Novel Isoindigo-Based Organic Semiconductors End Capped with 1,1-Dicyanomethylene-3-Indanone: Effect of the Bromination and Position of Bromine Substituents on the Chemical–Physical and Electrical Properties
Abstract
1. Introduction
2. Results and Discussion
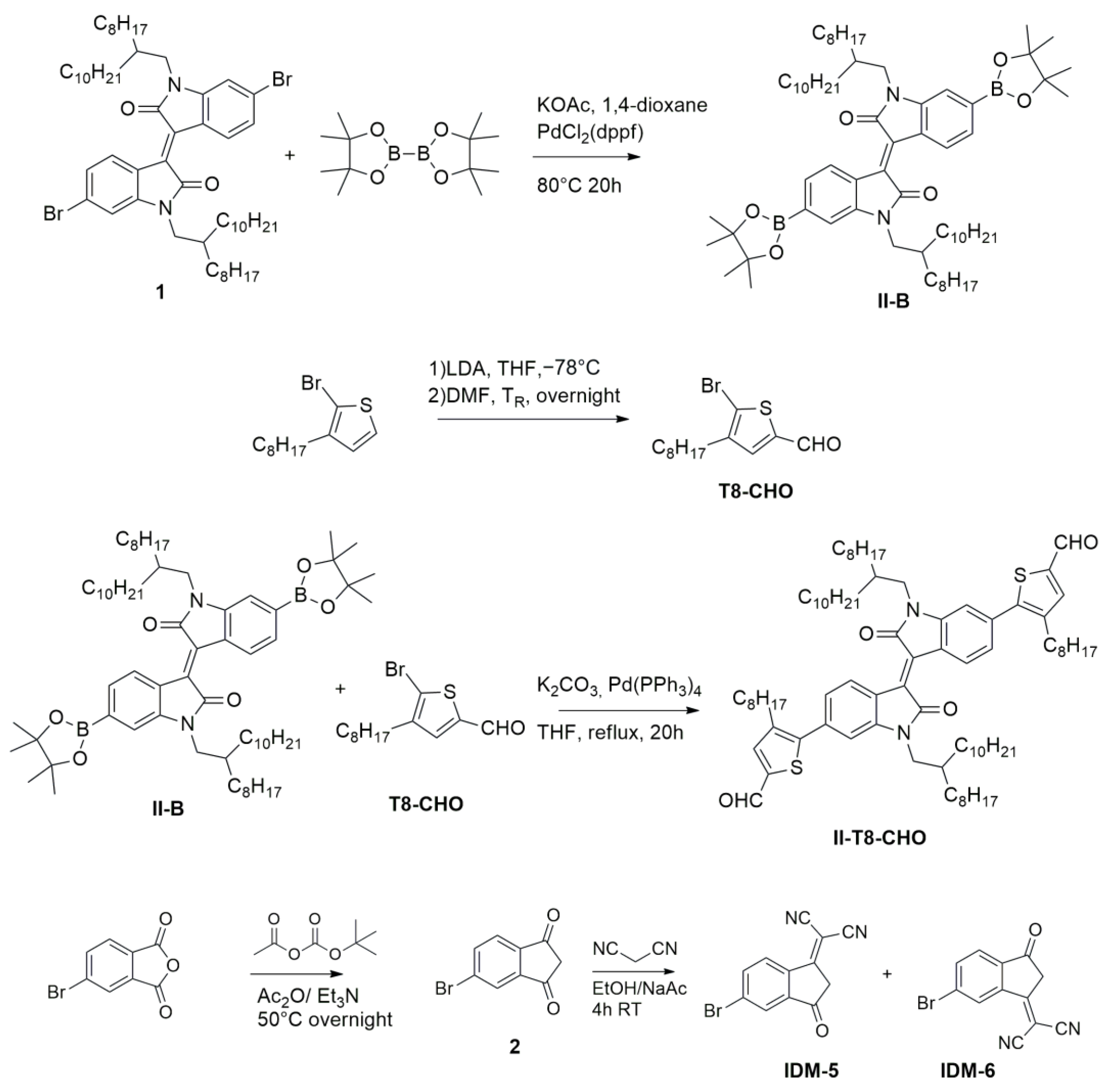
2.1. Synthesis and Thermal Characterization
2.2. Optical Characterization
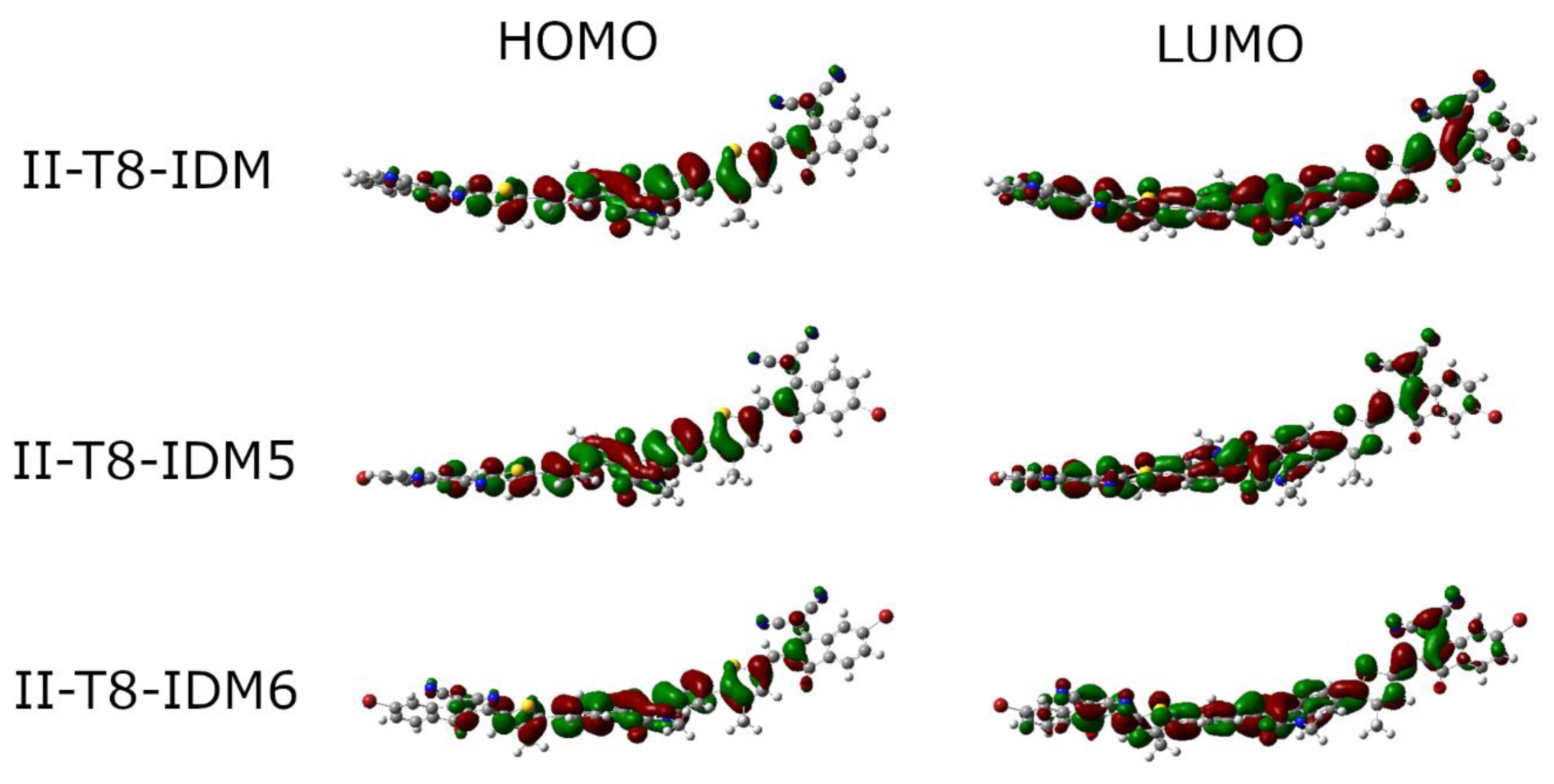
2.3. DFT Analysis
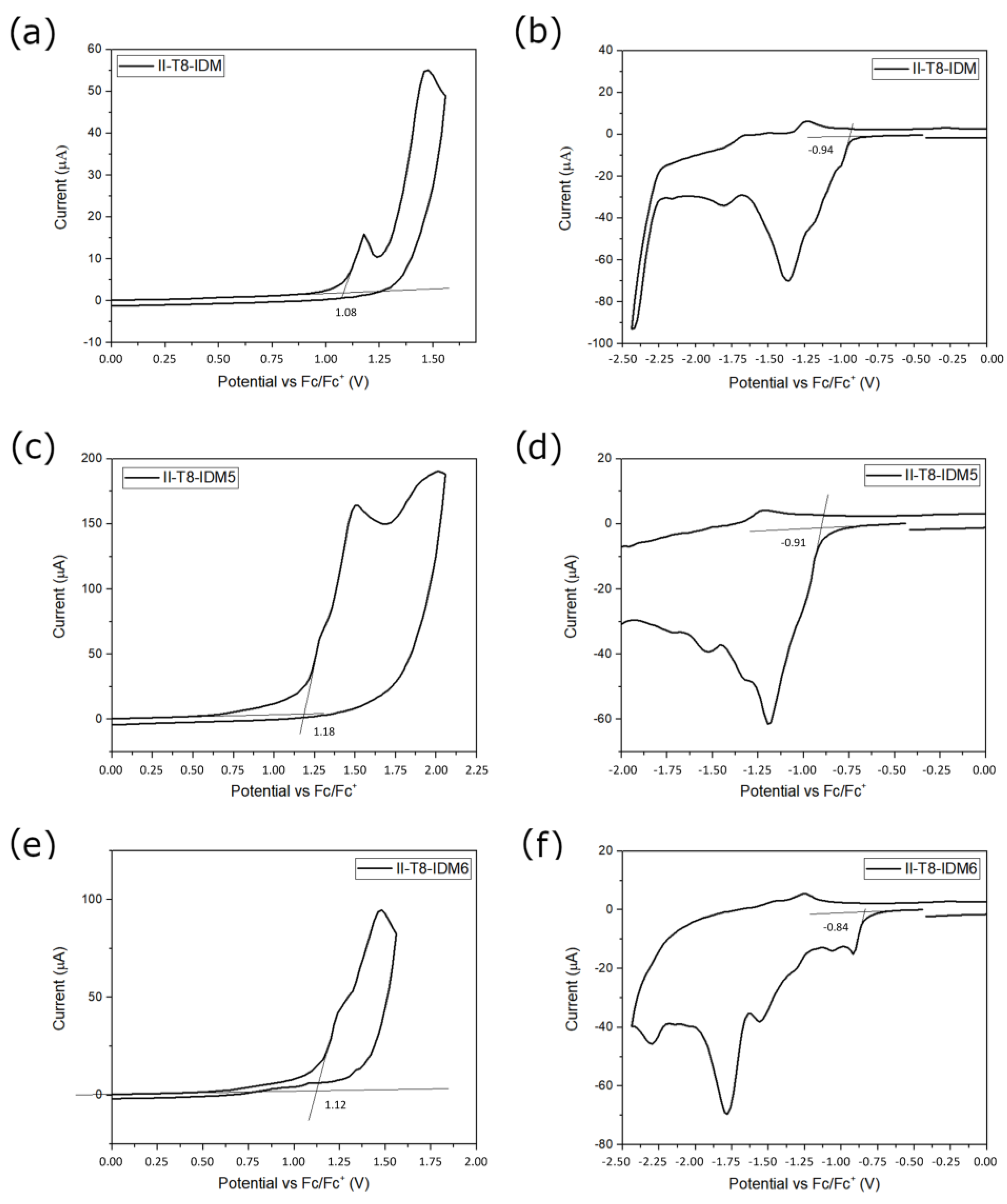
2.4. Electrochemical Characterization
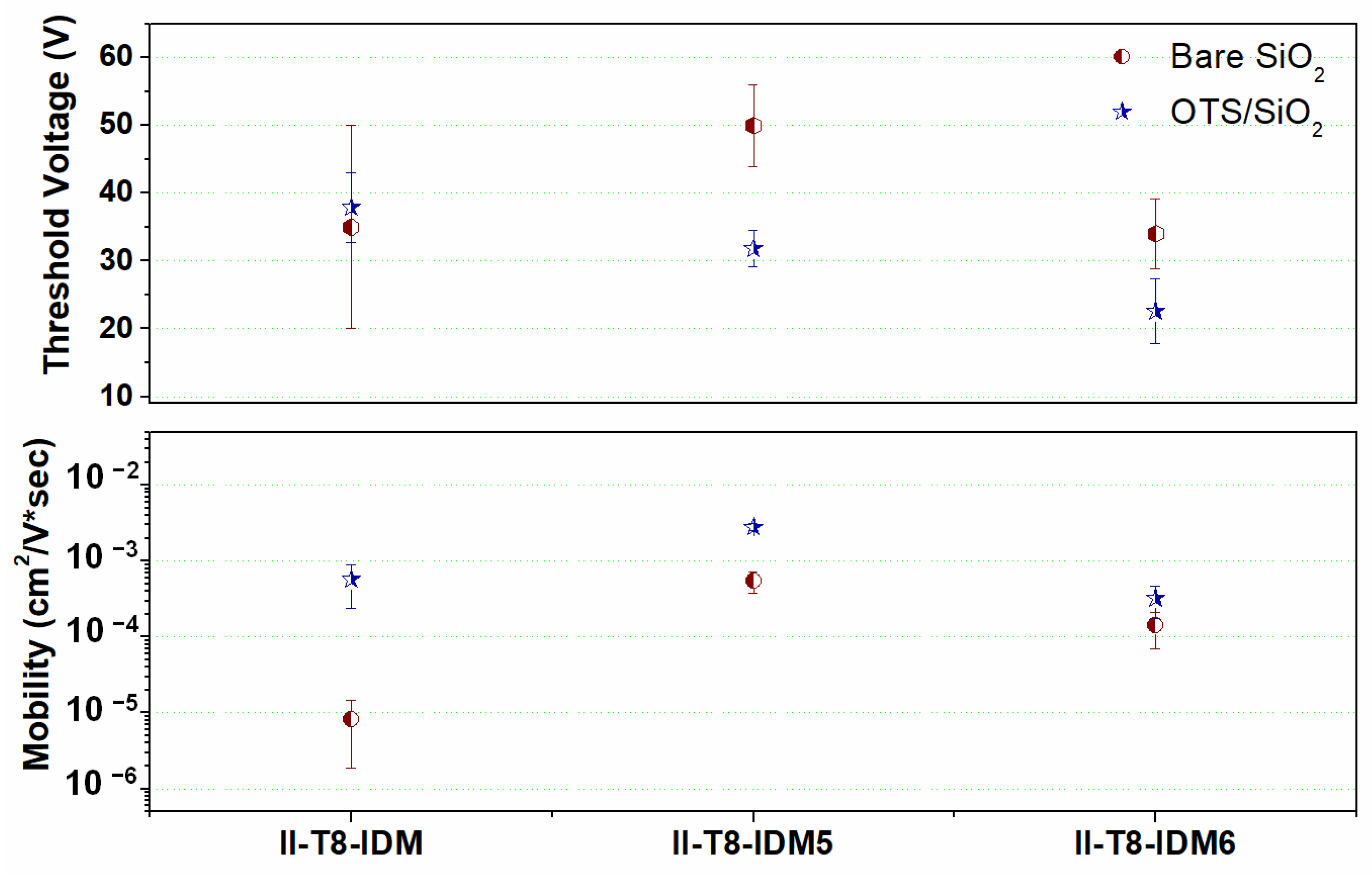
2.5. OFET Fabrication and Electrical Characterization
3. Materials and Methods
3.1. General Information
3.2. Synthesis
3.2.1. Synthesis of (E)-1,1′-Bis(2-Octyldodecyl)-6,6′-Bis(4,4,5,5-Tetramethyl-1,3,2-Dioxaborolan-2-Yl)-[3,3′-Biindolinylidene]-2,2′-Dione (II-B)
3.2.2. Synthesis of 5-Bromo-3-Octylthiophene-2-Carbaldehyde (T8-CHO)
3.2.3. Synthesis of (E)-5,5′-(1,1′-Bis(2-Octyldodecyl)-2,2′-Dioxo-[3,3′-Biindolinylidene]-6,6′-Diyl)bis(4-Octylthiophene-2-Carbaldehyde) (II-T8-CHO)
3.2.4. Synthesis of 5-Bromo-1H-Indene-1,3(2H)-Dione and 6-Bromo-1H-Indene-1,3(2H)-Dione (Compound 2)
3.2.5. Synthesis of 2-(5-Bromo-3-Oxo-2,3-Dihydro-1H-Inden-1-Ylidene)Malononitrile (IDM-5) and 2-(6-Bromo-3-Oxo-2,3-Dihydro-1H-Inden-1-Ylidene)Malononitrile (IDM-6)
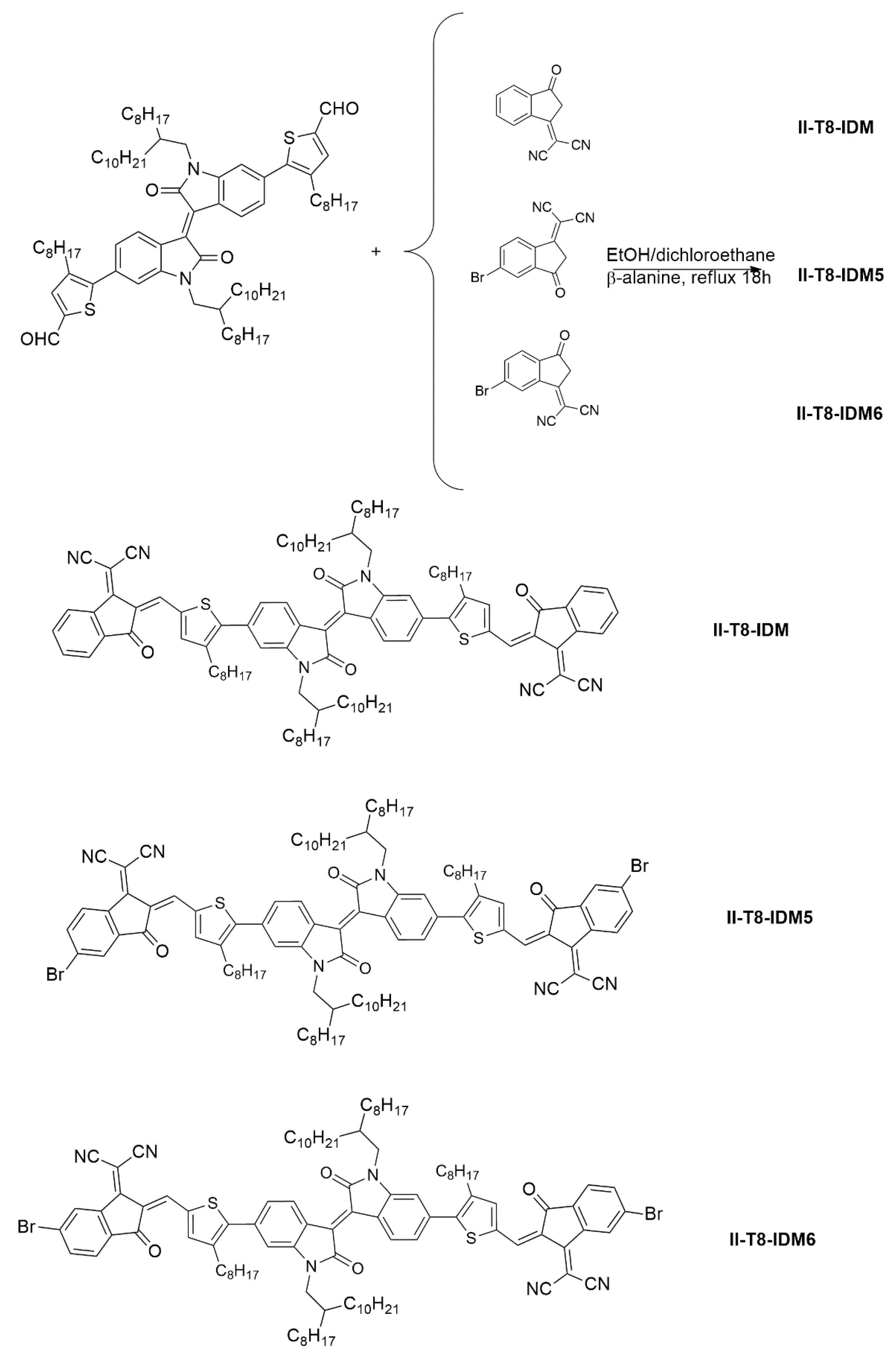
3.2.6. Synthesis of 2,2′-((2Z,2′Z)-((((E)-1,1′-Bis(2-Octyldodecyl)-2,2′-Dioxo-[3,3′-Biindolinylidene]-6,6′-Diyl)Bis(4-Octylthiophene-5,2-Diyl))Bis(Methaneylylidene))Bis(3-Oxo-2,3-Dihydro-1H-Indene-2,1-Diylidene))Dimalononitrile (II-T8-IDM)
3.2.7. Synthesis of 2,2′-((2Z,2′Z)-((((E)-1,1′-Bis(2-Octyldodecyl)-2,2′-Dioxo-[3,3′-Biindolinylidene]-6,6′-Diyl)Bis(4-Octylthiophene-5,2-Diyl))Bis(Methaneylylidene))Bis(-Bromo-3-Oxo-2,3-Dihydro-1H-Indene-2,1-Diylidene))Dimalononitrile (II-T8-IDM5)
3.2.8. Synthesis of 2,2′-((2Z,2′Z)-((((E)-1,1′-Bis(2-Octyldodecyl)-2,2′-Dioxo-[3,3′-Biindolinylidene]-6,6′-Diyl)Bis(4-Octylthiophene-5,2-Diyl))Bis(Methaneylylidene))Bis(6-Bromo-3-Oxo-2,3-Dihydro-1H-Indene-2,1-Diylidene))Dimalononitrile (II-T8-IDM6)
3.3. DFT Analysis
3.4. OFET Fabrication and Electrical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Facchetti, A. Semiconductors for organic transistors. Mater. Today 2007, 10, 28–37. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, W.; Sirringhaus, H.; Müllen, K. Recent Progress in Emerging Organic Semiconductors. Adv. Mater. 2022, 34, e2108701. [Google Scholar] [CrossRef] [PubMed]
- Forrest, S.R. Organic Electronics: Foundations to Applications; Oxford University Press: Oxford, UK, 2020; pp. 1–1014. [Google Scholar] [CrossRef]
- Basiricò, L.; Mattana, G.; Mas-Torrent, M. Editorial: Organic Electronics: Future Trends in Materials, Fabrication Techniques and Applications. Front. Phys. 2022, 10, 888155. [Google Scholar] [CrossRef]
- Root, S.E.; Savagatrup, S.; Printz, A.D.; Rodriquez, D.; Lipomi, D.J. Mechanical Properties of Organic Semiconductors for Stretchable, Highly Flexible, and Mechanically Robust Electronics. Chem. Rev. 2017, 117, 6467–6499. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, Y.; Liu, Y. Wearable Electronics Based on Stretchable Organic Semiconductors. Small 2023, 19, e2206309. [Google Scholar] [CrossRef]
- O’Connor, T.F.; Zaretski, A.V.; Savagatrup, S.; Printz, A.D.; Wilkes, C.D.; Diaz, M.I.; Sawyer, E.J.; Lipomi, D.J. Wearable organic solar cells with high cyclic bending stability: Materials selection criteria. Sol. Energy Mater. Sol. Cells 2016, 144, 438–444. [Google Scholar] [CrossRef]
- Yang, A.; Yan, F. Flexible Electrochemical Biosensors for Health Monitoring. ACS Appl. Electron. Mater. 2021, 3, 53–67. [Google Scholar] [CrossRef]
- Hong, G.; Gan, X.; Leonhardt, C.; Zhang, Z.; Seibert, J.; Busch, J.M.; Bräse, S. A Brief History of OLEDs—Emitter Development and Industry Milestones. Adv. Mater. 2021, 33, e2005630. [Google Scholar] [CrossRef]
- Kubo, T.; Häusermann, R.; Tsurumi, J.; Soeda, J.; Okada, Y.; Yamashita, Y.; Akamatsu, N.; Shishido, A.; Mitsui, C.; Okamoto, T.; et al. Suppressing molecular vibrations in organic semiconductors by inducing strain. Nat. Commun. 2016, 7, 11156. [Google Scholar] [CrossRef]
- Paulsen, B.D.; Tybrandt, K.; Stavrinidou, E.; Rivnay, J. Organic mixed ionic–electronic conductors. Nat. Mater. 2020, 19, 13–26. [Google Scholar] [CrossRef]
- Landi, A.; Reisjalali, M.; Elliott, J.D.; Matta, M.; Carbone, P.; Troisi, A. Simulation of polymeric mixed ionic and electronic conductors with a combined classical and quantum mechanical model. J. Mater. Chem. C 2023, 11, 8062–8073. [Google Scholar] [CrossRef]
- Liu, K.; Ouyang, B.; Guo, X.; Guo, Y.; Liu, Y. Advances in flexible organic field-effect transistors and their applications for flexible electronics. npj Flex. Electron. 2022, 6, 1. [Google Scholar] [CrossRef]
- Schwartz, G.; Tee, B.C.-K.; Mei, J.; Appleton, A.L.; Kim, D.H.; Wang, H.; Bao, Z. Flexible polymer transistors with high pressure sensitivity for application in electronic skin and health monitoring. Nat. Commun. 2013, 4, 1859. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.T.E.; Zhu, J.; Li, X.; Wang, J.; Li, Y. Recent progress in the development of n-type organic semiconductors for organic field effect transistors. J. Mater. Chem. C 2017, 5, 8654–8681. [Google Scholar] [CrossRef]
- Meng, B.; Liu, J.; Wang, L. Recent development of n-type thermoelectric materials based on conjugated polymers. Nano Mater. Sci. 2021, 3, 113–123. [Google Scholar] [CrossRef]
- Gao, X.; Hu, Y. Development of n-type organic semiconductors for thin film transistors: A viewpoint of molecular design. J. Mater. Chem. C 2014, 2, 3099–3117. [Google Scholar] [CrossRef]
- Liu, Y. Advantages of CMOS Technology in Very Large Scale Integrated Circuits. In Proceedings of the AIEE‘21: Proceedings of the 2021 2nd International Conference on Artificial Intelligence in Electronics Engineering, Phuket, Thailand, 15–17 January 2021; pp. 82–88. [Google Scholar] [CrossRef]
- Xie, Y.; Ding, C.; Jin, Q.; Zheng, L.; Xu, Y.; Xiao, H.; Cheng, M.; Zhang, Y.; Yang, G.; Li, M.; et al. Organic transistor-based integrated circuits for future smart life. SmartMat 2024, 5, e1261. [Google Scholar] [CrossRef]
- Yeh, N.; Yeh, P. Organic solar cells: Their developments and potentials. Renew. Sustain. Energy Rev. 2013, 21, 421–431. [Google Scholar] [CrossRef]
- Liu, W.; Xu, X.; Yuan, J.; Leclerc, M.; Zou, Y.; Li, Y. Low-Bandgap Non-fullerene Acceptors Enabling High-Performance Organic Solar Cells. ACS Energy Lett. 2021, 6, 598–608. [Google Scholar] [CrossRef]
- Kim, M.; Ryu, S.U.; Park, S.A.; Pu, Y.-J.; Park, T. Designs and understanding of small molecule-based non-fullerene acceptors for realizing commercially viable organic photovoltaics. Chem. Sci. 2021, 12, 14004–14023. [Google Scholar] [CrossRef]
- Li, S.; Zhang, H.; Yue, S.; Yu, X.; Zhou, H. Recent advances in non-fullerene organic photovoltaics enabled by green solvent processing. Nanotechnology 2021, 33, 072002. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, J.; Chow, P.C.Y.; Jiang, K.; Zhang, J.; Zhu, Z.; Zhang, J.; Huang, F.; Yan, H. Nonfullerene Acceptor Molecules for Bulk Heterojunction Organic Solar Cells. Chem. Rev. 2018, 118, 3447–3507. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Huang, Y.; Zhang, R.; Mou, H.; Ding, J.; Zhou, J.; Wang, Z.; Li, H.; Chen, W.; Zhu, J.; et al. Organic solar cells with 20.82% efficiency and high tolerance of active layer thickness through crystallization sequence manipulation. Nat. Mater. 2025, 24, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Wang, G.; Lian, Q.; Gámez-Valenzuela, S.; Li, B.; Ding, R.; Yang, W.; Wang, K.; Zeng, J.; Zhang, Y.; et al. Non-fullerene electron-transporting materials for high-performance and stable perovskite solar cells. Nat. Mater. 2025, 24, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Chen, W.; Yang, W.; Li, Y.; Zhou, Y.; Larson, B.W.; Johnson, J.C.; Lu, Y.-H.; Zhong, W.; Xu, J.; et al. Improving Efficiency and Stability of Perovskite Solar Cells Enabled by A Near-Infrared-Absorbing Moisture Barrier. Joule 2020, 4, 1575–1593. [Google Scholar] [CrossRef]
- Jiang, J.; Jin, Z.; Lei, J.; Wang, Q.; Zhang, X.; Zhang, J.; Gao, F.; Liu, S. ITIC surface modification to achieve synergistic electron transport layer enhancement for planar-type perovskite solar cells with efficiency exceeding 20%. J. Mater. Chem. A 2017, 5, 9514–9522. [Google Scholar] [CrossRef]
- Forti, G.; Nitti, A.; Osw, P.; Bianchi, G.; Po, R.; Pasini, D. Recent Advances in Non-Fullerene Acceptors of the IDIC/ITIC Families for Bulk-Heterojunction Organic Solar Cells. Int. J. Mol. Sci. 2020, 21, 8085. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, J.; Zhang, Z.; Bai, H.; Li, Y.; Zhu, D.; Zhan, X. An Electron Acceptor Challenging Fullerenes for Efficient Polymer Solar Cells. Adv. Mater. 2015, 27, 1170–1174. [Google Scholar] [CrossRef]
- Yuan, J.; Zou, Y. The history and development of Y6. Org. Electron. 2022, 102, 106436. [Google Scholar] [CrossRef]
- Ge, J.; Xie, L.; Peng, R.; Ge, Z. Organic Photovoltaics Utilizing Small-Molecule Donors and Y-Series Nonfullerene Acceptors. Adv. Mater. 2023, 35, e2206566. [Google Scholar] [CrossRef]
- Feng, L.; Yuan, J.; Zhang, Z.; Peng, H.; Zhang, Z.-G.; Xu, S.; Liu, Y.; Li, Y.; Zou, Y. Thieno[3,2-b]pyrrolo-Fused Pentacyclic Benzotriazole-Based Acceptor for Efficient Organic Photovoltaics. ACS Appl. Mater. Interfaces 2017, 9, 31985–31992. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Brabec, C.J.; Kyaw, A.K.K. Non-fused ring electron acceptors for high-performance and low-cost organic solar cells: Structure-function, stability and synthesis complexity analysis. Nano Energy 2023, 114, 108661. [Google Scholar] [CrossRef]
- Carella, A.; Franzini, M.; Fusco, S.; Centore, R.; Barra, M.; Chiarella, F.; Cassinese, A.; Bonomo, M.; Nejrotti, S.; Carbone, M.; et al. Isoindigo dyes functionalized with terminal electron-withdrawing groups: Computational, optical and electrical characterization. Dye. Pigment. 2022, 208, 110866. [Google Scholar] [CrossRef]
- Maglione, C.; Carella, A.; Centore, R.; Fusco, S.; Velardo, A.; Peluso, A.; Colonna, D.; Di Carlo, A. Tuning optical absorption in pyran derivatives for DSSC. J. Photochem. Photobiol. A Chem. 2016, 321, 79–89. [Google Scholar] [CrossRef]
- Lu, S.; Li, F.; Zhang, K.; Zhu, J.; Cui, W.; Yang, R.; Yu, L.; Sun, M. Halogenation on terminal groups of ITIC based electron acceptors as an effective strategy for efficient polymer solar cells. Sol. Energy 2020, 195, 429–435. [Google Scholar] [CrossRef]
- Landi, A.; Landi, A.; Velardo, A.; Peluso, A. Efficient Charge Dissociation of Triplet Excitons in Bulk Heterojunction Solar Cells. ACS Appl. Energy Mater. 2022, 5, 10815–10824. [Google Scholar] [CrossRef]
- Cardona, C.M.; Li, W.; Kaifer, A.E.; Stockdale, D.; Bazan, G.C. Electrochemical Considerations for Determining Absolute Frontier Orbital Energy Levels of Conjugated Polymers for Solar Cell Applications. Adv. Mater. 2011, 23, 2367–2371. [Google Scholar] [CrossRef]
- Chiarella, F.; Barra, M.; Carella, A.; Parlato, L.; Sarnelli, E.; Cassinese, A. Contact-resistance effects in PDI8-CN 2 n-type thin-film transistors investigated by Kelvin-probe potentiometry. Org. Electron. 2016, 28, 299–305. [Google Scholar] [CrossRef]
- Lai, H.; Chen, H.; Zhou, J.; Qu, J.; Chao, P.; Liu, T.; Chang, X.; Zheng, N.; Xie, Z.; He, F. Isomer-free: Precise Positioning of Chlorine-Induced Interpenetrating Charge Transfer for Elevated Solar Conversion. iScience 2019, 17, 302–314. [Google Scholar] [CrossRef]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Crystallogr. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Carella, A.; Landi, A.; Bonomo, M.; Chiarella, F.; Centore, R.; Peluso, A.; Nejrotti, S.; Barra, M. Asymmetrical Diketopyrrolopyrrole Derivatives with Improved Solubility and Balanced Charge Transport Properties. Molecules 2024, 29, 2805. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W. Gaussian 16, Revision, A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Miertuš, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilizaion of AB initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Paternò, G.M.; Barbero, N.; Galliano, S.; Barolo, C.; Lanzani, G.; Scotognella, F.; Borrelli, R. Excited state photophysics of squaraine dyes for photovoltaic applications: An alternative deactivation scenario. J. Mater. Chem. C 2018, 6, 2778–2785. [Google Scholar] [CrossRef]
- Padula, D.; Landi, A.; Prampolini, G. Assessing alkyl side chain effects on electron transport properties of Y6-derived non-fullerene acceptors. Energy Adv. 2023, 2, 1215–1224. [Google Scholar] [CrossRef]
- Chiarella, F.; Toccoli, T.; Barra, M.; Aversa, L.; Ciccullo, F.; Tatti, R.; Verucchi, R.; Iannotta, S.; Cassinese, A. High mobility n-type organic thin-film transistors deposited at room temperature by supersonic molecular beam deposition. Appl. Phys. Lett. 2014, 104, 143302. [Google Scholar] [CrossRef]

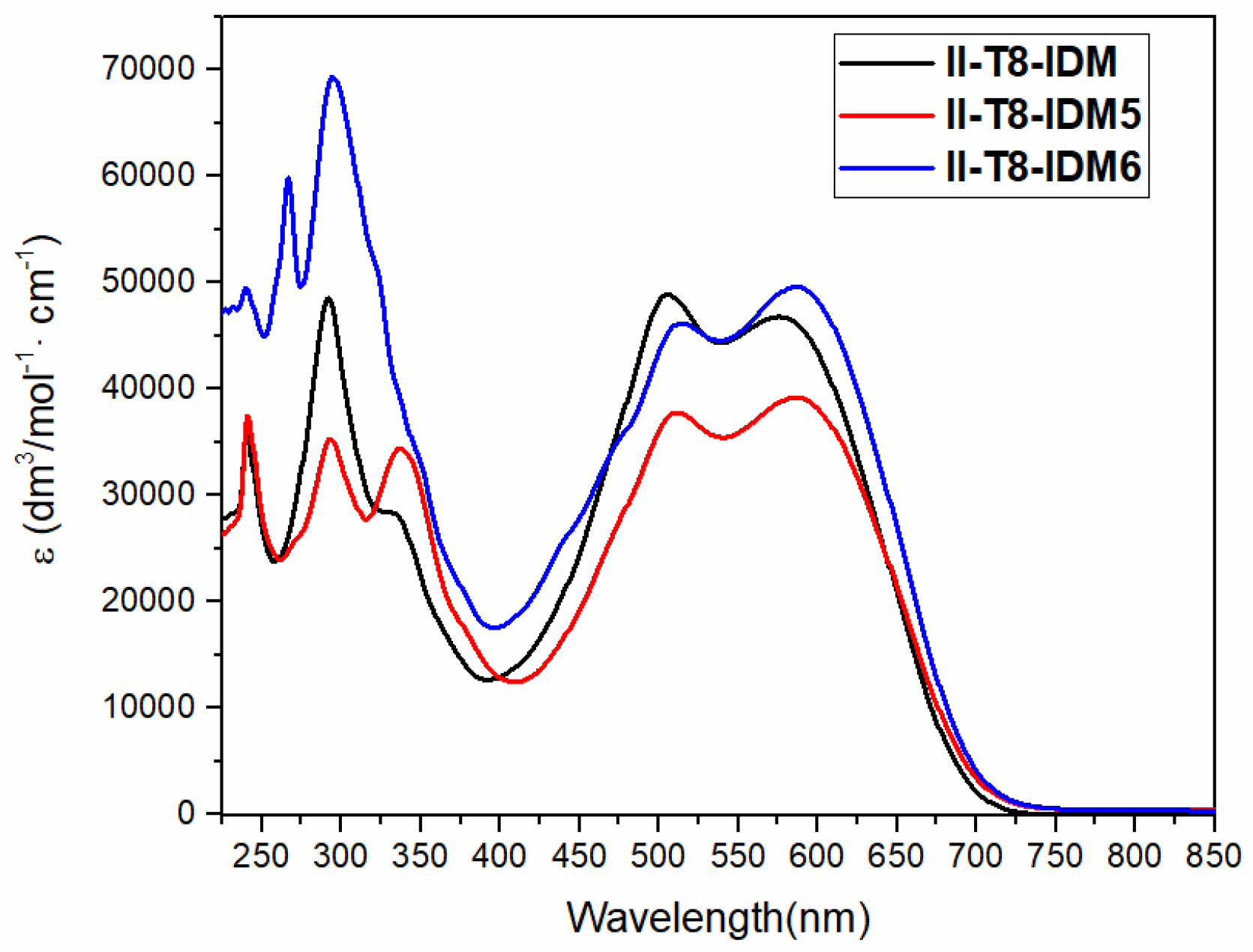
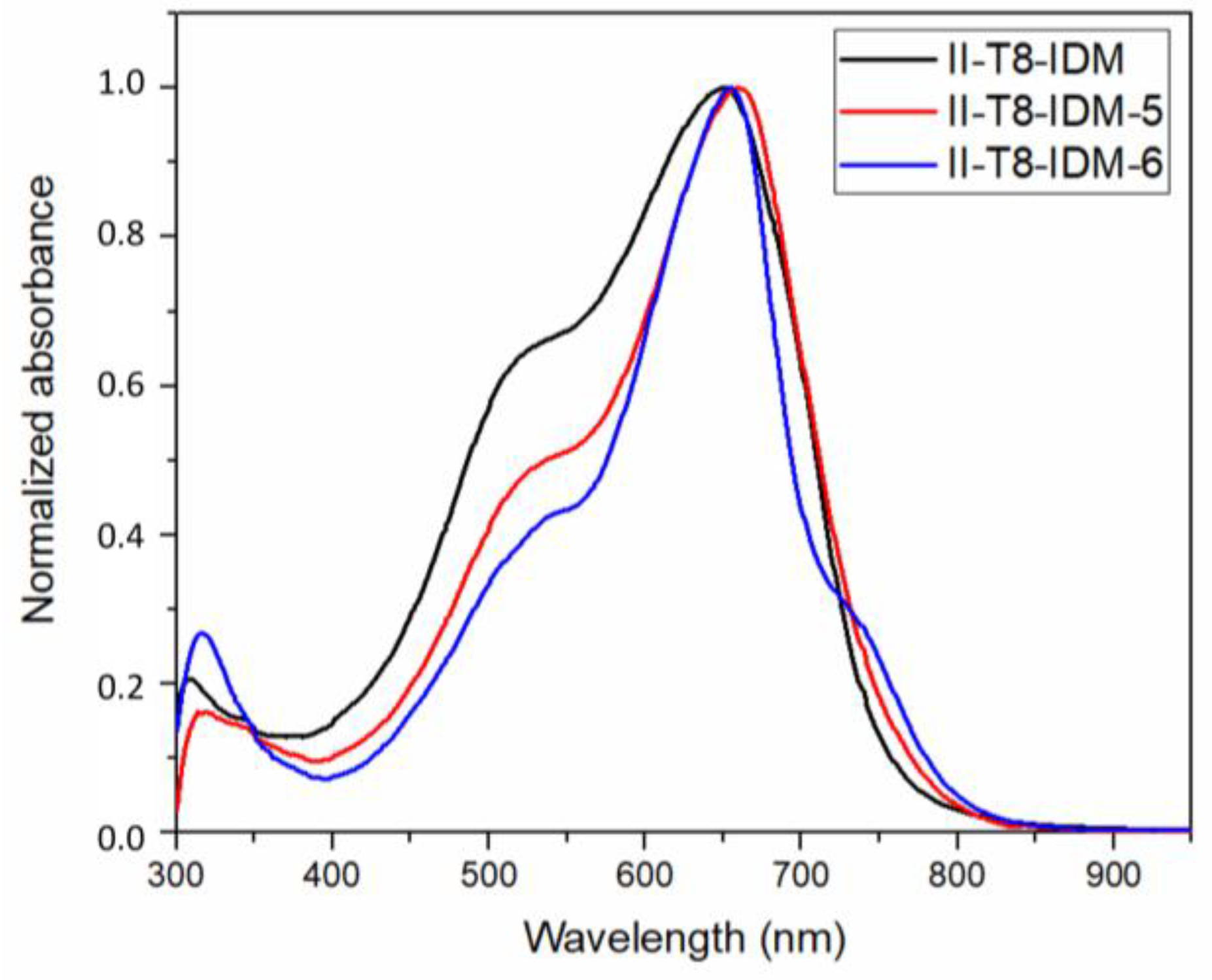


| Dyes | λ1/ε1a | λ2/ε2a | λ3/ε3a | λ4/εa | λ5/ε5a |
|---|---|---|---|---|---|
| II-T8-IDM | 577/4.7 × 104 | 506/4.9 × 104 | 333/2.8 × 104 | 292/4.8 × 104 | 242/3.50 × 104 |
| II-T8-IDM5 | 587/3.9 × 104 | 512/3.8 × 104 | 338/3.4 × 104 | 293/3.5 × 104 | 242/3.53 × 104 |
| II-T8-IDM6 | 587/4.9 × 104 | 515/4.6 × 104 | - | 295/6.8 × 104 | 241/4.70 × 104 |
| Dyes | λ (nm) | Eg (eV) b |
|---|---|---|
| II-T8-IDM | 650/552(sh) | 1.66 |
| II-T8-IDM5 | 660/553(sh) | 1.64 |
| II-T8-IDM6 | 657/555(sh)/722(sh) | 1.55 |
| Dyes | D1 (°) | D1′ (°) | D2 (°) | D2′ (°) | D3 (°) |
|---|---|---|---|---|---|
| II-T8-IDM | −10.10 | −13.77 | 37.41 | −38.86 | 12.78 |
| II-T8-IDM5 | −8.29 | −11.10 | 37.44 | −38.97 | 14.77 |
| II-T8-IDM6 | −10.49 | −10.49 | 37.62 | −38.96 | 14.89 |
| λabs (nm) | F a | μGS (D) b | μES (D) c | Eg (eV) d | Egexp (eV) e | |
|---|---|---|---|---|---|---|
| II-T8-IDM | 637 | 1.97 | 6.07 | 4.95 | 1.95 | 1.80 |
| II-T8-IDM5 | 642 | 2.06 | 6.82 | 5.72 | 1.93 | 1.78 |
| II-T8-IDM6 | 644 | 2.06 | 8.85 | 7.35 | 1.93 | 1.77 |
| Dyes | Eox (V) a | HOMO (eV) b | HOMO (eV) c | Ered (V) a | LUMO (eV) b | LUMO (eV) c |
| II-T8-IDM | 1.08 | −6.18 | −6.14 | −0.94 | −4.16 | −3.63 |
| II-T8-IDM5 | 1.18 | −6.28 | −6.18 | −0.91 | −4.19 | −3.69 |
| II-T8-IDM6 | 1.12 | −6.22 | −6.18 | −0.84 | −4.26 | −3.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mocerino, F.; Barra, M.; Borbone, F.; Carella, A.; Centore, R.; Chiarella, F.; Landi, A.; Peluso, A. Novel Isoindigo-Based Organic Semiconductors End Capped with 1,1-Dicyanomethylene-3-Indanone: Effect of the Bromination and Position of Bromine Substituents on the Chemical–Physical and Electrical Properties. Molecules 2025, 30, 3672. https://doi.org/10.3390/molecules30183672
Mocerino F, Barra M, Borbone F, Carella A, Centore R, Chiarella F, Landi A, Peluso A. Novel Isoindigo-Based Organic Semiconductors End Capped with 1,1-Dicyanomethylene-3-Indanone: Effect of the Bromination and Position of Bromine Substituents on the Chemical–Physical and Electrical Properties. Molecules. 2025; 30(18):3672. https://doi.org/10.3390/molecules30183672
Chicago/Turabian StyleMocerino, Fabio, Mario Barra, Fabio Borbone, Antonio Carella, Roberto Centore, Fabio Chiarella, Alessandro Landi, and Andrea Peluso. 2025. "Novel Isoindigo-Based Organic Semiconductors End Capped with 1,1-Dicyanomethylene-3-Indanone: Effect of the Bromination and Position of Bromine Substituents on the Chemical–Physical and Electrical Properties" Molecules 30, no. 18: 3672. https://doi.org/10.3390/molecules30183672
APA StyleMocerino, F., Barra, M., Borbone, F., Carella, A., Centore, R., Chiarella, F., Landi, A., & Peluso, A. (2025). Novel Isoindigo-Based Organic Semiconductors End Capped with 1,1-Dicyanomethylene-3-Indanone: Effect of the Bromination and Position of Bromine Substituents on the Chemical–Physical and Electrical Properties. Molecules, 30(18), 3672. https://doi.org/10.3390/molecules30183672









