Google Scholar]
- Zebarjadi, A.; Rostami Ahmadvandi, H.; Kahrizi, D.; Cheghamirza, K. Assessment of genetic diversity by application of Inter Simple Sequence Repeat (ISSR) primers on Iranian harmal (Peganum harmala L.) germplasm as an important medicinal plant. J. Appl. Biotechnol. Rep. 2016, 3, 441–445. [Google Scholar]
- Sheydaei, P.; Duarte, A.P. The Genus Tripleurospermum Sch. Bip.(Asteraceae): A Comprehensive Review of Its Ethnobotanical Utilizations, Pharmacology, Phytochemistry, and Toxicity. Life 2023, 13, 1323. [Google Scholar] [CrossRef] [PubMed]
- Yaşar, A.; Üçüncü, O.; Güleç, C.; İnceer, H.; Ayaz, S.; Yayl, N. GC-MS Analysis of Chloroform Extracts in Flowers, Stems, and Roots of Tripleurospermum callosum. Pharm. Biol. 2005, 43, 108–112. [Google Scholar] [CrossRef]
- Ghoran, S.H.; Babaei, E.; Seresht, H.R.; Karimzadeh, Z. Cytotoxic constituents and molecular docking study of the active triterpenoids from Tripleurospermum disciforme (C.A. Mey.) Schultz-Bip. Jundishapur J. Nat. Pharm. Prod. 2019, 15, e65760. [Google Scholar]
- Saleem, M. Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett. 2009, 285, 109–115. [Google Scholar] [CrossRef]
- Amico, V.; Barresi, V.; Condorelli, D.; Spatafora, C.; Tringali, C. Antiproliferative terpenoids from almond hulls (Prunus dulcis): Identification and structure–activity relationships. J. Agric. Food Chem. 2006, 54, 810–814. [Google Scholar] [CrossRef]
- Lee, S.M.; Min, B.S.; Lee, C.-G.; Kim, K.-S.; Kho, Y.H. Cytotoxic triterpenoids from the fruits of Zizyphus jujuba. Planta Medica 2003, 69, 1051–1054. [Google Scholar] [CrossRef]
- Minaiyan, M.; Ghassemi-Dehkordi, N.; Mohammadzadeh, B. Anti-ulcer effect of Tripleurospermum disciforme (CA Mey) Shultz Bip on pylorus ligated (Shay) rats. Res. Pharm. Sci. 2007, 1, 15–21. [Google Scholar]
- Tofighi, Z.; Molazem, M.; Doostdar, B.; Taban, P.; Shahverdi, A.R.; Samadi, N.; Yassa, N. Antimicrobial activities of three medicinal plants and investigation of flavonoids of Tripleurospermum disciforme. Iran. J. Pharm. Res. 2015, 14, 225. [Google Scholar] [PubMed]
- Chehregani, A.; Mohsenzadeh, F.; Mirazi, N.; Hajisadeghian, S.; Baghali, Z. Chemical composition and antibacterial activity of essential oils of Tripleurospermum disciforme in three developmental stages. Pharm. Biol. 2010, 48, 1280–1284. [Google Scholar] [CrossRef] [PubMed]
- Souri, E.; Sarkhail, P.; Kaymanesh, P.; Amini, M.; Farsam, H. Antioxidant activity of extract and a new isolated dioxaspiran derivative of Tripleurospermum disciforme. Pharm. Biol. 2005, 43, 620–623. [Google Scholar] [CrossRef]
- Ghasemi, D.N.; Amin, G.R.; Rahiminezhad, M.; Salehi, M.; Jafarpisheh, A. Morphological and phytochemical study of Tripleurospermum disciforme (CA Mey) Schultz Bip. Nat. Resour. 2003, 16, 42–46. [Google Scholar]
- Heinrich, M. Ethnobotany and its role in drug development. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2000, 14, 479–488. [Google Scholar] [CrossRef]
- Santos, E.S.; Luís, Â.; Gonçalves, J.; Rosado, T.; Pereira, L.; Gallardo, E.; Duarte, A.P. Julbernardia paniculata and Pterocarpus angolensis: From ethnobotanical surveys to phytochemical characterization and bioactivities evaluation. Molecules 2020, 25, 1828. [Google Scholar] [CrossRef]
- Bouasla, A.; Bouasla, I. Ethnobotanical survey of medicinal plants in northeastern of Algeria. Phytomedicine 2017, 36, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Jouad, H.; Haloui, M.; Rhiouani, H.; El Hilaly, J.; Eddouks, M. Ethnobotanical survey of medicinal plants used for the treatment of diabetes, cardiac and renal diseases in the North centre region of Morocco (Fez–Boulemane). J. Ethnopharmacol. 2001, 77, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Sarper, F.; Akaydin, G.; Şimşek, I.; Yeşilada, E. An ethnobotanical field survey in the Haymana district of Ankara province in Turkey. Turk. J. Biol. 2009, 33, 79–88. [Google Scholar] [CrossRef]
- Jamzad, Z. A survey of Lamiaceae in the flora of Iran. Rostaniha 2013, 14, 59–67. [Google Scholar]
- Naghibi, F.; Mosadegh, M.; Mohammadi, M.S.; Ghorbani, A. Labiatae family in folk medicine in Iran: From ethnobotany to pharmacology. Iran. J. Pharm. Res. 2005, 2, 63–79. [Google Scholar]
- Khaleghi, B. Traditional ecological knowledge of medicinal shrubs and herbaceous plants in Arasbaran Forest. Indig. Knowl. 2016, 2, 205–236. [Google Scholar]
- Nasab, F.K.; Khosravi, A.R. Ethnobotanical study of medicinal plants of Sirjan in Kerman Province, Iran. J. Ethnopharmacol. 2014, 154, 190–197. [Google Scholar] [CrossRef]
- Sadeghi, Z.; Kuhestani, K.; Abdollahi, V.; Mahmood, A. Ethnopharmacological studies of indigenous medicinal plants of Saravan region, Baluchistan, Iran. J. Ethnopharmacol. 2014, 153, 111–118. [Google Scholar] [CrossRef]
- Nadaf, M.; Joharchi, M.; Amiri, M.S. Ethnomedicinal uses of plants for the treatment of nervous disorders at the herbal markets of Bojnord, North Khorasan Province, Iran. Avicenna J. Phytomed. 2019, 9, 153. [Google Scholar]
- Lotfi, S.; Gholizadeh, H.; Naqinezhad, A.; Amirzadeh, M.; Davarpanah, S.J. An ethnobotanical survey of medicinal plants of Rudsar and Amlash province. J. Appl. Plant Biol. 2023, 1, 1–11. [Google Scholar]
- Mosaddegh, M.; Naghibi, F.; Moazzeni, H.; Pirani, A.; Esmaeili, S. Ethnobotanical survey of herbal remedies traditionally used in Kohghiluyeh va Boyer Ahmad province of Iran. J. Ethnopharmacol. 2012, 141, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Ghadimi Joboni, M.; Ghavam, M. Ethnobotany of medicinal and edible plants in Jubon village of Guilan province using descriptive statistics. Iran. J. Med. Aromat. Plants Res. 2021, 37, 127–144. [Google Scholar]
- Akaydin, G.; Şimşek, I.; Arituluk, Z.C.; Yeşilada, E. An ethnobotanical survey in selected towns of the Mediterranean subregion (Turkey). Turk. J. Biol. 2013, 37, 230–247. [Google Scholar] [CrossRef]
- Özüdoğru, B.; Akaydın, G.; Erik, S.; Yesilada, E. Inferences from an ethnobotanical field expedition in the selected locations of Sivas and Yozgat provinces (Turkey). J. Ethnopharmacol. 2011, 137, 85–98. [Google Scholar] [CrossRef]
- Amiri, M.S.; Joharchi, M.R. Ethnobotanical knowledge of Apiaceae family in Iran: A review. Avicenna J. Phytomed. 2016, 6, 621. [Google Scholar]
- Bibi, T.; Ahmad, M.; Tareen, R.B.; Tareen, N.M.; Jabeen, R.; Rehman, S.-U.; Sultana, S.; Zafar, M.; Yaseen, G. Ethnobotany of medicinal plants in district Mastung of Balochistan province-Pakistan. J. Ethnopharmacol. 2014, 157, 79–89. [Google Scholar] [CrossRef]
- Amiri, M.S.; Jabbarzadeh, P.; Akhondi, M. An ethnobotanical survey of medicinal plants used by indigenous people in Zangelanlo district, Northeast Iran. J. Med. Plants Res. 2012, 6, 749–753. [Google Scholar] [CrossRef]
- Mehrnia, M.; Akaberi, M.; Amiri, M.; Nadaf, M.; Emami, S. Ethnopharmacological studies of medicinal plants in central Zagros, Lorestan Province, Iran. J. Ethnopharmacol. 2021, 280, 114080. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.). Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2006, 20, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, P.; Pigera, S.; Premakumara, G.S.; Galappaththy, P.; Constantine, G.R.; Katulanda, P. Medicinal properties of ‘true’cinnamon (Cinnamomum zeylanicum): A systematic review. BMC Complement. Altern. Med. 2013, 13, 275. [Google Scholar] [CrossRef]
- Sharangi, A. Medicinal and therapeutic potentialities of tea (Camellia sinensis L.)—A review. Food Res. Int. 2009, 42, 529–535. [Google Scholar] [CrossRef]
- Naghibi, F.; Esmaeili, S.; Malekmohammadi, M.; Hassanpour, A.; Mosaddegh, M. Ethnobotanical survey of medicinal plants used traditionally in two villages of Hamedan, Iran. Res. J. Pharmacogn. 2014, 1, 7–14. [Google Scholar]
- Mollarafie, P.; Khadiv Parsi, P.; Zarghami, R.; Amini Fazl, M.; Ghafarzadegan, R. Antibacterial and wound healing properties of thymol (Thymus vulgaris Oil) and its application in a novel wound dressing. J. Med. Plants 2015, 14, 69–81. [Google Scholar]
- Hajimehdipoor, H.; Shekarchi, M.; Khanavi, M.; Adib, N.; Amri, M. A validated high performance liquid chromatography method for the analysis of thymol and carvacrol in Thymus vulgaris L. volatile oil. Pharmacogn. Mag. 2010, 6, 154. [Google Scholar] [CrossRef]
- Liolios, C.; Gortzi, O.; Lalas, S.; Tsaknis, J.; Chinou, I. Liposomal incorporation of carvacrol and thymol isolated from the essential oil of Origanum dictamnus L. and in vitro antimicrobial activity. Food Chem. 2009, 112, 77–83. [Google Scholar] [CrossRef]
- Ahvazi, M.; Khalighi-Sigaroodi, F.; Charkhchiyan, M.M.; Mojab, F.; Mozaffarian, V.-A.; Zakeri, H. Introduction of medicinal plants species with the most traditional usage in Alamut region. Iran. J. Pharm. Res. 2012, 11, 185. [Google Scholar]
- Safaei, A. Identification and quantitative determination of luteolin and apigenin in the aerial parts and an extract of Stachys lavandulifolia by HPLC. Iran. J. Pharm. Res. 2004, 3, 90. [Google Scholar]
- Zeka, K.; Ruparelia, K.; Arroo, R.R.; Budriesi, R.; Micucci, M. Flavonoids and their metabolites: Prevention in cardiovascular diseases and diabetes. Diseases 2017, 5, 19. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Singh, A.K.; Kumar, R.; Croley, C.R.; Pandey, A.K.; Coy-Barrera, E.; Kumar Patra, J.; Das, G.; Kerry, R.G.; Annunziata, G. Targeting inflammation by flavonoids: Novel therapeutic strategy for metabolic disorders. Int. J. Mol. Sci. 2019, 20, 4957. [Google Scholar] [CrossRef]
- Zeka, K.; Marrazzo, P.; Micucci, M.; Ruparelia, K.C.; Arroo, R.R.; Macchiarelli, G.; Annarita Nottola, S.; Continenza, M.A.; Chiarini, A.; Angeloni, C. Activity of antioxidants from Crocus sativus L. petals: Potential preventive effects towards cardiovascular system. Antioxidants 2020, 9, 1102. [Google Scholar] [CrossRef] [PubMed]
- Akhondzadeh, S. Herbal medicines in the treatment of psychiatric and neurological disorders. In Low-Cost Approaches to Promote Physical and Mental Health: Theory, Research, and Practice; Springer: Berlin/Heidelberg, Germany, 2007; pp. 119–138. [Google Scholar]
- Akhondzadeh, S.; Fallah-Pour, H.; Afkham, K.; Jamshidi, A.-H.; Khalighi-Cigaroudi, F. Comparison of Crocus sativus L. and imipramine in the treatment of mild to moderate depression: A pilot double-blind randomized trial [ISRCTN45683816]. BMC Complement. Altern. Med. 2004, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Suroowan, S.; Mahomoodally, M.F. A comparative ethnopharmacological analysis of traditional medicine used against respiratory tract diseases in Mauritius. J. Ethnopharmacol. 2016, 177, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Wahyuningrum, R.; Pangestu, D.; Budiman, A. Ethnomedical Study of Plants as a Traditional Medicine on Respiratory System Disease in Cilongok, Banyumas, Indonesia. Maj. Obat Tradis. 2022, 27, 41–50. [Google Scholar] [CrossRef]
- Ali, B.H.; Blunden, G.; Tanira, M.O.; Nemmar, A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem. Toxicol. 2008, 46, 409–420. [Google Scholar] [CrossRef]
- Mashhadi, N.S.; Ghiasvand, R.; Askari, G.; Hariri, M.; Darvishi, L.; Mofid, M.R. Anti-oxidative and anti-inflammatory effects of ginger in health and physical activity: Review of current evidence. Int. J. Prev. Med. 2013, 4, S36. [Google Scholar]
- Mahluji, S.; Attari, V.E.; Mobasseri, M.; Payahoo, L.; Ostadrahimi, A.; Golzari, S.E. Effects of ginger (Zingiber officinale) on plasma glucose level, HbA1c and insulin sensitivity in type 2 diabetic patients. Int. J. Food Sci. Nutr. 2013, 64, 682–686. [Google Scholar] [CrossRef]
- Tabuti, J.R.; Kukunda, C.B.; Waako, P.J. Medicinal plants used by traditional medicine practitioners in the treatment of tuberculosis and related ailments in Uganda. J. Ethnopharmacol. 2010, 127, 130–136. [Google Scholar] [CrossRef]
- Nguta, J.M.; Appiah-Opong, R.; Nyarko, A.K.; Yeboah-Manu, D.; Addo, P.G. Medicinal plants used to treat TB in Ghana. Int. J. Mycobacteriol. 2015, 4, 116–123. [Google Scholar] [CrossRef]
- Palatty, P.L.; Haniadka, R.; Valder, B.; Arora, R.; Baliga, M.S. Ginger in the prevention of nausea and vomiting: A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 659–669. [Google Scholar] [CrossRef]
- Delaveau, P.; Guillemain, J.; Narcisse, G.; Rousseau, A. Neuro-depressive properties of essential oil of lavender. Comptes Rendus Seances Soc. Biol. Ses Fil. 1989, 183, 342–348. [Google Scholar]
- Akhondzadeh, S.; Kashani, L.; Fotouhi, A.; Jarvandi, S.; Mobaseri, M.; Moin, M.; Khani, M.; Jamshidi, A.H.; Baghalian, K.; Taghizadeh, M. Comparison of Lavandula angustifolia Mill. tincture and imipramine in the treatment of mild to moderate depression: A double-blind, randomized trial. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2003, 27, 123–127. [Google Scholar] [CrossRef]
- Farhadi, A.; Hassanzad-Azar, H.; Pour-Anbari, P.; Joudaki, Y.; Shahsavari, F.; Bahmani, M.; Rafieian-Kopaei, M. The most important medicinal plants affecting the brain and nerves: An overview on Iranian ethnobotanical sources. Der Pharma Chem. 2016, 8, 269–274. [Google Scholar]
- Akbarzadeh, A.; Jaimand, K.; Hemmati, A.; Khanjani Shiraz, B. Medicinal plants of Gilan province and their applications. Iran. J. Med. Aromat. Plants Res. 2010, 26, 326–347. [Google Scholar]
- Chen, M.; He, X.; Sun, H.; Sun, Y.; Li, L.; Zhu, J.; Xia, G.; Guo, X.; Zang, H. Phytochemical analysis, UPLC-ESI-Orbitrap-MS analysis, biological activity, and toxicity of extracts from Tripleurospermum limosum (Maxim.) Pobed. Arab. J. Chem. 2022, 15, 103797. [Google Scholar] [CrossRef]
- Ćavar Zeljković, S.; Ayaz, F.A.; Inceer, H.; Hayirlioglu-Ayaz, S.; Colak, N. Evaluation of chemical profile and antioxidant activity of Tripleurospermum insularum, a new species from Turkey. Nat. Prod. Res. 2015, 29, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Abbasian, S.; Karimi, F.; Moghaddam, G.; Soroush, A.; Moloudian, H.; Ahosseini, M.S. Antioxidant properties of different black tea samples and some Iranian native plants. Pharm. Glob. 2013, 4, 1–5. [Google Scholar]
- Özgen, U.; Mavi, A.; Terzi, Z.; Coflkun, M.; Ali, Y. Antioxidant activities and total phenolic compounds amount of some Asteraceae species. Turk. J. Pharm. Sci. 2004, 1, 203–216. [Google Scholar]
- Didier, F.; Catherine, F.; Odile, T.; Jean-Louis, L. Caffeoyl derivatives: Major antioxidant compounds of some wild herbs of the Asteraceae family. Food Nutr. Sci. 2011, 2. [Google Scholar] [CrossRef]
- Li, X.; Li, K.; Xie, H.; Xie, Y.; Li, Y.; Zhao, X.; Jiang, X.; Chen, D. Antioxidant and cytoprotective effects of the Di-O-Caffeoylquinic acid family: The mechanism, structure–activity relationship, and conformational effect. Molecules 2018, 23, 222. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; Xia, F.; Modarresi-Ghazani, F. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef]
- Alcázar Magaña, A.; Kamimura, N.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Caffeoylquinic acids: Chemistry, biosynthesis, occurrence, analytical challenges, and bioactivity. Plant J. 2021, 107, 1299–1319. [Google Scholar] [CrossRef]
- Tajner-Czopek, A.; Gertchen, M.; Rytel, E.; Kita, A.; Kucharska, A.Z.; Sokół-Łętowska, A. Study of antioxidant activity of some medicinal plants having high content of caffeic acid derivatives. Antioxidants 2020, 9, 412. [Google Scholar] [CrossRef]
- Colak, N.; Inceer, H.; Gruz, J.; Strnad, M.; Hayirlioglu-Ayaz, S.; Aksu Kalmuk, N.; Ayaz, F. Antioxidant capacity of phenolics in some representatives of the tribe Anthemideae (Asteraceae) from Turkey. Int. J. Pharm. Sci. Res. 2017, 8, 3265–3277. [Google Scholar]
- Oloumi, H.; Hassibi, N. Study the correlation between some climate parameters and the content of phenolic compounds in roots of Glycyrrhiza glabra. J. Med. Plants Res. 2011, 5, 6011–6016. [Google Scholar]
- Ghasemi, K.; Ghasemi, Y.; Ehteshamnia, A.; Nabavi, S.M.; Nabavi, S.F.; Ebrahimzadeh, M.A.; Pourmorad, F. Influence of environmental factors on antioxidant activity, phenol and flavonoids contents of walnut (Juglans regia L.) green husks. J. Med. Plants Res. 2011, 5, 1128–1133. [Google Scholar]
- Sheydaei, P.; Amaral, M.E.; Duarte, A.P. Genus echium L.: Phytochemical Characterization and Bioactivity Evaluation for Drug Discovery. Plants 2025, 14, 2548. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Kumar, N. Synthesis and Evaluation of Novel 4-hydroxycoumarin Derivatives as Potential Anti-microbial Agents. Orient. J. Chem. 2021, 37, 1132. [Google Scholar] [CrossRef]
- Alejo-Armijo, A.; Cobo, A.; Alejo-Armijo, A.; Altarejos, J.; Salido, S.; Ortega-Morente, E. Evaluation of Antibacterial and Antibiofilm Properties of Phenolics with Coumarin, Naphthoquinone and Pyranone Moieties Against Foodborne Microorganisms. Molecules 2025, 30, 944. [Google Scholar] [CrossRef]
- Završnik, D.; Muratović, S.; Špirtović, S.; Softić, D.; Medić-Šarić, M. The synthesis and antimicrobial activity of some 4-hydroxycoumarin derivatives. Bosn. J. Basic Med. Sci. 2008, 8, 277. [Google Scholar] [CrossRef]
- Ooi, L.S.; Wang, H.; He, Z.; Ooi, V.E. Antiviral activities of purified compounds from Youngia japonica (L.) DC (Asteraceae, Compositae). J. Ethnopharmacol. 2006, 106, 187–191. [Google Scholar] [CrossRef]
- Chakraborty, M.; Mitra, A. The antioxidant and antimicrobial properties of the methanolic extract from Cocos nucifera mesocarp. Food Chem. 2008, 107, 994–999. [Google Scholar] [CrossRef]
- Wu, Y.; Liang, S.; Zhang, M.; Wang, Z.; Wang, Z.; Ren, X. The effect of chlorogenic acid on Bacillus subtilis based on metabolomics. Molecules 2020, 25, 4038. [Google Scholar] [CrossRef]
- Ghavam, M. Tripleurospermum disciforme (C.A. Mey.) Sch. Bip., Tanacetum parthenium (L.) Sch. Bip, and Achillea biebersteinii Afan.: Efficiency, chemical profile, and biological properties of essential oil. Chem. Biol. Technol. Agric. 2021, 8, 45. [Google Scholar] [CrossRef]
- Ghavam, M.; Afzali, A.; Manconi, M.; Bacchetta, G.; Manca, M.L. Variability in chemical composition and antimicrobial activity of essential oil of Rosa × damascena Herrm. from mountainous regions of Iran. Chem. Biol. Technol. Agric. 2021, 8, 22. [Google Scholar] [CrossRef]
- Fard, M.B.; Hamedani, A.; Ebadi, M.; Hamidi, D.; Motlaghzadeh, K.; Emarati, M.; Wu, D.; Mckay, G. Sustainable waste-to-energy plant site selection by a hybrid method of geographic information system and evidential reasoning: A case study Guilan province. Process Saf. Environ. Prot. 2023, 176, 316–331. [Google Scholar] [CrossRef]
- Akhani, H.; Djamali, M.; Ghorbanalizadeh, A.; Ramezani, E. Plant biodiversity of Hyrcanian relict forests, N Iran: An overview of the flora, vegetation, palaeoecology and conservation. Pak. J. Bot. 2010, 42, 231–258. [Google Scholar]
- Pourbabaei, H.; Ebrahimi, S.S.; Torkaman, J.; Pothier, D. Comparison in woody species composition, diversity and community structure as affected by livestock grazing and human uses in beech forests of northern Iran. Forestry 2014, 20, 1–11. [Google Scholar]
- Shahriar, F.; Montazeri, M.; Momeni, M.; Freidooni, A. Regionalization of the climatic areas of Qazvin Province using multivariate statistical methods. Mod. Appl. Sci. 2015, 9, 123. [Google Scholar] [CrossRef]
- Alijani, B.; Harman, J.R. Synoptic climatology of precipitation in Iran. Ann. Assoc. Am. Geogr. 1985, 75, 404–416. [Google Scholar] [CrossRef]
- Rechinger, K. Flora Iranica; Akademishe Druck University: Graz, Austria, 2005; Volume 176. [Google Scholar]
- Assadi, M.; Maassoumi, A.; Khatamsaz, M.; Mozaffarian, V. Flora of Iran; Research Institute of Forest Publication: Tehran, Iran, 1988; Volume 149. (In Persian) [Google Scholar]
- Luís, Â.; Neiva, D.; Pereira, H.; Gominho, J.; Domingues, F.; Duarte, A.P. Stumps of Eucalyptus globulus as a source of antioxidant and antimicrobial polyphenols. Molecules 2014, 19, 16428–16446. [Google Scholar] [CrossRef] [PubMed]
- Scherer, R.; Godoy, H.T. Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chem. 2009, 112, 654–658. [Google Scholar] [CrossRef]
- Luís, Â.; Duarte, A.; Gominho, J.; Domingues, F.; Duarte, A.P. Chemical composition, antioxidant, antibacterial and anti-quorum sensing activities of Eucalyptus globulus and Eucalyptus radiata essential oils. Ind. Crops Prod. 2016, 79, 274–282. [Google Scholar] [CrossRef]
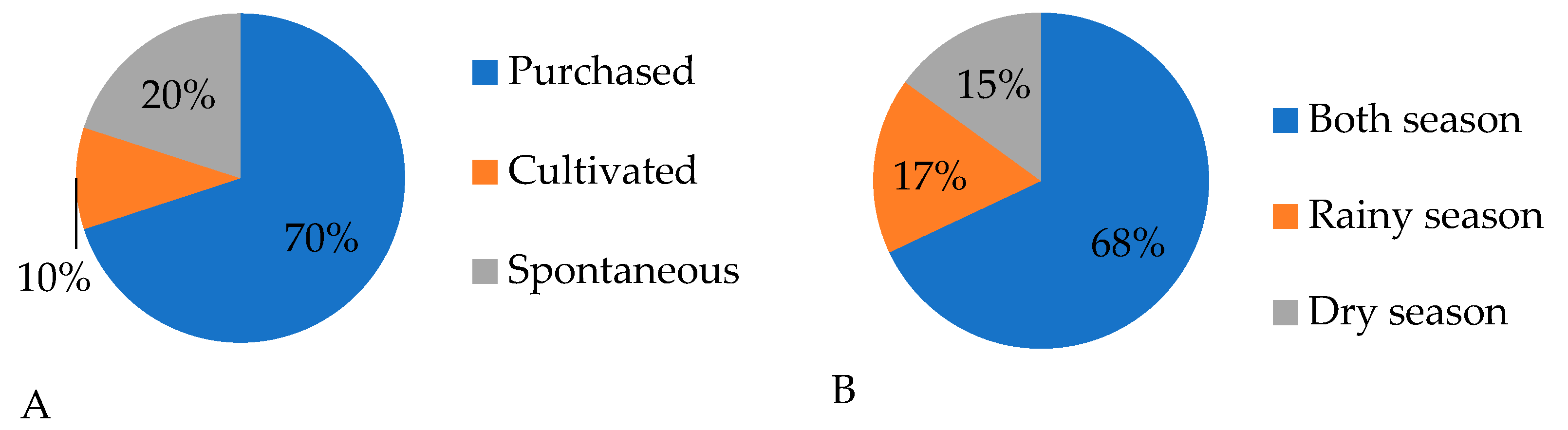


| Therapeutic Indications | Number of Used Reports (Nur) | Number of Taxa (Nt) | ICF Value |
|---|---|---|---|
| Cardiac system | 37 | 11 | 0.67 |
| Urinary system | 26 | 10 | 0.58 |
| Nervous system | 74 | 11 | 0.82 |
| Respiratory disorder | 9 | 3 | 0.63 |
| Skin disorders | 49 | 11 | 0.74 |
| Digestive system | 95 | 11 | 0.85 |
| Wound healing | 41 | 8 | 0.77 |
| Therapeutic Indications | Plant Species | FL (%) |
|---|---|---|
| Digestive system | Tripleurospermum disciforme | 62% |
| Camellia sinensis | 76% | |
| Echium amoenum | 25% | |
| Mentha spp. | 100% | |
| Cinnamomum zeylanicum | 85% | |
| Skin disorders | Tripleurospermum disciforme Thymus kotschyanus | 37% 30% |
| Wound healing | Thymus kotschyanus | 50% |
| Nervous system | Tripleurospermum disciforme Echium amoenum Stachys lavandulifolia | 38% 68% 77% |
| Urinary system | Echium amoenum | 31% |
| Cardiac disorder | Crocus haussknechtii | 73% |
| Respiratory disorder | Zingiber officinale | 77% |
| Plant Species | Number of Participants That Cited the Species | RFC | UV |
|---|---|---|---|
| Tripleurospermum disciforme | 42 | 0.42 | 1.72 |
| Thymus kotschyanus | 40 | 0.4 | 1.75 |
| Echium amoenum | 32 | 0.32 | 1.31 |
| Mentha spp. | 30 | 0.3 | 1 |
| Crocus sativus (Saffron) | 15 | 0.15 | 1 |
| Camellia sinensis | 14 | 0.14 | 1 |
| Cinnamomum zeylanicum | 14 | 0.14 | 1 |
| Lavandula angustifolia | 10 | 0.1 | 1 |
| Stachys lavandulifolia | 9 | 0.09 | 1 |
| Malva sylvestris L. | 8 | 0.08 | 1 |
| Rosmarinus officinalis | 7 | 0.07 | 1 |
| Zingiber officinale | 7 | 0.07 | 1 |
| Ziziphora clinopodoides | 7 | 0.07 | 1 |
| Salvia spp. | 6 | 0.06 | 1 |
| Rosa spp. | 5 | 0.05 | 1 |
| Curcuma longa | 4 | 0.04 | 1 |
| Aloysia citrodora | 4 | 0.04 | 1 |
| Cichorium intybus | 2 | 0.02 | 1 |
| Eryngium planum | 2 | 0.02 | 1 |
| Salix aegyptiaca | 1 | 0.01 | 1 |
| Achillea spp. | 1 | 0.01 | 1 |
| Cuscuta epithymum | 1 | 0.01 | 1 |
| Ziziphus vulgaris | 1 | 0.01 | 1 |
| Citrus aurantium | 1 | 0.01 | 1 |
| Nigella arvensis | 1 | 0.01 | 1 |
| Elettaria cardamomum | 1 | 0.01 | 1 |
| Adiantum capillus-veneris L. | 1 | 0.01 | 1 |
| Foeniculum vulgare Mill. | 1 | 0.01 | 1 |
| Juniperus communis | 1 | 0.01 | 1 |
| Urtica urens | 1 | 0.01 | 1 |
| Cuminum cyminum | 1 | 0.01 | 1 |
| Syzygium aromaticum | 1 | 0.01 | 1 |
| Cassia angustifolia | 1 | 0.01 | 1 |
| Glycyrrhiza glabra L. | 1 | 0.01 | 1 |
| Portulaca oleracea L. | 1 | 0.01 | 1 |
| Compound Name | Formula | Measured m/z | Retention Time (min) | CCS (Å2) | Detected in These Solvents |
|---|---|---|---|---|---|
| 2′,4′,6′-Trihydroxyacetophenone | C8H8O4 | 169.04974 | 6.54 | 120.8 | Ethyl acetate, Hexane, Methanol |
| 2-Acetylbenzoic acid | C9H8O3 | 163.04001 | 8.46 | 125.8 | Ethyl acetate, Hexane, Methanol |
| 3,4-Dicaffeoylquinic acid | C25H24O12 | 515.11879 | 9.57 | 211.5 | Ethyl acetate |
| 3-Feruloylquinic acid | C17H20O9 | 367.10357 | 8.85 | 188.7 | Ethyl acetate, Hexane, Methanol |
| 4-(3,4-Dimethoxyphenyl)-3-buten-1-ol | C12H16O3 | 209.11765 | 8.33 | 130.8 | Ethyl acetate, Methanol |
| 4-(4-Hydroxyphenyl)-2-butanone | C10H12O2 | 165.09078 | 11.66 | 121.0 | Ethyl acetate, Hexane, Methanol |
| 4-Hydroxycoumarin | C9H6O3 | 163.03905 | 6.2 | 116.2 | Ethyl acetate, Hexane, Methanol |
| 5-Hydroxymethyl-7-methoxybenzofuran | C10H10O3 | 179.07166 | 12.19 | 119.9 | Ethyl acetate, Hexane, Methanol |
| 6-Gingerol | C17H26O4 | 295.19226 | 14.38 | 155.9 | Ethyl acetate, Hexane, Methanol |
| 6-Hydroxyluteolin | C15H10O7 | 303.05216 | 9.26 | 153.2 | Ethyl acetate, Hexane, Methanol |
| 6-Hydroxyluteolin 7-O-rhamnoside | C21H20O11 | 447.09297 | 8.6 | 198.6 | Ethyl acetate, Hexane, Methanol |
| 7,3′,4′-Trihydroxyflavone | C15H10O5 | 269.04531 | 11.38 | 153.4 | Ethyl acetate, Hexane, Methanol |
| 9-Dehydroxyeurotinone | C15H12O5 | 271.06046 | 11.57 | 154.4 | Ethyl acetate, Hexane, Methanol |
| Agrostophyllidin | C17H16O4 | 283.0973 | 13.03 | 171.6 | Ethyl acetate, Hexane, Methanol |
| Altechromone A | C11H10O3 | 191.07067 | 10.22 | 123.7 | Ethyl acetate, Hexane, Methanol |
| Caffeic acid | C9H8O4 | 179.03505 | 5.92 | 127.8 | Ethyl acetate, Hexane, Methanol |
| Chrysin | C15H10O4 | 253.05016 | 12.84 | 152.2 | Ethyl acetate, Hexane, Methanol |
| Deoxyarbutin | C11H14O3 | 195.10202 | 10.04 | 126.7 | Ethyl acetate, Hexane, Methanol |
| Eriodictyol | C15H12O6 | 287.05448 | 10.72 | 161.3 | Methanol |
| Kaempherol | C15H10O6 | 287.0567 | 11.17 | 148.6 | Ethyl acetate, Methanol |
| Myricetin 3-O-rhamnoside | C21H20O12 | 463.08758 | 9.96 | 200.0 | Ethyl acetate, Hexane, Methanol |
| N-Phenylacetylaminoacetic acid | C10H11NO3 | 194.08138 | 7.13 | 124.0 | Ethyl acetate, Methanol |
| Neochlorogenic acid | C16H18O9 | 353.08764 | 0.83 | 180.7 | Ethyl acetate, Hexane, Methanol |
| Olivetol | C11H16O2 | 181.1228 | 12.28 | 115.8 | Hexane, Methanol |
| Scutellarein | C15H10O6 | 287.05658 | 10.79 | 128.5 | Hexane, Methanol |
| p-HPEA-AC | C10H12O3 | 181.08631 | 4.25 | 108.7 | Ethyl acetate |
| Samples | * DPPH Free Radical Scavenging Assay | ||
|---|---|---|---|
| IC50 (μg/mL) | ** AAI | Antioxidant Activity | |
| n-Hexane | 290.020 ± 80.639 | 0.183 ± 0.013 | No activity |
| Ethyl acetate | 12.496 ± 4.153 | 4.211 ± 0.339 | Very strong |
| Chloroform | 71.984 ± 20.112 | 0.720 ± 0.050 | Moderate |
| Methanol | 64.774 ± 24.764 | 0.834 ± 0.068 | Moderate |
| Gallic acid | 3.923 ± 1.259 | 13.001 ± 0.672 | Very strong |
| MIC (µg/mL) | |||||
|---|---|---|---|---|---|
| Strains | Hexane | Ethyl Acetate | Chloroform | Methanol | Tetracycline |
| Staphylococcus aureus ATCC 25923 | 1250 | 5000 | 5000 | 5000 | 2 |
| Escherichia coli ATCC 25922 | >5000 | >5000 | >5000 | >5000 | 4 |
| Klebsiella pneumoniae ATCC 13883 | >5000 | >5000 | >5000 | >5000 | 8 |
| Acinetobacter baumannii LMG 1025 | 5000 | 5000 | 5000 | >5000 | 2 |
| Bacillus cereus ATCC 11778 | 2500 | 312 | 625 | 625 | 0.25 |
| Pseudomonas aeruginosa ATCC 27853 | >5000 | >5000 | >5000 | >5000 | 16 |
| Salmonella Typhimurium ATCC 13311 | >5000 | >5000 | >5000 | >5000 | 8 |
| Enterococcus faecalis ATCC 29212 | >5000 | 5000 | >5000 | >5000 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheydaei, P.; Ferreira, S.; Almeida, M.; Coimbra, A.; Yousefbeyk, F.; Gallardo, E.; Breitenfeld, L.; Amaral, M.E.; Duarte, A.P. Bioactive Compounds and Traditional Uses of Tripleurospermum disciforme (C.A.Mey.) Sch.Bip.: A Comprehensive Study on Its Therapeutic Potential. Molecules 2025, 30, 3685. https://doi.org/10.3390/molecules30183685
Sheydaei P, Ferreira S, Almeida M, Coimbra A, Yousefbeyk F, Gallardo E, Breitenfeld L, Amaral ME, Duarte AP. Bioactive Compounds and Traditional Uses of Tripleurospermum disciforme (C.A.Mey.) Sch.Bip.: A Comprehensive Study on Its Therapeutic Potential. Molecules. 2025; 30(18):3685. https://doi.org/10.3390/molecules30183685
Chicago/Turabian StyleSheydaei, Parvaneh, Susana Ferreira, Micaela Almeida, Alexandra Coimbra, Fatemeh Yousefbeyk, Eugenia Gallardo, Luiza Breitenfeld, Maria Emília Amaral, and Ana Paula Duarte. 2025. "Bioactive Compounds and Traditional Uses of Tripleurospermum disciforme (C.A.Mey.) Sch.Bip.: A Comprehensive Study on Its Therapeutic Potential" Molecules 30, no. 18: 3685. https://doi.org/10.3390/molecules30183685
APA StyleSheydaei, P., Ferreira, S., Almeida, M., Coimbra, A., Yousefbeyk, F., Gallardo, E., Breitenfeld, L., Amaral, M. E., & Duarte, A. P. (2025). Bioactive Compounds and Traditional Uses of Tripleurospermum disciforme (C.A.Mey.) Sch.Bip.: A Comprehensive Study on Its Therapeutic Potential. Molecules, 30(18), 3685. https://doi.org/10.3390/molecules30183685









