Optimizing the Hydrophobic Environment of Zeolite-Based Catalysts for Promoted Application in Heterogeneous Catalysis
Abstract
1. Introduction
2. Improving Hydrophobicity by Tuning Framework Composition and Surface Defect of Zeolites
2.1. Aluminosilicate Zeolites
2.2. Metallosilicate Zeolites
3. Hydrophobic Modification of Zeolites
3.1. Liquid-Mediated Post-Synthetic Modification
3.2. Silanization Post-Modification of Zeolites
3.3. Direct Hydrothermal Synthesis of Hydrophobic Zeolite-Based Catalysts
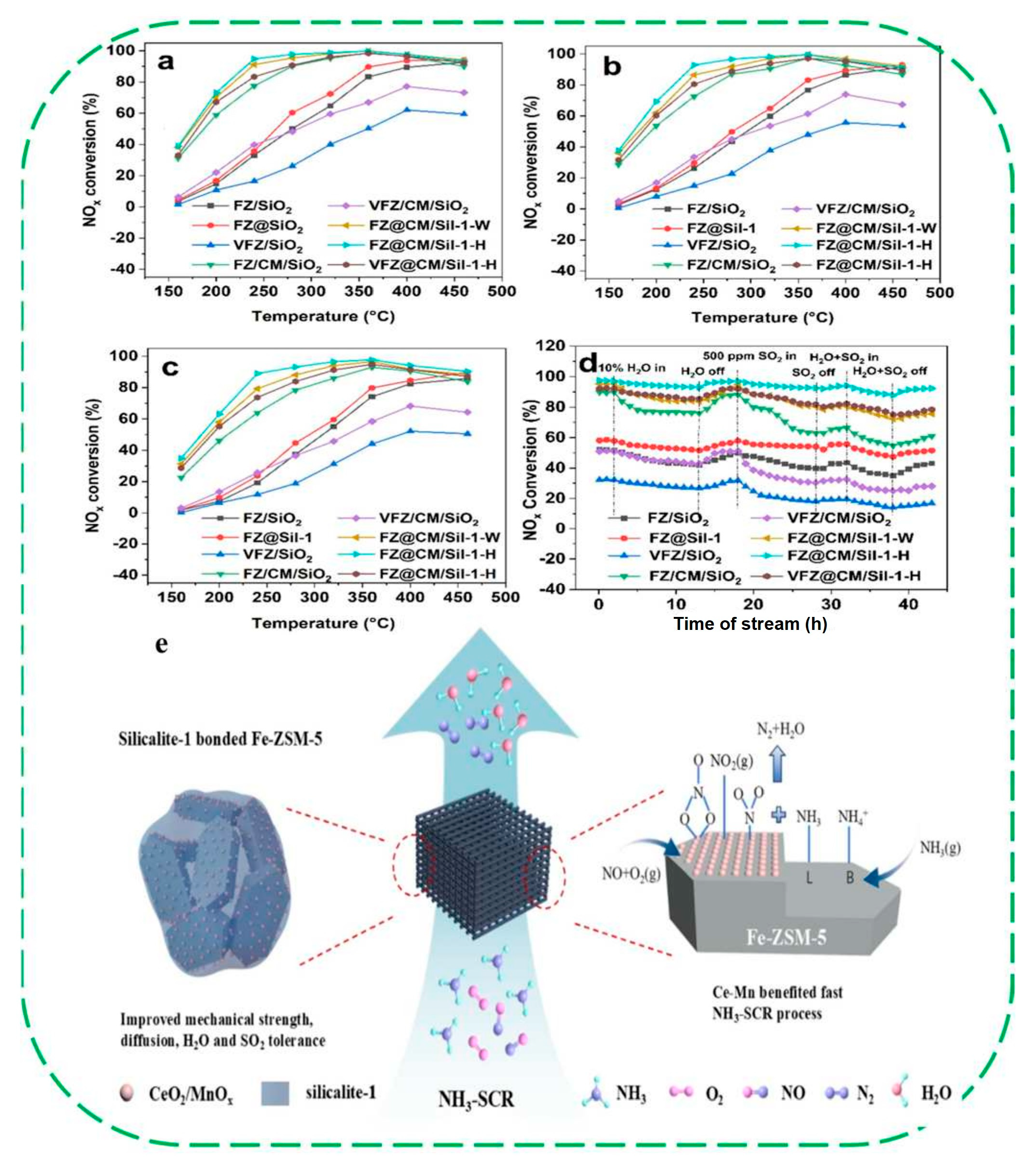
4. Constructing Hydrophobic Zeolite-Based Composites
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Gao, M.; Yan, W.; Yu, J. Regulation of the Si/Al ratios and Al distributions of zeolites and their impact on properties. Chem. Sci. 2023, 14, 1935–1959. [Google Scholar] [CrossRef]
- Zhang, Q.; Gao, S.; Yu, J. Metal sites in zeolites: Synthesis, characterization, and catalysis. Chem. Rev. 2022, 123, 6039–6106. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Bi, C.; Du, X.; Liu, T.; Jia, M. Morphology and microstructural optimization of zeolite crystals utilizing polymer growth modifiers for enhanced catalytic application. Catalysts 2024, 14, 375. [Google Scholar] [CrossRef]
- Hui, Y.; Wang, L.; Xiao, F.-S. Catalysis enhanced by catalyst wettability. ACS Nano 2025, 19, 7617–7633. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, Y.; Wu, Q.; Wen, Y.; Xiao, F.-S. Zeolite nanosheets for catalysis. Chem. Soc. Rev. 2022, 51, 2431–2443. [Google Scholar] [CrossRef]
- Zhan, J.; Wang, Y.; Li, J.; Bi, C.; He, T.; Zhang, C.; Liu, Q.; Li, K.; Zhang, Y.; Jia, M. Hierarchical Beta aggregates assembled from sheet-like nanocrystals: Synthesis and enhanced catalytic application in acylation and alkylation reactions. J. Colloid Interface Sci. 2025, 694, 137677. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Wang, Y.; He, T.; Sheng, L.; Wu, B.; Liu, Q.; Jia, M.; Zhang, Y. Nonionic polymer and amino acid-assisted synthesis of ZSM-5 nanocrystals and their catalytic application in the alkylation of 2-methylnaphthalene. Dalton Trans. 2024, 53, 7384–7396. [Google Scholar] [CrossRef]
- Liu, F.; Huang, K.; Zheng, A.; Xiao, F.-S.; Dai, S. Hydrophobic solid acids and their catalytic applications in green and sustainable chemistry. ACS Catal. 2017, 8, 372–391. [Google Scholar] [CrossRef]
- Wang, L.; Xiao, F.-S. A significant enhancement of catalytic performance by adjusting catalyst wettability. Sci. China Mater. 2018, 61, 1137–1142. [Google Scholar] [CrossRef]
- Wang, C.; Guo, H.; Leng, S.; Yu, J.; Feng, K.; Cao, L.; Huang, J. Regulation of hydrophilicity/hydrophobicity of aluminosilicate zeolites: A review. Crit. Rev. Solid State Mater. Sci. 2020, 46, 330–348. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, Y.; Qin, Q.; Liu, H.; Zhu, J. Development on hydrophobic modification of aluminosilicate and titanosilicate zeolite molecular sieves. Appl. Catal. A 2021, 611, 117952. [Google Scholar] [CrossRef]
- Ponge, C.A.; Corbin, D.R.; Sabolay, C.M.; Shiflett, M.B. Designing zeolites for the removal of aqueous PFAS: A perspective. Ind. Chem. Mater. 2024, 2, 270–275. [Google Scholar] [CrossRef]
- Jiang, N.; Shang, R.; Heijman, S.G.J.; Rietveld, L.C. High-silica zeolites for adsorption of organic micro-pollutants in water treatment: A review. Water Res. 2018, 144, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Algieri, C.; Drioli, E. Zeolite membranes: Synthesis and applications. Sep. Purif. Technol. 2021, 278, 119295. [Google Scholar] [CrossRef]
- Wang, J.T.; Huang, Z.; Zhu, Y.T.; Wang, S.N. Pervaporative poly (vinyl alcohol)/H-β zeolite mixed matrix membranes for dewatering C2–C3 alcohols. J. Appl. Polym. Sci. 2023, 140, e54454. [Google Scholar] [CrossRef]
- Tao, T.-L.; Chang, C.-K.; Kang, Y.-H.; Chen, J.-J.; Kang, D.-Y. Enhanced pervaporation performance of zeolite membranes treated by atmospheric-pressure plasma. J. Taiwan Inst. Chem. Eng. 2020, 116, 112–120. [Google Scholar] [CrossRef]
- Bandehali, S.; Parvizian, F.; Ruan, H.; Moghadassi, A.; Shen, J.; Figoli, A.; Adeleye, A.S.; Hilal, N.; Matsuura, T.; Drioli, E.; et al. A planned review on designing of high-performance nanocomposite nanofiltration membranes for pollutants removal from water. J. Ind. Eng. Chem. 2021, 101, 78–125. [Google Scholar] [CrossRef]
- Wu, X.; Li, Y.; Li, Y.; Peng, M.; Yang, Z.; Gui, T.; Chen, X.; Kita, H. Fabrication of hydrophilic and durable Beta zeolite membranes for dehydration of fuel n-butanol by adjusting Al spatial distribution. Sep. Purif. Technol. 2024, 351, 128047. [Google Scholar] [CrossRef]
- Okuhara, T. Water-tolerant solid acid catalysts. Chem. Rev. 2002, 102, 3641–3666. [Google Scholar] [CrossRef] [PubMed]
- Moliner, M. State of the art of Lewis acid-containing zeolites: Lessons from fine chemistry to new biomass transformation processes. Dalton Trans. 2014, 43, 4197–4208. [Google Scholar] [CrossRef] [PubMed]
- Gounder, R. Hydrophobic microporous and mesoporous oxides as Brønsted and Lewis acid catalysts for biomass conversion in liquid water. Catal. Sci. Technol. 2014, 4, 2877–2886. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, Z. Synthesis and catalytic applications of advanced Sn- and Zr- zeolites materials. Adv. Sci. 2023, 11, 2306533. [Google Scholar] [CrossRef]
- Khechfe, A.A.; Matha, T.B.M.; Román-Leshkov, Y. Solvent polarity and framework hydrophobicity of Hf-BEA zeolites influence aldol addition rates in organic media. ACS Catal. 2023, 13, 6474–6485. [Google Scholar] [CrossRef]
- Yao, X.; Zhuang, H.; Guo, H.; Lu, X.; Xia, Q.; Zhou, D. Catalytic hydration/hydrolysis of bulky and hydrophobic organic compounds in aqueous phase over zeolite-based catalyst efficiently. Appl. Surf. Sci. 2025, 705, 163514. [Google Scholar] [CrossRef]
- Namgung, Y.; Li, Y.; Kim, S.; Park, H.Y.; Saqlain, S.; Kim, Y.D. A simple heat-treatment strategy to prepare hydrophobic zeolite ZSM-5 for efficient adsorption of toluene under humid conditions. Chem. Eng. J. 2025, 507, 160580. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.; Li, Y.; Li, H.; Zhang, L.; Zhai, J.; Song, Y.; Liu, B.; Jiang, L.; Zhu, D. Super-hydrophobic surfaces: From natural to artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Bohn, H.F.; Federle, W. Insect aquaplaning: Nepenthes pitcher plants capture prey with the peristome, a fully wettable water-lubricated anisotropic surface. Proc. Natl. Acad. Sci. USA 2004, 28, 14138–14143. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, P.; Zhang, L.; Liu, H.; Jiang, Y.; Zhang, D.; Han, Z.; Jiang, L. Continuous directional water transport on the peristome surface of Nepenthes alata. Nature 2016, 532, 85–89. [Google Scholar] [CrossRef]
- Parker, A.R.; Lawrence, C.R. Water capture by a desert beetle. Nature 2001, 414, 33–34. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yin, T.; Li, Y.; Liu, N.; Shi, L.; Meng, X. Synthesis of high hydrophobicity USY zeolite with excellent VOCs adsorption performance under humid condition: Combined strategy of high temperature steam and acid treatment. Sep. Purif. Technol. 2024, 329, 124914. [Google Scholar] [CrossRef]
- Sun, K.; Kong, F.; Gong, X.; Shi, Y.; Jin, D.; Jin, H.; Guo, X.; Zhou, R. Hierarchical porous S-1 zeolite supported Pt catalyst for catalytic oxidation of various VOCs at low temperature with strong water resistance and excellent stability. Appl. Catal. A Gen. 2025, 699, 120284. [Google Scholar] [CrossRef]
- Gao, X.; Feng, A.; Mi, L.; Yu, Y.; Yu, Y. Fabrication and adsorption properties of hierarchical NaY zeolite with mesopores in the range of 2–5 nm. Chem. Phys. Lett. 2025, 858, 141732. [Google Scholar] [CrossRef]
- Jin, S.; Luo, Q.; Fan, X.; Sun, H.; Yang, W. Effect of secondary acid treatment on the structure of Ti-MWW zeolite and its catalytic performance. Microporous Mesoporous Mater. 2025, 389, 113572. [Google Scholar] [CrossRef]
- Liu, Z.; Gong, Y.; Jiang, T.; Xiao, C.; Pang, Y.; Chen, B.; Song, J.; Wang, T. Enhancing carbon chain extension of higher alcohols to jet fuels by surface hydrophobicity modification of HZSM-5. Energy 2025, 318, 134866. [Google Scholar] [CrossRef]
- Boer, D.G.; Asgar Pour, Z.; Poli, S.; Langerak, J.; Bakker, B.; Pescarmona, P.P. ZSM-5/Silicalite-1 core-shell beads as CO2 adsorbents with increased hydrophobicity. Mater. Today Chem. 2023, 32, 101621. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Yu, S.; Zhang, B.; Xie, J.; Chen, J.; Wang, D.; Feng, B.; Zhong, C.; Zhou, L.; et al. Insight into the hydrogen mobility upon Pt/ZSM-5 and its catalytic function during liquid-phase hydrogen isotopes exchange. J. Catal. 2024, 430, 115345. [Google Scholar] [CrossRef]
- Lari, G.M.; Dapsens, P.Y.; Scholz, D.; Mitchell, S.; Mondelli, C.; Pérez-Ramírez, J. Deactivation mechanisms of tin-zeolites in biomass conversions. Green Chem. 2016, 18, 1249–1260. [Google Scholar] [CrossRef]
- Catuzo, G.L.; Santilli, C.V.; Martins, L. Hydrophobic-hydrophilic balance of ZSM-5 zeolites on the two-phase ketalization of glycerol with acetone. Catal. Today 2021, 381, 215–223. [Google Scholar] [CrossRef]
- Zhu, X.; He, X.; Guo, L.; Shi, Y.; Zhao, N.; Qiao, C.; Dai, L.; Tian, Y. Hydrophobic modification of ZSM-5-encapsulated uniform Pt nanoparticles for catalytic oxidation of volatile organic compounds. ACS Appl. Nano Mater. 2022, 5, 3374–3385. [Google Scholar] [CrossRef]
- Zapata, P.A.; Faria, J.; Ruiz, M.P.; Jentoft, R.E.; Resasco, D.E. Hydrophobic zeolites for biofuel upgrading reactions at the liquid–liquid interface in water/oil emulsions. J. Am. Chem. Soc. 2012, 134, 8570–8578. [Google Scholar] [CrossRef]
- Guo, Q.; Qiao, Y.; Xiao, Y.; Pan, Y.; Li, Y.; Liu, X.; He, G. Synthesis of hydrophobic CuCl/LaA modified by butyltrichlorosilane towards enhanced CO adsorption under humid environment. Appl. Surf. Sci. 2024, 659, 159882. [Google Scholar] [CrossRef]
- Li, R.; Xue, T.; Li, Z.; Wang, Q. Hierarchical structure ZSM-5/SBA-15 composite with improved hydrophobicity for adsorption-desorption behavior of toluene. Chem. Eng. J. 2020, 392, 124861. [Google Scholar] [CrossRef]
- Liu, H.; Wei, K.; Long, C. Enhancing adsorption capacities of low-concentration VOCs under humid conditions using NaY@meso-SiO2 core–shell composite. Chem. Eng. J. 2022, 442, 136108. [Google Scholar] [CrossRef]
- Güemes, L.; Navarro, M.; Cacho-Bailo, F.; Jaimes-Paez, C.D.; Cazorla-Amorós, D.; Téllez, C.; Coronas, J. Zeolite@Metal-organic framework core-shell synthesized from the aluminum of the zeolite with accessible internal surface for CO2 adsorption. Chem. Eng. J. 2025, 518, 164314. [Google Scholar] [CrossRef]
- Bae, J.; Dusselier, M. Synthesis strategies to control the Al distribution in zeolites: Thermodynamic and kinetic aspects. Chem. Commun. 2023, 59, 852–867. [Google Scholar] [CrossRef]
- Medeiros-Costa, I.C.; Dib, E.; Nesterenko, N.; Dath, J.-P.; Gilson, J.-P.; Mintova, S. Silanol defect engineering and healing in zeolites: Opportunities to fine-tune their properties and performances. Chem. Soc. Rev. 2021, 50, 11156–11179. [Google Scholar] [CrossRef]
- Sultana Poly, S.; Hakim Siddiki, S.M.A.; Touchy, A.S.; Yasumura, S.; Toyao, T.; Maeno, Z.; Shimizu, K.-i. High-silica Hβ zeolites for catalytic hydration of hydrophobic epoxides and alkynes in water. J. Catal. 2018, 368, 145–154. [Google Scholar] [CrossRef]
- Camblor, M.A.; Corma, A.; Iborra, S.; Miquel, S.; Primo, J.; Valencia, S. Beta zeolite as a catalyst for the preparation of alkyl glucoside surfactants: The role of crystal size and hydrophobicity. J. Catal. 1997, 172, 76–84. [Google Scholar] [CrossRef]
- Lee, W.-S.; Majumdar, P.; Dinh, K.T.; Naik, S.; Gu, B.; Kang, J.H.; Qiu, X.; Yusuf, S.; Anaya, D.; Klann, J.; et al. Catalyst site requirements for olefin etherification over H-Beta zeolites. ACS Catal. 2024, 14, 13973–13985. [Google Scholar] [CrossRef]
- Zhang, L.; Li, M.; Li, G.; Liu, W.; Ye, L.; Liu, K.; Ma, Q.; Peng, H. Catalytic cycle network: Boosting CO2 hydrogenation to propane. Appl. Catal. B Environ. Energy 2024, 358, 124372. [Google Scholar] [CrossRef]
- Chen, Z.; Tao, Q.; Yang, L.; Qin, Y.; Wang, C.; Guo, Y. Charge shielding effect of sodium ions in improving the hydrophobicity and performance of Co/Na-SSZ-13 zeolite for passive NOx adsorber. Adv. Funct. Mater. 2024, 34, 2310682. [Google Scholar] [CrossRef]
- Shi, X.; Yang, G.; Zhang, M.; Liu, X.; Zhang, W.; Liu, Y. Tuning hydrogenation catalysis via pH-responsive microenvironments in hydrophobic zeolite encapsulating palladium. ACS Catal. 2025, 15, 10819–10827. [Google Scholar] [CrossRef]
- Inagaki, S.; Takeyama, M.; Asanuma, K.; Yokose, Y.; Zhang, S.; Okuda, T.; Nakamura, K.; Nishi, Y.; Kubota, Y. Preparation of hydrophobic Ti-beta catalyst derived from Al-rich zeolite beta synthesized without using organic structure-directing agent and its enhanced catalytic performance for phenol oxidation. Microporous Mesoporous Mater. 2022, 346, 112323. [Google Scholar] [CrossRef]
- Yao, Y.; Yang, Z.; Zheng, P.; Diao, Z. Enhancing the accessible TiO6 concentration and the hydrophobicity of hierarchical TS-1 zeolites for alkene epoxidation and oxidative desulfurization. Chem. Eng. J. 2023, 475, 146053. [Google Scholar] [CrossRef]
- Sheng, N.; Lin, D.; Liang, W.; Zhao, C.; Liu, Y.; Zhu, Y.; Song, Z.; Jiang, J.; Xu, W.; Yang, Z.; et al. Intracrystalline diffusion regulation of Au/hierarchical TS-1 for simultaneously enhanced stability and selectivity in propene epoxidation with H2 and O2. Chem. Eng. Sci. 2024, 300, 120538. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, Y.; Li, Y.; Tian, Y.; Jia, H.; Xu, W.; Yang, Z.; Sun, B. Alkaline-assisted excessive impregnation for enhancing Au nanoparticle loading on the TS-1 zeolite. Langmuir 2025, 41, 804–810. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, Q.; Wang, H.; Ge, Q.; Zhu, X. Ketonization of propionic acid over TS-1 and Ti-Beta zeolites: Mechanism and effects of topology and hydrophobicity. J. Catal. 2024, 429, 115247. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, H.; Jiang, J.; Wu, H.; Wu, P. Structural reconstruction: A milestone in the hydrothermal synthesis of highly active Sn-Beta zeolites. Chem. Commun. 2017, 53, 12516–12519. [Google Scholar] [CrossRef]
- Tang, B.; Li, S.; Song, W.-C.; Yang, E.-C.; Zhao, X.-J.; Guan, N.; Li, L. Fabrication of hierarchical Sn-Beta zeolite as efficient catalyst for conversion of cellulosic sugar to methyl lactate. ACS Sustain. Chem. Eng. 2020, 8, 3796–3808. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, Y.; Tang, K.; Xu, W.; Lu, X.; Ma, R.; Fu, Y.; Zhu, W. Role of the pore-opening structure and hydrophobicity of stannosilicate zeolites in Baeyer–Villiger oxidation. J. Catal. 2021, 394, 8–17. [Google Scholar] [CrossRef]
- Zhang, J.; Bo, S.; Liao, W.; Yang, K.; Su, T.; Lü, H.; Zhu, Z. Pore-engineering-dependence and hydrophobicity-dependence on stannosilicate zeolite catalyst for efficient Baeyer-Villiger oxidation. Fuel 2024, 357, 129662. [Google Scholar] [CrossRef]
- Li, X.; Liu, L. Hydrothermally stable zeolite-encapsulated metal catalyst promoted by framework Sn species. ACS Catal. 2024, 15, 403–421. [Google Scholar] [CrossRef]
- Grand, J.; Talapaneni, S.N.; Vicente, A.; Fernandez, C.; Dib, E.; Aleksandrov, H.A.; Vayssilov, G.N.; Retoux, R.; Boullay, P.; Gilson, J.-P.; et al. One-pot synthesis of silanol-free nanosized MFI zeolite. Nat. Mater. 2017, 16, 1010–1015. [Google Scholar] [CrossRef]
- Wang, X.; You, Q.; Wu, Y.; Bi, C.; Chen, H.; Dai, C.; Hao, Q.; Zhang, J.; Ma, X. Tungsten-substituted Silicalite-1 with an interconnected hollow structure for catalytic epoxidation of cyclohexene. Microporous Mesoporous Mater. 2021, 317, 111028. [Google Scholar] [CrossRef]
- Wang, X.; Li, N.; Yang, F.; Yang, X.; Chun, W.; He, G.; Chen, H. Efficient epoxidation of cyclohexene catalyzed by W-substituted self-pillared pentasil zeolite nanosheets. ACS Appl. Nano Mater. 2025, 8, 10526–10536. [Google Scholar] [CrossRef]
- Li, G.; Gao, L.; Sheng, Z.; Zhan, Y.; Zhang, C.; Ju, J.; Zhang, Y.; Tang, Y. A Zr-Al-Beta zeolite with open Zr(IV) sites: An efficient bifunctional Lewis-Brønsted acid catalyst for a cascade reaction. Catal. Sci. Technol. 2019, 9, 4055–4065. [Google Scholar] [CrossRef]
- Zhang, H.; Jaenicke, S.; Okumura, K.; Tan, H.-R.; Chuah, G.-K. Mechanochemistry-based liquid-assisted synthesis of hydrophobic Zr-Beta with high metal loading for Meerwein-Ponndorf-Verley reduction. J. Catal. 2024, 431, 115398. [Google Scholar] [CrossRef]
- Dubray, F.; Moldovan, S.; Kouvatas, C.; Grand, J.; Aquino, C.; Barrier, N.; Gilson, J.-P.; Nesterenko, N.; Minoux, D.; Mintova, S. Direct evidence for single molybdenum atoms incorporated in the framework of MFI zeolite nanocrystals. J. Am. Chem. Soc. 2019, 141, 8689–8693. [Google Scholar] [CrossRef]
- Konnov, S.V.; Dubray, F.; Clatworthy, E.B.; Kouvatas, C.; Gilson, J.P.; Dath, J.P.; Minoux, D.; Aquino, C.; Valtchev, V.; Moldovan, S.; et al. Novel strategy for the synthesis of ultra-stable single-site Mo-ZSM-5 zeolite nanocrystals. Angew. Chem. Int. Ed. 2020, 59, 19553–19560. [Google Scholar] [CrossRef]
- Dib, E.; Yue, Q.; Confalonieri, G.; Salusso, D.; Vayssilov, G.N.; Barreau, M.; Gramatikov, S.P.; Arletti, R.; Dalena, F.; Lomachenko, K.A.; et al. Germanium atoms exceed the tetrahedral coordination in MFI zeolite. J. Am. Chem. Soc. 2025, 147, 3274–3282. [Google Scholar] [CrossRef]
- Mal, N.K.; Ramaswamy, V.; Ganapathy, S.; Ramaswamy, A.V. Synthesis and characterization of crystalline, Tin-silicate molecular sieves with MFI structure. J. Chem. Soc. Chem. Commun. 1994, 17, 1933–1934. [Google Scholar] [CrossRef]
- Zhu, Y.; Chuah, G.; Jaenicke, S. Al-free Zr-zeolite beta as a regioselective catalyst in the Meerwein–Ponndorf–Verley reaction. Chem. Commun. 2003, 21, 2734–2735. [Google Scholar] [CrossRef]
- Dalena, F.; Yue, Q.; Dib, E.; Aitblal, A.; Piva, D.H.; Qin, Z.; Mintova, S. Enhanced catalytic ring-opening of propylene oxide using a Germanium-containing MFI zeolite catalyst with high selectivity. ACS Catal. 2025, 15, 8024–8035. [Google Scholar] [CrossRef]
- Cordon, M.J.; Harris, J.W.; Vega-Vila, J.C.; Bates, J.S.; Kaur, S.; Gupta, M.; Witzke, M.E.; Wegener, E.C.; Miller, J.T.; Flaherty, D.W.; et al. Dominant role of entropy in stabilizing sugar isomerization transition states within hydrophobic zeolite pores. J. Am. Chem. Soc. 2018, 140, 14244–14266. [Google Scholar] [CrossRef]
- Vega-Vila, J.C.; Gounder, R. Quantification of intraporous hydrophilic binding sites in Lewis acid zeolites and consequences for sugar isomerization catalysis. ACS Catal. 2020, 10, 12197–12211. [Google Scholar] [CrossRef]
- Di Iorio, J.R.; Johnson, B.A.; Román-Leshkov, Y. Ordered hydrogen-bonded alcohol networks confined in Lewis acid zeolites accelerate transfer hydrogenation turnover rates. J. Am. Chem. Soc. 2020, 142, 19379–19392. [Google Scholar] [CrossRef]
- Palčić, A.; Moldovan, S.; El Siblani, H.; Vicente, A.; Valtchev, V. Defect sites in zeolites: Origin and healing. Adv. Sci. 2021, 9, 2104414. [Google Scholar] [CrossRef]
- Iyoki, K.; Kikumasa, K.; Onishi, T.; Yonezawa, Y.; Chokkalingam, A.; Yanaba, Y.; Matsumoto, T.; Osuga, R.; Elangovan, S.P.; Kondo, J.N.; et al. Extremely stable zeolites developed via designed liquid-mediated treatment. J. Am. Chem. Soc. 2020, 142, 3931–3938. [Google Scholar] [CrossRef] [PubMed]
- Spanos, A.P.; Parulkar, A.; Brunelli, N.A. Enhancing hydrophobicity and catalytic activity of nano-Sn-Beta for alcohol ring opening of epoxides through post-synthetic treatment with fluoride. J. Catal. 2021, 404, 430–439. [Google Scholar] [CrossRef]
- Li, B.; Iyoki, K.; Techasarintr, P.; Elangovan, S.P.; Simancas, R.; Okubo, T.; Yokoi, T.; Wakihara, T. Hydrophobicity manipulation of titanium Silicalite-1 with enhanced catalytic performance via liquid-mediated defect healing treatment. ACS Catal. 2023, 13, 15155–15163. [Google Scholar] [CrossRef]
- Hu, P.; Oishi, R.; Ya, H.; Yonezawa, Y.; Matsukura, M.; Iyoki, K.; Okubo, T.; Wakihara, T. Development of zeolite adsorbent with low water sensitivity for CO2 capture. Chem. Eng. J. 2025, 508, 161054. [Google Scholar] [CrossRef]
- Castro, M.; Losch, P.; Park, W.; Haouas, M.; Taulelle, F.; Loerbroks, C.; Brabants, G.; Breynaert, E.; Kirschhock, C.E.A.; Ryoo, R.; et al. Unraveling direct formation of hierarchical zeolite Beta by dynamic light scattering, small angle X-ray scattering, and liquid and solid-state NMR: Insights at the supramolecular level. Chem. Mater. 2018, 30, 2676–2686. [Google Scholar] [CrossRef]
- Lee, K.M.; Kim, N.S.; Numan, M.; Kim, J.-C.; Cho, H.S.; Cho, K.; Jo, C. Postsynthetic modification of zeolite internal surface for sustainable capture of volatile organic compounds under humid conditions. ACS Appl. Mater. Interfaces 2021, 13, 53925–53934. [Google Scholar] [CrossRef]
- Tian, H.; Liu, S.; Liu, Q. HZSM-5 zeolite modification and catalytic reaction mechanism in the reaction of cyclohexene hydration. RSC Adv. 2022, 12, 24654–24669. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.S.D.; Silva, L.L.; Zapelini, I.W.; Mintova, S.; Martins, L. Tailoring the accessibility and amphiphilicity of MWW zeolites for two-phase glycerol ketalization. Inorg. Chem. Front. 2023, 10, 5649–5661. [Google Scholar] [CrossRef]
- Yin, T.; Xu, J.; Li, Y.; Liu, N.; Meng, X.; Shi, L. Study of the inner surface silanization of zeolite to improve the toluene capture ability in humid environment and fixed-bed adsorption kinetics analysis. Fuel 2024, 371, 131881. [Google Scholar] [CrossRef]
- Wang, K.; Sun, Z.; Zhao, Y.; Guo, W.; Zhu, C.; Zhu, Y.; He, H.; Cao, Y.; Liu, Y.; Bao, X. Hydrophobic nanosized H-Beta catalysis for biomass furanic compound valorization. J. Phys. Chem. C 2023, 128, 146–156. [Google Scholar] [CrossRef]
- Ma, X.; Guo, H.; Sui, Y.; Li, Y.; Cao, J. Fabrication of zeolite Beta with hydrophobic pore for synthesis of lactide from lactic acid. Asia-Pac. J. Chem. Eng. 2025, 20, e70045. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, L.; Hu, Q.; Zhang, L.; Xu, S.; Dong, X.; Gao, X.; Ma, R.; Meng, X.; Xiao, F.-S. Hydrophobic zeolite containing Titania particles as wettability-selective catalyst for formaldehyde removal. ACS Catal. 2018, 8, 5250–5254. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, L.; Zuidema, E.; Mondal, K.; Zhang, M.; Zhang, J.; Wang, C.; Meng, X.; Yang, H.; Mesters, C.; et al. Hydrophobic zeolite modification for in situ peroxide formation in methane oxidation to methanol. Science 2020, 367, 193–197. [Google Scholar] [CrossRef]
- Tian, J.; Wang, C.; Wu, J.; Sun, D.; Li, Q. Enhancing water resistance of Pt nanoparticles by tailoring microenvironment of hollow ZSM-5 for efficient benzene oxidation. Chem. Eng. J. 2023, 451, 138351. [Google Scholar] [CrossRef]
- Wang, S.; Hu, R.; Ren, J.; Lv, Y.; Song, L.; Zhao, H.; Jiang, X.; Gao, D.; Chen, G. Surface hydrophobization of zeolite enables mass transfer matching in gas-liquid-solid three-phase hydrogenation under ambient pressure. Nat. Commun. 2024, 15, 2076. [Google Scholar] [CrossRef]
- Na, K.; Choi, M.; Park, W.; Sakamoto, Y.; Terasaki, O.; Ryoo, R. Pillared MFI zeolite nanosheets of a single-unit-cell thickness. J. Am. Chem. Soc. 2010, 132, 4169–4177. [Google Scholar] [CrossRef] [PubMed]
- Na, K.; Jo, C.; Kim, J.; Cho, K.; Jung, J.; Seo, Y.; Messinger, R.J.; Chmelka, B.; Ryoo, R. Directing zeolite structures into hierarchically nanoporous architectures. Science 2011, 333, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liang, H.; Li, R.; Zhang, Z.; Qin, C.; Xu, D.; Fan, H.; Hou, B.; Wang, J.; Gu, X.K.; et al. Insights into the diffusion behaviors of water over hydrophilic/hydrophobic catalysts during the conversion of syngas to high-quality gasoline. Angew. Chem. Int. Ed. 2023, 62, e202306786. [Google Scholar] [CrossRef]
- Wang, S.; Bai, P.; Wei, Y.; Liu, W.; Ren, X.; Bai, J.; Lu, Z.; Yan, W.; Yu, J. Three-dimensional-printed core–shell structured MFI-type zeolite monoliths for volatile organic compound capture under humid conditions. ACS Appl. Mater. Interfaces 2019, 11, 38955–38963. [Google Scholar] [CrossRef]
- Miyake, K.; Inoue, R.; Miura, T.; Nakai, M.; Al-Jabri, H.; Hirota, Y.; Uchida, Y.; Tanaka, S.; Miyamoto, M.; Inagaki, S.; et al. Improving hydrothermal stability of acid sites in MFI type aluminosilicate zeolite (ZSM-5) by coating MFI type all silica zeolite (silicalite-1) shell layer. Microporous Mesoporous Mater. 2019, 288, 109523. [Google Scholar] [CrossRef]
- Du, T.; Qu, H.; Liu, Q.; Zhong, Q.; Ma, W. Synthesis, activity and hydrophobicity of Fe-ZSM-5@silicalite-1 for NH3-SCR. Chem. Eng. J. 2015, 262, 1199–1207. [Google Scholar] [CrossRef]
- Tian, X.; Li, J.; Yang, Z.; Tang, W.; Diao, Z.; Wang, W.; Tian, Y. Tailoring Ce Mn doped Fe-ZSM-5 monoliths with enhanced mechanical strength and hydrothermal stability for efficient NH3-SCR. Chem. Eng. J. 2025, 519, 165458. [Google Scholar] [CrossRef]
- Jia, Y.; Li, S.; Peng, L.; Xie, Z.; Yu, F.; Huang, C.; Wang, W.; Liu, R.; Wang, Z.; Liu, C.; et al. Rational design of silicalite-1-encapsulated W-ZSM-5 core-shell catalysts for optimizing selectivity and stability in methane dehydro-aromatization reaction. Mol. Catal. 2025, 584, 115314. [Google Scholar] [CrossRef]
- Javed, M.; Cheng, S.; Zhang, G.; Dai, P.; Cao, Y.; Lu, C.; Yang, R.; Xing, C.; Shan, S. Complete encapsulation of zeolite supported Co based core with silicalite-1 shell to achieve high gasoline selectivity in Fischer-Tropsch synthesis. Fuel 2018, 215, 226–231. [Google Scholar] [CrossRef]
- Javed, M.; Zhang, G.; Gao, W.; Cao, Y.; Dai, P.; Ji, X.; Lu, C.; Yang, R.; Xing, C.; Sun, J. From hydrophilic to hydrophobic: A promising approach to tackle high CO2 selectivity of Fe-based Fischer-Tropsch microcapsule catalysts. Catal. Today 2019, 330, 39–45. [Google Scholar] [CrossRef]

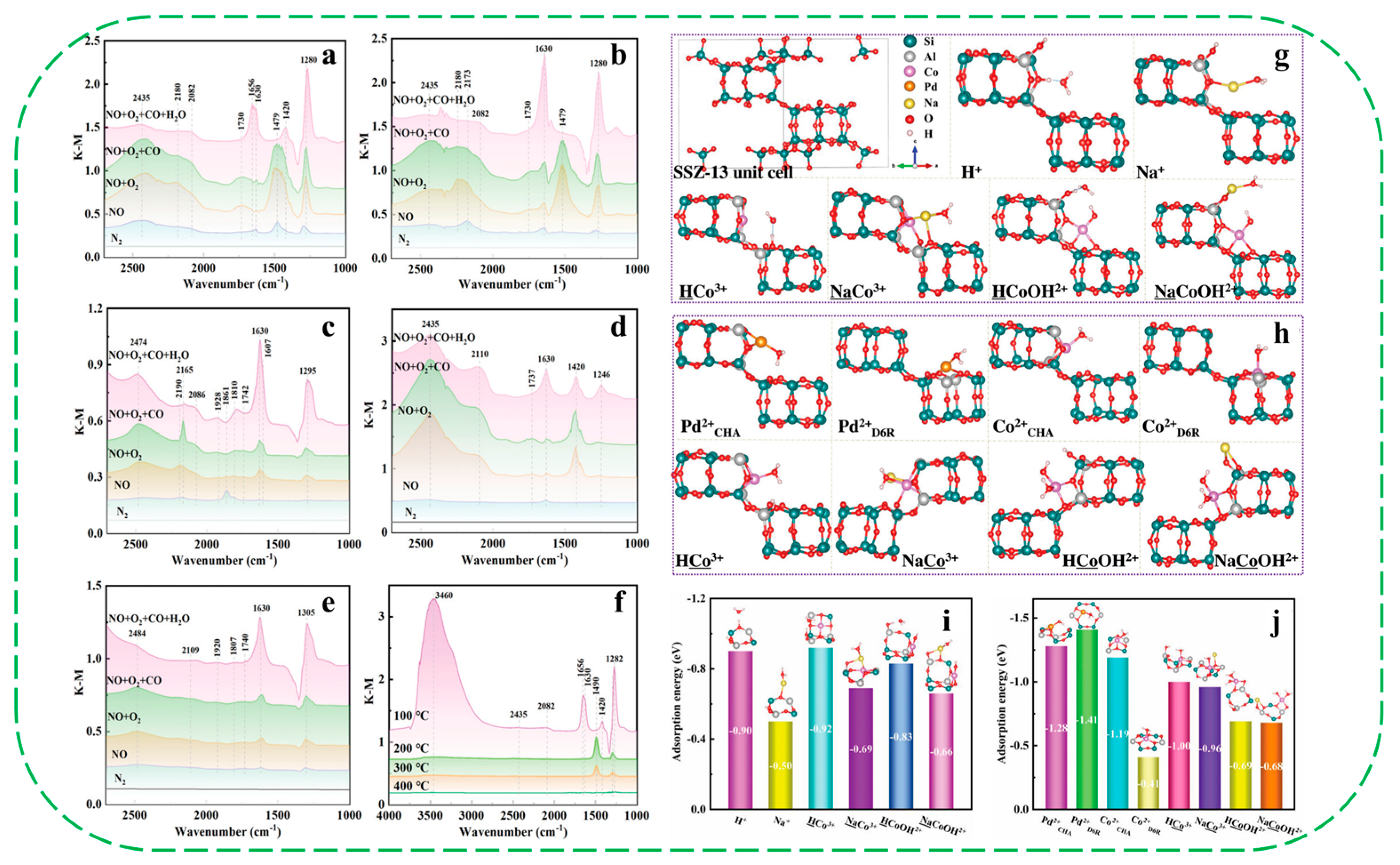
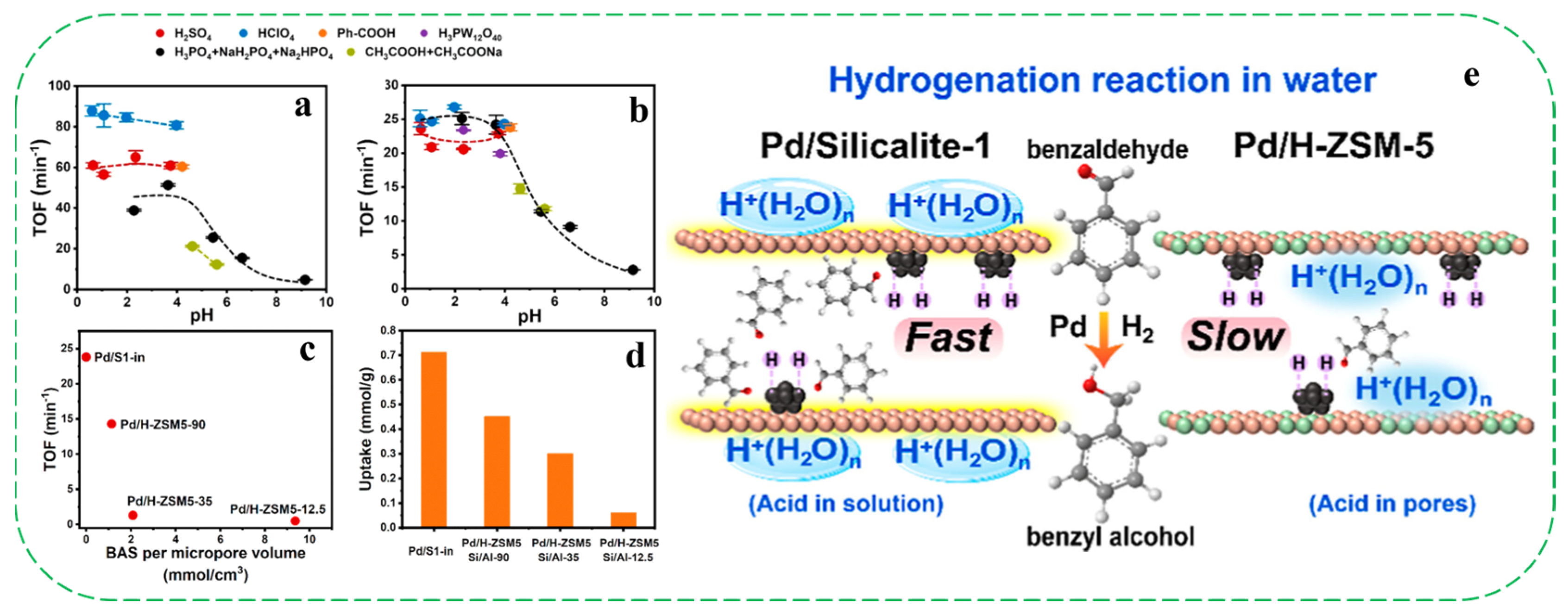
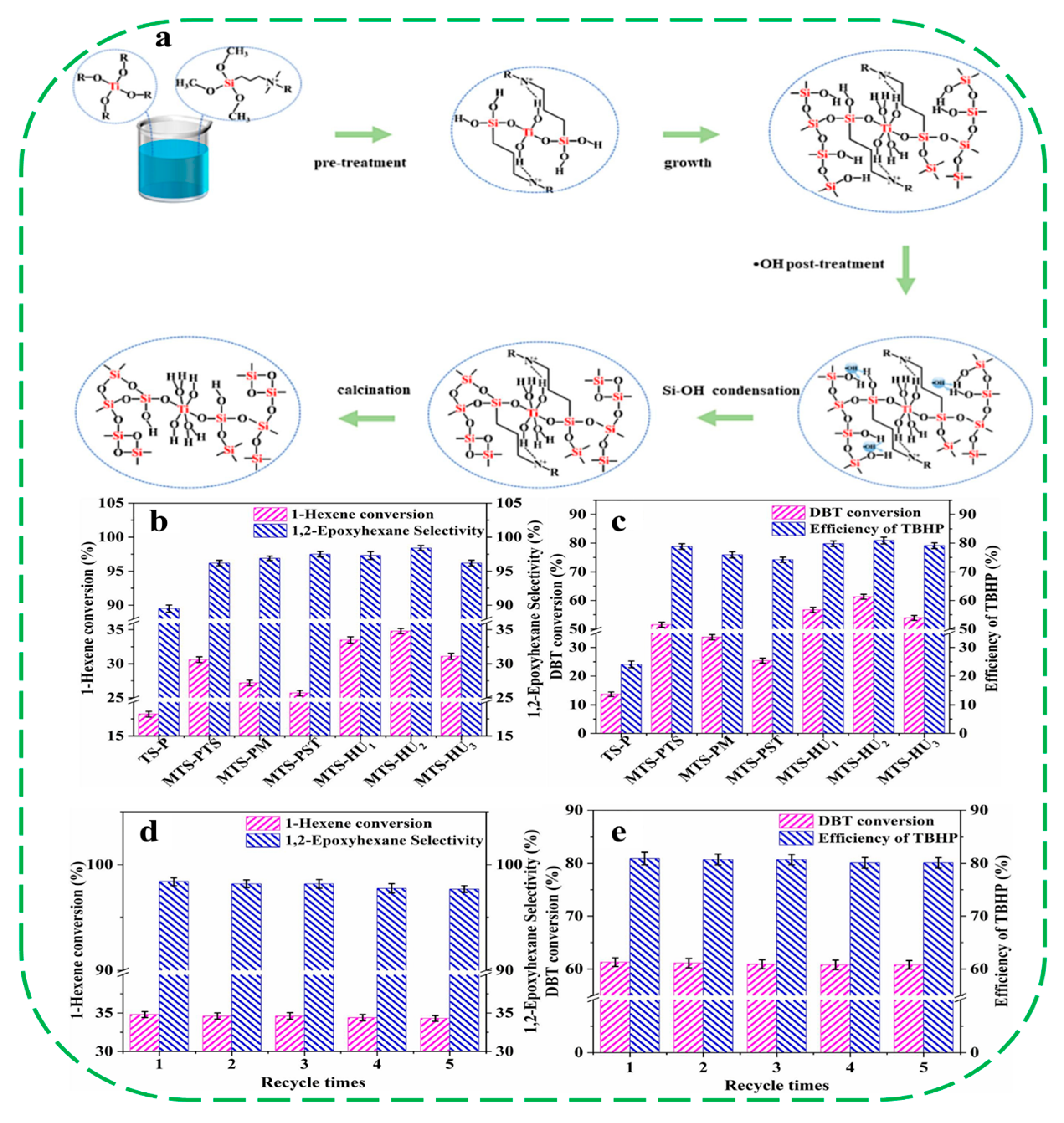
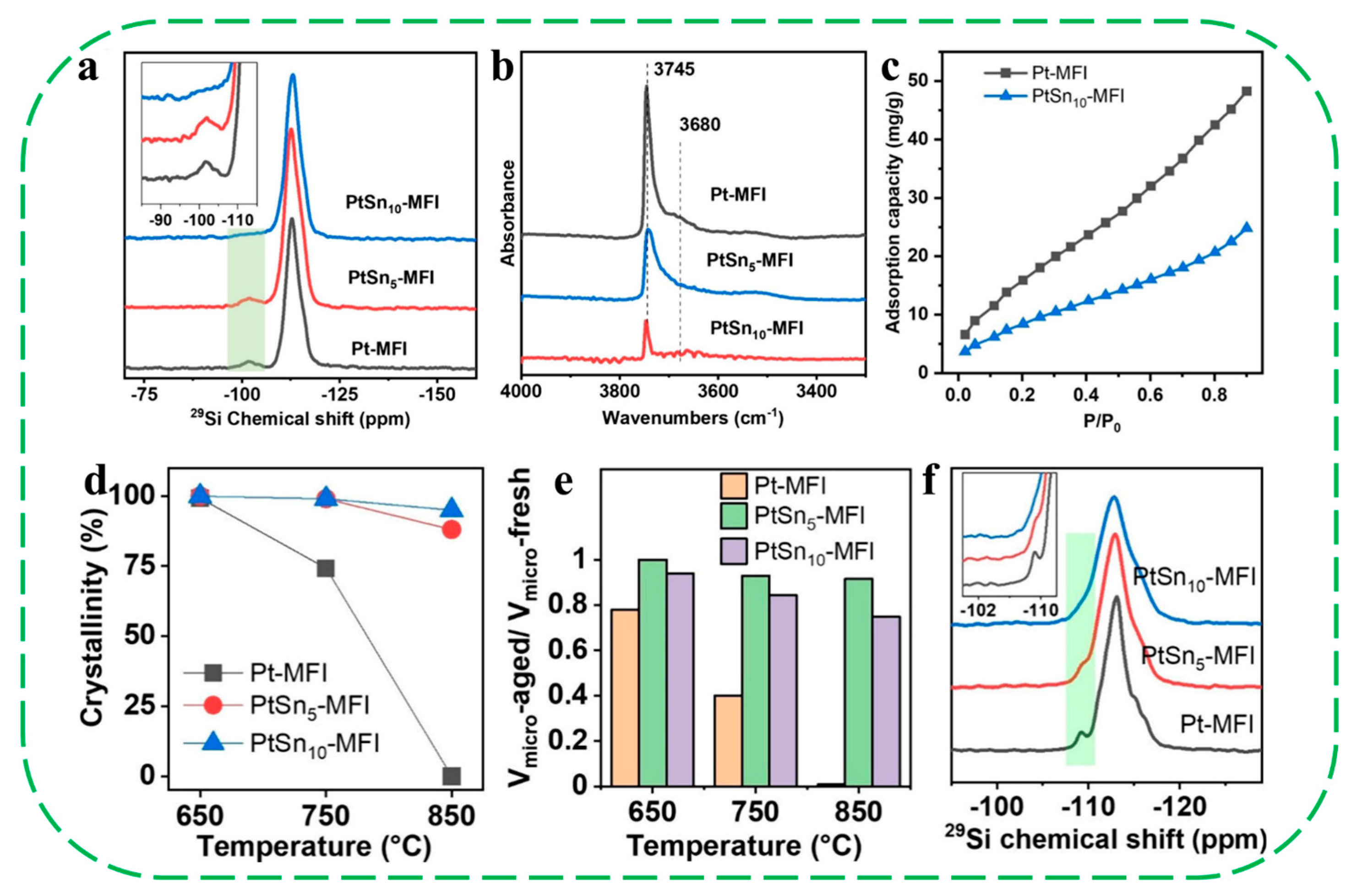
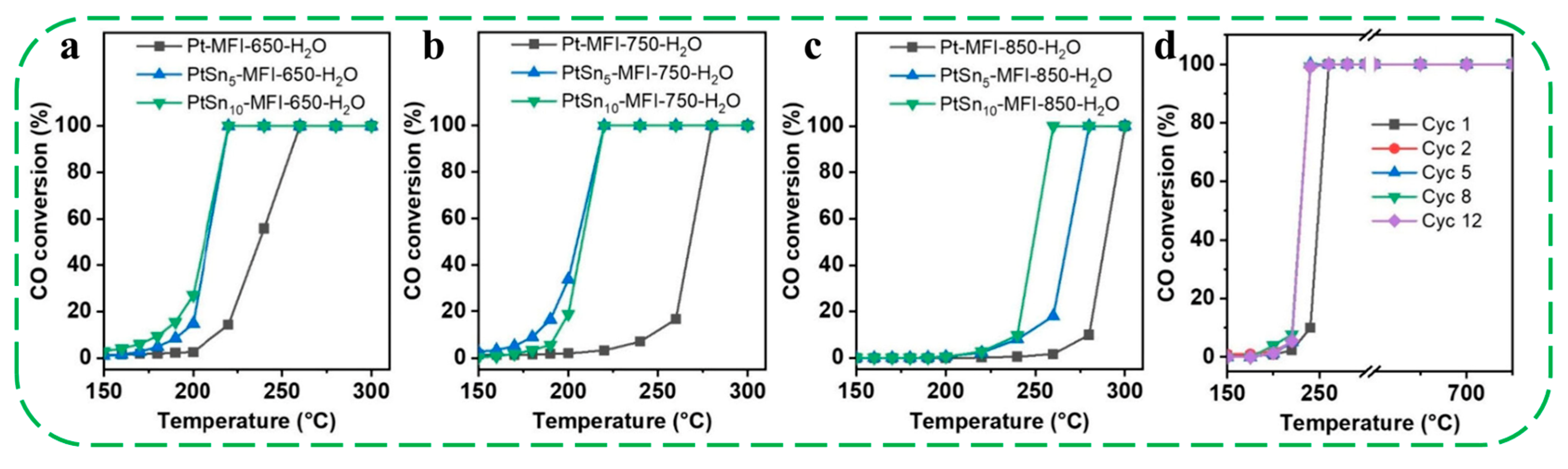
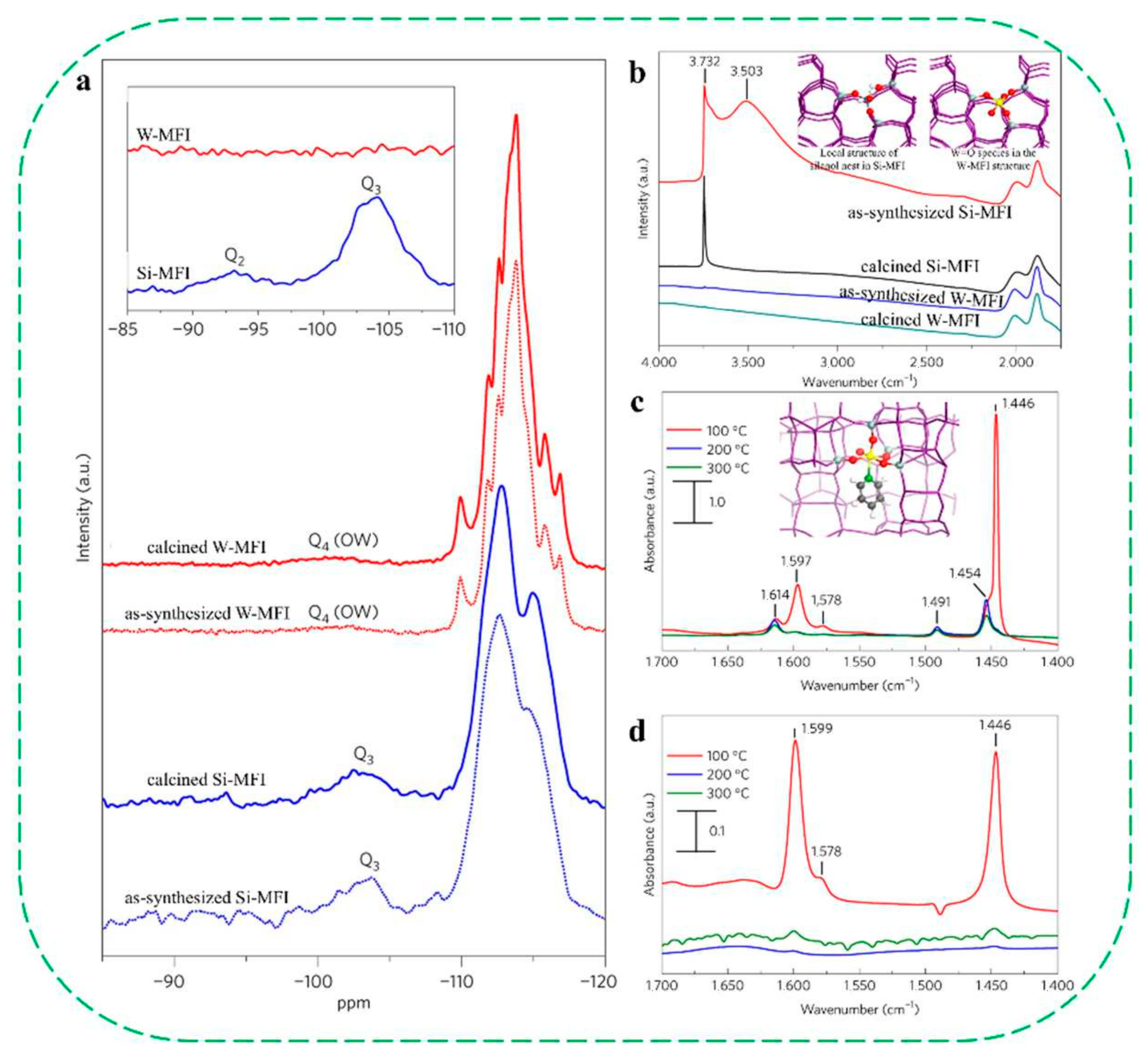
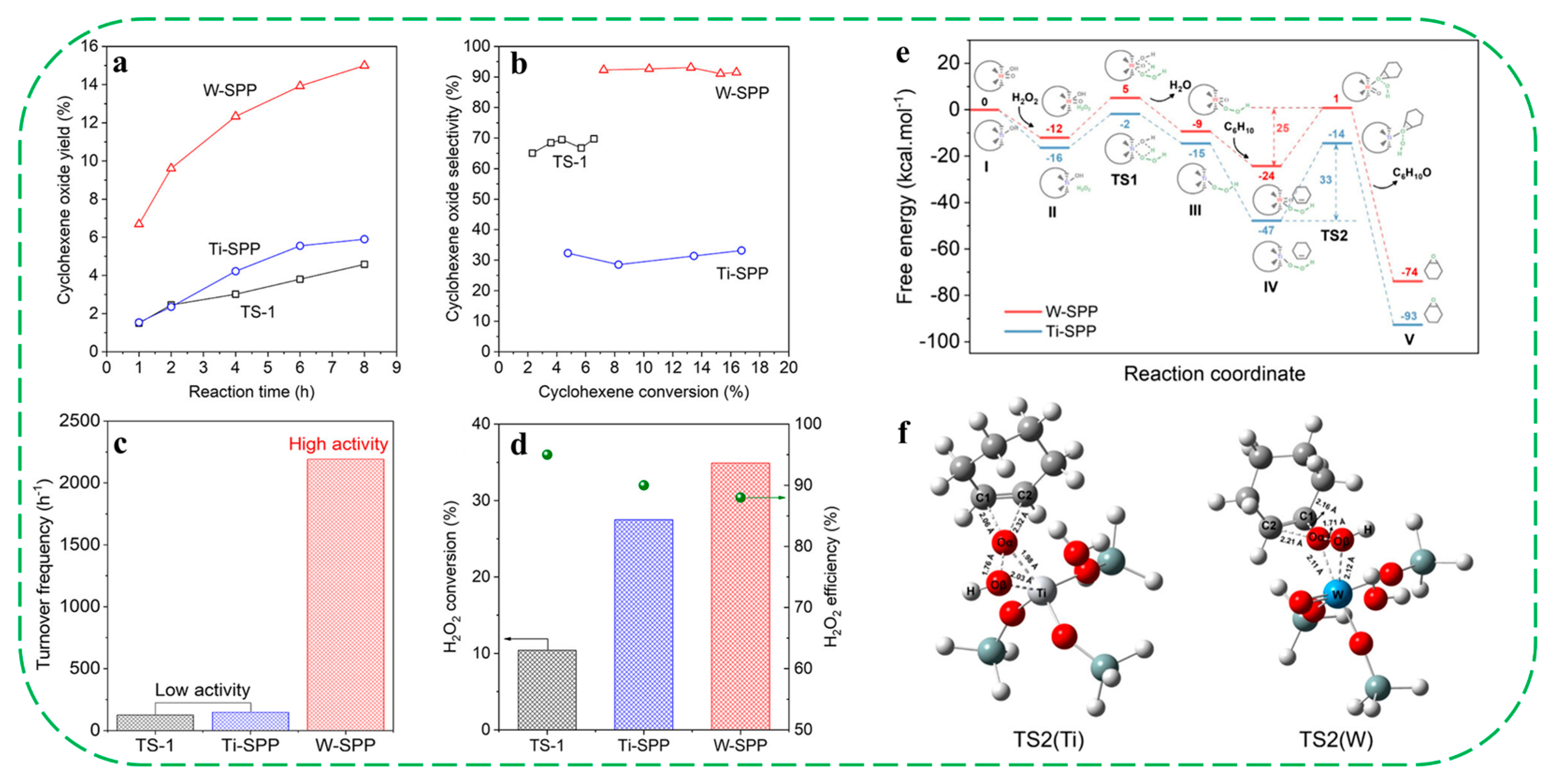
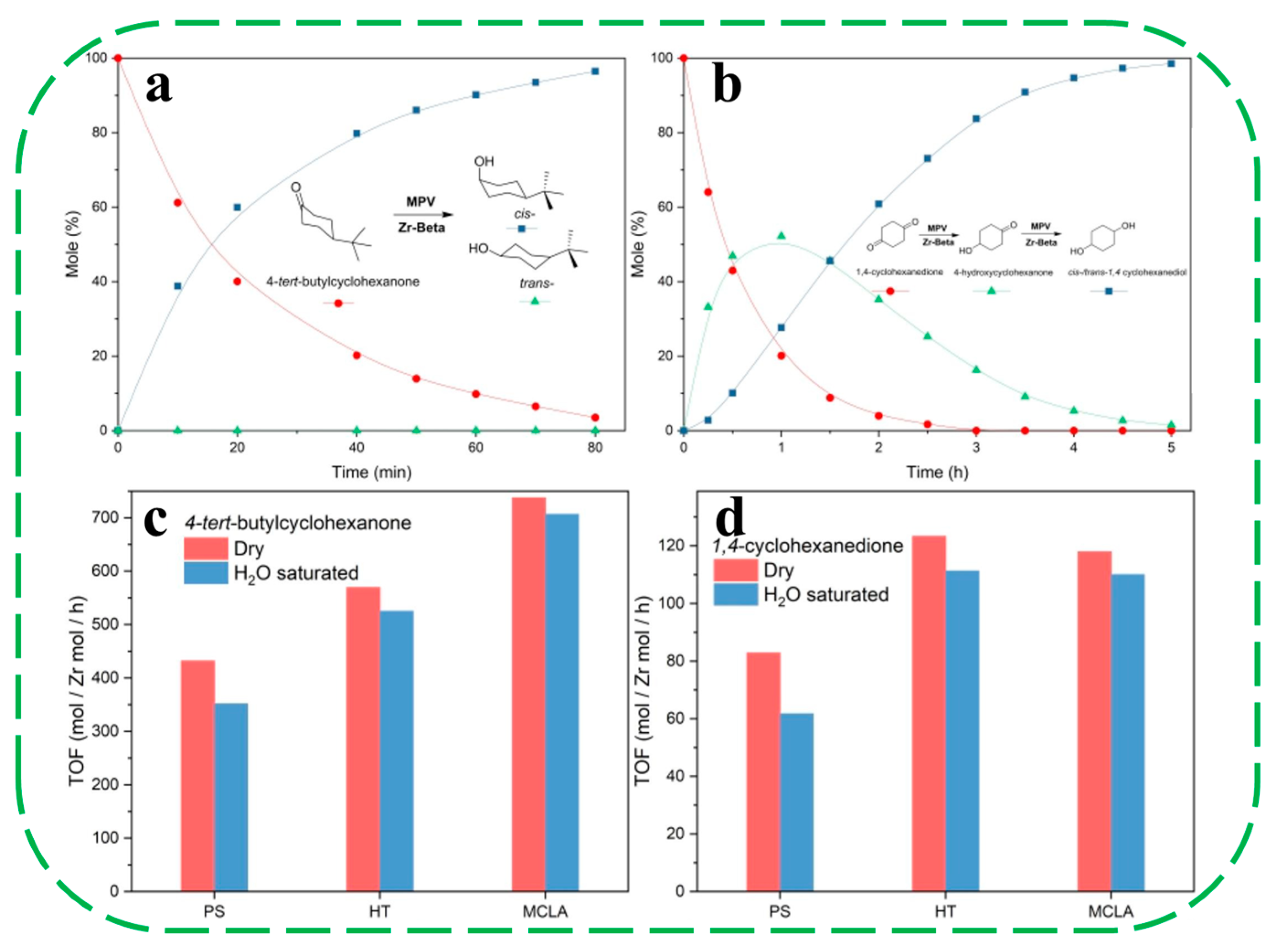
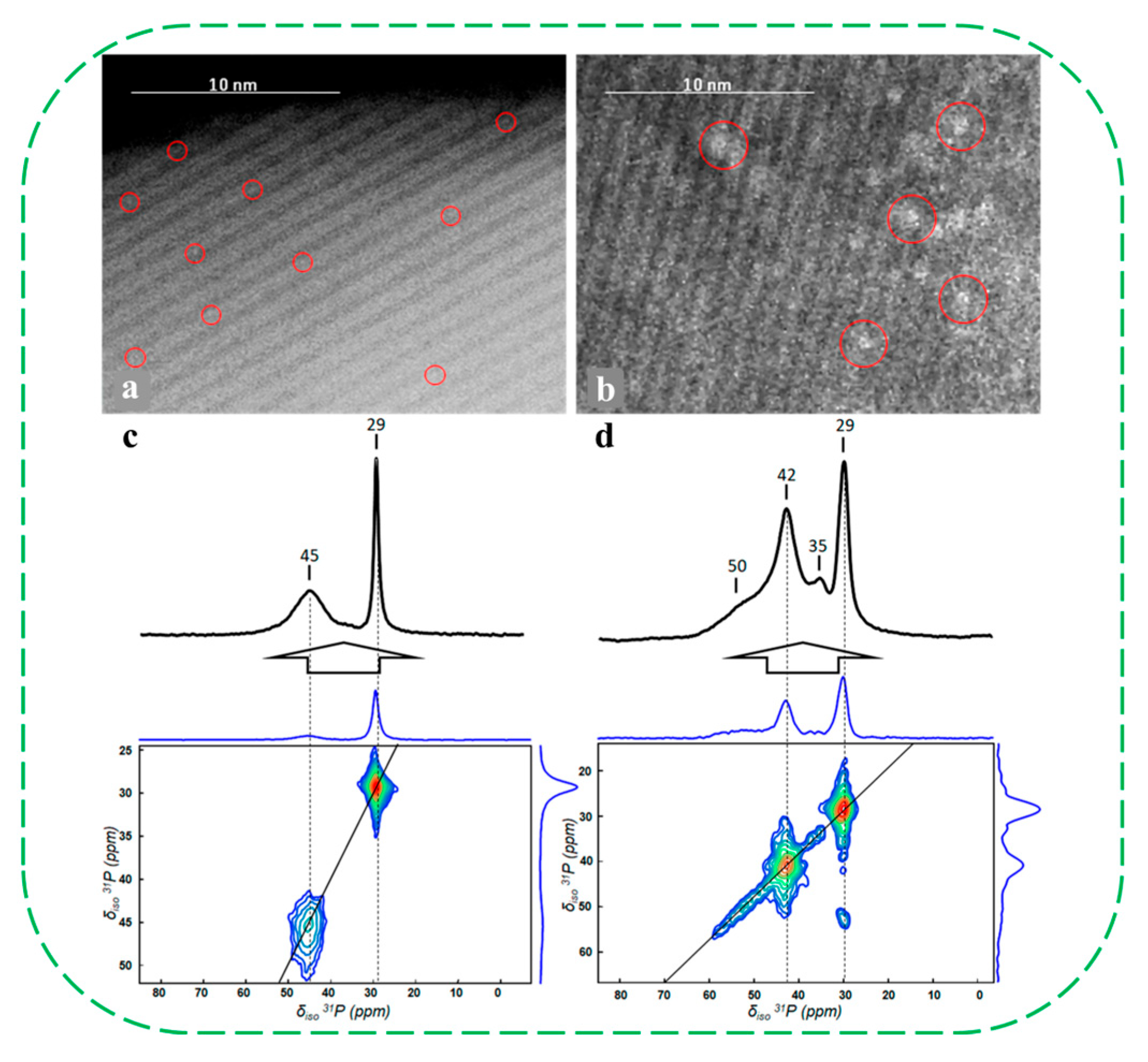
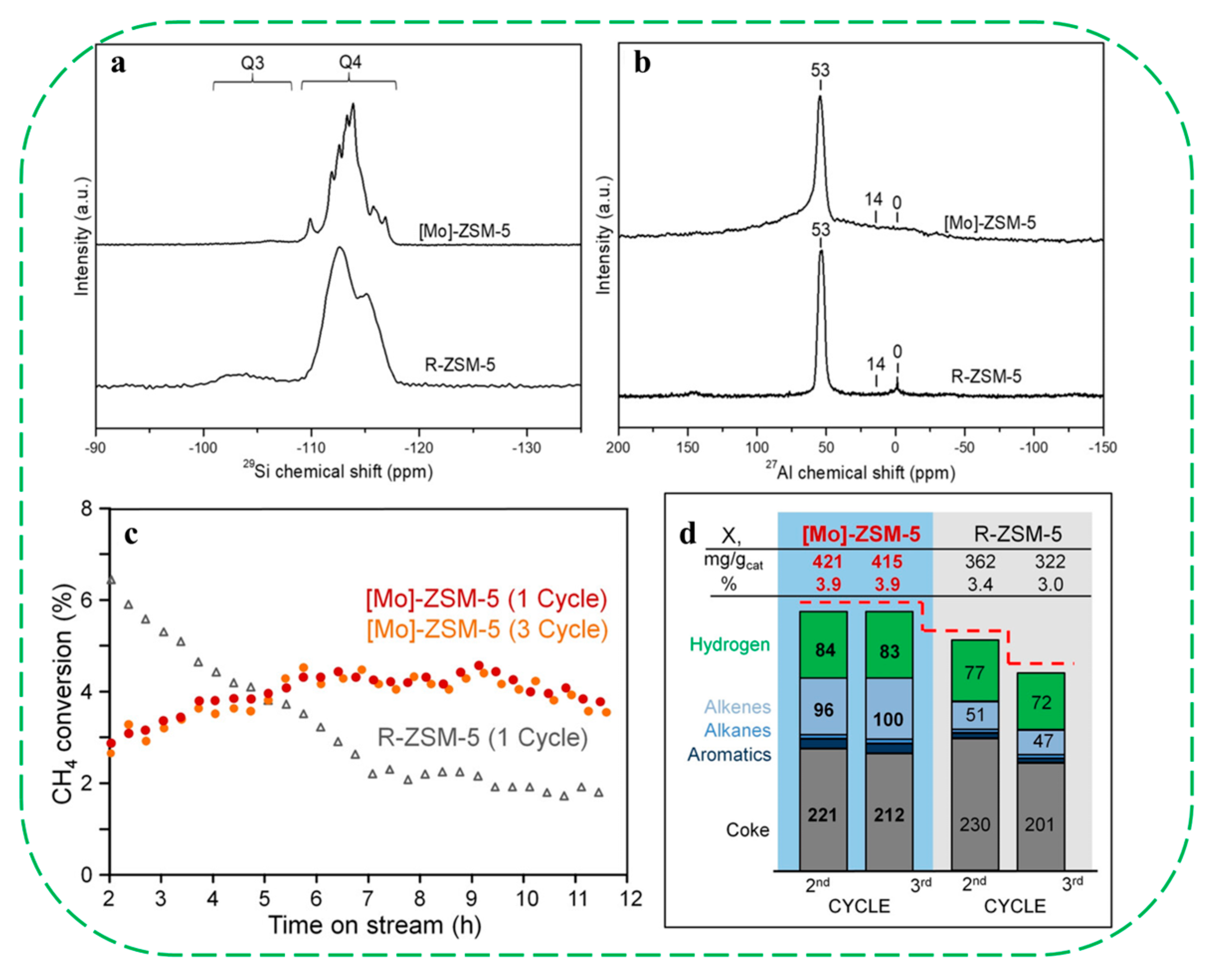
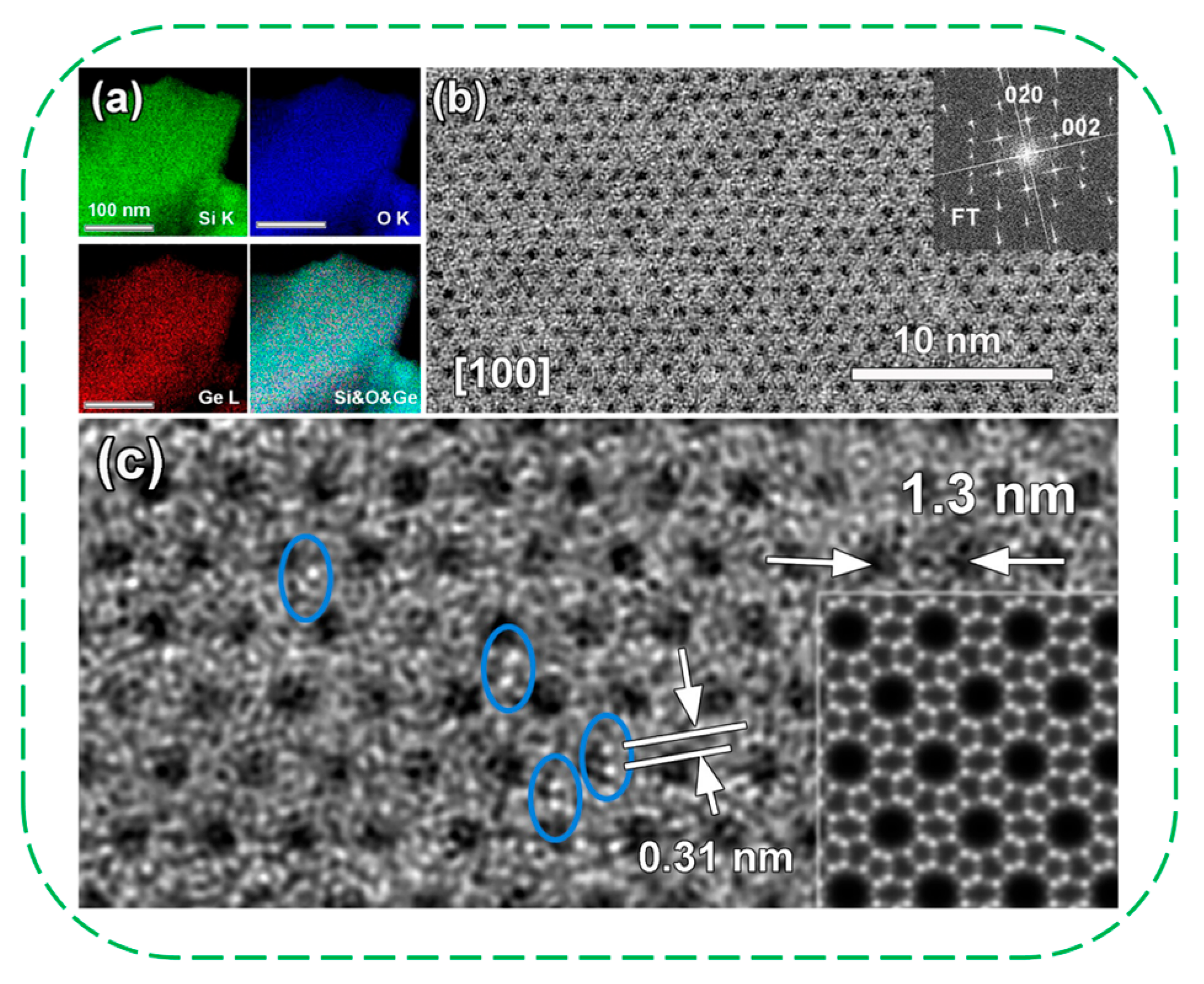
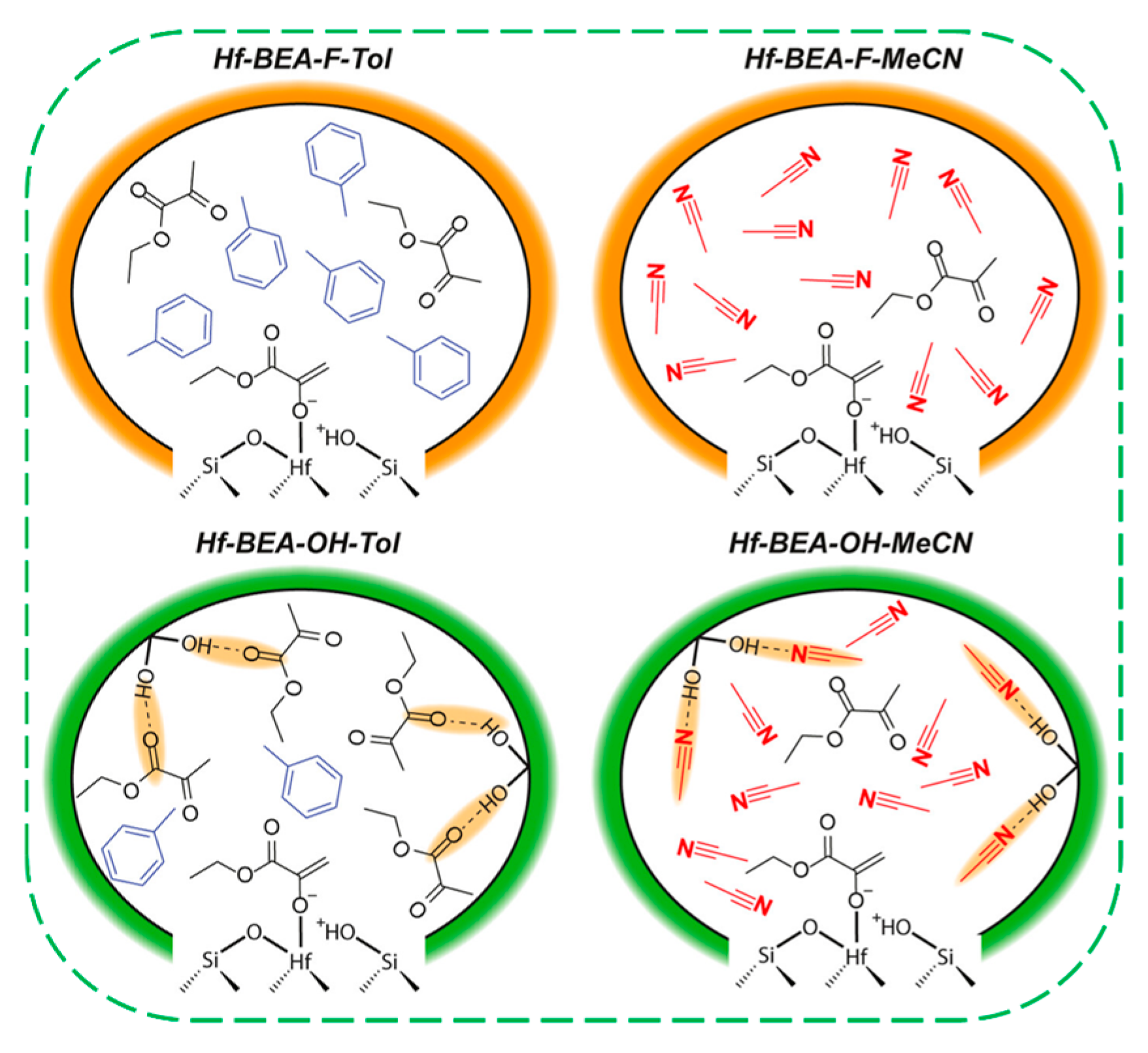
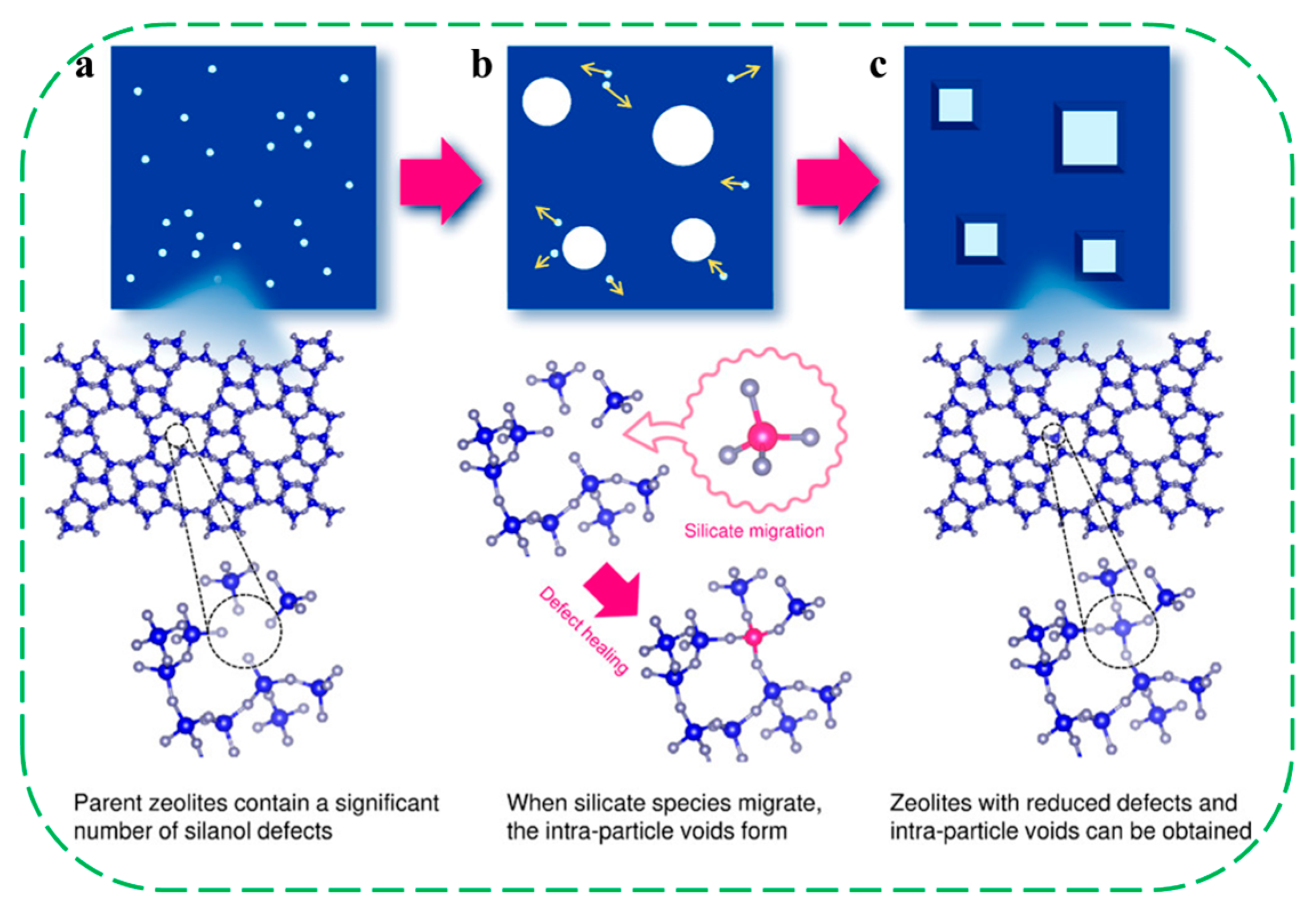
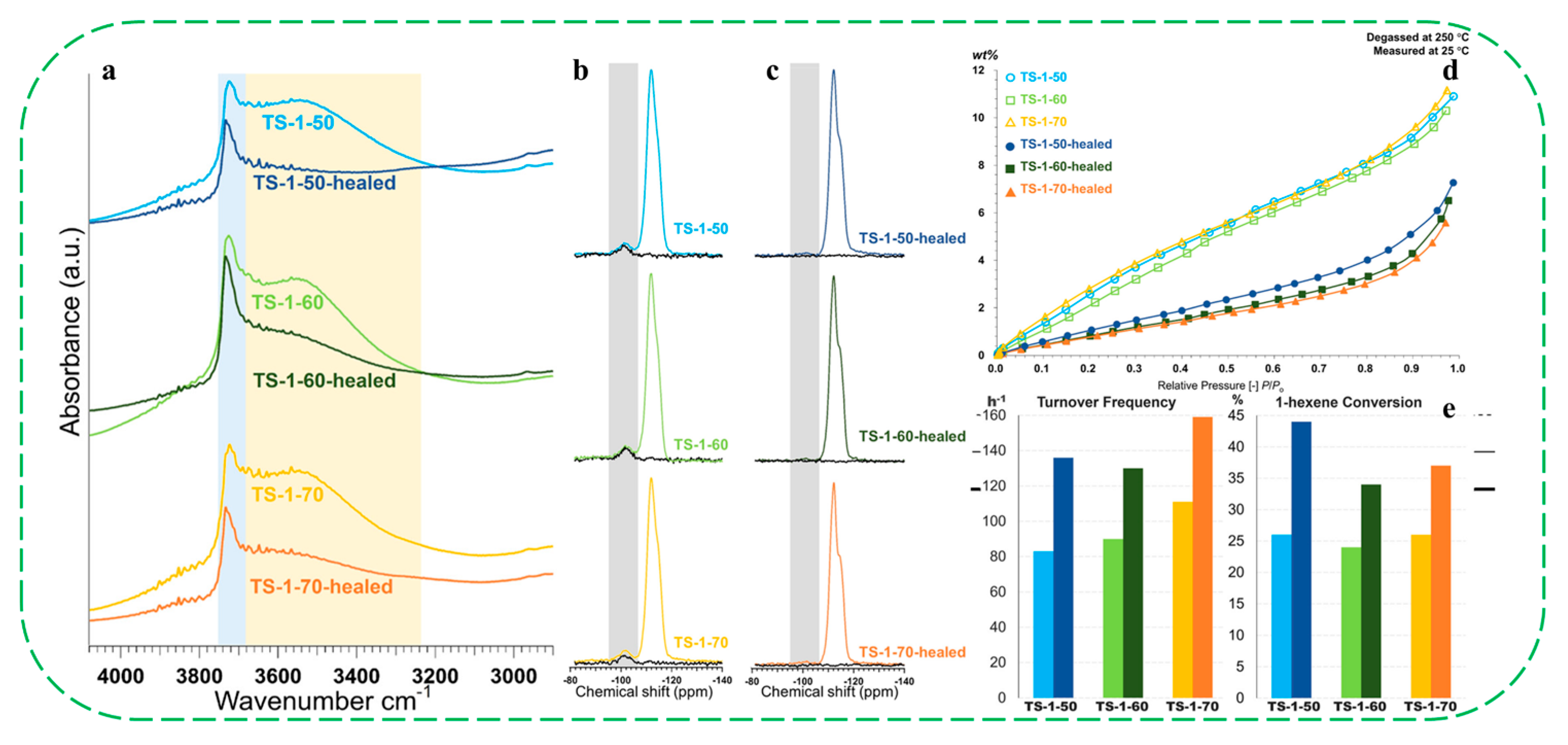

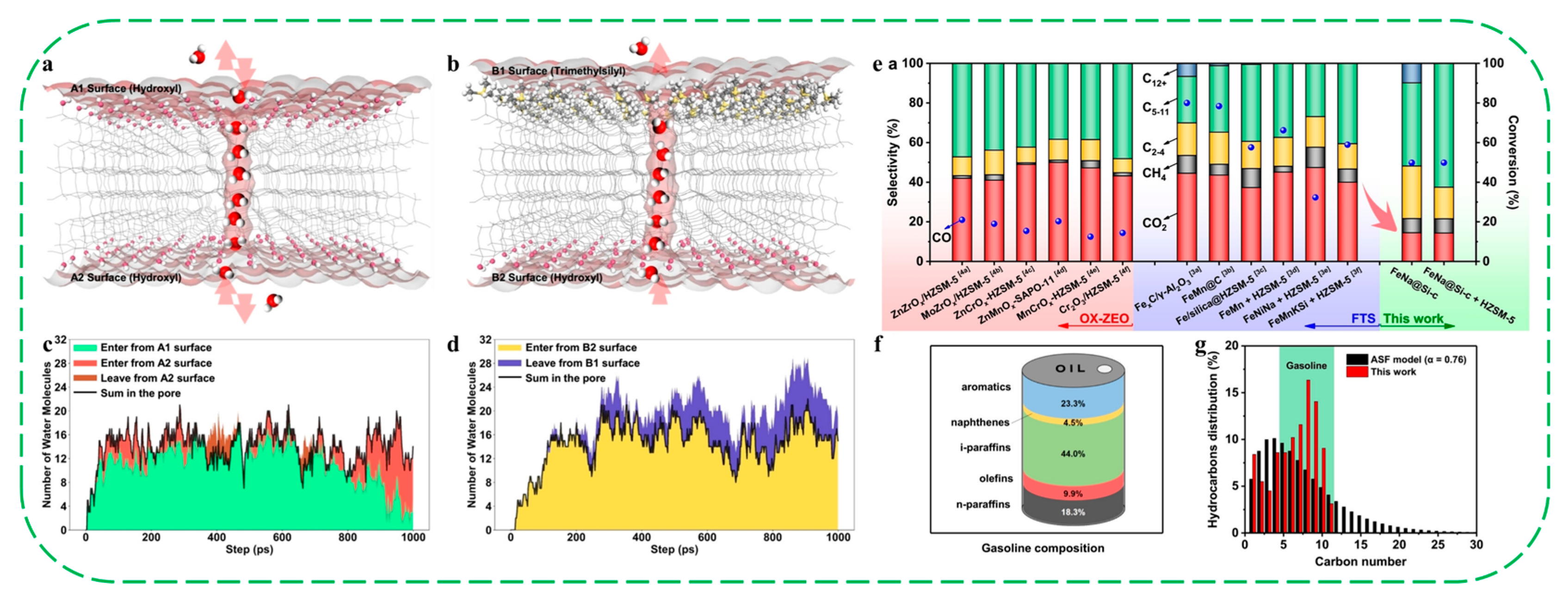
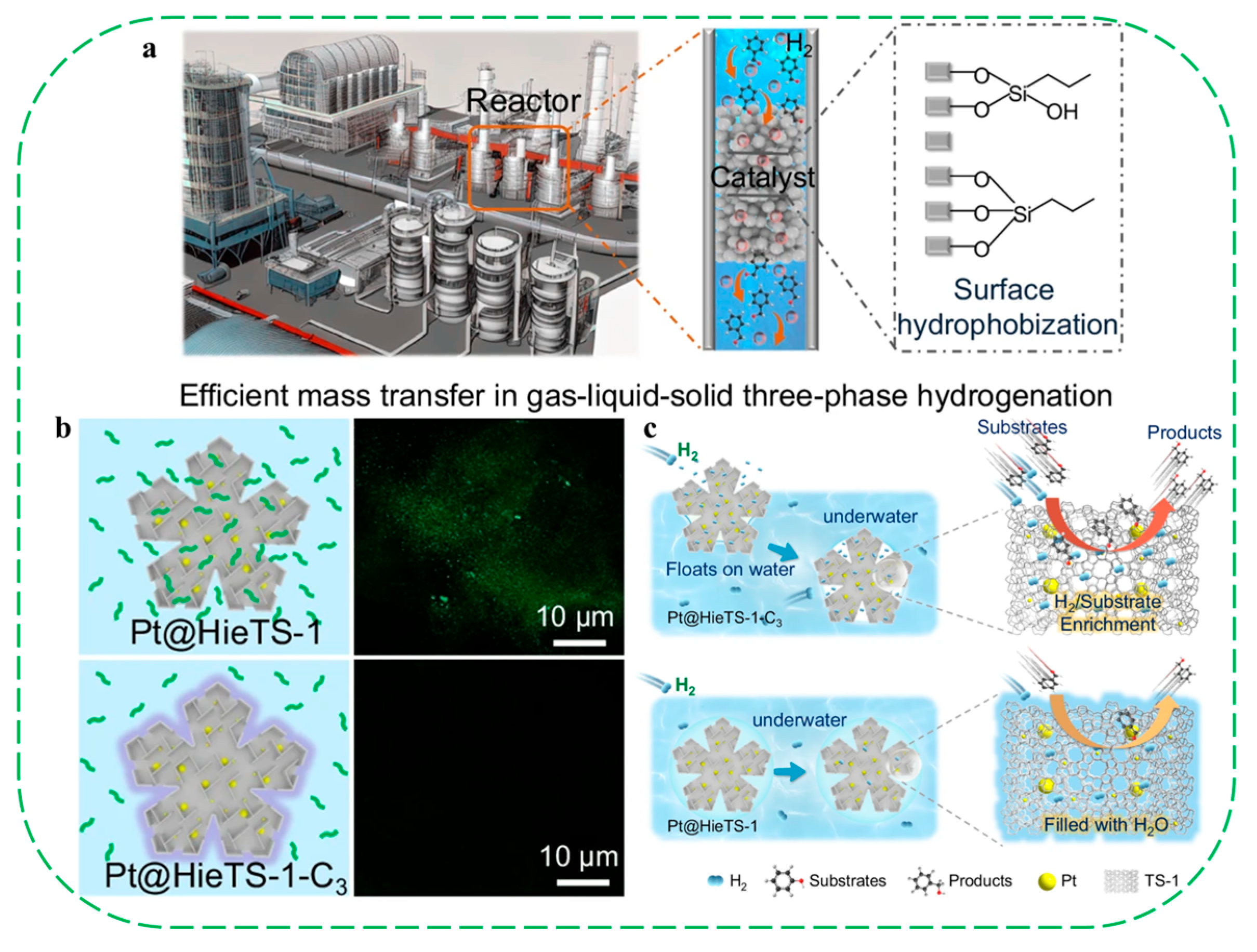

| Catalysts | HF a | Carbon Deposition (%) b | Stability (h) | Surface Hydroxyl Groups (Q3/Q4,%) c | PO Selectivity (%) d |
|---|---|---|---|---|---|
| Au/MTS-1-8 | 0.056 | 5.65 | 6 | 14.68 | ~60 |
| Au/MTS-1-10 | 0.067 | 3.52 | 13 | 11.18 | ~60 |
| Au/MTS-1-12 | 0.074 | 3.10 | 17 | 9.15 | ~78 |
| Au/MTS-1-16 | 0.084 | 1.94 | 25 | 8.92 | ~90 |
| Catalyst | Si/Sn b | Cover. (%) c | Lactone sel. (%) | TOF (h–1) d | STY (h–1) e | Regeneration Methods | Cycles | Cover. (%) f | Activity Retention (%) g |
|---|---|---|---|---|---|---|---|---|---|
| blank | - | 0 | - | 0 | 0 | - | - | - | - |
| Beta–Re | ∞ | 0 | - | 0 | 0 | - | - | - | - |
| SnO2/Beta–Re | 30 | 0.3 | 91.1 | 0.4 | 0.04 | - | - | - | - |
| Sn–Beta–Re-150 | 152 | 26.9 | 99.5 | 195 | 3.6 | - | - | - | - |
| Sn–Beta–Re-60 | 62 | 49.7 | 99.3 | 147 | 6.7 | - | - | - | - |
| Sn–Beta–Re-30 | 33 | 61.6 | 99.6 | 98 | 8.3 | calcination | 4 | ~59 | 96 |
| Sn–Beta–F-150 | 153 | 16.6 | 99.1 | 123 | 2.2 | calcination | 4 | ~15 | 90 |
| Sn–Beta–GPS-36 | 36 | 48.6 | 99.5 | 84 | 6.4 | calcination | 4 | ~38 | 78 |
| Sn–Beta–SSIE-30 | 30 | 47.3 | 99.3 | 73 | 6.2 | calcination | 4 | ~34 | 72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, J.; Zhang, C.; Zhang, Y.; Jia, M. Optimizing the Hydrophobic Environment of Zeolite-Based Catalysts for Promoted Application in Heterogeneous Catalysis. Molecules 2025, 30, 3670. https://doi.org/10.3390/molecules30183670
Zhan J, Zhang C, Zhang Y, Jia M. Optimizing the Hydrophobic Environment of Zeolite-Based Catalysts for Promoted Application in Heterogeneous Catalysis. Molecules. 2025; 30(18):3670. https://doi.org/10.3390/molecules30183670
Chicago/Turabian StyleZhan, Junling, Chi Zhang, Yu Zhang, and Mingjun Jia. 2025. "Optimizing the Hydrophobic Environment of Zeolite-Based Catalysts for Promoted Application in Heterogeneous Catalysis" Molecules 30, no. 18: 3670. https://doi.org/10.3390/molecules30183670
APA StyleZhan, J., Zhang, C., Zhang, Y., & Jia, M. (2025). Optimizing the Hydrophobic Environment of Zeolite-Based Catalysts for Promoted Application in Heterogeneous Catalysis. Molecules, 30(18), 3670. https://doi.org/10.3390/molecules30183670








