Innovative and Healthy Cookies Enriched with Blueberry Leaf Powder
Abstract
1. Introduction
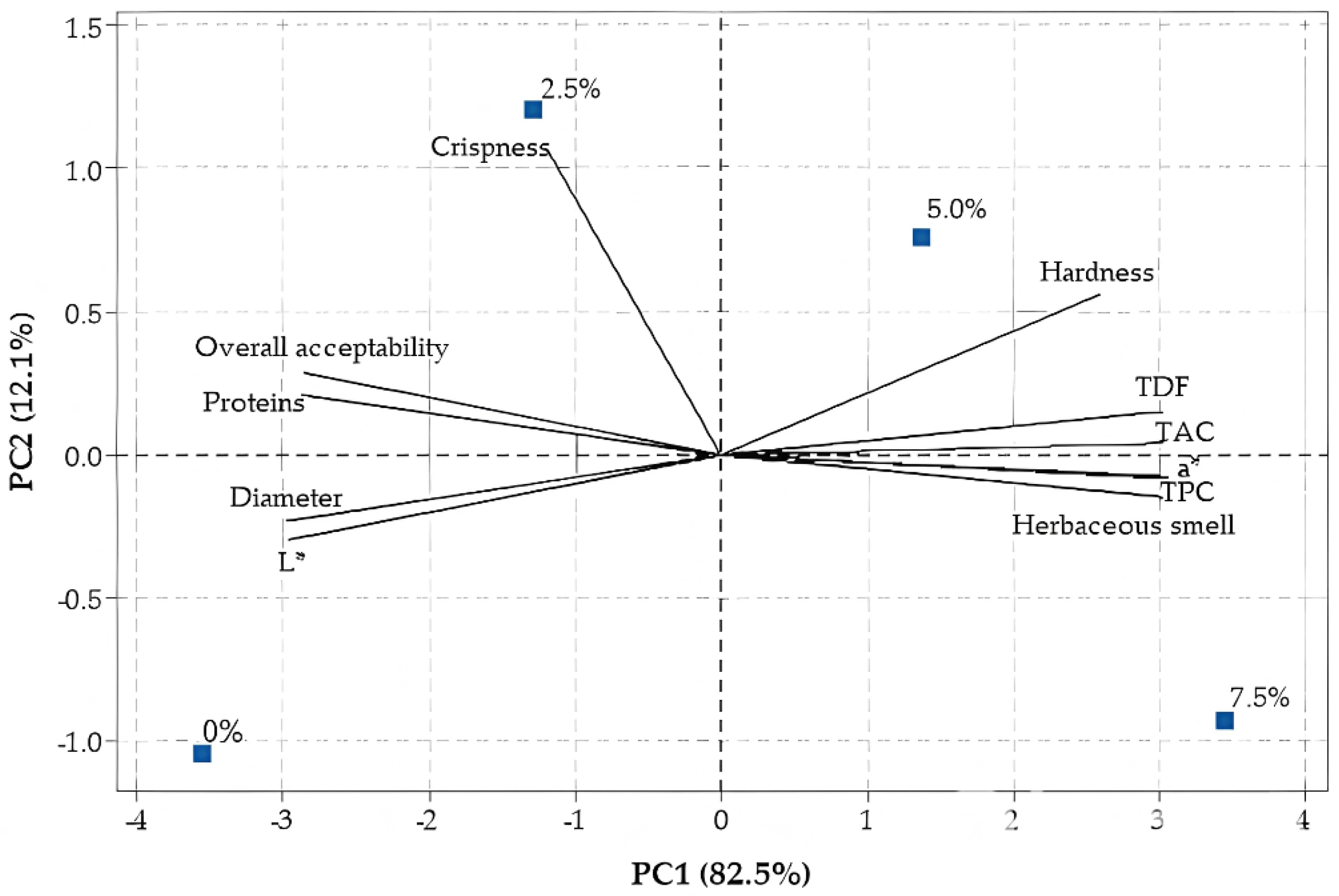
2. Results and Discussion
2.1. BBLP Characterization
2.2. Cookies Characterization
2.2.1. Chemical Composition
2.2.2. Physical Analyses
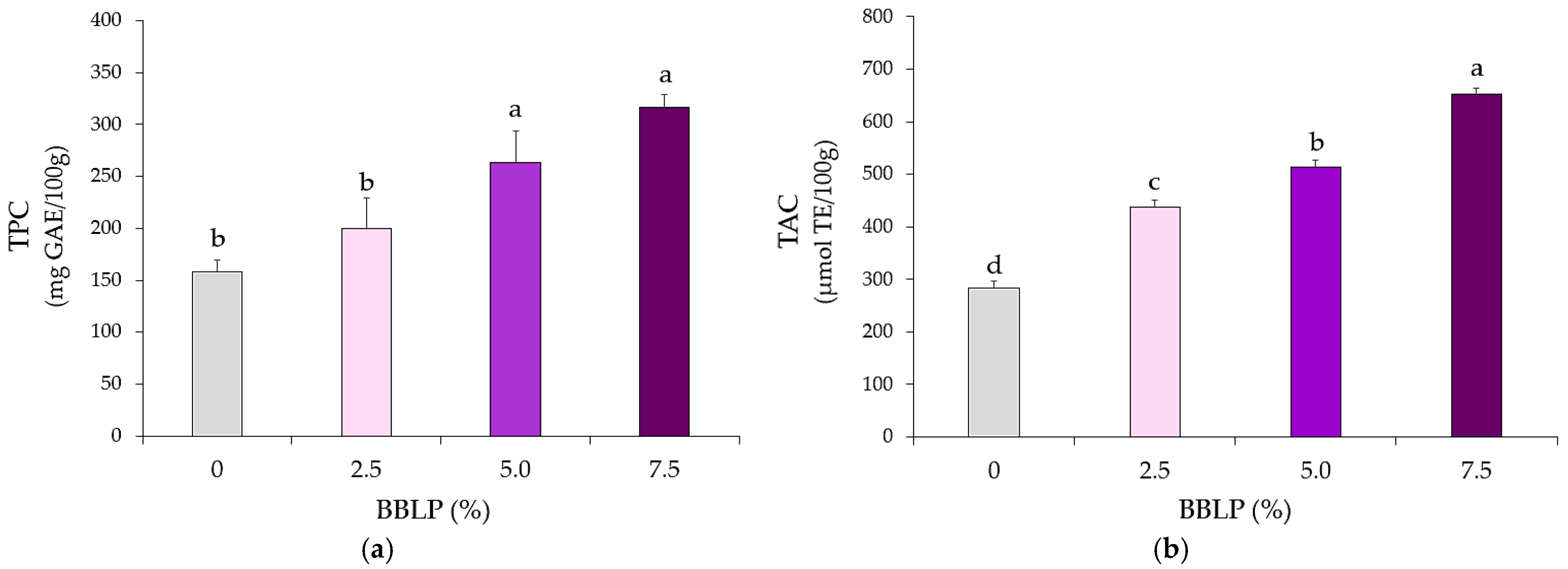
2.2.3. Antioxidant Activity
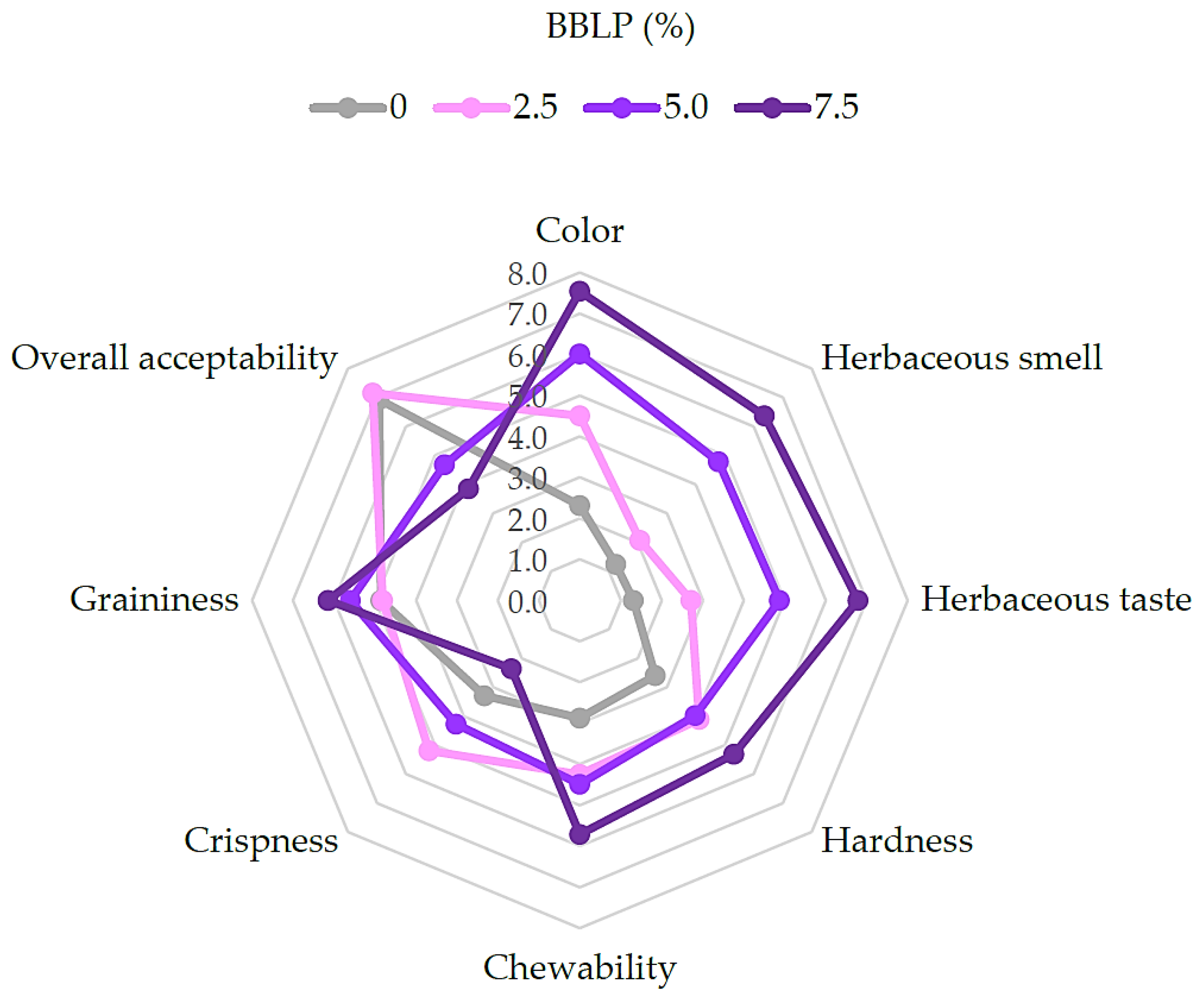
2.2.4. Sensory Analysis
2.3. Limitations and Future Perspectives
3. Materials and Methods
3.1. Ingredients
3.2. BBLP Characterization
3.2.1. Chemical Analyses
3.2.2. Polyphenol Extraction and Antioxidant Assays
3.3. Experimental Protocol, Formulation, and Preparation of Cookies
3.4. Characterization of Cookies
3.4.1. Chemical Analyses
3.4.2. Physical Analyses
3.4.3. Antioxidant Capacity
3.4.4. Descriptive Sensory Analysis
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Blueberry Organization. Global State of the Blueberry Industry Report. Available online: https://www.internationalblueberry.org (accessed on 23 July 2025).
- Arora, J.; Ramawat, K.G.; Mérillon, J.M. Disposal of agricultural waste and its effects on the environment, production of useful metabolites and energy: Potential and challenges. In Agricultural Waste: Environmental Impact, Useful Metabolites and Energy Production; Ramawat, K., Mérillon, J.M., Arora, J., Eds.; Springer: Singapore, 2023; Volume 31, pp. 1–28. [Google Scholar] [CrossRef]
- Okan, O.T.; Ulusoy, E.; Öz, M.; Deniz, I. Evaluation of antioxidant properties of wild and cultivated blueberry leaves and their phenolic compounds. Kastamonu Univ. J. For. 2024, 24, 104–114. [Google Scholar] [CrossRef]
- Değirmencioğlu, N.; Gürbüz, O.; Karatepe, G.E.; Irkin, R. Influence of hot air drying on phenolic compounds and antioxidant capacity of blueberry (Vaccinium myrtillus) fruit and leaf. J. Appl. Bot. Food Qual. 2017, 90, 115–125. [Google Scholar] [CrossRef]
- Debnath-Canning, M.; Unruh, S.; Vyas, P.; Daneshtalab, N.; Igamberdiev, A.U.; Weber, J.T. Fruits and leaves from wild blueberry plants contain diverse polyphenols and decrease neuroinflammatory responses in microglia. J. Funct. Foods. 2020, 68, 103906. [Google Scholar] [CrossRef]
- Ștefănescu, B.E.; Szabo, K.; Mocan, A.; Crișan, G. Phenolic compounds from five Ericaceae species leaves and their related bioavailability and health benefits. Molecules 2019, 24, 2046. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Tenuta, M.C.; Loizzo, M.R.; Bonesi, M.; Finetti, F.; Trabalzini, L.; Deguin, B. Vaccinium Species (Ericaceae): From chemical composition to bio-functional activities. Appl. Sci. 2021, 11, 5655. [Google Scholar] [CrossRef]
- Wang, J.; Tian, J.; Li, D.; Gao, N.; Deng, J.; Yang, X.; Wang, L.; He, Y.; Li, B.; Wang, L. Blueberry leaves as a promising sustainable source of polyphenols: Chemical composition, functional activities and future application perspectives. Food Res. Int. 2025, 207, 116110. [Google Scholar] [CrossRef]
- Czernicka, M.; Sowa-Borowiec, P.; Puchalski, C.; Czerniakowski, Z.W. Content of bioactive compounds in highbush blueberry Vaccinium corymbosum L. leaves as a potential raw material for food technology or pharmaceutical industry. Foods 2024, 13, 246. [Google Scholar] [CrossRef]
- Teleszko, M.; Wojdyło, A. Comparison of phenolic compounds and antioxidant potential of different berry leaves and fruits. J. Funct. Foods. 2015, 14, 736–746. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wojdyło, A.; Gorzelany, J.; Kapusta, I. Identification and characterization of low molecular weight polyphenols in berry leaf extracts by HPLC-DAD and LC-ESI/MS. J. Agric. Food Chem. 2021, 59, 12830–12835. [Google Scholar] [CrossRef]
- Ferlemi, A.V.; Lamari, F.N. Berry leaves: An alternative source of bioactive natural products of nutritional and medicinal value. Antioxidants 2016, 5, 17. [Google Scholar] [CrossRef]
- Biel, W.; Jaroszewska, A. The nutritional value of leaves of selected berry species. Sci. Agric. 2017, 74, 405–410. [Google Scholar] [CrossRef]
- Koh, W.Y.; Lim, X.X.; Tan, T.C.; Mamat, H.; Kobun, R.; Rasti, B. Utilising spent tea leaves powder as functional ingredient to enhance the quality of non-gluten shortbread cookies. Foods 2023, 12, 1557. [Google Scholar] [CrossRef]
- Hefnawy, H.T.M.; El-Shourbagy, G.A.; Ramadan, M.F. Phenolic extracts of carrot, grape leaf and turmeric powder: Antioxidant potential and application in biscuits. J. Food Meas. Charact. 2016, 10, 576–583. [Google Scholar] [CrossRef]
- Nguyen, N.D.T.; Phan, T.T.H.; Tran, T.T.T.; Ton, N.M.N.; Vo, D.L.T.; Le, V.V.M. Enzymatic treatment of spent green tea leaves and their use in high-fibre cookie production. FTB 2022, 60, 396–405. [Google Scholar] [CrossRef]
- Abdel-Moemin, A.R. Healthy cookies from cooked fish bones. Food Biosci. 2015, 12, 114–121. [Google Scholar] [CrossRef]
- Walker, S.; Seetharaman, K.; Goldstein, A. Characterizing physicochemical changes of cookies baked in a commercial oven. Food Res. Int. 2012, 48, 249–256. [Google Scholar] [CrossRef]
- Žilić, S.; Kocadağlı, T.; Vančetović, J.; Gökmen, V. Effects of baking conditions and dough formulations on phenolic compound stability, antioxidant capacity and color of cookies made from anthocyanin-rich corn flour. LWT–Food Sci. Technol. 2016, 65, 597–603. [Google Scholar] [CrossRef]
- Kozlov, A.V.; Javadov, S.; Sommer, N. Cellular ROS and antioxidants: Physiological and pathological role. Antioxidants 2024, 13, 602. [Google Scholar] [CrossRef] [PubMed]
- Kalogerakou, T.; Antoniadou, M. The role of dietary antioxidants, food supplements and functional foods for energy enhancement in healthcare professionals. Antioxidants 2024, 13, 1508. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chai, Z.; Hutabarat, R.P.; Zeng, Q.; Niu, L.; Li, D.; Yu, H.; Huang, W. Blueberry leaves from 73 different cultivars in Southeastern China as nutraceutical supplements rich in antioxidants. Food Res. Int. 2019, 122, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Páscoa, R.N.M.J.; Gomes, M.J.; Sousa, C. Antioxidant activity of blueberry (Vaccinium spp.) cultivar leaves: Differences across the vegetative stage and the application of near infrared spectroscopy. Molecules 2019, 24, 3900. [Google Scholar] [CrossRef]
- Saura-Calixto, F. Concept and health-related properties of nonextractable polyphenols: The missing dietary polyphenols. J. Agric. Food Chem. 2012, 60, 11195–11200. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Chen, J.; Yang, J.; Hao, Y.; Fan, Y.; Wang, C.; Li, N. Free, soluble-bound and insoluble-bound phenolics and their bioactivity in raspberry pomace. LWT–Food Sci. Technol. 2021, 135, 109995. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J. Insoluble-bound phenolics in food. Molecules 2016, 21, 1216. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Guidelines for Use of Nutrition and Health Claims (CAC/GL 23-1997). Available online: https://www.fao.org/ag/humannutrition/32443-04352e8311b857c57caf5ffc4c5c4a4cd.pdf (accessed on 30 June 2025).
- Aljobair, M.O. Physicochemical properties and sensory attributes of cookies prepared from sorghum and millet composite flour. Food Sci. Nutr. 2022, 10, 3415–3423. [Google Scholar] [CrossRef]
- Sinaki, N.Y.; Koksel, F. Effects of dietary fibre source and content and extrusion conditions on the physicochemical composition and physical quality of fibre-enriched lentil snacks. Int. J. Food Sci. Technol. 2024, 59, 2236–2248. [Google Scholar] [CrossRef]
- Kaur, A.; Singh, B.; Yadav, M.P.; Bhinder, S.; Singh, N. Isolation of arabinoxylan and cellulose-rich arabinoxylan from wheat bran of different varieties and their functionalities. Food Hydrocoll. 2021, 112, 106287. [Google Scholar] [CrossRef]
- Arias, F.; Zavala, J.A.; Ciancia, M. Pectins and hemicelluloses from cell walls of hulls from developing soybean seeds. Int. J. Biol. Macromol. 2025, 305, 140882. [Google Scholar] [CrossRef] [PubMed]
- Alba, J.; Campbell, G.M.; Kontogiorgos, V. Dietary fibre from berry-processing waste and its impact on bread structure: A review. J. Sci. Food Agric. 2019, 99, 4189–4199. [Google Scholar] [CrossRef]
- Opperman, C.; Majzoobi, M.; Farahnaky, A.; Shah, R.; Van, T.T.H.; Ratanpaul, V.; Blanch, E.W.; Brennan, C.; Eri, R. Beyond soluble and insoluble: A comprehensive framework for classifying dietary fibre’s health effects. Food Res. Int. 2025, 206, 115843. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Guidelines on Nutrition Labelling (CAC/GL 2-1985, revised 2017; amended 2024). Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXG%2B2-1985%252FCXG_002e.pdf (accessed on 30 June 2025).
- Li, M.; Li, L.; Sun, B.; Ma, S. Interaction of wheat bran dietary fiber–gluten protein affects dough product: A critical review. Int. J. Biol. Macromol. 2023, 255, 128199. [Google Scholar] [CrossRef]
- Chouaibi, M.; Rezig, L.; Boussaid, A.; Hamdi, S. Insoluble tomato-fiber effect on wheat dough rheology and cookies’ quality. Ital. J. Food Sci. 2019, 31, 1–13. [Google Scholar] [CrossRef]
- Mancebo, C.M.; Rodríguez, P.; Martínez, M.M.; Gómez, M. Effect of the addition of soluble (nutriose, inulin and polydextrose) and insoluble (bamboo, potato and pea) fibres on the quality of sugar-snap cookies. Int. J. Food Sci. Technol. 2018, 53, 129–136. [Google Scholar] [CrossRef]
- Gruppi, A.; Giuberti, G.; Duserm Garrido, G.; Spigno, G. Effect of different fibre addition on cookie dough and texture. Food Sci. Technol. Int. 2024, 30, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.; Oliveira, R.; Ribeiro, C.; Magalhães, C.; Ferreira, H.; Ferreira, P.; Freire, C. The potential of anthocyanins from blueberries as a natural dye for cotton: A combined experimental and theoretical study. Dyes Pigm. 2020, 176, 108180. [Google Scholar] [CrossRef]
- Sui, X.; Yap, P.Y.; Zhou, W. Anthocyanins during baking: Their degradation kinetics and impacts on color and antioxidant capacity of bread. In Impact of Food Processing on Anthocyanins, 1st ed.; Sui, X., Ed.; Springer: Singapore, 2016; pp. 67–86. [Google Scholar] [CrossRef]
- Borrelli, R.C.; Fogliano, V. Bread crust melanoidins as potential prebiotic ingredients. Mol. Nutr. Food Res. 2005, 49, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Zhu, M.; Wan, X.; Zhai, X.; Ho, C.T.; Zhang, L. Food polyphenols and Maillard reaction: Regulation effect and chemical mechanism. Crit. Rev. Food Sci. Nutr. 2022, 64, 4904–4920. [Google Scholar] [CrossRef]
- Kruczek, D.; Gumul, K.; Korus, A.; Buksa, K.; Ziobro, R. Phenolic compounds and antioxidant status of cookies supplemented with apple pomace. Antioxidants 2023, 12, 324. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The role of polyphenols in human health and food systems: A mini-review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Drewnowski, A.; Gomez-Carneros, C. Bitter taste, phytonutrients, and the consumer: A review. Am. J. Clin. Nutr. 2000, 72, 1424–1435. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, N.; Shimizu, T.; Fujii, Y.; Fushimi, T.; Calabrese, V. Sensory nutrition and bitterness and astringency of polyphenols. Biomolecules 2024, 14, 234. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. Official Methods of Analysis of AOAC International, 16th ed., 5th rev.; AOAC International: Gaithersburg, MD, USA, 1998. [Google Scholar]
- AOAC International. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Pérez, M.; Domínguez-López, I.; Lamuela-Raventós, R.M. The chemistry behind the Folin–Ciocalteu method for the estimation of (poly)phenol content in food: Total phenolic intake in a Mediterranean dietary pattern. J. Agric. Food Chem. 2023, 71, 17543–17553. [Google Scholar] [CrossRef]
- Pukalskas, A.; Van Beek, T.A.; Venskutonis, R.P.; Linssen, J.P.H.; Van Veldhuizen, A.; De Groot, Æ. Identification of radical scavengers in sweet grass (Hierochloe odorata). J. Agric. Food Chem. 2002, 50, 2914–2919. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture, Agricultural Research Service. FoodData Central: FDC ID 169761. Whole-wheat flour. Available online: https://fdc.nal.usda.gov/food-details/169761/nutrients (accessed on 20 July 2025).
- U.S. Department of Agriculture, Agricultural Research Service. FoodData Central: FDC ID 174275. Soy Flour, Defatted. Available online: https://fdc.nal.usda.gov/food-details/174275/nutrients (accessed on 20 July 2025).
- Yam, K.L.; Papadakis, S.E. A simple digital imaging method for measuring and analyzing color of food surfaces. J. Food Eng. 2004, 61, 137–142. [Google Scholar] [CrossRef]
- Stone, H.; Bleibaum, R.N.; Thomas, H.A. Descriptive Analysis. In Sensory Evaluation Practices, 3rd ed.; Megan, B., Ed.; Elsevier Academic Press: San Diego, CA, USA, 2000; pp. 235–294. [Google Scholar]
- Palmay-Paredes, J.; Paz-Yépez, C.; Medina-Galarza, G.; Guerra, R.; Campuzano-Vera, A.; Hernández-Maya, C. Training of a sensory panel and its correlation with instrumental methods: Texture of a pseudo-plastic dressing. Curr. Res. Nutr. Food Sci. 2023, 11, 1374–1385. [Google Scholar] [CrossRef]

| Component | Content |
|---|---|
| Moisture (% W/W) | 7.2 ± 0.4 |
| Ashes (% W/W) | 4.8 ± 0.1 |
| Proteins (% W/W) | 8.2 ± 0.1 |
| Fat (% W/W) | 2.2 ± 0.1 |
| TDF (% W/W) | 44 ± 1 |
| IDF (% W/W) | 39 ± 1 |
| SDF (% W/W) | 5 ± 2 |
| TPC (mg GAE/100 g) | 2109 ± 20 |
| TAC (µmol Trolox/100 g) | 6251 ± 42 |
| Component | BBLP (%) | |||
|---|---|---|---|---|
| 0 | 2.5 | 5.0 | 7.5 | |
| Moisture | 9.5 ± 0.1 a | 9.3 ± 0.08 ab | 9.2 ± 0.1 b | 8.7 ± 0.1 c |
| Ash | 3.7 ± 0.1 a | 3.6 ± 0.1 a | 3.7 ± 0.2 a | 3.6 ± 0.1 a |
| Proteins | 12.0 ± 0.1 a | 11.6 ± 0.1 b | 11.5 ± 0.1 b | 10.7 ± 0.1 c |
| Fats | 21.5 ± 0.4 a | 21.2 ± 0.1 a | 21.7 ± 0.2 a | 21.2 ± 0.4 a |
| SFAs | 11.2 ± 0.3 a | 10.3 ± 0.1 a | 10.7 ± 0.1 a | 10.3 ± 0.1 a |
| UFAs | 10.3 ± 0.3 a | 10.9 ± 0.1 a | 11.0 ± 0.1 a | 10.9 ± 0.1 a |
| TFAs | 0.40 ± 0.01 a | 0.39 ± 0.02 a | 0.40 ± 0.02 a | 0.40 ± 0.01 a |
| TDF | 5.6 ± 0.4 b | 6.5 ± 0.5 ab | 7.2 ± 0.5 ab | 7.8 ± 0.4 a |
| IDF | 3.4 ± 0.4 b | 4.5 ± 0.5 ab | 5.1 ± 0.6 ab | 5.6 ± 0.1 a |
| SDF | 2.2 ± 0.1 a | 2.0 ± 0.4 a | 2.1 ± 0.4 a | 2.2 ± 0.5 a |
| Carbohydrates | 47.9 ± 0.5 a | 48.0 ± 0.4 a | 46.6 ± 0.6 a | 48.0 ± 0.4 a |
| Calcium * | 1182 ± 141 a | 1174 ± 40 a | 1162 ± 81 a | 1108 ± 42 a |
| Sodium * | 96 ± 7 a | 97 ± 5 a | 103 ± 6 a | 102 ± 3 a |
| Parameter | BBLP (%) | |||
|---|---|---|---|---|
| 0 | 2.5 | 5.0 | 7.5 | |
| Individual weight (g) | 13.0 ± 1.0 a | 13.5 ± 0.3 a | 12 ± 1.0 a | 11.9 ± 0.2 a |
| Diameter (cm) | 5.30 ± 0.02 a | 5.14 ± 0.03 b | 5.09 ± 0.05 b | 5.01 ± 0.02 bc |
| Thickness (cm) | 0.90 ± 0.01 a | 0.86 ± 0.01 b | 0.82 ± 0.01 c | 0.81 ± 0.01 c |
| Spread Ratio | 5.8 ± 0.1 b | 6.0 ± 0.1 b | 6.20 ± 0.03 a | 6.15 ± 0.03 a |
| Volume (mL) | 20 ± 2 a | 18 ± 1 b | 16 ± 1 bc | 17 ± 1 bc |
| Hardness (N) | 9 ± 1 b | 12 ± 1 ab | 14 ± 2 a | 13 ± 2 a |
| L* | 49 ± 1 a | 36 ± 1 b | 28 ± 1 c | 25 ± 1 d |
| a* | 8.2 ± 0.5 b | 8.6 ± 0.3 b | 9.6 ± 0.5 a | 10.1 ± 0.4 a |
| b* | 25 ± 1 a | 24.6 ± 0.3 b | 23.6 ± 0.4 c | 22.1 ± 0.3 d |
| BI | 82 ± 4 d | 124 ± 7 c | 170 ± 8 b | 191 ± 10 a |
| Ingredients (% W/W) | BBLP (%) | |||
|---|---|---|---|---|
| 0 | 2.5 | 5.0 | 7.5 | |
| Wheat flour | 26.8 | 25.2 | 23.6 | 21.9 |
| Soy flour | 13.9 | 13.1 | 12.2 | 11.4 |
| Margarine | 20.4 | 20.4 | 20.4 | 20.4 |
| Water | 10.6 | 10.6 | 10.6 | 10.6 |
| Egg powder | 3.1 | 3.1 | 3.1 | 3.1 |
| Baking powder | 0.8 | 0.8 | 0.8 | 0.8 |
| Vanilla extract | 0.3 | 0.3 | 0.3 | 0.3 |
| Sugar | 20.4 | 20.4 | 20.4 | 20.4 |
| Calcium carbonate | 3.6 | 3.6 | 3.6 | 3.6 |
| BBLP | 0.0 | 2.5 | 5.0 | 7.5 |
| Descriptor | References | |
|---|---|---|
| Color: color intensity | 1 = light Reference: alfajor cookie layers FANTOCHE® | 9 = dark Reference: cookie “Chocolinas” ARCOR® |
| Herbaceous smell: herbaceous notes intensity in smell | 1 = light Reference: water | 9 = very Reference: blueberry leaf powder |
| Herbaceous taste: herbaceous notes intensity in taste | 1 = light Reference: water | 9 = very Reference: blueberry leaf powder |
| Hardness: force applied at first bite, evaluated by front teeth | 1 = light Reference: alfajor cookie layers FANTOCHE® | 9 = very Reference: cookie “Muesli” MURKE® |
| Chewability: number of times that is necessary masticate to allow the deglutition | 1 = light Reference: water | 9 = very Reference: cookie “Muesli” MURKE® |
| Crispness: quantifies the food shattering in mouth | 1 = light Reference: water | 9 = very Reference: cookie “Muesli” MURKE® |
| Graininess: granules perception by pressing masticated food between tongue and palate | 1 = light Reference: water | 9 = very Reference: cookie “Muesli” MURKE® |
| Overall acceptability: harmony level of all mentioned parameters | 1 = light | 9 = very |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santuccione, F.A.; Soazo, M.; Llopart, E.; Rossi, M.; Verdini, R.A.; Pittia, P.; Pérez, L.M. Innovative and Healthy Cookies Enriched with Blueberry Leaf Powder. Molecules 2025, 30, 3671. https://doi.org/10.3390/molecules30183671
Santuccione FA, Soazo M, Llopart E, Rossi M, Verdini RA, Pittia P, Pérez LM. Innovative and Healthy Cookies Enriched with Blueberry Leaf Powder. Molecules. 2025; 30(18):3671. https://doi.org/10.3390/molecules30183671
Chicago/Turabian StyleSantuccione, Francesco Antonio, Marina Soazo, Emilce Llopart, Matías Rossi, Roxana Andrea Verdini, Paola Pittia, and Leonardo Martín Pérez. 2025. "Innovative and Healthy Cookies Enriched with Blueberry Leaf Powder" Molecules 30, no. 18: 3671. https://doi.org/10.3390/molecules30183671
APA StyleSantuccione, F. A., Soazo, M., Llopart, E., Rossi, M., Verdini, R. A., Pittia, P., & Pérez, L. M. (2025). Innovative and Healthy Cookies Enriched with Blueberry Leaf Powder. Molecules, 30(18), 3671. https://doi.org/10.3390/molecules30183671









