Construction of Hierarchical Fe-MFI Nanosheets with Enhanced Fenton-like Degradation Performance
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis of the Fe-MFI Nanosheets with Different b-Axis Thicknesses
2.2. Fenton-like Oxidation Degradation of Rhodamine B by Fe-x Catalysts
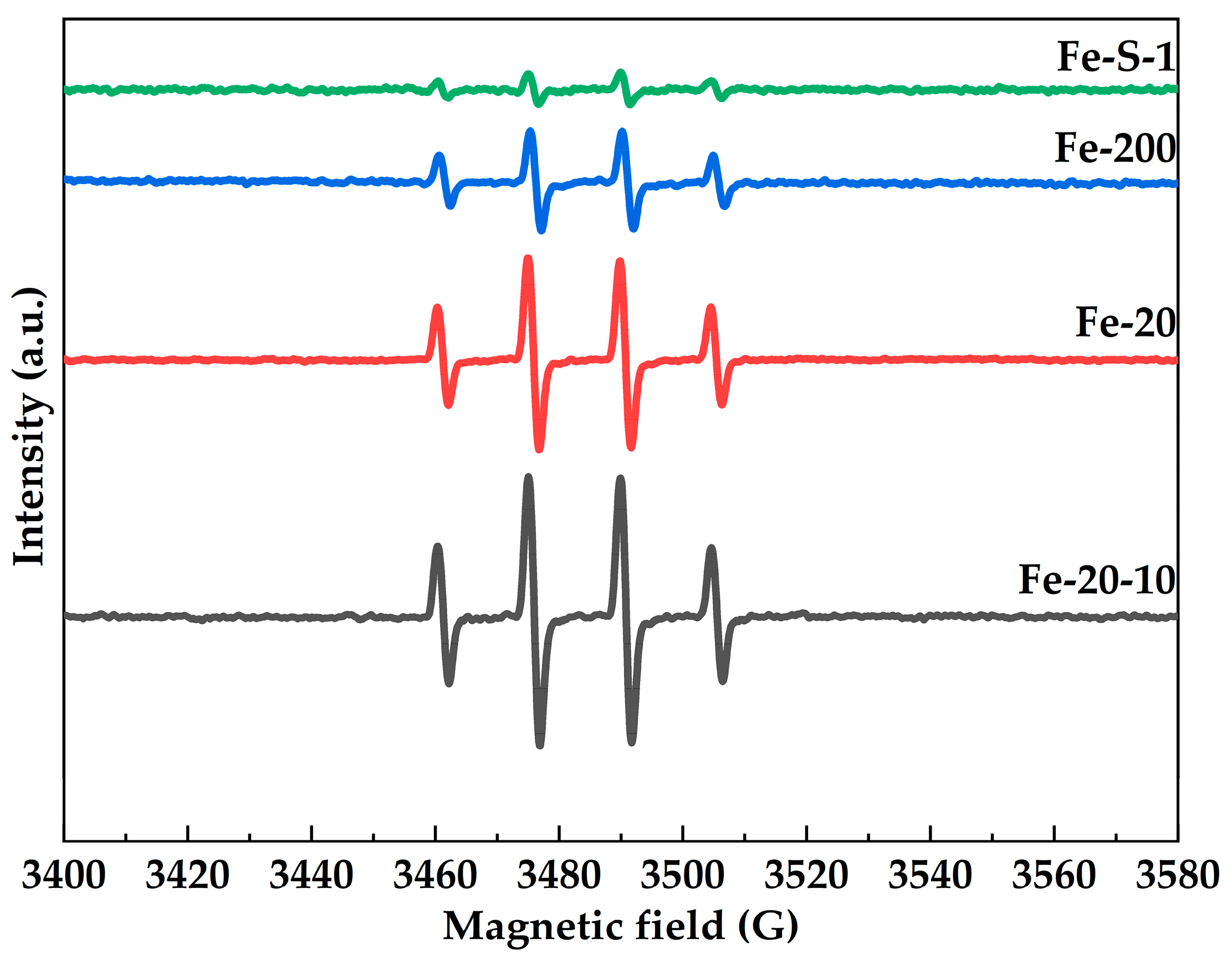
2.3. Synthesis of the Hierarchical Fe-MFI Nanosheets
2.4. Fenton-like Oxidation Degradation of Rhodamine B by Hierarchical of Fe-MFI Nanosheets
2.5. Identifying of the Most Efficient Active Sites and Possible Reaction Process
3. Materials and Methods
3.1. Materials
3.2. Preparation of Seed Suspension
3.3. Preparation of Fe-MFI Nanosheets with Different Thicknesses
3.4. Preparation of Hierarchical Fe-MFI Nanosheets
3.5. Characterizations Instruments
3.6. Heterogenous Fenton-like Degradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shanker, U.; Rani, M.; Jassal, V. Degradation of Hazardous Organic Dyes in Water by Nanomaterials. Environ. Chem. Lett. 2017, 15, 623–642. [Google Scholar] [CrossRef]
- Caliman, F.A.; Gavrilescu, M. Pharmaceuticals, Personal Care Products and Endocrine Disrupting Agents in the Environment—A Review. CLEAN Soil Air Water 2009, 37, 277–303. [Google Scholar] [CrossRef]
- Chadelaud, T.; Zeghioud, H.; Reynoso De La Garza, A.; Fuerte, O.; Benítez-Rico, A.; Revel, M.; Chávez-Miyauchi, T.E.; Djelal, H. Comparative Study of Rhodamine B Treatment: Assessing of Efficiency Processes and Ecotoxicity of By-Products. Processes 2023, 11, 2671. [Google Scholar] [CrossRef]
- Akinnawo, S.O.; Ayadi, P.O.; Oluwalope, M.T. Chemical Coagulation and Biological Techniques for Wastewater Treatment. Ovidius Univ. Ann. Chem. 2023, 34, 14–21. [Google Scholar] [CrossRef]
- Solayman, H.M.; Hossen, M.A.; Abd Aziz, A.; Yahya, N.Y.; Leong, K.H.; Sim, L.C.; Monir, M.U.; Zoh, K.-D. Performance Evaluation of Dye Wastewater Treatment Technologies: A Review. J. Environ. Chem. Eng. 2023, 11, 109610. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Liang, D.; Li, N.; An, J.; Ma, J.; Wu, Y.; Liu, H. Fenton-Based Technologies as Efficient Advanced Oxidation Processes for Microcystin-LR Degradation. Sci. Total Environ. 2021, 753, 141809. [Google Scholar] [CrossRef] [PubMed]
- Villota, N.; Duoandicoechea, U.; Lombraña, J.I.; De Luis, A.M. Kinetic Modelling of Aromaticity and Colour Changes during the Degradation of Sulfamethoxazole Using Photo-Fenton Technology. Catalysts 2024, 14, 718. [Google Scholar] [CrossRef]
- Zhang, M.; Dong, H.; Zhao, L.; Wang, D.; Meng, D. A Review on Fenton Process for Organic Wastewater Treatment Based on Optimization Perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef]
- Hussain, S.; Aneggi, E.; Goi, D. Catalytic Activity of Metals in Heterogeneous Fenton-like Oxidation of Wastewater Contaminants: A Review. Environ. Chem. Lett. 2021, 19, 2405–2424. [Google Scholar] [CrossRef]
- Fajardo-Puerto, E.; Elmouwahidi, A.; Amaro-Gahete, J.; Pérez-Cadenas, M.; Bailón-García, E.; Pérez-Cadenas, A.F.; Carrasco-Marín, F. Bifunctional Catalysts Based on Carbon-Coated Manganese Microspheres Applied in the Heterogeneous Electro-Fenton Process for Tetracycline Degradation. J. Environ. Chem. Eng. 2025, 13, 115725. [Google Scholar] [CrossRef]
- Wang, J.; Tang, J. Fe-Based Fenton-like Catalysts for Water Treatment: Preparation, Characterization and Modification. Chemosphere 2021, 276, 130177. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ding, J.; Zhong, Q. Catalytic Decomposition of H2O2 over Fe-Based Catalysts for Simultaneous Removal of NOX and SO2. Appl. Surf. Sci. 2015, 326, 66–72. [Google Scholar] [CrossRef]
- Barranco-López, A.; Moral-Rodríguez, A.I.; Fajardo-Puerto, E.; Elmouwahidi, A.; Bailón-García, E. Highly Graphitic Fe-Doped Carbon Xerogels as Dual-Functional Electro-Fenton Catalysts for the Degradation of Tetracycline in Wastewater. Environ. Res. 2023, 228, 115757. [Google Scholar] [CrossRef]
- Zhang, F.; Ke, R.; Liu, M.; Zhang, X.; Wang, Y.; Wang, Y. Improved Electrocatalytic Performance of Fe/CeO2 Bifunctional Electrocatalyst by Simultaneous H2O2 in-Situ Generation and Activation. Chem. Eng. J. Adv. 2022, 9, 100231. [Google Scholar] [CrossRef]
- González-Bahamón, L.F.; Hoyos, D.F.; Benítez, N.; Pulgarín, C. New Fe-Immobilized Natural Bentonite Plate Used as Photo-Fenton Catalyst for Organic Pollutant Degradation. Chemosphere 2011, 82, 1185–1189. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, M.; Hu, S.; Wang, L.; Yao, H. Characteristics and Mechanisms of 4A Zeolite Supported Nanoparticulate Zero-Valent Iron as Fenton-like Catalyst to Degrade Methylene Blue. Toxicol. Environ. Chem. 2014, 96, 227–242. [Google Scholar] [CrossRef]
- Chen, J. Immobilisation of Iron-Containing Materials onto Supporting Materials in Heterogeneous Fenton System: A Review. Adv. Mater. Res. 2014, 955–959, 569–580. [Google Scholar] [CrossRef]
- Ramírez-Valencia, L.D.; Bailón-García, E.; Carrasco-Marín, F.; Álvarez-Merino, M.A.; Pérez-Cadenas, A.F. Advanced Fe-Doped Carbon Xerogels as Bifunctional Electro-Catalysts for Targeted Hydroxyl Radical Production and Superior Electro-Fenton Pollutant Removal in Water. Chem. Eng. J. 2025, 519, 165565. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, H.; Kang, L.; Gao, Z.; Ren, F. Fe-Based Metal-Organic Frameworks as Fenton-like Catalysts for Highly Efficient Degradation of Tetracycline Hydrochloride over a Wide pH Range: Acceleration of Fe(II)/ Fe(III) Cycle under Visible Light Irradiation. Appl. Catal. B Environ. 2020, 263, 118282. [Google Scholar] [CrossRef]
- Saifuddin, M.; Bae, J.; Kim, K.S. Role of Fe, Na and Al in Fe-Zeolite-A for Adsorption and Desorption of Phosphate from Aqueous Solution. Water Res. 2019, 158, 246–256. [Google Scholar] [CrossRef]
- Simancas, R.; Chokkalingam, A.; Elangovan, S.P.; Liu, Z.; Sano, T.; Iyoki, K.; Wakihara, T.; Okubo, T. Recent Progress in the Improvement of Hydrothermal Stability of Zeolites. Chem. Sci. 2021, 12, 7677–7695. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Zhang, X.; Lv, G.; Zhai, Y.; Yang, Z.; Zhu, Y.; Li, H.; Wang, F. Influence of Zeolite Carriers on the Dyes Degradation for Framework Fe-Doped Zeolite Catalysts. J. Sol-Gel Sci. Technol. 2019, 91, 54–62. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, Y.; Zhang, H.; Wu, X. Catalytic Wet Peroxide Oxidation of Phenol on Fe-ZSM-5/PSSF Membrane Catalysts: Effect of Framework Fe by Hydrothermal Synthesis. Sep. Purif. Technol. 2020, 237, 116452. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Z.; Xiong, J.; Yu, Z.; Lu, X. Metal Stabilization of Metal-Supported Catalysts: Anchoring Strategies and Catalytic Applications in Carbon Resources Conversion. ChemCatChem 2025, 17, e00182. [Google Scholar] [CrossRef]
- Sashkina, K.A.; Labko, V.S.; Rudina, N.A.; Parmon, V.N.; Parkhomchuk, E.V. Hierarchical Zeolite FeZSM-5 as a Heterogeneous Fenton-Type Catalyst. J. Catal. 2013, 299, 44–52. [Google Scholar] [CrossRef]
- Wei, Y.; Feng, J.; Guan, B.; Yu, J. Structural Engineering of Hierarchical Zeolite-Based Catalysts. Acc. Mater. Res. 2024, 5, 857–871. [Google Scholar] [CrossRef]
- Ji, Y.; Xu, F.; Wei, W.; Gao, H.; Zhang, K.; Zhang, G.; Xu, Y.; Zhang, P. Efficient and Fast Adsorption of Methylene Blue Dye onto a Nanosheet MFI Zeolite. J. Solid State Chem. 2021, 295, 121917. [Google Scholar] [CrossRef]
- Roth, W.J.; Gil, B.; Tarach, K.A.; Góra-Marek, K. Top-down Engineering of Zeolite Porosity. Chem. Soc. Rev. 2025, 54, 7484–7560. [Google Scholar] [CrossRef]
- Meng, L.; Zhu, X.; Hensen, E.J.M. Stable Fe/ZSM-5 Nanosheet Zeolite Catalysts for the Oxidation of Benzene to Phenol. ACS Catal. 2017, 7, 2709–2719. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, Y.; Wu, Q.; Wen, Y.; Xiao, F.-S. Zeolite Nanosheets for Catalysis. Chem. Soc. Rev. 2022, 51, 2431–2443. [Google Scholar] [CrossRef]
- Yabushita, M.; Kobayashi, H.; Osuga, R.; Nakaya, M.; Matsubara, M.; Maki, S.; Kanie, K.; Muramatsu, A. Mechanochemical Approach to Preparation of MFI Zeolites Substituted Isomorphously by Both Al and Fe as Durable Catalysts for the Dimethyl Ether to Olefin Reaction. Ind. Eng. Chem. Res. 2021, 60, 2079–2088. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, L.; Zhou, A.; Li, W.; Shang, S.; Liu, Y.; Jia, Z.; Liu, W.; Zhang, A.; Guo, X.; et al. Tailored Synthesis of ZSM-5 Nanosheets with Controllable b -Axis Thickness and Aspect Ratio: Strategy and Growth Mechanism. Chem. Mater. 2022, 34, 3217–3226. [Google Scholar] [CrossRef]
- Verheyen, E.; Jo, C.; Kurttepeli, M.; Vanbutsele, G.; Gobechiya, E.; Korányi, T.I.; Bals, S.; Van Tendeloo, G.; Ryoo, R.; Kirschhock, C.E.A.; et al. Molecular Shape-Selectivity of MFI Zeolite Nanosheets in n-Decane Isomerization and Hydrocracking. J. Catal. 2013, 300, 70–80. [Google Scholar] [CrossRef]
- Kim, M.S.; Park, E.D. Aqueous-Phase Partial Oxidation of Methane with H2O2 over Fe-ZSM-5 Catalysts Prepared from Different Iron Precursors. Microporous Mesoporous Mater. 2021, 324, 111278. [Google Scholar] [CrossRef]
- Naghmash, M.A.; Saif, M.; Mahmoud, H.R. Transition Metal Ions Doped Bi12SiO20 as Novel Catalysts for the Decomposition of Hydrogen Peroxide (H2O2). J. Taiwan Inst. Chem. Eng. 2021, 121, 268–275. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Cai, P.; Wen, Z. An Electrochemically Neutralized Energy-Assisted Low-Cost Acid-Alkaline Electrolyzer for Energy-Saving Electrolysis Hydrogen Generation. J. Mater. Chem. A 2018, 6, 4948–4954. [Google Scholar] [CrossRef]
- Kolesnichenko, N.V.; Pavlov, V.S.; Stashenko, A.N.; Yashina, O.V.; Ezhova, N.N.; Konnov, S.V.; Khadzhiev, S.N. Dimethyl Ether Conversion to Olefins in a Slurry Reactor: The Effect of MFI Zeolite Catalyst Acidity and Selectivity Control. React. Kinet. Mech. Catal. 2018, 124, 825–838. [Google Scholar] [CrossRef]
- Qin, Z.; Hafiz, L.; Shen, Y.; Daele, S.V.; Boullay, P.; Ruaux, V.; Mintova, S.; Gilson, J.-P.; Valtchev, V. Defect-Engineered Zeolite Porosity and Accessibility. J. Mater. Chem. A 2020, 8, 3621–3631. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, X.; Yi, H.; Yu, Q.; Zhang, Y.; Wei, J.; Yuan, Y. Synthesis, Characterization and Application of Fe-Zeolite: A Review. Appl. Catal. A Gen. 2022, 630, 118467. [Google Scholar] [CrossRef]
- Su, X.; Hu, Z.-P.; Han, J.; Wei, Y.; Liu, Z. Selective Incorporation of Iron Sites into MFI Zeolite Framework by One-Pot Synthesis. Cryst. Growth Des. 2023, 23, 2644–2651. [Google Scholar] [CrossRef]
- Rajendran, C.; Thirumoorthy, K.; Satishkumar, G.; Landau, M.V. Alumina as Solid-State Ligand in Enhancing the Redox Catalytic Property of Iron Oxide Grafted AlSBA-15 towards Arylation of Arene. ChemCatChem 2018, 10, 4768–4776. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J. Metal Organic Framework with Coordinatively Unsaturated Sites as Efficient Fenton-like Catalyst for Enhanced Degradation of Sulfamethazine. Environ. Sci. Technol. 2018, 52, 5367–5377. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, X.; Wu, X.; Kang, Y.; Huang, Y.; Zhao, Z.; Li, Z.; Zhou, T.; Wang, J.; Zhu, W.; et al. Enhanced Photo-Fenton Catalysis via Bandgap Engineering of Metalloporphyrin-Based Covalent Organic Framework Shells on Bimetallic Metal–Organic Frameworks: Accelerating Fe(iii)/Fe(ii) Loop Activation. J. Mater. Chem. A 2025, 13, 9952–9962. [Google Scholar] [CrossRef]
- Li, M.; Song, J.; Han, W.; Yeung, K.L.; Zhou, S.; Mo, C.-H. Iron-Organic Frameworks as Effective Fenton-like Catalysts for Peroxymonosulfate Decomposition in Advanced Oxidation Processes. NPJ Clean Water 2023, 6, 37. [Google Scholar] [CrossRef]
- Segura, Y.; Martínez, F.; Melero, J.A.; Molina, R.; Chand, R.; Bremner, D.H. Enhancement of the Advanced Fenton Process (Fe0/H2O2) by Ultrasound for the Mineralization of Phenol. Appl. Catal. B Environ. 2012, 113–114, 100–106. [Google Scholar] [CrossRef]
- Zhu, Q.; Yan, J.; Dai, Q.; Wu, Q.; Cai, Y.; Wu, J.; Wang, X.; Zhan, W. Ethylene Glycol Assisted Synthesis of Hierarchical Fe-ZSM-5 Nanorods Assembled Microsphere for Adsorption Fenton Degradation of Chlorobenzene. J. Hazard. Mater. 2020, 385, 121581. [Google Scholar] [CrossRef]
- Magomedova, A.; Isaev, A.; Orudzhev, F.; Sobola, D.; Murtazali, R.; Rabadanova, A.; Shabanov, N.S.; Zhu, M.; Emirov, R.; Gadzhimagomedov, S.; et al. Magnetically Separable Mixed-Phase α/γ-Fe2O3 Catalyst for Photo-Fenton-like Oxidation of Rhodamine B. Catalysts 2023, 13, 872. [Google Scholar] [CrossRef]
- Corrêa, C.R.R.; De Siqueira, A.B.; Matos Lopes, P.R.; Ambrosio, J.A.R.; Simioni, A.R.; De Vasconcelos, L.G.; De Morais, E.B. Green Synthesis of Iron Nanoparticles from the Baru (Dipteryx alata) Endocarp Extract for the Efficient Removal of Rhodamine B and Caffeine from Water through the Heterogeneous Fenton Process. AQUA—Water Infrastruct. Ecosyst. Soc. 2024, 73, 771–789. [Google Scholar] [CrossRef]
- Oliveira Aguiar Perez, J.; Tuono Martins Xavier, G.; Felix Bitencourt, G.; Dos Santos Andrade, L.; Alves Carvalho, W.; Jesus Olortiga Asencios, Y. Fe-Supported Perlite Applied for the Degradation of Rhodamine B Dye by Heterogeneous Fenton Reaction. JSSE 2025, 2, 14. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.-Q.; Xu, H.-Y.; Dong, L.-M.; Cao, M.-C.; Shan, L.-W.; Jin, L.-G.; He, X.-L.; Qi, S.-Y. Comparative Studies on Fenton-like Reactions Catalyzed by Fe3O4 Loaded inside and Outside Halloysite Nanotubes for the Removal of Organic Pollutants. Front. Mater. Sci. 2024, 18, 240673. [Google Scholar] [CrossRef]
- Zhang, C.; Zeng, J.; Ouyang, Z.; Li, X.; Lin, L.; Peng, Y.; Gong, X. Reuse of Microalgae Residue after Oil Production as a Fenton-like Catalyst in Wastewater Treatment: Catalytic Performance and Mechanism. J. Water Process Eng. 2023, 55, 104092. [Google Scholar] [CrossRef]
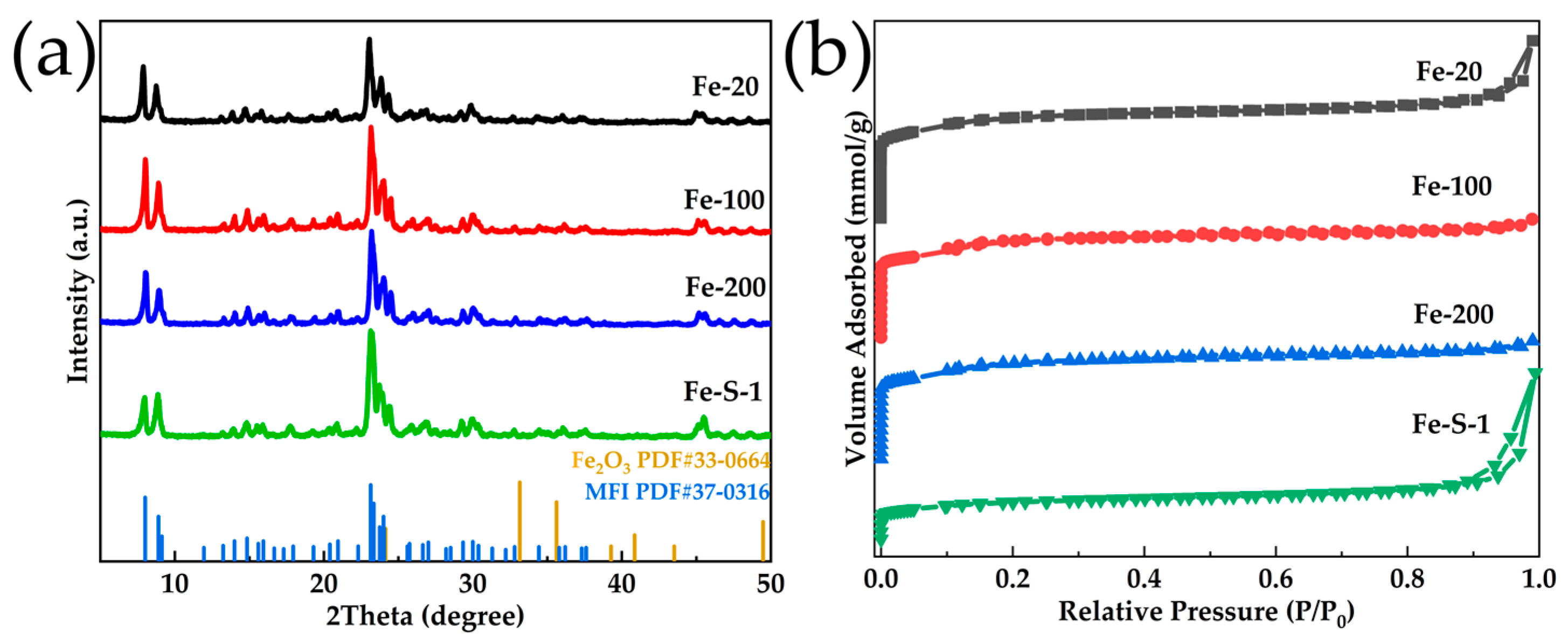
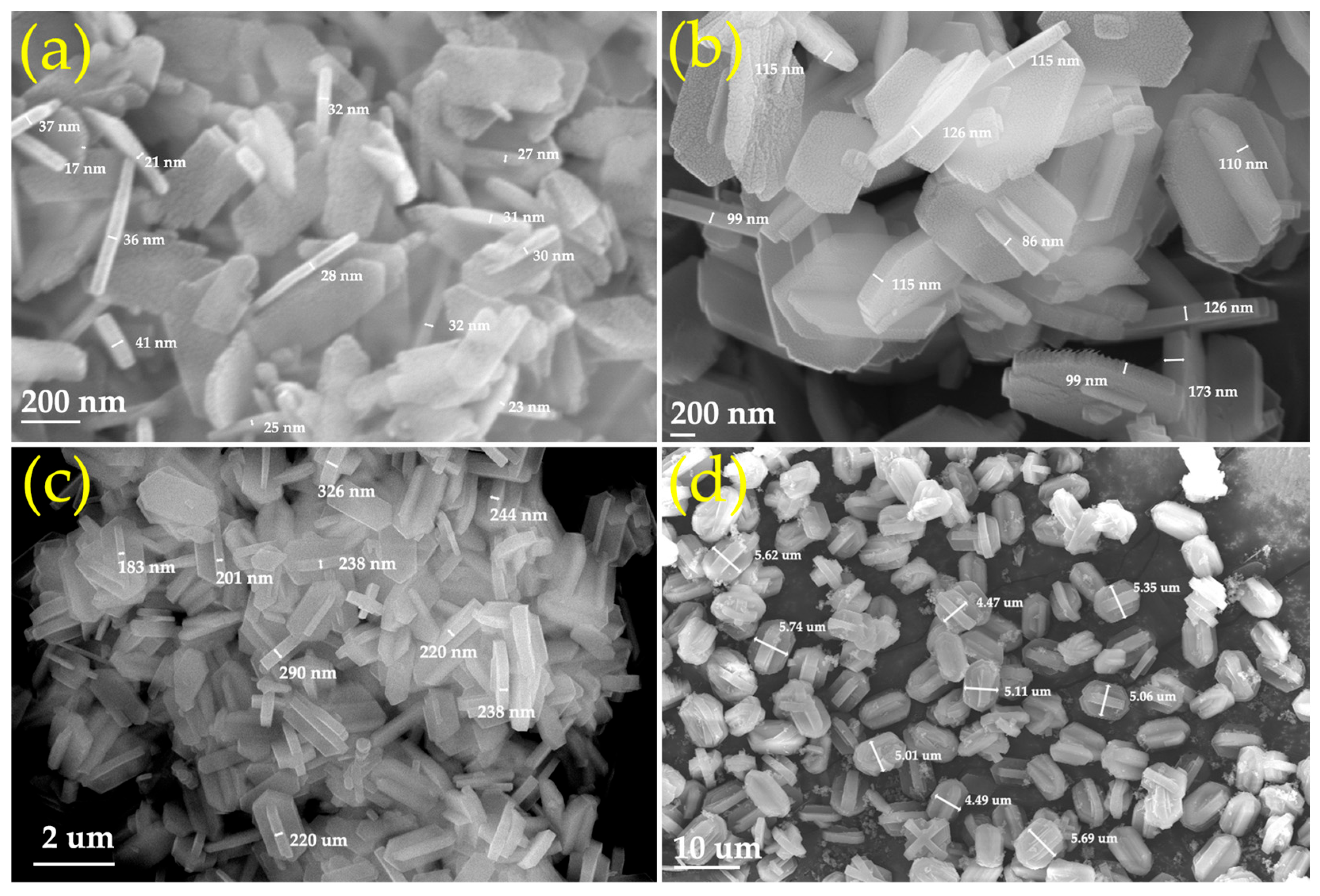
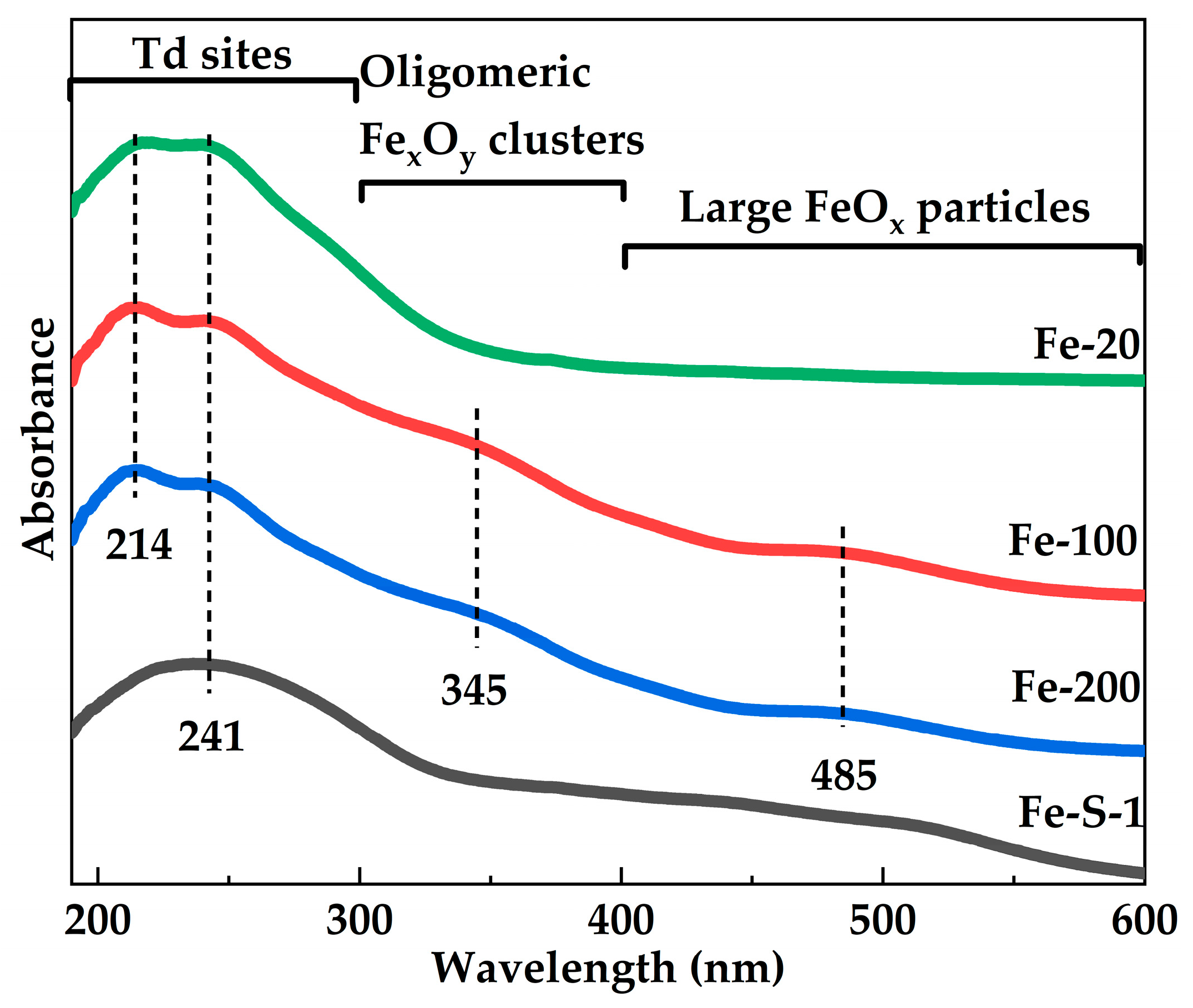

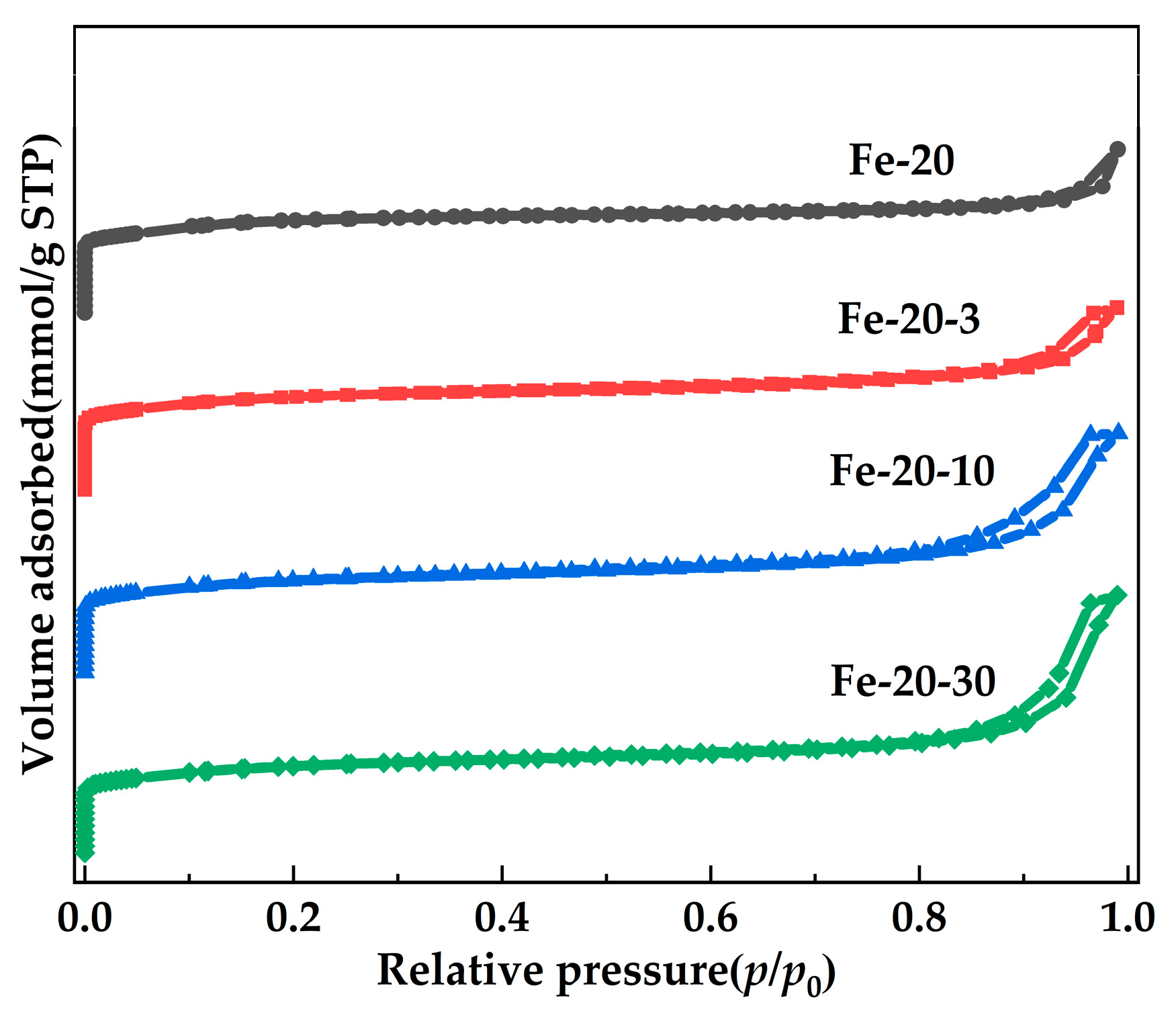
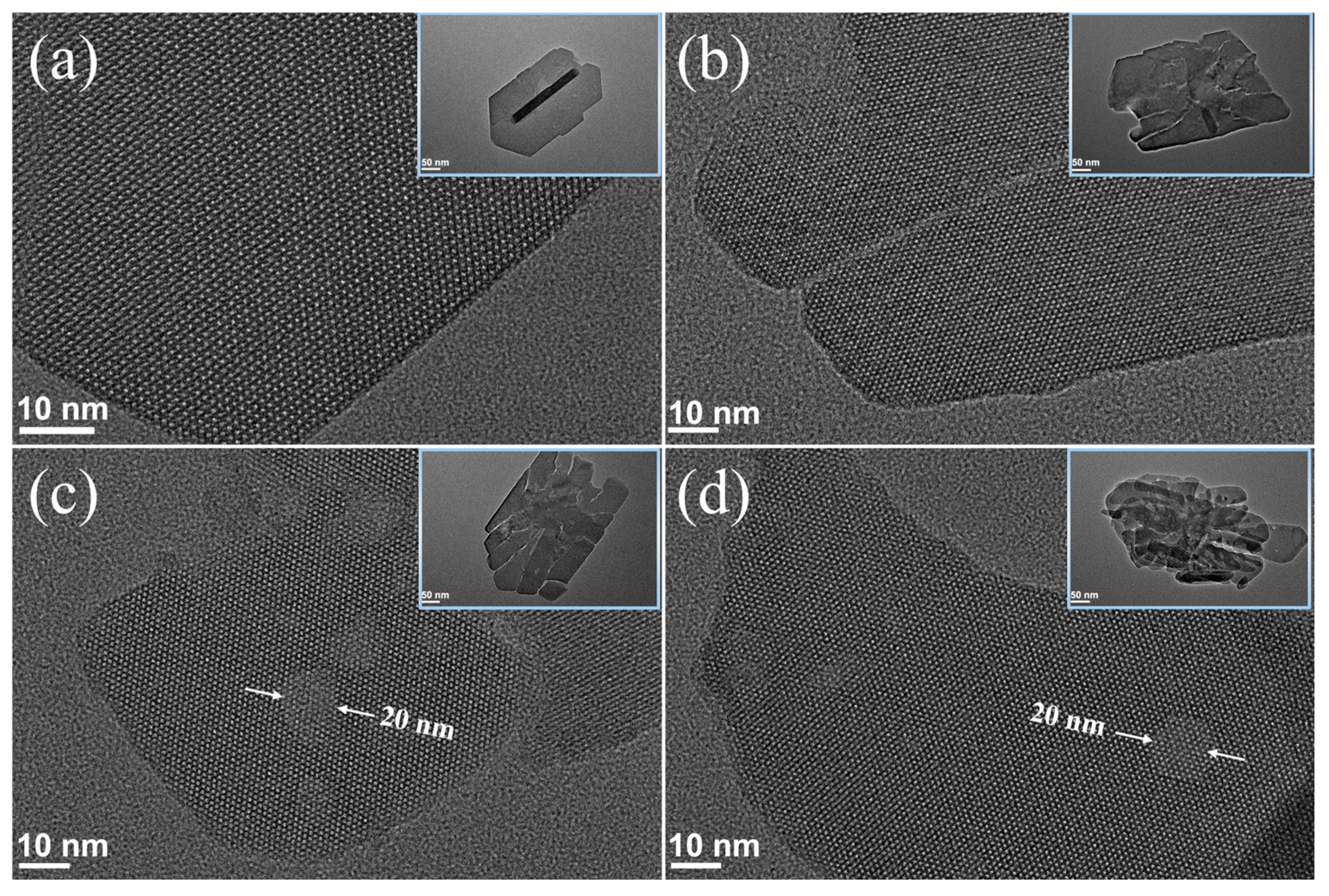
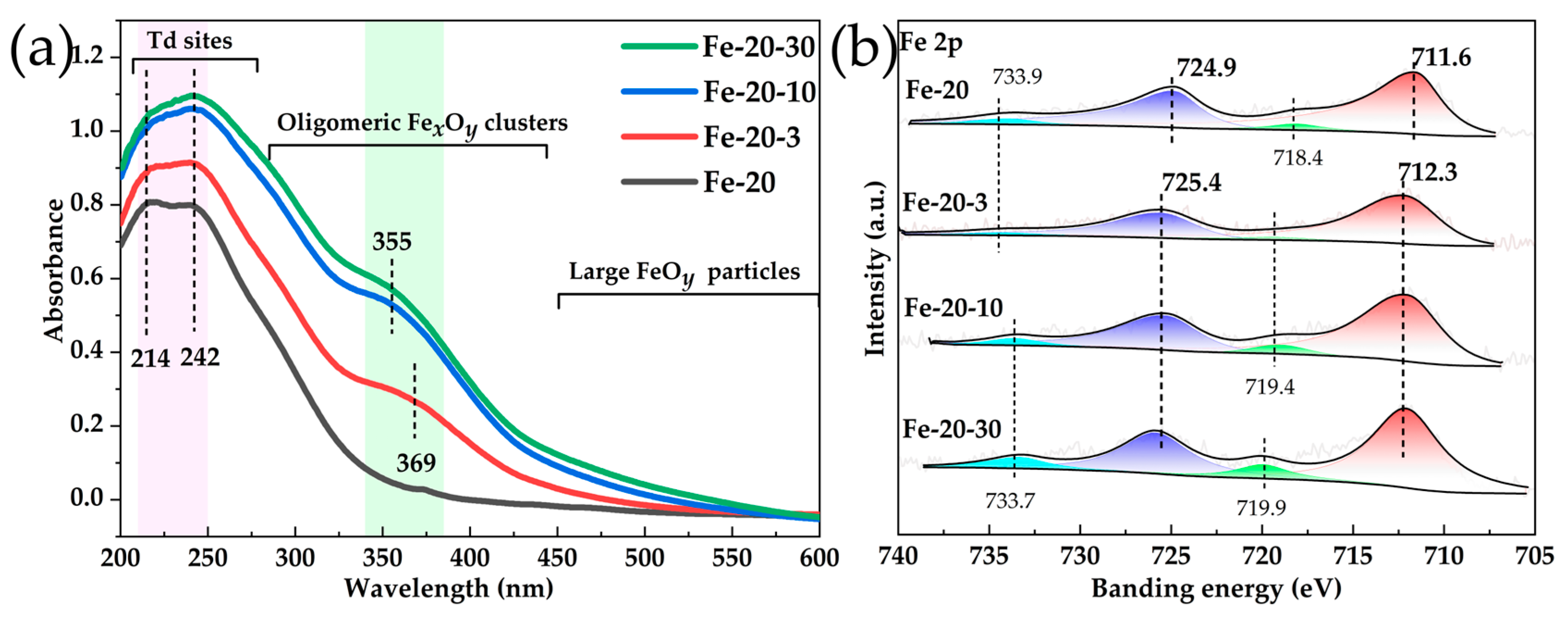
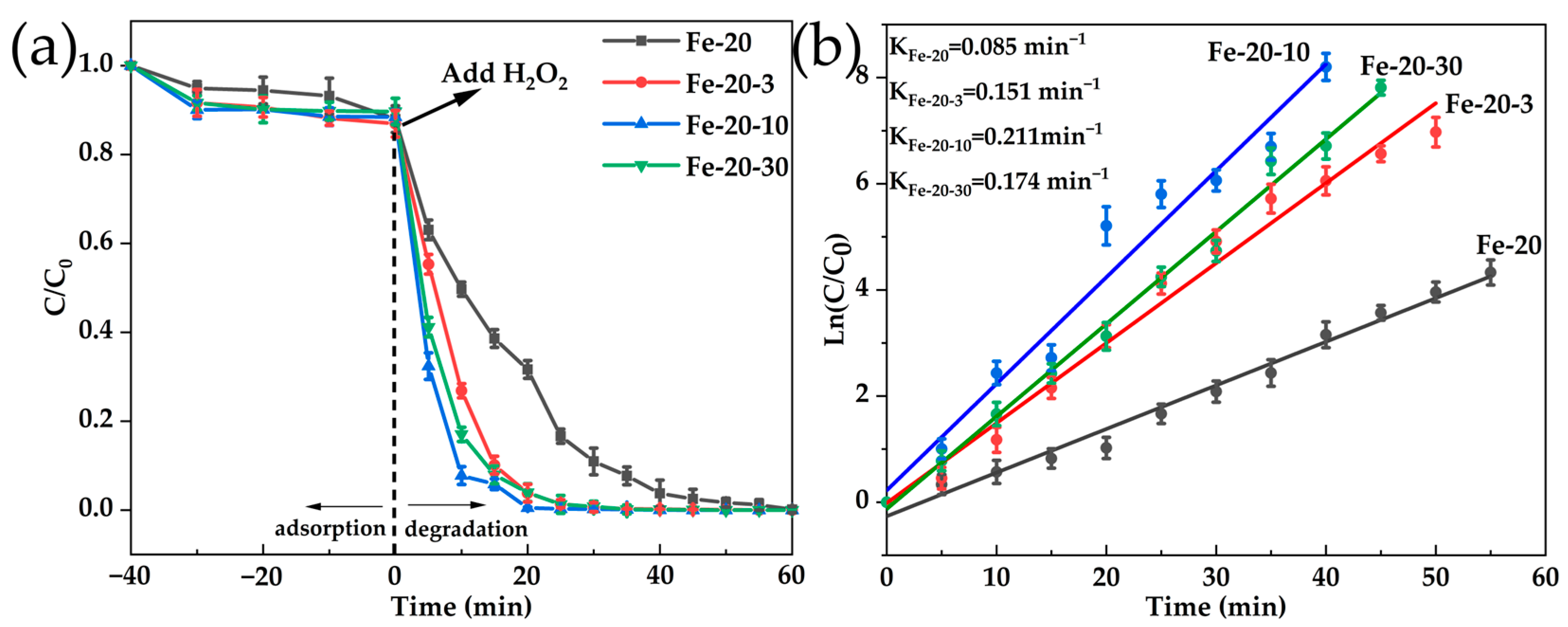
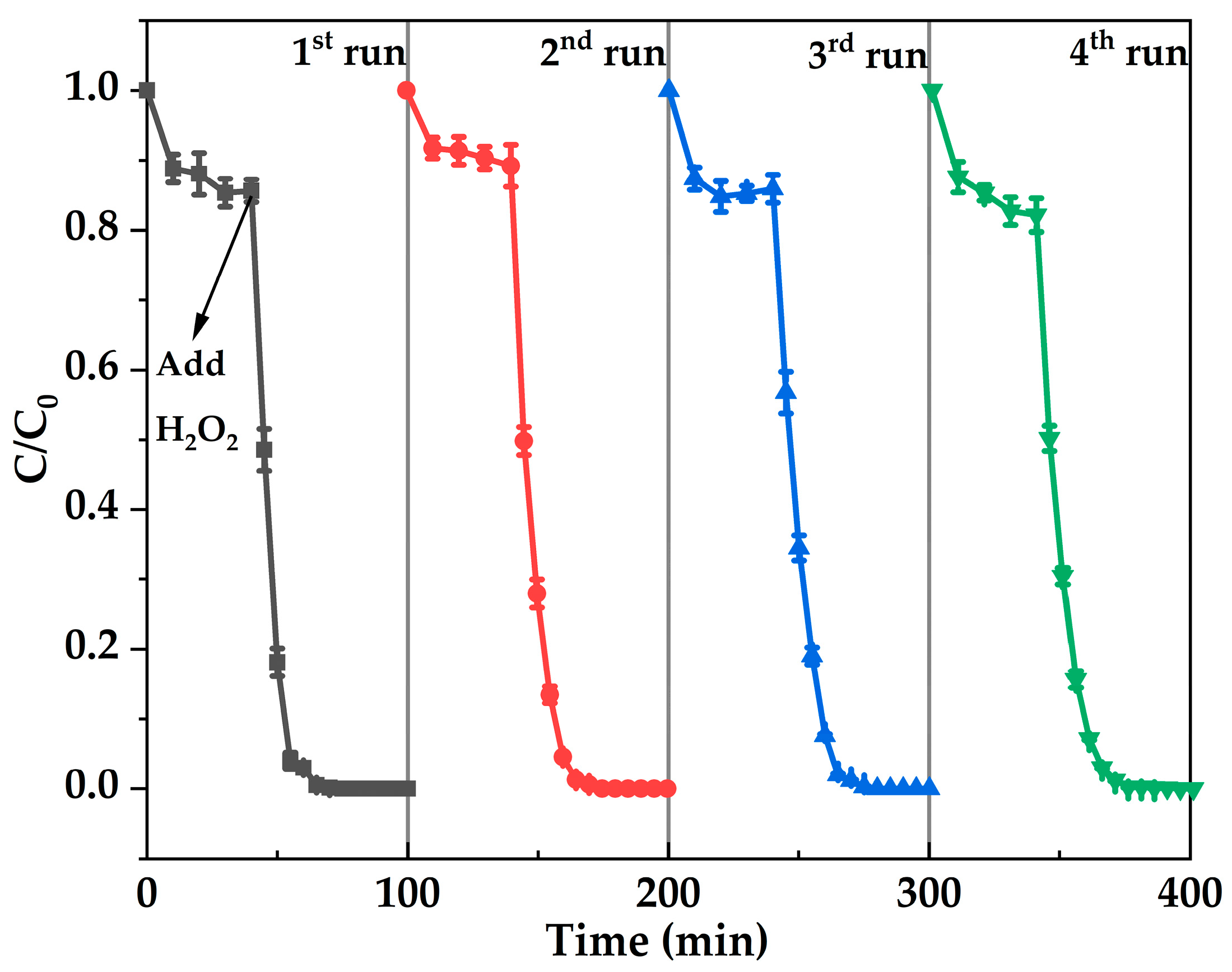

| Sample | Si/Fe Ratio a | Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | |||
|---|---|---|---|---|---|---|
| Total b | Exter c | Total c | Micro c | Meso d | ||
| Fe-20 | 36 | 425 | 79 | 0.31 | 0.14 | 0.17 |
| Fe-100 | 36 | 396 | 89 | 0.21 | 0.13 | 0.08 |
| Fe-200 | 37 | 396 | 87 | 0.21 | 0.13 | 0.08 |
| Fe-S-1 | 32 | 171 | 56 | 0.28 | 0.05 | 0.23 |
| Sample | Si/Fe Ratio a | Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | |||
|---|---|---|---|---|---|---|
| Total b | Exter c | Total c | Micro c | Meso d | ||
| Fe-20 | 36 | 425 | 79 | 0.31 | 0.14 | 0.17 |
| Fe-20-3 | 31 | 426 | 96 | 0.34 | 0.13 | 0.21 |
| Fe-20-10 | 26 | 426 | 110 | 0.45 | 0.13 | 0.32 |
| Fe-20-30 | 25 | 404 | 104 | 0.48 | 0.12 | 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.; Xu, L.; Meng, Q.; Feng, X.; Wang, J.; Li, Y.; Li, J. Construction of Hierarchical Fe-MFI Nanosheets with Enhanced Fenton-like Degradation Performance. Molecules 2025, 30, 4030. https://doi.org/10.3390/molecules30194030
Jiang H, Xu L, Meng Q, Feng X, Wang J, Li Y, Li J. Construction of Hierarchical Fe-MFI Nanosheets with Enhanced Fenton-like Degradation Performance. Molecules. 2025; 30(19):4030. https://doi.org/10.3390/molecules30194030
Chicago/Turabian StyleJiang, Haibo, Lin Xu, Qingrun Meng, Xu Feng, Junxuan Wang, Yankai Li, and Junjie Li. 2025. "Construction of Hierarchical Fe-MFI Nanosheets with Enhanced Fenton-like Degradation Performance" Molecules 30, no. 19: 4030. https://doi.org/10.3390/molecules30194030
APA StyleJiang, H., Xu, L., Meng, Q., Feng, X., Wang, J., Li, Y., & Li, J. (2025). Construction of Hierarchical Fe-MFI Nanosheets with Enhanced Fenton-like Degradation Performance. Molecules, 30(19), 4030. https://doi.org/10.3390/molecules30194030







